A Bridge between the Breath and the Brain: Synchronization of Respiration, a Pupillometric Marker of the Locus Coeruleus, and an EEG Marker of Attentional Control State
Abstract
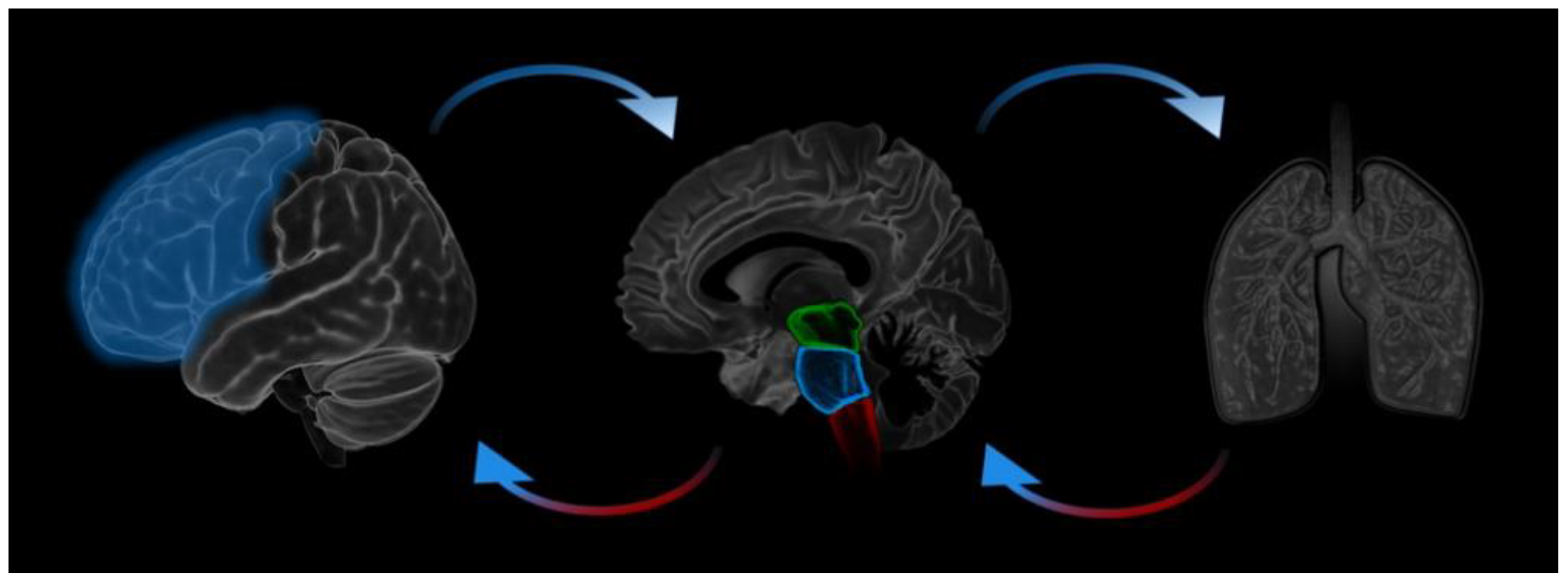
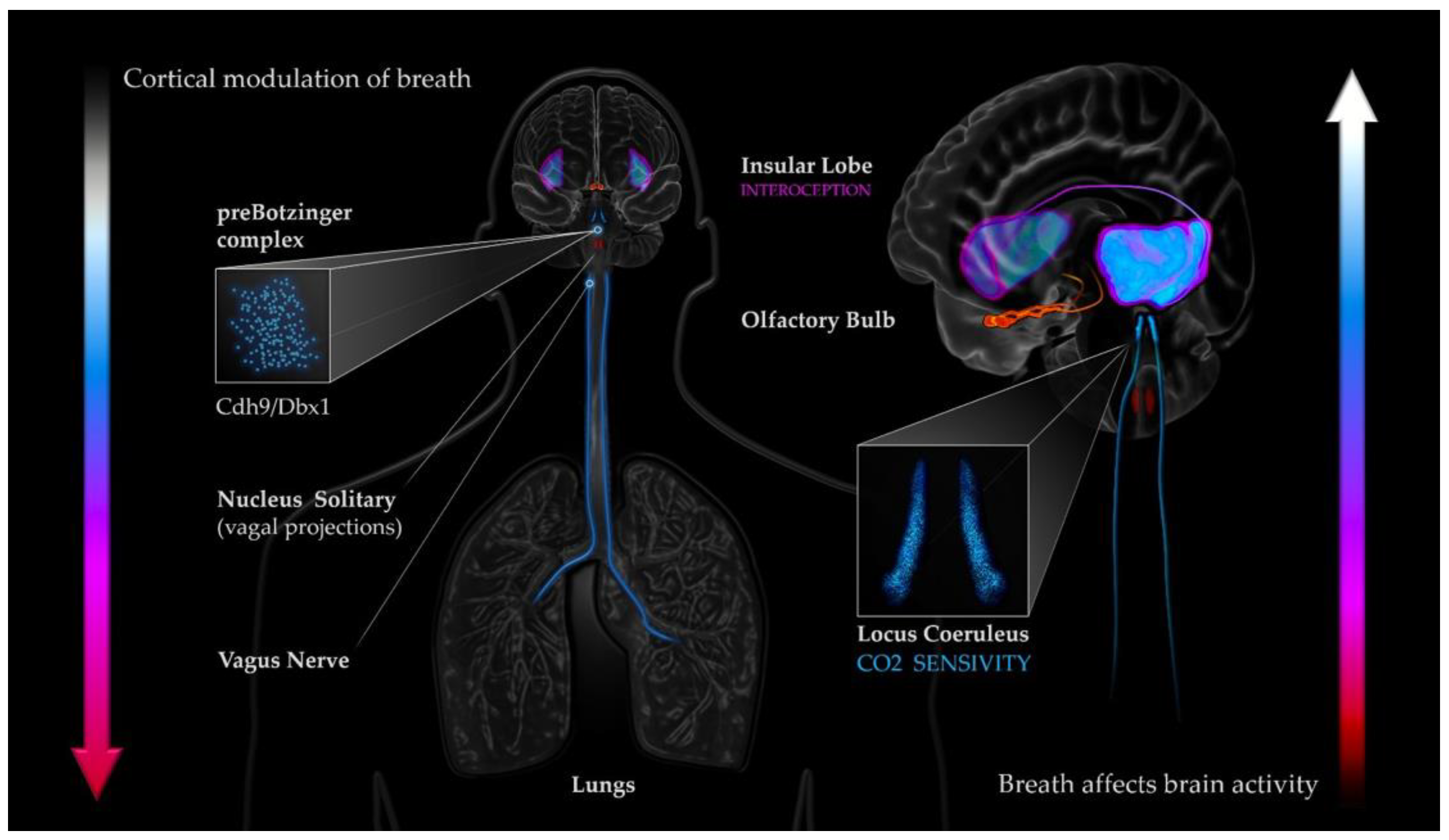
1. Introduction
2. Methods
2.1. Participants
2.2. Data Recording
2.3. Data Preparation
2.4. Data Analysis
3. Results
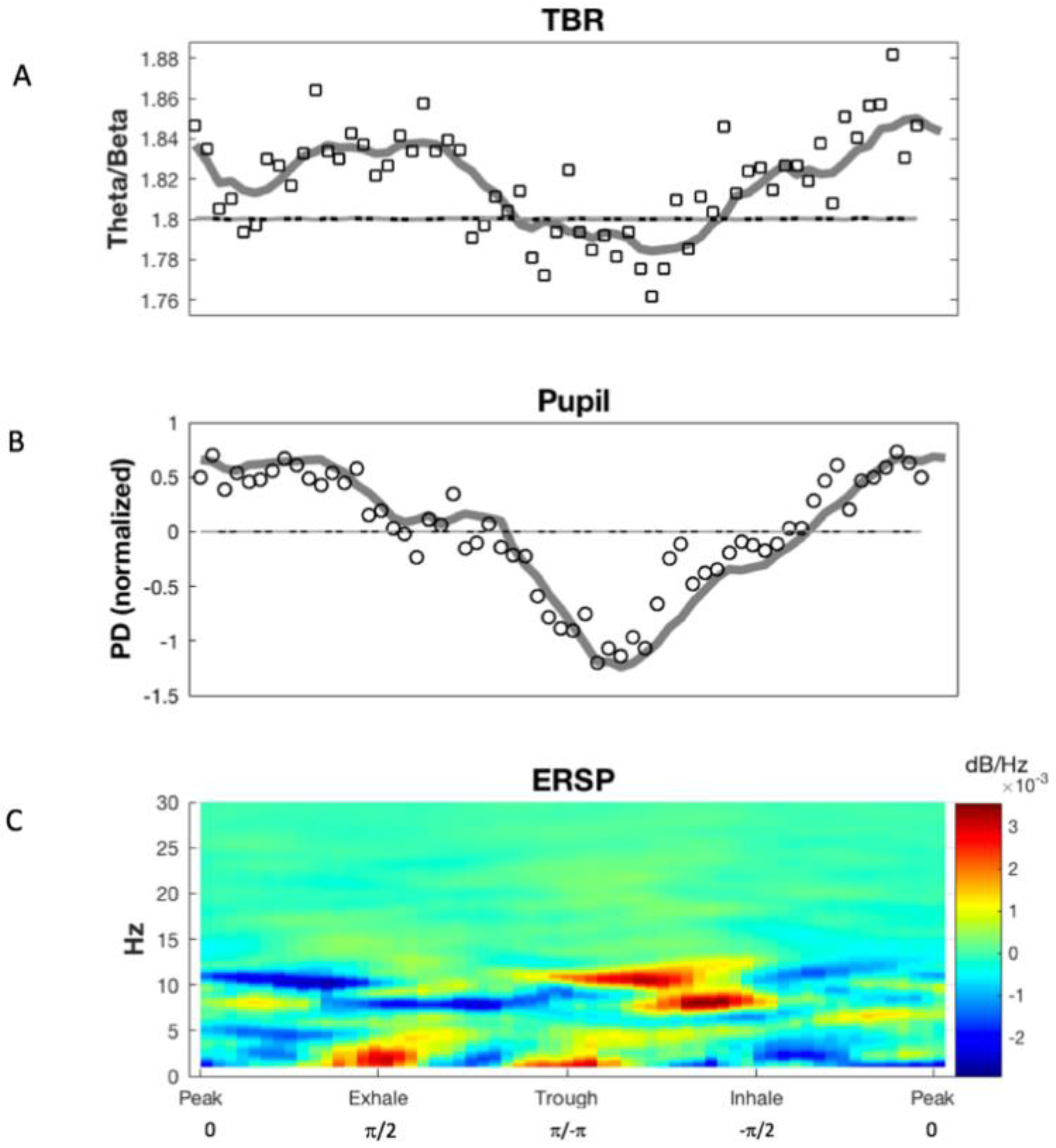
3.1. Phase Synchronization Analysis
3.2. Multivariate Granger-Causality Analysis
4. Discussion
5. Study Limitations
6. Conclusions
7. Future Research Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adrian, E. The electrical activity of the mammalian olfactory bulb. Electroencephalogr. Clin. Neurophysiol. 1950, 2, 377–388. [Google Scholar] [CrossRef]
- Alnaes, D.; Sneve, M.H.; Espeseth, T.; Endestad, T.; Van De Pavert, S.H.P.; Laeng, B. Pupil size signals mental effort deployed during multiple object tracking and predicts brain activity in the dorsal attention network and the locus coeruleus. J. Vis. 2014, 14, 1. [Google Scholar] [CrossRef]
- Angelidis, A.; Hagenaars, M.; van Son, D.; van der Does, W.; Putman, P. Do not look away! Spontaneous frontal EEG theta/beta ratio as a marker for cognitive control over attention to mild and high threat. Biol. Psychol. 2018, 135, 8–17. [Google Scholar] [CrossRef]
- Muktibhodananda, S. Hatha Yoga Pradapika, 3rd ed.; Bihar School of Yoga Publishers: Bihar, India, 2013. [Google Scholar]
- Sai, V. Ananda Sutra; Sri Vasantha Sai Books and Publications Trust: Andhra Pradesh, India, 2010. [Google Scholar]
- Satchidananda, S.S. The Yoga Sutras of Patanjali: Reprint Edition; Integral Yoga Publications: Buckingham, VA, USA, 2012. [Google Scholar]
- Biskamp, J.; Bartos, M.; Sauer, J.-F. Organization of prefrontal network activity by respiration-related oscillations. Sci. Rep. 2017, 7, srep45508. [Google Scholar] [CrossRef]
- Hobson, J.A. Respiration and EEG Synchronization in the Frog. Nature 1967, 213, 988–989. [Google Scholar] [CrossRef]
- Melnychuk, M.C.; Dockree, P.M.; O’Connell, R.G.; Murphy, P.R.; Balsters, J.H.; Robertson, I.H. Coupling of respiration and attention via the locus coeruleus: Effects of meditation and pranayama. Psychophysiology 2018, 55, e13091. [Google Scholar] [CrossRef] [PubMed]
- Stankovski, T.; Petkoski, S.; Raeder, J.; Smith, A.F.; McClintock, P.; Stefanovska, A. Alterations in the coupling functions between cortical and cardio-respiratory oscillations due to anaesthesia with propofol and sevoflurane. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150186. [Google Scholar] [CrossRef] [PubMed]
- Tort, A.B.; Brankačk, J.; Draguhn, A. Respiration-Entrained Brain Rhythms Are Global but Often Overlooked. Trends Neurosci. 2018, 41, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Yackle, K.; Schwarz, L.A.; Kam, K.; Sorokin, J.M.; Huguenard, J.R.; Feldman, J.L.; Luo, L.; Krasnow, M.A. Breathing control center neurons that promote arousal in mice. Science 2017, 355, 1411–1415. [Google Scholar] [CrossRef]
- Zelano, C.; Jiang, H.; Zhou, G.; Arora, N.; Schuele, S.; Rosenow, J.; Gottfried, J.A. Nasal Respiration Entrains Human Limbic Oscillations and Modulates Cognitive Function. J. Neurosci. 2016, 36, 12448–12467. [Google Scholar] [CrossRef]
- Gellhorn, E.; Kraines, S.H. The influence of hyperpnea and of variations in the o2-and co2-tension of the inspired air on word-associations. Science 1936, 83, 266–267. [Google Scholar] [CrossRef]
- Lehmann, A. Revue Philosophique de la France Et de l’Etranger. 1893. Available online: https://gallica.bnf.fr/ark:/12148/cb34349223n/date (accessed on 31 August 2021).
- Macdougall, R.; Munsterberg, H. Studies from the Harvard Psychological Laboratory (IV): The physical characteristics of attention. Psychol. Rev. 1896, 3, 158–180. [Google Scholar] [CrossRef][Green Version]
- Porges, S.W.; Raskin, D.C. Respiratory and heart rate components of attention. J. Exp. Psychol. 1969, 81, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.W. The Effect of Certain Stimuli upon the Attention Wave. Am. J. Psychol. 1901, 12, 335. [Google Scholar] [CrossRef]
- Winkler, C. Attention and respiration. K. Ned. Akad. Van Wet. Proc. Ser. B Phys. Sci. 1898, 1, 1898–1899. [Google Scholar]
- Christoff, K.; Gordon, A.M.; Smallwood, J.; Smith, R.; Schooler, J.W. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc. Natl. Acad. Sci. USA 2009, 106, 8719–8724. [Google Scholar] [CrossRef] [PubMed]
- Mason, M.F.; Norton, M.I.; Van Horn, J.D.; Wegner, D.M.; Grafton, S.T.; Macrae, C.N. Wandering Minds: The Default Network and Stimulus-Independent Thought. Science 2007, 315, 393–395. [Google Scholar] [CrossRef]
- Barron, E.; Riby, L.M.; Greer, J.; Smallwood, J. Absorbed in thought: The effect of mind wandering on the processing of relevant and irrelevant events. Psychol. Sci. 2011, 22, 596–601. [Google Scholar] [CrossRef]
- Smallwood, J.; Beach, E.; Schooler, J.W.; Handy, T.C. Going AWOL in the brain: Mind wandering reduces cortical analysis of external events. J. Cogn. Neurosci. 2008, 20, 458–469. [Google Scholar] [CrossRef]
- Arnau, S.; Löffler, C.; Rummel, J.; Hagemann, D.; Wascher, E.; Schubert, A. Inter-trial alpha power indicates mind wandering. Psychophysiology 2020, 57, e13581. [Google Scholar] [CrossRef]
- Baldwin, C.L.; Roberts, D.M.; Barragan, D.; Lee, J.D.; Lerner, N.; Higgins, J.S. Detecting and Quantifying Mind Wandering during Simulated Driving. Front. Hum. Neurosci. 2017, 11, 406. [Google Scholar] [CrossRef]
- Compton, R.J.; Gearinger, D.; Wild, H. The wandering mind oscillates: EEG alpha power is enhanced during moments of mind-wandering. Cogn. Affect. Behav. Neurosci. 2019, 19, 1184–1191. [Google Scholar] [CrossRef]
- Braboszcz, C.; Delorme, A. Lost in thoughts: Neural markers of low alertness during mind wandering. NeuroImage 2010, 54, 3040–3047. [Google Scholar] [CrossRef]
- Van Son, D.; De Blasio, F.M.; Fogarty, J.S.; Angelidis, A.; Barry, R.J.; Putman, P. Frontal EEG theta/beta ratio during mind wandering episodes. Biol. Psychol. 2018, 140, 19–27. [Google Scholar] [CrossRef]
- Angelidis, A.; Van der Does, W.; Schakel, L.; Putman, P. Frontal EEG theta/beta ratio as an electrophysiological marker for attentional control and its test-retest reliability. Biol. Psychol. 2016, 121, 49–52. [Google Scholar] [CrossRef]
- Putman, P.; Verkuil, B.; Arias-Garcia, E.; Pantazi, I.; Van Schie, C. EEG theta/beta ratio as a potential biomarker for attentional control and resilience against deleterious effects of stress on attention. Cogn. Affect. Behav. Neurosci. 2013, 14, 782–791. [Google Scholar] [CrossRef]
- Schutter, D.J.; Van Honk, J. Electrophysiological ratio markers for the balance between reward and punishment. Cogn. Brain Res. 2005, 24, 685–690. [Google Scholar] [CrossRef]
- Barry, R.J.; Clarke, A.R.; Johnstone, S.; McCarthy, R.; Selikowitz, M. Electroencephalogram θ/β Ratio and Arousal in Attention-Deficit/Hyperactivity Disorder: Evidence of Independent Processes. Biol. Psychiatry 2009, 66, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Barry, R.J.; Clarke, A.R.; McCarthy, R.; Selikowitz, M.; A Rushby, J.; Ploskova, E. EEG differences in children as a function of resting-state arousal level. Clin. Neurophysiol. 2004, 115, 402–408. [Google Scholar] [CrossRef]
- Barry, R.J.; Clarke, A.R.; Johnstone, S.J. A review of electrophysiology in attention deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clin. Neurophysiol. 2003, 114, 171–183. [Google Scholar] [CrossRef]
- Clarke, A.R.; Barry, R.J.; Dupuy, F.E.; McCarthy, R.; Selikowitz, M.; Johnstone, S.J. Excess beta activity in the EEG of children with attention-deficit/hyperactivity disorder: A disorder of arousal? Int. J. Psychophysiol. 2013, 89, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.R.; Barry, R.J.; Karamacoska, D.; Johnstone, S.J. The EEG theta/beta ratio: A marker of arousal or cognitive processing capacity? Appl. Psychophysiol. Biofeedback 2019, 44, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Lubar, J.F. Discourse on the development of EEG diagnostics and biofeedback for attention-deficit/hyperactivity disorders. Appl. Psychophysiol. Biofeedback 1991, 16, 201–225. [Google Scholar] [CrossRef] [PubMed]
- Mann, C.A.; Lubar, J.F.; Zimmerman, A.W.; Miller, C.A.; Muenchen, R.A. Quantitative analysis of EEG in boys with attention-deficit-hyperactivity disorder: Controlled study with clinical implications. Pediatr. Neurol. 1992, 8, 30–36. [Google Scholar] [CrossRef]
- Clayton, E.; Rajkowski, J.; Cohen, J.D.; Aston-Jones, G. Phasic Activation of Monkey Locus Ceruleus Neurons by Simple Decisions in a Forced-Choice Task. J. Neurosci. 2004, 24, 9914–9920. [Google Scholar] [CrossRef] [PubMed]
- Derryberry, D.; Reed, M.A. Anxiety-related attentional biases and their regulation by attentional control. J. Abnorm. Psychol. 2002, 111, 225–236. [Google Scholar] [CrossRef]
- Putman, P.; van Peer, J.; Maimari, I.; van der Werff, S. EEG theta/beta ratio in relation to fear-modulated response-inhibition, attentional control, and affective traits. Biol. Psychol. 2010, 83, 73–78. [Google Scholar] [CrossRef]
- Massar, S.; Rossi, V.; Schutter, D.; Kenemans, J. Baseline EEG theta/beta ratio and punishment sensitivity as biomarkers for feedback-related negativity (FRN) and risk-taking. Clin. Neurophysiol. 2012, 123, 1958–1965. [Google Scholar] [CrossRef]
- Massar, S.A.; Kenemans, J.L.; Schutter, D.J. Resting-state EEG theta activity and risk learning: Sensitivity to reward or punishment? Int. J. Psychophysiol. 2014, 91, 172–177. [Google Scholar] [CrossRef]
- Schutter, D.J.L.G.; Knyazev, G.G. Cross-frequency coupling of brain oscillations in studying motivation and emotion. Motiv. Emot. 2011, 36, 46–54. [Google Scholar] [CrossRef]
- Schutter, I.; Kenemans, J.L.; Schutter, D.J.L.G. Resting-state theta/beta EEG ratio is associated with reward- and punishment-related reversal learning. Cogn. Affect. Behav. Neurosci. 2017, 17, 754–763. [Google Scholar] [CrossRef]
- Van Son, D.; de Rover, M.; De Blasio, F.M.; van der Does, W.; Barry, R.J.; Putman, P. Electroencephalography theta/beta ratio covaries with mind wandering and functional connectivity in the executive control network. Ann. N. Y. Acad. Sci. 2019, 1452, 52. [Google Scholar] [CrossRef] [PubMed]
- Hasenkamp, W.; Wilson-Mendenhall, C.D.; Duncan, E.; Barsalou, L.W. Mind wandering and attention during focused meditation: A fine-grained temporal analysis of fluctuating cognitive states. NeuroImage 2011, 59, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Fontanini, A.; Bower, J.M. Slow-waves in the olfactory system: An olfactory perspective on cortical rhythms. Trends Neurosci. 2006, 29, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Heck, D.H.; McAfee, S.; Liu, Y.; Babajani-Feremi, A.; Rezaie, R.; Freeman, W.J.; Wheless, J.W.; Papanicolaou, A.C.; Ruszinkó, M.; Sokolov, Y.; et al. Breathing as a Fundamental Rhythm of Brain Function. Front. Neural Circuits 2017, 10, 115. [Google Scholar] [CrossRef]
- Ito, J.; Roy, S.K.; Liu, Y.; Cao, Y.; Fletcher, M.L.; Lu, L.; Boughter, J.D.; Grün, S.; Heck, D.H. Whisker barrel cortex delta oscillations and gamma power in the awake mouse are linked to respiration. Nat. Commun. 2014, 5, 3572. [Google Scholar] [CrossRef]
- Moberly, A.H.; Schreck, M.; Bhattarai, J.P.; Zweifel, L.S.; Luo, W.; Ma, M. Olfactory inputs modulate respiration-related rhythmic activity in the prefrontal cortex and freezing behavior. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Jerath, R.; Edry, J.W.; Barnes, V.A.; Jerath, V. Physiology of long pranayamic breathing: Neural respiratory elements may provide a mechanism that explains how slow deep breathing shifts the autonomic nervous system. Med. Hypotheses 2006, 67, 566–571. [Google Scholar] [CrossRef]
- Farb, N.A.S.; Segal, Z.V.; Anderson, A.K. Attentional Modulation of Primary Interoceptive and Exteroceptive Cortices. Cereb. Cortex 2012, 23, 114–126. [Google Scholar] [CrossRef]
- Fuxe, K.; Hökfelt, T.; Ungerstedt, U. Morphological and Functional Aspects of Central Monoamine Neurons. Int. Rev. Neurobiol. 1970, 13, 93–126. [Google Scholar] [CrossRef]
- Loughlin, S.; Foote, S.; Bloom, F. Efferent projections of nucleus locus coeruleus: Topographic organization of cells of origin demonstrated by three-dimensional reconstruction. Neuroscience 1986, 18, 291–306. [Google Scholar] [CrossRef]
- Carter, M.; Yizhar, O.; Chikahisa, S.; Nguyen, H.; Adamantidis, A.; Nishino, S.; Deisseroth, K.; De Lecea, L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat. Neurosci. 2010, 13, 1526–1533. [Google Scholar] [CrossRef]
- Aston-Jones, G.; Cohen, J.D. An integrative theory of locus coeruleus-norepinephrine function: Adaptive Gain and Optimal Performance. Annu. Rev. Neurosci. 2005, 28, 403–450. [Google Scholar] [CrossRef]
- Aston-Jones, G.; Rajkowski, J.; Kubiak, P. Conditioned responses of monkey locus coeruleus neurons anticipate acquisition of discriminative behavior in a vigilance task. Neuroscience 1997, 80, 697–715. [Google Scholar] [CrossRef]
- Bouret, S.; Sara, S.J. Reward expectation, orientation of attention and locus coeruleus-medial frontal cortex interplay during learning. Eur. J. Neurosci. 2004, 20, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Rajkowski, J.; Majczyński, H.; Clayton, E.; Aston-Jones, G. Activation of Monkey Locus Coeruleus Neurons Varies With Difficulty and Performance in a Target Detection Task. J. Neurophysiol. 2004, 92, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Usher, M.; Cohen, J.D.; Servan-Schreiber, D.; Rajkowski, J.; Aston-Jones, G. The Role of Locus Coeruleus in the Regulation of Cognitive Performance. Science 1999, 283, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Aston-Jones, G.; Rajkowski, J.; Lu, W.; Zhu, Y.; Cohen, J.D.; Morecraft, R.J. Prominent projections from the orbital prefrontal cortex to the locus coeruleus in monkey. J. Neurosci. 2002, 28, 86–89. [Google Scholar]
- Chandler, D.J.; Gao, W.-J.; Waterhouse, B.D. Heterogeneous organization of the locus coeruleus projections to prefrontal and motor cortices. Proc. Natl. Acad. Sci. USA 2014, 111, 6816–6821. [Google Scholar] [CrossRef]
- Rajkowski, J.; Lu, W.; Zhu, Y.; Cohen, J.; Aston-Jones, G. Prominent projections from the anterior cingulate cortex to the locus coeruleus in Rhesus monkey. Soc. Neurosci. Abstr. 2000, 26, 2230. [Google Scholar]
- Zhu, Y.; Iba, M.; Rajkowski, J.; Aston-Jones, G. Projection from the orbitofrontal cortex to the locus coeruleus in monkeys revealed by anterograde tracing. Soc. Neurosci. Abstr. 2004, 30, 211–213. [Google Scholar]
- Aston-Jones, G.; Rajkowski, J.; Cohen, J. Role of locus coeruleus in attention and behavioral flexibility. Biol. Psychiatry 1999, 46, 1309–1320. [Google Scholar] [CrossRef]
- Ivanova, S.; Rajkowski, J.; Silakov, V.; Watanabe, T.; Aston-Jones, G. Local chemomanipulations of locus coeruleus (LC) activity in monkeys alter cortical event-related potentials (ERPs) and task performance. Soc. Neurosci. Abstr. 1997, 23, 1587. [Google Scholar]
- Biancardi, V.; Bicego, K.; Almeida, M.C.; Gargaglioni, L.H. Locus coeruleus noradrenergic neurons and CO2 drive to breathing. Pflügers Arch.-Eur. J. Physiol. 2007, 455, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Filosa, J.A.; Dean, J.B.; Putnam, R.W. Role of intracellular and extracellular pH in the chemosensitive response of rat locus coeruleus neurones. J. Physiol. 2002, 541, 493–509. [Google Scholar] [CrossRef] [PubMed]
- Gargaglioni, L.H.; Hartzler, L.K.; Putnam, R.W. The locus coeruleus and central chemosensitivity. Respir. Physiol. Neurobiol. 2010, 173, 264–273. [Google Scholar] [CrossRef]
- Noronha-De-Souza, C.R.; Bícego, K.C.; Michel, G.; Glass, M.L.; Branco, L.G.S.; Gargaglioni, L.H. Locus coeruleus is a central chemoreceptive site in toads. Am. J. Physiol. Integr. Comp. Physiol. 2006, 291, R997–R1006. [Google Scholar] [CrossRef]
- Oyamada, Y.; Ballantyne, D.; Mückenhoff, K.; Scheid, P. Respiration-modulated membrane potential and chemosensitivity of locus coeruleus neurones in thein vitrobrainstem-spinal cord of the neonatal rat. J. Physiol. 1998, 513, 381–398. [Google Scholar] [CrossRef]
- Pineda, J.; Aghajanian, G. Carbon dioxide regulates the tonic activity of locus coeruleus neurons by modulating a proton- and polyamine-sensitive inward rectifier potassium current. Neuroscience 1997, 77, 723–743. [Google Scholar] [CrossRef]
- Band, D.M.; Cameron, I.R.; Semple, S.J. Oscillations in arterial pH with breathing in the cat. J. Appl. Physiol. 1969, 26, 261–267. [Google Scholar] [CrossRef]
- Band, D.M.; Wolff, C.B.; Ward, J.; Cochrane, G.M.; Prior, J. Respiratory oscillations in arterial carbon dioxide tension as a control signal in exercise. Nature 1980, 283, 84–85. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Ueda, M. Fluctuations of arterial PH associated with the respiratory cycle in dogs. Jpn. J. Physiol. 1961, 11, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Li, Y.; Kalwani, R.M.; Gold, J.I. Relationships between Pupil Diameter and Neuronal Activity in the Locus Coeruleus, Colliculi, and Cingulate Cortex. Neuron 2015, 89, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Rodenkirch, C.; Moskowitz, N.; Schriver, B.; Wang, Q. Dynamic Lateralization of Pupil Dilation Evoked by Locus Coeruleus Activation Results from Sympathetic, Not Parasympathetic, Contributions. Cell Rep. 2017, 20, 3099–3112. [Google Scholar] [CrossRef] [PubMed]
- Reimer, J.; McGinley, M.J.; Liu, Y.; Rodenkirch, C.; Wang, Q.; A McCormick, D.; Tolias, A.S. Pupil fluctuations track rapid changes in adrenergic and cholinergic activity in cortex. Nat. Commun. 2016, 7, 13289. [Google Scholar] [CrossRef]
- Unsworth, N.; Robison, M.K. Pupillary correlates of lapses of sustained attention. Cogn. Affect. Behav. Neurosci. 2016, 16, 601–615. [Google Scholar] [CrossRef]
- Varazzani, C.; San-Galli, A.; Gilardeau, S.; Bouret, S. Noradrenaline and Dopamine Neurons in the Reward/Effort Trade-Off: A Direct Electrophysiological Comparison in Behaving Monkeys. J. Neurosci. 2015, 35, 7866–7877. [Google Scholar] [CrossRef]
- Lachaux, J.P.; Rodriguez, E.; Martinerie, J.; Varela, F.J. Measuring phase synchrony in brain signals. Hum. Brain Mapp. 1999, 8, 194–208. [Google Scholar] [CrossRef]
- Barnett, L.; Seth, A.K. The MVGC multivariate Granger causality toolbox: A new approach to Granger-causal inference. J. Neurosci. Methods 2014, 223, 50–68. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Berkovits, I.; Hancock, G.R.; Nevitt, J. Bootstrap Resampling Approaches for Repeated Measure Designs: Relative Robustness to Sphericity and Normality Violations. Educ. Psychol. Meas. 2000, 60, 877–892. [Google Scholar] [CrossRef]
- Wilson, D.J. The harmonic mean p-value for combining dependent tests. Proc. Natl. Acad. Sci. USA 2019, 116, 1195–1200. [Google Scholar] [CrossRef]
- Field, A. Discovering Statistics Using IBM SPSS Statistics; Sage: Los Angeles, CA, USA, 2013. [Google Scholar]
- Lehmann, A. Uber die Beziehung swischen Athmung und Aufmerksamkeit. Phil. Stud. 1894, 9, 66–91. [Google Scholar]
- Lange, N. Beitrige zur Theorie der Aufmerksamkeit und der activen Apperception. Phil. Stud. 1888, 4, 390–422. [Google Scholar]
- Urbantschitsch, V. Ueber eine Eigenthiimlichkeit der Schallempfindungen geringster Intensittit, Centralbl. f. d. med. Wiss. 1875. Available online: https://dejure.org/dienste/vernetzung/rechtsprechung?Gericht=BVerfG&Datum=15.01.1958&Aktenzeichen=1%20BvR%20400%2F51 (accessed on 31 August 2021).
- Langner, R.; Eickhoff, S.B. Sustaining attention to simple tasks: A meta-analytic review of the neural mechanisms of vigilant attention. Psychol. Bull. 2013, 139, 870–900. [Google Scholar] [CrossRef] [PubMed]
- Robertson, I.H.; Ridgeway, V.; Greenfield, E.; Parr, A. Motor recovery after stroke depends on intact sustained attention: A 2-year follow-up study. Neuropsychology 1997, 11, 290–295. [Google Scholar] [CrossRef]
- Stadler, M.; Erke, H. Über einige periodische Vorgänge in der Figuralwahrnehmung. Vis. Res. 1968, 8, 1081–1092. [Google Scholar] [CrossRef]
- Sripada, C.S. An Exploration/Exploitation Trade-off between Mind-Wandering and Goal-Directed Thinking. Oxf. Handb. Spontaneous Thought Mind-Wander. Creat. Dreaming 2018, 34, 23–34. [Google Scholar] [CrossRef]
- Foxe, J.J.; Snyder, A.C. The Role of Alpha-Band Brain Oscillations as a Sensory Suppression Mechanism during Selective Attention. Front. Psychol. 2011, 2, 154. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W.; Sauseng, P.; Hanslmayr, S. EEG alpha oscillations: The inhibition–timing hypothesis. Brain Res. Rev. 2007, 53, 63–88. [Google Scholar] [CrossRef] [PubMed]
- Mathewson, K.E.; Lleras, A.; Beck, D.M.; Fabiani, M.; Ro, T.; Gratton, G. Pulsed Out of Awareness: EEG Alpha Oscillations Represent a Pulsed-Inhibition of Ongoing Cortical Processing. Front. Psychol. 2011, 2, 99. [Google Scholar] [CrossRef]
- Pfurtscheller, G. Induced Oscillations in the Alpha Band: Functional Meaning. Epilepsia 2003, 44, 2–8. [Google Scholar] [CrossRef]
- Uusberg, A.; Uibo, H.; Kreegipuu, K.; Allik, J. EEG alpha and cortical inhibition in affective attention. Int. J. Psychophysiol. 2013, 89, 26–36. [Google Scholar] [CrossRef]
- Canteroa, J.L.; Atienzaa, M.; Salasa, R.M. Alpha EEG coherence in different brain states: An electrophysiological index of the arousal level in human subjects. Neurosci. Lett. 1999, 271, 170. [Google Scholar] [CrossRef]
- Mikutta, C.; Altorfer, A.; Strik, W.; Koenig, T. Emotions, arousal, and frontal alpha rhythm asymmetry during Beethoven’s 5th symphony. Brain Topogr. 2012, 25, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Eysenck, M. Attention and Arousal: Cognition and Performance; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Yerkes, R.M.; Dodson, J.D. The relation of strength of stimulus to rapidity of habit-formation. J. Comp. Neurol. Psychol. 1908, 18, 459–482. [Google Scholar] [CrossRef]
- Garey, J.; Goodwillie, A.; Frohlich, J.; Morgan, M.; Gustafsson, J.A.; Smithies, O.; Korach, K.; Ogawa, S.; Pfaff, D.W. Genetic contributions to generalized arousal of brain and behavior. Proc. Natl. Acad. Sci. USA 2003, 100, 11019–11022. [Google Scholar] [CrossRef] [PubMed]
- Hebb, D.O. Drives and the c.n.s. (conceptual nervous system)*. Psychol. Rev. 1966, 62, 67–84. [Google Scholar] [CrossRef]
- Pfaff, D.W. Brain Arousal and Information Theory: Neural and Genetic Mechanisms; Harvard University Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Bradley, M.M.; Miccoli, L.; Escrig, M.A.; Lang, P.J. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology 2008, 45, 602–607. [Google Scholar] [CrossRef]
- Bradshaw, J.L. Pupil Size as a Measure of Arousal during Information Processing. Nat. Cell Biol. 1967, 216, 515–516. [Google Scholar] [CrossRef]
- Hess, E.H.; Polt, J.M. Pupil Size as Related to Interest Value of Visual Stimuli. Science 1960, 132, 349–350. [Google Scholar] [CrossRef]
- Huijbers, W.; Pennartz, C.; Beldzik, E.; Domagalik, A.; Vinck, M.; Hofman, W.F.; Cabeza, R.; Daselaar, S.M. Respiration phase-locks to fast stimulus presentations: Implications for the interpretation of posterior midline “deactivations”. Hum. Brain Mapp. 2014, 35, 4932–4943. [Google Scholar] [CrossRef]
- Glover, G.H.; Li, T.Q.; Ress, D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn. Reson. Med. 2000, 44, 162–167. [Google Scholar] [CrossRef]
- Birn, R.M. The role of physiological noise in resting-state functional connectivity. NeuroImage 2012, 62, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zotev, V.; Phillips, R.; Bodurka, J. Correlated slow fluctuations in respiration, EEG, and BOLD fMRI. NeuroImage 2013, 79, 81–93. [Google Scholar] [CrossRef]
- Lorenz, E.N. Deterministic nonperiodic flow. J. Atmos. Sci. 1963, 20, 130–141. [Google Scholar] [CrossRef]
- Kox, M.; van Eijk, L.T.; Zwaag, J.; Wildenberg, J.V.D.; Sweep, F.; van der Hoeven, J.G.; Pickkers, P. Voluntary activation of the sympathetic nervous system and attenuation of the innate immune response in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 7379–7384. [Google Scholar] [CrossRef] [PubMed]
- Muzik, O.; Reilly, K.T.; Diwadkar, V.A. “Brain over body”—A study on the willful regulation of autonomic function during cold exposure. NeuroImage 2018, 172, 632–641. [Google Scholar] [CrossRef]
- Berwian, I.M.; Wenzel, J.G.; Collins, A.G.E.; Seifritz, E.; Stephan, K.E.; Walter, H.; Huys, Q.J.M. Computational Mechanisms of Effort and Reward Decisions in Patients With Depression and Their Association With Relapse After Antidepressant Discontinuation. JAMA Psychiatry 2020, 77, 513–522. [Google Scholar] [CrossRef]
- Grueschow, M.; Stenz, N.; Thörn, H.; Ehlert, U.; Breckwoldt, J.; Maeder, M.B.; Exadaktylos, A.K.; Bingisser, R.; Ruff, C.C.; Kleim, B. Real-world stress resilience is associated with the responsivity of the locus coeruleus. Nat. Commun. 2021, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kaldewaij, R.; Koch, S.B.J.; Hashemi, M.M.; Zhang, W.; Klumpers, F.; Roelofs, K. Anterior prefrontal brain activity during emotion control predicts resilience to post-traumatic stress symptoms. Nat. Hum. Behav. 2021, 5, 1055–1064. [Google Scholar] [CrossRef]
- Kurniawan, I.T.; Grueschow, M.; Ruff, C.C. Anticipatory Energization Revealed by Pupil and Brain Activity Guides Human Effort-Based Decision Making. J. Neurosci. 2021, 41, 6328–6342. [Google Scholar] [CrossRef] [PubMed]
- Maier, S.U.; Grueschow, M. Pupil dilation predicts individual self-regulation success across domains. Sci. Rep. 2021, 11, 1–18. [Google Scholar] [CrossRef]
- Marzo, A.; Bai, J.; Otani, S. Neuroplasticity regulation by noradrenaline in mammalian brain. Curr. Neuropharmacol. 2009, 7, 286–295. [Google Scholar] [CrossRef]
- Chen, M.J.; Nguyen, T.V.; Pike, C.J.; Russo-Neustadt, A.A. Norepinephrine induces BDNF and activates the PI-3K and MAPK cascades in embryonic hippocampal neurons. Cell. Signal. 2007, 19, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Ivy, A.S.; Rodriguez, F.G.; Garcia, C.; Chen, M.J.; Russo-Neustadt, A.A. Noradrenergic and serotonergic blockade inhibits BDNF mRNA activation following exercise and antidepressant. Pharmacol. Biochem. Behav. 2003, 75, 81–88. [Google Scholar] [CrossRef]
- Russo-Neustadt, A.A.; Alejandre, H.; Garcia, C.; Ivy, A.S.; Chen, M.J. Hippocampal brain-derived neurotrophic factor expression following treatment with reboxetine, citalopram, and physical exercise. Neuropsychopharmacology 2004, 29, 2189–2199. [Google Scholar] [CrossRef] [PubMed]

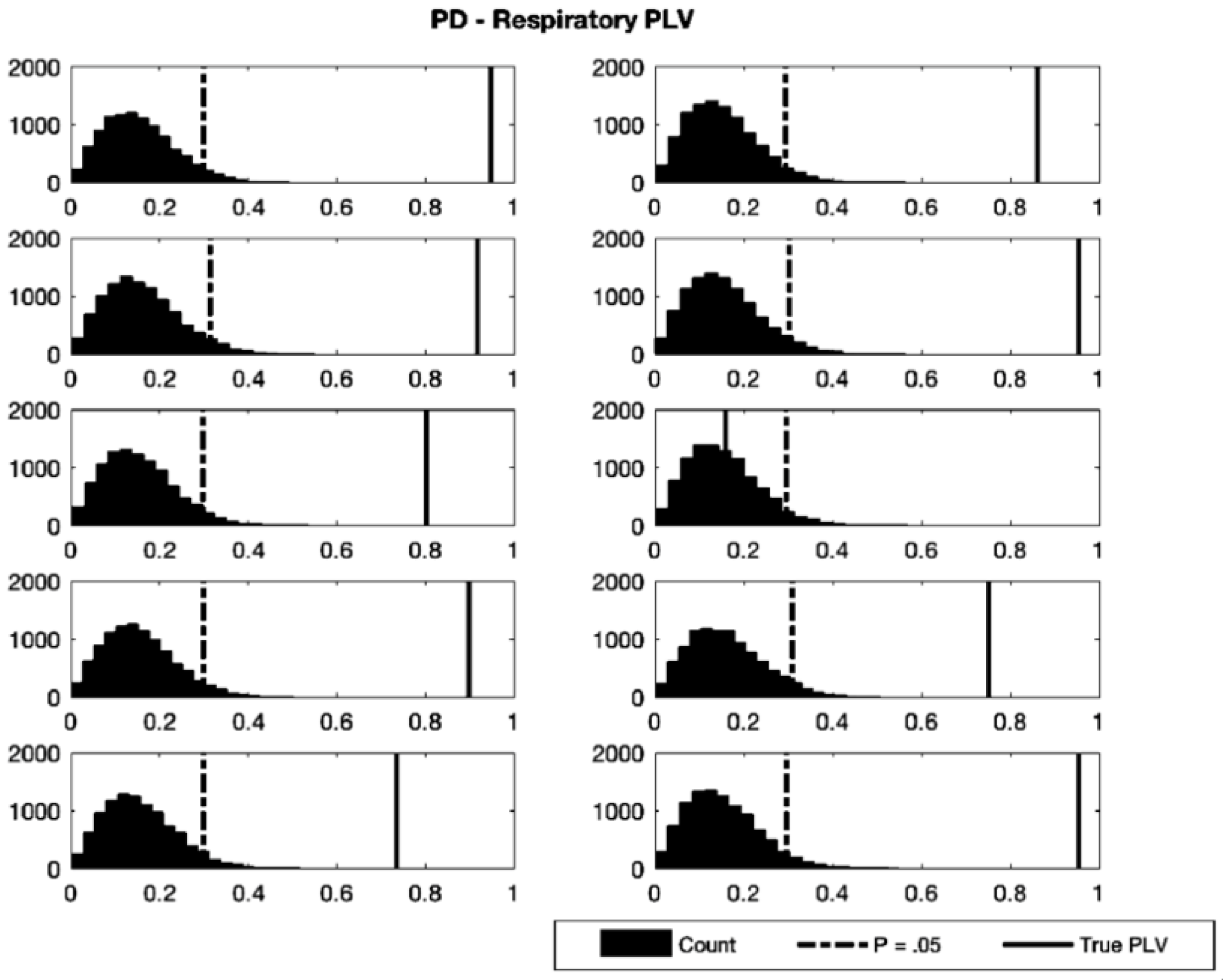
| Participant | Test PLV | Critical PLV | p-Value |
|---|---|---|---|
| 1 | 0.500 | 0.294 | 0.0001 |
| 2 | 0.568 | 0.295 | <0.0001 |
| 3 | 0.523 | 0.296 | 0.0002 |
| 4 | 0.644 | 0.300 | <0.0001 |
| 5 | 0.239 | 0.297 | 0.147 |
| 6 | 0.400 | 0.297 | 0.005 |
| 7 | 0.291 | 0.295 | 0.046 |
| 8 | 0.588 | 0.304 | <0.0001 |
| 9 | 0.390 | 0.302 | 0.005 |
| 10 | 0.103 | 0.295 | 0.036 |
| 11 | 0.079 | 0.296 | 0.371 |
| 0.0003 |
| Participant | Test PLV | Critical PLV | p-Value |
|---|---|---|---|
| 1 | 0.948 | 0.296 | <0.0001 |
| 2 | 0.862 | 0.295 | <0.0001 |
| 3 | 0.917 | 0.305 | <0.0001 |
| 4 | 0.955 | 0.302 | <0.0001 |
| 5 | 0.803 | 0.299 | <0.0001 |
| 6 | 0.160 | 0.297 | 0.414 |
| 7 | 0.899 | 0.298 | <0.0001 |
| 8 | 0.753 | 0.306 | <0.0001 |
| 9 | 0.736 | 0.303 | <0.0001 |
| 10 | 0.955 | 0.297 | <0.0001 |
| < 0.0001 |
| Model Order | A.C. Lags | Epochs | Mean MCD | Harmonic Mean (p-Value) | |
|---|---|---|---|---|---|
| P1 | 17 | 7052 | 1 | 0.0002 | 0.49 |
| P2 | 63 | 20,000 | 1 | 0.0090 | 0.00020 |
| P3 | 50 | 10,000 | 41 | 0.0023 | 0.00015 |
| P4 | 15 | 3144 | 20 | 0.0061 | 0.00012 |
| P5 | 17 | 10,000 | 1 | 0.0480 | 0.00005 |
| P6 | 12 | 10,000 | 1 | 0.0093 | 0.00020 |
| P7 | 40 | 10,000 | 1 | 0.0010 | 0.00055 |
| P8 | 40 | 10,000 | 1 | 0.0017 | 0.00020 |
| P9 | 60 | 20,000 | 1 | 0.0090 | 0.00030 |
| P10 | 40 | 10,000 | 1 | 0.0109 | 0.00028 |
| Grand Mean | 0.0097 | 0.00017 |
| Resp Pupil | Resp Fz | Pupil Resp | Pupil Fz | Fz Resp | Fz Pupil | |
|---|---|---|---|---|---|---|
| P1 | 0.00021 (0.61) | 0.00027 (0.32) | 0.00025 (0.43) | 0.00023 (0.51) | 0.00018 (0.74) | 0.00022 (0.54) |
| P2 | 0.0017 (<0.0001) * | 0.0016 (<0.0001) * | 0.048 (<0.0001) * | 0.0009 (0.27) | 0.0011 (0.036) | 0.0007 (0.79) |
| P3 | 0.0009 (0.23) | 0.0018 (<0.0001) * | 0.006 (<0.0001) * | 0.0009 (0.28) | 0.0024 (<0.0001) * | 0.0016 (0.0001) * |
| P4 | 0.0005 (0.01) * | 0.0065 (<0.0001) * | 0.0019 (<0.0001) * | 0.0245 (<0.0001) * | 0.0022 (<0.0001) * | 0.0010 (<0.0001) * |
| P5 | 0.0005 (0.001) * | 0.0002 (0.41) | <0.0001 (>0.99) | 0.0003 (0.08) | 0.2870 (<0.00001) * | 0.0005 (<0.0001) * |
| P6 | 0.0003 (0.03) | 0.0112 (<0.0001) * | 0.0062 (<0.0001) * | 0.0003 (0.03) | 0.0373 (<0.0001) * | 0.0004 (0.004) * |
| P7 | 0.0005 (0.95) | 0.0013 (0.001) * | 0.0006 (0.69) | 0.0007 (0.45) | 0.002 (<0.0001) * | 0.001 (0.08) |
| P8 | 0.0005 (0.44) | 0.0017 (<0.0001) * | 0.0015 (<0.0001) * | 0.0005 (0.33) | 0.0059 (<0.0001) * | 0.0005 (0.42) |
| P9 | 0.0030 (<0.0001) * | 0.0008 (0.098) | 0.0483 (<0.0001) * | 0.0002 (>0.99) | 0.0007 (0.24) | 0.0009 (0.01) * |
| P10 | 0.0011 (<0.001) * | 0.0028 (<0.0001) * | 0.0008 (0.06) | 0.0005 (0.66) | 0.0590 (<0.0001) * | 0.0010 (0.002) * |
| Mean MCD | 0.00106 | 0.002817 | 0.011365 | 0.002903 | 0.039778 | 0.000782 |
| Fisher’s χ2 (p-value) | 86.3 (<0.0001) * | 133.05 (<0.0001) * | 118.60 (<0.0001) * | 41.64 (0.0031) * | 143.65 (<0.0001) * | 96.43 (<0.0001) * |
| Resp | Fz | Pupil | |
|---|---|---|---|
| P1 | 2.0001 (0.99) | 2.0040 (0.60) | 2.0040 (0.18) |
| P2 | 1.9979 (0.77) | 2.0017 (0.82) | 1.9797 (<0.01) |
| P3 | 2.0037 (0.65) | 2.0064 (0.43) | 1.9965 (0.66) |
| P4 | 1.9830 (0.05) | 1.9244 (<0.01) | 2.0004 (0.97) |
| P5 | 1.9778 (<0.01) | 1.9999 (0.98) | 2.0015 (0.82) |
| P6 | 1.9881 (0.10) | 1.9997 (0.96) | 1.9306 (<0.01) |
| P7 | 2.0197 (0.0213) | 2.0028 (0.75) | 2.0017 (0.85) |
| P8 | 1.9961 (0.57) | 2.0007 (0.92) | 1.9120 (< 0.01) |
| P9 | 2.0017 (0.80) | 2.0005 (0.95) | 1.7753 * (<0.01) |
| P10 | 2.0128 (0.09) | 1.9996 (0.96) | 1.9997 (0.96) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melnychuk, M.C.; Robertson, I.H.; Plini, E.R.G.; Dockree, P.M. A Bridge between the Breath and the Brain: Synchronization of Respiration, a Pupillometric Marker of the Locus Coeruleus, and an EEG Marker of Attentional Control State. Brain Sci. 2021, 11, 1324. https://doi.org/10.3390/brainsci11101324
Melnychuk MC, Robertson IH, Plini ERG, Dockree PM. A Bridge between the Breath and the Brain: Synchronization of Respiration, a Pupillometric Marker of the Locus Coeruleus, and an EEG Marker of Attentional Control State. Brain Sciences. 2021; 11(10):1324. https://doi.org/10.3390/brainsci11101324
Chicago/Turabian StyleMelnychuk, Michael Christopher, Ian H. Robertson, Emanuele R. G. Plini, and Paul M. Dockree. 2021. "A Bridge between the Breath and the Brain: Synchronization of Respiration, a Pupillometric Marker of the Locus Coeruleus, and an EEG Marker of Attentional Control State" Brain Sciences 11, no. 10: 1324. https://doi.org/10.3390/brainsci11101324
APA StyleMelnychuk, M. C., Robertson, I. H., Plini, E. R. G., & Dockree, P. M. (2021). A Bridge between the Breath and the Brain: Synchronization of Respiration, a Pupillometric Marker of the Locus Coeruleus, and an EEG Marker of Attentional Control State. Brain Sciences, 11(10), 1324. https://doi.org/10.3390/brainsci11101324






