Abstract
Alzheimer’s disease (AD) represents the most common neurodegenerative disorder, with 47 million affected people worldwide. Current treatment strategies are aimed at reducing the symptoms and do slow down the progression of the disease, but inevitably fail in the long-term. Induced pluripotent stem cells (iPSCs)-derived neuronal cells from AD patients have proven to be a reliable model for AD pathogenesis. Here, we have conducted an in silico analysis aimed at identifying pathogenic gene-expression profiles and novel drug candidates. The GSE117589 microarray dataset was used for the identification of Differentially Expressed Genes (DEGs) between iPSC-derived neuronal progenitor (NP) cells and neurons from AD patients and healthy donors. The Discriminant Analysis Module (DAM) algorithm was used for the identification of biomarkers of disease. Drugs with anti-signature gene perturbation profiles were identified using the L1000FWD software. DAM analysis was used to identify a list of potential biomarkers among the DEGs, able to discriminate AD patients from healthy people. Finally, anti-signature perturbation analysis identified potential anti-AD drugs. This study set the basis for the investigation of potential novel pharmacological strategies for AD. Furthermore, a subset of genes for the early diagnosis of AD is proposed.
1. Introduction
Alzheimer’s disease (AD) represents the most common neurodegenerative disorder, with 47 million affected people worldwide. AD is characterized by several neuropathological changes—including cerebral atrophy, intense synaptic loss, and neuronal death—in regions of the prefrontal cortex and hippocampus that are responsible for cognitive functions. The disease shows a prodromal period that can last for decades and is characterized by a preclinical asymptomatic phase before cognitive impairment occurs [1]. Mild cognitive impairment (MCI) represents the first clinical phase of AD, characterized by an alteration in episodic memory. It is reasonable to believe that treatment with disease-modifying agents would likely be most effective in this stage of AD, before neurodegeneration is too marked and widespread. Thus, studies aimed at identifying potential AD biomarkers for early diagnosis is warranted. However, MCI can derive from a variety of causes (e.g., vascular, presence of Lewy bodies) and only approximately half of cases is associated to AD, with consequent obvious diagnostic difficulties. Indeed, magnetic resonance imaging (MRI)-based regional brain volumes, cerebrospinal fluid (CSF) analytes, and positron emission tomography (PET) imaging of cerebral fibrillar β-amyloid along ad hoc cognitive tests have been investigated as biomarkers of disease, they are useful only for the late stages of the disease, and may not be sensitive enough to detect initial neuropathophysiological processes occurring in AD patients who show mild cognitive impairment [2,3]. Hence for most cases, definite diagnosis is only possible with the post-mortem analysis of the brain and with the observation of severe brain atrophy and neuronal loss, as well as the presence of dense extracellular deposits and intracellular aggregates within neurons, identified as amyloid plaques and neurofibrillary tangles, respectively.
As regards AD therapy, up to now, only five drugs have been approved by FDA for human use. However, none of them are able to cure the disease and are only modestly able to slow down AD progression and improve the cognitive abilities of the patients. The reason for the lack of an effective treatment for AD likely relies on the multifactorial pathology of this disease, as well as the heterogeneous patient population [4]. Therefore, there is a strong need to develop novel anti-AD therapies. However, traditional drug development is burdened by the requirement of long time, high financial investments, and low success rate. In recent years, a large number of in vitro and in vivo studies, as well as some clinical studies, have been carried out with the aim of evaluating protective effects of some known multitarget molecules with antioxidant, anti-inflammatory, and neuroprotective potential on neurodegenerative processes [5]. On the other hand, drug repurposing can be used to redirect approved drugs for treating different disorders and seems an attractive strategy in AD, as it may expedite the design of phase II-III clinical trials, reduce the risks associated with early stages of drug development, while being cost-effective. Fessel et al. proposed that the combination of eight drugs that are already approved for different clinical indications and with limited or null overlapping activities that may warrant preclinical studies in animal models or Phase II PoC studies in humans [6]. Clearly, daily combination of eight drugs is clinically difficult and a trial of this kind would require adequate compliance of patients and also considerable economic supports that may be difficult to obtain in view of the lack of adequate patent protection of these drugs in the area of AD [6].
With the aim to identify possible diagnostic and therapeutic (e.g., theranostic) markers of AD development and progression, we have presently used a machine learning approach to identify a subset of genes that may predict AD in Induced Pluripotent Stem Cells (iPSC)-derived neuronal cells from dermal fibroblasts. The generation of iPSCs derived neuronal cells from patients with AD represent a unique opportunity to create a relevant in vitro model for mechanistic studies and preclinical drug discovery, and have been widely exploited in AD [7,8,9,10,11,12], as well as in other diseases such as amyotrophic lateral sclerosis [13], Parkinson’s disease [14], Rett syndrome [15], schizophrenia [16], Duchenne muscular dystrophy, Becker muscular dystrophy, Down syndrome, Juvenile diabetes mellitus, Huntington disease and Lesch-Nyhan syndrome [17].
Furthermore, we have performed a computational analysis of candidate drugs, based on their ability to modulate in an opposite manner the transcriptional profiles characterizing AD, in order to shortlist promising anti-AD drugs. A diagram showing the study plan is presented as Figure 1.
Figure 1.
Study plan.
2. Material and Methods
2.1. Dataset Selection
The publicly available microarray dataset GSE117589, originally generated and analyzed by Meyer and collaborators [1] was used for the identification of Differentially Expressed Genes (DEGs) between Induced Pluripotent Stem Cells (iPSC)-derived Neural Progenitor cells (NPCs) and neurons from AD patients and healthy donors. GSE117589 was retrieved from the Gene Expression Omnibus (GEO) databank (https://www.ncbi.nlm.nih.gov/gds) [1]. Briefly, for the generation of the dataset, iPSCs were obtained by retroviral transduction of KLF4, SOX2, c-MYC, and OCT4 in human dermal fibroblasts (Coriell Cell Repository, Camden, NJ, USA) from 5 healthy donors and 5 individuals with sporadic AD (SAD). Cells were then differentiated into NPCs and neurons, as described in the Meyer et al., 2019 [1]. The age of the healthy donors was 72.2 ± 13.3 and the age of the SAD patients was 69.6 ± 11.1. The female to male ratio was 2/3 and 3/2 in the healthy controls and SAD patients, respectively. All healthy donors had the E3/E3 APOE genotype, with the exception for one subject, who had the E3/E4 genotype. Two SAD patients had the E3/E3 genotype, two had the E4/E4 genotype and one the E2/E3 genotype [1]. Transcriptomic profiling was performed using the Affymetrix U133 Plus 2.0 arrays. The submitter-supplied pre-preprocessed and normalized gene expression matrix was used for the analysis [1]. Briefly, the probesets from the U133 Plus 2.0 platform were first converted into Ensembl genes and gene ids without annotation were removed [1]. Raw data were then preprocessed using the Robust Multi-array Average (RMA) algorithm [1].
2.2. Identification of Biomarkers of Disease and Validation
For the identification of the Differentially Expressed Genes (DEGs) in the cells from SAD individuals and Healthy donors, the LIMMA (Linear models for microarray data) parametric test was used. An adjusted p-value < 0.1 was considered to indicate a statistically significant difference. Gene Ontology (GO) analysis was performed for the DEGs, using the Metascape web-based tool, using default settings [18].
In order to identify a specific transcriptomic signature able to discriminate healthy subjects from AD patients, we used the Discriminant Analysis Module (DAM) algorithm [19]. DEGs were used as input data. DAM performs first a gene dimensional reduction method, the Multivariate Partial Least Squares (MPLS). Afterwards, the Polychotomous Discriminant Analysis (PDA) was applied as classification method. Hierarchical Clustering (HCL) was performed using the identified predictors in order to determine the relative distance of samples using Pearson’s correlation as similarity comparison.
In order to validate the results from the biomarkers prediction, we interrogated the GSE118553 microarray dataset [20]. This dataset was chosen as it included whole-genome expression data of brain areas known to be affected by AD pathology (i.e., entorhinal cortex, temporal cortex, and frontal cortex) and an area partially spared by the disease (i.e., cerebellum) from healthy controls (n = 27) and AD patients (n = 52) [20]. Not all subjects had tissue samples extracted from all four brain regions [20]. Entorhinal cortex AD patients were 83.9 ± 9.7 years old (vs. 71.9 ± 15.6 of control subjects), had a Braak stage of 4.9 ± 1 and a disease duration of 11.8 ± 5.2 years. Temporal cortex AD patients were 82.7 ± 9.8 years old (vs. 71.5 ± 16.9 of controls subjects), had a Braak stage of 4.9 ± 0.9 and a disease duration of 9.7 ± 5.4 years. Frontal cortex AD patients were 82.5 ± 4.7 years old (vs. 69.8 ± 15.4 of controls subjects), had a Braak stage of 4.9 ± 1 and a disease duration of 10.5 ± 5.7 years. Cerebellum AD patients were 82.6 ± 10.6 years old (vs. 69.4 ± 16 of controls subjects), had a Braak stage of 5.1 ± 0.3 and a disease duration of 9.4 ± 5.6 years. Principal Component Analysis (PCA) was used to evaluate the segregation of the samples using the predicted biomarkers.
2.3. Drug Prediction Analysis
The L1000FDW web-based utility [21] was used to identify potential novel pharmacological strategies for the treatment of AD. L1000FWD calculates the similarity between an input gene expression signature vector and the LINCS-L1000 data, in order to rank drugs potentially able to reverse the transcriptional signature [21]. The L1000 transcriptomic database is part of the Library of Integrated Network-based Cellular Signatures (LINCS) project, a NIH Common Fund program, that extended the Connectivity Map project and includes the transcriptional profiles of approximately 50 human cell lines upon exposure to about 20,000 compounds, over a range of concentrations and time [21]. An adjusted p-value (q-value) of 0.05 has been considered as threshold for statistical significance.
2.4. Statistical Analysis
GraphPad Prism (v. 8) and MeV (v. 4.9) software programs were used for the statistical analysis and the generation of the graphs. Differentially expression analysis, PCA and DAM have been performed using the MeV 4.9 software, which used R v.2.11.1 and LIMMA v3.4.5.
3. Results
3.1. Machine Learning-Identified Genes for the Diagnosis of AD
In order to identify a specific gene signature characterizing AD, we first interrogated the GSE117589 microarray dataset. LIMMA analysis identified 65 DEGs in NP cells from SAD patients as compared to Healthy controls, 30 upregulated and 35 downregulated. When analyzing iPSC-derived neurons, 386 DEGs were found, 131 upregulated and 255 downregulated in SAD patients as compared to Healthy controls. Gene Ontology analysis revealed a partial overlapping of enriched biological processes among the upregulated DEGs in AD NP cells and neurons, that included “regulation of ion transport”, “regulation of neuron differentiation”, “chemical synaptic transmission”, “neuron projection morphogenesis”, “negative regulation of cell differentiation” and “axon guidance” (Figure 2A,B).
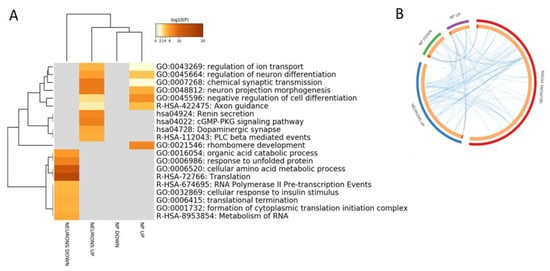
Figure 2.
(A) Hierarchical clustering of the top 20 most enriched terms by genes significantly modulated in Induced pluripotent stem cells (iPSCs)-derived neuronal progenitors cells (NP) and iPSCs-derived neurons from sporadic Alzheimer’s disease patients vs. healthy donors. The heatmap is colored by the p-values, and grey cells indicate the lack of significant enrichment; (B) Circos plot showing overlapping between the genes significantly modulated in iPSCs-derived neuronal progenitors cells (NP) and iPSCs-derived neurons from sporadic Alzheimer’s disease patients vs. healthy donors. Purple lines link the same genes that are shared by the input lists. Blue lines link the different genes that fall in the same ontology term.
Among the DEGs identified for the iPSC-derived neurons, five have been associated to AD by GWAS: SPON1, ANKRD55, RHOBTB22, TTLL7 and MRPL10. With the exception of MRPL10, which is downregulated, all of the other genes are upregulated in AD samples. None of the DEGs identified in iPSC-derived NP cells have been associated to AD.
Next, we employed the DAM analysis, in order to identify the lowest number of genes able to differentiate SAD patients from healthy individuals. A total of 10 predictors out of the 65 NP cells DEGs were identified from the DAM analysis. Consistent with these findings, HCL accurately segregated iPSC-derived NP cells from SAD patients from those obtained from non-demented controls (Figure 3A). The 10 identified predictors are presented in Table 1. In order to validate the reliability of the identified biomarkers, we performed a PCA on the entorhinal, frontal and temporal cortex, as well as on cerebellum, from healthy controls and AD patients. As shown in Figure 3B–D, a discrete separation of samples from healthy and AD subjects was observed for the entorhinal cortex. Only a partial segregation was observed for the temporal and frontal cortex (Figure 3B). An overlapping distribution of samples was instead observed for the cerebellum (Figure 3B).
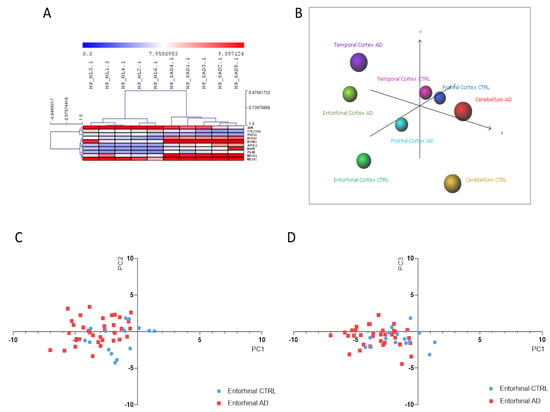
Figure 3.
(A) Hierarchical clustering of the Alzheimer’s disease (AD) biomarkers identified using the Discriminant Analysis Module (DAM) algorithm in the Induced pluripotent stem cells (iPSCs)-derived neuronal progenitors (NP) cells from sporadic Alzheimer’s disease patients vs. healthy donors (CTRL); (B) Principal Component Analysis (PCA) using the identified AD biomarkers on the samples from the GSE118553 dataset; (C) Scatterplot showing Principal Component (PC)1 and PC2 for the entorhinal samples from the GSE118553 dataset; (D) Scatterplot showing PC1 and PC3 for the entorhinal samples from the GSE118553 dataset.

Table 1.
List of biomarkers identified by DAM analysis in iPSC-derived NP cells 1.
As regards iPSC-derived neurons, DAM analysis identified 12 predictors out of 386 DEGs. Consistent with these findings, HCL accurately segregated iPSC-derived neurons from SAD patients from those obtained from non-demented controls (Figure 4A). The 12 identified predictors are presented in Table 2 and were used to perform a PCA analysis on samples of entorhinal, frontal and temporal cortex, as well as of cerebellum, from healthy controls and AD patients, obtained from the GSE118553 dataset. As shown in Figure 4B–D, a discrete separation of samples from healthy and AD subjects was observed.
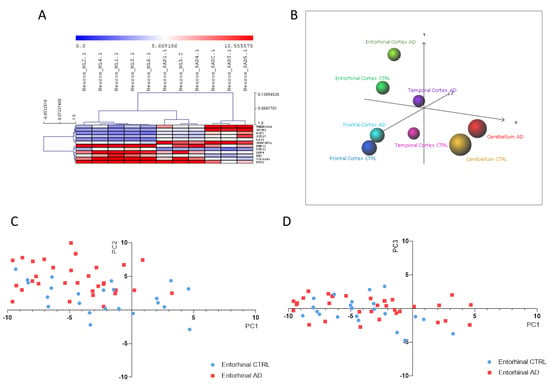
Figure 4.
(A) Hierarchical clustering of the Alzheimer’s disease (AD) biomarkers identified using the Discriminant Analysis Module (DAM) algorithm in the Induced pluripotent stem cells (iPSCs)-derived neurons from sporadic Alzheimer’s disease patients (SAD) vs. healthy donors; (B) Principal Component Analysis (PCA) using the identified AD biomarkers on the samples from the GSE118553 dataset; (C) Scatterplot showing PC1 and PC2 for the entorhinal samples from the GSE118553 dataset; (D) Scatterplot showing PC1 and PC3 for the entorhinal samples from the GSE118553 dataset.

Table 2.
List of biomarkers identified by DAM analysis in iPSC-derived neuronal cells 1.
3.2. Prediction of Novel Chemotherapeutics for AD
Anti-signature perturbation analysis was performed using the DEGs identified for the iPSCs-derived NP cells and neurons (Figure 5A,B, respectively). Among the significant predicted drugs, we have prioritized those already in clinical use. In Table 3, we have enlisted the potential anti-AD drugs identified by the L1000FWD analysis using the iPSC-derived NP cells model of AD. Among them, the top three drugs are: etacrynic-acid, a diuretic; cytarabine, a chemotherapy medication used to treat acute myeloid leukemia, acute lymphocytic leukemia, chronic myelogenous leukemia and non-Hodgkin’s lymphoma; and betamethasone, a corticosteroid. Table 4 contains a list of the potential anti-AD drugs identified using the iPSC-derived neuronal cells model of AD. Among them, the top three drugs are: cyclosporin-a, an immunesuppressive agent; dabrafenib, a B-raf inhibition used to treat melanoma; and penfluridol, indicated for antipsychotic treatment of schizophrenia and psychotic disorders. Interestingly, from our analysis, cyclosporin-a is the only drug that has been convergently predicted using both iPSC-derived NP and neuronal cells (Table 3 and Table 4).
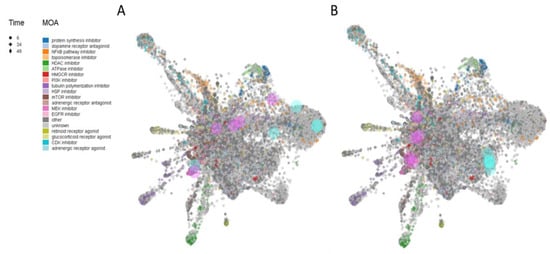
Figure 5.
L1000FDW visualization of drug-induced signature. Input genes are represented by the significantly upregulated and downregulated genes obtained from the analysis of the GSE117589 dataset, for iPSC-derived NP cells (A) and neuronal cells (B). Blue and red circles identify drugs with similar and anti-similar signatures. Dots are color-coded based on the Mode of Action (MOA) of the respective drug.

Table 3.
Potential anti-AD drugs identified by the L1000FWD analysis using the iPSC-derived NP cells model of AD.

Table 4.
Potential anti-AD drugs identified by the L1000FWD analysis using the iPSC-derived neuronal cells model of AD.
4. Discussion
Given the limited access to brain-derived neuronal cells, little information is still available on the pathogenic processes that characterize the initial phases of sporadic AD. Therefore, the use of in-vitro-based models that reflect AD-affected neurons may allow for early diagnosis, and to test preventive approaches for patient treatment. Recently, independent groups have differentiated cells from AD patients into neuronal progenitors and neuronal cells using iPSC-based methods, and evaluated them for the molecular mechanisms underlying disease development. These studies are thought to give valuable insights regarding AD molecular phenotypes, and could represent predictive models to be used in the future in a clinical setting.
We have here identified a gene-signature that could be used for the diagnosis of AD, by using a publicly available whole-genome transcriptomic dataset on iPSC-derived NP cells and neurons from AD patients and non-demented controls. Our analysis followed a more conservative approach than those used by Meyer and collaborators [1], resulting in a lower number of prioritized DEGs.
Interestingly, we observed that the identified AD biomarkers allowed to differentially segregate brain samples from healthy subjects and AD patients. When using the biomarkers identified using the NP cells, a better segregation was observed for the entorhinal cortex, while a poor segregation was observed for frontal cortex, temporal cortex and cerebellum. This is in line with the observation from Patel and collaborators [20] who described a higher percentage of perturbed genes in the entorhinal cortex, followed by progressively reduced numbers of DEGs in the temporal cortex, frontal cortex and, finally in the cerebellum [20]. This seems to reflect the pattern of AD progression and suggests that the iPSC-based model used in the present analysis may better mirror ab initio transcriptional defects underlying AD pathogenesis. Furthermore, gene ontology analysis revealed that these genes are involved in the regulation of cell differentiation and neurogenesis. These data support the hypothesis that the early identification of susceptible individuals is possible using iPSCs-based models. Furthermore, the biomarkers predicted using the iPSC-derived neurons showed a similar ability to discriminate AD from non-demented patients.
It is believed that early interventions that tackle factors that are associated and increase the relative risk of AD development, could drastically reduce the burden of dementia associated with AD at the population level. Indeed, such interventions could reduce the development of signs and symptoms of AD, preventing the progression from MCI to AD, and reducing the subclinical deficits in dementia individuals.
Typically, development of a new drug takes up to 15 years and requires between 2 to 3 billion dollars of investment. In addition, on average, only 10% of drugs entering phase I trials obtain approval for human use. The rest of the molecules are dropped because of toxicity issues or lack of efficacy [22,23]. Drug repurposing, i.e., finding novel indications for already approved drugs, overcomes these limitations, as toxicity, pharmacokinetic and pharmacodynamic are already available and consequently, the drugs can rapidly be tested in phase II-III trials, dramatically reducing development risk, time and cost. Nowadays, almost 30% of new drugs are repositioned drugs [22]. Drug repurposing can be investigated both experimentally and computationally (in silico) [24]. The latter is based on the evaluation of the anti-similarity between drugs and a disease [25,26,27]. To this aim, gene expression signatures obtained from -omics data [28] are used to discover novel mechanisms of disease and searches inverse drug–disease relationships by matching gene expression profiles. We and others have used whole-genome expression databases for the better understanding of pathogenic pathways and the prediction of diagnostic and therapeutic strategies for a series of disorders—e.g., immunoinflammatory and autoimmune diseases [29,30,31,32,33,34,35,36,37], and cancer [38,39]—which has led to the identification of potential novel therapeutic targets [40,41,42,43,44,45,46,47,48,49,50,51]. However, gene perturbation alone cannot accurately predict treatment options due to variability related to disease genetics and epigenetics, as well as, experimental settings. For instance, although arginase inhibitor was expected to increase neurotoxicity, in preclinical model of AD, it has been shown to exert protection in mice [52]. Limitations of our work rely on the fact that our model does not account for epigenetic and post-transcription modifications affecting the final phenotype. Furthermore, although drug gene perturbation signatures come from genes ubiquitously modulated across a series of cell lines, however, they are constructed on cell types strikingly different from those found in the central nervous system, and treatments are limited in terms of concentrations and time points. Finally, drug candidates for AD, and neurodegenerative disease in general, should also be selected on the basis on their ability to cross the blood–brain barrier.
Interestingly, in our study, cyclosporine-a was predicted to be a potential anti-AD drug, when using both the iPSC-derived NP cells and neurons. This is in line with recent data from Stallings’ group, showing that cyclosporine-a blocked dendritic spine loss in Aβ42-treated cells [53]. Furthermore, cyclosporine-a inhibited amyloid synthesis and improved amyloid induced neurotoxicity in neuroblastoma cells [54]. Finally, a pilot open-label study of tacrolimus, which shares the same mode of action with cyclosporin-a in AD (ClinicalTrials.gov Identifier: NCT04263519) is expected to be completed by December 2021.
In addition, our analysis has identified the corticosteroids, betamethasone and triamcinolone, as potential anti-AD drugs. This seems consistent with a post mortem study conducted by Beeri et al. [55] on 694 brains of subjects who did not have neuropathologies other than neuritic plaques (NPs), neurofibrillary tangles (NFTs), or cerebrovascular disease, that patients receiving corticosteroids had significantly lower ratings and counts of NPs for all neuropathological measures, and NFTs overall and in the cerebral cortex and amygdala. In contrast, no significance was observed for subjects who received NSAIDs. AD has been linked to neuroinflammation [56], and biochemical and neuropathological studies on AD brains provide evidence for the activation of inflammatory pathways and glial inflammation [57]. Notably, women are more susceptible to developing immunoinflammatory disorders than men [58], and accordingly, the estimated lifetime risk of developing AD shows a female to male ratio of 1.8. Based on these observations, the nasal administration of corticosteroids has been proposed for the early stages of AD [59]. On the other hand, preclinical studies have shown conflicting effects of glucocorticoids on CNS, as hypersecretion was shown to contribute to age-related hippocampal degeneration [60].
5. Conclusions
Our study set the basis for the identification of biomarkers for the early diagnosis of AD, using the low invasive model of iPSC-derived neuronal cells. Indeed, the use of imaging techniques or the measurement of CSF markers is difficult to achieve for the costs and invasive procedures. Furthermore, blood biomarkers have not yet given satisfactory results as diagnostic tools in AD. However, this is an exploratory study and future studies on larger cohorts of patients with SAD are needed to validate the data here generated. Furthermore, since other cellular types—including astrocytes and microglia—are likely to be directly involved in the etiopathogenesis of AD, future studies aimed at investigating potential glial-related biomarkers are warranted. Finally, the single and combined administration of the potential anti-AD drugs that has emerged from our study seems worthy being evaluated in preclinical models of AD to exploit the translatability of these findings to the clinical setting.
Author Contributions
Conceptualization, P.F., F.N., and K.M.; methodology, P.F.; formal analysis, E.C., M.P., M.S.B., S.D.L. and M.C.P.; investigation, M.S.B., S.D.L. and M.C.P.; resources, F.N.; writing—original draft preparation, E.C., M.S.B., and M.C.P; writing—review and editing, G.B., V.B., R.K., L.T., P.F., F.N. and K.M..; visualization, E.C., M.S.B. and P.F.; supervision, P.F., F.N. and K.M.; project administration, F.N. All the authors significantly contributed to the preparation of the manuscript and approved the submitted version of the paper.
Funding
This study was supported by current research funds 2019 of IRCCS “Neuromed”, Pozzilli, Italy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Meyer, K.; Feldman, H.M.; Lu, T.; Drake, D.; Lim, E.T.; Ling, K.-H.; Bishop, N.A.; Pan, Y.; Seo, J.; Lin, Y.-T.; et al. REST and Neural Gene Network Dysregulation in iPSC Models of Alzheimer’s Disease. Cell Rep. 2019, 26, 1112–1127. [Google Scholar] [CrossRef]
- Lashley, T.; Schott, J.M.; Weston, P.; Murray, C.E.; Wellington, H.; Keshavan, A.; Foti, S.C.; Foiani, M.; Toombs, J.; Rohrer, J.D.; et al. Molecular biomarkers of Alzheimer’s disease: Progress and prospects. Dis. Model. Mech. 2018, 11, dmm031781. [Google Scholar] [CrossRef]
- Joe, E.; Ringman, J.M. Cognitive symptoms of Alzheimer’s disease: Clinical management and prevention. BMJ 2019, 367, l6217. [Google Scholar] [CrossRef]
- L Ferreira, I.; Resende, R.; Ferreiro, E.; Rego, C.A.; Pereira, F.C. Multiple Defects in Energy Metabolism in Alzheimers Disease. Curr. Drug Targets 2010, 11, 1193–1206. [Google Scholar] [CrossRef]
- Abarova, S.; Koynova, R.; Tancheva, L.; Tenchov, B. A novel DSC approach for evaluating protectant drugs efficacy against dementia. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2934–2941. [Google Scholar] [CrossRef] [PubMed]
- Fessel, J. Prevention of Alzheimer’s disease by treating mild cognitive impairment with combinations chosen from eight available drugs. Alzheimer Dement. 2019, 5, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Israel, M.A.; Yuan, S.H.; Bardy, C.; Reyna, S.M.; Mu, Y.; Herrera, C.; Hefferan, M.P.; Van Gorp, S.; Nazor, K.L.; Boscolo, F.S.; et al. Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature 2012, 482, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Muratore, C.R.; Rice, H.C.; Srikanth, P.; Callahan, D.G.; Shin, T.; Benjamin, L.N.P.; Walsh, D.M.; Selkoe, D.J.; Young-Pearse, T.L. The familial Alzheimer’s disease APPV717I mutation alters APP processing and Tau expression in iPSC-derived neurons. Hum. Mol. Genet. 2014, 23, 3523–3536. [Google Scholar] [CrossRef]
- Kondo, T.; Asai, M.; Tsukita, K.; Kutoku, Y.; Ohsawa, Y.; Sunada, Y.; Imamura, K.; Egawa, N.; Yahata, N.; Okita, K.; et al. Modeling Alzheimer’s Disease with iPSCs Reveals Stress Phenotypes Associated with Intracellular Aβ and Differential Drug Responsiveness. Cell Stem Cell 2013, 12, 487–496. [Google Scholar] [CrossRef]
- Sproul, A.A.; Vensand, L.B.; Dusenberry, C.R.; Jacob, S.; Vonsattel, J.P.G.; Paull, D.J.; Shelanski, M.L.; Crary, J.F.; Noggle, S.A. Generation of iPSC lines from archived non-cryoprotected biobanked dura mater. Acta Neuropathol. Commun. 2014, 2, 4. [Google Scholar] [CrossRef]
- Yagi, T.; Ito, D.; Okada, Y.; Akamatsu, W.; Nihei, Y.; Yoshizaki, T.; Yamanaka, S.; Okano, H.; Suzuki, N. Modeling familial Alzheimer’s disease with induced pluripotent stem cells. Hum. Mol. Genet. 2011, 20, 4530–4539. [Google Scholar] [CrossRef] [PubMed]
- Knoferle, J.; Yoon, S.Y.; Walker, D.; Leung, L.; Gillespie, A.K.; Tong, L.M.; Bien-Ly, N.; Huang, Y. Apolipoprotein E4 Produced in GABAergic Interneurons Causes Learning and Memory Deficits in Mice. J. Neurosci. 2014, 34, 14069–14078. [Google Scholar] [CrossRef]
- Dimos, J.T.; Rodolfa, K.T.; Niakan, K.K.; Weisenthal, L.M.; Mitsumoto, H.; Chung, W.; Croft, G.F.; Saphier, G.; Leibel, R.; Goland, R.; et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science 2008, 321, 1218–1221. [Google Scholar] [CrossRef] [PubMed]
- Soldner, F.; Hockemeyer, D.; Beard, C.; Gao, Q.; Bell, G.W.; Cook, E.G.; Hargus, G.; Blak, A.; Cooper, O.; Mitalipova, M.; et al. Parkinson’s Disease Patient-Derived Induced Pluripotent Stem Cells Free of Viral Reprogramming Factors. Cell 2009, 136, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Marchetto, M.C.N.; Carromeu, C.; Acab, A.; Yu, D.; Yeo, G.W.; Mu, Y.; Chen, G.; Gage, F.H.; Muotri, A.R. A Model for Neural Development and Treatment of Rett Syndrome Using Human Induced Pluripotent Stem Cells. Cell 2010, 143, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Brennand, K.J.; Simone, A.; Jou, J.; Gelboin-Burkhart, C.; Tran, N.; Sangar, S.; Li, Y.; Mu, Y.; Chen, G.; Yu, D.; et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature 2011, 473, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Park, I.-H.; Arora, N.; Huo, H.; Maherali, N.; Ahfeldt, T.; Shimamura, A.; Lensch, M.W.; Cowan, C.; Hochedlinger, K.; Daley, G.Q. Disease-specific induced pluripotent stem cells. Cell 2008, 134, 877–886. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Nguyen, D.V.; Rocke, D.M. Tumor classification by partial least squares using microarray gene expression data. Bioinformatics 2002, 18, 39–50. [Google Scholar] [CrossRef]
- Patel, H.; Hodges, A.K.; Curtis, C.; Lee, S.H.; Troakes, C.; Dobson, R.J.B.; Newhouse, S.J. Transcriptomic analysis of probable asymptomatic and symptomatic alzheimer brains. Brain Behav. Immun. 2019, 80, 644–656. [Google Scholar] [CrossRef]
- Wang, Z.; Lachmann, A.; Keenan, A.B.; Ma’Ayan, A. L1000FWD: Fireworks visualization of drug-induced transcriptomic signatures. Bioinformatics 2018, 34, 2150–2152. [Google Scholar] [CrossRef] [PubMed]
- Plenge, R.M.; Scolnick, E.M.; Altshuler, D. Validating therapeutic targets through human genetics. Nat. Rev. Drug Discov. 2013, 12, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Scannell, J.W.; Blanckley, A.; Boldon, H.; Warrington, B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat. Rev. Drug Discov. 2012, 11, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.S.; Liu, J.O. Recent Advances in Drug Repositioning for the Discovery of New Anticancer Drugs. Int. J. Biol. Sci. 2014, 10, 654–663. [Google Scholar] [CrossRef]
- Liu, Z.; Fang, H.; Reagan, K.; Xu, X.; Mendrick, D.L.; Slikker, W.; Tong, W. In silico drug repositioning–What we need to know. Drug Discov. Today 2013, 18, 110–115. [Google Scholar] [CrossRef]
- Hodos, R.A.; Kidd, B.A.; Shameer, K.; Readhead, B.P.; Dudley, J.T. In silico methods for drug repurposing and pharmacology. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 186–210. [Google Scholar] [CrossRef]
- Jin, G.; Wong, S.T.C. Toward better drug repositioning: Prioritizing and integrating existing methods into efficient pipelines. Drug Discov. Today 2014, 19, 637–644. [Google Scholar] [CrossRef]
- Jadamba, E.; Shin, M. A Systematic Framework for Drug Repositioning from Integrated Omics and Drug Phenotype Profiles Using Pathway-Drug Network. BioMed Res. Int. 2016, 2016, 7147039. [Google Scholar] [CrossRef]
- Lombardo, S.D.; Mazzon, E.; Mangano, K.; Basile, M.S.; Cavalli, E.; Mammana, S.; Fagone, P.; Nicoletti, F.; Petralia, M.C. Transcriptomic Analysis Reveals Involvement of the Macrophage Migration Inhibitory Factor Gene Network in Duchenne Muscular Dystrophy. Genes 2019, 10, 939. [Google Scholar] [CrossRef]
- Mangano, K.; Cavalli, E.; Mammana, S.; Basile, M.S.; Caltabiano, R.; Pesce, A.; Puleo, S.; Atanasov, A.G.; Magro, G.; Nicoletti, F.; et al. Involvement of the Nrf2/HO-1/CO axis and therapeutic intervention with the CO-releasing molecule CORM-A1, in a murine model of autoimmune hepatitis. J. Cell. Physiol. 2018, 233, 4156–4165. [Google Scholar] [CrossRef]
- Cavalli, E.; Mazzon, E.; Basile, M.S.; Mangano, K.; Di Marco, R.; Bramanti, P.; Nicoletti, F.; Fagone, P.; Petralia, M.C. Upregulated Expression of Macrophage Migration Inhibitory Factor, Its Analogue D-Dopachrome Tautomerase, and the CD44 Receptor in Peripheral CD4 T Cells from Clinically Isolated Syndrome Patients with Rapid Conversion to Clinical Defined Multiple Sclerosis. Medicina 2019, 55, 667. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.D.; Mazzon, E.; Basile, M.S.; Cavalli, E.; Bramanti, P.; Nania, R.; Fagone, P.; Nicoletti, F.; Petralia, M.C. Upregulation of IL-1 Receptor Antagonist in a Mouse Model of Migraine. Brain Sci. 2019, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, F.; Mazzon, E.; Fagone, P.; Mangano, K.; Mammana, S.; Cavalli, E.; Basile, M.S.; Bramanti, P.; Scalabrino, G.; Lange, A.; et al. Prevention of clinical and histological signs of MOG-induced experimental allergic encephalomyelitis by prolonged treatment with recombinant human EGF. J. Neuroimmunol. 2019, 332, 224–232. [Google Scholar] [CrossRef]
- Cavalli, E.; Mazzon, E.; Basile, M.S.; Mammana, S.; Pennisi, M.; Fagone, P.; Kalfin, R.; Martinovic, V.; Ivanovic, J.; Andabaka, M.; et al. In Silico and In Vivo Analysis of IL37 in Multiple Sclerosis Reveals Its Probable Homeostatic Role on the Clinical Activity, Disability, and Treatment with Fingolimod. Molecules 2019, 25, 20. [Google Scholar] [CrossRef] [PubMed]
- Basile, M.S.; Mazzon, E.; Mangano, K.; Pennisi, M.; Petralia, M.C.; Lombardo, S.D.; Nicoletti, F.; Fagone, P.; Cavalli, E. Impaired Expression of Tetraspanin 32 (TSPAN32) in Memory T Cells of Patients with Multiple Sclerosis. Brain Sci. 2020, 10, 52. [Google Scholar] [CrossRef]
- Lombardo, S.D.; Mazzon, E.; Basile, M.S.; Campo, G.; Corsico, F.; Presti, M.; Bramanti, P.; Mangano, K.; Petralia, M.C.; Nicoletti, F.; et al. Modulation of Tetraspanin 32 (TSPAN32) Expression in T Cell-Mediated Immune Responses and in Multiple Sclerosis. Int. J. Mol. Sci. 2019, 20, 4323. [Google Scholar] [CrossRef]
- Petralia, M.C.; Mazzon, E.; Fagone, P.; Falzone, L.; Bramanti, P.; Nicoletti, F.; Basile, M.S. Retrospective follow-up analysis of the transcriptomic patterns of cytokines, cytokine receptors and chemokines at preconception and during pregnancy, in women with post-partum depression. Exp. Ther. Med. 2019, 18, 2055–2062. [Google Scholar] [CrossRef]
- Cavalli, E.; Mazzon, E.; Mammana, S.; Basile, M.S.; Lombardo, S.D.; Mangano, K.; Bramanti, P.; Nicoletti, F.; Fagone, P.; Petralia, M.C. Overexpression of Macrophage Migration Inhibitory Factor and Its Homologue D-Dopachrome Tautomerase as Negative Prognostic Factor in Neuroblastoma. Brain Sci. 2019, 9, 284. [Google Scholar] [CrossRef]
- Petralia, M.C.; Mazzon, E.; Fagone, P.; Russo, A.; Longo, A.; Avitabile, T.; Nicoletti, F.; Reibaldi, M.; Basile, M.S. Characterization of the Pathophysiological Role of CD47 in Uveal Melanoma. Molecules 2019, 24, 2450. [Google Scholar] [CrossRef]
- Fagone, P.; Mangano, K.; Quattrocchi, C.; Cavalli, E.; Mammana, S.; Lombardo, G.A.G.; Pennisi, V.; Zocca, M.-B.; He, M.; Al-Abed, Y.; et al. Effects of NO-Hybridization on the Immunomodulatory Properties of the HIV Protease Inhibitors Lopinavir and Ritonavir. Basic Clin. Pharmacol. Toxicol. 2015, 117, 306–315. [Google Scholar] [CrossRef]
- Maksimovic-Ivanic, D.; Mojic, M.; Bulatovic, M.; Radojkovic, M.; Kuzmanovic, M.; Ristic, S.; Stosic-Grujicic, S.; Miljkovic, D.; Cavalli, E.; Libra, M.; et al. The NO-modified HIV protease inhibitor as a valuable drug for hematological malignancies: Role of p70S6K. Leuk. Res. 2015, 39, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Mammana, S.; Cavalli, E.; Gugliandolo, A.; Silvestro, S.; Pollastro, F.; Bramanti, P.; Mazzon, E. Could the Combination of Two Non-Psychotropic Cannabinoids Counteract Neuroinflammation? Effectiveness of Cannabidiol Associated with Cannabigerol. Medicina 2019, 55, 747. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, E.; Mammana, S.; Nicoletti, F.; Bramanti, P.; Mazzon, E. The neuropathic pain: An overview of the current treatment and future therapeutic approaches. Int. J. Immunopathol. Pharmacol. 2019, 33. [Google Scholar] [CrossRef] [PubMed]
- Paskas, S.; Mazzon, E.; Basile, M.S.; Cavalli, E.; Al-Abed, Y.; He, M.; Rakocevic, S.; Nicoletti, F.; Mijatovic, S.; Maksimovic-Ivanic, D. Lopinavir-NO, a nitric oxide-releasing HIV protease inhibitor, suppresses the growth of melanoma cells in vitro and in vivo. Investig. New Drugs 2019, 37, 1014–1028. [Google Scholar] [CrossRef] [PubMed]
- Basile, M.S.; Mazzon, E.; Krajnovic, T.; Draca, D.; Cavalli, E.; Al-Abed, Y.; Bramanti, P.; Nicoletti, F.; Mijatovic, S.; Maksimovic-Ivanic, D. Anticancer and Differentiation Properties of the Nitric Oxide Derivative of Lopinavir in Human Glioblastoma Cells. Molecules 2018, 23, 2463. [Google Scholar] [CrossRef]
- Mammana, S.; Bramanti, P.; Mazzon, E.; Cavalli, E.; Basile, M.S.; Fagone, P.; Petralia, M.C.; McCubrey, J.A.; Nicoletti, F.; Mangano, K. Preclinical evaluation of the PI3K/Akt/mTOR pathway in animal models of multiple sclerosis. Oncotarget 2018, 9, 8263–8277. [Google Scholar] [CrossRef]
- Mangano, K.; Mazzon, E.; Basile, M.S.; Di Marco, R.; Bramanti, P.; Mammana, S.; Petralia, M.C.; Fagone, P.; Nicoletti, F. Pathogenic role for macrophage migration inhibitory factor in glioblastoma and its targeting with specific inhibitors as novel tailored therapeutic approach. Oncotarget 2018, 9, 17951. [Google Scholar] [CrossRef]
- Mammana, S.; Fagone, P.; Cavalli, E.; Basile, M.S.; Petralia, M.C.; Nicoletti, F.; Bramanti, P.; Mazzon, E. The role of macrophages in neuroinflammatory and neurodegenerative pathways of alzheimer’s disease, amyotrophic lateral sclerosis, and multiple sclerosis: Pathogenetic cellular effectors and potential therapeutic targets. Int. J. Mol. Sci. 2018, 19, 831. [Google Scholar] [CrossRef]
- Petralia, M.C.; Battaglia, G.; Bruno, V.; Pennisi, M.; Mangano, K.; Lombardo, S.D.; Fagone, P.; Cavalli, E.; Saraceno, A.; Nicoletti, F.; et al. The Role of Macrophage Migration Inhibitory Factor in Alzheimer’s Disease: Conventionally Pathogenetic or Unconventionally Protective? Molecules 2020, 25, 291. [Google Scholar] [CrossRef]
- Petralia, M.C.; Mazzon, E.; Fagone, P.; Basile, M.S.; Lenzo, V.; Quattropani, M.C.; Bendtzen, K.; Nicoletti, F. Pathogenic contribution of the Macrophage migration inhibitory factor family to major depressive disorder and emerging tailored therapeutic approaches. J. Affect. Disord. 2020, 263, 15–24. [Google Scholar] [CrossRef]
- Fagone, P.; Mangano, K.; Mammana, S.; Cavalli, E.; Di Marco, R.; Barcellona, M.L.; Salvatorelli, L.; Magro, G.; Nicoletti, F. Carbon monoxide-releasing molecule-A1 (CORM-A1) improves clinical signs of experimental autoimmune uveoretinitis (EAU) in rats. Clin. Immunol. 2015, 157, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Kan, M.J.; Lee, J.E.; Wilson, J.G.; Everhart, A.L.; Brown, C.M.; Hoofnagle, A.N.; Jansen, M.; Vitek, M.P.; Gunn, M.D.; Colton, C.A. Arginine deprivation and immune suppression in a mouse model of Alzheimer’s disease. J. Neurosci. 2015, 35, 5969–5982. [Google Scholar] [CrossRef] [PubMed]
- Stallings, N.R.; O’Neal, M.A.; Hu, J.; Kavalali, E.T.; Bezprozvanny, I.; Malter, J.S. Pin1 mediates Aβ42-induced dendritic spine loss. Sci. Signal. 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, S.; Mujeeb, A.A.; Owais, M. Cyclic undecapeptide Cyclosporin A mediated inhibition of amyloid synthesis: Implications in alleviation of amyloid induced neurotoxicity. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Beeri, M.S.; Schmeidler, J.; Lesser, G.T.; Maroukian, M.; West, R.; Leung, S.; Wysocki, M.; Perl, D.P.; Purohit, D.P.; Haroutunian, V. Corticosteroids, but not NSAIDs, are associated with less Alzheimer neuropathology. Neurobiol. Aging 2012, 33, 1258–1264. [Google Scholar] [CrossRef]
- McManus, R.M.; Heneka, M.T. Role of neuroinflammation in neurodegeneration: New insights. Alzheimer Res. Ther. 2017, 9, 14. [Google Scholar] [CrossRef]
- D’Andrea, M.R.; Cole, G.M.; Ard, M.D. The microglial phagocytic role with specific plaque types in the Alzheimer disease brain. Neurobiol. Aging 2004, 25, 675–683. [Google Scholar] [CrossRef]
- Desai, M.K.; Brinton, R.D. Autoimmune Disease in Women: Endocrine Transition and Risk across the Lifespan. Front. Endocrinol. 2019, 10, 265. [Google Scholar] [CrossRef]
- Lehrer, S.; Rheinstein, P.H. Nasal steroids as a possible treatment for Alzheimer’s disease. Discov. Med. 2017, 24, 147–152. [Google Scholar]
- Conrad, C.D. Chronic Stress-induced Hippocampal Vulnerability: The Glucocorticoid Vulnerability Hypothesis. Rev. Neurosci. 2008, 19, 395–412. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).