Improving Electrochemical Performance at Graphite Negative Electrodes in Concentrated Electrolyte Solutions by Addition of 1,2-Dichloroethane
Abstract
:1. Introduction
2. Materials and Methods
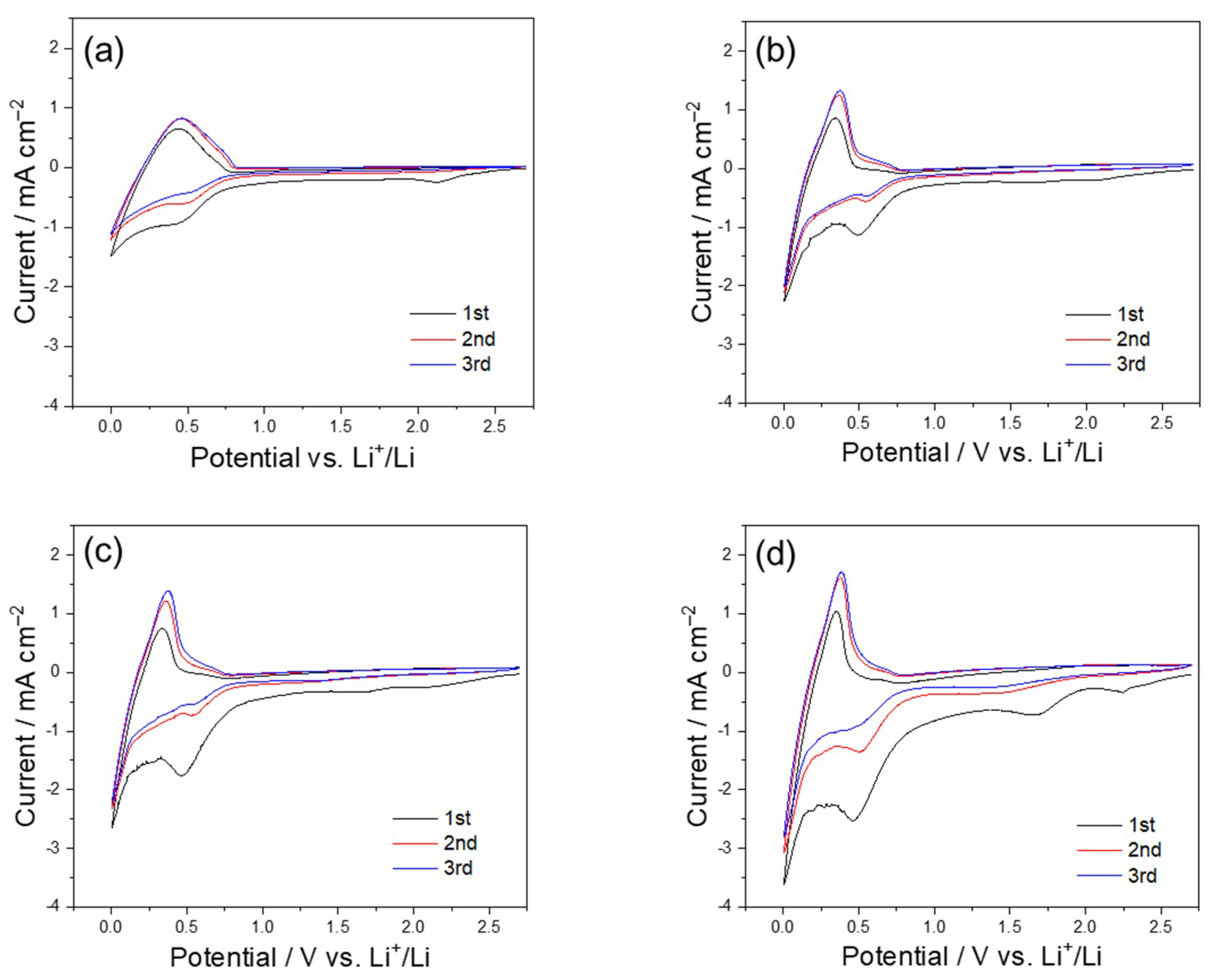
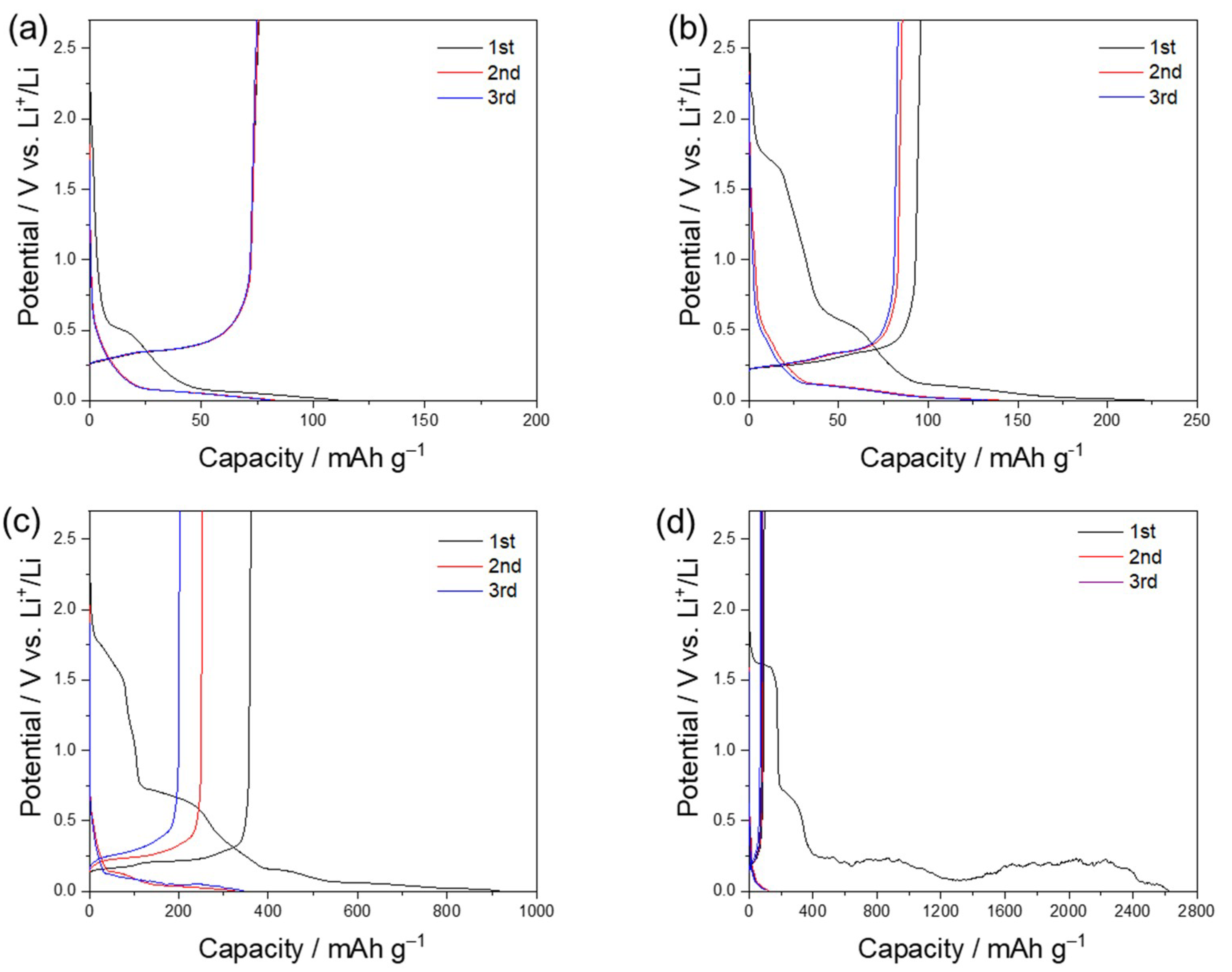
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef] [PubMed]
- Peled, E. The electrochemical behavior of alkali and alkaline earth metals in nonaqueous battery systems—The solid electrolyte interphase model. J. Electrochem. Soc. 1979, 126, 2047–2051. [Google Scholar] [CrossRef]
- Guyomard, D.; Tarascon, J.M. The carbon/Li1+xMn2O4 system. Solid State Ion. 1994, 69, 222–237. [Google Scholar] [CrossRef]
- Guyomard, D.; Tarascon, J.M. Li metal-free rechargeable LiMn2O4/carbon cells: Their understanding and optimization. J. Electrochem. Soc. 1992, 139, 937–948. [Google Scholar] [CrossRef]
- Aurbach, D.; Levi, M.D.; Levi, E.; Schechter, A. failure and stabilization mechanisms of graphite electrodes. J. Phys. Chem. B 1997, 101, 2195–2206. [Google Scholar] [CrossRef]
- Song, H.-Y.; Jeong, S.-K. Investigating continuous co-intercalation of solvated lithium ions and graphite exfoliation in propylene carbonate-based electrolyte solutions. J. Power Sources 2018, 373, 110–118. [Google Scholar] [CrossRef]
- Jache, B.; Binder, J.O.; Abe, T.; Adelhelm, P. A comparative study on the impact of different glymes and their derivatives as electrolyte solvents for graphite co-intercalation electrodes in lithium-ion and sodium-ion batteries. Phys. Chem. Chem. Phys. 2016, 18, 14299–14316. [Google Scholar] [CrossRef]
- Jeong, S.-K.; Inaba, M.; Iriyama, Y.; Abe, T.; Ogumi, Z. Interfacial reactions between graphite electrodes and propylene carbonate-based solutions: Electrolyte-concentration dependence of electrochemical lithium intercalation reaction. J. Power Sources 2008, 175, 540–546. [Google Scholar] [CrossRef]
- Jeong, S.-K.; Inaba, M.; Iriyama, Y.; Abe, T.; Ogumi, Z. Electrochemical intercalation of lithium ion within graphite from propylene carbonate solutions. Elecrochem. Solid-State Lett. 2003, 6, A13–A15. [Google Scholar] [CrossRef]
- Yamada, Y.; Yaegashi, M.; Abe, T.; Yamada, A. A superconcentrated ether electrolyte for fast-charging Li-ion batteries. Chem. Commun. 2013, 49, 11194–11196. [Google Scholar] [CrossRef]
- Sodeyama, K.; Yamada, Y.; Aikawa, K.; Yamada, A.; Tateyama, Y. Sacrificial anion reduction mechanism for electrochemical stability improvement in highly concentrated Li-salt electrolyte. J. Phys. Chem. C 2014, 118, 14091–14097. [Google Scholar] [CrossRef]
- Song, H.-Y.; Fukutsuka, T.; Miyazaki, K.; Abe, T. Suppression of co-intercalation reaction of propylene carbonate and lithium ion into graphite negative electrode by addition of diglyme. J. Electrochem. Soc. 2016, 163, A1265–A1269. [Google Scholar] [CrossRef]
- Fukutsuka, T.; Kokumai, R.; Song, H.-Y.; Takeuchi, S.; Miyazaki, K.; Abe, T. In situ AFM observation of surface morphology of highly oriented pyrolytic graphite in propylene carbonate-based electrolyte solutions containing lithium and bivalent cations. J. Electrochem. Soc. 2017, 164, A48–A53. [Google Scholar] [CrossRef]
- Takeuchi, S.; Kokumai, R.; Nagata, S.; Fukutsuka, T.; Miyazaki, K.; Abe, T. Effect of the addition of bivalent ions on electrochemical lithium-ion intercalation at graphite electrodes. J. Electrochem. Soc. 2016, 163, A1693–A1696. [Google Scholar] [CrossRef]
- Kasnatscheew, J.; Wagner, R.; Winter, M.; Cekic-Laskovic, I. Interfaces and materials in lithium ion batteries: Challenges for theoretical electrochemistry. Top. Curr. Chem. 2018, 376, 16. [Google Scholar] [CrossRef] [PubMed]
- Siffert, B.; Eleli-Letsango, J.; Jada, A.; Papirer, E. Experimental determination of the electron donor and acceptor numbers of oxides by zetametry in organic media. Colloids Surf. A 1994, 92, 107–111. [Google Scholar] [CrossRef]
- Kondo, K.; Sano, M.; Hiwara, A.; Omi, T.; Fujita, M.; Kuwae, A.; Iida, M.; Mogi, K.; Yokoyama, H. Conductivity and solvation of Li+ ions of LiPF6 in propylene carbonate solutions. J. Phys. Chem. B 2000, 104, 5040–5044. [Google Scholar] [CrossRef]
- Battisti, D.; Nazri, A.; Klassen, B.; Aroca, R. Vibrational studies of lithium perchlorate in propylene carbonate solutions. J. Phys. Chem. 1993, 97, 5826–5830. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, W.; Huang, X.; Mo, Y.; Chen, L. Spectroscopic studies on interactions and microstructures in propylene carbonate—LiTFSI electrolytes. J. Raman Spectrosc. 2001, 32, 900–905. [Google Scholar] [CrossRef]
- Huang, W.; Frech, R.; Johansson, P.; Lindgren, J. Cation-polymer interaction and ionic association in diglyme-LiCF3SO3 and diglyme-propylene carbonate-LiCF3SO3 complexes. Electrochim. Acta 1995, 40, 2147–2151. [Google Scholar] [CrossRef]
- Mizushima, S.; Shimanouchi, T.; Harada, I.; Abe, Y.; Takeuchi, H. Infrared and Raman spectra of 1,2-dichloroethane and its deuterium compound in the gaseous, liquid, and solid states. Can. J. Phys. 1975, 53, 2085–2094. [Google Scholar] [CrossRef]
- Kato, M.; Abe, I.; Taniguchi, Y. Raman study of the trans–gauche conformational equilibrium of 1,2-dichloroethane in water: Experimental evidence for the hydrophobic effect. J. Chem. Phys. 1999, 110, 11982–11986. [Google Scholar] [CrossRef]

| Electrolyte Solution | Concentration (mol kg−1) | Molar Ratio (PC/LiPF6/DCE) | Viscosity (Pa s) | Ionic Conductivity (mS cm−1) |
|---|---|---|---|---|
| PLD310 | 3.27 | 3:1:0 | 0.580 | 0.41 |
| PLD621 | 2.81 | 6:2:1 | 0.117 | 0.85 |
| PLD311 | 2.47 | 3:1:1 | 0.059 | 1.31 |
| PLD312 | 1.98 | 3:1:2 | 0.030 | 2.84 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, H.-Y.; Jung, M.-H.; Jeong, S.-K. Improving Electrochemical Performance at Graphite Negative Electrodes in Concentrated Electrolyte Solutions by Addition of 1,2-Dichloroethane. Appl. Sci. 2019, 9, 4647. https://doi.org/10.3390/app9214647
Song H-Y, Jung M-H, Jeong S-K. Improving Electrochemical Performance at Graphite Negative Electrodes in Concentrated Electrolyte Solutions by Addition of 1,2-Dichloroethane. Applied Sciences. 2019; 9(21):4647. https://doi.org/10.3390/app9214647
Chicago/Turabian StyleSong, Hee-Youb, Moon-Hyung Jung, and Soon-Ki Jeong. 2019. "Improving Electrochemical Performance at Graphite Negative Electrodes in Concentrated Electrolyte Solutions by Addition of 1,2-Dichloroethane" Applied Sciences 9, no. 21: 4647. https://doi.org/10.3390/app9214647
APA StyleSong, H.-Y., Jung, M.-H., & Jeong, S.-K. (2019). Improving Electrochemical Performance at Graphite Negative Electrodes in Concentrated Electrolyte Solutions by Addition of 1,2-Dichloroethane. Applied Sciences, 9(21), 4647. https://doi.org/10.3390/app9214647







