Abstract
Tannic acid (TA) is a major pollutant present in the wastewater generated from vegetable tanneries process and food processing. This work studied TA degradation by two advanced oxidation processes (APOs): UV irradiation at the wavelength of 254 nm in the presence of hydrogen peroxide (H2O2) and ferrous iron (photo-Fenton) and in the presence of potassium persulfate. The influence of certain experimental parameters such as K2S2O8, H2O2, Fe2+, and TA concentrations, initial pH and temperature was evaluated in order to obtain the highest efficiency in terms of aromatics (decay in UV absorbance at 276 nm) and TOC removals. Chemical oxidation of TA (0.1 mM) by UV/persulfate achieved 96.32% of aromatics removal and 54.41% of TOC removal under optimized conditions of pH = 9 and 53.10 mM of K2S2O8 after 60 min. The treatment of TA by photo-Fenton process successfully led to almost complete aromatics removal (99.32%) and high TOC removal (94.27%) from aqueous solutions containing 0.1 mM of TA at natural pH = 3 using 29.4 mM of H2O2 and 0.18 mM of Fe2+ at 25 °C after 120 min. More efficient degradation of TA by photo-Fenton process than UV/persulfate was obtained, which confirms that hydroxyl radicals are more powerful oxidants than sulfate radicals. The complete removal of organic pollution from natural waters can be accomplished by direct chemical oxidation via hydroxyl radicals generated from photocatalytic decomposition of H2O2.
1. Introduction
Tannic acid (penta-m-digallolyl glucose) (TA) is the simplest principal member of a specific group of hydrolysable tannins [1,2,3]. The formula of commercial TA, C76H52O46 (Figure 1), involves a large number of reactive functional groups, such as hydroxyl and phenolic hydroxyl. It is a water-soluble polyphenolic material with high molecular weights, between 500 and 3000 Da [4,5]. TA is a natural compound found in bananas, sorghum, coffee, and tea [6,7]. It is also found in industrial wastewaters, which is an emerging problem because of their harmful influence on natural ecosystems [5,8]. It causes liver, kidney, and central nervous system problems due to its high toxicity [9,10,11].
Figure 1.
Structure and properties of Tannic Acid.
TA is recalcitrant to conventional biological treatments and other methods [8,12,13,14,15,16]. In our laboratory, electrocoagulation process has been successfully used as a method for treating wastewaters contaminated with TA, but large amounts of precipitates were discharged at the end of the treatment [17]. Recently, advanced oxidation processes (AOPs) based on the generation of hydroxyl radicals (HO•) from H2O2 have been suggested to eliminate TA [18]. The photochemical system UV/H2O2 has also been recently used for TA degradation. This process generates good quality of treated water in comparison with other AOPs, but the amounts of hydroxyl radicals are obtained mainly by H2O2 decomposition with UV irradiation, which is a high-energy consumption process. Therefore, it is essential to find more efficient and low-cost alternative technology processes to eliminate TA from water. To achieve this goal, AOPs such as UV/persulfate and photo-Fenton processes have been considered as useful options. AOPs, based on the formation of sulfate radical SO4•– and hydroxyl radical HO•, have been used in in the treatment of wastewater containing bio-recalcitrant organic pollutants/toxicants recalcitrant compounds to convert them into biodegradable products [18,19,20,21].
Persulfate (K2S2O8) salts decompose in water into persulfate anion (S2O82−). It is a very strong oxidant (E° = 2.05 V/NHE), which is not selectively reactive and is relatively stable at room temperature [22,23]. However, it is kinetically slow in reacting with many organics at ambient temperatures [24,25]. The photochemical activated degradation of S2O82− ion to sulfate radical (SO4•−) has been used as a technique to accelerate the procedure [24,26,27]. Sulfate radical (SO4•−) is a very strong oxidant for its high standard redox potential (2.5–3.1 V) with a kinetically fast reacting tendency [20,28]. In general, SO4•− can be generated via photolysis, ultrasonic thermolysis, or activation by transition metals of persulfate or peroxymonosulfate for the oxidation of various organic pollutants [29,30,31,32,33,34]. UV/S2O82− has been considered as a highly efficient method to destruct different organic pollutants [35,36]. The peroxydisulfate chemistry can be demonstrated by the following equations [24,37,38]:
S2O82− + hν → 2SO4•−
SO4•− + H2O → H+ + SO42− + OH− (at all pH values)
SO4•− + OH− → HO• + SO42− (at alkaline pH values)
SO4•− or HO• + pollutant → degradation of pollutant
HO• are powerful oxidants (E° = 2.80 V/NHE), which react immediately and non-selectively with organic pollutants in water. It can be generated by a chemical process (Fenton (H2O2/Fe2+) [39], photochemical processes UV/TiO2 [40], UV/H2O2 [41,42], photo-Fenton (UV/H2O2/Fe2+) [43,44], and electrochemical (anodic oxidation, electro-Fenton) [45,46]. This process represents the decomposition of hydrogen peroxide into HO•, which occurs through two routes: (1) Catalytic decomposition by Fe2+ (Equations (5)) and (2) photodecomposition by UV irradiation at 254 nm (Equation (6)). Additionally, there is the production of further HO• by photo-reduction of ferric to ferrous iron (Equation (7)), combined with H2O2 photolysis (Equation (6)), or by photolysis of complexes formed between the organic compounds or their intermediates with Fe3+ (Equation (8)) [41,47,48].
Fe2+ + H2O2 → Fe3+ + HO•+ HO−
H2O2 + hν → 2HO•
Fe(OH)2+ + hν → Fe2+ + HO•
Fe(RCO2)2+ + hν → Fe2+ + CO2 + R•
As a result, the photo-Fenton process is reported to be effective in removing several varieties of pollutants such as pesticides, dyes, and pharmaceuticals [49,50,51,52]. The effectiveness of these techniques is greatly improved by the addition of a catalyst or an oxidant.
The aim of this study is to realize the degradation of TA aqueous solution by UV/persulfate and photo-Fenton processes. Besides, the effect of different factors such as initial pH, oxidant dosage, ferrous iron dose, initial TA content, and temperature on the performance of degradation and mineralization by UV/persulfate and photo-Fenton processes was also evaluated.
2. Materials and Methods
2.1. Chemicals
Tannic acid (C76H52O46) was of analytical grade supplied from Sigma-Aldrich Hydrogen peroxide was a 30% solution (w/w) (AR grade, Fluka). All the other chemicals including FeSO4. 7H2O, K2S2O8, H2SO4, NaOH, EtOH, t-BuOH, and Na2SO3 were of analytical grade and were ordered from Sigma-Aldrich or Fluka.
2.2. Analytical Methods
The total organic carbon (TOC) content of the samples was measured with a TOC-5050 analyzer (Shimadzu Corporation, Kyoto, Japan). Samples taken from treated solutions were filtered with 0.20 μm polytetrafluoroethylene (PTFE) filters before analysis. The UV absorbance of TA aqueous samples was measured using a UV-Visible spectrophotometer by a 1 cm quartz cells at 276 nm. The solution pH was determined with a Micronal pH meter (model B474). TA was analyzed by HPLC using a Nucleosil C18 column (mobile phase, 60% water 40% acetonitrile; flow rate, 0.50 mL min−1) with UV detector at 276 nm.
2.3. UV/S2O82− and UV/H2O2/Fe2+ Processes
The photochemical experiments were carried out in the photo-reactor (pyrex) of 2 L capacity equipped with a 125-W Heraeus Noblelight (TNN 15/32) mercury vapor lamp, a magnetic stirrer, and a thermometer. The lamp was located in a quartz sleeve at the center of the reactor in an axial position and emitting at 254 nm. 1 L of TA aqueous solution was added into the reactor in each experiment. The solution pH was adjusted to the desired values by addition of sodium hydroxide or sulfuric acid. Then, K2S2O8 (UV/persulfate) or FeSO4.7H2O (photo-Fenton) were directly added to the photoreactor at the beginning of each experiment. After switching on the lamp, a precise amount of hydrogen peroxide (30%) was immediately added to 1 L of TA aqueous solution (for photo-Fenton process). All the experiments were performed in duplicate. Samples of 10 mL were withdrawn at predetermined time intervals and immediately quenched with Na2SO3. The samples were filtered through 0.20 μm polytetrafluoroethylene (PTFE) filters and then tested to determine the pH, TOC, and absorbance at wavelengths of 276 nm in duplicate.
3. Results and Discussion
The efficiency of UV/persulfate (UV/PS) and photo-Fenton (UV/PF) methods was evaluated by following monitoring UV absorbance at 276 nm (a typical UV-visible spectrum of TA aqueous solution presents two bands at 210 nm and 276 nm) and TOC. The absorbance at 276 nm is used to follow the degradation of TA and its aromatic intermediates (, where and are absorbencies measured at 276 nm at t = 0 s and instant t). TOC is used to evaluate the mineralization of the organic content (, where and are TOC measured at t = 0 s and instant t). Persulfate, hydrogen peroxide, ferrous iron and TA concentrations, temperature, and initial pH are the main factors to achieve the highest TA degradation and mineralization yields.
3.1. Comparitive Study of TA Degradation under Various Processes
An initial comparative study on the degradation of TA aqueous solution by six different processes including chemical oxidation by S2O82− alone, chemical oxidation by H2O2 alone, direct UV irradiation, UV/H2O2, UV/S2O82−, and UV/H2O2/Fe2+ was performed at the same operating conditions (temperature, pH and initial concentrations). The experimental results were compared in Figure 2. According to Figure 2, only 4.22%, 5.35%, and 9.86% of aromatics were reduced by S2O82− alone, H2O2 alone and direct UV irradiation, respectively during 120 min. These results indicate that the TA degradation by S2O82− alone, H2O2 alone and direct photolysis irradiation was very inefficient, which reveals the direct reactions of persulfate or H2O2 with TA in water are generally slow oxidative kinetics. However, combining UV irradiation with H2O2 oxidation (UV/H2O2) achieved more than 29% of aromatics removal after 120 min.
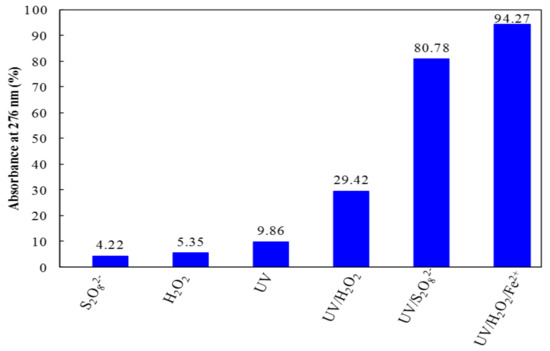
Figure 2.
Comparison of TA degradation for 120 min reaction time by different processes including S2O82− alone ([S2O82−] = 53.1 mM), H2O2 alone ([H2O2] = 29.4 mM), UV irradiation alone (λ = 254 nm), UV/H2O2 (λ = 254 nm, [H2O2] = 29.4 mM), UV/S2O82 (λ = 254 nm, [S2O82−] = 53.1 mM), and UV/H2O2/Fe2+ (λ = 254 nm, [Fe2+] = 0.18 mM and [H2O2] = 29.4 mM). Experimental conditions: [TA] = 0.1 mM, initial pH (pH = 3.4), T = 25 °C.
This indicates that the combination of hydrogen peroxide with UV radiation enhanced TA degradation. This is because of the HO• radicals produced from UV photolysis of H2O2. The above results implied that UV/H2O2 process is more efficient than direct UV irradiation and H2O2 oxidation alone, but it was not very effective to reach complete removal of TA and its degradation intermediates.
As can be observed from Figure 2, high TA degradation yields can be reached by UV/PS and photo-Fenton oxidation processes, respectively. For a reaction time of 120 min, the highest aromatics removal efficiency was measured as 80.78% for UV/PS and 94.27% for photo-Fenton. This result indicates that TA degradation could be improved by incorporating persulfate (K2S2O8) or H2O2 and ferrous iron (H2O2/Fe2+) to TA aqueous solutions under UV radiation. This enhancement is due to the generated radical species from UV photolysis of persulfate (Equations (1)) or from UV photolysis of the H2O2 and from the Fenton reaction (Equations (5) and (6)). In this study, UV/PS and photo-Fenton processes have a higher efficiency to degrade TA than the other four processes (S2O82− alone, H2O2 alone, UV irradiation, and UV/H2O2).
3.2. Influence of Radical Species
To realize the contribution of the oxidizing species (SO4•− and HO•) in the efficiency of UV/PS and photo-Fenton processes, two alcoholic radical scavengers, ethanol (EtOH) and tert-butyl alcohol (t-BuOH), were added to TA aqueous solutions. So, alcohol with alpha H (EtOH) can quench both hydroxyl and sulfate radicals; whereas, alcohol with no alpha H (t-BuOH) can mainly scavenge hydroxyl radicals and poorly react with sulfate radicals. In this study, alcohol (EtOH, t-BuOH) concentration used was 50 mM. Figure 3 illustrates the influence of EtOH and t-BuOH as radical scavengers on TA degradation efficiency during UV/PS and UV/H2O2/Fe2+ processes. As Figure 3 shows, TA degradation was significantly decreased by the addition of alcohols in both processes. The degradation of TA in UV/PS process was 96% without any radical scavenger (see Figure 3a). However, the addition of EtOH and t-BuOH has reduced the efficiencies to 11% and 60%, respectively, within 60 min, compared to the degradation observed in the absence of both scavengers. The results confirmed that the addition of EtOH has diminished the degradation efficiency much more than the same amount t-BuOH.
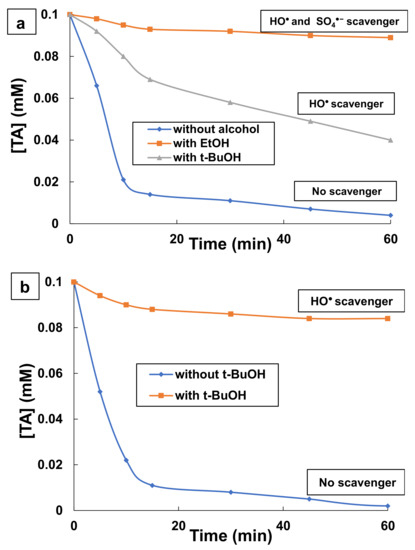
Figure 3.
Effect of EtOH and t-BuOH as radical scavengers on TA degradation efficiency during (a) UV/persulfate and (b) photo-Fenton oxidation. Experimetal conditions: ((a) UV/PS: [TA] = 0.1 mM, [EtOH] = 50 mM, [t-BuOH] = 50 mM, [K2S2O8] = 53.1 mM, pH = 9 and T = 25 °C; (b) UV/H2O2/Fe2+: [TA] = 0.1 mM, [t-BuOH] = 10 mM, [H2O2] = 29.4 mM, [Fe2+] = 0.18 mM, pH = 3, and T = 25 °C).
These observations imply that in the case of t-BuOH addition, hydroxyl radical HO• was scavenged and TA was degraded by sulfate radical SO4•−, while in the case of EtOH addition, both SO4•− and HO• were scavenged, which lead to a low TA degradation yield. In order to evaluate the contribution of HO• radicals into TA degradation, t-BuOH was introduced into the photo-reactor during photo-Fenton degradation of TA. As can be seen in Figure 3b, the addition of 50 mM t-BuOH decreased TA degradation yield from 98% (without scavengers) to 16% after 60 min reaction. This confirms that TA degradation occurs mainly via chemical oxidation with hydroxyl radicals during photo-Fenton process.
These results confirmed that the higher aromatics removals obtained in the previous experiments, when US/PS and photo-Fenton processes were used, is mainly due to the oxidation of TA and its aromatics intermediates by radical oxidizing species (SO4•− and HO•). Furthermore, it is clear that when UV/PS process is used, not only sulfate radicals, SO4•− contribute in the degradation of TA, but also hydroxyl radicals, HO• are involved in TA degradation since the addition of EtOH and t-BuOH decreased TA degradation yield. Considering that t-BuOH is generally used as HO• scavenger and EtOH can scavenge both SO4•− and HO• radical species, it can be concluded that 60% of TA molecules are degraded by HO• radicals, and only 30–40% of TA molecules are degraded by SO4•− radicals.
3.3. Influence of Ph
It is noteworthy to mention that pH effectively controls the behavior of AOPs [42,53,54]. The UV/persulfate and photo-Fenton processes are heavily dependent on the pH of a solution [44,55,56,57]. In this study, the effect of initial pH on the photo-degradation of TA through both UV/persulfate (UV/PS) and photo-Fenton (UV/H2O2/Fe2+) processes was examined at pH 3, 5, 7, 9, and 10. Figure 4 represents the changes of aromatics and TOC removals during the treatment of aqueous solutions containing 0.1 mM TA by UV/PS and photo-Fenton processes at different initial pH values.
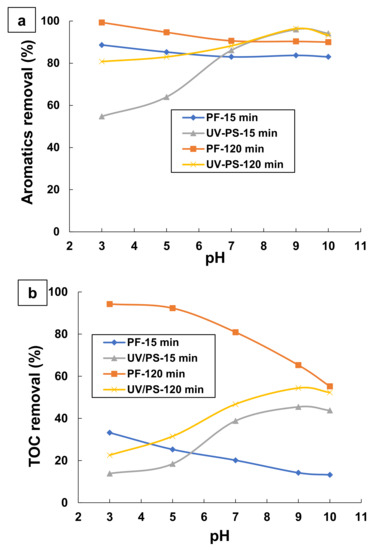
Figure 4.
Influence of initial pH on the changes of: (a) aromatics removal, and (b) TOC removal, during the treatment of 0.1 mM TA by UV/PS and UV/H2O2/Fe2+ processes. Experimental conditions: (UV/PS: [K2S2O8] = 53.1 mM, pH = 3–10 and T = 25 °C; UV/H2O2/Fe2+: [H2O2] = 29.4 mM, [Fe2+] = 0.18 mM, pH = 3–10, and T = 25 °C).
In the UV/PS process, aromatics and TOC removals were influenced by the initial pH as demonstrated in (Figure 4a,b). The highest efficiency is obtained at an initial pH of 9 and 10. The results suggested that the alkaline conditions were more favorable than neutral and acidic conditions during the degradation of TA. A similar finding was reported in other studies relevant to these observations [57,58,59]. An increase of pH from three to nine, the photo-degradation efficiency of TA at 120 min irradiation increased from 80.78 to 96.38% and from 22.55 to 54.41%, respectively, for aromatics removal and TOC removal. In contrast, the rise of pH higher than pH 9 does not have any significant influence on the kinetics and TOC removals. This indicates that the highest degradation and mineralization efficiencies of TA by the UV/PS process can be reached at pH nine. These results confirm that very strict conditions (strongly acidic or basic) are less favorable to complete mineralization of TA [57,58,59].
Thus, the photo-degradation of TA requires a big amount of persulfate radicals and it cannot be carried out at any pH value (see Figure 4b). This may be relative to the abundance of radicals and their nature in the aqueous medium. The literature indicates that increasing the pH increases the concentration of hydroxyl anions in basic medium [56,60]. The pH effect on the TA photo-degradation by UV/persulfate can be explained by, firstly, base-activated persulfate favored the production of SO4•− under alkaline condition. SO4•− doubled, as demonstrated in Equation (1). Second, a high pH, sulfate radicals SO4•− react with OH− or H2O to generate hydroxyl radicals HO• (E° = 2.7 V) based on Equations (2) and (3) [29,61,62,63]. Thus, HO• is a more powerful oxidant than SO4•−. It should be observed that HO• and sulfates radicals SO4•− oxidize organic compounds mainly in three ways: (i) Hydrogen abstraction, (ii) addition and substitution of alkanes and aromatics, and (iii) electron transfer from carboxylate groups [64]. On the other hand, in acidic pH, SO4•− is the predominant radical species in the UV/PS process [60,65]. In our work, the lowest TOC removal efficiency (22%) was observed at pH = 3 after 120 min of treatment (Figure 4b). This can be due to the more selective and less reactive nature of SO4•− radicals compared to hydroxyl radicals HO•. Moreover, at pH < 7, SO4•− radicals are consumed by S2O82− to form SO42− and S2O8•−, as shown in (Equation (9)). Thus, SO4•− anions easily degrade TA but cannot complete its mineralization under the acidic conditions. Also, along with radical SO4•−, there are several other sulfur intermediates such as HS2O8−, HSO4−, SO4, and H2SO5, which are generated and more dominant in the reaction medium according to the following equations (Equations (10)–(13)) [56]:
SO4•− + S2O82− → SO42− + S2O8•−
S2O82− + H+ → HS2O8−
HS2O8− → SO4•− + SO42− + H+
S2O82− + H+ → HSO4− + SO4
SO4 + H2O → H2SO5
In alkaline medium, hydroxyl radicals HO• are formed (Equation (3)). Being very powerful and non-selective, hydroxyl radicals are capable to mineralize TA and its aromatic intermediates. Hence, the maximum aromatics and TOC removals occurred at pH = 9.
For photo-Fenton (UV/H2O2/Fe2+) process, removal efficiency reaches a maximum at initial pH of 3. This is similar to a previous investigation [42,48]. Several studies reported that optimal pH for the production of hydroxyl radicals by the photo-Fenton must be in the range three–five [66,67]. It was also demonstrated that at low acid medium (pH > 5), the Fe2+ catalyst regeneration becomes very difficult because of Fe(OH)3 precipitation. However, in high acid conditions (pH < 3) protons can function as scavengers for hydroxyl radicals [42]. Additionally, there may be inhibition for the radical forming activity of iron. In this study, the pH is varied from three to 10. UV/H2O2/Fe2+ process performance was significantly influenced by varying the pH as shown in Figure 4. From Figure 4a, it is obvious that the change in pH had no significant effect on aromatics removal since aromatics removals of 99.3 and 90% at pH values three and 10 after 2 h photo-Fenton treatment of TA. However, TOC removal during UV/H2O2/Fe2+ process decreased as pH increased (see Figure 4b). As shown in Figure 4b, TOC removal remains almost constant with the increasing of initial pH from pH = 3 to pH = 5. In contrast, when the pH of the reaction was higher than five (neutral or alkaline pH), the TOC removal efficiency rapidly decreased as pH increased. After 2 h of treatment, the obtained percentage of TOC removal was 94.27, 92.29, 80.9, 65.28, and 55.2% at pH 3, 5, 7, 9, and 10, respectively. The findings demonstrated that aromatics were completely degraded into aliphatic intermediates by the photo-Fenton process for acid and neutral mediums, but the degradation was incomplete in the alkaline medium. These results can be interpreted the easier and rapid decomposition of H2O2 hydroxyl radical in acidic medium. In addition, the competitive reactions to H2O2 photo-reduction such as the consumption of hydroxyl radicals by the protons, the dismutation of hydrogen peroxide and the duplication of hydroxyl radicals are negligible under these conditions. This indicated that the majority of H2O2 is decomposed into an important amount of hydroxyl radicals. Consequently, this condition is the most favorable for complete treatment of TA by the photo-Fenton process. However, if the solution pH is too high (basic medium), H2O2 reacts with hydroxide anions to generate water and hydroperoxide anion (HO2−) (Equation (14)). At neutral and alkaline pH, precipitation of ferric ions limits the regeneration of Fe2+ catalyst. These results are in good agreement with those mentioned in previous reports [42,53]. In this work, pH = 3 was selected as the optimal pH in order to provide a favorable condition for higher mineralization efficiency.
H2O2 + HO− → H2O + HO2−
From the above results, the pH values of three and nine were found to be the optimum pH values for photo-Fenton and UV/PS and processes, respectively.
3.4. Influence of Persulfate Dose
The initial persulfate concentration is an important parameter in the destruction of organic pollutants by UV/PS [24,68]. For this approach, some experiments were done with various PS doses in the range of 17.70–70.80 mM using 0.1 mM TA at pH 9 and a temperature of 25 °C. The results are shown in Figure 5. As it can be seen in Figure 5a, high aromatics removals (>94%) were achieved independently of PS concentration. This indicates that persulfate concentrations higher than 17.70 mM are capable to degrade almost completely TA and its aromatic intermediates.
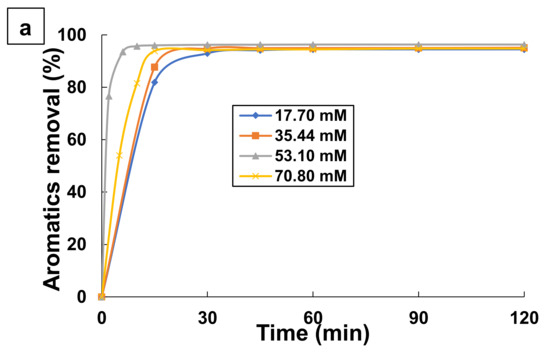
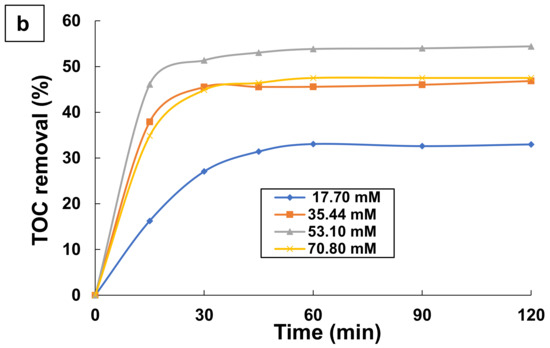
Figure 5.
Effect of persulfate dose on the changes with time of: (a) Aromatics removal, and (b) TOC removal by UV/PS process. Experimental conditions: [TA] = 0.1 mM; [PS] = 17.70–70.80 mM; T = 25 °C; and pH = 9.
However, TOC removal depends on PS concentration as demonstrated in Figure 5b. The increase of PS content from 17.70 to 53.10 mM led to increase the TOC removal from 32.97 to 54.41% after a 1 h treatment. PS is a source of sulfate and hydroxyl radicals in the UV/PS process, and more reactive radicals would be generated to degrade TA at higher PS doses. This indicates that an increase of PS dose up to 53.1 mM was able to mineralize more organics. A dose of PS higher than 53.10 mM led to the decrease in TOC removal. It seems that PS dose of 53.10 mM is considered optimal for the treatment of 0.1 mM TA aqueous solution by the UV/PS process. Increasing the persulfate dose above 53.10 mM (optimal dose) resulted in a decrease in TA degradation efficiency. This can be explained by the fact that excessive amount of persulfate (S2O82−) in the solution can react with sulfate radicals (SO4•−) to form less reactive species such as persulfate radicals [56,61,69,70,71]. This, in turn, reduces the efficiency of the process, as explained in Equations (9) and (15).
SO4•− + SO4•− → S2O82−
These experimental results are similar to the results obtained by Hori et al. [72] for the degradation of perfluorooctanoic acid (PFOA) using UV/PS process (50 mM persulfate were used).
3.5. Influence of Hydrogen Peroxide and Ferrous Iron Concentrations
The amount of hydrogen peroxide and ferrous ions are the main factors affecting the cost of the operation and the process efficiency for many wastewater treatment facilities by Fenton and phot-Fenton processes [73,74]. Photo-Fenton process efficiency can be assessed readily in terms of both the absolute concentration of reagents (hydrogen peroxide and ferrous ions) and the weight ratio ([H2O2]/[Fe2+]). Several researches affirm that the performance of photo-Fenton process to degrade organic matter present in water is mainly related to the production of hydroxyl radicals by chemical and photochemical decomposition of hydrogen peroxide [41,43]. For this subject, some experiments were conducted at different initial oxidant concentrations using 0.1 mM TA at optimum pH 3 and at a room temperature of 25 °C. Ferrous iron concentration and hydrogen peroxide concentration were examined in the range of 0–0.27 mM and 14.7–58.8 mM, respectively, for TA degradation. Figure 6 and Figure 7 show the effect of ferrous iron and hydrogen peroxide concentrations on aromatics and TOC removals during the treatment of TA by photo-Fenton process.
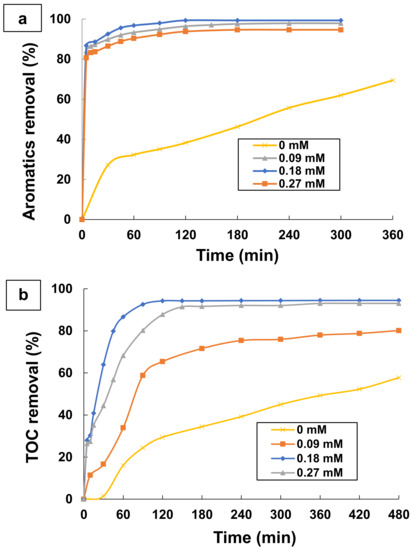
Figure 6.
Effect of Fe2+ amount on (a) Aromatics removal and (b) TOC removal by photo-Fenton process (UV/H2O2/Fe2+). Experimental conditions: [TA] = 0.1 mM; [Fe2+] = 0–0.27 mM; [H2O2] = 29.4 mM; T = 25 °C; and pH = 3.
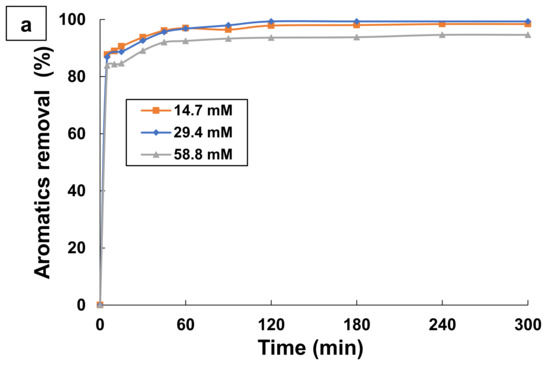
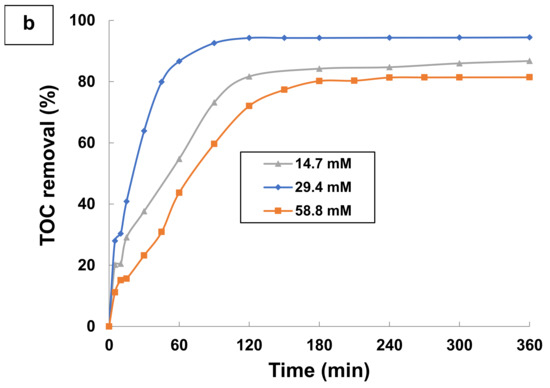
Figure 7.
Effect of H2O2 dose on (a) Aromatics removal and (b) TOC removal by photo-Fenton process (UV/H2O2/Fe2+). Experimental conditions: [TA] = 0.1 mM; [H2O2] = 14.7–58.8 mM; [H2O2]/[Fe2+] = 164; T = 25 °C; and pH = 3.
As it can be observed in Figure 6a and Figure 7a, aromatics removal during the treatment of TA does not depend on the initial contents of ferrous ions and hydrogen peroxide. There is no effect on both the kinetic and the efficiency of absorbance percentage removal. Indeed, over 94% of absorbance reduction was reached in any concentration of ferrous ions and hydrogen peroxide. Thus, it can be concluded that a total disappearance of TA was obtained with the addition of ferrous ions and hydrogen peroxide within the first 60 min of treatment by photo-Fenton process. However, without the addition of a catalyst, 69.42% of absorbance removal could be achieved in UV/H2O2 process after 6 h of treatment (Figure 6a). It should be noted that a partial mineralization was obtained under these experimental conditions. These results indicate that ferrous ions are excellent catalysts and a small catalytic amount may be sufficient to decompose H2O2 efficiently into hydroxyl radicals HO•. That is why the H2O2 concentration is higher than Fe2+ in all the experiments. In contrast, Figure 6b and Figure 7b show that the ferrous iron and hydrogen peroxide concentrations have an important effect on the rate of TOC removal during the photo-Fenton treatment. In fact, the incorporation of Fe2+ enhanced the efficiency of UV/H2O2 for TA degradation (see Figure 6b). It is noticed that, in the presence of Fe2+ catalyst, the degradation efficiency is more than 80% after 120 min treatment, while in the absence of Fe2+ catalyst, the efficiency of the organic compounds degradation is less than 58% for 420 min of reaction time. Thus, the absence of ferrous iron during the mineralization of TA resulted in the latter’s slow degradation. This could be due to the retarding production of excessive generated reactive radicals. However, by adding ferrous iron, the TOC removal increases from 80.15 to 94.27% after increasing the Fe2+ dose from 0.09 mM to 0.18 mM, and then it remains constant above this latter concentration.
Moreover, the kinetic of TOC removal decreases to a dose of 0.27 mM. Hence, an almost complete conversion of TA was achieved after 120 min of the treatment of TA by photo-Fenton process for 0.18 mM of ferrous iron. These results can be interpreted by the fact that the addition of low amounts of ferrous iron (0.09 mM) does not enhance the performance of photo-Fenton process in wastewater treatment. In this case, a small content of hydroxyl radicals results mainly from the photodecomposition of hydrogen peroxide by UV light and it causes a slow removal in the mineralization. Besides, a gradual increase in the concentration of ferrous ions to 0.18 mM increases the concentration of hydroxyl radicals HO• in the aqueous medium through the photo Fenton process, under the Fenton reaction, to form ferric ions (Equation (5)) and by the photo-reduction of Fe(OH)2+ complex (Equation (7)). Moreover, when high amounts of Fe2+ are added to the wastewater, the regeneration of the catalyst (Fe2+ ions) from Fe3+ ions becomes slow due to the precipitation of ferric hydroxide Fe(OH)3, which prevents the penetration of UV light into the reaction medium and therefore it reduces the quantum efficiency of the UV lamp. Consequently, this inhibits the generation of hydroxyl radicals by ferrous iron. In addition, the use of a much higher concentration of Fe2+ could lead to the self-scavenging of hydroxyl radical HO• by converting it to hydroxyl ions HO− during the oxidation of Fe2+ (Equation (16)). From these results, we can deduce that a 0.18 mM dose of ferrous ions is optimal for a better treatment of aqueous solutions containing 0.1 mM of TA at pH 3.0 and 29.4 mM of H2O2, using the photo-Fenton process.
Fe2+ + HO• → Fe3+ + HO−
Similar behavior is noticed in the case of hydrogen peroxide dosage (Figure 7b). The addition of initial hydrogen peroxide concentration from 14.7 to 29.4 mM resulted in an increase in mineralization extents from 84.22% to 94.27% of TOC removal after 120 min of treatment, as seen in Figure 7b. A low concentration of H2O2 did not allow achieving complete mineralization of TA in the photo-Fenton treatment. This can be explained by Equation (6). Increasing the amount of H2O2 up to 29.4 mM caused considerable efficiency improvement because of more reactive radical generation (Equations (5) and (6)), which strongly enhanced the efficiency of TA degradation. Further increase of the H2O2 dose, greater than 29.4 mM, decreased the mineralization efficiency from 94.27% to 80.19% after 120 min of the treatment of TA. After reaching an optimal value, a further boost of H2O2 concentration negatively influences the efficiency of the process due to the OH• scavenging effect of H2O2 and recombination of hydroxyl radicals (Equation (17)). In addition, when hydrogen peroxide concentration is elevated, the competitive reactions of hydroxyl radicals with H2O2 excess in order to produce HO2• (Equations (18) and (19)) consume a considerable amount of HO• radicals, which leads to a decline in the process efficiency.
HO• + HO• → H2O2
H2O2 + HO• → H2O + HO2•
HO2• + HO• → H2O + O2
Therefore, a H2O2 dose of 29.4 mM was found to be optimal for a complete disappearance of TA in 60 min and total TOC removal in 120 min in the photo-Fenton oxidation for treating TA wastewater. It was found that both parameters Fe2+ and H2O2 had a positive effect on the percentage of TOC removal over the studied range. The optimal conditions for the decay absorbance at 276 nm (aromatics removal) and TOC removal of TA were determined as [Fe2+] = 0.18 mM and [H2O2] = 29.4 mM. This corresponds to a mass ratio of H2O2 dose to Fe2+ dose ([H2O2]/[Fe2+]) equal to 164.
3.6. Influence of Initial Dose of TA
To study the effect of this parameter, four TA concentrations of 0.05, 0.1, 0.2, and 0.4 mM were tested at optimum conditions determined in previous steps (UV/PS: [K2S2O8]/[TA] = 531, pH = 9 and T = 25 °C; UV/H2O2/Fe2+: [H2O2]/[Fe2+] = 164, [H2O2]/[TA] = 294, pH = 3, and T = 25 °C). Results presenting final percentage of absorbance and TOC removals for 120 min treatment are illustrated in Figure 8. As shown in Figure 8a, the initial concentration of TA has no significant effect on either the rate or the percentage of aromatics removal during the treatment by UV/persulfate and photo-Fenton processes. In both processes, there is more than 90% of absorbance removal for the different initial concentration of TA in the range 0.05–0.4 mM. However, TOC removal was strongly influenced by the initial concentration of TA, as seen in Figure 8b. An increase in TA concentration from 0.05 to 0.1 mM resulted in an increase in removal efficiency from 42.95 to 54.41% and from 85.44 to 94.27% for the UV/persulfate and photo-Fenton processes, respectively, at 120 min of treatment. Increasing initial concentration of TA from 0.1 to 0.4 mM decreases TOC removal efficiency.
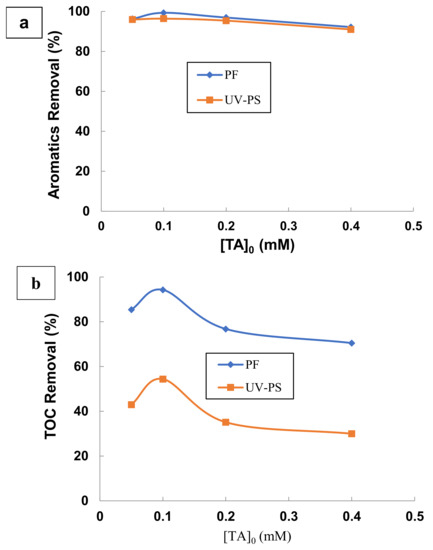
Figure 8.
Effect of TA initial concentration on (a) aromatics removal and (b) TOC removal by UV/persulfate (UV-PS) and photo-Fenton (UV/H2O2/Fe2+) processes under optimal conditions. (UV/PS: [TA] = 0.05–0.4 mM, [K2S2O8]/[TA] = 531, pH = 9 and T = 25 °C; UV/H2O2/Fe2+: [TA] = 86–680 mg L−1, [H2O2]/[Fe2+] = 164, [H2O2]/[TA] = 294, pH = 3, and T = 25 °C).
The results obtained can be interpreted as follows: (i) A higher TA dose requires a big amount of oxidants, and thus, the process efficiency decreased with a constant amount of oxidant. The greater of amount in the reaction medium decreases the quantum yield of production of sulfates radicals SO4•− from K2S2O8 and hydroxyl radicals OH• from H2O2. This is mainly due to the absorption of a significant amount of UV radiation by the organic molecules, besides, by the coloring of the solution and the formation of a precipitate layer of the jacket of the reactor, which makes difficult the passage of UV light in organic molecules. It is also due to competition between TA molecules and intermediates compounds generated during the reaction. (ii) A low concentrations of TA and oxidants (K2S2O8, Fe2+, and H2O2) decrease the probability of collusion between sulfates or hydroxyl radicals and aliphatic intermediates, which makes difficult their mineralization into CO2 and H2O. Several authors have found similar observation in their study [44,75,76,77].
From these results, it is concluded that the best performance for the mineralization and degradation of TA by UV/persulfate and photo-Fenton processes is obtained for an initial dose of TA equal to 0.1 mM. This corresponds to a ratio weight equal to 531 and 294 successively for [K2S2O8]/[TA] and [H2O2]/[TA]. The two ratios are relative to the degradation and the mineralization of TA. It is also clear that aromatics were decomposed into aliphatic intermediates by the photo-Fenton process, but it is more difficult to mineralize TA by the UV/persulfate process. Therefore, the mineralization and degradation of TA by the photo-Fenton process are more effective than by UV/persulfate process.
3.7. Temperature Influence
Table 1 shows the effect of temperature on absorbance decay percentage and TOC removal percentage during the treatment of TA by UV/PS and photo-Fenton processes within 2 h UV irradiation. Temperature was examined in the range of 20–35 °C. The work of this range of temperature does not cause any risk for either UV lamp or the photo-reactor. Results indicated that the increase of temperature from 20 °C to 25 °C increases the percentage of absorbance and TOC removals at 120 min of the treatment. However, the increase of this temperature from 25 °C to 35 °C does not have any significant effect on aromatics and TOC removals during the treatment of aqueous solutions of TA by the UV/PS and photo-Fenton processes.

Table 1.
Effect of temperature on UV/PS and photo-Fenton efficiencies during the degradation of TA for 2 h UV irradiation. (UV/PS: [K2S2O8]/[TA] = 531, pH = 9 and T = 20–35 °C; UV/H2O2/Fe2+: [H2O2]/[Fe2+] = 164, [H2O2]/[TA] = 294, pH = 3, and T = 20–35 °C).
Thus, the optimum temperature deduced in this study is 25 °C for the treatment of this effluent, so less heating energy should be consumed. This phenomenon can be attributed to increasing the temperature higher than the optimum value (25 °C) accelerates the rate of competitive reactions which consumes sulfates and hydroxyl radicals. Besides, for a low temperature, the sulfates and hydroxyl radicals generated in the reaction medium are not able to degrade TA. Many reports discussed the temperature influence and have found similar results [74,78]. From these results, it can be concluded that UV/persulfate process cannot be a viable option for the treatment of TA in water taking into account its low mineralization yield (54.41%). Further investigations are needed to enhance the performance of UV/PS in the mineralization of organic matter. The photo-Fenton (UV/H2O2/Fe2+) process achieved more than 99% of aromatics removal and more than 94% of TOC removal. The combination of UV/PS and photo-Fenton can be a good cost-effective method that benefits from the advantages of persulfates (low cost) and the high efficiency of photo-Fenton (high mineralization).
4. Conclusions
The efficiency and operating parameters for the treatment of TA by the UV/persulfate and photo-Fenton were evaluated and compared. The results from this study showed that the performance of photo-Fenton process is significantly superior to that of UV/persulfate process in the degradation of TA aqueous solutions. The optimum conditions obtained for the best degradation were a pH = 9, a persulfate concentration of about 53.10 mM, a TA concentration of 0.1 mM and a temperature of 25 °C for UV/persulfate process and a pH 3, a ferrous iron concentration of 0.18 mM, a hydrogen peroxide concentration of 29.4 mM, a TA concentration of 0.1 mM, and a temperature of 25 °C for the photo-Fenton process. Under optimal conditions, removal efficiency for UV/persulfate and photo-Fenton were 96.38% and 99.32% for absorbance removal and 54.41% and 94.27% for TOC removal, respectively. The results obtained have shown that a more complete mineralization was obtained in the photo-Fenton process. It can be summarized that photo-Fenton process more effective than UV/persulfate process. The efficiency of photo-Fenton can be explained by the catalytic decomposition of H2O2 by Fe2+ ions, the photo reduction of hydrogen peroxide by irradiation UV and the photo-reduction of hydroxyl iron complex, which lead to the production of higher contents of hydroxyl radicals. Of those hydroxyl radicals, HO• is a more powerful oxidant than sulfates radicals SO4•−. Even though, PS is widely known to produce less powerful radicals, the fact of having an optimal pH at nine may be easily applied to real cases and the costs of adjusting the pH can be fewer.
Author Contributions
N.B. and A.B. designed the experimental part; S.D., M.M.Z. and M.E. performed the experiments; N.B. and A.B supervised experimental work and data analysis; S.D., N.B., M.M.Z. and M.E. wrote the manuscript.
Funding
This research received no external funding. The publication of this article was funded by the Qatar National Library.
Conflicts of Interest
All authors of this article declare no conflicts of interest.
References
- Buzzini, P.; Arapitsas, P.; Goretti, M.; Branda, E.; Turchetti, B.; Pinelli, P.; Ieri, F.; Romani, A. Antimicrobial and antiviral activity of hydrolysable tannins. Mini-Rev. Med. Chem. 2008, 8, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Coppo, E.; Marchese, A. Antibacterial activity of polyphenols. Curr. Pharm. Biotechnol. 2014, 15, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, P.; Soliwoda, K.; Emilia, T.; Bien, K.; Fruba, A.; Gniadek, M.; Labedz, O.; Nowak, Z.; Celichowski, G.; Grobelny, J.; et al. Toxicity of tannic acid-modified silver nanoparticles in keratinocytes: Potential for immunomodulatory applications. Toxicol. In Vitro 2016, 35, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Erdelyi, K.; Kiss, A.; Bakondi, E.; Bai, P.; Szabo, C.; Gergely, P.; Erdodi, F.; Virag, L. Gallotannin inhibits the expression of chemokines and inflammatory cytokines in A549 cells. Mol. Pharmacol. 2005, 68, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Xiong, B.; Pan, Y.; Cui, H. Adsorption removal of tannic acid from aqueous solution by polyaniline: Analysis of operating parameters and mechanism. J. Colloid Interface Sci. 2017, 487, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, E.; Dobrowolski, P.; Winiarska-Mieczan, A.; Kwiecien, M.; Tomczyk, A.; Muszynski, S. The effect of tannic acid on the bone tissue of adult male Wistar rats exposed to cadmium and lead. Exp. Toxicol. Pathol. 2017, 69, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, T.; Pérez-Manríquez, L.; Neelakanda, P.; Peinemann, K.V. Bioinspired tannic acid-copper complexes as selective coating for nanofiltration membranes. Sep. Purif. Rev. 2017, 184, 188–194. [Google Scholar] [CrossRef]
- Wang, J.H.; Zheng, S.R.; Liu, J.L.; Xu, Z.Y. Tannic acid adsorption on aminofunctionalized magnetic mesoporous silica. Chem. Eng. J. 2010, 165, 10–16. [Google Scholar] [CrossRef]
- Varanka, Z.; Rojik, I.; Varanka, I.; Nemcsók, J.; Ábrahám, M. Biochemical and morphological changes in carp (Cyprinus carpio L.) liver following exposure to copper sulfate and tannic acid. Comp. Biochem. Physiol. C 2001, 128, 467–477. [Google Scholar] [CrossRef]
- De Nicola, E.; Meriç, S.; Gallo, M.; Iaccarino, M.; Della, R.C.; Lofrano, G.; Russo, T.; Pagano, G. Vegetable and synthetic tannins induce hormesis/toxicity in sea urchin early development and in algal growth. Environ. Pollut. 2007, 146, 46–54. [Google Scholar] [CrossRef]
- Deng, Y.H.; Wang, L.; Hu, X.B.; Liu, B.Z.; Wei, Z.B.; Yang, S.G.; Sun, C. Highly efficient removal of tannic acid from aqueous solution by chitosan-coated attapulgite. Chem. Eng. J. 2012, 181–182, 300–306. [Google Scholar] [CrossRef]
- Buso, A.; Balbo, L.; Giomo, M.; Farnia, G.; Sandonà, G. Electrochemical removal of tannins from aqueous solutions. Ind. Eng. Chem. Res. 2000, 39, 494–499. [Google Scholar] [CrossRef]
- Cañizares, P.; Pérez, Á.; Camarillo, R.; Llanos, J. Tannic acid removal from aqueous effluents using micellar enhanced ultrafiltration at pilot scale. Desalination 2006, 200, 310–312. [Google Scholar] [CrossRef]
- Rodríguez, H.; de las Rivas, B.; Gómez-Cordovés, C.; Muñoz, R. Degradation of tannic acid by cell–free extracts of Lactobacillus plantarum. Food Chem. 2008, 107, 664–670. [Google Scholar] [CrossRef]
- Pepi, M.; Lampariello, L.R.; Altieri, R.; Esposito, A.; Perra, G.; Renzi, M.; Lobianco, A.; Feola, A.; Gasperini, S.; Focardi, S.E. Tannic acid degradation by bacterial strains Serratia spp. and Pantoea sp. isolated from olive mill waste mixtures. Int. Biodeterior. Biodegrad. 2010, 64, 73–80. [Google Scholar] [CrossRef]
- Goel, G.; Kumar, A.; Beniwal, V.; Raghav, M.; Puniya, A.K.; Singh, K. Degradation of tannic acid and purification and characterization of tannase from Enterococcus faecalis. Int. Biodeterior. Biodegrad. 2011, 65, 1061–1065. [Google Scholar] [CrossRef]
- Mansouri, K.; Elsaid, K.; Bedoui, A.; Bensalah, N.; Abdel-Wahabb, A. Application of electrochemically dissolved iron in the removal of tannic acid from water. Chem. Eng. J. 2011, 172, 970–976. [Google Scholar] [CrossRef]
- Bensalah, N.; Chair, K.; Bedoui, A. Efficient degradation of tannic acid in water by UV/H2O2 process. Sustain. Environ. Res. 2018, 28, 1–11. [Google Scholar] [CrossRef]
- Adak, A.; Mangalgiri, K.P.; Lee, J.; Blaney, L. UV irradiation and UV-H2O2 advanced oxidation of the roxarsone and nitarsone organoarsenicals. Water Res. 2015, 70, 74–85. [Google Scholar] [CrossRef]
- Avetta, P.; Pensato, A.; Minella, M.; Malandrino, M.; Maurino, V.; Minero, C.; Hanna, K.; Vione, D. Activation of persulfate by irradiated magnetite: Implications for the degradation of phenol under heterogene heterogeneous photo-fentonlike conditions. Environ. Sci. Technol. 2015, 49, 1043–1050. [Google Scholar] [CrossRef]
- Khandarkhaeva, M.; Batoeva, A.; Aseev, D.; Sizykh, M.; Tsydenova, O. Oxidation of atrazine in aqueous media by solar- enhanced Fenton-like process involving persulfate and ferrous ion. Ecotoxicol. Environ. Saf. 2017, 137, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Pérez, S.; Eichhorn, P.; Celiz, M.D.; Aga, D.S. Structural characterization of metabolites of the X-ray contrast agent iopromide in activated sludge using ion trap mass spectrometry. Anal. Chem. 2006, 78, 1866–1874. [Google Scholar] [CrossRef] [PubMed]
- Osgerby, I.T. ISCO technology overview: Do you really understand the chemistry? In Contaminated Soils, Sediments and Water; Calabrese, E.J., Kostecki, P.T., Dragun, J., Eds.; Springer: Boston, MA, USA, 2006; Volume 10, pp. 287–308. [Google Scholar]
- Saien, J.; Soleymani, A.R.; Sun, J.H. Parametric optimization of individual and hybridized AOPs of Fe2+/H2O2 and UV/S2O82− for rapid dye destruction in aqueous media. Desalination 2011, 279, 298–305. [Google Scholar] [CrossRef]
- Velo-Gala, I.; López-Peñalver, J.J.; Sánchez-Polo, M.; Rivera-Utrilla, J. Comparative study of oxidative degradation of sodium diatrizoate in aqueous solution by H2O2/Fe2+, H2O2/Fe3+, Fe (VI) and UV, H2O2/UV, K2S2O8/UV. Chem. Eng. J. 2014, 241, 504–512. [Google Scholar] [CrossRef]
- Guan, Y.H.; Ma, J.; Ren, Y.M.; Liu, Y.L.; Xiao, J.Y.; Lin, L.Q.; Zhang, C. Efficient degradation of atrazine by magnetic porous copper ferrite catalyzed peroxymonosulfate oxidation via the formation of hydroxyl and sulfate radicals. Water Res. 2013, 47, 5431–5438. [Google Scholar] [CrossRef] [PubMed]
- Lutze, H.V.; Bircher, S.; Rapp, I.; Kerlin, N.; Bakkour, R.; Geisler, M.; Schmidt, T.C. Degradation of chlorotriazine pesticides by sulfate radicals and influence of organic matter. Environ. Sci. Technol. 2015, 49, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Ma, J.; Jiang, J.; Liu, Y.; Song, Y.; Yang, Y.; Guan, Y.; Wu, D. Simulation and comparative study on the oxidation kinetics of atrazine by UV/H2O2, UV/HSO5− and UV/S2O82−. Water Res. 2015, 80, 99–108. [Google Scholar] [CrossRef]
- Gao, Y.Q.; Gao, N.Y.; Deng, Y.; Yang, Y.Q.; Ma, Y. Ultraviolet (UV) light-activated persulfate oxidation of sulfamethazine in water. Chem. Eng. J. 2012, 195–196, 248–253. [Google Scholar] [CrossRef]
- Hori, H.; Nagano, Y.; Murayama, M.; Koike, K.; Kutsuna, S. Efficient decomposition of perfluoroether carboxylic acids in water with a combination of persulfate oxidant and ultrasonic irradiation. J. Fluor. Chem. 2012, 141, 5–10. [Google Scholar] [CrossRef]
- Guo, Y.G.; Zhou, J.; Lou, X.Y.; Liu, R.L.; Xiao, D.X.; Fang, C.L.; Wang, Z.H.; Liu, J.S. Enhanced degradation of Tetrabromobisphenol A in water by a UV/base/persulfate system: Kinetics and intermediates. Chem. Eng. J. 2014, 254, 538–544. [Google Scholar] [CrossRef]
- Johnson, R.L.; Tratnyek, P.G.; Johnson, R.O. Persulfate persistence under thermal activation conditions. Environ. Sci. Technol. 2008, 42, 9350–9356. [Google Scholar] [CrossRef] [PubMed]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical generation by the interaction of transition metals with common oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, M.G.; Cruz, A.A.; Dionysiou, D.D. Degradation of microcystin-LR using sulfate radicals generated through photolysis, thermolysis and e-transfer mechanisms. Appl. Catal. B 2010, 96, 290–298. [Google Scholar] [CrossRef]
- Shah, N.S.; He, X.; Khan, H.M.; Khan, J.A.; O’shea, K.E.; Boccelli, D.L.; Dionysiou, D.D. Efficient removal of endosulfan from aqueous solution by UV-C/peroxides: A comparative study. J. Hazard. Mater. 2013, 263, 584–592. [Google Scholar] [CrossRef]
- He, X.; Mezyk, S.P.; Michael, I.; Fatta-Kassinos, D.; Dionysiou, D.D. Degradation Kinetics and mechanism of -lactam antibiotics by the activation of H2O2 and Na2S2O8 under UV-254 nm irradiation. J. Hazard. Mater. 2014, 279, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, A.R.; Saien, J.; Bayat, H. Artificial neural networks developed for prediction of dye decolorization efficiency with UV/K2S2O8 process. Chem. Eng. J. 2011, 170, 29–35. [Google Scholar] [CrossRef]
- Xiaoyang, C.; Xue, Z.; Yanlai, Y.; Weiping, W.; Fengxiang, Z.; Chunlai, H. Oxidation Degradation of Rhodamine B in Aqueous by UV/S2O8 Treatment System. Int. J. Photoenergy 2012, 2012, 754691. [Google Scholar] [CrossRef]
- Bensalah, N.; Khodary, A.; Abdel-Wahab, A. Kinetic and Mechanistic Investigations of Mesotrione Degradation in Aqueous Medium by Fenton Process. J. Hazard. Mater. 2011, 189, 479–485. [Google Scholar] [CrossRef]
- Hua, W.; Bennett, E.R.; Letcher, R.J. Ozone Treatment and the Depletion of Detectable Pharmaceuticals and Atrazine Herbicide in Drinking Water Sourced from the Upper Detroit River, Ontario, Canada. Water Res. 2006, 40, 259–2266. [Google Scholar] [CrossRef]
- Ahmed, B.; Mohamed, H.; Limem, E.; Nasr, B. Degradation and Mineralization of Organic Pollutants Contained in Actual Pulp and Paper Mills Wastewaters by a UV/H2O2 Process. Ind. Eng. Chem. Res. 2009, 48, 3370–3379. [Google Scholar] [CrossRef]
- Bedoui, A.; Elsaid, K.; Bensalah, N.; Abdel-Wahab, A. Treatment of Pharmaceutical-Manufacturing Wastewaters by UV Irradiation/Hydrogen Peroxide Process. J. Adv. Oxid. Technol. 2011, 14, 226–234. [Google Scholar] [CrossRef]
- Konstantinou, K.I.; Albanis, A.T. Photocatalytic Transformation of Pesticides in Aqueous Titanium Dioxide Suspensions Using Artificial and Solar Light: Intermediates and Degradation Pathways. Appl. Catal. B Environ. 2003, 42, 319–335. [Google Scholar] [CrossRef]
- Dbira, S.; Bedoui, A.; Bensalah, N. Investigations on the Degradation of Triazine Herbicides in Water by Photo-Fenton Process. Am. J. Anal. Chem. 2014, 5, 500–517. [Google Scholar] [CrossRef]
- Bedoui, A.; Elalaoui, L.; Ahmed, A.W.; Bensalah, N. Photo-Fenton Treatment of Actual Agro-Industrial Wastewaters. Ind. Eng. Chem. Res. 2011, 50, 6673–6680. [Google Scholar]
- Dbira, S.; Bensalah, N.; Cañizares, P.; Rodrigo, M.A.; Bedoui, A. The electrolytic treatment of synthetic urine using DSA electrodes. J. Electroanal. Chem. 2015, 744, 62–68. [Google Scholar] [CrossRef]
- Kavitha, V.; Palanivelu, K. The role of ferrous ion in Fenton and photo-Fenton processes for the degradation of phenol. Chemosphere 2004, 55, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Hermosilla, D.; Cortijo, M.; Huang, C.P. Optimizing the treatment of landfill leachate by conventional Fenton and photo-Fenton processes. Sci. Total Environ. 2009, 407, 3473–3481. [Google Scholar] [CrossRef]
- Huston, P.L.; Pignatello, J.J. Degradation of selected pesticide active ingredients and commercial formulations in water by the photo-assisted Fenton reaction. Water Res. 1999, 33, 1238–1246. [Google Scholar] [CrossRef]
- Monteagudo, J.M.; Duran, A.; Lopez-Almodovar, C. Homogeneous ferrioxalate assisted solar photo-Fenton degradation of Orange II aqueous solutions. Appl. Catal. B 2008, 83, 46–55. [Google Scholar] [CrossRef]
- Maezono, T.; Tokumura, M.; Sekine, M.; Kawase, Y. Hydroxyl radical concentration profile in photo-Fenton oxidation process: Generation and consumption of hydroxyl radicals during the discoloration of azo-dye Orange II. Chemosphere 2011, 82, 1422–1430. [Google Scholar] [CrossRef]
- Alalm, M.G.; Tawfik, A.; Ookawara, S. Degradation of four pharmaceuticals by solar photo-Fenton process: Kinetics and costs estimation. J. Environ. Chem. Eng. 2015, 3, 46–51. [Google Scholar] [CrossRef]
- Dehghani, M.; Shahsavani, E.; Farzadkia, M.; Reza, S.M. Optimizing photo-Fenton like process for the removal of diesel fuel from the aqueous phase. J. Environ. Health Sci. Eng. 2014, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Epold, I.; Dulova, N. Oxidative degradation of levofloxacin in aqueous solution by S2O82-/Fe2+, S2O82-/H2O2 and S2O82-/OH- processes: A comparative study. J. Environ. Chem. Eng. 2015, 3, 1207–1214. [Google Scholar] [CrossRef]
- María, C.Y.; Díaz, L.; Fernández, J. Catalytic activity of the SO4•- radical for photodegradation of the azo dye Cibacron Brilliant Yellow 3 and 3,4-dichlorophenol: Optimization by application of response surface methodology. J. Photochem. Photobiol. A Chem. 2010, 215, 90–95. [Google Scholar]
- Chia-Chang, L.; Li-Ting, L.; Ling-Jung, H. Performance of UV/S2O82− process in degrading polyvinyl alcohol in aqueous solutions. J. Photochem. Photobiol. A Chem. 2013, 252, 1–7. [Google Scholar]
- Guan, Y.H.; Jun, M.; Li, X.C.; Fang, J.Y.; Chen, L.W. Influence of pH on the Formation of Sulfate and Hydroxyl Radicals in the UV/Peroxymonosulfate System. Environ. Sci. Technol. 2011, 45, 9308–9314. [Google Scholar] [CrossRef] [PubMed]
- Furman, O.S.; Teel, A.L.; Watts, R.J. Mechanism of base activation of persulfate. Environ. Sci. Technol. 2010, 44, 6423–6428. [Google Scholar] [CrossRef]
- Liu, Y.; He, X.; Fu, Y.; Dionysiou, D.D. Kinetics and mechanism investigation on the destruction of oxytetracycline by UV-254 nm activation of persulfate. J. Hazard. Mater. 2016, 305, 229–239. [Google Scholar] [CrossRef]
- Lin, Y.T.; Liang, C.; Chen, J.H. Feasibility study of ultraviolet activated persulfate oxidation of phenol. Chemosphere 2011, 82, 1168–1172. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lo, S.L.; Kuo, J.; Lin, Y.L. Persulfate oxidation of perfluorooctanoic acid under the temperatures of 20–40 °C. Chem. Eng. J. 2012, 198–199, 27–30. [Google Scholar] [CrossRef]
- Lu, X.; Shao, Y.; Gao, N.; Chen, J.; Zhang, Y.; Xiang, H.; Guo, Y. Degradation of diclofenac by UV-activated persulfate process: Kinetic studies, degradation pathways and toxicity assessments. Ecotoxicol. Environ. Saf. 2017, 141, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Saien, J.; Osali, M.; Soleymani, A.R. UV/persulfate and UV/hydrogen peroxide processes for the treatment of salicylic acid: Effect of operating parameters, kinetic, and energy consumption. Desalin. Water Treat. 2014, 56, 3087–3095. [Google Scholar] [CrossRef]
- Liang, C.; Wang, Z.S.; Bruell, C.J. Influence of pH on persulfate oxidation of TCE at ambient temperatures. Chemosphere 2007, 66, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Wang, Z.; Hu, Y.; Wang, B.; Gao, S. Probing the radical chemistry in UV/persulfate-based saline wastewater treatment: Kinetics modeling and by products identification. Chemosphere 2014, 109, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Rathi, A.; Rajor, H.K.; Sharma, R.K. Photodegradation of Direct Yellow-12 Using UV/H2O2/Fe2+. J. Hazard. Mater. 2003, 102, 231–241. [Google Scholar] [CrossRef]
- Ravichandran, L.; Selvam, K.; Swaminathan, M. Photo-Fenton Defluoridation of Pentafluorobenzoic Acid with UV-C Light. J. Photochem. Photobiol. A Chem. 2007, 188, 392–398. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, N.; Wu, S.; Zhang, Q.; Yang, Z. Modeling the oxidation kinetics of sono-activated persulfate’s process on the degradation of humic acid. Ultrason. Sonochem. 2015, 23, 128–134. [Google Scholar] [CrossRef]
- Li, B.; Li, L.; Lin, K.; Zhang, W.; Lu, S.; Luo, Q. Removal of 1,1,1-trichloroethane from aqueous solution by a sono-activated persulfate process. Ultrason. Sonochem. 2013, 20, 855–863. [Google Scholar] [CrossRef]
- Chu, W.; Li, D.; Gao, N.; Templeton, M.R.; Tan, C.; Gao, Y. The control of emerging haloacetamide DBP precursors with UV/Persulfate treatment. Water Res. 2015, 72, 340–348. [Google Scholar] [CrossRef]
- Pengchao, X.; Jun, M.; Wei, L.; Jing, Z.; Siyang, Y.; Xuchun, L.; Wiesner, R.M.; Jingyun, F. Removal of 2-MIB and geosmin using UV/Persulfate: Contributions of hydroxyl and sulfate radicals. Water Res. 2015, 69, 223–233. [Google Scholar]
- Hori, H.; Yamamoto, A.; Hayakawa, E.; Taniyasu, S.; Yamashita, N.; Kutsuna, S.; Kiatagawa, H.; Arakawa, R. Efficient decomposition of environmentally persistent perfluorocarboxylic acids by use of persulfate as a photochemical oxidant. Environ. Sci. Technol. 2005, 39, 2383–2388. [Google Scholar] [CrossRef]
- Gulsen, H.; Turan, M. Treatment of sanitary landfill leachate using a combined anaerobic fluidized bed reactor and Fenton’s oxidation. Environ. Eng. Sci. 2004, 21, 627–636. [Google Scholar] [CrossRef]
- Zhang, H.; Choi, H.J.; Huang, C.P. Optimization of Fenton process for the treatment of landfill leachate. J. Hazard. Mater. B 2005, 125, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Asgari, G.; Mohammadi, A.S.; Poormohammadi, A.; Ahmadian, M. Removal of cyanide from aqueous solution by adsorption onto bone charcoal, Fresenius. Environ. Bull. 2006, 23, 720–727. [Google Scholar]
- Muruganandham, M.; Swaminathan, M. TiO2–UV photocatalytic oxidation of Reactive Yellow 14: Effect of operational parameters. J. Hazard. Mater. 2006, 135, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.Y.; Huang, S.W.; Tsai, M.K. Comparative study of acid blue 113 wastewater degradation and mineralization by UV/persulfate and UV/Oxone processes. Desalin. Water Treat. 2016, 57, 29517–29530. [Google Scholar] [CrossRef]
- Rivas, F.J.; Beltrán, F.; Gimeno, O.; Carvalho, F. Fenton-like oxidation of landfill leachate. J. Environ. Sci. Health A 2003, 38, 371–379. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).