Abstract
Ionic liquids are considered environmentally friendly media for various industrial applications. Basic data on physicochemical properties are significant for a new material, in terms of developing its potential applications. In this work, 1-ethyl-3-methylimidazolium fluoride ([EMIm]F) ionic liquid was synthesized via an anion metathesis process. Physical properties including the density, viscosity, electrical conductivity, and thermal stability of the product were measured. The results show that the density of [EMIm]F decreases linearly with temperature increases, while dynamic viscosity decreases rapidly below 320 K and the temperature dependence of electrical conductivity is in accordance with the VFT (Vogel–Fulcher–Tammann) equation. The temperature dependence of the density, conductivity, and viscosity of [EMIm]F can be expressed via the following equations: ρ = 1.516 − 1.22 × 10−3 T, σm = 4417.1exp[−953.17/(T − 166.65)] and η = 2.07 × 10−7exp(−5.39 × 104/T), respectively. [EMIm]F exhibited no clear melting point. However, its glass transition point and decomposition temperature are −71.3 °C and 135 °C, respectively.
1. Introduction
Ionic liquids (ILs) are molten salts comprised entirely of cations and anions with a melting point below 100 °C. Generally, cations are organic, such as alkyl-substituted imidazolium, pyridinium, pyrrolidinium, tetraalkylammonium, and tetraalkylphosphonium. Anions can be organic or inorganic, such as halide, tetrafluoroborate, hexafluorophosphate, and bistriflimide. In earlier studies, ethylpyridinium cation was reported as an IL in the 1950s [1], N-butylpyridinium cation was reported as an IL in the 1970s [2], and 1-ethyl-3-methyl-imidazolium cation was reported as an IL in the 1980s [3]. Subsequently, ILs received increasing attention due to their extraordinary physicochemical properties including low melting point, low vapor pressure, high conductivity, and wide potential window. In recent decades in particular, ILs have been considered a promising, green medium for reactive metal electrodeposition, replacing high-temperature molten salts [4,5,6,7,8,9,10].
Numerous studies have focused on designing new ILs and on the relationship between the phase behavior and molecular structure, in order to clarify the nature of ILs and expand their applications [11,12,13,14,15].
Fundamental data on physical and chemical properties are very important for IL design and for the exploration of new applications. So far, many experimental or simulated studies on the density, viscosity, conductivity, thermal behavior, and solubility of pure ILs and their mixtures have appeared in the literature [16,17,18,19,20]. Tokuda and co-workers [21,22,23] systematically studied the effect of ionic structures, including anionic species, cationic species, and alkyl chain, in imidazolium cation on the physicochemical characteristics of ILs.
Alkyl-substituted imidazolium cation-based ILs have been studied extensively due to their advantages in terms of superior fluidity and conductivity. The generic structure of alkyl-substituted imidazolium-based ILs is shown in Figure 1.
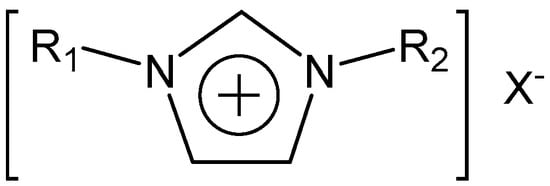
Figure 1.
The generic structure of alkyl-substituted imidazolium=based ILs. R1 = alkyl (methyl typically), R2 = alkyl, X− = anion (halide, BF4−, PF6−, Tf2N−, typically).
For the molecular structure of alkyl-substituted imidazolium-based ILs, as shown in Figure 1, R1 is typically methyl and R2 can generally be butyl [24,25,26,27,28,29,30,31], ethyl [32,33,34,35], or octyl [36]. Common anionic species include halide, tetrafluoroborate (BF4−), tetrafluorophosphate (PF6−), bis(trifluoromethylsulfonyl)imide (Tf2N−), dicyanamide (DCA), ethylsulfate (EtSO4), etc. Among them, ILs with a single fluoride anion are rarely reported because the syntheses of such ILs are challenging, unlike those of other halide ILs. As is known, the enhanced basicity of metal fluorides can lead to Hoffman elimination on side chains of the cationic part of the system. However, anion metathesis from other halides is accessible. Hagiwara and co-workers [37] prepared 1-ethyl-3-methyl imidazolium fluoride (EMIF∙2.3HF) via the reaction of hydrogen fluoride and homologous chloride. Pagoria et al. [38] reported a method that included putting a strongly hydrogen bonded organic material in contact with an ionic liquid having a fluoride anion for the solubilization of the strongly hydrogen bonded organic material. [EMIm]F and other similar imidazolium fluoride ionic liquids could be prepared using imidazolium chloride and silver fluoride as raw materials. Zhu et al. [39] synthesized a series of 1-akyl-3-methylimidazolium fluorides via the reaction of silver fluoride with the corresponding imidazolium iodides; weak interactions between the fluoride anion and sp3-hybridized C–H groups were observed.
So far, few studies exist on the synthesis of [EMIm]F ionic liquid, and knowledge of its physical properties is lacking. We therefore prepared [EMIm]F ionic liquid via an anion metathesis process. It could potentially be used as fluoride source for nanoparticle synthesis [40,41], as an electrolyte for metal deposition, and as a solvent for chemical processing [42]. We focused on its physical properties including density, viscosity, conductivity, and thermal stability. We investigated the temperature effects.
2. Materials and Methods
2.1. Materials
1-ethyl-3-methylimidazolium chloride ([EMIm]Cl) with 99% purity was provided by Lanzhou Institute of Chemical Physics (Lanzhou, China). Silver fluoride with 98% purity and analytical grade silver nitrate was purchased from Sigma Aldrich (St. Louis, MO, USA). Analytical grade sodium chloride, ethyl acetate, and methanol were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shenyang, China). All the chemicals were used as received without further purification.
2.2. Synthesis of [EMIm]F
The reaction principle of synthesizing [EMIm]F is based on the different solubility of silver halide. The reaction equations from [EMIm]Cl are shown in Figure 2.
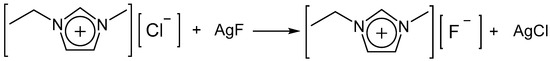
Figure 2.
Synthesis process of [EMIm]F.
The synthesis procedure of [EMIm]F is as follows. (1) Twenty-five milliliters of 2.76 M [EMIm]Cl aqueous solution and 20 mL of 3.45 M AgF aqueous solution (mole ratio of [EMIm]Cl/AgF is 1:1) were mixed. A white precipitate was observed, indicating that the metathesis reaction began rapidly. (2) The mixture was kept for 24 h under stirring. Subsequently, the liquid was separated from the precipitate via filtration. (3) The filtrate was dried for 48 h under vacuum conditions and a viscous liquid, [EMIm]F, was obtained. (4) The product was weighed, and the yield of [EMIm]F was found to be 95~98%. (5) In order to check the impurities removal and the purity of [EMIm]F, two samples of the final product were titrated via AgNO3 and NaCl, respectively. No precipitation indicated that Cl− and Ag+ ions were removed completely.
2.3. Physical Properties of [EMIm]F
The physical properties of [EMIm]F were also examined. To avoid the influence of water, the measurements of density and conductivity were carried out in a glove box full of argon gas. The density was measured via 10 mL pycnometer and the uncertainty of the measurements was estimated to be better than ±5 × 10−5 g·cm−3. The conductivity was measured via the fixed conductivity cell method, with an uncertainty of ±3% calibrated by a standard sodium chloride solution. Furthermore, viscosity was measured via the rotation method on a Brookfield DV−2T viscometer (rotor model SC 4–18, vertical cryostat tank DC0506N), with a nominal uncertainty of ±2%. The thermal stability of [EMIm]F was measured via a differential scanning calorimeter (DSC, NETZSCH, STA449 F3) in a nitrogen atmosphere at a temperature range of −160 °C to 200 °C and a heating rate of 10 °C/min.
3. Results and Discussion
All the experimental data are available in the Supplementary Materials. Tables S1–S3 show density, viscosity and electrical conductivity of [EMIm]F at different temperatures, respectively. The density of [EMIm]F was measured for temperatures between 303.15 K and 353.15 K. The temperature dependence of the density of [EMIm]F is shown in Figure 3. Meanwhile, the densities of ILs with the same cationic group and different anionic groups were also compared [43,44,45,46]. Figure 3 shows that the density of [EMIm]F decreases linearly with the increase of temperature for the other ILs. At a certain temperature, the density of [EMIm]F is higher than that of [EMIm][OAc] and [EMIm][DCA], and is lower than that of [EMIm][Tf2N], [EMIm][BF4] and [EMIm][EtSO4]. Correspondingly, the molar volume, calculated by dividing the molar mass by the density, increases linearly with the increase of temperature. The relationships between the density, molar volume, and temperature can be described by the following fitting equations:
ρ = 1.516 − 1.22 × 10−3 T
Vm = 76.21 + 0.1296 T.
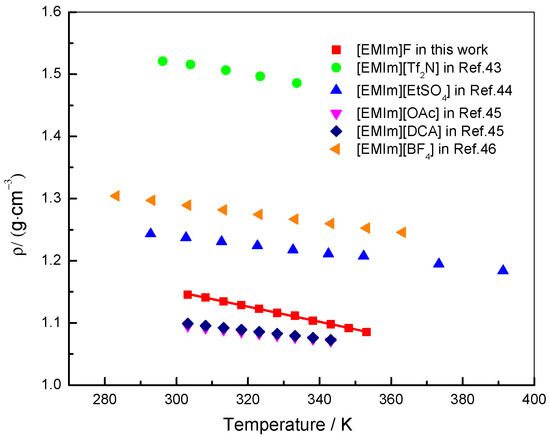
Figure 3.
Temperature dependence of the densities of [EMIm]F and similar ILs.
To study the volume changes of a pure liquid caused by the temperature at a constant pressure, a parameter, α, known as the thermal expansion coefficient, is defined as
where the subscript p indicates constant pressure.
According to the calculus principle, Equation (3) can also be expressed as
As we discussed above, both the density and molar volume have a linear relation with the temperature. Namely, and are constant. The thermal expansion coefficient α varies with the density according to Equation (3). However, α is a constant from Equation (4) because the plot of lnρ versus T is also linear. Actually, the two perspectives are not so conflicting due to different linear relations supposed in a rational scope (R2 > 0.99) for the two equations. In the literature, both alternatives were reported in order to evaluate the volume expansivity for ILs [44,47,48]. For simplification, our work considers the thermal expansion coefficient of [EMIm]F a constant from Equation (4): 1.09 × 10−3 K−1.
The dynamic viscosity of [EMIm]F was measured via a DV-2T viscometer. The viscosities of ILs with the same cationic group and different anionic groups were compared, as shown in Figure 4. The viscosity of [EMIm]F is obviously larger than that of other ILs below 320 K, and decreases sharply with the temperature increase. The viscosity of [EMIm]F approaches that of other ILs above 320 K. As is known, the viscosity of ILs is mainly dependent on the combination of hydrogen bonds and van der Waals forces. The viscosity variation of [EMIm]F indicates that the interaction forces between the cation and anion are susceptible to temperature. In general, the relationship between temperature and viscosity can be expressed as an exponential equation, commonly known as an Arrhenius-type equation:
where R is the ideal gas constant, η∞ is the viscosity at the infinite temperature, and Ea is the apparent activation energy. According to the fitting results, the characteristic parameters of [EMIm]F can be obtained: η∞ = 2.07 × 10−7 mPa·s, Ea = −5.39 × 104 J·mol−1.
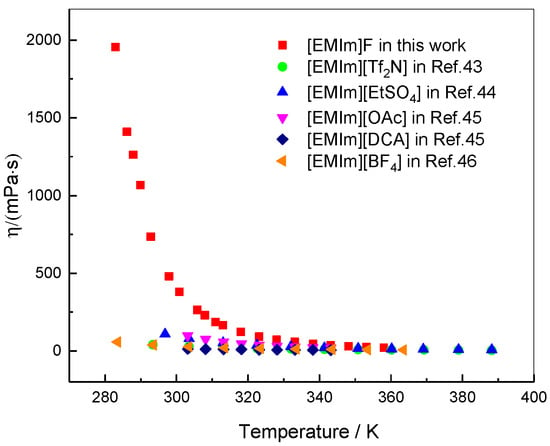
Figure 4.
Viscosity of [EMIm]F and similar ILs as a function of temperature.
The conductivity of ionic liquids is a parameter reflecting the current transporting capacity. The conductivity of [EMIm]F as a function of temperature was studied, as shown in Figure 5. As can be seen, the conductivity of [EMIm]F increases with the increase in temperature. We fitted the relationship between the conductivity and temperature using a VFT (Vogel–Fulcher–Tammann) equation:
where σ0 (mS·cm−1), B (K), and T0 (K) are constants. The fitting parameters are obtained as follows: σ0 = 4417.1 mS·cm−1, B = 953.17 K, T0 = 166.65 K.
σ = σ0exp[−B/(T − T0)],
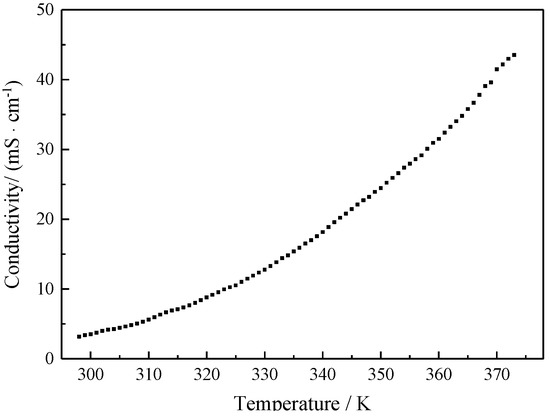
Figure 5.
Electrical conductivity of [EMIm]F as a function of temperature.
The thermal behavior of [EMIm]F was studied via differential scanning calorimetry. The DSC curve of [EMIm]F at a cooling and heating rate of 10 K/min is shown in Figure 6. During cooling from 50 to −150 °C, we could only observe an exothermic peak at −71.3 °C, corresponding to the glass transition. In the heating process from −150 to 200 °C, two endothermic peaks were found. One is the glass transition point, which was also observed during the cooling process. The other is assigned to the decomposition temperature around 135 °C. No melting point on the DSC curve was observed, as is the case for many other ionic liquids. It has been reported that the melting point of many ionic liquids is uncertain because they can undergo considerable supercooling [49,50,51]. The temperature of the phase change can differ considerably depending on whether the sample is heated or cooled [52].
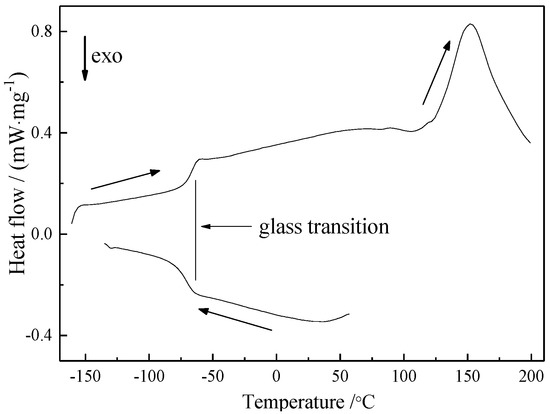
Figure 6.
Differential scanning calorimeter (DSC) curve of [EMIm]F at the cooling and heating rate of 10 K/min.
4. Conclusions
[EMIm]F was synthesized via an anion metathesis process using [EMIm]Cl and AgF as raw materials. The density, conductivity, dynamic viscosity, and the thermal stability of [EMIm]F were studied. With the increase of temperature, the density of [EMIm]F decreased linearly, while the viscosity and conductivity of [EMIm]F increased. The corresponding temperature dependence equations were summarized. The thermal analysis indicated that [EMIm]F had no clear melting point, while its glass transition point and decomposition temperature were −71.3 °C and 135 °C, respectively.
Supplementary Materials
The following are available online at http://www.mdpi.com/2076-3417/8/3/356/s1, Table S1: Density of [EMIm]F at different temperatures. Table S2: Viscosity of [EMIm]F at different temperatures. Table S3: Conductivity of [EMIm]F at different temperatures.
Acknowledgments
The authors acknowledge financial support from the National Key R & D Program of China (No. 2017YFC0805101), the National Natural Science Foundation of China (No. 51574071, 51322406), the Fundamental Research Funds for the Central Universities (No. N162503001), and the NEU Postdoctoral Foundation (No. 20170307).
Author Contributions
Zhongning Shi. conceived and designed the experiments; Fengguo Liu and Xiongwei Zhong performed the experiments; Fengguo Liu, Ali Kamali, and Junli Xu analyzed the data; Fengguo Liu wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hurley, F.H.; Weir, T.P. The Electrodeposition of Aluminum from Nonaqueous Solutions at Room Temperature. J. Electrochem. Soc. 1951, 98, 207–212. [Google Scholar] [CrossRef]
- Chum, H.L.; Koch, V.R.; Miller, L.L.; Osteryoung, R.A. Electrochemical scrutiny of organometallic iron complexes and hexamethylbenzene in a room temperature molten salt. J. Am. Chem. Soc. 1975, 97, 3264–3265. [Google Scholar] [CrossRef]
- Wilkes, J.S.; Levisky, J.A.; Wilson, R.A.; Hussey, C.L. Dialkylimidazolium chloroaluminate melts: A new class of room-temperature ionic liquids for electrochemistry, spectroscopy and synthesis. Inorg. Chem. 1982, 21, 1263–1264. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Keim, W. Ionic liquids-new solution for transition metal catalysis. Angew. Chem. Int. Ed. 2000, 39, 3772–3789. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Q.; Zhang, S.; Lu, X.; Zhang, X. Electrodeposition in Ionic Liquids. Chem. Phys. Chem. 2016, 17, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, J.; Tu, J.; Dreisinger, D. Ionic liquid electrodeposition of reactive metals. Miner. Process. Extr. Metall. 2008, 117, 113–117. [Google Scholar] [CrossRef]
- Xu, C.; Hua, Y.; Zhang, Q.; Li, J.; Lei, Z.; Lu, D. Electrodeposition of Al-Ti alloy on mild steel from AlCl3-BMIC ionic liquid. J. Solid State Electr. 2017, 21, 1349–1356. [Google Scholar] [CrossRef]
- Zhang, M.; Kamavarum, V.; Reddy, R.G. New electrolytes for aluminum production: Ionic liquids. JOM 2003, 55, 54–57. [Google Scholar] [CrossRef]
- Liu, A.; Shi, Z.; Reddy, R. Electrodeposition of Pb from PbO in urea and 1-butyl-3-methylimidazolium chloride deep eutectic solutions. Electrochim. Acta 2017, 251, 176–186. [Google Scholar] [CrossRef]
- Li, M.; Gao, B.; Liu, C. AlCl3/amide ionic liquids for electrodeposition of aluminium. J. Solid State Electr. 2016, 21, 1–8. [Google Scholar]
- Zou, F.; Yu, X.; Zhang, J.; Cheng, N.; Huang, X. Electropolymerization in a novel proton functionalized room temperature ionic liquid anilinium acetate. Synth. Met. 2015, 204, 76–83. [Google Scholar] [CrossRef]
- Huddleston, J.G.; Willauer, H.D.; Swatloski, R.P.; Visser, A.E.; Rogers, R.D. Room temperature ionic liquids as novel media for ‘clean’ liquid–liquid extraction. Chem. Commun. 1998, 16, 1765–1766. [Google Scholar] [CrossRef]
- Björling, M.; Bair, S.; Mu, L.; Zhu, J.; Shi, Y. Elastohydrodynamic Performance of a Bio-Based, Non-Corrosive Ionic Liquid. Appl. Sci. 2017, 7, 996. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, Y.; Wang, Q.; Wang, Z.; Tian, D. Density and Viscosity of Binary Mixtures of 1-Ethyl-3-methylimidazolium Heptachlorodialuminate and Tetrachloroaluminate Ionic Liquids. J. Chem. Eng. Data 2017, 62, 4006–4014. [Google Scholar] [CrossRef]
- Jacquemin, J.; Husson, P.; Mayer, V.; Cibulka, I. High-Pressure Volumetric Properties of Imidazolium-Based Ionic Liquids: Effect of the Anion. J. Chem. Eng. Data 2007, 52, 2204–2211. [Google Scholar] [CrossRef]
- Anuar, N.K.; Subban, R.H.Y.; Mohamed, N.S. Properties of PEMA-NH4CF3SO3 Added to BMATSFI Ionic Liquid. Materials 2012, 5, 2609–2620. [Google Scholar] [CrossRef]
- Grothe, D.C.; Meyer, W.; Janietz, S. Acrylate Functionalized Tetraalkylammonium Salts with Ionic Liquid Properties. Molecules 2012, 17, 6593–6604. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.H.; Chen, Y.P.; Su, C.S. Density and Viscosity Measurements for Binary Mixtures of 1-Ethyl-3-methylimidazolium Tetrafluoroborate ([Emim][BF4]) with Dimethylacetamide, Dimethylformamide, and Dimethyl Sulfoxide. J. Chem. Eng. Data 2016, 61, 920–927. [Google Scholar] [CrossRef]
- Ghatee, M.H.; Zare, M.; Moosavi, F.; Zolghadr, A.R. Temperature-Dependent Density and Viscosity of the Ionic Liquids 1-Alkyl-3-methylimidazolium Iodides: Experiment and Molecular Dynamics Simulation. J. Chem. Eng. Data 2010, 55, 3084–3088. [Google Scholar] [CrossRef]
- Jacquemin, J.; Nancarrow, P.; Rooney, D.W.; Costa Gomes, M.F.; Husson, P.; Majer, V.; Pádua, A.A.; Hardacre, C. Prediction of Ionic Liquid Properties. II. Volumetric Properties as a Function of Temperature and Pressure. J. Chem. Eng. Data 2008, 53, 716–726. [Google Scholar] [CrossRef]
- Tokuda, H.; Hayamizu, K.; Ishii, K.; Susan, M.A.B.H.; Watanabe, M. Physicochemical Properties and Structures of Room Temperature Ionic Liquids. 1. Variation of Anionic Species. J. Phys. Chem. B 2004, 108, 16593–16600. [Google Scholar] [CrossRef]
- Tokuda, H.; Hayamizu, K.; Ishii, K.; Susan, M.A.B.H.; Watanabe, M. Physicochemical Properties and Structures of Room Temperature Ionic Liquids. 2. Variation of Alkyl Chain Length in Imidazolium Cation. J. Phys. Chem. B 2005, 109, 6103–6110. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, H.; Ishii, K.; Susan, M.A.B.H.; Tsuzuki, S.; Hayamizu, K.; Watanabe, M. Physicochemical Properties and Structures of Room-Temperature Ionic Liquids. 3. Variation of Cationic Structures. J. Phys. Chem. B 2006, 110, 2833–2839. [Google Scholar] [CrossRef] [PubMed]
- Yadav, J.S.; Reddy, B.V.S.; Basak, A.K.; Narsaiah, A.V. [Bmim]BF4 Ionic Liquid: A Novel Reaction Medium for the Synthesis of β-Amino Alcohols. Tetrahedron Lett. 2003, 44, 1047–1050. [Google Scholar] [CrossRef]
- Chang, H.C.; Wang, T.H.; Burba, C.M. Probing Structures of Interfacial 1-Butyl-3-Methylimidazolium Trifluoromethanesulfonate Ionic Liquid on Nano-Aluminum Oxide Surfaces Using High-Pressure Infrared Spectroscopy. Appl. Sci. 2017, 7, 855. [Google Scholar] [CrossRef]
- Zhou, Z.; He, D.L.; Yang, R.H.; Guo, Y.N.; Zhong, J.F.; Li, G.X. Electropolymerization of benzotriazole in room temperature ionic liquid [bmim]PF6. J. Appl. Electrochem. 2008, 38, 1757–1761. [Google Scholar] [CrossRef]
- Harris, K.R.; Kanakubo, M. Self-diffusion, velocity cross-correlation, distinct diffusion and resistance coefficients of the ionic liquid [BMIM][Tf2N] at high pressure. Phys. Chem. Chem. Phys. 2015, 17, 23977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; An, M.; Chen, Q.; Liu, A.; Jiang, X.; Ji, S.; Lian, Y.; Wen, X. Electrochemical Study of the Diffusion and Nucleation of Gallium(III) in [Bmim][TfO] Ionic Liquid. Electrochim. Acta 2016, 190, 1066–1077. [Google Scholar] [CrossRef]
- Larriba, M.; Navarro, P.; García, J.; Rodríguez, F. Liquid–Liquid Extraction of Toluene from Heptane Using [emim][DCA], [bmim][DCA], and [emim][TCM] Ionic Liquids. Ind. Eng. Chem. Res. 2013, 52, 2714–2720. [Google Scholar] [CrossRef]
- Fernández, A.; García, J.; Torrecilla, J.S.; Oliet, M.; Rodríguez, F. Volumetric, Transport and Surface Properties of [bmim][MeSO4] and [emim][EtSO4] Ionic Liquids As a Function of Temperature. J. Chem. Eng. Data 2008, 53, 1518–1522. [Google Scholar] [CrossRef]
- Tagiuri, A.; Sumon, K.Z.; Henni, A. Solubility of carbon dioxide in three [Tf2N] ionic liquids. Fluid Phase Equilib. 2014, 380, 39–47. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, L.; Geng, Y.; Wang, Y. The structure of acidified ionic liquid [emim]BF4 and its catalytic performance in the reaction for 4,4′-MDC synthesis. J. Mol. Catal. A Chem. 2007, 276, 168–173. [Google Scholar] [CrossRef]
- Lin, Y.F.; Sun, I.W. Electrodeposition of zinc from a Lewis acidic zinc chloride-1-ethyl-3-methylimidazolium chloride molten salt. Electrochim. Acta 1999, 46, 1169–1177. [Google Scholar] [CrossRef]
- Horikawa, Y.; Tokushima, T.; Takahashi, O.; Hoke, H.; Takamuku, T. Correlation between Soft X-ray Absorption and Emission Spectra of the Nitrogen Atoms within Imidazolium-Based Ionic Liquids. J. Phys. Chem. B 2016, 120, 7480–7487. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.; Thompson, D.L. Molecular dynamics studies of melting and some liquid-state properties of 1-ethyl-3-methylimidazoliumhexafluorophosphate [emim][PF6]. J. Chem. Phys. 2005, 122, 154704–154715. [Google Scholar] [CrossRef] [PubMed]
- Afsharpour, A.; Kheiri, A. Implementation of CPA EoS for simultaneous solubility modeling of CO2 and H2S in ionic liquid [OMIM][Tf2N]. Pet. Sci. Technol. 2017, 36, 280–286. [Google Scholar] [CrossRef]
- Hagiwara, R.; Hirashige, T.; Tsuda, T.; Ito, Y. Acidic 1-ethyl-3-methylimidazolium fluoride: A new room temperature ionic liquid. J. Fluor. Chem. 1999, 99, 1–3. [Google Scholar] [CrossRef]
- Pagoria, P.F.; Maiti, A.; Gash, A.; Han, T.Y.; Orme, C.; Fried, L. Ionic Liquids as Solvents. US 20090012297, 8 January 2009. [Google Scholar]
- Zhu, Z.Q.; Jiang, M.Y.; Zheng, C.G.; Xiao, J.C. Efficient synthesis of 1-alkyl-3-methylimidazolium fluorides and possibility of the existence of hydrogen bonding between fluoride anion and C(sp3)–H. J. Fluor. Chem. 2012, 133, 160–162. [Google Scholar] [CrossRef]
- Nuñez, N.O.; Ocaña, M. An ionic liquid based synthesis method for uniform luminescent lanthanide fluoride nanoparticles. Nanotechnology 2007, 18, 455606. [Google Scholar] [CrossRef]
- Olchowka, J.; Suta, M.; Wickleder, C. Green synthesis of small nanoparticles of A2SiF6 (A = Li-Cs) using Ionic Liquids as Solvent and Fluorine Source: A novel simple approach without HF. Chem. Eur. J. 2017, 23, 12092–12095. [Google Scholar] [CrossRef] [PubMed]
- Brennecke, J.F.; Maginn, E.J. Ionic liquids: Innovative fluids for chemical processing. Aiche J. 2001, 47, 2384–2389. [Google Scholar] [CrossRef]
- Fredlake, C.P.; Crosthwaite, J.M.; Hert, D.G.; Aki, S.N. Thermophysical Properties of Imidazolium-Based Ionic Liquids. J. Chem. Eng. Data 2004, 49, 954–964. [Google Scholar] [CrossRef]
- Jacquemin, J.; Husson, P.; Padua, A.A.H.; Majer, V. Density and viscosity of several pure and water-saturated ionic liquids. Green Chem. 2006, 8, 172–180. [Google Scholar] [CrossRef]
- Quijada-Maldonado, E.; Boogaart, S.V.D.; Lijbers, J.H.; Meindersma, G.W.; De Haan, A.B. Experimental densities, dynamic viscosities and surface tensions of the ionic liquids series 1-ethyl-3-methylimidazolium acetate and dicyanamide and their binary and ternary mixtures with water and ethanol at T = (298.15 to 343.15 K). J. Chem. Thermodyn. 2012, 51, 51–58. [Google Scholar] [CrossRef]
- Shamsipur, M.; Beigi, A.A.M.; Teymouri, M.; Pourmortazavi, S.M.; Irandoust, M. Physical and electrochemical properties of ionic liquids 1-ethyl-3-methylimidazolium tetrafluoroborate, 1-butyl-3-methylimidazolium trifluoromethanesulfonate and 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide. J. Mol. Liq. 2010, 157, 43–50. [Google Scholar] [CrossRef]
- Yang, J.Z.; Lu, X.M.; Gui, J.S.; Xu, W.G. A new theory for ionic liquids-the Interstice Model Part 1. The density and surface tension of ionic liquid EMISE. Green Chem. 2004, 6, 541–543. [Google Scholar] [CrossRef]
- Gu, Z.; Brennecke, J.F. Volume Expansivities and Isothermal Compressibilities of Imidazolium and Pyridinium-Based Ionic Liquids. J. Chem. Eng. Data 2002, 47, 339–345. [Google Scholar] [CrossRef]
- Nishikawa, K.; Wang, S.; Katayanagi, H.; Hayashi, S.; Hamaguchi, H.; Koga, Y.; Tozaki, K. Melting and Freezing Behaviors of Prototype Ionic Liquids, 1-Butyl-3-methylimidazolium Bromide and Its Chloride, Studied by Using a Nano-Watt Differential Scanning Calorimeter. J. Phys. Chem. B 2007, 111, 4894–4900. [Google Scholar] [CrossRef] [PubMed]
- Huddleston, J.G.; Visser, A.E.; Reichert, W.M.; Willauer, H.D.; Broker, G.A.; Rogers, R.D. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazoliumcation. Green Chem. 2001, 3, 156–164. [Google Scholar] [CrossRef]
- Imanari, M.; Fujii, K.; Endo, T.; Seki, H.; Tozaki, K.; Nishikawa, K. Ultraslow Dynamics at Crystallization of a Room Temperature Ionic Liquid, 1-Butyl-3-methylimidazolium Bromide. J. Phys. Chem. B 2012, 116, 3991–3997. [Google Scholar] [CrossRef] [PubMed]
- Ngo, H.L.; Lecompte, K.; Hargens, L.; Mcewen, A.B. Thermal properties of imidazolium ionic liquids. Thermochim. Acta 2000, 357, 97–102. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).