Abstract
In this paper, we review recent progress and new challenges in the area of oxyfluoride perovskite, especially layered systems including Ruddlesden-Popper (RP), Dion-Jacobson (DJ) and Aurivillius (AV) type perovskite families. It is difficult to synthesize oxyfluoride perovskite using a conventional solid-state reaction because of the high chemical stability of the simple fluoride starting materials. Nevertheless, persistent efforts made by solid-state chemists have led to a major breakthrough in stabilizing such a mixed anion system. In particular, it is known that layered perovskite compounds exhibit a rich variety of O/F site occupation according to the synthesis used. We also present the synthetic strategies to further extend RP type perovskite compounds, with particular reference to newly synthesized oxyfluorides, Sr2CoO3F and Sr3Fe2O5+xF2−x (x ~ 0.44).
1. Introduction
Since the discovery of a high-TC superconducting cuprate by Bednorz and Muller [], there has been considerable progress in the field of solid-state chemistry and physics. In particular, we have deepened the understanding of metal oxides, while making improvements and development of experimental techniques and theories. It is well known that a perovskite structure formulated as ABO3 (A = large s-, d-, or f-block cation; B = smaller transition metal cation) has rich variety in structural, electronic and magnetic properties, ranging from superconductivity, through ferroelectricity, to photocatalytic activity. Figure 1(a) shows the ideal perovskite structure in which A cation occupies an interstitial site of an eight corner-sharing BO6 octahedra. This type of structure can be extended to the layered perovskite intergrowth system termed the Ruddlesden-Popper (RP) phase, A2A’n−1BnO3n+1 (n = the number of perovskite block), the structures with n = 1 and 2 which are depicted in Figure 1(b) and (c). Each perovskite block is intervened with double rock-salt AO layers. Thanks to the ability of the A and B sites to adopt various metal cations, we are able to finely control the chemical compositions as well as the physical properties, as exemplified by magnetoresistive manganite [,,] and superconducting cuprate [,]. While the majority of studies have concentrated on the influence of cation substitution, little effort has been made to control the structural and physical properties by manipulating the anion lattices. Considering that the anion strongly affects the crystal field and electronic state of the metal center, we can expect that substitution of anions with different bonding nature, valence state or ionic radius from oxygen in a metal oxide can enhance the original physical properties or induce new exotic phenomena. In fact, LaTiO2N [] and Sr2CuO2F2+δ [] exhibit visible-light photocatalytic activity and superconductivity, respectively.
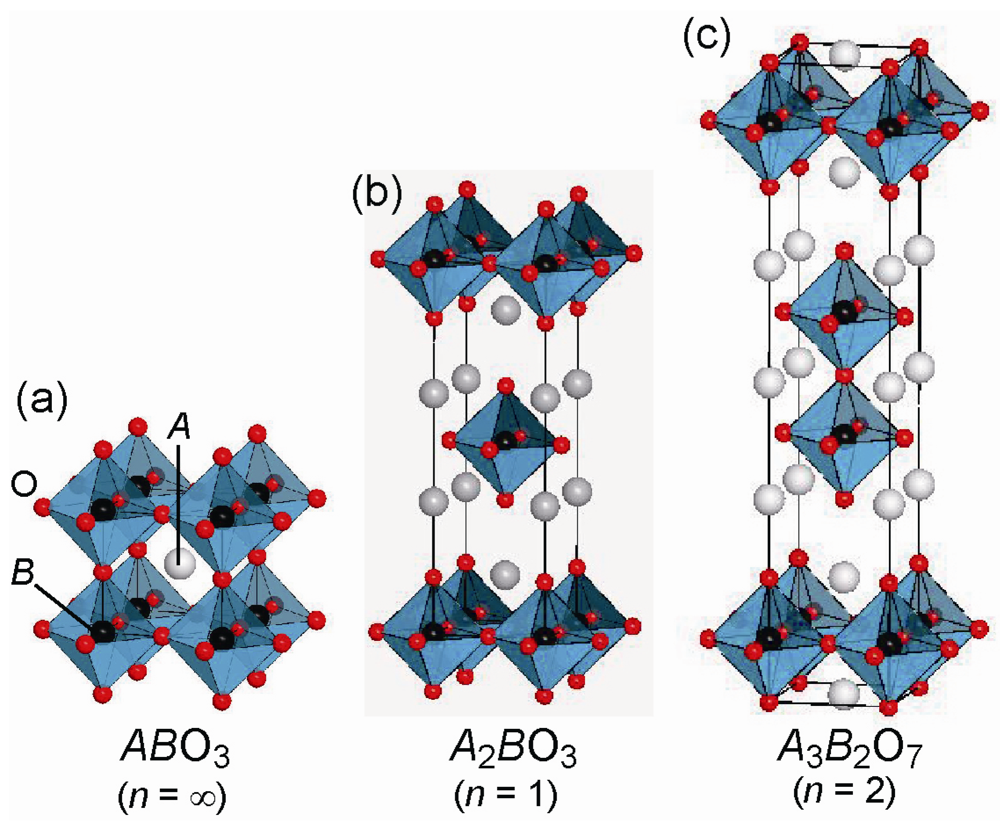
Figure 1.
Structure of the ideal perovskite structure, An−1BnO3n+1 (n = 1, 2, ∞).
In general, it is very difficult to stabilize two kinds of hetero anions in one structure using conventional solid-state reactions, in comparison with compounds with a single anion, such as an oxide, sulfide or nitride. As for the oxyfluoride compounds, several synthetic approaches are employed to overcome the problem, namely a low-temperature reaction using fluorinating agents [,,,], hydrothermal reaction [] or high-pressure synthesis [,]. Interestingly, O/F site order and/or F contents inserted, which are closely correlated with the structural and physical properties, depend on the reaction method used. In particular, layered perovskite structures exhibit three types of anion distribution patterns; (i) regular or random anion occupation pattern in the perovskite blocks, (ii) fluorine insertion into only interstitial sites between the perovskite blocks, or (iii) fluorine occupation of both the terminal apical sites and the interstitial sites. In this paper, we first review recent progress in the oxyfluoride chemistry of perovskite-based compounds, then present a synthesis strategy to further extend layered perovskite systems.
2. Fluorine Occupation Patterns in Layered Oxyfluoride Perovskite
2.1. Regular or Random Anion Occupation Pattern in the Perovskite Blocks
Early in the study on the layered oxyfluoride perovskite, high temperature solid-state reaction, one of the easiest synthetic methods, was commonly employed. However, the variety of transition metals in the reported oxyfluoride compounds is quite limited, mainly due to the high chemical stability of the simple starting fluoride materials. To the best of our knowledge, the first example of the RP-type layered perovskite compound is K2NbO3F, which was reported in 1962 by Galasso and Darby []. As shown in Figure 2(a), the niobium oxyfluoride adopts the tetragonal structure in the space group of I4/mmm with octahedral coordination around Nb atom and O/F site disorder at the apical sites. The preferential occupation of the fluoride anion at the apical sites elongated the Nb-O/F bonds (2.0642 Å) along the c axis compared with those (1.9780 Å) in the ab plane. Subsequently, the same authors reported an isostructural iron oxyfluoride, Sr2FeO3F [] and determined the crystal structure to be I4/mmm. However, Weller and his collaborators demonstrated that the exact crystal symmetry could be described as P4/nmm []. In contrast to K2NbO3F, the Fe counterpart possesses the O/F site order at the apical sites leading to a strong distortion of the FeO5F octahedron (see Figure 2(b)). In fact, the bond length between Fe and O or F is 1.904 or 2.730 Å. Therefore, the iron metal center effectively takes a square pyramidal coordination against five O2− anions. Weller et al. also extended the layered oxyfluoride system to Ba2BO3F (B = Sc and In) []. Both compounds exhibit preferential occupation by F− anions at the apical sites, but the anion-site order/disorder in B = In/Sc.
In addition to the RP-type layered perovskite, Dion-Jacobson (DJ) and Aurivillius (AV) -type layered oxyfluoride phases are reported. The formulas of DJ and AV phases are expressed as AA’n−1BnO3n+1 and (Bi2O2)(A’n−1BnO3n+1), respectively. ASrNb2O6F (A = Li, Na, Rb) [] (Figure 3) and RbLnTiNbO6F (Ln = La, Pr, Nd) [] were synthesized by conventional solid-state reaction. In contrast to the above RP phases, the F atoms prefer to occupy the equatorial and central apical anion sites in the double-layered perovskite block, not the terminal apical ones, because the covalency of the chemical bond between Nb and the terminal apical oxygen is incompatible with the ionicity of the Nb-F bond. On the other hand, Kobayashi et al. reported the reductive fluorination of DJ-RbLaNb2O7 and RP-NaYTiO4 into RbLaNb2O7−xFx and NaYTiO4−xFx using poly(vinylidene)fluoride (PVDF) []. PVDF or poly(tetrafluoroethylene) (PTFE) are effective fluorinating agents, utilized by Slater for the first time []. This fluorination proceeds in a topotactic manner; the framework of the precursor is maintained through the reaction. While ASrNb2O6F and RbLnTiNbO6F with the non-magnetic B cations are insulating, RbLaNb2O7−xFx possesses mixed valence states between Nb4+ and Nb5+ cations, which makes it electrically conductive.
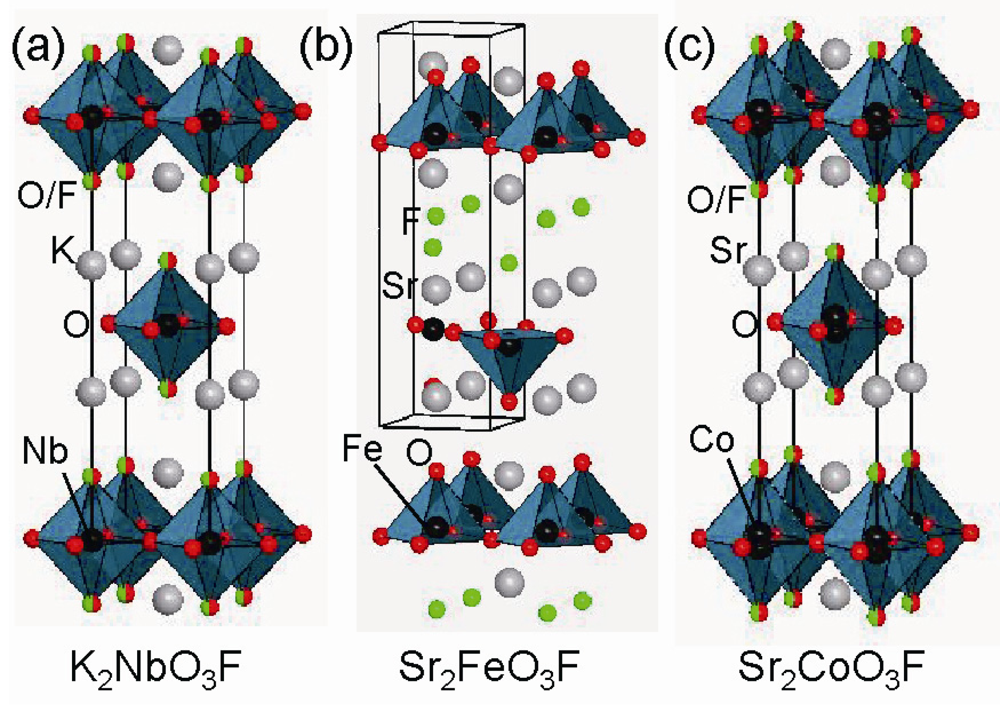
Figure 2.
Crystal structure of (a) K2NbO3F, (b) Sr2FeO3F, and (c) Sr2CoO3F. Solid line represents the unit cell.
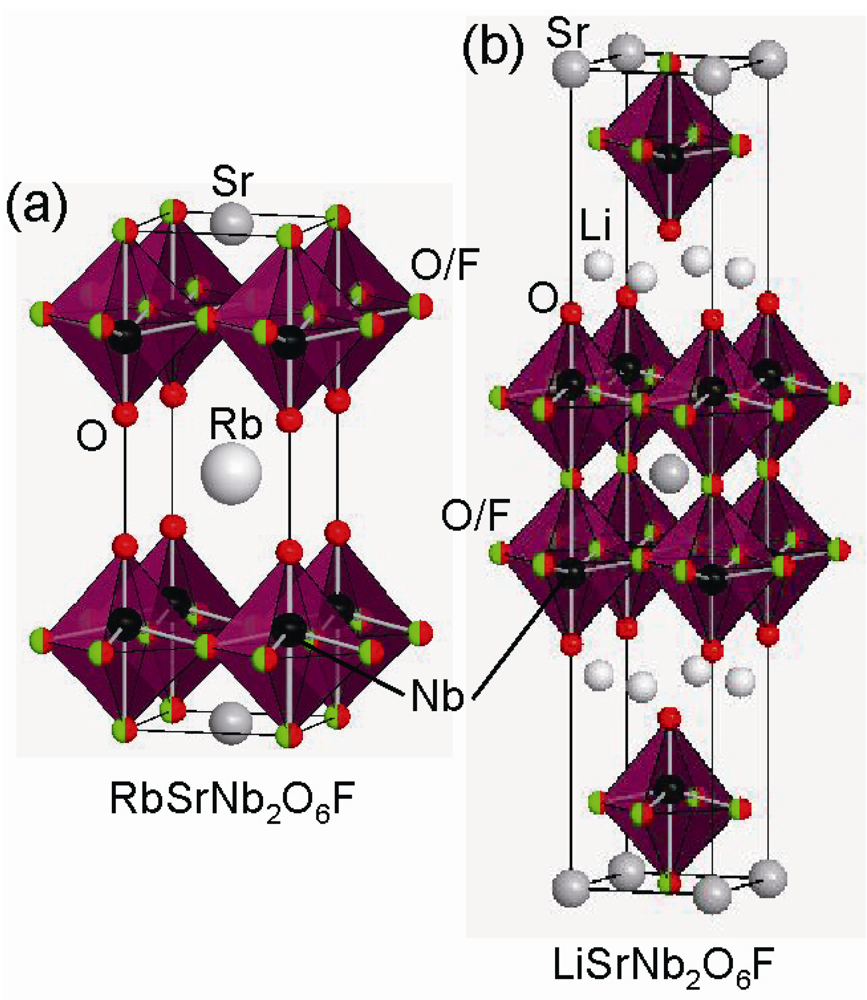
Figure 3.
Crystal structure of (a) RbSrNb2O6F and (b) LiSrNb2O6F.
For n = 2 RP-type layered perovskite, Ba3In2O5F2 [] and Sr3Fe2O6F0.68 [] are known. Common to both compounds, fluorine atoms occupy the terminal apical sites. In comparison with Ba3In2O5F2, synthesized by conventional high-temperature reaction, Sr3Fe2O6F0.68 is obtained by a low-temperature fluorination using F2 gas. As shown in Figure 4, the precursor Sr3Fe2O6 has an oxygen vacancy at the central apical site, but its oxidative fluorination causes local migration of the terminal apical site to the original oxygen vacant site. Because the intercalated F atoms occupy the terminal apical sites with O, the deviation of the O-Fe-O bond angle in the plane from the ideal 180° is only 7.6°, much smaller than the corresponding value of 15.28° in Ba3In2O5F2 with full fluorine occupation of the terminal apical sites.
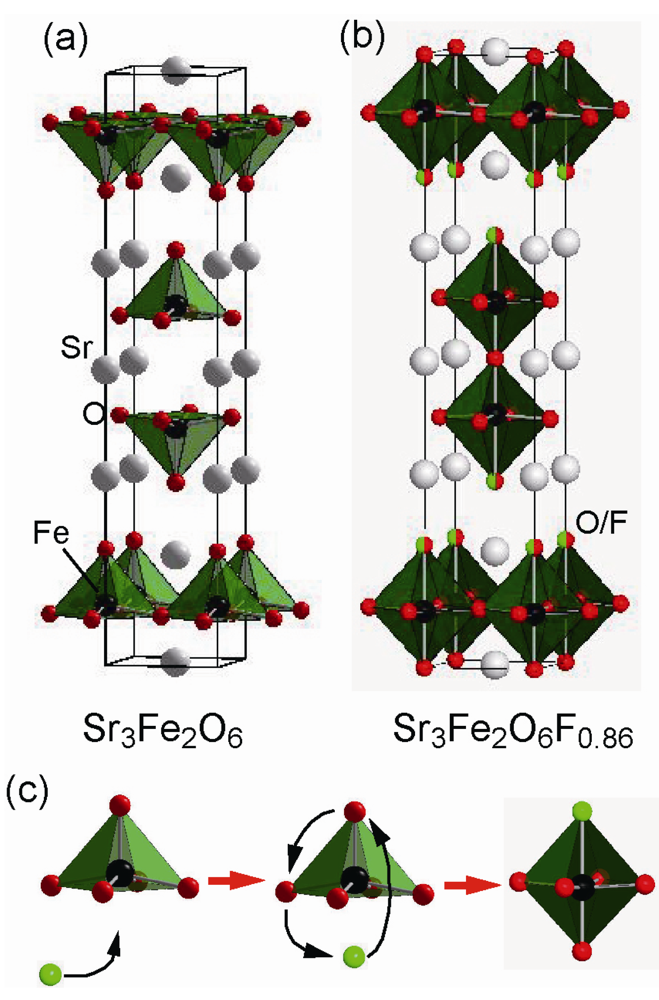
Figure 4.
Crystal structure of (a) Sr3Fe2O6 and (b) Sr3Fe2O6F0.86. (c) Fluorination process of Sr3Fe2O6 to Sr3Fe2O6F0.86, showing rearrangement of oxide and fluoride anions.
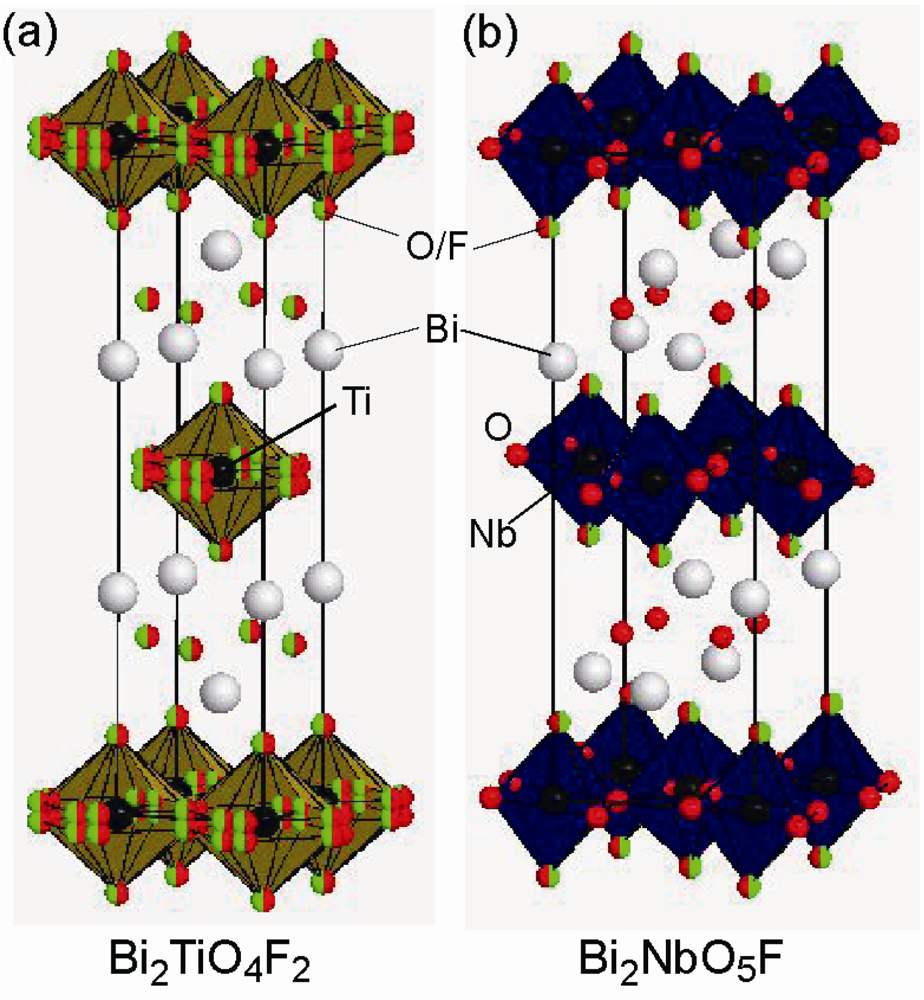
Figure 5.
Crystal structure of (a) Bi2TiO4F2, and (b) Bi2NbO5F.
Three kinds of AV-type layered oxyfluoride perovskites, Bi2BO5F (B = Nb, Ta) and Bi2TiO4F2 (Figure 5), were synthesized by Aurivillius []. Later work presented the ferroelectric phase transitions at TC = 303, 283, and 284 K, respectively, but the relationship between the crystal structure and the ferroelectricity is still controversial. Hydrothermal synthesis yields better sample quality than conventional solid-state reaction []. Bi2TiO4F2 adopts the simple body-centered tetragonal structure in the space group I4/mmm [] while Bi2NbO5F is proposed to adopt Pbca symmetry []. These centrosymmetric crystal structures contradict the requirement for the ferroelectricity. In fact, the reexamination of electrical properties in Bi2NbO5F demonstrated neither second-harmonic generation nor a permittivity anomaly associated with ferroelectric phase transition reported previously. It is believed from bond-valence-sum calculations that F atoms tend to occupy the equatorial sites for Bi2TiO4F2 or the apical sites for Bi2NbO5F.
We would like to show a new class of anion ordered perovskite materials, KNaNbOF5 and KNaMO2F4 (M = Mo6+, W6+) expressed as the general formula ABB’(O,F)6 []. Poeppelmeier et al. successfully synthesized these three compounds by hydrothermal reaction. Layers of K+ cations and cation vacancies are alternately located in the A site along the c axis, and Na+ and B (Nb5+, Mo6+, W6+) cations are ordered in a rock salt configuration. More interestingly, fluoride anions are located in K+ cation layers while apical oxide anions are located in the adjacent A-site layer containing the A-site vacancies. Similar to DJ-ASrNb2O6F, the Nb5+ cation form short Nb=O bonds and one long Nb-F bond opposite the O2− anion, leading to strong distortion of the Nb5+-centered octahedron. This type of O/F anion order has never been seen in any other oxyfluorides. It is apparent that the cation order in both A and B sites influences the O/F anion order.
2.2. Fluorine Insertion into Only Interstitial Sites between the Perovskite Blocks
There are a few examples that involve fluorine insertion into only interstitial sites between the perovskite block layers. The RP-type layered manganese oxides, LaSrMnO4 (n = 1) and Ln1.2Sr1.8Mn2O7 (Ln = Pr, Nd, Sm, Eu, and Gd) (n = 2), accommodate fluorine in the (La/Sr)O rock-salt layers between the Mn-O perovskite blocks, followed by large expansion of the c-axis of 1 ~ 3 Å (see Figure 6). Utilization of F2 gas, NH4F, CuF2 or PVDF as a fluorination agent at low temperatures yields LaSrMnO4F2 [,] and Ln1.2Sr1.8Mn2O7F2 [,] where fluorine is inserted in each perovskite block. In addition, heating these fluorinated compounds with the corresponding precursors in appropriate ratios results in a staged intercalation structure, namely LaSrMnO4F [,] and La1.2Sr1.8Mn2O7F [] where F is inserted between alternate rock-salt layers. It should be noted that the F sites in the rock-salt layers are different between LaSrMnO4F and LaSrMnO4F2, and La1.2Sr1.8Mn2O7F and Ln1.2Sr1.8Mn2O7F2; the F atoms in LaSrMnO4F and La1.2Sr1.8Mn2O7F are located in the interlayer space so as to bridge between La/Sr and the apical oxygen, while the F atoms in LaSrMnO4F2 and Ln1.2Sr1.8Mn2O7F2 occupy tetrahedral sites of (La/Sr)4 in the rock-salt layers. In spite of the valences of Mn cations close to 4+, no long-range magnetic order is observed down to 5 K.
The n = 2 RP phase Sr3Ru2O7 is also fluorinated using CuF2 to give the oxyfluoride Sr3Ru2O7F2 []. In the fluorinated phase, fluorine is inserted in the tetrahedral sites of Sr4 in rock-salt layers between perovskite blocks (see Figure 7). The precursor crystallizes in the tetragonal structure with I4/mmm, but the fluorine insertion lowers the crystal symmetry to orthorhombic symmetry (Pbam), which is associated with rotation and tilting of the RuO6 octahedra. The magnetic properties also change after the fluorination reaction, from the ferromagnetic state with TC = 105 K to weak ferromagnetic state with TN = 185 K.
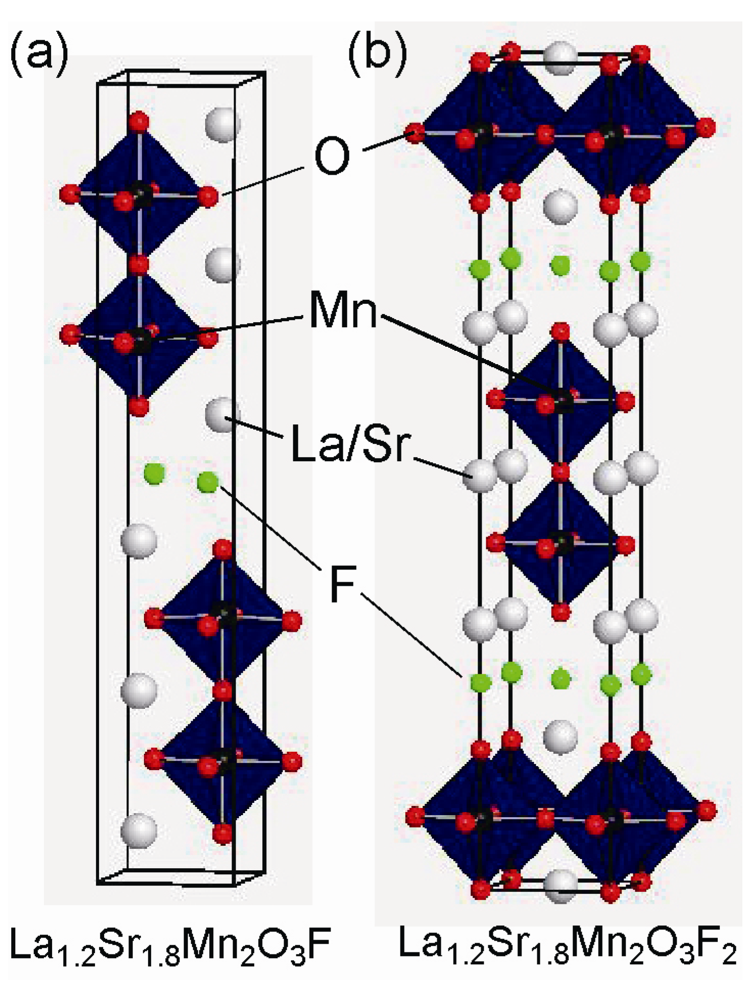
Figure 6.
Crystal structure of (a) La1.2Sr1.8Mn2O3F, and (b) La1.2Sr1.8Mn2O3F2.
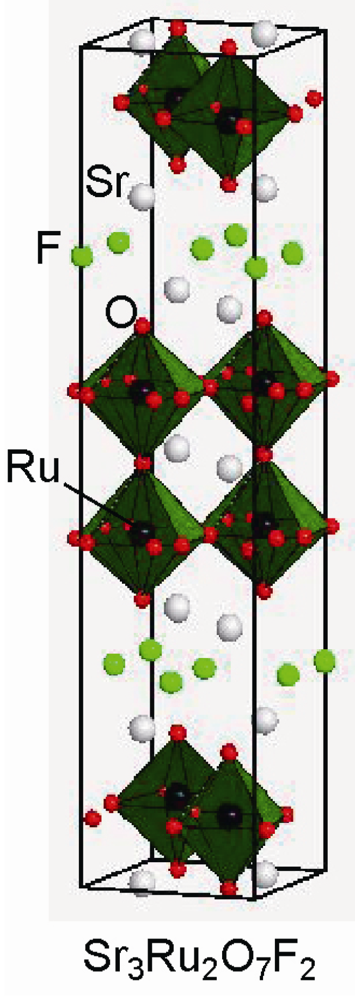
Figure 7.
Crystal structure of Sr3Ru2O7F2.
Fluorination of the RP-Ba2−xSrxPdO3 (0 ≤ x ≤ 1.5) by PVDF involves unusual oxygen displacement through anion exchange [,], which is different from the case of the fluorination of Sr3Fe2O6 described above []. As shown in Figure 8, the structure of Ba2−xSrxPdO3 comprises corner-linked chains of PdO4 squares along the a-axis, similar to the one-dimensional structure in Sr2CuO3. Fluorination to Ba2−xSrxPdO2F2 involves structural conversion to T’-structure (isostructural with Nd2CuO4), namely rearrangement of the PdO4 network from a 1-D chain to a 2-D plane. The remaining O2− anions in the apical site move to the original vacant site and the two inserted F− anions build fluorite block layer with Ba/Sr cations. Because the Pd2+ cation exhibits a strong preference for square lattice geometry, no additional fluorine insertion at the apical sites is allowed.
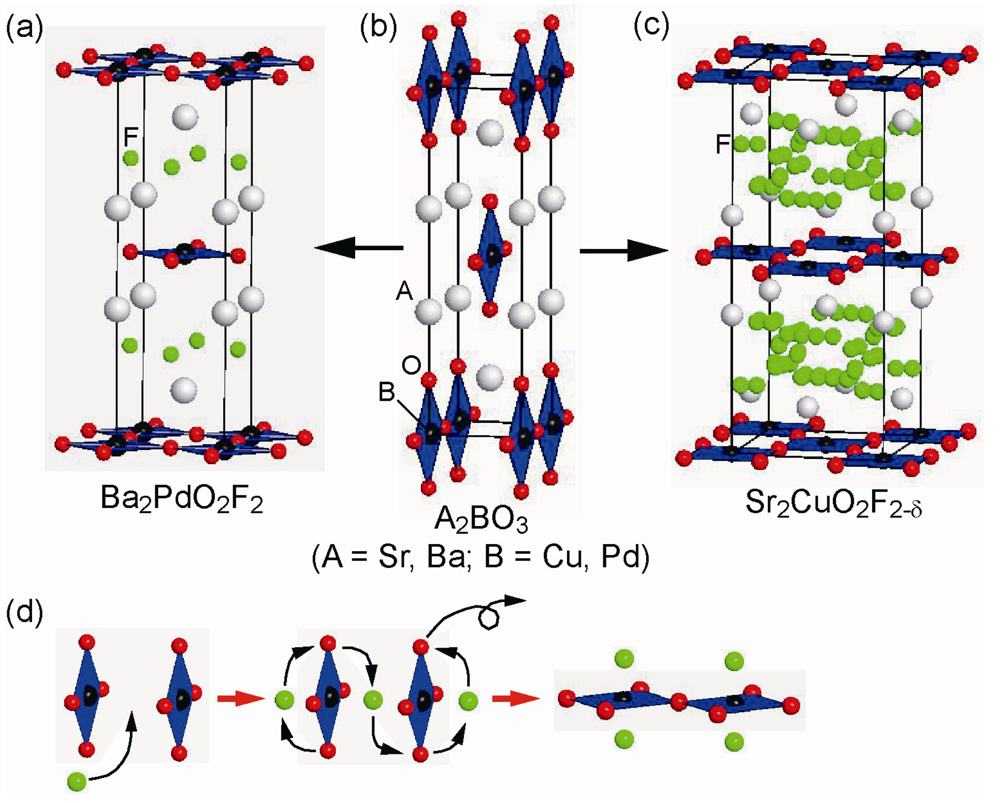
Figure 8.
(a), (b), (c) Fluorination of A2BO3 to A2BO2F2−δ (A = Sr, Ba; B = Cu, Pd). (d) Rearrangement of oxide and fluoride anions during fluorination reaction.
2.3. Fluorine Occupation of Both the Terminal Apical Sites and the Interstitial Sites
In most cases, low-temperature fluorination in layered perovskite structure proceeds by anion substitution at the apical sites and fluorine insertion in interstitial sites between perovskite blocks. This type of fluorination, especially in Cu oxides, has already been reviewed in detail by Greaves et al. and Wiley et al., independently [,,], so we will present the following four examples in this paper, namely Sr2CuO2F2+δ [,], Ba2ZrO3F2∙xH2O [], Sr2TiO3F2 [], and nitride-fluoride Ce2MnN3F2−δ [].
The discovery of a superconducting copper oxyfluoride Sr2CuO2F2+δ by Greaves et al. triggered the search for new oxyfluoride perovskite materials by a low-temperature route []. Other fluorinating agents, such as NH4F and XeF2, were shown to be useful in later work [,]. This compound was initially obtained by reaction of a 1-D structure Sr2CuO3 with F2 gas. As observed in Ba2−xSrxPdO2F2 [], the network of corner-sharing CuO4 units drastically changes from a 1-D chain to a 2-D layer in the fluorination process (see Figure 8). However, fluorine anions partially occupy not only interstitial sites between the perovskite blocks but also the apical sites. The superconducting temperature takes a maximum value of TC = 46 K at δ ~ 0.3. Interestingly, reduction of Sr2CuO2F2+δ in a flowing H2/N2 gas atmosphere yields an insulating T’-structure Sr2CuO2F2 []; fluorine removal at the apical sites and rearrangement of the remaining F− anions form a fluorite Sr2F2 layer and square planar coordinated CuO4.
Sr2TiO3F2 or Ba2ZrO3F2∙xH2O can be prepared by the reaction of n = 1RP Sr2TiO4 or Ba2ZrO4 with NH4F, CuF2, ZnF2 or PVDF [,]. In both cases, fluorination occurs by substitution of two F− anions for one O2− anion, but the fluorine insertion manner and coordination environment around the metal center are different between these two compounds (see Figure 9). Fluoride anions in Sr2TiO3F2 occupy both the apical sites and interstitial sites in alternate rock-salt layers. Additionally, the TiO5F octahedron is highly distorted, probably due to O/F site order at the apical sites. In contrast, Ba2ZrO3F2∙xH2O possesses fluorine, located at the apical sites and in each rock-salt layer. The Zr metal center takes an octahedral coordination, with O/F anions being disordered at the apical sites.
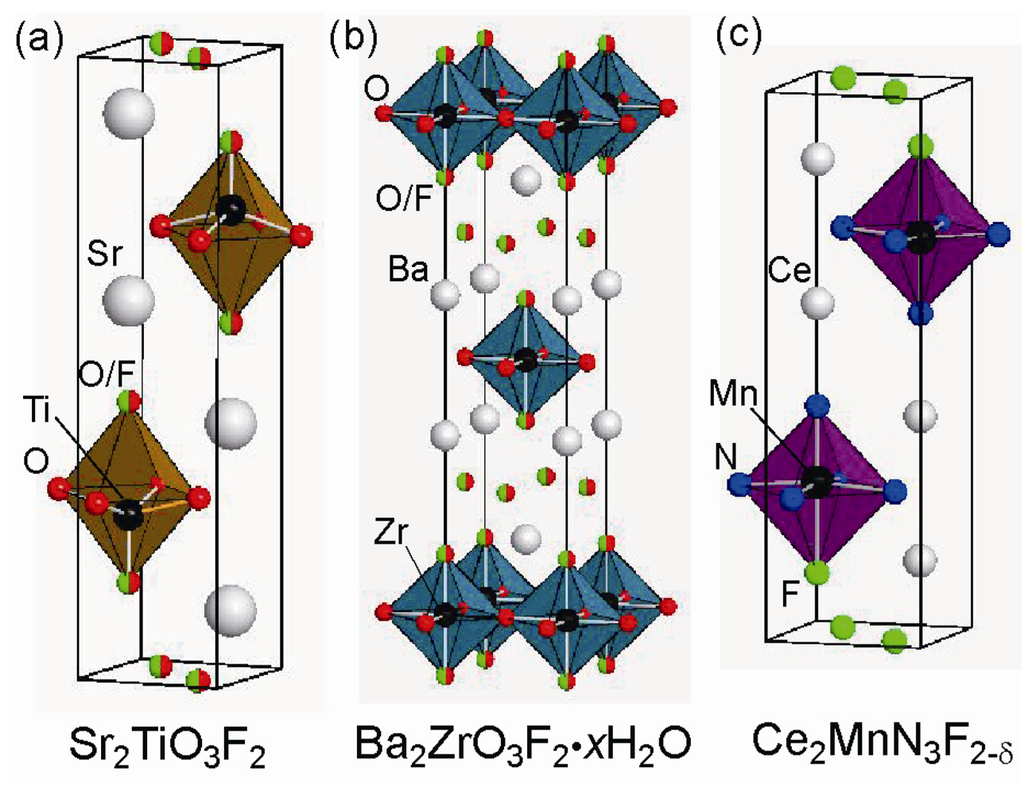
Figure 9.
Crystal structure of (a) Sr2TiO3F2, (b) Ba2ZrO3F2•xH2O, and (c) Ce2MnN3F2−δ.
It is important to note that a RP-type layered manganese nitride Ce2MnN3, which is isostructural with Sr2CuO3, accommodates fluorine in a different way []. Unlike Sr2CuO2F2+δ and Ba2−xSrxPdO2F2, fluorination involves local migration of the original apical oxygen to the equatorial anion vacant sites, but anion substitution or oxygen removal does not take place. Moreover, additional fluorine atoms are incorporated in alternate rock-salt layers. The resultant structure resembles that of Sr2TiO3F2 and the N/F site order results in deformation of the MnN4F octahedron. Upon fluorination, the magnetic properties changes the Pauli paramagnetic behavior to a paramagnetic one.
3. Recent Results on New Layered Iron and Cobalt Oxyfluoride Compounds
3.1. Unusual O/F Site Disorder in Layered Cobalt Oxyfluoride
As reviewed above, a variety of fluorinating agents greatly contributes to oxyfluoride chemistry. Considering the limited variety of transition metals, however, a further search for new oxyfluoride phase is necessary in order to better understand oxyfluoride. Co is among the 3d transition metals studied to a lesser extent. In fact, only one Co-based perovskite compound has been reported: LaSrCoFeO5F [], where O/F sites are randomly distributed as well as Fe/Co sites. High-pressure synthesis is an effective alternative approach to low-temperature fluorination, although expensive apparatus required for the reaction method is necessary. Recently, we have achieved the synthesis of the first example of RP-type layered cobalt oxyfluoride Sr2CoO3F, under a pressure of 6 GPa at 1,700 °C []. This compound adopts a simple body-centered tetragonal structure with the space group I4/mmm (Figure 2(c)). Magnetic susceptibility measurements revealed an antiferromagnetic phase transition at around 320 K, which is different from the ferromagnetic behavior in the corresponding oxide Sr2CoO4 []. Furthermore, neutron powder diffraction study characterized the S = 2 high-spin state at Co cations. The structural features in the cobalt oxyfluoride are also worthy of attention. The O/F anions are disordered at the apical sites, and the cobalt cation shifted from the basal plane takes a square pyramidal coordination. We noticed that the coordination environment around Co center is unusual when compared with related oxyfluoride compounds (see Figure 2). As described above, a similar O/F site disorder is observed in Ba2ScO3F and K2NbO3F, but each B cation with d0 electronic configuration takes octahedral coordination without shifting from the basal plane. In comparison, Sr2FeO3F and Ba2InO3F have a square-pyramidal coordinated metal center, but O/F site occupation occurs in an ordered manner. Thus, coexistence of anion disorder and square-pyramidal coordination, which has never seen in related oxyfluoride, is realized in the new cobalt oxyfluoride. It was initially assumed that square-pyramidal coordination stabilizes the O/F site ordered state, but this is not the case in Sr2CoO3F. Square-pyramidal coordination of Co cations is commonly seen in cobalt-based materials with d6 high spin configuration, such as BiCoO3 [] and Sr2CoO3Cl []. The observed O/F disorder, however, is non-trivial. The role played in the unusual coordination environment around Co is probably related to the reaction condition employed: high-pressure and high-temperature synthesis. A denser environment at high pressure or entropic effects associated with high temperature are likely to stabilize the anion-disordered phase.
3.2. Highly Fluorinated Iron Oxides
Various fluorinating agents have been reported so far, for example, F2 gas, NH4F, XeF2, CuF2 and PVDF and PTFE [,,,,]. Each fluorinating agent exhibits a distinct fluorinating power and reaction pathway. Thus, the fluorine contents in the resultant material depend on the choice, not only of the fluorinating agent, but also the anion lattice of the precursors. For example, the fluorine contents incorporated by XeF2 in YBa2Cu3O7−δ increase with decreasing the oxygen deficient contents []. The fluorination of perovskite SrFeO3 using PVDF yields SrFeO2F while the reaction of brownmillerite Sr2Fe2O5 with F2 gas results in the formation of two cubic phases different from SrFeO2F [,].
On the other hand, as shown above, the n = 2 RP-type layered iron oxide Sr3Fe2O6 was fluorinated with F2 gas to give the oxyfluoride Sr3Fe2O6O0.87 []. This fluorination did not involve anion substitution. There are two approaches to extending the fluorine contents; a precursor with smaller oxygen concentrations is reacted with F2 gas, or a fluorinating agent with higher reducing power is employed. According to such perspectives, we successfully synthesized a more highly fluorinated iron oxide, Sr3Fe2O5.44F1.56, by reaction of Sr3Fe2O7−δ (δ~0.25) []. As is the case in Sr3Fe2O6O0.87, fluoride anions preferentially occupy the terminal apical anion sites with oxide anions, but the O/F sites are displaced from the ideal 4e (0, 0, z) to more general sites 16m (x, x, z). Moreover, fluorination with PTFE results in more significant expansion of the c-axis (21.406(2) Å) and the deviation of the O-Fe-O bond angle in the plane from 180° is 15.28° nearly close to that in Ba3In2O5F2, reflecting the increased fluorine content in Sr3Fe2O5.44F1.56. And, the antiferromagnetic phase transition temperature greatly differs from below r.t. to 390 K.
Interestingly, work that is more recent has demonstrated further extended fluorination. Slater et al. have successfully synthesized three more fluorinated phases, Sr3Fe2O5.28F1.72, Sr3Fe2O4F4, and Sr3Fe2O3F6, by changing the molar ratios of Sr3Fe2O7−δ to PVDF []. While Sr3Fe2O5.28F1.72 has structural features similar to Sr3Fe2O5.44F1.56, fluorine atoms in Sr3Fe2O4F4 occupy half interstitial sites between rock-salt layers as well as terminal and central anion sites (see Figure 10). These two compounds exhibit antiferromagnetic order at r.t. In contrast, Sr3Fe2O3F6, which is assumed to correspond to complete filling of both apical sites and interstitial sites by fluorine, magnetically orders below r.t.
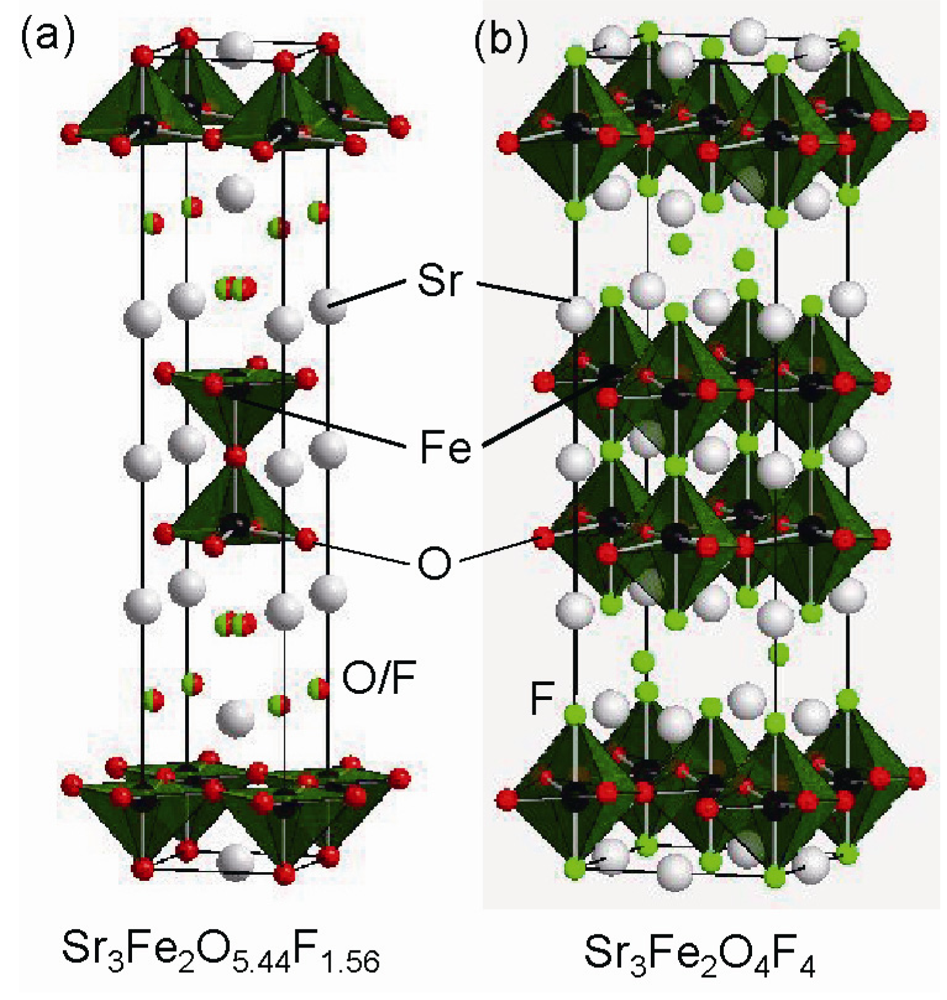
Figure 10.
Crystal structure of (a) Sr3Fe2O5.44F1.56, and (b) Sr3Fe2O4F4.
5. Conclusions
We have reviewed how layered oxyfluoride perovskite compounds have a rich variety of fluorine occupation, depending on reaction route, namely, low-temperature reaction, hydrothermal reaction, and high-pressure synthesis. Low temperature topotactic reaction is an attractive and straightforward technique to synthesize kinetically stable phases; Not only anion substitution but also fluorine insertion in interstitial sites between perovskite blocks occur, in some cases involving a rearrangement of anions around the metal center. In particular, the high capacity for fluorine insertion observed in Sr3Fe2O7−δ is quite unusual compared with other metal oxides. High-pressure synthesis can also provide good opportunities for extending oxyfluoride chemistry. Indeed, an unusual coordination environment in Sr2CoO3F was realized under extreme experimental conditions. This result should contribute not only to further syntheses of new oxyfluoride compounds, but also to controlling the structural and physical properties through anion order/disordering.
Acknowledgments
The work on Sr2CoO3F and Sr3Fe2O5.44F1.56 was conducted in collaboration with J. J. Li, Y. Matsushita, Y. Katsuya, M. Tanaka, Y. Shirako, M. Akaogi, K. Kodama, N. Igawa, and N. Hayashi. Our work was supported by the World Premier International Research Center (WPI) initiative on Materials Nanoarchitechtonics (MANA), a Grant-in-Aid for transformative Research-Project on Iron Pnictides (TRIP) from JSPS and Grants-in-Aid for Research Activity (22850019 and 21540330) from MEXT in Japan, and NIMS-RIKEN-JAEA Cooperative Research Program on Quantum Beam Science and Technology.
References
- Bednorz, J.G.; Müller, K.A. Possible high Tc superconductivity in the Ba-La-Cu-O. Z. Phys. B 1986, 64, 189–193. [Google Scholar] [CrossRef]
- Urushibara, A.; Moritomo, Y.; Arima, T.; Asamitsu, A.; kido, G.; Tokura, Y. Insulator-metal transition and giant magnetoresistance in La1−xSrxMnO3. Phys. Rev. B 1995, 51, 14103–14109. [Google Scholar]
- Moritomo, Y.; Asamitsu, A.; Kuwahara, H.; Tokura, Y. Giant magnetoresistance of manganese oxides with a layered perovskite structure. Nature 1996, 380, 141–144. [Google Scholar] [CrossRef]
- Tokura, Y.; Nagaosa, N. Orbital physics in transition-metal oxides. Science 2000, 288, 462–468. [Google Scholar] [CrossRef]
- Cava, R.J. Oxide superconductors. J. Am. Ceram. Soc. 2000, 83, 1–28. [Google Scholar]
- Dagotto, E. Correlated electrons in high-temperature superconductors. Rev. Mod. Phys. 1994, 66, 763–841. [Google Scholar] [CrossRef]
- Kasahara, A.; Nukumizu, K.; Hitoki, G.; Takata, T.; Kondo, J.N.; Hara, M.; Kobayashi, H.; Domen, K. Photoreaction on LaTiO2N under visible light irradiation. J. Phys. Chem.A 2002, 106, 6750–6753. [Google Scholar]
- Al-Mamouri, M.; Edwards, P.P.; Greaves, C.; Slaski, M. Synthesis and superconducting properties of the strontium copper oxy-fluoride Sr2CuO2F2+δ. Nature 1994, 369, 382–384. [Google Scholar] [CrossRef]
- Slater, P.R.; Hodges, J.P.; Francesconi, M.G.; Edwards, P.P.; Greaves, C.; Gameson, I.; Slaski, M. An improved route to the synthesis of superconducting copper oxyfluorides Sr2−xAxCuO2F2+δ (A = Ca, Ba) using transition metal difluorides as fluorinating reagents. Phys. C 1995, 253, 16–22. [Google Scholar] [CrossRef]
- Ardashnikova, E.I.; Lubarsky, S.V.; Denisenko, D.I.; Shpanchenko, R.V.; Antipov, E.V.; van Tendeloo, G. A new way of synthesis and characterization of superconducting oxyfluoride Sr2Cu(O, F)4+δ. Phys. C 1995, 253, 259–265. [Google Scholar] [CrossRef]
- Slater, P.R. Poly(vinylidene fluoride) as a reagent for the synthesis of K2NiF4-related inorganic oxide fluorides. J. Fluor. Chem. 2002, 117, 43–45. [Google Scholar] [CrossRef]
- Pinlac, R.A.; Stern, C.L.; Poeppelmeier, K.R. New layered oxide-fluoride perovskites: KNaNbOF5 and KNaMO2F4 (M = Mo6+, W6+). Crystals 2011, 1, 3–14. [Google Scholar] [CrossRef]
- Troyanchuk, I.O.; Kasper, N.V.; Mantytskaya, O.S.; Shapovalova, E.F. High-pressure synthesis of some perovskite-like compounds with a mixed anion type. Mater. Res. Bull. 1995, 30, 421–425. [Google Scholar] [CrossRef]
- Katsumata, T.; Nakashima, M.; Umemoto, H.; Inaguma, Y. Synthesis of the novel perovskite-type oxyfluoride PbScO2F under high pressure and high temperature. J. Solid State Chem. 2008, 181, 2737–2740. [Google Scholar] [CrossRef]
- Galasso, F.; Darby, W. Preparation, structure, and properties of K2NbO3. J. Phys. Chem. 1962, 66, 1318–1320. [Google Scholar] [CrossRef]
- Galasso, F.; Darby, W. Preparation and properties of Sr2FeO3F. J. Phys. Chem. 1963, 67, 1451–1453. [Google Scholar] [CrossRef]
- Case, G.S.; Hector, A.L.; Levason, W.; Needs, R.L.; Thomas, M.F.; Weller, M.T. Synthesis powder neutron diffraction structures and Mössbauer studies of some complex iron oxyfluorides: Sr3Fe2O6F0.87, Sr2FeO3F and Ba2InFeO5F0.68. J. Mater. Chem. 1999, 9, 2821–2827. [Google Scholar] [CrossRef]
- Needs, R.L.; Weller, M.T.; Scheler, U.; Harris, R.K. Synthesis and structure of Ba2InO3X (X = F, Cl, Br) and Ba2ScO3F; oxide/halide ordering in K2NiF4-type structures. J. Mater. Chem. 1996, 6, 1219–1224. [Google Scholar] [CrossRef]
- Choy, J.H.; Kim, J.Y.; Kim, S.J.; Sohn, J.S. New Dion-Jacobson-type layered perovskite oxyfluorides, ASrNb2O6F (A = Li, Na, and Rb). Chem. Mater. 2001, 13, 906–912. [Google Scholar] [CrossRef]
- Caruntu, G.; Spinu, L.; Wiley, J.B. New rare-earth double-layered-perovskite oxyfluorides, RbLnTiNbO6F (Ln = La, Pr, Nd). Mater. Res. Bull. 2002, 37, 133–140. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Tian, M.; Eguchi, M.; Mallouk, T. Ion-exchangeable, electronically conducting layered perovskite oxyfluorides. J. Am. Chem. Soc. 2009, 131, 9849–9855. [Google Scholar]
- Needs, R.L.; Weller, M.T. Structure of Ba3In2O5F2 by combined powder analysis; oxide/fluoride ordering in a Ruddlesden-Popper phase. J. Chem. Soc. Dalton Trans. 1995, 18, 3015–3017. [Google Scholar]
- Aurivillius, B. The structure of Bi2NbO5F and isomorphous compounds. Ark. Kemi. 1952, 5, 39–47. [Google Scholar]
- Needs, R.L.; Dann, S.E.; Weller, M.T. Cherryman, J.C.; Harris, R.K. The structure and oxide/fluoride ordering of the ferroelectrics Bi2TiO4F2 and Bi2NbO5F. J. Mater. Chem. 2005, 15, 2399–2407. [Google Scholar]
- McCabe, E.E.; Jones, I.P.; Zhang, D.; Hyatt, N.C.; Greaves, C. Crystal structure and electrical characterization of Bi2NbO5F: An Aurivillius oxide fluoride. J. Mater. Chem. 2007, 17, 1193–1200. [Google Scholar] [CrossRef]
- Greaves, C.; Kissick, J. L.; Francesconi, M.G.; Aikens, L.D. Gillie, L.J. Synthetic strategies for new inorganic oxide fluorides and oxide sulfates. J. Mater. Chem. 1999, 9, 111–116. [Google Scholar] [CrossRef]
- Aikens, L.D.; Gillie, L.J.; Li, R.K.; Greaves, C. Staged fluorine insertion into manganese oxides with Ruddlesden-Popper structures: LaSrMnO4F and La1.2Sr1.8MnO2O7F. J. Mater. Chem. 2002, 12, 264–267. [Google Scholar] [CrossRef]
- Sivakumar, T.; Wiley, J.B. Topotactic route for new layered perovskite oxides containing fluorine: Ln1.2Sr1.8Mn2O7F2 (Ln = Pr, Nd, Sm, Eu, and Gd). Mater. Res. Bull. 2009, 44, 74–77. [Google Scholar] [CrossRef]
- Aikens, L.D.; Li, R.K.; Greaves, C. The synthesis and structure of a new oxide fluoride, LaSrMnO4F, with stated fluorine insertion. Chem. Commun. 2000, 2149–2130. [Google Scholar]
- Li, R.K.; Greaves, V. Double-layered ruthenate Sr3Ru2O7F2 formed by fluorine insertion into Sr3Ru2O7. Phys. Rev. B 2000, 62, 3811–13815. [Google Scholar]
- Baikie, T.; Dixon, E.L.; Rooms, J.F.; Young, N.A.; Francesconi, M.G. Ba2−xSrxPdO2F2 (0 ≤ x ≤ 1.5): The first palladium-oxide-fluoride. Chem. Commun. 2003, 1580–1581. [Google Scholar]
- Baikie, T.; Islam, M.S.; Francesconi, M.G. Defects in the new oxide-fluoride Ba2PdO2F2: The search for fluoride needles in an oxide haystack. J. Mater. Chem. 2005, 15, 119–123. [Google Scholar] [CrossRef]
- Greaves, C.; Fracesconi, M.G. Fluorine insertion in inorganic materials. Curr. Opni. Solid State Mater. Sci. 1998, 3, 132–136. [Google Scholar] [CrossRef]
- McCabe, E.E.; Greaves, C. Fluorine insertion reactions into preformed metal oxides. J. Fluor. Chem. 2007, 128, 448–458. [Google Scholar] [CrossRef]
- Sanjaya Ranmohotti, K.G.; Josepha, E.; Choi, J.; Zhang, J.; Wiley, J.B. Topochemical manipulation of perovskites: Low-temperature reaction strategies for directing structure and properties. Adv. Mater. 2011, 23, 442–460. [Google Scholar]
- Kissick, J.L.; Greaves, C.; Edwards, P.P.; Cherkashenko, V.M.; Kurmaev, E.Z.; Bartkowski, S.; Neumann, M. Synthesis, structure, and XPS characterization of the stoichiometric phase Sr2CuO2F2. Phys. Rev. B 1997, 56, 2831–2835. [Google Scholar]
- Slater, P.R.; Gover, R.K.B. Synthesis and structure of the new oxide fluoride Ba2ZrO3F2∙xH2O. J. Mater. Chem. 2001, 11, 2035–2038. [Google Scholar] [CrossRef]
- Slater, P.R.; Gover, R.K.B. Synthesis and structure of the new oxide fluoride Sr2TiO3F2 from the low temperature fluorination of Sr2TiO4: An example of a staged fluorine substitution/insertion reaction. J. Mater. Chem. 2002, 12, 291–294. [Google Scholar] [CrossRef]
- Headspith, D.A.; Sullivan, E.; Greaves, C.; Francesconi, M.G. Synthesis and characterization of the quaternary nitride-fluoride Ce2MnN3F2−δ. Dalton Trans. 2009, 9273–9279. [Google Scholar]
- El Shinawi, H; Marco, J.F.; Berry, F.J.; Greaves, C. LaSrCoFeO5, LaSrCoFeO5F and LaSrCoFeO5.5: New La-Sr-Co-Fe perovskites. J. Mater. Chem. 2010, 20, 3253–3259. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Li, J.J.; Yamaura, K.; Matsushita, Y.; Katsuya, Y.; Tanaka, M.; Shirako, Y.; Akaogi, M.; Takayama-Muromachi, E. New layered cobalt oxyfluoride, Sr2CoO3F. Chem. Commun. 2011, 47, 3263–3265. [Google Scholar]
- Wang, X.L.; Takayama-Muromachi, E. Magnetic and transport properties of the layered perovskite system Sr2−yYyCoO4 (0 ≤ y ≤ 1). Phys. Rev. B 2005, 72. [Google Scholar] [CrossRef]
- Belik, A.A.; Iikubo, S.; Kodama, K.; Igawa, N.; Shamoto, S.; Niitaka, S.; Azuma, M.; Shimakawa, Y.; Takano, M.; Izumi, F.; et al. Neutron powder diffraction study on the crystal and magnetic structures of BiCoO3. Chem. Mater. 2006, 18, 798–803. [Google Scholar] [CrossRef]
- Loureiro, S.M.; Felser, C.; Huang, Q.; Cava, R.J. Refinement of the crystal structures of strontium cobalt oxychlorides by neutron powder diffraction. Chem. Mater. 2000, 12, 3181. [Google Scholar]
- Shpanchenko, R.V.; Rozova, M.G.; Abakumov, A.M.; Ardashnikova, E.I.; Kovba, M.L.; Putilin, S.N.; Antipov, E.V.; Lebedev, O.I.; Tendeloo, G.V. Inducing superconductivity and structural transformations by fluorination of reduced YBCO. Phys. C 1997, 280, 272–280. [Google Scholar] [CrossRef]
- Berry, F.J.; Ren, X.; Hea, R.; Slater, P.; Thomas, M.F. Fluorination of perovskite-related SrFeO3−δ. Solid State Commun. 2005, 134, 621–624. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Yamaura, K.; Hayashi, N.; Kodama, K.; Igawa, N.; Matsushita, Y.; Katsuya, Y.; Shirako, Y.; Akaogi, M.; Takayama-Muromachi, E. Topotactic synthesis and crystal structure of a highly fluorinated Ruddlesden–Popper-type Iron oxide, Sr3Fe2O5+xF2–x (x ≈ 0.44). Chem. Mater. 2011, 23, 3652–3658. [Google Scholar] [CrossRef]
- Hancock, C.A.; Herranz, T.; Marco, J.F.; Berry, F.J.; Slater, P.R. Low temperature fluorination of Sr3Fe2O7−δ with polyvinylidine fluoride: An X-ray powder diffraction and Mössbauer spectroscopy study. J. Solid State Chem. 2012, 186, 195–203. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).