Abstract
Background: Silybin, the primary active constituent of the milk thistle extract silymarin, has been historically recognized for its hepatoprotective properties. More recently, its potential effects on blood coagulation have garnered attention, suggesting a broader pharmacological profile. Methods: This study aimed to investigate silybin’s impact on hemostasis using rotational thromboelastometry (ROTEM) in normal human plasma. ROTEM enables the dynamic assessment of clot formation, providing a detailed analysis of coagulation processes in real-time. We specifically focused on the effects of silybin concentrations of 10 µM, 50 µM, and 100 µM on the ROTEM parameters compared to controls using normal human plasma with 0.1% dimethyl sulfoxide (DMSO). The parameters derived from the tests included clotting time (CT), α-angle (α), and amplitude at 10 and 20 min (A10 and A20) for each of the three channels: intrinsic pathway thromboelastometry (INTEM), extrinsic pathway thromboelastometry (EXTEM), and fibrinogen thromboelastometry (FIBTEM). Each measurement was performed four times. Results: Analysis of the INTEM assay results demonstrated that silybin at concentrations of 10 µM and 50 µM significantly reduced clotting time (CT) compared to the control. Additionally, all tested silybin concentrations significantly decreased the α-angle in the INTEM test. In the EXTEM assay, no significant effect on CT was observed at any silybin concentration. However, consistent with the INTEM findings, all silybin concentrations resulted in a significant reduction in the α-angle. In the FIBTEM assay, silybin at 10 µM and 50 µM significantly shortened CT. Furthermore, all tested concentrations led to a significant decrease in the α-angle and A20, while a reduction in A10 was observed only at the 50 µM concentration compared to the control. Conclusions: This study demonstrates that silybin modulates ROTEM parameters in a manner that tends to vary with concentration, with the strongest effects observed at lower concentrations (10–50 µM), notably reducing CT, α-angle, and clot firmness (A10, A20). These findings suggest a potential role of silybin in influencing coagulation dynamics.
1. Introduction
Silybin, also known as silibinin, is the principal active constituent of silymarin, a flavonolignan complex extracted from the fruit of the milk thistle plant (Silybum marianum). Although silymarin has been used for centuries in traditional medicine for the treatment of liver disorders, including cirrhosis, chronic hepatitis, and toxin-induced liver injury, recent research has shifted focus specifically toward silybin as the most abundant and biologically active component of silymarin [1,2,3,4]. These studies have not only confirmed its hepatoprotective effects but also revealed additional antineoplastic, neuroprotective and anti-inflammatory properties [2,4,5,6].
The intriguing aspect of silybin’s therapeutic potential is its influence on blood coagulation processes, an area of growing interest in recent wide-ranging research. Silybin has been identified as an inhibitor of activated factor X (FXa), a key enzyme in the coagulation cascade, and has also been shown to modulate thrombin activity by impairing both its amidolytic and proteolytic functions [7,8,9]. In addition, silybin affects platelet function, reducing their responsiveness to physiological agonists and inhibiting aggregation [10,11,12,13,14]. These combined actions suggest a multifaceted anticoagulant mechanism, positioning silybin as a promising candidate for further investigation in thrombosis-related disorders. However, most of the available evidence is derived from in vitro and cell-based studies, which limits the direct extrapolation of results to clinical settings [7,8,9,10,11,12,13,14]. Beyond its direct effects on coagulation factors and platelets, silybin has been shown to protect endothelial cells from high-glucose-induced oxidative stress and to improve their functional viability, suggesting broader vascular actions that may influence hemostatic balance in a context-dependent manner [15].
Hemostasis is a complex and tightly regulated process involving multiple cellular and plasma components, including coagulation factors, platelets, and the vascular endothelium. To date, most studies on silybin have focused on selective aspects of this system, such as its effects on coagulation and vascular cells, without evaluating its integrated impact across the full spectrum of hemostatic pathways. This fragmented evidence highlights a clear gap and provides a rationale for investigating how silybin may modulate coagulation in a more holistic manner.
In our previous study, we confirmed the effect of silybin on extrinsic and common pathway of coagulation by demonstrating a level-dependent reduction in prothrombin time (PT) in normal human plasma [16]. Notably, all observed PT values remained within standard reference values, indicating that the effect of silybin was subtle and physiologically relevant. This finding suggests a potential influence of silybin on the coagulation cascade. However, our previous study was preliminary in nature, and the assessment of PT alone is insufficient to delineate which specific component of hemostasis is affected by silybin. Nonetheless, our pilot study served as the foundation for further investigation.
To gain a more comprehensive understanding of coagulation dynamics, we employed rotational thromboelastometry (ROTEM), a viscoelastic method that provides real-time assessment of clot formation and dissolution. Unlike conventional coagulation tests, which measure isolated endpoints such as prothrombin time or activated partial thromboplastin time, ROTEM captures the entire coagulation process, including initiation, propagation, and stabilization [17,18]. In this study, ROTEM experiments were performed in plasma, which allows precise control of coagulation components, although it does not fully replicate the cellular and vascular contributions present in whole blood. Using this approach, we were able to evaluate the effect of silybin on clot dynamics. Our results provide important insights into how silybin may influence hemostasis and lay the groundwork for further studies under more physiologically representative conditions. To the best of our knowledge, this is the first study examining silybin’s effects using this viscoelastic test, which evaluates the mechanical properties of clot formation in a controlled experimental system.
2. Results
Thromboelastometry was performed with three levels of silybin prepared in 0.1% dimethyl sulfoxide (DMSO) (10 µM, 50 µM, and 100 µM). The results were compared to the ROTEM parameters of normal human plasma with the addition of 0.1% DMSO alone. For each condition, measurements were performed in four technical replicates. Three ROTEM assays, intrinsic pathway thromboelastometry (INTEM), extrinsic pathway thromboelastometry (EXTEM), and fibrinogen thromboelastometry (FIBTEM), were conducted, each providing four parameters describing the hemostatic process, resulting in a total of 192 ROTEM values used for the final analysis (64 INTEM, 64 EXTEM, and 64 FIBTEM) (Table 1, Figure 1). All experiments were performed using a single commercial plasma lot.

Table 1.
ROTEM® parameter values depending on the silybin level in the sample.
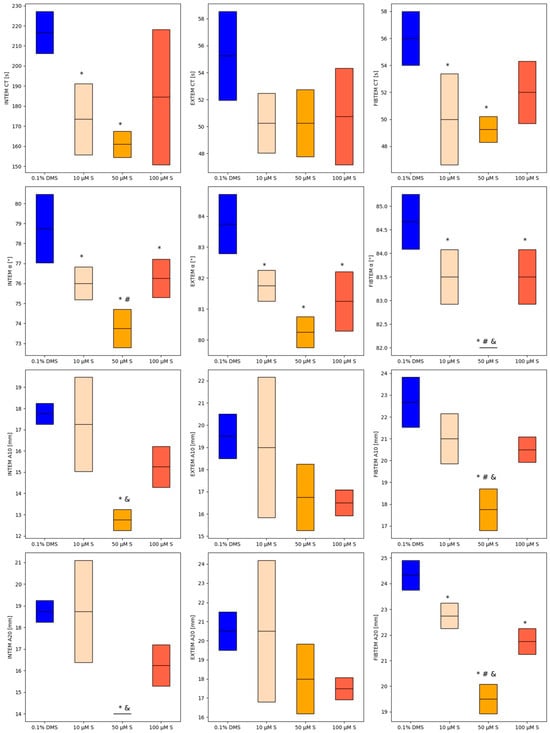
Figure 1.
Changes in ROTEM test parameters for different concentrations of silybin. Mean values are represented by solid lines, with standard deviations indicated by the box. * p < 0.05 versus control; # p < 0.05 versus 100 µM silybin; and & p < 0.05 versus 10 µM silybin.
2.1. Examination of the Impact of Silybin on INTEM Parameters
Direct comparison of INTEM parameters between control sample and 10 µM silybin solution revealed statistically significant differences in CT (216.75 ± 10.44 s vs. 173.50 ± 17.69 s; p = 0.0429) and α-angle (78.75 ± 1.71° vs. 76 ± 0.82°; p = 0.0262). Further statistical analysis showed statistically significant differences between control sample and 50 µM silybin solution across all INTEM parameters: CT (216.75 ± 10.44 s vs. 161 ± 6.48 s; p = 0.0092), α-angle (78.75 ± 1.71° vs. 73.75 ± 0.96°; p = 0.0005), A10 (17.75 ± 0.5 mm vs. 12.75 ± 0.5 mm; p = 0.0007), and A20 (18.75 ± 0.5 mm vs. 14 ± 0 mm; p = 0.0013). In the sample with a 100 µM silybin solution, only the α-angle value (76.25 ± 0.96°) was significantly decreased compared to the control sample (78.75 ± 1.71°), with p = 0.0444.
2.2. Examination of the Impact of Silybin on EXTEM Parameters
Among EXTEM parameters, statistically significant differences were observed only for the α-angle parameter. After adding the silybin solutions, we demonstrated a statistically significant decrease in α-angle values (0.1% DMSO—83.75 ± 0.96°; 10 µM silybin–81.75 ± 0.5°; 50 µM silybin—80.25 ± 0.5°; 100 µM silybin—81.25 ± 0.96°; p = 0.0002) in normal human plasma samples. No difference was found between CT, A10, and A20 values of compared samples (p > 0.05).
2.3. Examination of the Impact of Silybin on FIBTEM Parameters
Initial comparison showed significant difference in FIBTEM CT between the control sample and those with 10 µM and 50 µM silybin solutions (p = 0.0149). Comparing FIBTEM parameters between the control sample and 10 µM silybin solution revealed significant differences in CT (56 ± 2 s vs. 50 ± 3.37 s; p = 0.0287), α-angle (84.67 ± 0.58° vs. 83.50 ± 0.58°; p = 0.0430) and A20 (24.33 ± 0.58 mm vs. 22.75 ± 0.50 mm; p = 0.0121). Further analysis indicated significant differences between the control sample and 50 µM silybin solution in CT (56 ± 2 s vs. 49.25 ± 0.96 s; p = 0.0144), α-angle (84.67 ± 0.58° vs. 82 ± 0°; p = 0.0003), A10 (22.67 ± 1.15 mm vs. 17.75 ± 0.96 mm; p = 0.0031), and A20 (24.33 ± 0.58 mm vs. 19.50 ± 0.58 mm; p = 0.0002). In the sample with a 100 µM silybin solution, the α-angle value (83.5 ± 0.58°) and A20 (21.75 ± 0.5 mm) were significantly decreased compared to control sample (α = 84.67 ± 0.58°; A20 = 24.33 ± 0.58 mm), with p = 0.0429 and p = 0.0005, respectively.
3. Discussion
This is the first study to examine the effects of silybin on the hemostatic profile of normal human plasma using rotational thromboelastometry (ROTEM). The key findings from this study include the following: (1) silybin significantly influences ROTEM parameters, (2) the effect of silybin tends to vary with concentration, and (3) silybin leads to notable reductions in several measured parameters, including CT, alpha angle, and the clot firmness at 10 and 20 min (A10 and A20). These results provide crucial insights into the modulatory effects of silybin on blood coagulation.
Rotational thromboelastometry, employed in this study, is an advanced diagnostic tool providing real-time, comprehensive insights into the hemostatic process, from clot initiation to fibrinolysis, supporting rapid clinical decision-making in critical-care, as emphasized in recent studies [19,20]. The decision to employ ROTEM on human plasma in this investigation was influenced by our prior research, which explored the impact of silybin on prothrombin time (PT) [16]. Building on these findings, the current study further investigates the effects of silybin and its various concentrations on coagulation processes. We continued to use silybin and normal human plasma sourced from the same manufacturers as in our earlier work. Silybin employed in this study is a pure, equimolar mixture of silybin A and silybin B, which enhances the validity of our results. The exploration of silybin’s effects on hemostasis is pivotal, promising to enhance our understanding of its therapeutic potential in coagulation management. As a primary active constituent of the milk thistle plant, silybin has shown intriguing interactions with the blood clotting mechanisms, indicating a capacity to influence coagulation pathways significantly. Experimental research on the potential role of silybin as a modulator of hemostasis is still in progress. Previous studies have indicated that silybin may act predominantly as an anticoagulant, exerting inhibitory effects on both coagulation enzymes and platelet function [7,9,10,11,12,13,14]. Our study contributes to this area of investigation and helps address the existing gap in understanding the effects of silybin on hemostasis as assessed in vivo using viscoelastic testing.
The analysis of INTEM assay results demonstrated that silybin at concentrations of 10 µM and 50 µM significantly reduced CT compared to the control. Additionally, all tested concentrations of silybin significantly decreased the α-angle, indicating an effect on clot propagation. An influence on clot firmness at 10 and 20 min (A10 and A20) was also observed. In the EXTEM assay, no significant changes in CT were noted at any silybin concentration. However, similar to the INTEM results, all silybin concentrations led to a significant reduction in the α-angle. In the FIBTEM assay, silybin at 10 µM and 50 µM significantly shortened CT. Furthermore, all tested concentrations resulted in a significant decrease in the α-angle compared to the control. The most pronounced reduction was observed at 50 µM, which was also significantly lower than the values recorded for 10 µM and 100 µM. Clot amplitude at both 10 and 20 min was significantly reduced in all experimental conditions. The lowest A10 and A20 values were again observed for the 50 µM silybin concentration, suggesting a dose-dependent effect.
Interpreting the ROTEM data collectively, the observed shortening of CT may indicate an accelerated initiation of fibrin formation, potentially suggesting that silybin does not inhibit the activity of plasma coagulation factors involved in the early phase of thrombin generation. However, this interpretation should be approached with caution, as the viscoelastic assessment was performed in platelet-poor plasma, which does not fully replicate physiological conditions. Even if our findings contrast with previous experimental studies reporting predominantly anticoagulant effects of silybin, this may reflect differences in experimental models and endpoints. In platelet-poor plasma, CT mainly reflects the initiation of fibrin formation, which can occur independently of thrombin generation or coagulation enzyme activity, and the absence of platelets may further reduce observable anticoagulant effects. Clinical evidence, including the case report by Lindner et al. (2025) and recent porcine studies by Sharma P. et al. (2025), highlights that silymarin’s effects are context-dependent [21,22]. Together, these observations support the idea that silybin’s action on hemostasis is multifaceted and may vary depending on experimental conditions, dose, and biological context.
The consistent reduction in the α-angle across assays points to impaired clot propagation dynamics, which may be attributable either to a direct effect of silybin or, alternatively, to the absence of platelets that normally contribute to the amplification and stabilization of clot formation. The observed decrease in clot amplitude at 10 and 20 min may also reflect altered fibrin polymerization, potentially due to silybin’s modulatory influence on fibrinogen function. While these findings provide novel insights into the hemostatic profile of silybin, further studies involving more physiologically relevant models are warranted to better characterize its mechanisms of action and clinical significance.
The results of our studies cannot be directly related to any available literature data. Studies by Bijak et al. have indicated that silybin exhibits an inhibitory effect on thrombin [7] and factor X [9], suggesting a potential anticoagulant effect of silybin. In their follow-up studies, Bijak and colleagues concentrated on the effects of silybin and other flavonolignans on platelet function [10,11,12,13,14]. They revealed silybin as a significant inhibitor, not only of critical coagulation enzymes but also of platelet aggregation and activation. Bijak and his team were not the only ones to explore the impact of silybin on platelet aggregation. Pourová et al. [23] also conducted research on the effects of silymarin flavonolignans on this process. Their findings indicated that silybin modestly inhibits platelet aggregation; however, this inhibitory effect is negated at concentrations below 100 µM. Nevertheless, we cannot directly compare our findings to the cited works due to fundamentally different methodologies used in our studies. Our results certainly complement the current state of knowledge and are consistent with the observations of other authors that silybin may affect the hemostasis process, and the biological effect may depend on the dose of silybin or a mixture of various flavonoids often present together.
In this study, several limitations need to be considered. First, it is an experimental study performed in standard human plasma instead of a whole blood sample, as a continuation of our previous work [16]. For this reason, we may not clearly identify the exact biological effect of silybin on platelets as part of thrombus formation. However, several experimental studies, including those by Christian Schoergenhofer et al. (2017) [24] and Christoph J. Schlimp et al. (2013) [25], indicated that thromboelastometry can be performed in both fresh and frozen plasma, and that the differences observed in ROTEM measurements between whole blood and plasma were considered acceptable. Silybin itself appears to exhibit various effects on hemostasis. Therefore, in the initial phase, we aimed to assess its possible action on standard (healthy) human plasma before employing a more advanced experimental model. We believe that the tests could also be conducted with higher concentrations of silybin and the results should be correlated with standard laboratory tests of hemostasis. Nevertheless, our study should be considered preliminary, and further evaluation in a larger cohort is warranted to validate our results.
4. Materials and Methods
Thromboelastometry was performed using the ROTEM® Delta (TEM Innovations GmbH, Munich, Germany) analyzer according to the manufacturer’s instructions. The ROTEM® device measures changes in viscoelastic properties of blood during clot formation in a small sample of blood after the addition of clotting factors. A blood sample is heated to 37 °C in a stationary disposable cup, which is subjected to the constant rotational force of an oscillating pin.
Three standard tests were performed: the INTEM test (assessment of the intrinsic coagulation pathway), the EXTEM test (assessment of the extrinsic coagulation pathway), and the FIBTEM test (assessment of the fibrinogen level and fibrin polymerization).
The ROTEM® parameters were obtained as follows:
- -
- The clotting time (CT), which is the time from test start until an amplitude of 2 mm is reached;
- -
- α-angle, the angle between the baseline and a tangent to the clotting curve through the 2 mm point;
- -
- Clot firmness at time, namely 10 min (A10) and 20 min (A20) (Figure 2).
 Figure 2. Schematic ROTEM Delta tracing illustrating clot formation. For illustrative purposes only.
Figure 2. Schematic ROTEM Delta tracing illustrating clot formation. For illustrative purposes only.
All reagents used for each test are summarized in Table 2.

Table 2.
Characteristics of the thromboelastometry tests.
According to the manufacturer of the reagents and analyzer, the reference values for ROTEM parameters in whole blood from healthy individuals were as follows. For INTEM, clotting time (CT) ranged from 100 to 240 s, the alpha angle (α) ranged from 70° to 83°, clot amplitude at 10 min (A10) ranged from 44 to 66 mm, and clot amplitude at 20 min (A20) ranged from 50 to 71 mm. For EXTEM, CT ranged from 38 to 79 s, α from 63° to 83°, A10 from 43 to 65 mm, and A20 from 50 to 71 mm. For FIBTEM, CT ranged from 38 to 62 s, A10 from 7 to 23 mm, and A20 from 8 to 24 mm; the alpha angle was not defined.
The effect of silybin on ROTEM parameters was measured with a mixture of two diastereomers, silybin A and silybin B, in an equimolar ratio (product #: 89280, PhytoLab GmbH & Co. KG, Vestenbergsgreuth, Germany). In reference to our previous findings, we prepared three levels of silybin, 10 µM, 50 µM, and 100 µM, using 0.1% dimethylsulfoxide (product #: D2650-5X5ML, DMSO, Sigma-Aldrich, Co., St. Louis, MO, USA). For each level, 60 µL of silybin was mixed with 240 µL of normal human plasma, which is pooled plasma obtained from healthy donors (product #: 91020, Dia-CONT I, DIAGON Kft., Budapest, Hungary) and incubated for 2 min at 37 °C. A control sample was then prepared as a mixture of 60 µL of 0.1% DMSO with 240 µL of plasma. All the samples were analyzed in parallel channels for INTEM, EXTEM and FIBTEM tests after warming the reagents and pipetting, as recommended by the manufacturer. The parameters derived from the tests included clotting time (CT), α-angle (α), and amplitude at 10 and 20 min (A10 and A20) for the three channels. Each measurement was performed four times.
5. Statistical Analysis
The database and statistical analysis were prepared using Statistica 13.3 software (TIBCO Software Inc., Palo Alto, CA, USA). The distribution of quantitative variables was assessed by Shapiro–Wilk test. Normally distributed quantitative variables were expressed as mean (X) and standard deviation (SD). One-way ANOVA and post hoc tests were performed to compare means of normally distributed variables. A p value below 0.05 was statistically significant.
6. Conclusions
This study represents the first investigation into the impact of varying concentrations of silybin on the hemostatic profile using ROTEM in normal human plasma. Specifically, silybin caused a shortening of CT, a decrease in the alpha angle, and reductions in A10 and A20 compared to the control. The impact of silybin on FIBTEM parameters was most notable at a concentration of 50 µM. Our findings indicate that silybin demonstrates concentration-dependent effects at lower concentrations on coagulation dynamics.
Author Contributions
Conceptualization, J.M., A.S. and E.Ż.; methodology, J.M., J.B. and G.S.; investigation, J.M., G.S., K.S.-G.; formal analysis, J.B. and K.S.-G.; data curation, J.M. and A.S.; writing—original draft preparation, J.M., J.B. and A.S.; writing—review and editing, E.Ż.; visualization, J.B.; supervision, D.Z. and A.S.; project administration, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bijak, M. Silybin, a Major Bioactive Component of Milk Thistle (Silybum marianum L. Gaernt.)-Chemistry, Bioavailability, and Metabolism. Molecules 2017, 22, 1942. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Dallio, M.; Loguercio, C. Silymarin/Silybin and Chronic Liver Disease: A Marriage of Many Years. Molecules 2017, 22, 191. [Google Scholar] [CrossRef]
- Loguercio, C.; Festi, D. Silybin and the liver: From basic research to clinical practice. World J. Gastroenterol. 2011, 17, 2288–2301. [Google Scholar] [CrossRef]
- Abenavoli, L.; Izzo, A.A.; Milić, N.; Cicala, C.; Santini, A.; Capasso, R. Milk thistle (Silybum marianum): A concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. Phytother. Res. 2018, 32, 2202–2213. [Google Scholar] [CrossRef]
- Agarwal, C.; Wadhwa, R.; Deep, G.; Biedermann, D.; Gažák, R.; Křen, V.; Agarwal, R. Anti-cancer efficacy of silybin derivatives–a structure-activity relationship. PLoS ONE 2013, 8, e60074. [Google Scholar] [CrossRef]
- Romanucci, V.; Pagano, R.; Lembo, A.; Capasso, D.; Di Gaetano, S.; Zarrelli, A.; Di Fabio, G. Phosphodiester Silybin Dimers Powerful Radical Scavengers: A Antiproliferative Activity on Different Cancer Cell Lines. Molecules 2022, 27, 1702. [Google Scholar] [CrossRef]
- Bijak, M.; Ziewiecki, R.; Saluk, J.; Ponczek, M.; Pawlaczyk, I.; Krotkiewski, H.; Wachowicz, B.; Nowak, P. Thrombin inhibitory activity of some polyphenolic compounds. Med. Chem. Res. 2014, 23, 2324–2337. [Google Scholar] [CrossRef] [PubMed]
- Jedinák, A.; Maliar, T.; Grancai, D.; Nagy, M. Inhibition activities of natural products on serine proteases. Phytother. Res. 2006, 20, 214–217. [Google Scholar] [CrossRef]
- Bijak, M.; Ponczek, M.B.; Nowak, P. Polyphenol compounds belonging to flavonoids inhibit activity of coagulation factor X. Int. J. Biol. Macromol. 2014, 65, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M.; Szelenberger, R.; Dziedzic, A.; Saluk-Bijak, J. Inhibitory Effect of Flavonolignans on the P2Y12 Pathway in Blood Platelets. Molecules 2018, 23, 374. [Google Scholar] [CrossRef]
- Bijak, M.; Saluk-Bijak, J. Flavonolignans inhibit the arachidonic acid pathway in blood platelets. BMC Complement. Altern. Med. 2017, 17, 396. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M.; Dziedzic, A.; Saluk-Bijak, J. Flavonolignans reduce the response of blood platelet to collagen. Int. J. Biol. Macromol. 2018, 106, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M.; Szelenberger, R.; Saluk, J.; Nowak, P. Flavonolignans inhibit ADP induced blood platelets activation and aggregation in whole blood. Int. J. Biol. Macromol. 2017, 95, 682–688. [Google Scholar] [CrossRef]
- Bijak, M.; Dziedzic, A.; Synowiec, E.; Sliwinski, T.; Saluk-Bijak, J. Flavonolignans Inhibit IL1-β-Induced Cross-Talk between Blood Platelets and Leukocytes. Nutrients 2017, 9, 1022. [Google Scholar] [CrossRef] [PubMed]
- Palomino, O.M.; Gouveia, N.M.; Ramos, S.; Martín, M.A.; Goya, L. Protective Effect of Silybum marianum and Silibinin on Endothelial Cells Submitted to High Glucose Concentration. Planta Medica 2017, 83, 97–103. [Google Scholar] [CrossRef]
- Mlicka, A.; Siemiątkowska, K.; Plaku, I.; Żekanowska, E.; Slomka, A. Silybin, the main active component of Silybum marianum, affects blood coagulation: An in vitro pilot study. Med. Sci. Forum 2023, 21, 22. [Google Scholar] [CrossRef]
- Crochemore, T.; Piza, F.M.T.; Rodrigues, R.D.R.; Guerra, J.C.C.; Ferraz, L.J.R.; Corrêa, T.D. A new era of thromboelastometry. Einstein 2017, 15, 380–385. [Google Scholar] [CrossRef]
- Whiting, D.; DiNardo, J.A. TEG and ROTEM: Technology and clinical applications. Am. J. Hematol. 2014, 89, 228–232. [Google Scholar] [CrossRef]
- Kleinveld, D.J.B.; Curry, N.; Levy, J.H. Coagulation support during perioperative bleeding management. Intensive Care Med. 2023, 49, 1110–1113. [Google Scholar] [CrossRef]
- Peng, H.T.; Nascimento, B.; Tien, H.; Callum, J.; Rizoli, S.; Rhind, S.G.; Beckett, A. A comparative study of viscoelastic hemostatic assays and conventional coagulation tests in trauma patients receiving fibrinogen concentrate. Clin. Chim. Acta 2019, 495, 253–262. [Google Scholar] [CrossRef]
- Lindner, E.; Alhmouz, M.M.; Abuhalimeh, R.; Abuhalimeh, L.; Chin, J.; Qadura, M.; Younes, H.; Abuhalimeh, B.J. Is Milk Thistle a Cardioprotective Ally or a Thrombotic Threat? JACC Case Rep. 2025, 30, 104999. [Google Scholar] [CrossRef]
- Sharma, P.; Asediya, V.; Kalra, G.; Sultana, S.; Purohit, N.; Kibitlewska, K.; Kozera, W.; Czarnik, U.; Karpiesiuk, K.; Lecewicz, M.; et al. Hepatoprotective Effect of Silymarin Herb in Prevention of Liver Dysfunction Using Pig as Animal Model. Nutrients 2025, 17, 3278. [Google Scholar] [CrossRef] [PubMed]
- Pourová, J.; Applová, L.; Macáková, K.; Vopršalová, M.; Migkos, T.; Bentanachs, R.; Biedermann, D.; Petrásková, L.; Tvrdý, V.; Hrubša, M.; et al. The Effect of Silymarin Flavonolignans and Their Sulfated Conjugates on Platelet Aggregation and Blood Vessels Ex Vivo. Nutrients 2019, 11, 2286. [Google Scholar] [CrossRef] [PubMed]
- Schoergenhofer, C.; Buchtele, N.; Schwameis, M.; Bartko, J.; Jilma, B.; Jilma-Stohlawetz, P. The use of frozen plasma samples in thromboelastometry. Clin. Exp. Med. 2017, 17, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Schlimp, C.J.; Solomon, C.; Hochleitner, G.; Zipperle, J.; Redl, H.; Schöchl, H. Thromboelastometric maximum clot firmness in platelet-free plasma is influenced by the assay used. Anesth. Analg. 2013, 117, 23–29. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.