Abstract
The rehydroxylation (RHX) dating technique offers a promising method for determining the ages of ceramic materials, leveraging the time-dependent mass gain from water reabsorption after high-temperature firing. However, the reliability of RHX dating is under discussion in many cases, with its accuracy depending on the various component materials in ceramics. In the present study, we considered the incomplete removal of weakly bonded water molecules during the conventional preheating step at 105 °C, a phenomenon that may lead to inaccurate mass measurements and overestimates of age. In this study, we propose an enhanced experimental protocol incorporating thermogravimetric analysis (TGA) to identify and quantify interstitial water fractions within ceramics. For samples exhibiting significant water retention (>1%), we recommend preheating at relatively higher temperatures (up to 300 °C) to ensure complete water removal and a more accurate mass determination. This approach was tested on five archaeological samples, yielding improved consistency and agreement with independently known dates. The method highlights the importance of tailored preheating protocols in RHX dating of ancient ceramics.
1. Introduction
In archaeology and the study of the history of art, dating of ceramic artefacts and bricks is extremely important, supporting stylistic studies in determining the chronology of art pieces or the construction phases of historical buildings [1]. The current established technique is thermoluminescence (TL) [2], which is based on the luminescence properties of heated clays. Although it has been successfully used for dating in many cases [3,4], it relies on many parameters that are not always available or easily extrapolated (e.g., the measurement of environmental gamma dose-rate or the water content over the centuries in the soil and the sample [2]). Another property of ceramic materials that can be exploited for dating is the rehydroxylation phenomenon [5], which can be examined in tandem with the correlation between mass increase and time. In fact, when clay is subjected to high-temperature heating, creating ceramic material, it undergoes a complete dehydroxylation, losing all the water molecules and OH groups. Subsequently, when the ceramic material is cooled at ambient temperature, it adsorbs water molecules (rehydration, RH) and OH groups (rehydroxylation, RHX), increasing its volume and mass as time passes. Specifically, the phenomenon consists mainly of two stages: in the first (Stage I), which has a very short duration compared to Stage II, the material adsorbs unbonded water by capillarity and weakly bonded water (interlayer) by diffusion. This step lasts until the pores and interlayer spaces are saturated. As diffusion continues, only the second process (Stage II) is involved: hydroxyl groups interact with the lattice structure to form strong bonds (rehydroxylation). The kinetics of this later, much slower, process form the basis of the RHX dating technique [6]. This method was first proposed following studies about the expansive properties of fired clay bricks [7] and has since undergone considerable investigation (for example, [8,9,10]). In [7], Wilson et al. studied the volume and mass increase as a function of time, finding that the mass gain of a ceramic sample as a function of time follows a power law with exponent 1/4 in Stage II. The application of RHX as a dating method resulted in a few promising results from ceramic artefacts, bricks and tiles of known ages on the order of many centuries [5]. Rehydroxylation (RHX) dating is a gravimetric technique: in principle, weighing a piece of ceramic under controlled environmental conditions, before and after reheating, its mass increases due to water adsorption. The mass the ceramic has gained since it was originally or last fired above 500 °C can be estimated. Then, the rehydration/rehydroxylation (mass gain) rate can be calculated by measuring the mass gain of this reheated sample as a function of time in an aging environment equivalent to that of the ceramic’s burial or depositional position. Knowing both the rate of mass gain and the mass gained during its lifetime, the elapsed time since the ceramic was fired can be calculated. Since its first applications, the need for a precise evaluation of the mass-gain rate has been clear, as it depends on the material mineralogy and the initial firing conditions. Other problems come from the mentioned experimental steps. They will be concisely presented below. A more detailed discussion can be found in the papers by Wilson et al. [5,6,11]. The phenomenon begins when inorganic clays are fired at temperatures in the range 450–900 °C; under those conditions, hydroxyls of the clay minerals are converted to water and subsequently evaporate (a process accompanied by a collapse of the crystal structure of the clay). A state of complete dihydroxylation (DHX) should be reached. Following cooling, a reversal of this process takes place in the hardened ceramic; water from the environment is absorbed, first via the absorption of water molecules from the environment (rehydration, RH), then as OH groups fixed in the ceramic structure (rehydroxylation, RHX). The rate at which rehydroxylation occurs has been described by a well-defined equation, as a function of time1/4 [7]. It is assumed that reheating the ceramic to a sufficiently high temperature (typically > 500 °C) for a sufficient duration will dehydroxylate the ceramic and that any subsequent mass gain will replicate the original mass gain following firing, provided the environmental conditions, mainly the temperature, are the same. Dating by RHX requires the acquisition of the initial mass of the sample (m2), which is the sum of the ceramic mass, the masses associated with the three types of water adsorbed during the sample lifetime (mw0, mw1, ma, see below) and that masses of possible nonrefractory components. Here, we use the same terms as in [6], as follows:
where mcer is the mass of the total inorganic mineral assemblage; mnrc is the mass of the non-refractory components, including any substances other than water present in the as-received sample; ma is the mass of water gained during RHX; mw0 is the physisorbed water, i.e., water molecules easily eliminated by heating at 105–110 °C; and mw1 represents all the remaining water molecules present in the ceramic in interstitial positions, which also should be partly eliminated by the lower-temperature heating, although this often does not happen [12], as it will be discussed below. This lower-temperature drying procedure is generally carried out for a period that varies in length from a few hours to several days. After the lower-temperature heating step at 105–110 °C, m2 is measured (see Figure 1). Then, after the material has been heated at 500 °C, the amount of absorbed water in the sample structure (mw0, mw1, ma) is reset, as is mnrc, the non-refractory fraction; the mass-gain curve is acquired, and from this curve, a dry-mass value (m4) and a growth rate α are derived. These are the parameters required for dating a sample following the equation below:
m2 = mcer + mw0 + mw1 + mnrc + ma
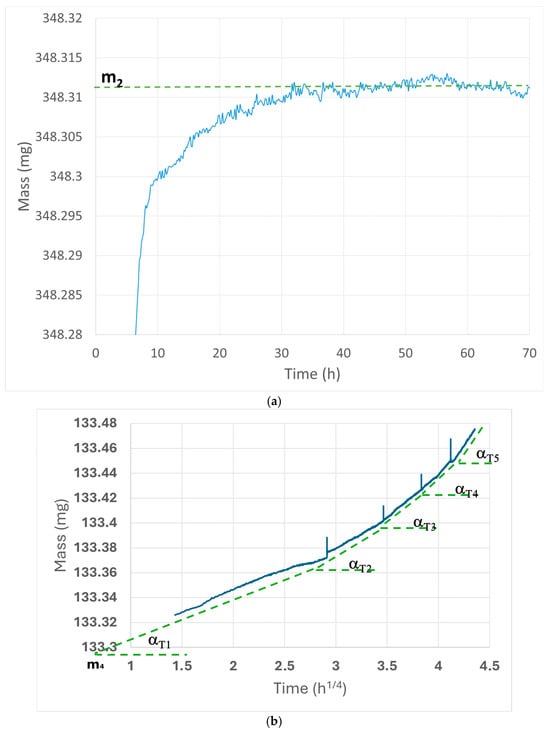
Figure 1.
(a) Brick_1 m2 mass evaluation; blue curve represents the experimental data, dotted green line represents the extrapolation of the mass value. (b) Brick_1 m4 mass and α evaluation; blue curve represents the experimental data, dotted green lines represent the linear regression of the different steps.
Since the first works by Wilson et al. [5,7], the complexity of the molecular-diffusion processes that influence RH/RHX have been evident.
They underlined that the temperature at which an artefact was submitted in its life should be known: a mean temperature from tabulated values should be considered [13].
The effect of humidity on the instantaneous mass of the ceramic sample may be significant, but this point is still a matter of debate [14,15]. RHX is also strongly affected by the mineralogical composition of the samples to be dated because the phenomenon is known to be differently important across different mineral components. It could be useful to determine the ceramic’s composition [16]. The complex picture of DHX/RHX can be investigated using different techniques: infrared spectroscopy (IR) [12], nuclear magnetic resonance (NMR) [17], differential thermal analysis (DTA) and thermogravimetric analysis (TGA) [18]. It is clear that a single, simple mechanism cannot be established also because the RHX can be different in different mineral components.
Of course, it is not always possible to investigate the mineralogical composition of ceramic samples to be dated. However, the behaviors of some minerals during hydroxylation have been studied, and some interesting results have been published [19]. The amounts of H2O and OH can be measured by IR spectroscopy, and changes in these values as a function of heating treatment can be measured by TGA. Shoval et al., in 1991 [12], reported some IR spectra that evidenced very different behavior in the release of water molecules and OH groups as a function of temperature: after heating to a series of temperatures, from 110 °C to 550 °C, for 24 h, the movements of water molecules and OH groups were measured, yielding very different results for samples coming from the same place. While the results obtained in quite a few experiments indicate that ancient ceramic artefacts can be dated by the RHX technique [11], some experimental groups have declared that RHX cannot generally give reliable results [10,20] due to various causes that must be separately analyzed.
In [10], the authors claim that the duration of heating at both 105 °C and 500 °C is a critical parameter for the RHX dating method. In some samples, they noted a systematic lack of stabilization of the mass of the samples after their heating at 105 °C, even after several weeks of monitoring.
In such cases, they observed that mass-gain data do not allow us to conclude that the (re)hydroxylation process acting in archaeological or industrial fired-clay materials systematically and accurately follows a t1/4 power law [20].
In a recent work [21], we have shown that in a specific case, treatment in the first step with preheating to temperatures increasingly higher than 105 °C could yield correct dating results.
Specifically, the dating of this sample yielded an age much greater than the expected value after heating to 105 °C, as is usual in the first heating step, while the dating was correct when the first heating step was carried out at 300 °C. The validity of this procedure will be discussed in this work, testing it on well-dated archaeological and historical materials such as building bricks and pottery remains (see Section 2.1 for details). The Lambda laboratory in Milano−Bicocca University started studying RH/RHX in well-dated samples in 2016; about 50 RHX dating measurements have been carried out. The dating process proved successful in about half of the attempts. Following the obtaining of the above-mentioned results [21], it was proposed that in some types of ceramics, a material parameter possibly dependent on mineralogical composition could be critical for the precision of the analysis.
This parameter was identified, in the samples examined in the present work, to be associated with the partial removal of weakly bonded water molecules during the first preheating step, which may lead to an incorrect evaluation of the mass analysis. Here, we propose an experimental assessment of the preheating temperatures and the use of thermogravimetric analysis (TGA) to identify the temperature at which the differently absorbed molecules are released.
As is well known, TGA is a method used to characterize the control of the reaction processes and the properties of the materials.
The proposed procedure has been applied to date to five samples whose ages had already been independently determined [22,23], matching experimental and historical data. Gaps remain in our understanding of the fundamental processes that drive hydroxylation in ceramics, a phenomenon crucial to RHX dating. As mentioned above, this study is designed to address these inconsistencies. In particular, the focus was on the presence of interstitial water molecules not eliminated in the lower-temperature heating step because these molecules can contribute to the RHX mechanism, possibly giving unreliable dating results.
2. Materials and Methods
2.1. Samples Description
Five ceramic samples were selected; they originate from different historical periods and were produced with different raw materials and different technologies. The five samples that were selected included both examples that had yielded reliable results when analyzed using the commonly applied method (to serve as reference samples) and examples that had given evidently wrong dates when they were analyzed with the same method (see Table 1).

Table 1.
Expected and experimental dates for the five samples analyzed.
PV18 is a brick taken from the perimeter wall of the Certosa di Pavia structure (Italy) and was selected from a group of independently (historical records and thermoluminescence [22]) dated samples. D2500F is a brick taken from the church of San Vittore al Corpo in Milano, Italy [24]. It comes from the external N-E foundation wall. D2643A is a brick from a medieval archaeological site in Stromboli, Italy [25]. Lc and Lk are Renaissance lustred decorated majolica [23] from Umbria (central Italy). Henceforth, samples will be labelled according to the second column of Table 1.
2.2. Methods
The samples were analyzed using thermogravimetric analysis (TGA). A thermogravimetric analyzer by Mettler Toledo (Greifensee, Switzerland) was used to perform the analysis in a dry-air atmosphere (temperature rate 10 °C /min, temperature range 25–1000 °C). The results were matched with literature data to determine the material components that evaporated during the analysis. RHX dating involves the measurement of very small mass variations (~10−7 g) and requires an extremely precise device to maintain constant environmental conditions during the experiment. For this purpose, a dynamic vapor sorption analyzer (Aquadyne DVS2, AquaDyne, Kent, WA, USA) was used. The two balance plates in the device allow the weighing of two samples simultaneously. The plates are placed in a conditioned room with controlled temperature and relative humidity (RH). For the purpose of this study, RH was set to a stable value of 40%, while temperature was varied according to experimental conditions, as explained further. For this analysis, the samples were cut to a mass of ~200–300 mg. Every experimental step involving recording of mass change was divided into two parts, following the explanation of Stage I and Stage II given in the Introduction. Stage I is transient and is not associated with any experimental effect.
The data analysis focuses on the trend in Stage II mass values vs. time (see Figure 1). To study the effect of the preheating temperature on the reliability of the dating process, the commonly accepted dating procedure [6] was changed as follows:
- A preliminary TGA spectrum was acquired for every sample; this provided general insight into the behavior of the material mass at different temperatures. The temperature range was set between 25 and 10,000 °C, with the region of interest between 25 and 600 °C; the curves were acquired in air, with a temperature-change rate of 100 °C/min.
- The samples that showed a significant mass loss in the dehydration range (100–400 °C) were subjected to different preheating temperatures: the same samples were sequentially preheated at room temperature (RT), 105, 200, 300, and 400 °C for 12 h; after each heating, they were processed according to Step 3.
- The samples that did not show a significant mass loss were processed following the commonly accepted procedure [14], skipping the multiple-preheatings sequence.
- The samples were placed in the dynamic vapor sorption balance (DVS) at a constant temperature and relative humidity (200 °C, 40% RH). Once the mass variation recorded on the mass vs. time curve reached a value <10−4 mg/min, meaning that there were no more significant changes in the mass loss, the mass vs. time data related to Stage II were used to generate linear regression parameters, with the mass m2 (see Equation (1)) as the intercept and the mass change (negligible) as the slope (Figure 1a).
- All samples (both the ones that had been sequentially preheated and the ones that had been preheated only at 100 °C) were fired at 500 °C for 24 h. During this step, all the water fractions were released and the ceramic material returned to its original condition after the first firing.
- The mass-gain data were acquired for every sample. Since the activation energy of RHX is hypothesized to follow an Arrhenius-like law, several mass-gain curves were measured at different, increasing temperatures (20, 30, 35, 40 and 50 °C) to find the correlation between temperature and rate of mass gain (Figure 1b) [26].
- The data related to Stage II for every experimental temperature (20, 30, 35, 40 and 50 °C) were graphed against t1/4. In Step 3, the linear regression of the data was used to evaluate the intercept of the first curve (m4), which corresponds to the sum of the ceramic mass mcer and the physiosorbed water mw0 (see Equation (1)). The slopes of the curves correspond to the α(T) values, following the Arrhenius law, as given below:
α(T) = α0e−E/RT
The lnα(T) values were plotted against T−1, and the corresponding linear fit parameters were evaluated. Once the α/T correlation had been established, α(ELT), the growth rate at the estimated lifetime temperature (ELT), a value that depends on historical climatic temperatures, was obtained.
3. Results
The proposed experimental sequence, beginning with an initial TGA screening, allowed for effective qualitative discrimination between samples that were suitable for the standard procedure and those that were not. As shown in Figure 2, the TGA curves of the five samples clearly segregate the samples into two groups. Samples Brick_1 and Cer_2 underwent continuous mass loss starting at approximately 100 °C, with no apparent discontinuities until about 400 °C, thereby losing 2–3% of their weight. In contrast, samples Brick_2, Brick_3 and Cer_1 underwent more limited mass loss (<1%) in the 100 °C dehydration range, coupled with a stepped behavior in the mass-loss profile.
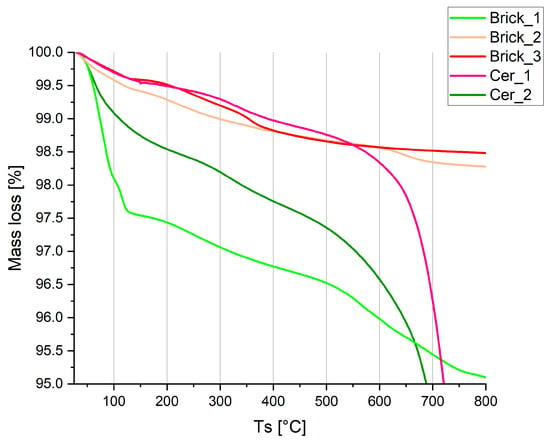
Figure 2.
TGA curves for the five samples. The different mass losses in the 100–400 °C range are clearly visible.
The mass difference, ∆m = m2 − m4, was interpreted as an indicator of the weakly bonded water fraction that remains after the standard low-temperature preheating (100 °C). This residual moisture leads to an overestimation of the sample age when using the conventional protocol. As an illustrative case, Figure 3 shows the Brick_1 data: as the preheating temperature was raised from 100 °C toward 300 °C, the normalized mass gain decreased, and the estimated ages converged toward the expected historical age. Table 2 further confirms that for the Brick_1 and Cer_2 samples, a higher preheating temperature significantly improved the accuracy of the dating process.
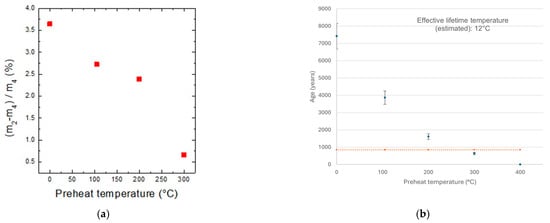
Figure 3.
(a) Normalized mass-gain contribution calculated for different preheating temperatures. (b) Estimated ages from data obtained at different preheating temperatures vs. expected age for the sample Brick_1.

Table 2.
Results obtained from Brick_1 and Cer_2 samples preheated at different temperatures.
Furthermore, the reliability of rehydroxylation (RHX) dating critically depends on the complete removal of weakly bonded water. The experimental evidence presented here indicates that a standard 100 °C preheating is insufficient for samples with significant interstitial water retention. Our data demonstrate that raising the preheating temperature to around 300 °C effectively clears these water molecules, thereby yielding more accurate dating results.
In addition, the kinetic parameters extracted from the mass-gain curves, especially the growth rate α as a function of temperature, support the hypothesis that the rehydroxylation process follows an Arrhenius-like behavior. The linear regression of the lnα(T) versus T−1 plot (Figure 1b) not only confirms the temperature dependence but also facilitates the determination of the activation energy for the RHX process.
A critical examination of previous work in this field reveals both similarities and improvements in our approach. Wilson et al. [5,7] originally established that the mass gain in ceramic samples follows a t1/4 power law, attributing this behavior to the slow kinetics of hydroxyl incorporation after initial rapid water absorption adsorption. While their work laid the foundation for RHX dating, subsequent studies (e.g., [10,20]) raised concerns regarding the reproducibility and the influence of incomplete dehydration on dating accuracy.
Our study addresses these concerns by incorporating an initial TGA step to identify water-fraction retention and by systematically varying the preheating temperature. The observed improvement in dating accuracy, particularly in samples Brick_1 and Cer_2, demonstrates that tailoring the preheating conditions (up to 300 °C) can mitigate the overestimation error. Moreover, while Shoval et al. [12] reported variability in water-release behavior among different ceramics, our comparative analysis shows that a carefully controlled preheating protocol can standardize the dehydration process across heterogeneous samples.
Additionally, our results are consistent with the broader literature, which underlines the importance of mineralogical composition in RHX kinetics [16,17]. Unlike some earlier studies that focused solely on the power law behavior, our approach integrates both the qualitative TGA screening and the quantitative kinetic analysis, thereby offering a more robust framework for addressing the challenges inherent in RHX dating.
4. Discussion
The proposed experimental sequence, with the initial TGA step, allowed for qualitative discrimination of the samples not suitable for analysis by the standard procedure. Figure 2 shows the TGA curves of the five samples, which can be clustered into two groups. In the first group are Brick_2, Brick_3 and Cer_1, which underwent limited weight loss in the TGA curves (mass fraction <1%) in the 100 °C dehydration range and a stepped behavior in the mass loss.
However, the samples in the second group, Brick_1 and Cer_2 (for which we obtained an age overestimation after a 105 °C preheating) decreased in weight starting, as expected, at around 100 °C; it is remarkable that after this temperature, there were no discontinuity points, but the mass continued to decrease at least until 400 °C, with the samples losing a considerable mass fraction (2–3%).
Brick_1 and Cer_2 seem to retain a non-negligible fraction of water after low-temperature heating, as discussed below, leading to a wrong estimate of the adsorbed water content and, as a result, to a wrong estimate of the sample age, and it suggests that there is an incomplete dehydration of the weakly bonded water fraction with a low-temperature step.
The most probable explanation for this behavior seemed to be the mass difference, which is calculated as follows:
∆m = m2− m4
Step 2 (100°C preheat) makes the physiosorbed molecular water in pores dehydrate, leaving on site the weakly bound adsorbed water
The firing in Step 4 removes all water and hydroxyl groups from the sample, leaving only the ceramic mass. It is clear, then, that the relative amount of weakly bonded water in the structure is crucial for the reliability of the dating technique. In fact, the presence of a considerable amount of weakly bonded water molecules in the matrix can lead to an overestimation of the m2 mass. Raising the preheating temperature completely empties the adsorption channels, as well as the interstitial water sites, mimicking the initial firing conditions of dehydration and dehydroxylation of the material. Considering this experimental evidence, it is necessary to find the proper preheating temperature (see Step 2) to ensure a more reliable result or at least to pay particular attention to probable overaging effects. An increase in preheating temperature to about 300 °C for the two overaged samples in this study improved the accuracy of the dating process (see Table 2).
As an example of the effect of the preheating temperature, the results obtained from the Brick_1 sample are reported in Figure 3: the mass difference decreases, and the age estimate is more accurate with preheating in a temperature range between 200 and 300 °C. The discrepancy between experimental and expected ages is due to the continuity of mass loss between 200 and 300 °C, as discussed before: the optimal temperature lies in this interval, but it is not easily determined.
Finally, our attention focused on the reproducibility of the proposed procedure by testing the dating sequence on four subsamples of Cer_2: the results were in good agreement (Table 3 and Figure 4), even if only one sample was in perfect agreement with the expected age.

Table 3.
Data for the Cer_2 sample.
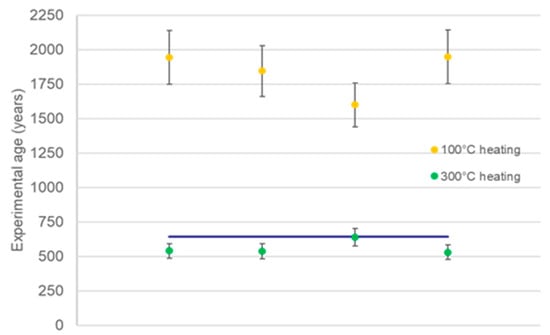
Figure 4.
Comparison of 100 °C and 300 °C preheating results for the Cer_2 sample.
5. Conclusions
In this study, we propose a novel experimental approach to improve the accuracy of ceramic dating via rehydroxylation. At present, the key innovation involves the preliminary use of thermogravimetric analysis (TGA) to quantify the mass-loss fraction associated with interstitial water within the ceramic matrix. If this fraction exceeds 1%, our results suggest that a consistent amount of water molecules is still present in interstitial sites and a consequent overestimation of the age of the sample results. We have seen that those ceramics with a limited mass loss in the range 100–500 °C, as detected by TGA, give dating results in good agreement with the known ages. In contrast, when TGA curves show significant mass loss in the range 100–500 °C, the first step in RHX procedure should involve preheating the sample to a higher temperature, on the order of 300 °C; the resulting dates appear to be correct, within the range of experimental error. A further in-depth analysis could involve acquisition of FTIR spectra at different heating temperatures to study the behavior in the release of water molecules and OH groups as a function of temperature. We recommend selecting a preheating temperature tailored to the specific characteristics of the material studied. This step ensures optimal removal of loosely bound water, minimizing variability in rehydroxylation kinetics and enhancing the accuracy of the dating results. This methodology provides a more robust framework for addressing the challenges posed by heterogeneous ceramic samples, possibly supporting the broader application of rehydroxylation dating to ancient materials.
Author Contributions
Conceptualization, M.M., A.G. and F.M.; Methodology, M.M., A.G., L.P. and F.M.; Validation, F.M.; Investigation, F.M.; Data curation, F.M.; Writing—original draft, M.M. and F.M.; Writing—review & editing, M.M., A.G., L.P. and F.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Galli, A.; Sibilia, E.; Martini, M. Ceramic chronology by luminescence dating: How and when it is possible to date ceramic artefacts. Archaeol. Anthropol. Sci. 2020, 12, 190. [Google Scholar] [CrossRef]
- Aitken, M.J. Thermoluminescence Dating. In Quaternary Research; Academic Press: London, UK, 1985; Volume 26. [Google Scholar] [CrossRef]
- Bailiff, I.K. Luminescence Dating of Pottery and Bricks. In Encyclopedia of Geoarchaeology; Gilbert, A.S., Ed.; Springer: Dordrecht, The Netherlands, 2017; pp. 494–498. [Google Scholar] [CrossRef]
- Martini, M.; Sibilia, E. Absolute dating of historical buildings: The contribution of thermoluminescence (TL). J. Neutron Res. 2006, 14, 69–74. [Google Scholar] [CrossRef]
- Wilson, M.A.; Carter, M.A.; Hall, C.; Hoff, W.D.; Ince, C.; Savage, S.D.; Mckay, B.; Betts, I.M. Dating fired-clay ceramics using long-term power law rehydroxylation kinetics. Proc. R. Soc. A Math. Phys. Eng. Sci. 2009, 465, 2407–2415. [Google Scholar] [CrossRef]
- Wilson, M.A.; Hamilton, A.; Ince, C.; Carter, M.A.; Hall, C. Rehydroxylation (RHX) dating of archaeological pottery. Proc. R. Soc. A Math. Phys. Eng. Sci. 2012, 468, 3476–3493. [Google Scholar] [CrossRef]
- Wilson, M.A.; Hoff, W.D.; Hall, C.; McKay, B.; Hiley, A. Kinetics of moisture expansion in fired clay ceramics: A (time)(1/4) law. Phys. Rev. Lett. 2003, 90, 125503. [Google Scholar] [CrossRef]
- Savage, S.D.; Wilson, M.A.; Carter, M.A.; Hoff, W.D.; Hall, C.; Mckay, B. Moisture expansion and mass gain in fired clay ceramics: A two-stage (time)(1/4) process. J. Phys. D-Appl. Phys. 2008, 41, 055402. [Google Scholar] [CrossRef]
- Savage, S.D.; Wilson, M.A.; Carter, M.A.; Mckay, B.; Hoff, W.D.; Hall, C. Mass Gain due to the Chemical Recombination of Water in Fired Clay Brick. J. Am. Ceram. Soc. 2008, 91, 3396–3398. [Google Scholar] [CrossRef]
- Le Goff, M.; Gallet, Y. Evaluation of the rehydroxylation dating method: Insights from a new measurement device. Quat. Geochronol. 2014, 20, 89–98. [Google Scholar] [CrossRef]
- Wilson, M.A.; Clelland, S.; Carter, M.A.; Ince, C.; Hall, C.; Hamilton, A.; Batt, C.M. Rehydroxylation of Fired-Clay Ceramics: Factors Affecting Early-Stage Mass Gain in Dating Experiments. Archaeometry 2014, 56, 689–702. [Google Scholar] [CrossRef]
- Shoval, S.; Beck, P.; Kirsh, Y.; Levy, D.; Gafttand, M.; Yadin, E. Rehydroxylation of clay minerals and hydration in ancient pottery from the “Land of Geshur”. Joumal Thermal Anal. 1991, 37, 1579–1592. [Google Scholar] [CrossRef]
- Hall, C.; Hamilton, A.; Wilson, M.A. The influence of temperature on rehydroxylation [RHX] kinetics in archaeological pottery. J. Archaeol. Sci. 2013, 40, 305–312. [Google Scholar] [CrossRef]
- Bowen, P.K.; Ranck, H.J.; Scarlett, T.J.; Drelich, J.W. Rehydration/Rehydroxylation Kinetics of Reheated XIX-Century Davenport (Utah) Ceramic. J. Am. Ceram. Soc. 2011, 94, 2585–2591. [Google Scholar] [CrossRef]
- Drelich, J.; Bowen, P.K.; Scarlett, T.J. Effect of humidity instability on rehydroxylation in fired clay ceramics. J. Am. Ceram. Soc. 2013, 96, 1047–1050. [Google Scholar] [CrossRef]
- Hamilton, A.; Hall, C. A Review of Rehydroxylation in Fired-Clay Ceramics. J. Am. Ceram. Soc. 2012, 95, 673–2678. [Google Scholar] [CrossRef]
- Avramovska, M.; Freude, D.; Schwieger, W.; Fey, T.; Kärger, J.; Rgen Haase, J. NMR Studies of the Rehydroxylation of Ceramic Materials with Respect to Rehydroxylation Dating. J. Phys. Chem. C 2023, 127, 19833–19840. [Google Scholar] [CrossRef]
- Shoval, S.; Paz, Y. A study of the mass-gain of ancient pottery in relation to archeological ages using thermal analysis. Appl. Clay Sci. 2013, 82, 113–120. [Google Scholar] [CrossRef]
- Stevenson, C.M.; Gurnick, M. Structural collapse in kaolinite, montmorillonite and illite clay and its role in the ceramic rehydroxylation dating of low-fired earthenware. J. Archaeol. Sci. 2016, 69, 54–63. [Google Scholar] [CrossRef]
- Le Goff, M.; Gallet, Y. Evidence for complexities in the RHX dating method. Archaeometry 2015, 57, 897–910. [Google Scholar] [CrossRef]
- Maspero, F.; Galli, A.; Panzeri, L.; Martini, M. Rehydroxylation: A promising technique for ceramic dating. J. Phys. Conf. Ser. 2022, 2204, 012031. [Google Scholar] [CrossRef]
- Galli, A.; Martini, M.; Maspero, F.; Panzeri, L.; Sibilia, E. Surface dating of bricks, an application of luminescence techniques. Eur. Phys. J. Plus 2013, 129, 101. [Google Scholar] [CrossRef]
- Galli, A.; Martini, M.; Sibilia, E.; Padeletti, G.; Fermo, P. Luminescence properties of lustre decorated majolica. Appl. Phys. A Mater. Sci. Process. 2004, 79, 293–297. [Google Scholar] [CrossRef]
- Sannazaro, M. Lo sviluppo urbanistico di Milano in età paleocristiana. LANX 2014, 19, 79–94. [Google Scholar]
- Rosi, M.; Levi, S.T.; Pistolesi, M.; Bertagnini, A.; Brunelli, D.; Cannavò, V.; Di Renzoni, A.; Ferranti, F.; Renzulli, A.; Yoon, D. Geoarchaeological Evidence of Middle-Age Tsunamis at Stromboli and Consequences for the Tsunami Hazard in the Southern Tyrrhenian Sea. Sci. Rep. 2019, 9, 677. [Google Scholar] [CrossRef] [PubMed]
- Clelland, S.J.; Wilson, M.A.; Carter, M.A.; Batt, C.M. Rhx Dating: Measurement of the Activation Energy of Rehydroxylation for Fired-Clay Ceramics. Archaeometry 2015, 57, 392–404. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).