Metal Recovery from Wastes: A Review of Recent Advances in the Use of Bioelectrochemical Systems
Abstract
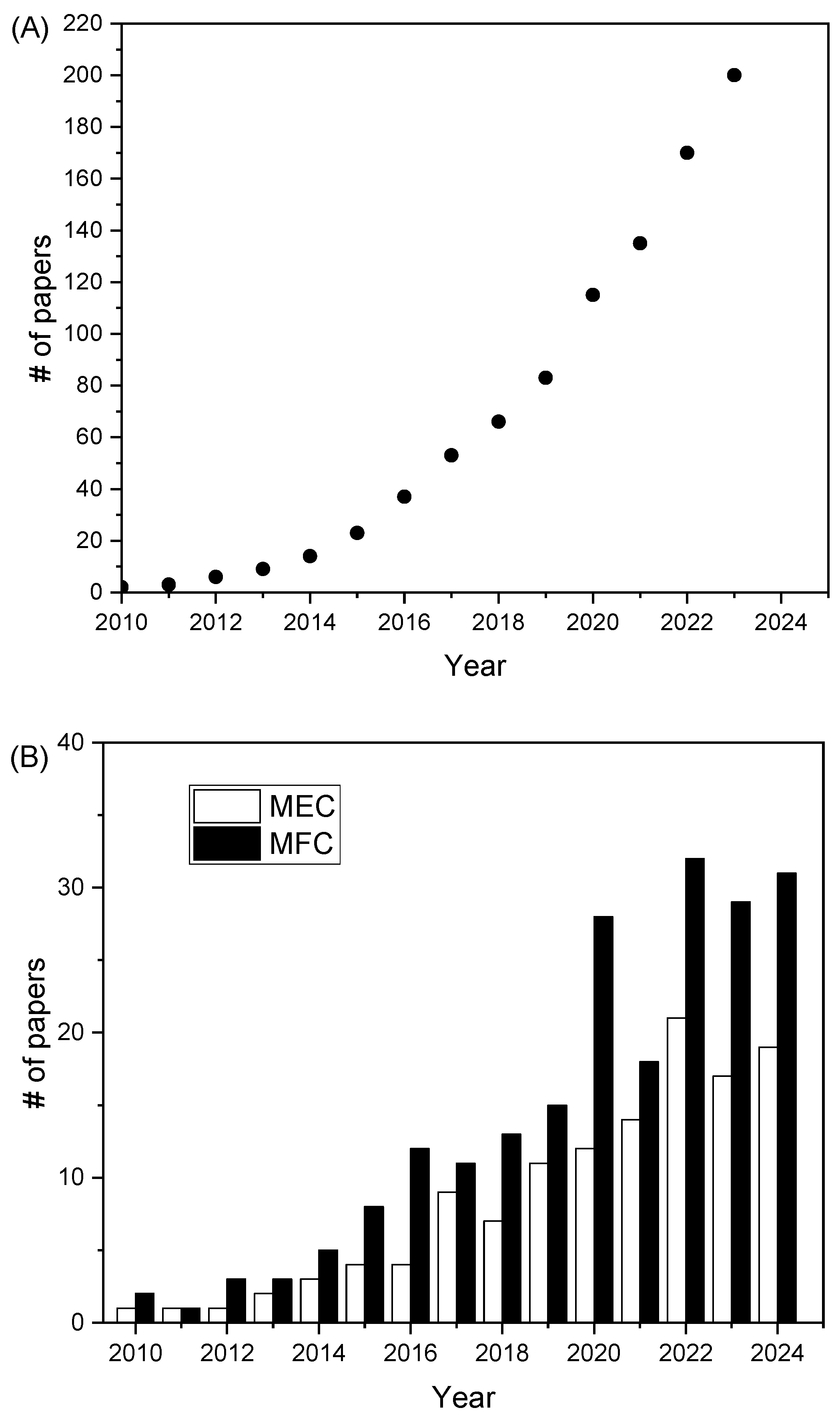
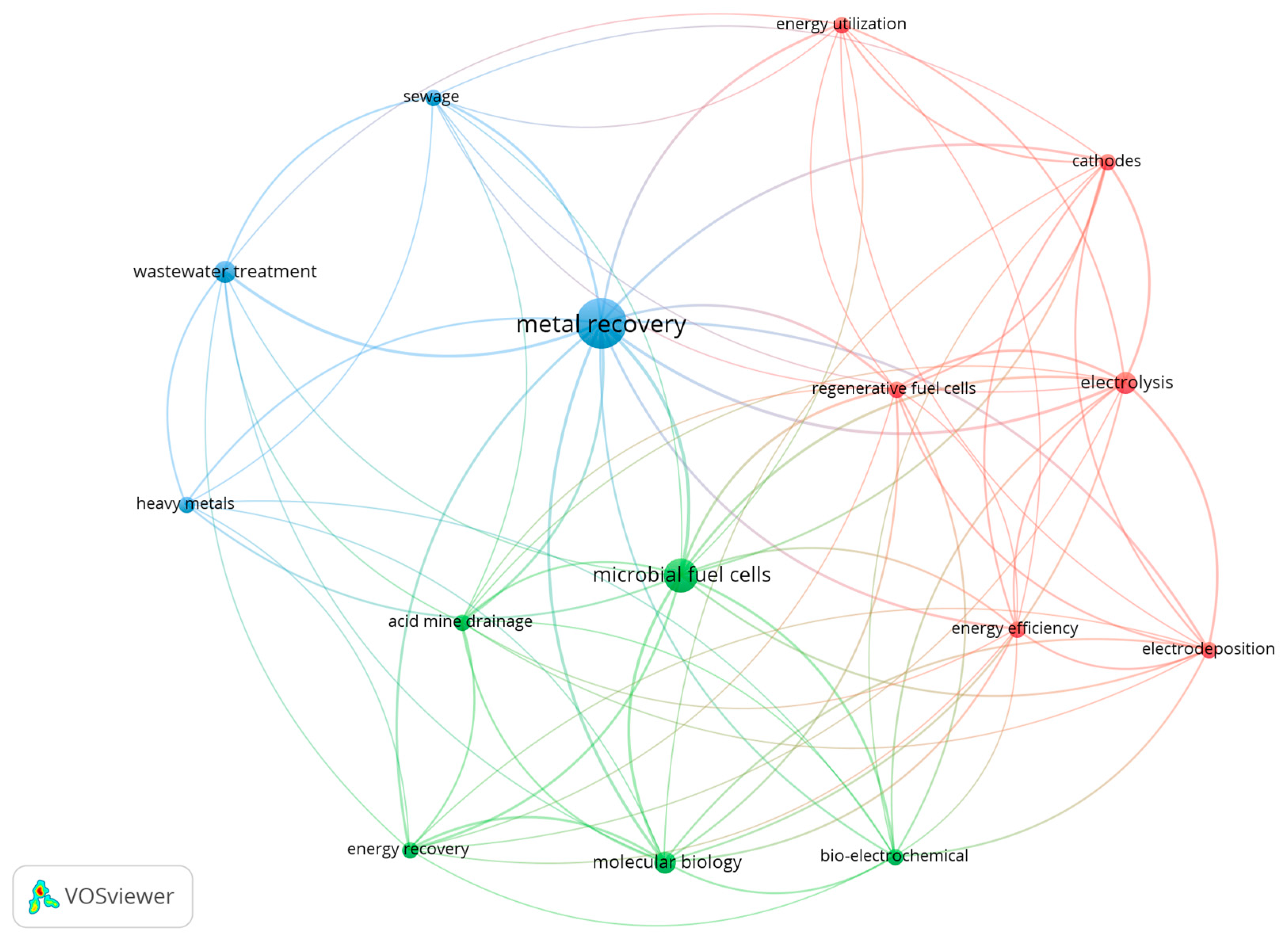
1. Introduction
| Technology | Process Description | Advantages | Disadvantages |
|---|---|---|---|
| Pyrometallurgy [23] | High-temperature processes to extract and refine metals from ores or waste (e.g., smelting, roasting). | - Minimal pre-treatment required - Rapid processing - Adaptable to different metal sources | - High energy consumption - Low selectivity - Loss of strategic metals - Emission of polluting gases |
| Hydrometallurgy [22,34] | Metal extraction via aqueous solutions primarily involving leaching, solvent extraction, and precipitation. | High purity, effective impurity control, lower energy costs | - Extensive use of chemicals - Secondary pollution and wastewater generation |
| Ion Exchange [35] | Metal ions are exchanged with ions on a solid resin, allowing for selective recovery. | - High selectivity - Reusable resins | - High operational cost - Requires regeneration of resins - Less effective for high metal concentrations |
| Electrochemical Recovery/electrowinning [36,37] | Metals are plated onto cathodes via redox reactions in electrochemical cells. | - High purity of recovered metals - Can be coupled with renewable energy | - Reduced efficiency for dilute solutions due to mass transport limitations. |
| Membrane Filtration [35,38] | Separates metals via physical barriers (e.g., reverse osmosis, nanofiltration, ultrafiltration). | - High removal efficiency - Suitable for a wide range of metals | - High CAPEX and OPEX - Prone to membrane fouling - Requires high pressure and energy input |
| Bioleaching [22,39] | Uses microorganisms to extract metals through biological oxidation/reduction processes. | - Environmentally friendly - Cost-effective for low-grade ores - Minimal energy input | - Slow process - Requires specific conditions (e.g., pH, temperature) - Limited to certain metals |
| Bioelectrochemical Systems (BES) [28,40] | Integrates bioprocesses with electrochemical techniques for metal solubilization and recovery. | - Energy-efficient or energy-generating - Simultaneous wastewater treatment and metal recovery | - Slow kinetics due to microbial activity - Limited scalability - Low technological readiness level |
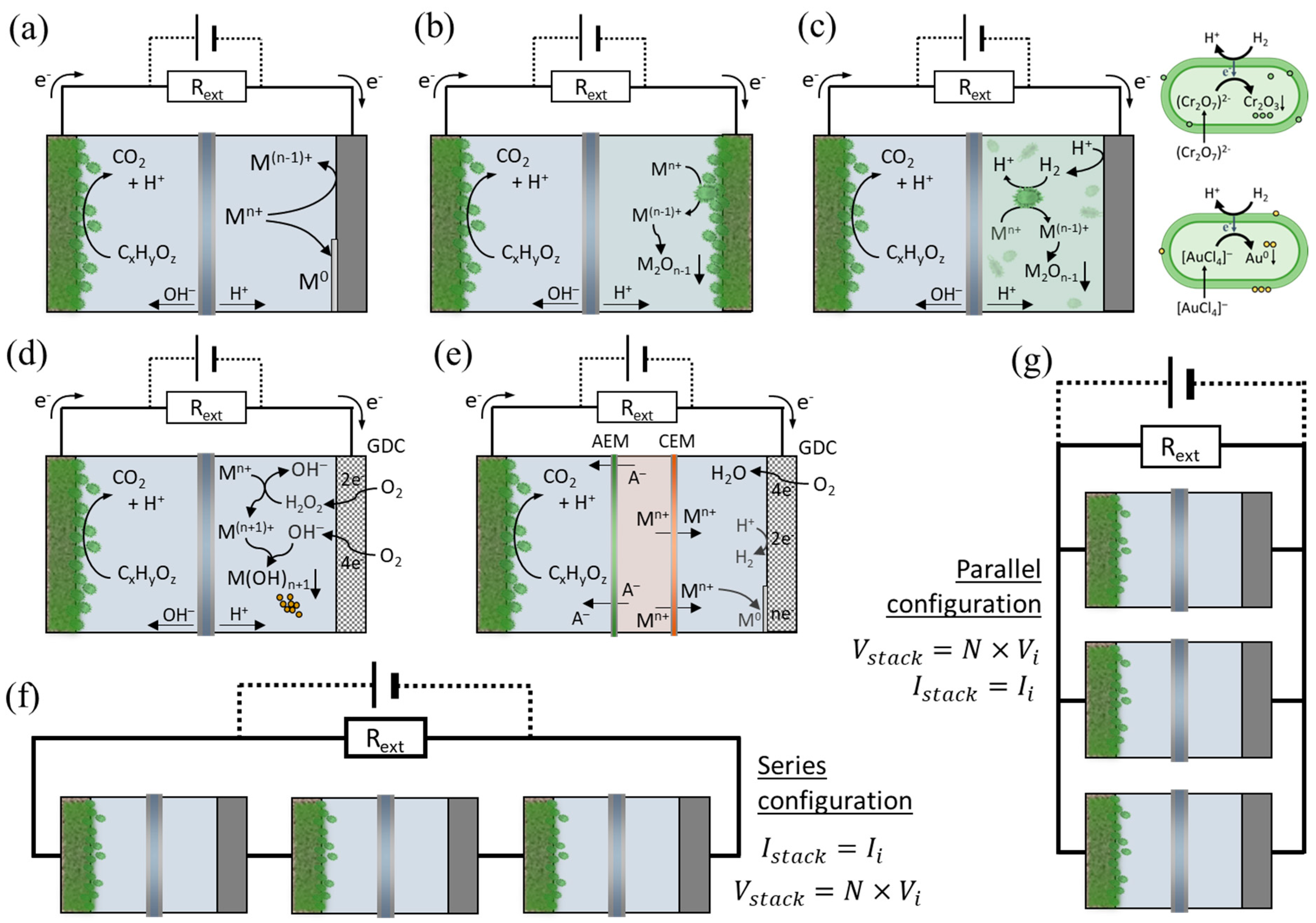
2. Bioelectrochemical Systems for Metal Recovery
| Objective | Reactor Setup | Operational Conditions | Milestones | Ref. |
|---|---|---|---|---|
| Use of MFC for recovery of metals from AMD | System: Dual-chamber MFC Anode: Carbon felt (CF) and doped variants with non-activated (CFnaH) or activated hydrochar (CFaH), electrode dimensions (2.5 × 2.5 × 0.8 cm) Cathode: Copper (2.5 × 2.5 cm) Membrane: Bipolar (Fumasep® FBM) | Anolyte: Synthetic medium with acetate (1000 mg/L, pH 7) Catholyte: Synthetic AMD (400 mg/L Cu2+, 400 mg/L Fe3+, 50 mg/L Ni2+, 50 mg/L Sn2+, pH 2.5) Rext: 120 Ω | - Cu recovery: 91% (CFaH), 85% (CFnaH), 48% (CF) - Faster Cu recovery (3 days vs. 4 days) with doped anodes - Fe3+ reduced from 400 to 10 mg/L - Current density: 1.2 A/m2 | [10] |
| Copper recovery by saline MFC with polypyrrole-based cathodes | System: Dual-chamber MFC Anode: Carbon felt (6 mm thick, 3 × 3 cm2) Cathode: Stainless steel with Polypyrrole (SS/PPy), Polypyrrole with Phytic Acid (SS/PPy-PA), Polypyrrole with Carbonised Cellulose (SS/PPy-CC), and combinations (SS/PPy-PA-CC) Membrane: Nafion | Anolyte: Synthetic wastewater with acetate (pH 7) Catholyte: CuSO4 solution (256 mg/L Cu) in 2% NaCl pH: 3.0 Temperature: 30 °C Rext: Various (10–20,000 Ω) | - 97% Cu recovery in 3 days—SS/PPy-PA cathode: higher efficiency but corroded over time - SS/PPy suitable for large-scale MFCs | [70] |
| Metal removal using algae and algal biochar as cathode catalysts in Photosynthetic dual-chamber MFC (PMFCs) | System: Photosynthetic dual-chamber MFC Anode: Carbon felt (CF) (110 cm2) Cathode: CF (130 cm2), CF coated with Cu-accumulated ABC700 (activated biochar prepared from algae biomass thermally activated at 700 °C), and CF coated with Co-accumulated ABC700 Membrane: Clayware cylinder wall as proton exchange separator | Anolyte: 125 mL cultivated algae solution (pH 7) Catholyte: 400 mL with Cu2+ or Co2+ (25, 50, 75, and 100 mg/L, pH 7) Rext: 100 Ω Temperature: Room temperature | - Cu2+ recovery: 94% - Co2+ recovery: 88% - Highest power density and COD removal achieved with Co-accumulated ABC700 cathode - Algal biochar cathodes contributed to reducing pollutants and supporting energy generation | [68] |
| Heavy metal removal and energy recovery using pyrite-enhanced MnCo/CNF anode | System: Dual-chamber “H”-Type MFC Anode: Carbon felt (CF), Carbon nanofibers (CNF), MnCo/CNF, MnCo/CNF with pyrite (5 × 2 cm) Cathode: Carbon felt (5 × 2 cm) Membrane: Nanofiber membrane | Anolyte: Acetate and glucose-based medium Catholyte: 25 mM K3[Fe(CN)6] solution Rext: 1000 Ω Temperature: 20 ± 5 °C | - 99% Sb removal with MnCoPy-MFC - Maximum power density hierarchy: CF-MFC < CNF-MFC < MnCoCNF-MFC < MnCoPy-MFC - MnCoPy-MFC exhibited the highest energy recovery performance | [71] |
| Cr6+ recovery using upflow MFC | System: Upflow MFC Anode: Carbon felt with granular activated carbon (45 g, 377 cm2) Cathode: Carbon cloth (1130 cm2) Membrane: Proton exchange (Nafion® 117) | Anolyte: Mineral medium (pH 7), 0.98 L Catholyte: Cr(VI)-containing medium (pH 4), 4.2 L Temperature: 34 °C Rext: 1000 Ω | - 97.7% Cr(VI) removal with optimal 800 mg/L influent, 2-day retention time, and pH 7 (anode)/pH 4 (cathode). - Cr(III) precipitation reduced MFC efficiency over time. - Parallel configuration outperformed series | [72] |
| Cr6+ recovery using a combined wetland and MFC system | System: Vertical upward-flow MFC Anode/Cathode: 100-mesh stainless steel wrapped with activated carbon Membrane: None | Structure: Bottom (zeolite), anode (activated carbon), middle (ceramsite), cathode (activated carbon), wetland plants Simulated Wastewater: Cr(VI) (40–120 mg/L), acetate, NH4Cl, NaHCO3, nutrients Rext: 1000 Ω | - 97.7% Cr(VI) removal efficiency - Effective for chromium pollution reduction in wastewater - Integrated system design leveraging wetland plants and vertical MFC layers | [73] |
| Environmental impact investigation on Cr6+ recovery and electricity generation | System: Dual-chamber MFC Anode: Carbon felt Cathode: Carbon cloth, carbon brush, carbon felt Membrane: Proton exchange membrane | System: Dual-chamber MFC Anode: Carbon felt Cathode: Carbon cloth, carbon brush, carbon felt Membrane: Proton exchange | System: Dual-chamber MFC Anode: Carbon felt Cathode: Carbon cloth, carbon brush, carbon felt Membrane: Proton exchange | [74] |
| Chromium nano-mining using electrified bioreactors (EBS) | System: Two-compartment EBS (0.125 L per compartment) separated by CEM (Ultrex CMI-700, 64 cm2) Electrodes: Stainless Steel mesh (4.8 cm2) | Catholyte: 250 mL K2Cr2O7, pH 7, inoculated with S. oneidensis MR-1 Cathode polarization: –0.8 VSHE Flow rate: Batch recirculation at 2.5 L/h | - Cr(VI) removal: 76% in 24 h - Total Cr removal: 61% - Cr nanoparticles (19–36 nm) observed around bacterial cells - Energy consumption: 0.8 kJ - Maximum removal capacity: 1121 mg Cr/g cells | [47] |
| Silver recovery from photovoltaic panels | System: Dual-chamber MFC Anode: Carbon felt (5 × 5 cm) Cathode: Graphite paper (3 × 3 cm) Membrane: Nafion® 115 | Anolyte: Phosphate buffer + glucose Catholyte: Synthetic extract solution (AgNO3, CuCl2, AlCl3) Rext: 100 Ω/Temperature: 30 °C | - Ag: 100% recovery (7 h) - Cu: 60% recovery (7 h) - Al: 15% recovery (7 h) | [75] |
| Investigating the effect of Ag concentrations on current production and recovery | System: Dual-chamber H-type MFC Anode: Carbon cloth (25 cm2) Cathode: Carbon cloth (25 cm2) with Pt (0.5 mg/cm2) Membrane: Cation exchange membrane (Nafion® 117) | Anolyte: Synthetic wastewater (acetate 1 g/L, trace elements, neutral pH) Catholyte: Ag solution (6–95 mg/L) Rext: 100 Ω Temperature: 25 °C | - Maximum Ag removal: 99.8% at 100 mg/L Ag - Ag recovery: 75% in 2 h at <30 mg/L, 90% in 6 h at >50 mg/L - Batch operation up to 8 h investigated for varying Ag concentrations | [76] |
| Feasibility of MFC technology for silver recovery from synthetic PV hydrometallurgical wastewater | System: Dual-chamber MFC Anode: Graphite paper (2.5 × 3.8 cm) Cathode: Plain graphite paper Membrane: Proton exchange membrane (Nafion® 117, 3.77 cm2) | Anolyte: Synthetic wastewater with 50 mg/L Ag Catholyte: Phosphate buffer (pH 7, 0.16 g/L KCl) Rext: 100 Ω/Temperature: 32 °C | - Ag recovery: >93% with NaClO4 as the supporting electrolyte - 100% Ag recovery at pH 2.00 in 3 h and pH 7.00 in 5 h - Lower efficiency when KCl was used as a supporting electrolyte | [77] |
| Gold Recovery and Nanoparticle Synthesis in Microbial Systems Using Fractional Factorial Design | Serum bottle reactors (120 mL), 50 mL solution, sealed with butyl rubber stoppers; Shewanella oneidensis MR-1 and Cupriavidus metallidurans CH34 as bacterial strains Electrochemical tests: Two-compartment BES Electrodes: Carbon electrodes Electron donor: In situ hydrogen from cathodic water reduction | Factors: cell concentration (OD 0.5 or 1), temperature (28 °C or 37 °C), anoxic/oxic, pH (1 or 5), Au3+ concentration (0.2 mM or 2 mM), electron donor (electrogenerated H2 or lactate), bacterial species; batch incubation for 72 h. Electrochemical tests for H2 generation: Carbon cathode poised at −0.3 V vs. Ag/AgCl | - Highest Au removal (88.2%) achieved with S. oneidensis MR-1 at 0.2 mM Au3+, pH 5, anoxic conditions, and (electrogenerated) H2 as the electron donor. - Targeted Au nanoparticle size (50 nm) obtained under optimal conditions - Significant role of pH and Au3+ concentration in removal efficiency | [48] |
| Alternative approach for Au recovery using microbial electrochemical snorkel (MES) | System: Dual-chamber MFC Anode: Bioanode Cathode: Gold foil and graphitized paper (8 cm2) Membrane: Proton exchange (Nafion® 117) | Anolyte: Aqueous phase above freshwater sediment Catholyte: Gold-containing solution (1 g/L Au3+) Rext: 510 Ω Temperature: 25 °C | - In MES-mode (short-circuited), >50% Au recovered in 2 h, 100% recovered in 24 h - Gold deposited as an elemental state - Similar performance for both cathodes, with graphite offering a cost advantage | [78] |
| Assessing Castellaniella inoculum for cathodic metal recovery in BES | System: Dual-chamber MFC Anode: Biofilm of mixed Castellaniella inoculum Cathode: Carbon cloth Membrane: Anion exchange membrane | Anolyte: 50 mM PBS with Wolfe’s vitamins and trace elements Catholyte: Pb–Zn smelting wastewater (COD: 2.26 mg/L, Cu2+: 183.19 mg/L, Hg2+: 78.44 mg/L, Pb2+: 206.94 mg/L, Zn2+: 126.92 mg/L, pH 2.38) Rext: 10 Ω/Temperature: 30 °C | Mode MFC: - Cu2+: 99.86% recovery in 60 h - Hg2+: 99.98% recovery in 17 h - Pb2+, Zn2+ unchanged Mode MEC: - 1 V: Pb2+: 93.49% in 36 h, Zn2+ unchanged - 2 V: Pb2+: 99.98% in 24 h, Zn2+: 99.17% in 24 h | [79] |
| Investigating heavy metal removal with varying metal concentrations and dissolved oxygen conditions | System: Dual-chamber MFC Anode: Carbon felt (diagonal placement) Cathode: Titanium sheet and graphite plate (vertical placement) Membrane: Proton exchange (Nafion® 117) | Anolyte: Anaerobic sludge with nutrient solution (acetate 1.0 g/L, trace elements) Catholyte: CuCl2 (100 mg/L) and K2Cr2O7 (10–150 mg/L) Rext: 200 Ω Volume: 125 cm3 (both chambers) | - Cu2+ recovery: 98.34% at 10 mg/L Cr6+ with graphite cathode - Cr6+ recovery: 99.92% at 10 mg/L Cr6+ with graphite cathode - Graphite cathode outperformed titanium: Cu2+: 98.09% vs. 88.79%, Cr6+: 86.13% vs. 51.13% - Higher Cr6+ conc. reduced recovery efficiency | [80] |
| Recovery of metals from AMD via electrodialysis followed by MFC and MEC modes | System: Dual-chamber MFC Anode: Carbon felt (KFA 10, 2.5 × 2.5 × 1.1 cm) Cathode: Titanium (2.5 × 2.5 cm) Membrane: Bipolar membrane (Fumasep® FBM) | Anolyte: 100 mL activated sludge (50%) mixed with nutrient medium (acetate 1 g/L, Na2HPO4 3 g/L, trace elements) Catholyte: 100 mL concentrated AMD Rext: 120 Ω (MFC) | MFC mode: - Fe3+ reduced to Fe2+ in 2 days - Cu2+ reduced to Cu0 in 8 days MEC mode: - Ec = –0.5 V: Negligible recovery in 48 h - Ec = –1.0 V: Mixed metal recovery (Cd2+, Ni2+, Fe2+, Zn2+) in 96 h - Ec = –1.5 V: Remaining Fe2+ and Zn2+ recovered in 96 h | [81] |
| Model comparison of factors affecting Cu2+ deposition in MFCs | System: Dual-chamber MFC Anode: Carbon fiber brush (2.5 cm diameter, 2.5 cm length) Cathode: Rough graphite plate (4 cm2) Membrane: Bipolar membrane (BPM-I) | Anolyte: Acetate with 50 mM phosphate buffer (27 mL) Catholyte: Simulated Cu2+ wastewater (17.5 mL) Rext: 50 Ω/Temperature: 30 °C | - Cu2+ recovery: 84.59% after 16 h. - Electromigration identified as a significant factor in Cu2+ mass transfer - Mathematical model was successfully validated | [82] |
| Simultaneous bioelectricity and Cu recovery | System: Dual-chamber MFC Anode: Carbon felt (16 cm2) Cathode: Carbon felt (16 cm2) Membrane: Nafion® 117 | Anolyte: Culture medium with anaerobic sludge Catholyte: Artificial Cu2+ solution (1 g/L) pH: 7.0 (anode), 3.0 (cathode) Temperature: 35 °C/Rext: 1000 Ω | - 99.7% Cu recovery in 192 h - 50% recovery achieved within 48 h | [69] |
| Purification of fracturing flowback water (FFW) and Cu2+ removal using dual-anode MFCs | System: Dual-anode MFC (DA-MFC) with two glass chambers (180 mL total volume) Anode: Bioanode, circular carbon felt (Φ4 cm, 3 mm thick) Cathode: Wet-proofed carbon cloth (Φ5 cm) with Pt (0.5 mg/cm2) Membrane: Proton exchange membrane (Nafion® 117) | Anolyte: Synthetic FFW (1298.78 ± 1.22 mg/L COD, 247.5 ± 23.0 mg/L SO42−, 2 mg/L Cu2+, 12,000 mg salinity, pH 7) Catholyte: Phosphate buffer (50 mM, pH 7, 150 mL) Voltage: 0.05–0.2 V Rext: 100 Ω | - Cu2+ removal: 99.9 ± 0.5% in 5.5 days at 0.1 V - Removal at other voltages: 99.5 ± 0.3% at 0.05 V, 99.8 ± 0.7% at 0.2 V - Optimum performance at 0.1 V | [83] |
| Simultaneous mineralization of organic compounds and heavy metal recovery using photo-assisted BES | System: Dual-chamber MFC and MEC (MFC in situ-MEC and MFC1-MFC2-MEC) Anode: Porous graphite felt (1 × 1 × 1 cm) Cathode: Graphite felt with WO3/MoO3/g-C3N4 heterojunctions (2 × 2 × 0.25 cm) Membrane: None | Anolyte: 26 mL with 5 mM NaH2PO4, acetate, NH4NO3, KNO3, and trace elements Catholyte: 13–26 mL; soluble COD: 306 mg/L, Cu2+: 25.1 mg/L, Zn2+: 5.1 mg/L, Ni2+: 8.2 mg/L, pH 2.98 Rext: 10 Ω | - Optimal setup: 2 MFCs in series (13 mL cathodes) feeding a 26 mL cathode MEC, 6 h HRT Cu2+ reduced on MFC cathodes, Zn2+ precipitated in MEC–Metal removal: Metal removal: Cu2+, Ni2+ nearly 100%; Zn2+: 86% - Absence of light enhanced removal efficiency | [84] |
| Recovery of Zn from bioleachate using a MEC | System: Dual-chamber H-cell MEC Anode: Carbon felt (12.5 cm2) Cathode: Graphite foil (12.5 cm2) Membrane: Proton exchange membrane | Anolyte: 220 mL nutrient medium with COD: 675 mg/L (acetate, glucose, peptone, yeast extract, sewage sludge, pH 7.2) Catholyte: 220 mL, 36 mM phosphate buffer (pH 7.2) Potential: –100 mV | - Zn2+ reduced from 444 mg/L to 245 mg/L in 4 days - Al3+ reduced from 270 mg/L to 10 mg/L in 4 days - Simultaneous Fe reduction observed - Lower energy consumption compared to electrowinning | [85] |
| Recovery of Cr, Cu, and Cd from industrial water using Castellaniella species in MFC and MEC | System: Dual-chamber MFC and MEC Anode: Electroactive biofilms of Castellaniella species Cathode: Rectangular carbon cloth (2.5 × 0.9 cm) Membrane: Cation exchange membrane | Anolyte: 28 mL (2.5 g/L NaCl, 1.25 g/L yeast extract, 2.5 g/L peptone, pH 7) Catholyte: 15 mL simulated industrial wastewater (Cr6+: 134.88 mg/L, Cd2+: 130.18 mg/L, Cu2+: 130.78 mg/L, pH 1.8) Rext: 10 Ω/Temperature: 30 °C | MFC mode removal efficiency: - Cr6+: 99.6%, Cu2+: 99.9% MEC mode removal efficiency: - Cd2+: 99.9% - Mixed culture biofilm outperformed pure strains. | [86] |
| Wastewater treatment and elemental telluride recovery in a dual-chamber MFC | System: Dual-chamber MFC Anode: Graphite (38 cm2) Cathode: Graphite (38 cm2) Membrane: Proton exchange (Nafion® 117, 4 × 4 cm) | Anolyte: Acid-pretreated inoculum (10% v/v, VSS: 2.0 g/L) in nutrient medium (glucose: 3 g/L, pH 6) Catholyte: Sodium tellurite (0.011–0.044 g/L, pH 7) Rext: 30–0.05 kΩ/Temperature: 28 ± 2 °C | - Highest elemental tellurium (Te0) recovery: 45.3%. - Simultaneous Te4+ removal: 54.7% - Cathodic terminal electron acceptors enhanced microbial anodic oxidation and metal detoxification | [71] |
| Recovery and separation of Cu2+, Ni2+, and Zn2+ from Etching Terminal Wastewater (ETW) using photocomposition-assisted BES | System: Dual-chamber MFC (MFCCu) and MEC (MECNi, MECZn) Anode: Graphite felt Cathode: Graphite felt with WO3/MoO3/g-C3N4 heterojunctions Membrane: Cation exchange membrane (CM1-7000) | Anolyte: Nutrient solution (acetate 1 g/L, KH2PO4 4.4 g/L, trace elements, 26 mL) Catholyte: Actual ETW (SCOD 306 mg/L, Cu2+ 25.2 mg/L, Zn2+ 5.1 mg/L, Ni2+ 8.2 mg/L, Cr6+ 0.2 mg/L, pH 2.98) Power Source: 0.3 V and 0.6 V (MEC) Rext: 10 Ω (MFC) Temperature: 25 ± 3 °C | - Metal recovery: Cu2+ 85.8% (MFCCu), Ni2+ 71.6% (MECNi), Zn2+ 67.7% (MECZn) - Complete removal of Cr6+ and total chromium - Light irradiation and current application worked synergistically for mineralizing recalcitrant organic compounds and metals present in ETW - Current application improved metal recovery by 35.8–112.8% compared to light irradiation alone. | [87] |
| Investigating Cu, Ni, and Zn removal using BES under MFC and MEC modes | System: Dual-chamber MFC and MEC Anode: Carbon felt (25 cm2, Sigracell® KFD2.5) Cathode: Carbon fiber fabric (25 cm2, Sigratex® C U200) with PMF-011904 catalyst (2 mg/cm2) Membrane: Anion exchange membrane (AMI-7001) | Anolyte: Acetate-based mineral medium (ABMM, 0.5 L) Catholyte: 1 L solutions: Cu2+ (1.1 mM), Ni2+ (1.1 mM), Zn2+ (1.6 mM) Modes: Short-circuit MFC (Ecell = 0 V), MEC (Ec = –0.4 V vs. Ag/AgCl) | - Copper (24 h): MEC: 97.1% (64.1 g/d·m3), MFC: 88.1% (53.3 g/d·m3) - Nickel (48 h): MFC: 50.7% (17.4 g/d·m3), MEC: 41.0% (13.1 g/d·m3) - Zinc (24 h): MEC: 73.2% (40.25 g/d·m3), MFC: 74.5% (39.03 g/d·m3) | [88] |
| Recovery of cadmium and nickel from wastewater using a biocathode in MFC | System: Dual-chamber MFC Anode: Graphite (15 × 9 cm) Cathode: Graphite (15 × 9 cm) Membrane: Proton exchange membrane (Nafion® 117, 4 cm diameter) | Anolyte: Distillery wastewater (33,750 mg/L COD, pH 6, inoculum from Nazafgarh wetland) Catholyte: Ni2+, Cd2+, or Ni2+–Cd2+ solutions (10–25 mg/L, pH neutral) Rext: 200 Ω/Temperature: 25 ± 3 °C | - Abiotic cathode: Ni recovery: 71.5% (10 mg/L), 28.6% (25 mg/L); Cd recovery: 68.2% (10 mg/L), 20.6% (25 mg/L) - Biocathode: Ni recovery: 91.7% (10 mg/L), 48% (25 mg/L); Cd recovery: 86.9% (10 mg/L), 33% (25 mg/L) | [89] |
| Removal of zinc from industrial effluents using MFCs | System: Half-cell MFC Anode: Plain carbon felt (3 × 3 cm2) Cathode: Plain carbon felt (3 × 3 cm2) Membrane: Anion exchange membrane (FAB-PK-130, Fumatech) | Anolyte: 1.0 g/L CH3COONa, 3.0 g/L NaCl (7 × 7 × 2 cm3 working volume) Catholyte: 1.9 mM ZnCl2, 3.0 g/L NaCl (7 × 7 × 2 cm3 working volume) Rext: 10 Ω Temperature: 22 ± 3 °C | - Zn2+ removal: 96% for synthetic and industrial samples (<2.0 mM Zn2+) in 22 h - Cathode recovery: 83% (synthetic), 46% (industrial) - Electrodeposition predominated over chemical deposition | [90] |
| Recovery of metals (Fe, Cu, Sn, Ni) from synthetic AMD using BES in MFC and MEC modes | System: Dual-chamber MFC and MEC Anode: Carbon felt (KFA10, 2.5 × 2.5 × 0.8 cm, porosity: 0.95) Cathode: Copper (MFC), Titanium (MEC) Membrane: Bipolar membrane (Fumasep® FBM) | Anolyte: 0.1 L (acetate-based medium, pH 7.48) Catholyte: 0.1 L synthetic AMD (Fe3+: 500 mg/L, Cu2+: 500 mg/L, Sn2+: 50 mg/L, Ni2+: 50 mg/L, pH 2.5) Rext: 120 Ω Temperature: 25 °C | MFC mode: - Fe3+ reduced to Fe2+ within 1 day - Cu2+ to Cu0 recovery: 100% in 4 days MEC mode: - At –0.7 V: Sn2+ recovery: >80% in 0.3 days - After 72 h: Ni2+: 77%, Fe2+: 60% - BES required 0.5 V, significantly less than the 1.8 V needed in the abiotic blank system | [91] |
| Synchronized recovery of high-purity Fe and S from sulfide tailings using triple-chamber MFC | System: Triple-chamber MFC (two cathode chambers flanking the anode chamber) Anode: Pretreated carbon felt (7.1 cm2) Cathode: Pretreated carbon felt (7.1 cm2) Membranes: CEM (first cathode chamber) and CEM (second cathode chamber), area: 7.1 cm2 | Anolyte: 28 mL (25 g/L sulfide tailing model, 1 g/L NaHCO3) Catholyte: 28 mL (Na2SO4: 0.67 g, simulating ferrous- and sulfate-laden streams) Rext: 500 Ω Temperature: 30 ± 1 °C | - Recovery in 50 h: Fe: 80% as Fe(OH)3, S: 22.1% as S0 - Purities: Fe(OH)3: 93.1%, S0: 90.2% - Facilitates simultaneous removal of iron and sulfate ions, reducing waste and enabling direct reuse of recovered materials in metallurgical processes | [92] |
| Modeling and validating metal removal efficiencies and energy consumption in BES vs. conventional techniques | Model: “Grey-box” combining Monod kinetics (MFC) and Butler-Volmer equations (MEC), predicting concentration profiles, current generation, and metal recovery. System: Dual-chamber MFC and MEC Anode: Carbon felt (2.5 × 2.5 × 0.8 cm) Cathode: Copper (MFC), Titanium (MEC) Membrane: Bipolar membrane | Anolyte: 0.1 L (acetate-based medium, pH 7.48) Catholyte: 0.1 L (Cu2+: 500 mg/L, Fe3+: 500 mg/L, Sn2+: 50 mg/L, Ni2+: 50 mg/L, pH 2.5) Rext: 120 Ω Temperature: 25 °C | - Model Validation: High accuracy for Cu2+ recovery, slight deviations for Fe3+. Captures concentration profiles and current density trends - MFC Mode: Complete Cu2+ and Fe3+ removal in 4 days, linking recovery with electricity generation - MEC Mode: >80% Sn2+ recovery in 1 day; Ni2+ (15 mg/L) and Fe2+ (30 mg/L) recovered over 4 days | [93] |
| Recovery of Se4+ oxyanion at the cathode of a dual-chamber BES | System: Dual-chamber MFC Anode: Graphite (38 cm2) Cathode: Graphite (38 cm2) Membrane: Nafion 117 (4 × 4 cm) | Anolyte: 10% inoculum, 3 g/L glucose, pH 6 ± 0.01 Catholyte: Na2SeO3∙5H2O (0.025–0.102 g/L), pH 7 ± 0.01 Rext: 30 to 0.05 kΩ/Temperature: 28 ± 2 °C | - Highest Se0 recovery: 26.4% in BES-SeC with 73.6% selenium removal from catholyte - Selenium formed at the cathode was amorphous. - Increased selenium concentration enhanced microbial dehydrogenase activity | [94] |
| Recovery of Cr6+, Cu2+, and V5+ contaminants using MFC technology | System: Dual-chamber MFC Anode: Carbon cloth (1.5 cm2) Cathode: Carbon cloth (12 cm2) Membrane: Bipolar (Fumasep®, 7 cm2) | Anolyte: Domestic wastewater with nutrient medium, 150 mL, pH 7.00 Catholyte: Metal solutions (K2Cr2O7, CuCl2, NaVO3), 150 mL, pH 7.00 Rext: 500 Ω/Temperature: 30 °C | - Metal concentrations reduced from 1 g/L to 0.02 g/L in 8 days - Highest recovery: Cr6+ (80%) > Cu2+ (74%) > V5+ (70%). - Current density decreased with metal concentration reduction | [95] |
| Use of Pseudomonas sp. E8 in MFC and MEC mode for Cu2+ and Cd2+ recovery in simulated AMD | MFC (single chamber): - Anode: Carbon brush - Cathode: Carbon cloth (7.07 cm2) - Membrane: None MEC (dual chamber): - Anode: Carbon felt - Cathode: Carbon cloth (2.5 × 0.9 cm) - Membrane: Anionic exchange membrane | MFC & MEC Anolyte: 28 mL, peptone (2.5 g/L), yeast extract (1.25 mg/L), NaCl (2.5 g/L), pH 1.80. Pseudomonas sp. E8 biofilm MEC Catholyte: 15 mL, cadmium sulfate with leachate of chalcopyrite Rext: MFC: 1000 Ω, MEC: 10 Ω Temperature: 30 °C | - MFC mode: Cu2+ rapidly reduced from 184.78 to 56.43 mg/L in 10 h, achieving 99.95 ± 0.09% recovery in 48 h - MEC mode: Cd2+ recovery of 99.86 ± 0.04% at 1.20 V applied voltage - Sequential recovery enabled by switching from MFC to MEC mode | [96] |
| Removal of Cu2+ and Zn2+ from industrial wastewater using MDC | System: Batch-operated dual-chamber MDC Anode: Carbon graphite (4 × 1 × 14 cm) Cathode: Carbon graphite Membrane: Polyester-based CEM (Fumasep FTCM-E) and AEM (Fumasep FTAM-E) Chamber Volumes: 2.6 L each | Anolyte: Activated sludge and municipal wastewater Middle Chamber: Synthetic Cu2+ and Zn2+ solution (100 mg/L) at pH 7 Catholyte: 0.1 M phosphate buffer DO in cathode chamber: 4.4 mg/L Retention Time: 120 min Temperature: 26 °C (mesophilic conditions) | - Removal efficiency: Cu2+: 79.7%, Zn2+: 79.6% (synthetic samples) - Voltage: 0.9 V (Cu2+), 0.8 V (Zn2+) - Removal efficiency (real samples): Cu2+: 68.37%, Zn2+: 70.64% - Maximum removal at mesophilic phase, 4.4 mg/L DO, and 120 min retention time | [56] |
| Copper removal and desalination using an MDC-ED integrated system | System: Dual-chamber MDC-ED (electrodialysis) integrated system Anode: Carbon felt Cathode: Carbon felt Membrane: AEM and CEM | Anolyte: Synthetic wastewater with 1 g/L CH3COONa (pH 7) Catholyte: Cu2+ solution (100 mg/L, pH 2) Desalination Chamber: NaCl solution (5 g/L) Rext: 10 Ω/Temperature: 30 °C | - Cu recovery efficiency: 88% - Salt removal efficiency: 47% - Energy-efficient desalination with simultaneous Cu2+ recovery - Current density: 2.1 A/m2 | [55] |
| Simultaneous Cu and desalination in MDC | System: Four-chamber MDC (FMDC) Anode: Carbon felt Cathode: Carbon felt Membrane: AEM and CEM | Anolyte: Synthetic Cu wastewater (100–800 mg/L Cu2+, pH 7) Desalination chamber: 5 g/L NaCl solution Temperature: Room temperature | - 94% Cu recovery - 43% salt removal in desalination - Electricity generation with maximum current density: 2.0 A/m2 | [97] |
| Copper removal using ZIF-8 nanocomposite MDC | System: Dual-chamber MDC Anode: Carbon felt (3 × 3 cm) Cathode: Carbon felt Membrane: ZIF-8 nanocomposite | Anolyte: Acetate solution (1 g/L) (pH 7) Catholyte: Cu(NO3)2 solutions (pH 2) Temperature: Room temperature | - Cu removal efficiency increased to 292 mg/g due to ZIF-8 membrane adsorption capacity - Desalination efficiency of 56% achieved - Significant biofouling prevention due to reduced microbial adhesion on the ZIF-8 surface | [38] |
| Coupling MDC-MEC for Pb recovery | System: MDC-MEC integrated system MDC: Carbon brush anode, carbon cloth cathode, Nafion, and AEM membranes MEC: Carbon brush anode, carbon cloth cathode for Pb reduction | MDC and MEC Anolyte: Synthetic wastewater (acetate, pH 7) MDC Catholyte and middle compartment: Brine solution (NaCl) MEC Catholyte: Pb(NO3)2 solution (pH 3) | - Lead recovery: 96% in 48 h - Energy generated by MDC stored in capacitors to power MEC | [50] |
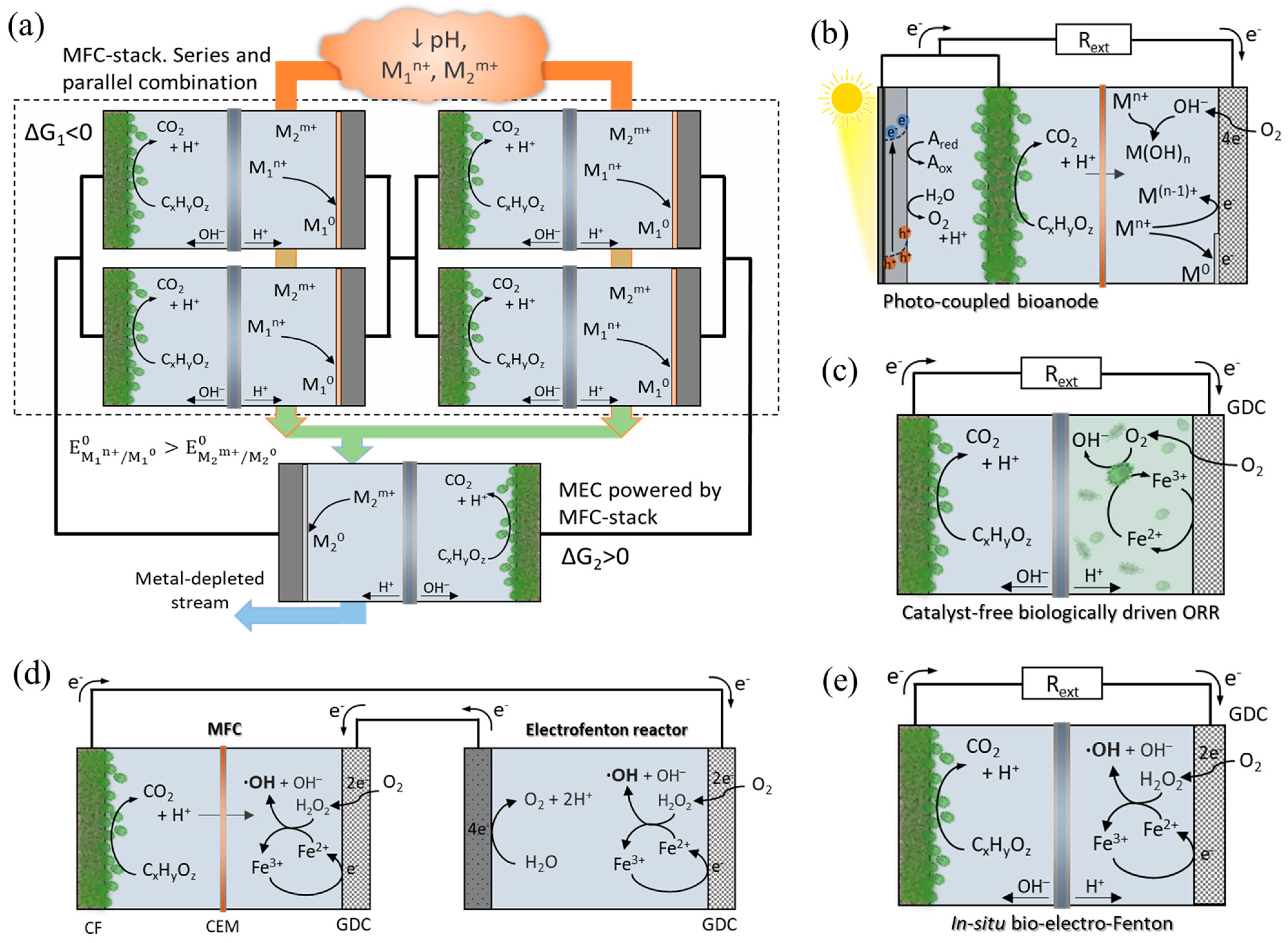
3. BES Coupling with Other Technologies for Metal Recovery
3.1. MFC–MEC Coupling System
3.2. Coupling MFC with Photocatalytic Fuel Cells
3.3. Coupled Redox Fuel Cells
3.4. Catalyst-Free Biologically Driven ORR
3.5. MFC-Fenton Hybrid Systems
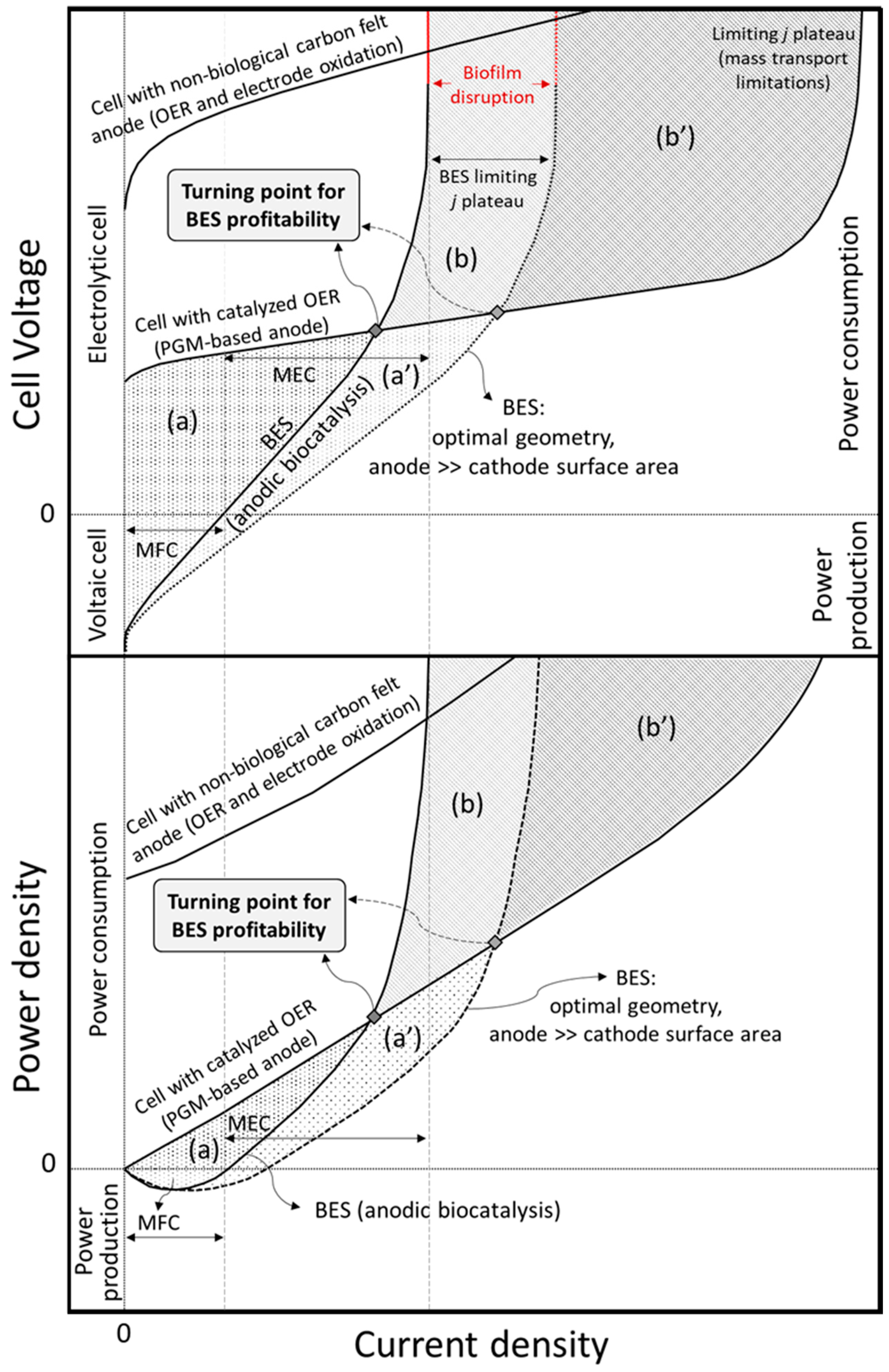
4. Techno-Economic Feasibility of BES for Metal Recovery
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barros, T.V.; Notario, V.A.; de Oliveira, J.A.; Bispo, D.F.; Freitas, L.d.S.; Jegatheesan, V.; Cardozo-Filho, L. Recovery of Lithium and Cobalt from Lithium Cobalt Oxide and Lithium Nickel Manganese Cobalt Oxide Batteries Using Supercritical Water. Environ. Pollut. 2024, 359, 124570. [Google Scholar] [CrossRef]
- Golzar-Ahmadi, M.; Bahaloo-Horeh, N.; Pourhossein, F.; Norouzi, F.; Schoenberger, N.; Hintersatz, C.; Chakankar, M.; Holuszko, M.; Kaksonen, A.H. Pathway to Industrial Application of Heterotrophic Organisms in Critical Metals Recycling from E-Waste. Biotechnol. Adv. 2024, 77, 108438. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Singh, S.; Gacem, A.; Yadav, K.K.; Bhutto, J.K.; Alreshidi, M.A.; Kumar, M.; Kumar, A.; Yadav, V.K.; Soni, S.; et al. A Review on E-Waste Contamination, Toxicity, and Sustainable Clean-up Approaches for Its Management. Toxicology 2024, 508, 153904. [Google Scholar] [CrossRef]
- Vishwakarma, S.; Kumar, V.; Arya, S.; Tembhare, M.; Rahul; Dutta, D.; Kumar, S. E-Waste in Information and Communication Technology Sector: Existing Scenario, Management Schemes and Initiatives. Environ. Technol. Innov. 2022, 27, 102797. [Google Scholar] [CrossRef]
- Jaiswal, M.; Srivastava, S. A Review on Sustainable Approach of Bioleaching of Precious Metals from Electronic Wastes. J. Hazard. Mater. Adv. 2024, 14, 100435. [Google Scholar] [CrossRef]
- Perdigones, B.; Ramírez, P.; Mazuelos, A. Adaptation of an Iron Oxidising Culture to Extremely High Fe Concentration by a Programmed Fed-Batch Bioreactor. Min. Eng. 2024, 206, 108531. [Google Scholar] [CrossRef]
- Mends, E.A.; Arthur, S.E.; Tita, A.M.; Hussaini, S.; Osho, B.; Timilsina, A.; Basyal, S.; Yang, Y.; Chu, P. Leaching Nickel Sulfide Tailings with Activated Carbon in Sulfuric Acid Medium. Sep. Purif. Technol. 2025, 353, 128520. [Google Scholar] [CrossRef]
- Zhong, Y.; Hu, S.; Xu, J.; Chen, Z.; Wang, S. Sustainable Upcycling of Copper from Waste Printed Circuit Boards with the Assistance of Tannic Acid and Fe3+ to a Magnetic Heterogeneous Catalyst. J. Environ. Manag. 2024, 370, 122391. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, W.M.; Jadhao, P.R.; Panda, R.; Pant, K.K.; Dua, V. Towards Circular Economy of Wasted Printed Circuit Boards of Mobile Phones Fuelled by Machine Learning and Robust Mathematical Optimization Framework. Resour. Conserv. Recycl. Adv. 2024, 23, 200226. [Google Scholar] [CrossRef]
- Delgado, Y.; Tapia, N.; López, E.; Llanos, J.; Vargas, I.; Fernández-Morales, F.J. Energy and Copper Recovery from Acid Mine Drainage by Microbial Fuel Cells. Effect of the Hydrochar Doping on Carbon Felt Anodes. Sep. Purif. Technol. 2025, 354, 129095. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Eloffy, M.G.; Priya, A.K.; Hammad, A.; Zahran, M.; Maged, A.; Elwakeel, K.Z. Revitalizing the Circular Economy: An Exploration of e-Waste Recycling Approaches in a Technological Epoch. Sustain. Chem. Environ. 2024, 7, 100124. [Google Scholar] [CrossRef]
- Qin, S.; Dou, S.; Ma, S.; Zhang, Z.; Hu, Y.; Li, Y.; Liu, P.; Lin, F.; Zhao, H. Enhanced Recovery of Low-Grade Copper Ore and Associated Precious Metals from Iron Tailings: A Case Study in China. Colloids Surf. A Physicochem. Eng. Asp. 2024, 699, 134656. [Google Scholar] [CrossRef]
- Firmansyah, M.L.; Ullah, N. Ionic Liquid-Containing Polymer Including Membrane for the Removal of Cobalt: Synthesis, Adsorption, Transport, and Modeling Studies. Sep. Purif. Technol. 2025, 353, 128332. [Google Scholar] [CrossRef]
- Jovanović, G.; Bugarčić, M.; Petronijević, N.; Stopic, S.R.; Friedrich, B.; Marković, B.; Stanković, S.; Sokić, M. A Multifocal Study Investigation of Pyrolyzed Printed Circuit Board Leaching. Metals 2022, 12, 2021. [Google Scholar] [CrossRef]
- Lee, H.; Coulon, F.; Wagland, S.T. Influence of PH, Depth and Humic Acid on Metal and Metalloids Recovery from Municipal Solid Waste Landfills. Sci. Total Environ. 2022, 806, 150332. [Google Scholar] [CrossRef]
- Ravi Raman, P.; Shanmugam, R.R.; Swaminathan, S. Review on the Role of Density-Based Separation in PCBs Recycling. Chem. Eng. J. 2024, 496, 154339. [Google Scholar] [CrossRef]
- Kwon, G.; Yoon, K.; Kwon, E.; Park, J.; Lee, H.; Song, H. Technical Advancement in Valorization of Electronic Waste and Its Contribution to Establishing Economic Value-Chain. Chem. Eng. J. 2024, 494, 153154. [Google Scholar] [CrossRef]
- Potysz, A.; Mikoda, B.; Napieraj, M. (Bio)Dissolution of Glassy and Diopside-Bearing Metallurgical Slags: Experimental and Economic Aspects. Minerals 2021, 11, 262. [Google Scholar] [CrossRef]
- Diallo, S.; Tran, L.H.; Larivière, D.; Blais, J.F. Mass Balance and Economic Study of a Treatment Chain for Rare Earths, Base Metals and Precious Metals Recovery from Used Smartphones. Min. Eng. 2024, 215, 108824. [Google Scholar] [CrossRef]
- Van Yken, J.; Boxall, N.J.; Cheng, K.Y.; Nikoloski, A.N.; Moheimani, N.R.; Kaksonen, A.H. Base Metals Recovery from Waste Printed Circuit Board Leachate Using Biogenic Hydrogen Sulfide Gas. Hydrometallurgy 2024, 228, 106341. [Google Scholar] [CrossRef]
- He, D.; Jiang, F.; Fu, X.; Liu, R.; Han, H.; Sun, W.; Niu, Z.; Yue, T. Recycling of Hazardous Jarosite Residues Based on Hydrothermal Crystal Transformation. Waste Manag. 2023, 172, 290–298. [Google Scholar] [CrossRef]
- Medina-Díaz, H.L.; Acosta, I.; Muñoz, M.; López Bellido, F.J.; Villaseñor, J.; Llanos, J.; Rodríguez, L.; Fernández-Morales, F.J. A Classical Modelling of Abandoned Mine Tailings’ Bioleaching by an Autochthonous Microbial Culture. J. Environ. Manag. 2022, 323, 116251. [Google Scholar] [CrossRef]
- Ebin, B.; Isik, M.I. Pyrometallurgical Processes for the Recovery of Metals from WEEE. In WEEE Recycling: Research, Development, and Policies; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Zhou, J.; Lv, J.; Li, Z.; Zheng, Y. Self-Sulfidation Roasting and Flotation of Electroplating Sludge: An Innovative Technology for Metal Recovery. Sep. Purif. Technol. 2025, 353, 128583. [Google Scholar] [CrossRef]
- Muscetta, M. Process Intensification in Metal Recovery from Solid Waste: Challenges, Opportunities and Recent Advances. Chem. Eng. Process.-Process Intensif. 2024, 204, 109937. [Google Scholar] [CrossRef]
- Pang, C.; Wu, Y.; Zhu, J.; Qin, B.; Ruan, J. The Study of Adsorption Mechanisms and Kinetics of Recovering Ag and Al on the Bioleaching of End-of-Life Crystalline Silicon Photovoltaic Cells. Sep. Purif. Technol. 2025, 354, 128999. [Google Scholar] [CrossRef]
- Mokarian, P.; Bakhshayeshi, I.; Taghikhah, F.; Boroumand, Y.; Erfani, E.; Razmjou, A. The Advanced Design of Bioleaching Process for Metal Recovery: A Machine Learning Approach. Sep. Purif. Technol. 2022, 291, 120919. [Google Scholar] [CrossRef]
- Hemdan, B.; Garlapati, V.K.; Sharma, S.; Bhadra, S.; Maddirala, S.; Varsha, K.M.; Motru, V.; Goswami, P.; Sevda, S.; Aminabhavi, T.M. Bioelectrochemical Systems-Based Metal Recovery: Resource, Conservation and Recycling of Metallic Industrial Effluents. Environ. Res. 2022, 204, 112346. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Jang, J.H.; Dempsey, B.A.; Logan, B.E. Efficient Recovery of Nano-Sized Iron Oxide Particles from Synthetic Acid-Mine Drainage (AMD) Water Using Fuel Cell Technologies. Water Res. 2011, 45, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Heijne, A.T.; Liu, F.; Van Der Weijden, R.; Weijma, J.; Buisman, C.J.N.; Hamelers, H.V.M. Copper Recovery Combined with Electricity Production in a Microbial Fuel Cell. Environ. Sci. Technol. 2010, 44, 4376–4381. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Lei, L. Electricity Production during the Treatment of Real Electroplating Wastewater Containing Cr6+ Using Microbial Fuel Cell. Process Biochem. 2008, 43, 1352–1358. [Google Scholar] [CrossRef]
- Huang, L.; Chen, J.; Quan, X.; Yang, F. Enhancement of Hexavalent Chromium Reduction and Electricity Production from a Biocathode Microbial Fuel Cell. Bioprocess. Biosyst. Eng. 2010, 33, 937–945. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Ji, Z.; Zhao, J.; Sun, H.M.; Ye, J.; Cheng, Z.; Kong, X.; Chen, J.; Chen, D. Configurations of Bioelectrochemical Reactor for Environmental Remediation: A Review. Chem. Eng. J. 2023, 471, 144325. [Google Scholar] [CrossRef]
- Boon, M.; Heijnen, J.J. Chemical Oxidation Kinetics of Pyrite in Bioleaching Processes. Hydrometallurgy 1998, 48, 27–41. [Google Scholar] [CrossRef]
- Stenina, I.; Golubenko, D.; Nikonenko, V.; Yaroslavtsev, A. Selectivity of Transport Processes in Ion-Exchange Membranes: Relationship with the Structure and Methods for Its Improvement. Int. J. Mol. Sci. 2020, 21, 5517. [Google Scholar] [CrossRef] [PubMed]
- Modin, O.; Fuad, N.; Rauch, S. Microbial Electrochemical Recovery of Zinc. Electrochim. Acta 2017, 248, 58–63. [Google Scholar] [CrossRef]
- Delgado, Y.; Fernández-Morales, F.J.; Llanos, J. An Old Technique with a Promising Future: Recent Advances in the Use of Electrodeposition for Metal Recovery. Molecules 2021, 26, 5525. [Google Scholar] [CrossRef] [PubMed]
- Miwornunyuie, N.; Jingyu, H.; Chen, L.; Ke, L.; Koomson, D.A.; Ewusi-Mensah, D.; Opoku, P.A. Application of ZIF-8 Nanocomposite Membrane in Microbial Desalination Cells for Simultaneous Heavy Metal Removal and Biofouling Prevention. Chemosphere 2022, 306, 135386. [Google Scholar] [CrossRef]
- Tributsch, H. Direct versus Indirect Bioleaching. Hydrometallurgy 2001, 59, 177–185. [Google Scholar] [CrossRef]
- Wang, S.; Adekunle, A.; Raghavan, V. Bioelectrochemical Systems-Based Metal Removal and Recovery from Wastewater and Polluted Soil: Key Factors, Development, and Perspective. J. Environ. Manag. 2022, 317, 115333. [Google Scholar] [CrossRef]
- Hubenova, Y.; Hubenova, E.; Chorbadzhiyska, E.; Sbirkova-Dimitrova, H.; Tsvetanova, L.; Slavcheva, E. Simultaneous Gold and Silver Recovery in Microbial Fuel Cells Operating in a Short-Circuited Mode. J. Power Sources 2025, 626, 235775. [Google Scholar] [CrossRef]
- Chhachhiya, N.; Tiwari, A.; Sharma, R.S.; Rai, P.K.; Anand, S.; Mishra, V. Transformative Potential of Optimized Microbial Fuel Cell Designs and Materials for Eco-Friendly Management of Hazardous Chemical Waste. J. Water Process Eng. 2025, 69, 106647. [Google Scholar] [CrossRef]
- Asefaw, B.K.; Chen, H.; Tang, Y. Removal of Selenate from Wastewater Using a Bioelectrochemical Reactor: The Importance of Measuring Selenide and the Role of Competing Anions. Biochem. Eng. J. 2024, 212, 109531. [Google Scholar] [CrossRef]
- Greenman, J.; Gajda, I.; You, J.; Mendis, B.A.; Obata, O.; Pasternak, G.; Ieropoulos, I. Microbial Fuel Cells and Their Electrified Biofilms. Biofilm 2021, 3, 100057. [Google Scholar] [CrossRef]
- Dessie, Y.; Tadesse, S. Advancements in Bioelectricity Generation Through Nanomaterial-Modified Anode Electrodes in Microbial Fuel Cells. Front. Nanotechnol. 2022, 4, 876014. [Google Scholar] [CrossRef]
- Wu, L.; Garg, S.; Waite, D. Progress and challenges in the use of electrochemical oxidation and reduction processes for heavy metals removal and recovery from wastewaters. J. Hazard. Mater. 2024, 479, 135581. [Google Scholar] [CrossRef]
- Anaya-Garzon, J.; Mosquera-Romero, S.; Leon-Fernandez, L.F.; Garcia-Timermans, C.; Van Dorpe, J.; Hoorens, A.; Thivel, P.-X.; Merlin, G.; Commenges-Bernole, N.; Rabaey, K.; et al. Chromium Nano-Mining with Electrified Bioreactors. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Mosquera-Romero, S.; Anaya-Garzon, J.; Garcia-Timermans, C.; Van Dorpe, J.; Hoorens, A.; Commenges-Bernole, N.; Verbeken, K.; Rabaey, K.; Varia, J. Combined Gold Recovery and Nanoparticle Synthesis in Microbial Systems Using Fractional Factorial Design. Nanomaterials 2023, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, S.G.F.; Prato, R.; Dominguez-Benetton, X.; Fransaer, J. Oxidation-Assisted Alkaline Precipitation of Nanoparticles Using Gas-Diffusion Electrodes. React. Chem. Eng. 2021, 6, 1031–1041. [Google Scholar] [CrossRef]
- Li, Y.; Styczynski, J.; Huang, Y.; Xu, Z.; McCutcheon, J.; Li, B. Energy-Positive Wastewater Treatment and Desalination in an Integrated Microbial Desalination Cell (MDC)-Microbial Electrolysis Cell (MEC). J. Power Sources 2017, 356, 529–538. [Google Scholar] [CrossRef]
- Dessì, P.; Rovira-Alsina, L.; Sánchez, C.; Dinesh, G.K.; Tong, W.; Chatterjee, P.; Tedesco, M.; Farràs, P.; Hamelers, H.M.V.; Puig, S. Microbial Electrosynthesis: Towards Sustainable Biorefineries for Production of Green Chemicals from CO2 Emissions. Biotechnol. Adv. 2021, 46, 107675. [Google Scholar] [CrossRef]
- Bian, B.; Bajracharya, S.; Xu, J.; Pant, D.; Saikaly, P.E. Microbial Electrosynthesis from CO2: Challenges, Opportunities and Perspectives in the Context of Circular Bioeconomy. Bioresour. Technol. 2020, 302, 122863. [Google Scholar] [CrossRef]
- Modestra, J.A.; Babu, M.L.; Mohan, S.V. Electro-Fermentation of Real-Field Acidogenic Spent Wash Effluents for Additional Biohydrogen Production with Simultaneous Treatment in a Microbial Electrolysis Cell. Sep. Purif. Technol. 2015, 150, 308–315. [Google Scholar] [CrossRef]
- Nagendranatha Reddy, C.; Kondaveeti, S.; Mohanakrishna, G.; Min, B. Application of Bioelectrochemical Systems to Regulate and Accelerate the Anaerobic Digestion Processes. Chemosphere 2022, 287, 132299. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, J.; Sui, M.; Qu, Y.; Ambuchi, J.J.; Wang, H.; Feng, Y. A Combined Microbial Desalination Cell and Electrodialysis System for Copper-Containing Wastewater Treatment and High-Salinity-Water Desalination. J. Hazard. Mater. 2017, 321, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Malakootian, M.; Mirzaienia, F.; Malakootian, M. Removal Efficiency of Cu2+ and Zn2+ from Industrial Wastewater by Using Microbial Desalination Cell. J. Water Chem. Technol. 2019, 41, 334–339. [Google Scholar] [CrossRef]
- Liu, S.Y.; Charles, W.; Ho, G.; Cord-Ruwisch, R.; Cheng, K.Y. Bioelectrochemical Enhancement of Anaerobic Digestion: Comparing Single- and Two-Chamber Reactor Configurations at Thermophilic Conditions. Bioresour. Technol. 2017, 245, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Bhaduri, S.; Behera, M. From Single-Chamber to Multi-Anodic Microbial Fuel Cells: A Review. J. Environ. Manag. 2024, 355, 120465. [Google Scholar] [CrossRef] [PubMed]
- Christwardana, M.; Yoshi, L.A. Performance and Techno-Economic Analysis of Scaling-up a Single-Chamber Yeast Microbial Fuel Cell as Dissolved Oxygen Biosensor. Int. J. Renew. Energy Dev. 2020, 9, 449–454. [Google Scholar] [CrossRef]
- Al Lawati, M.J.; Jafary, T.; Baawain, M.S.; Al-Mamun, A. A Mini Review on Biofouling on Air Cathode of Single Chamber Microbial Fuel Cell; Prevention and Mitigation Strategies. Biocatal. Agric. Biotechnol. 2019, 22, 101370. [Google Scholar] [CrossRef]
- Boas, J.V.; Oliveira, V.B.; Simões, M.; Pinto, A.M.F.R. Review on Microbial Fuel Cells Applications, Developments and Costs. J. Environ. Manag. 2022, 307, 114525. [Google Scholar] [CrossRef]
- Potrykus, S.; Nieznański, J.; Kutt, F.; Fernandez-Morales, F.J. Modeling the Effect of External Load Variations on Single, Serie and Parallel Connected Microbial Fuel Cells. Bioresour. Technol. 2025, 416, 131761. [Google Scholar] [CrossRef] [PubMed]
- Mateo, S.; Cantone, A.; Cañizares, P.; Fernández-Morales, F.J.; Scialdone, O.; Rodrigo, M.A. On the Staking of Miniaturized Air-Breathing Microbial Fuel Cells. Appl. Energy 2018, 232, 1–8. [Google Scholar] [CrossRef]
- Zadeh, P.G.; Rezania, S.; Fattahi, M.; Dang, P.; Vasseghian, Y.; Aminabhavi, T.M. Recent Advances in Microbial Fuel Cell Technology for Energy Generation from Wastewater Sources. Process Saf. Environ. Prot. 2024, 189, 425–439. [Google Scholar] [CrossRef]
- Cabrera, J.; Dai, Y.; Irfan, M.; Li, Y.; Gallo, F.; Zhang, P.; Zong, Y.; Liu, X. Novel Continuous Up-Flow MFC for Treatment of Produced Water: Flow Rate Effect, Microbial Community, and Flow Simulation. Chemosphere 2022, 289, 133186. [Google Scholar] [CrossRef]
- Bolognesi, S.; Cecconet, D.; Callegari, A.; Puig, S.; Capodaglio, A.G. Tubular Photo-MFC Reactors as Wastewater Polishing Treatment Step with Simultaneous Electricity Production. Bioresour. Technol. Rep. 2022, 18, 101059. [Google Scholar] [CrossRef]
- Prashanthi, R. A Review on Microbial Fuel Cell and Green Energy. Ionics 2023, 29, 1667–1697. [Google Scholar] [CrossRef]
- Das, S.; Kumar, S.; Kumar Mehta, A.; Ghangrekar, M.M. Heavy Metals Removal by Algae and Usage of Activated Metal-Enriched Biomass as Cathode Catalyst for Improving Performance of Photosynthetic Microbial Fuel Cell. Bioresour. Technol. 2024, 406, 131038. [Google Scholar] [CrossRef]
- Sobhani, D.; Rastegar, S.O.; Khamforoush, M.; Gu, T.; Khosravi, A. Copper Recovery from Printed Circuit Boards Leaching Solution with Bioelectricity Generation Using Microbial Fuel Cell. Bioprocess. Biosyst. Eng. 2023, 46, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Fathima, A.; Ilankoon, I.M.S.K.; Chong, M.N. Improving Scalability of Copper Recovery in Saline Microbial Fuel Cells with Microtubular Polypyrrole-Based Cathodic Electrocatalysts. Chemosphere 2024, 363, 142800. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Yan, M.; Li, Q.; Zheng, S.; Hu, Y.; Xu, X.; Wang, L.; Liu, Y.; Huang, M. Bioelectrocatalytic Reduction by Integrating Pyrite Assisted Manganese Cobalt-Doped Carbon Nanofiber Anode and Bacteria for Sustainable Antimony Catalytic Removal. Bioresour. Technol. 2024, 395, 130378. [Google Scholar] [CrossRef]
- Matsena, M.T.; Kholisa, B.; Chirwa, E.M.N. Key Operational Parameters Assessment for a Chromium (VI)-Reducing Annular Upflow Microbial Fuel Cell. J. Power Sources 2024, 590, 233794. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Y.; Liu, Q.; You, S. Optimization of a constructed wetland-microbial fuel cell system for Cr(VI) removal from wastewater and power generation performance. Environ. Prot. Eng. 2024, 50, 41–60. [Google Scholar] [CrossRef]
- Muazu, R.I.; Sadhukhan, J.; Venkata Mohan, S.; Gadkari, S. Hexavalent Chromium Waste Removal via Bioelectrochemical Systems—A Life Cycle Assessment Perspective. Environ. Sci. 2023, 9, 2487–2500. [Google Scholar] [CrossRef]
- Kanellos, G.; Tremouli, A.; Tsakiridis, P.; Remoundaki, E.; Lyberatos, G. Silver Recovery from End-of-Life Photovoltaic Panels Based on Microbial Fuel Cell Technology. Waste Biomass Valorization 2024, 15, 75–86. [Google Scholar] [CrossRef]
- Almatouq, A.; Webster, G.; Babatunde, A. Silver Removal and Microbial Community Structure in Microbial Fuel Cells. J. Chem. Technol. Biotechnol. 2022, 97, 3441–3452. [Google Scholar] [CrossRef]
- Kamperidis, T.; Tremouli, A.; Remoundaki, E.; Lyberatos, G. Silver Recovery from Wastewater, Simulating the Chemical Extract Originating from a PV Panel Using Microbial Fuel Cell Technology. Waste Biomass Valorization 2022, 13, 4073–4083. [Google Scholar] [CrossRef]
- Hubenova, Y.; Chorbadzhiyska, E.; Kostov, K.L.; Mitov, M. Efficient Gold Recovery by Microbial Electrochemical Technologies. Bioelectrochemistry 2023, 149, 108311. [Google Scholar] [CrossRef] [PubMed]
- Amanze, C.; Anaman, R.; Wu, X.; Alhassan, S.I.; Yang, K.; Fosua, B.A.; Yunhui, T.; Yu, R.; Wu, X.; Shen, L.; et al. Heterotrophic Anodic Denitrification Coupled with Cathodic Metals Recovery from On-Site Smelting Wastewater with a Bioelectrochemical System Inoculated with Mixed Castellaniella Species. Water Res. 2023, 231, 119655. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Y.; Mi, Y.; Wang, D.; Wang, Z.; Meng, H.; Jiang, C.; Dong, W.; Li, J.; Li, H. Cu(II) and Cr(VI) Removal in Tandem with Electricity Generation via Dual-Chamber Microbial Fuel Cells. Sustainability 2023, 15, 2388. [Google Scholar] [CrossRef]
- Delgado, Y.; Llanos, J.; Fernández-Morales, F.J. Coupling of Electrodialysis and Bio-Electrochemical Systems for Metal and Energy Recovery from Acid Mine Drainage. J. Chem. Technol. Biotechnol. 2023, 99, 2178–2185. [Google Scholar] [CrossRef]
- Cai, W.; Wang, Y. Investigation of a Two-Dimensional Model on Cu2+ Recovery in Bioemectrochemical System. IOP Conf. Ser. Earth Environ. Sci. 2023, 1135, 012013. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, X.; Lu, P.; Zhang, D. Copper Removal and Elemental Sulfur Recovery from Fracturing Flowback Water in a Microbial Fuel Cell with an Extra Electrochemical Anode. Chemosphere 2022, 303, 135128. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Kong, W.; Sun, S.; Wan, H.; Shi, Y.; Zhou, P. Photo-Assisted Self-Driven Bioelectrochemical Systems for Simultaneous Enhanced Treatment of Etching Terminal Wastewater and Selective Recovery of Heavy Metals. J. Power Sources 2023, 558, 232623. [Google Scholar] [CrossRef]
- Spiess, S.; Kucera, J.; Vaculovic, T.; Birklbauer, L.; Habermaier, C.; Conde, A.S.; Mandl, M.; Haberbauer, M. Zinc Recovery from Bioleachate Using a Microbial Electrolysis Cell and Comparison with Selective Precipitation. Front. Microbiol. 2023, 14, 1238853. [Google Scholar] [CrossRef] [PubMed]
- Amanze, C.; Zheng, X.; Man, M.; Yu, Z.; Ai, C.; Wu, X.; Xiao, S.; Xia, M.; Yu, R.; Wu, X.; et al. Recovery of Heavy Metals from Industrial Wastewater Using Bioelectrochemical System Inoculated with Novel Castellaniella Species. Environ. Res. 2022, 205, 112467. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Kong, W.; Huang, L.; Wang, Q.; Zhang, G.; Zhou, P. Synergistic Light Irradiation and Circuital Current for Efficient Mineralization of Recalcitrant Organics and Sequential Recovery of Heavy Metals from Etching Terminal Wastewater Using Photo-Assisted Bioelectrochemical Systems. J. Power Sources 2022, 522, 230991. [Google Scholar] [CrossRef]
- Aliaguilla, M.; Molognoni, D.; Bosch-Jimenez, P.; Borràs, E. Versatile Bioelectrochemical System for Heavy Metals Removal. E3S Web Conf. 2022, 334, 08006. [Google Scholar] [CrossRef]
- Singh, A.; Kaushik, A. Removal of Cd and Ni with Enhanced Energy Generation Using Biocathode Microbial Fuel Cell: Insights from Molecular Characterization of Biofilm Communities. J. Clean. Prod. 2021, 315, 127940. [Google Scholar] [CrossRef]
- Lim, S.S.; Fontmorin, J.M.; Pham, H.T.; Milner, E.; Abdul, P.M.; Scott, K.; Head, I.; Yu, E.H. Zinc Removal and Recovery from Industrial Wastewater with a Microbial Fuel Cell: Experimental Investigation and Theoretical Prediction. Sci. Total Environ. 2021, 776, 145934. [Google Scholar] [CrossRef] [PubMed]
- Leon-Fernandez, L.F.; Medina-Díaz, H.L.; Pérez, O.G.; Romero, L.R.; Villaseñor, J.; Fernández-Morales, F.J. Acid Mine Drainage Treatment and Sequential Metal Recovery by Means of Bioelectrochemical Technology. J. Chem. Technol. Biotechnol. 2021, 96, 1543–1552. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, L.; Zhu, Y.; Li, X.; Ren, Y. A Triple-Chamber Microbial Fuel Cell Enabled to Synchronously Recover Iron and Sulfur Elements from Sulfide Tailings. J. Hazard. Mater. 2021, 401, 123307. [Google Scholar] [CrossRef] [PubMed]
- León-Fernandez, L.F.; Rodríguez Romero, L.; Fernández-Morales, F.J.; Villaseñor Camacho, J. Modelling of a Bioelectrochemical System for Metal-Polluted Wastewater Treatment and Sequential Metal Recovery. J. Chem. Technol. Biotechnol. 2021, 96, 2033–2041. [Google Scholar] [CrossRef]
- Sravan, J.S.; Nancharaiah, Y.V.; Lens, P.N.L.; Mohan, S.V. Cathodic Selenium Recovery in Bioelectrochemical System: Regulatory Influence on Anodic Electrogenic Activity. J. Hazard. Mater. 2020, 399, 122843. [Google Scholar] [CrossRef] [PubMed]
- Aiyer, K.S. Recovery of Chromium, Copper and Vanadium Combined with Electricity Generation in Two-Chambered Microbial Fuel Cells. FEMS Microbiol. Lett. 2020, 367, fnaa129. [Google Scholar] [CrossRef] [PubMed]
- Ai, C.; Hou, S.; Yan, Z.; Zheng, X.; Amanze, C.; Chai, L.; Qiu, G.; Zeng, W. Recovery of Metals from Acid Mine Drainage by Bioelectrochemical System Inoculated with a Novel Exoelectrogen, Pseudomonas Sp. E8. Microorganisms 2020, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Zhang, H.; Wen, Q.; Chen, Z.; Du, M. Desalination Combined with Copper(II) Removal in a Novel Microbial Desalination Cell. Desalination 2014, 346, 115–121. [Google Scholar] [CrossRef]
- Siddiqui, S.; Bhatnagar, P.; Dhingra, S.; Upadhyay, U.; Sreedhar, I. Wastewater Treatment and Energy Production by Microbial Fuel Cells. Biomass-Convers. Biorefinery 2021, 13, 3569–3592. [Google Scholar] [CrossRef]
- Kwofie, M.; Amanful, B.; Gamor, S.; Kaku, F. Comprehensive Analysis of Clean Energy Generation Mechanisms in Microbial Fuel Cells. Int. J. Energy Res. 2024, 2024, 5866657. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, L.; Wu, D.; Huang, L.; Zhou, P.; Quan, X.; Chen, G. Dependency of Simultaneous Cr(VI), Cu(II) and Cd(II) Reduction on the Cathodes of Microbial Electrolysis Cells Self-Driven by Microbial Fuel Cells. J. Power Sources 2015, 273, 1103–1113. [Google Scholar] [CrossRef]
- Ezziat, L.; Elabed, A.; Ibnsouda, S.; El Abed, S. Challenges of Microbial Fuel Cell Architecture on Heavy Metal Recovery and Removal from Wastewater. Front. Energy Res. 2019, 7, 1. [Google Scholar] [CrossRef]
- Queiroz, B.D.; Fernandes, J.A.; Martins, C.A.; Wender, H. Photocatalytic Fuel Cells: From Batch to Microfluidics. J. Environ. Chem. Eng. 2022, 10, 107611. [Google Scholar] [CrossRef]
- Cao, T.N.D.; Wang, T.H.; Peng, Y.; Hsu, H.Y.; Mukhtar, H.; Yu, C.P. Photo-Assisted Microbial Fuel Cell Systems: Critical Review of Scientific Rationale and Recent Advances in System Development. Crit. Rev. Biotechnol. 2024, 44, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Lee, K.S.; Razzaq, A.; Lee, S.H.; Grimes, C.A.; In, S. Il Photocoupled Bioanode: A New Approach for Improved Microbial Fuel Cell Performance. Energy Technol. 2018, 6, 257–262. [Google Scholar] [CrossRef]
- Vinayak, V.; Khan, M.J.; Varjani, S.; Saratale, G.D.; Saratale, R.G.; Bhatia, S.K. Microbial Fuel Cells for Remediation of Environmental Pollutants and Value Addition: Special Focus on Coupling Diatom Microbial Fuel Cells with Photocatalytic and Photoelectric Fuel Cells. J. Biotechnol. 2021, 338, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Sarma, U.; Hoque, M.E.; Thekkangil, A.; Venkatarayappa, N.; Rajagopal, S. Microalgae in removing heavy metals from wastewater—An advanced green technology for urban wastewater treatment. J. Hazard. Mater. Adv. 2024, 15, 100444. [Google Scholar] [CrossRef]
- Mateo, S.; Cañizares, P.; Fernandez-Morales, F.J.; Rodrigo, M.A. A Critical View of Microbial Fuel Cells: What Is the Next Stage? ChemSusChem. 2018, 11, 4183–4192. [Google Scholar] [CrossRef] [PubMed]
- Andrade, T.S.; Dracopoulos, V.; Keramidas, A.; Pereira, M.C.; Lianos, P. Charging a Vanadium Redox Battery with a Photo(Catalytic) Fuel Cell. Sol. Energy Mater. Sol. Cells 2021, 221, 110889. [Google Scholar] [CrossRef]
- Yang, X.; Fan, Z.; Zhang, H.; Xu, W.; Wu, Z. Energy Harvest from Contaminants via Coupled Redox Fuel Cells. Energy Procedia 2017, 105, 1852–1857. [Google Scholar] [CrossRef]
- Wasmus, S.; Küver, A. Methanol Oxidation and Direct Methanol Fuel Cells: A Selective Review. J. Electroanal. Chem. 1999, 461, 14–31. [Google Scholar] [CrossRef]
- Sund, C.J.; McMasters, S.; Crittenden, S.R.; Harrell, L.E.; Sumner, J.J. Effect of Electron Mediators on Current Generation and Fermentation in a Microbial Fuel Cell. Appl. Microbiol. Biotechnol. 2007, 76, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.Y.; Karthikeyan, R.; Wong, J.W.C. Microbial Electrochemical Remediation of Organic Contaminants: Possibilities and Perspective. In Biomass, Biofuels, Biochemicals: Microbial Electrochemical Technology: Sustainable Platform for Fuels, Chemicals and Remediation; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Fathey, R.; Gomaa, O.M.; Ali, A.E.H.; El Kareem, H.A.; Zaid, M.A. Neutral Red as a Mediator for the Enhancement of Electricity Production Using a Domestic Wastewater Double Chamber Microbial Fuel Cell. Ann. Microbiol. 2016, 66, 695–702. [Google Scholar] [CrossRef]
- Montoya-Vallejo, C.; Gil Posada, J.O.; Quintero-Díaz, J.C. Effect of Glucose and Methylene Blue in Microbial Fuel Cells Using E. coli. Energies 2023, 16, 7901. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, D.; Xiao, Z. Application of Redox Mediators in Bioelectrochemical System. In Bioelectrochemistry Stimulated Environmental Remediation: From Bioelectrorespiration to Bioelectrodegradation; Springer Nature: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Cai, W.; Ma, Q.; Pu, K.; Jing, D.; Wang, Y. Incorporation of Silver Recovery with Electricity Generation through Methanol-Ag+ Coupled Redox Fuel Cell. Chem. Eng. Process.-Process Intensif. 2023, 183, 109222. [Google Scholar] [CrossRef]
- Izadi, P.; Fontmorin, J.-M.; Fernández, L.F.L.; Cheng, S.; Head, I.; Yu, E.H. High Performing Gas Diffusion Biocathode for Microbial Fuel Cells Using Acidophilic Iron Oxidizing Bacteria. Front. Energy Res. 2019, 7, 479252. [Google Scholar] [CrossRef]
- Sui, S.; Wang, X.; Zhou, X.; Su, Y.; Riffat, S.; Liu, C.J. A Comprehensive Review of Pt Electrocatalysts for the Oxygen Reduction Reaction: Nanostructure, Activity, Mechanism and Carbon Support in PEM Fuel Cells. J. Mater. Chem. A Mater. 2017, 5, 1808–1825. [Google Scholar] [CrossRef]
- Yang, X.; Zou, R.; Tang, K.; Andersen, H.R.; Angelidaki, I.; Zhang, Y. Degradation of Metoprolol from Wastewater in a Bio-Electro-Fenton System. Sci. Total Environ. 2021, 771, 145385. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, S.; Angelidaki, I.; Zhang, Y. Bio-Electro-Fenton Processes for Wastewater Treatment: Advances and Prospects. Chem. Eng. J. 2018, 354, 492–506. [Google Scholar] [CrossRef]
- Nordin, N.; Ho, L.N.; Ong, S.A.; Ibrahim, A.H.; Wong, Y.S.; Lee, S.L.; Oon, Y.S.; Oon, Y.L. Hybrid System of Photocatalytic Fuel Cell and Fenton Process for Electricity Generation and Degradation of Reactive Black 5. Sep. Purif. Technol. 2017, 177, 135–141. [Google Scholar] [CrossRef]
- Mahiroglu, A.; Tarlan-Yel, E.; Sevimli, M.F. Treatment of Combined Acid Mine Drainage (AMD)-Flotation Circuit Effluents from Copper Mine via Fenton’s Process. J. Hazard. Mater. 2009, 166, 782–787. [Google Scholar] [CrossRef]
- Wibowo, Y.G.; Imron, M.F.; Kurniawan, S.B.; Ramadan, B.S.; Taher, T.; Sudibya, A.H.; Syarifuddin, H.; Khairurrijal, K.; Jarwinda. Emerging Strategies for Mitigating Acid Mine Drainage Formation and Environmental Impacts: A Comprehensive Review of Recent Advances. Sci. Technol. Indones. 2023, 8, 516–541. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, S.; Shimko, J.; Dengler, R.W. Mine Drainage: Treatment Technologies and Rare Earth Elements. Water Environ. Res. 2019, 91, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Leon-Fernandez, L.F.; Dominguez-Benetton, X.; Villaseñor Camacho, J.; Fernandez-Morales, F.J. Coupling the Electrocatalytic Dechlorination of 2,4-D with Electroactive Microbial Anodes. Environ. Microbiol. Rep. 2023, 15, 512–529. [Google Scholar] [CrossRef]
- Aslam, T.; Masindi, V.; Ahmad, A.A.; Chatzisymeon, E. Valorization of Acid Mine Drainage into an Iron Catalyst to Initiate the Solar Photo-Fenton Treatment of Municipal Wastewater. Environments 2023, 10, 132. [Google Scholar] [CrossRef]
- Prato, R.A.; Van Vught, V.; Eggermont, S.; Pozo, G.; Marin, P.; Fransaer, J.; Dominguez-Benetton, X. Gas Diffusion Electrodes on the Electrosynthesis of Controllable Iron Oxide Nanoparticles. Sci. Rep. 2019, 9, 15370. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pines Pozo, M.T.; Lopez Fernandez, E.; Villaseñor, J.; Leon-Fernandez, L.F.; Fernandez-Morales, F.J. Metal Recovery from Wastes: A Review of Recent Advances in the Use of Bioelectrochemical Systems. Appl. Sci. 2025, 15, 1456. https://doi.org/10.3390/app15031456
Pines Pozo MT, Lopez Fernandez E, Villaseñor J, Leon-Fernandez LF, Fernandez-Morales FJ. Metal Recovery from Wastes: A Review of Recent Advances in the Use of Bioelectrochemical Systems. Applied Sciences. 2025; 15(3):1456. https://doi.org/10.3390/app15031456
Chicago/Turabian StylePines Pozo, María Teresa, Ester Lopez Fernandez, José Villaseñor, Luis F. Leon-Fernandez, and Francisco Jesus Fernandez-Morales. 2025. "Metal Recovery from Wastes: A Review of Recent Advances in the Use of Bioelectrochemical Systems" Applied Sciences 15, no. 3: 1456. https://doi.org/10.3390/app15031456
APA StylePines Pozo, M. T., Lopez Fernandez, E., Villaseñor, J., Leon-Fernandez, L. F., & Fernandez-Morales, F. J. (2025). Metal Recovery from Wastes: A Review of Recent Advances in the Use of Bioelectrochemical Systems. Applied Sciences, 15(3), 1456. https://doi.org/10.3390/app15031456







