Abstract
A rapid ultra-high-performance liquid chromatography method with diode-array (DAD) and tandem mass spectrometric (MS/MS) detection was developed and, for the first time, applied for the determination of potassium iodide in ophthalmic formulations. The approach is based on an indirect derivatization reaction in which iodide is oxidized by hydrogen peroxide to electrophilic iodine species that react with 4-hydroxybenzoic acid, forming 3-iodo-4-hydroxybenzoic acid as a stable and quantifiable product. Key reaction parameters, including oxidant concentration, temperature, and incubation time, were optimized to ensure selective mono-iodination and consistent analytical response. Chromatographic separation was performed on a C18-DE RP column using gradient elution with aqueous ammonium acetate and acetonitrile, while MS/MS detection was carried out in MRM mode under ESI(−) conditions. Both UHPLC–DAD and UHPLC–MS/MS methods were validated according to ICH Q2(R2), demonstrating linearity with r2 ≥ 0.998, recovery values of 97.5–107.1%, and intraday/interday RSD values up to 3.7%. UHPLC–MS/MS provided higher sensitivity (LOD 37.7 ng/mL; LOQ 114 ng/mL), whereas UHPLC–DAD reached LOD and LOQ values of 24.9 and 75.4 µg/mL. Comparative analysis showed that DAD is suitable for routine quantification, while MS/MS allows lower detection limits and improved selectivity. The developed method offers a practical and reliable tool for the quality control of potassium iodide in ophthalmic formulations.
1. Introduction
Potassium iodide (KI) has long been used in pharmacy and medicine, including ophthalmic preparations, where it exhibits antioxidant, mucolytic, and lens opacity-resolving properties. Its presence in eye drops results from documented anti-inflammatory effects and its ability to inhibit degenerative processes within ocular structures, particularly in conditions such as cataract and inflammatory states of the conjunctiva and cornea. Contemporary literature confirms that potassium iodide at concentrations of 0.3–3.3% most commonly occurs in ophthalmic formulations used in the prophylaxis and treatment of early cataract changes and lens transparency disorders [1]. Modern studies also indicate the protective role of potassium iodide in animal models of selenite-induced cataract, showing approximately 50% protection when administered at appropriate intervals [1].
Despite the widespread use of potassium iodide in pharmaceutical ophthalmic formulations, there is a clear gap in the literature regarding rapid and selective analytical methods for determining this substance using high-performance liquid chromatography coupled with diode array detector (HPLC–DAD) and mass spectrometry (MS), particularly in combination with indirect derivatization reactions involving 4-hydroxybenzoic acid (HBA) [2,3,4,5,6]. Such derivatization reactions rely on the oxidation of iodide ions (I−) by hydrogen peroxide to molecular iodine (I2) or hypoiodous acid (HOI), which then acts as an electrophilic iodinating agent reacting with the aromatic ring of HBA to form a stable 3-iodo-4-hydroxybenzoic acid derivative detectable by DAD and MS [4,5,6].
Previous reports have focused mainly on classical methods—such as manganometric titration, determination with ion-selective electrodes, ion-exchange chromatography with conductometric or amperometric detection, and spectrophotometric methods based on the reaction of iodide with oxidizing reagents [7,8,9,10]. Although these methods are effective for simple matrices, their selectivity is limited in complex ophthalmic formulations containing excipients such as preservatives, buffers, and stabilizers [11,12,13]. Over the past twenty years, iodide detection in mineral waters, food salts, and biological fluids has been carried out using ion-exchange chromatography coupled with electrochemical, UV, or ICP-OES detection [14,15,16,17,18,19,20,21], as well as HPLC–DAD employing phosphatidylcholine columns [22,23].
Some analytical approaches have employed derivatization reactions of iodide with compounds such as 2-iodosobenzoate in the presence of N,N-dimethylaniline, followed by GC–MS detection [24,25,26]; however, these procedures have not been adapted for ophthalmic preparations. Eye drops containing 0.5% KI require fast and precise analytical control, as excipients like benzalkonium chloride, boric acid, or sodium chloride may interfere with measurement accuracy [12,13]. Recent efforts have focused on adapting chromatographic techniques involving derivatization and detection methods such as DAD or MS for complex pharmaceutical matrices. Derivatization using benzoic acid derivatives, particularly 4-hydroxybenzoic acid, has been shown to enhance ionization efficiency and chromatographic separation in LC–MS systems [27,28,29,30].
However, no literature currently reports the application of a rapid UHPLC–DAD/MS/MS method involving indirect derivatization with 4-hydroxybenzoic acid for detecting potassium iodide in ophthalmic formulations, representing a significant research gap. Existing analytical approaches are largely limited to ion-exchange chromatography with UV, conductometric, or electrochemical detection, as well as classical spectrophotometric assays [14,15,16,17,20]. Despite the successful application of HPLC–DAD methods for the analysis of various ophthalmic active substances—such as tetrahydrozoline, gatifloxacin, ketorolac, naphazoline, pheniramine maleate, and olopatadine [29,30]—no studies have addressed potassium iodide determination in this matrix.
Although classical iodometric, titrimetric, or electrochemical methods are inexpensive and widely used for iodine analysis, they lack the selectivity required for complex pharmaceutical matrices and are strongly affected by excipients commonly present in ophthalmic formulations, such as benzalkonium chloride, buffers, or stabilizers [31,32]. These components interfere with redox-based measurements and make accurate quantification of iodide in eye drops essentially impossible using low-cost techniques. Chromatographic methods therefore remain the only reliable analytical option for this matrix. Within LC-based analytical approaches, UHPLC–DAD provides a practical and cost-effective solution for routine quality control, offering high precision, robustness, and compatibility with standard laboratory infrastructure. In contrast, UHPLC–MS/MS, although more expensive in operation, offers unmatched sensitivity and specificity, enabling not only accurate quantification of iodide but also monitoring of degradation products, trace impurities, and stability-related transformations that cannot be detected by classical methods. The complementary strengths of both detection systems highlight their practical relevance in ensuring regulatory-compliant, high-quality pharmaceutical analysis [33,34].
Therefore, the aim of this work was to develop, optimize, and fully validate a rapid UHPLC method employing two complementary detection systems: DAD and MS/MS—for the quantitative determination of potassium iodide in ophthalmic preparations containing 0.5% KI, based on indirect derivatization with 4-hydroxybenzoic acid. The study further included systematic optimization of key reaction parameters and a direct comparison of the analytical performance of UHPLC–DAD and UHPLC–MS/MS, thereby addressing a critical gap in the literature and providing a robust analytical tool for routine pharmaceutical quality control.
2. Materials and Methods
2.1. Reagents and Standards
All reagents were of analytical or LC–MS grade. 4-Hydroxybenzoic acid (≥99%, ReagentPlus®, Sigma-Aldrich, St. Louis, MO, USA) and potassium iodide (Pharmaceutical Secondary Standard, Sigma-Aldrich) were used as analytical standards. Acetonitrile (LC–MS grade), formic acid (LC–MS grade), ammonium acetate (LC–MS grade), acetone, sodium thiosulfate (ACS reagent grade), and deionized water were obtained from Sigma-Aldrich. Hydrogen peroxide (30% w/w) was purchased from a certified supplier and used without further purification. Stock standard solutions of potassium iodide were prepared at 0.005, 0.010, 0.025, 0.060, and 0.100 mg/mL in deionized water. Fresh standards were prepared on the day of analysis and stored in amber glass vials at 4 °C until use.
2.2. Sample Preparation
Sample description. Four commercial ophthalmic formulations as eye drops containing 0.5% (5 mg/mL) potassium iodide were purchased from local pharmacies in Wroclaw, Poland. Products originated from different manufacturers and production batches to ensure representative variability of the analyzed matrix. All samples were stored at room temperature according to label instructions and analyzed before their expiration dates. Samples were prepared as follows. Approximately 0.1 mL of the ophthalmic solution containing 0.5% (5 mg/mL) potassium iodide was transferred into a 10 mL volumetric flask and diluted to volume with deionized water. The solution was sonicated for 15 min in an ultrasonic bath to ensure complete homogenization. Subsequently, 1.0 mL of the diluted sample was transferred into a 10 mL glass-stoppered reaction tube, followed by the addition of 0.5 mL of 0.2% 4-hydroxybenzoic acid (HBA) solution in acetone. After thorough mixing, 0.2 mL of 3% hydrogen peroxide (H2O2) was added, corresponding to approximately 1.5–2.0 molar equivalents relative to HBA. For method optimization and robustness testing, the amount of hydrogen peroxide was intentionally varied by using four proportional volumes of the 3% H2O2 solution—0.16 mL (1.2 eq), 0.20 mL (1.5 eq), 0.27 mL (2.0 eq), and 0.31 mL (2.3 eq)—corresponding to the respective molar equivalents relative to HBA. The reaction tube was tightly stoppered to minimize air exposure and incubated at 65 °C for 90 min in a thermostated water bath. During incubation, iodide ions were oxidized to molecular iodine (I2) or hypoiodous acid (HOI), enabling electrophilic iodination of the aromatic ring of HBA to form 3-iodo-4-hydroxybenzoic acid. After the reaction, 1.0 mL of 0.01 M sodium thiosulfate (Na2S2O3) solution was added to reduce any residual iodine (I2) to iodide (I−), as indicated by the disappearance of the yellow coloration. The mixture was sonicated again for 15 min to ensure complete reduction and dissolution, and then diluted to 10 mL with deionized water. The resulting solution was filtered through a 0.22 µm PTFE syringe filter (13 mm, Millipore, Burlington, MA, USA) and transferred into autosampler vials for UHPLC–MS/MS analysis. Blank and spiked samples were prepared in the same manner, replacing the ophthalmic solution with deionized water (blank) or water spiked with known concentrations of potassium iodide (spiked controls) to evaluate accuracy and recovery. These samples were subjected to the same derivatization, reduction, and chromatographic conditions as the test solutions. The stabilizing effect of sodium thiosulfate was confirmed experimentally. The addition of 0.01 M Na2S2O3 effectively prevented oxidative degradation of iodide and maintained the stability of the derivatized product (3-iodo-4-hydroxybenzoic acid) for at least 24 h at room temperature, with no significant variation (<2%) in chromatographic peak area observed. This ensured reliable quantification and sample integrity during analytical runs.
2.3. Instrumentation and UHPLC–MS/MS Conditions
Chromatographic analyses were performed using a Shimadzu Nexera XR UHPLC system (Kyoto, Japan) equipped with an LC-40D XR quaternary pump, SIL-40C XR autosampler, CTO-40C column oven, and SPD-M40 photodiode array (DAD) detector. Separation was achieved on a C18-DE RP analytical column (Dr. Maisch GmbH, Ammerbuch-Entringen, Germany; 100 × 2.0 mm, 3 µm particle size) maintained at 40 °C. The mobile phase consisted of solvent A (0.1% ammonium acetate in water) and solvent B (0.1% formic acid in acetonitrile), delivered at a flow rate of 0.20 mL/min under the following gradient program: 0–3.4 min, 0–50% B; 3.4–6.8 min, 50–70% B; 6.8–10.2 min, 70–95% B; 10.2–13.6 min, 95% B (isocratic hold); and 13.6–17.0 min, re-equilibration to 5% B. The total run time was 17 min. The injection volume was 5 µL, and the autosampler temperature was maintained at 15 °C. The DAD detector was set to scan from 190 to 800 nm with an 8 nm slit width, a cell temperature of 40 °C, and a sampling frequency of 1.56 Hz.
Mass spectrometric detection was carried out using a Shimadzu LCMS-8040 triple quadrupole mass spectrometer (Kyoto, Japan) equipped with an electrospray ionization (ESI) interface operating in both positive and negative ionization modes. The interface voltage was set to –3.5 kV. The nebulizing gas flow was 3.0 L/min, and the drying gas flow was 15.0 L/min. The desolvation line (DL) and heat block temperatures were maintained at 250 °C and 400 °C, respectively. Data acquisition was performed in multiple reaction monitoring (MRM) mode using optimized transitions for parent and product ion.
2.4. Optimization
To establish reliable derivatization and chromatographic conditions, several key reaction and instrumental parameters were systematically optimized prior to method validation. The optimization of the derivatization step included evaluation of the influence of hydrogen peroxide concentration (1.2, 1.5, 2.0, and 2.3 molar equivalents relative to HBA), reaction temperature (50–65 °C), incubation time (30–120 min), and the heating mode (ultrasonic bath, water bath, and thermostated dry heating block). Each parameter was tested to determine its effect on the completeness of derivatization and selectivity toward mono-iodination of 4-hydroxybenzoic acid. Chromatographic conditions were optimized by assessing retention, resolution, and peak shape for both the derivatization reagent (HBA) and its iodinated product (3-iodo-4-hydroxybenzoic acid). For the UHPLC–MS/MS system, mass spectrometric parameters—including precursor ions, product ions, collision energies, and interface settings—were optimized to obtain the most sensitive and selective MRM transitions for HBA and its iodinated derivative. The optimized conditions identified through these experiments were subsequently used for all analyses described in Section 3.
2.5. Validation
The developed UHPLC–DAD and UHPLC–MS/MS methods were validated according to the ICH Q2(R2) guideline. The following parameters were evaluated: linearity, accuracy (recovery), precision (intraday and interday), limit of detection (LOD), limit of quantification (LOQ), and robustness. Linearity was assessed using five calibration levels prepared in duplicate within the concentration range 0.005–0.100 mg/mL. Accuracy was examined at three concentration levels corresponding to 80%, 100%, and 120% of the nominal sample concentration. Precision was evaluated as intraday repeatability (n = 3) and interday intermediate precision over three consecutive days (n = 3 per day). LOD and LOQ were determined based on signal-to-noise ratios of 3:1 and 10:1, respectively.
3. Results
3.1. Optimization of Chromatographic Method
The developed analytical procedure for iodide determination is based on an indirect derivatization reaction using 4-hydroxybenzoic acid (HBA) as an aromatic substrate. The principle of the reaction is illustrated schematically in Figure 1. Under mild heating conditions (65 °C, 1.5 h), iodide ions (I−) are oxidized by hydrogen peroxide (H2O2, 1.5–2.0 eq) to electrophilic iodine species—molecular iodine (I2) or hypoiodous acid (HOI)—which subsequently iodinate the aromatic ring of HBA to yield 3-iodo-4-hydroxybenzoic acid (m/z 263 [M–H]−) as the main product shown and description in Figure 2. This derivative serves as a stable and quantifiable compound directly proportional to the iodide concentration.
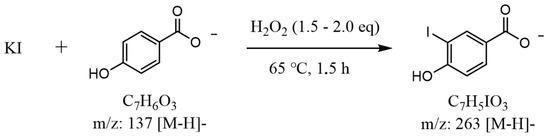
Figure 1.
Schematic representation of the indirect derivatization reaction between potassium iodide (KI) and 4-hydroxybenzoic acid (HBA) in the presence of hydrogen peroxide (H2O2) as an oxidizing agent.
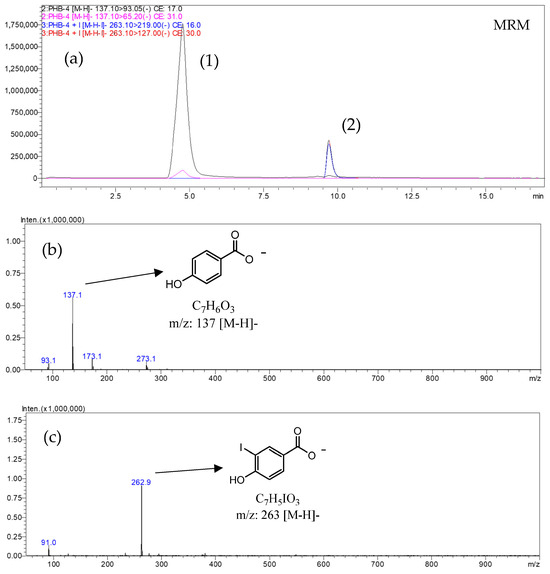
Figure 2.
UHPLC–MS/MS chromatogram and product-ion spectra obtained in ESI(−) mode after indirect derivatization of potassium iodide with 4-hydroxybenzoic acid (HBA). Chromatogram (a) shows the multiple reaction monitoring (MRM) mode, in which the peak assigned to HBA (1) (PHB-4; m/z 137 > 93) and the peak corresponding to its iodinated derivative 3-I-4-HBA (2) (PHB-4-I; m/z 263 > 219). Spectrum (b,c) present the ion of m/z 137 and m/z 263.
3.2. Chromatographic Confirmation
The chromatographic separation of the derivatization reagent (HBA) and its iodinated product is shown in Figure 3 and Figure 4. The UHPLC–DAD chromatograms recorded at 254 nm clearly demonstrate distinct retention peaks corresponding to 4-hydroxybenzoic acid and 3-iodo-4-hydroxybenzoic acid, confirming efficient derivatization and baseline separation of both compounds. The MS/MS data recorded in TIC mode confirmed the same chromatographic separation profile as observed with DAD detection, demonstrating full consistency between both detection systems. The stabilizing effect of sodium thiosulfate (Na2S2O3) on the temporal stability of derivatized samples is presented in Figure 5. The peak area of 3-iodo-4-hydroxybenzoic acid remained constant for at least 24 h, confirming that 0.01 M Na2S2O3 effectively prevents oxidative degradation and loss of iodine species, thereby maintaining the analytical stability of the samples during UHPLC–MS/MS analysis.
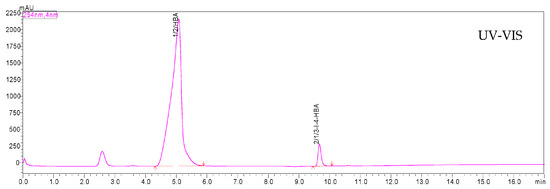
Figure 3.
UHPLC–DAD chromatogram recorded at 254 nm for the derivatized sample, showing the separation of 4-hydroxybenzoic acid and its iodinated derivative.
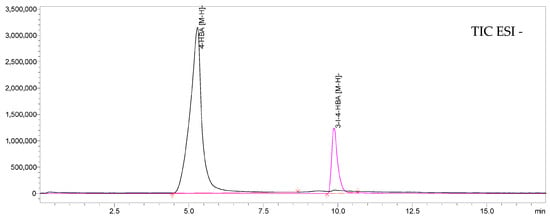
Figure 4.
UHPLC–MS/MS chromatogram in ESI(-) mode showing the TIC chromatogram for the monitored transitions of 4-hydroxybenzoic acid and its iodinated derivative 3-iodo-4-hydroxybenzoic acid.
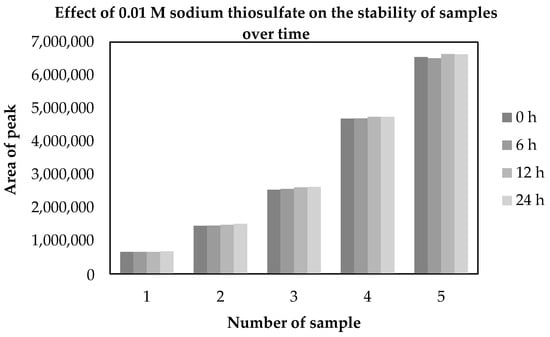
Figure 5.
Effect of 0.01 M sodium thiosulfate on the temporal stability of derivatized samples expressed as peak area variation over 24 h.
3.3. Optimization of Reaction Conditions
Reaction parameters such as temperature, oxidant concentration, heating mode, and reaction time were optimized to ensure complete conversion and high reproducibility. The influence of temperature on the iodination process is shown in Figure 6. Increasing the temperature from 50 °C to 65 °C significantly enhanced the iodination efficiency and linearity, with the correlation coefficient (R2) improving from 0.987 to 0.999. The peak area of 3-iodo-4-hydroxybenzoic acid increased proportionally with temperature, confirming that 65 °C provides optimal reaction kinetics.
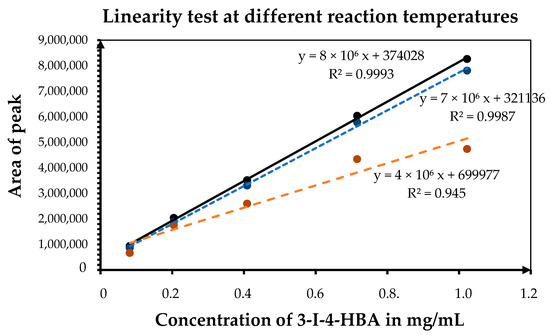
Figure 6.
Effect of temperature on the linearity of the derivatization process, highlighting that 65 °C yields the highest R2 values and most consistent analytical response.
The effect of hydrogen peroxide concentration on selectivity is illustrated in Figure 7. Concentrations above 2.0 eq led to over-oxidation and the formation of multi-iodinated by-products (m/z 344 and 470), whereas the range of 1.5–2.0 eq ensured selective mono-iodination and reproducible results. To further evaluate the robustness of the derivatization step, the volume of 3% H2O2 was intentionally varied and tested at four proportional levels corresponding to 1.2, 1.5, 2.0, and 2.3 molar equivalents relative to HBA. This additional robustness experiment confirmed that small deliberate changes in oxidant amount influenced conversion efficiency and selectivity in a predictable manner: 1.2 eq resulted in incomplete derivatization, 1.5–2.0 eq produced a single mono-iodinated product with high reproducibility, and 2.3 eq promoted the formation of over-iodinated species.
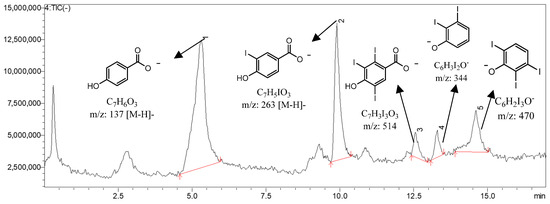
Figure 7.
UHPLC–MS/MS chromatogram illustrating the effect of oxidant concentration on the derivatization process. Increasing the amount of hydrogen peroxide (H2O2) promotes the formation of multi-iodinated derivatives of 4-hydroxybenzoic acid, as evidenced by the appearance of additional peaks corresponding to di- and tri-iodinated species (m/z 344, 470).
The influence of heating mode and reaction time is presented in Figure 6. Ultrasonic treatment at room temperature resulted in incomplete conversion, while heating in a water bath at 40 °C moderately improved signal intensity. The highest and most consistent response was obtained at 65 °C using a thermostated dry heating block, which provided uniform temperature distribution and minimized solvent loss. A 90-min incubation period was sufficient to reach a stable signal, and extending the reaction did not increase product yield. Therefore, the final derivatization conditions were established as 65 °C, 90 min, and 1.5–2.0 equivalents of H2O2, providing complete and reproducible mono-iodination of 4-hydroxybenzoic acid.
The chromatographic and mass spectrometric profiles illustrating the influence of oxidant concentration are shown in Figure 7. At the optimized oxidant level (1.5–2.0 equivalents of H2O2), a single dominant peak corresponding to 3-iodo-4-hydroxybenzoic acid (m/z 263 [M–H]−) was observed, confirming selective mono-iodination of the aromatic ring. Increasing the oxidant concentration beyond this range significantly altered the reaction profile, with additional peaks appearing at m/z 344 and m/z 470, corresponding to di- and tri-iodinated derivatives of HBA. This result indicates that excessive H2O2 promotes over-oxidation and formation of higher iodinated species through an increased concentration of electrophilic iodine. Consequently, the relative intensity of the mono-iodinated product decreases with increasing oxidant concentration, demonstrating a loss of selectivity.
Precise control of the oxidant-to-substrate ratio is therefore essential to maintain mono-iodination selectivity and ensure the accuracy and reproducibility of quantitative analysis. Maintaining H2O2 within 1.5–2.0 equivalents guarantees formation of the mono-iodinated derivative and optimal analytical performance of the UHPLC–DAD/MS/MS method.
4. Method Validation
The developed UHPLC–DAD/MS/MS method was validated in accordance with internationally recognized analytical guidelines to confirm its reliability, precision, and applicability for the determination of potassium iodide in ophthalmic formulations.
4.1. Optimization of MS Parameters
The optimized chromatographic and MS/MS acquisition conditions are summarized in Table 1. Both analytes, 4-hydroxybenzoic acid (4-HBA) and its iodinated derivative (3-I-4-HBA), were efficiently separated with retention times of 4.76 min and 9.70 min, respectively. Multiple reaction monitoring (MRM) transitions were optimized under negative ESI mode to ensure high sensitivity and selectivity. For 4-HBA, the precursor ion at m/z 137.1 produced major fragments at m/z 93.1 and 65.2, while 3-I-4-HBA (m/z 263.1) yielded product ions at m/z 219.0 and 127.0. Collision energies of 16–31 eV and pre-bias voltages between 12–28 V were selected to maximize signal intensity and minimize background noise, ensuring reproducible quantification of both compounds.

Table 1.
Retention time (RT) and multiple reaction monitoring (MRM) acquisition parameters for each analyte used in the UHPLC–MS/MS method. The table summarizes precursor and product ion transitions, collision energies, and pre-bias voltages optimized for 4-hydroxybenzoic acid (4-HBA) and its iodinated derivative (3-I-4-HBA) 1.
4.2. Validation Parameters
Linearity was established over the concentration range of 0.005–0.100 mg/mL for the iodinated derivative (HBA-4-I). Excellent linearity was achieved for both UHPLC–MS/MS and UHPLC–DAD, with correlation coefficients (r2) of 0.998 and 0.999, respectively (Table 2). The regression fits and low residual deviations confirmed a stable analytical response throughout the tested range.

Table 2.
Validation parameters of the developed UHPLC–MS/MS method for the determination of the iodinated derivative of 4-hydroxybenzoic acid (3-I-4-HBA) compared with the UHPLC–DAD method. The table summarizes linearity (r2), intra- and interday precision (RSD %), and sensitivity parameters (LOD and LOQ).
Precision was evaluated using spiked ophthalmic samples under intraday repeatability and interday intermediate precision conditions. UHPLC–MS/MS provided RSD values of 2.14% (intraday) and 3.72% (interday), while UHPLC–DAD showed 0.64% and 1.49%, respectively (Table 2), confirming excellent reproducibility.
Accuracy was assessed by recovery studies at three spiking levels (80%, 100%, 120%) relative to the target concentration (Table 3). For UHPLC–MS/MS, the recoveries ranged from 97.5% to 107.1%, while UHPLC–DAD recoveries were between 100.5% and 101.9%. Sensitivity was evaluated using signal-to-noise ratios of 3:1 and 10:1 for LOD and LOQ, respectively. UHPLC–MS/MS achieved LOD and LOQ values of 37.7 ng/mL and 114 ng/mL, respectively, whereas UHPLC–DAD exhibited 24.9 µg/mL and 75.4 µg/mL (Table 2). These results confirm that MS/MS detection is approximately three orders of magnitude more sensitive than UV detection.

Table 3.
Recovery study of the iodinated derivative of 4-hydroxybenzoic acid (3-I-4-HBA) obtained after derivatization of potassium iodide in ophthalmic formulations. The table summarizes the measured concentrations and calculated recoveries at three spiking levels.
The stabilizing effect of 0.01 M sodium thiosulfate on the iodide ion during derivatization was confirmed (Figure 5). No significant variation in peak area was observed within 24 h, demonstrating that thiosulfate effectively prevents oxidative degradation and ensures chemical stability of the iodinated product. Finally, the applicability of the validated method was demonstrated by analyzing four commercial ophthalmic formulations containing 0.5% potassium iodide. The determined concentrations showed excellent agreement with the declared content and recoveries between 98.5–104.6% for UHPLC–MS/MS and 98.8–102.7% for UHPLC–DAD (Table 4), confirming that the developed UHPLC–DAD/MS/MS method is precise, accurate, robust, and suitable for routine quality control of ophthalmic iodide formulations.

Table 4.
Quantitative determination of potassium iodide in commercial eye drops by UHPLC–MS/MS and UHPLC–DAD.
5. Comparison Between UHPLC–MS/MS and UHPLC–DAD Detection Systems
Both detection modes (UHPLC–MS/MS and UHPLC–DAD) were fully validated as part of the same derivatization-based UHPLC analytical method. To evaluate the applicability of the developed UHPLC–MS/MS procedure, its results were compared with those obtained using the conventional UHPLC–DAD method. Both techniques were applied to the quantitative determination of potassium iodide in four commercial ophthalmic formulations containing 5.0 mg/mL KI, and the results are summarized in Table 3 and Table 4. Analytical systems showed excellent agreement with the declared concentration of potassium iodide. The mean concentrations determined by UHPLC–MS/MS ranged from 4.98 to 5.31 mg/mL, while those obtained by UHPLC–DAD were 5.01–5.18 mg/mL, corresponding to recoveries of 98.5–104.6% and 98.8–102.7%, respectively (Table 4). These results confirm the accuracy and robustness of the indirect derivatization approach based on 4-hydroxybenzoic acid, as well as the stoichiometric correlation between iodide concentration and the formation of its iodinated derivative.
The recovery study performed at three concentration levels (Table 3) further highlighted differences between both detection systems. The UHPLC–MS/MS method yielded recoveries between 97.5% and 107.1%, with slightly higher variability at the mid-level concentration (5.0 mg/mL, 107.1%), while the UHPLC–DAD method provided highly consistent recoveries ranging from 100.5% to 101.9% across all tested levels. These results indicate that UHPLC–DAD offers superior repeatability and recovery stability, making it well suited for routine quality control applications. However, this improved precision comes at the expense of analytical sensitivity. The UHPLC–MS/MS system demonstrated markedly lower detection limits and a higher signal-to-noise ratio, allowing reliable quantification at sub-µg/mL levels. Moreover, the MS-based approach enables monitoring of potential degradation products of 4-hydroxybenzoic acid derivatives and the direct identification of iodide in complex matrices, providing an additional layer of selectivity and confirmation not achievable by UV detection alone. In summary, both analytical techniques produced accurate and reproducible quantification of potassium iodide in ophthalmic formulations. The UHPLC–MS/MS method offers higher analytical sensitivity, selectivity, and structural confirmation capability, making it particularly advantageous for trace-level analyses and stability studies. Conversely, the UHPLC–DAD method provides better recovery reproducibility and simplicity of operation, representing a robust and cost-effective solution for routine pharmaceutical quality control.
This study presents, for the first time, the development and validation of an indirect derivatization-based method for the quantitative determination of potassium iodide in ophthalmic formulations using UHPLC–MS/MS and UHPLC–DAD systems. The proposed approach introduces a novel analytical concept in iodide determination, relying on the in situ oxidation of iodide ions by hydrogen peroxide followed by the electrophilic iodination of 4-hydroxybenzoic acid (HBA) to yield a stable and highly detectable derivative—3-iodo-4-hydroxybenzoic acid. Unlike conventional titrimetric or ion-exchange procedures, this method allows the transformation of an inorganic analyte into an easily quantifiable organic species, enabling its detection by both UV and MS/MS techniques.
The derivatization mechanism, optimized under mild conditions (65 °C, 90 min, 1.5–2.0 eq H2O2), ensured reproducible and stoichiometric conversion of iodide to the mono-iodinated aromatic product. As demonstrated in Figure 6 and Figure 7, temperature and oxidant concentration significantly affected the yield and selectivity of iodination. Excess hydrogen peroxide promoted the formation of multi-iodinated derivatives (m/z 344 and 470), confirming the necessity of precise oxidant control to maintain mono-substitution and analytical reliability. The addition of sodium thiosulfate after the reaction effectively stabilized the system by reducing residual iodine and preventing oxidative degradation of the product (Figure 5).
Validation data confirmed excellent linearity (r2 = 0.998–0.999), precision (RSD up to 3.7%), and recovery within 97.5–107.1%, fulfilling the analytical performance criteria for pharmaceutical analysis. The UHPLC–MS/MS configuration, operated in MRM mode, exhibited superior sensitivity, with LOD and LOQ values approximately one order of magnitude lower than those of the UHPLC–DAD system. This higher sensitivity enables reliable trace-level detection of iodide even in complex ophthalmic matrices containing buffers and preservatives.
The comparative evaluation of UHPLC–MS/MS and UHPLC–DAD (Table 3 and Table 4) revealed complementary strengths of both systems. The UHPLC–DAD method demonstrated better repeatability and recovery consistency (100.5–101.9%) across all validation levels, confirming its robustness for routine use. In contrast, the UHPLC–MS/MS system offered superior sensitivity and selectivity, along with the unique capability to monitor degradation products of the iodinated HBA derivative and to detect iodide in complex pharmaceutical or biological matrices. This added structural information and confirmatory power make MS/MS indispensable for advanced analytical and stability studies.
The developed method also demonstrates high chemical and temporal stability due to the inclusion of sodium thiosulfate as a post-derivatization stabilizer, allowing reproducible chromatographic responses over 24 h. The consistent peak profiles of the iodinated derivative across multiple measurements confirm both the efficiency and robustness of the indirect derivatization concept.
In a broader analytical context, the present work establishes a new paradigm for iodide determination by extending the concept of aromatic derivatization—traditionally applied to organic molecules—to inorganic anions. The proposed method is compatible with routine LC–MS/MS workflows, and adaptable to a variety of pharmaceutical, environmental, or clinical samples. Importantly, it eliminates the limitations of classical iodometric and ion chromatography techniques, providing higher selectivity, lower detection limits, and improved reproducibility.
6. Conclusions
In this study, a rapid and highly selective UHPLC method coupled with DAD or MS/MS detection based on indirect derivatization with 4-hydroxybenzoic acid was successfully developed and applied for the first time to the quantitative determination of potassium iodide in ophthalmic formulations. The derivatization strategy enabled the transformation of iodide into a stable and readily detectable organic derivative, ensuring excellent chromatographic behavior and enhanced mass spectrometric sensitivity. Comparative evaluation against UHPLC–DAD detection confirmed the reliability of the developed approach and highlighted the complementary strengths of UV and MS/MS detection. The method was further validated through the analysis of commercial eye-drop formulations, demonstrating accurate recovery of the declared KI content and confirming its suitability for routine pharmaceutical quality control. Overall, this work provides a novel and effective analytical platform for iodide determination and establishes a valuable tool for the quality assessment of iodine-containing ophthalmic preparations.
Author Contributions
Conceptualization, J.S. and A.S.; methodology, J.S. and A.D.-M.; software, J.S.; validation, J.S., A.D.-M. and A.S.; formal analysis, J.S.; investigation, J.S.; resources, A.S.; data curation, J.S.; writing—original draft preparation, J.S.; writing—review and editing, A.D.-M. and A.S.; visualization, J.S.; supervision, A.S.; project administration, A.S.; funding acquisition, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out with the support of the Polish Ministry of Education and Science under the “Implementation Doctorate” program, agreement number DWD/5/0067/2021.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data cannot be made publicly available due to restrictions arising from the three-party agreement within the Implementation Doctorate program (agreement number DWD/5/0067/2021).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Muranov, K.; Poliansky, N.; Winkler, R.; Rieger, G.; Schmut, O.; Horwath-Winter, J. Protection by iodide of lens from selenite-induced cataract. Graefe’s Arch. Clin. Exp. Ophthalmol. 2004, 242, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Schwehr, K.A.; Santschi, P.H. Sensitive determination of iodine species, including organo-iodine, for freshwater and seawater samples using high performance liquid chromatography and spectrophotometric detection. Anal. Chim. Acta 2003, 482, 59–71. [Google Scholar] [CrossRef]
- Verma, K.K.; Jain, A.; Verma, A. Determination of iodide by high-performance liquid chromatography after precolumn derivatization. Anal. Chem. 1992, 64, 1484–1489. [Google Scholar] [CrossRef]
- Mishra, S.; Singh, V.; Jain, A.; Verma, K.K. Determination of iodide by derivatization to 4-iodo-N,N-dimethylaniline and gas chromatography–mass spectrometry. Analyst 2000, 125, 459–464. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, X. Total organic iodine measurement: A new approach with UPLC/ESI-MS for off-line iodide separation/detection. Water Res. 2013, 47, 163–172. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, X.; Li, Y. Identification, toxicity and control of iodinated disinfection byproducts in cooking with simulated chlor(am)inated tap water and iodized table salt. Water Res. 2016, 88, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.D.; Maiti, B.; Mathur, P.K. Determination of iodate and sulphate in iodized common salt by ion chromatography with conductivity detection. Talanta 2001, 53, 701–705. [Google Scholar] [CrossRef]
- Rebary, B.; Paul, P.; Ghosh, P.K. Determination of iodide and iodate in edible salt by ion chromatography with integrated am-perometric detection. Food Chem. 2010, 123, 529–534. [Google Scholar] [CrossRef]
- Shelor, C.P.; Dasgupta, P.K. Review of analytical methods for the quantification of iodine in complex matrices. Anal. Chim. Acta 2011, 702, 16–36. [Google Scholar] [CrossRef]
- Sandell, E.B.; Kolthoff, I.M. Chronometric catalytic method for the determination of micro quantities of iodine. J. Am. Chem. Soc. 1934, 56, 1426. [Google Scholar] [CrossRef]
- Judprasong, K.; Jongjaithet, N.; Chavasit, V. Comparison of methods for iodine analysis in foods. Food Chem. 2016, 193, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Desbenoit, N.; Schmitz-Afonso, I.; Baudouin, C.; Laprévote, O.; Touboul, D.; Brignole-Baudouin, F.; Brunelle, A. Localisation and quantification of benzalkonium chloride in eye tissue by TOF-SIMS imaging and liquid chromatography mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 4039–4049. [Google Scholar] [CrossRef] [PubMed]
- AlAani, H.; AlNukkary, Y. Determination of benzalkonium chloride in ophthalmic solutions by stability-indicating HPLC method: Application to a stability study. J. Appl. Pharm. Sci. 2016, 6, 80–89. [Google Scholar] [CrossRef]
- Cataldi, T.R.I.; Rubino, A.; Ciriello, R. Sensitive quantification of iodide by ion-exchange chromatography with electrochemi-cal detection at a modified platinum electrode. Anal. Bioanal. Chem. 2005, 382, 134–141. [Google Scholar] [CrossRef]
- Ito, K.; Hirokawa, T. Enhanced detection of iodide in seawater by ion chromatography using an ODS column coated with cetyltrimethyl ammonium. Anal. Sci. 2001, 17, 579–581. [Google Scholar] [CrossRef]
- Ito, K.; Nomura, R.; Fujii, T.; Tanaka, M.; Tsumura, T.; Shibata, H.; Hirokawa, T. Determination of nitrite, nitrate, bromide, and iodide in seawater by ion chromatography with UV detection using dilauryldimethylammonium-coated monolithic ODS columns. Anal. Bioanal. Chem. 2012, 404, 2513–2517. [Google Scholar] [CrossRef]
- Malongo, T.K.; Patris, S.; Macours, P.; Cotton, F.; Nsangu, J.; Kauffmann, J.-M. Highly sensitive determination of iodide by ion chromatography with amperometric detection at a silver-based carbon paste electrode. Talanta 2008, 76, 540–547. [Google Scholar] [CrossRef]
- Yamada, H.; Kajiyama, S.; Yonebayashi, K. Determination of trace amounts of iodine in soils by HPLC with fluorescence de-tection. Bunseki Kagaku 1995, 44, 1027–1032. [Google Scholar] [CrossRef]
- Hansen, V.; Yi, P.; Hou, X.; Aldahan, A.; Roos, P.; Possnert, G. Iodide and iodate in surface water of the Baltic Sea, Kattegat and Skagerrak. Sci. Total Environ. 2011, 412–413, 296–303. [Google Scholar] [CrossRef]
- Gilfedder, B.S.; Petri, M.; Biester, H. Iodine speciation and cycling in fresh waters: A case study from a humic rich headwater lake. J. Limnol. 2009, 68, 396–408. [Google Scholar] [CrossRef]
- Delange, F.; Bürgi, H.; Chen, Z.P.; Dunn, J.T. World status of monitoring of iodine deficiency disorders control programs. Thy-roid 2002, 12, 915–924. [Google Scholar] [CrossRef]
- Tatarczak-Michalewska, M.; Flieger, J.; Kawka, J.; Flieger, W.; Blicharska, E. HPLC-DAD determination of iodide in mineral waters on phosphatidylcholine column. Molecules 2019, 24, 1243. [Google Scholar] [CrossRef]
- Hu, W.; Haddad, P.R.; Tanaka, K.; Mori, M.; Tekura, K.; Hase, K.; Ohno, M.; Kamo, N. Creation and characteristics of phospha-tidylcholine stationary phases for the chromatographic separation of inorganic anions. J. Chromatogr. A 2003, 997, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Gupta, M.; Jain, A.; Verma, K.K. Single drop microextraction or solid phase microextraction–gas chromatography–mass spectrometry for the determination of iodine in pharmaceuticals, iodized salt, milk powder and vegetables involving conversion into 4-iodo-N,N-dimethylaniline. J. Chromatogr. A 2004, 1023, 33–39. [Google Scholar] [CrossRef]
- Cataldi, T.R.I.; Rubino, A.; Laviola, M.C.; Ciriello, R. Comparison of silver, gold and modified platinum electrodes for the elec-trochemical detection of iodide in urine samples following ion chromatography. J. Chromatogr. B 2005, 827, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Schwehr, K.A.; Ho, Y.F.; Xu, C.; Roberts, K.A.; Kaplan, D.I.; Brinkmeyer, R.; Yeager, C.M.; Santschi, P.H. A novel ap-proach for the simultaneous determination of iodide, iodate and organo-iodide for 127I and 129I in environmental samples using gas chromatography-mass spectrometry. Environ. Sci. Technol. 2010, 44, 9042–9048. [Google Scholar] [CrossRef]
- Bogos, L.G.; Pralea, I.E.; Moldovan, R.C.; Iuga, C.A. Indirect enantioseparations: Recent advances in chiral metabolomics for biomedical research. Int. J. Mol. Sci. 2022, 23, 7428. [Google Scholar] [CrossRef]
- Brichac, J.; Honzatko, A.; Picklo, M.J. Direct and indirect high-performance liquid chromatography enantioseparation of trans-4-hydroxy-2-nonenoic acid. J. Chromatogr. A 2007, 1149, 305–311. [Google Scholar] [CrossRef][Green Version]
- El Yazbi, F.A.; Hassan, E.M.; Khamis, E.F.; Ragab, M.A.A.; Hamdy, M.M.A. Stability indicating HPLC-DAD method for analysis of ketorolac binary and ternary mixtures in eye drops: Quantitative analysis in rabbit aqueous humor. J. Chromatogr. B 2017, 1068–1069, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Chen, N.; Wang, D.; Lai, Y.; Cao, Z. A validated stability-indicating HPLC method for the simultaneous determination of pheniramine maleate and naphazoline hydrochloride in pharmaceutical formulations. Chem. Cent. J. 2014, 8, 7. [Google Scholar] [CrossRef]
- Miranda, J.L.A.; António, M.R.S.; Segundo, M.A. Chip-based spectrofluorimetric determination of iodine in a multi-syringe flow platform with and without in-line digestion—Application to salt, pharmaceuticals, and algae samples. Molecules 2022, 27, 1325. [Google Scholar] [CrossRef] [PubMed]
- Riley, B.J.; Beck, C.L.; Evarts, J.S.; Chong, S.; Lines, A.M.; Felmy, H.M.; McFarlane, J.; Andrews, H.B.; Bryan, S.A.; McHugh, K.C.; et al. Analytical capabilities for iodine detection: Review of methods and applications. AIP Adv. 2024, 14, 080701. [Google Scholar] [CrossRef]
- Anghel, D.; Epuran, C.; Fringu, I.; Fratilescu, I.; Lascu, A.; Macsim, A.-M.; Chiriac, V.; Gherban, M.; Vlascici, D.; Fagadar-Cosma, E. Double Type Detection of Triiodide and Iodide Ions Using a Manganese(III) Porphyrin as Sensitive Compound. Sensors 2024, 24, 5517. [Google Scholar] [CrossRef] [PubMed]
- Rathod, R.H.; Chaudhari, S.R.; Patil, A.S.; Shirkhedkar, A.A. Ultra-high performance liquid chromatography-MS/MS (UHPLC-MS/MS) in practice: Analysis of drugs and pharmaceutical formulations. Future J. Pharm. Sci. 2019, 5, 6. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).