Abstract
Background: Asthma is a global public health challenge, and although guidelines recommend self-management programs, their implementation is limited. Digital health tools, such as mobile applications and web platforms, have emerged as promising solutions to improve self-management, adherence, and monitoring. However, the evidence on their effectiveness is heterogeneous and often presents methodological limitations. This systematic review and meta-analysis aimed to synthesize the current evidence on the efficacy of these tools in asthma management. Methods: A systematic search was conducted in six databases for randomized controlled trials (RCTs) published between 2010 and 2025. Twenty-six RCTs that evaluated digital interventions in pediatric and adult patients with asthma were included. The outcomes of interest were asthma control, pulmonary function, symptom-free days, and health-related quality of life (HRQoL). A meta-analysis was performed using a random-effects model. Results: Digital tools showed a statistically significant improvement in pulmonary function, specifically in FEV1 (SMD: 1.53; p = 0.007) and the FEV1/FVC ratio (SMD: 1.20; p = 0.02). No significant effects were found on asthma control, PEF, symptom-free days, or HRQoL in the overall analysis. However, subgroup analyses revealed that remote supervision significantly improved asthma control, and mobile applications improved HRQoL. Conclusions: Digital health interventions are a promising complement for asthma management, notably improving pulmonary function. Their effectiveness on other clinical outcomes appears to depend on factors such as the supervision mode and the type of tool. More standardized research is needed to confirm these findings.
1. Introduction
Asthma is a syndrome that encompasses multiple clinical phenotypes with similar manifestations but diverse etiologies []. It is defined as a chronic inflammatory disease of the airways, in which different cells and inflammatory mediators are involved, influenced in part by genetic factors []. Its clinical presentation is characterized by bronchial hyperreactivity and variable airflow obstruction, which can be totally or partially reversible, either spontaneously or through pharmacological treatment [].
Asthma represents a global public health challenge, affecting more than 260 million people and contributing significantly to morbidity, hospitalizations, and deterioration of quality of life, especially in low, and middle, income countries []. Despite therapeutic advances, asthma control remains insufficient for a considerable proportion of patients, which underscores the need to optimize management and self-care strategies []. Currently, major international clinical guidelines recommend including self-management programs as part of standard treatment, as these have been shown to improve patient outcomes [,]. However, their implementation in clinical practice and acceptance by patients remain limited [,].
In response to this problem, digital tools such as mobile applications, web platforms, and telemedicine programs have emerged as promising alternatives to improve self-management, facilitate continuous monitoring, and reinforce treatment adherence, thus empowering patients in the control of their disease [,,]. These tools allow patients to access education, remote follow-up, and medical advice, which helps to minimize exacerbations and improve their quality of life [,,].
Current evidence suggests that these interventions can improve symptom control and some clinical outcomes, although results remain variable and, in some cases, modest. Kandola et al. [] reported significant improvements in asthma control using a mobile application, with a mean increase of 1.9 points in the Asthma Control Test (ACT) and a higher probability of sustained clinical improvement. For their part, Ljungberg et al. [] evaluated AsthmaTuner in a crossover study and also found a significant improvement in the ACT (0.7 points). However, both studies showed relevant limitations, such as long-term adherence and the dependence of results on the frequent use of the tools. Although digital tools for asthma self-management show potential, the evidence is still limited and heterogeneous. There are conceptual and organizational barriers that hinder their adoption []. Conceptually, the advice provided by health professionals often does not match what patients really want to know about how to live with their asthma []. At the organizational level, the lack of flexible systems that facilitate effective communication between professionals and patients represents a significant obstacle [].
Despite advances in the development of digital interventions for asthma self-management, previous reviews have shown inconsistent results and methodological limitations, such as the heterogeneity of interventions, lack of standardization, and poor integration with routine clinical care, which hinders the generalizability of findings and the identification of the most effective components. Furthermore, most studies present differences in design, low platform standardization, and high dropout rates, which prevents drawing firm conclusions [,]. These limitations highlight the need for rigorous systematic reviews and meta-analyses that integrate the most recent evidence and evaluate the effectiveness of digital tools, considering the type of intervention and participant characteristics. In this context, the present meta-analysis seeks to provide updated evidence on the efficacy of digital tools for asthma self-management, through the inclusion of recent randomized controlled trials and the application of consistent methodological criteria. The study focuses on key clinical outcomes, with the purpose of offering a useful synthesis that contributes to the understanding of the clinical impact of these interventions and supports the design of future digital strategies. This review answers the following question: In adult or pediatric patients with asthma (P), what is the effectiveness of technological tools for asthma self-management (I), compared to standard care without technological interventions (C), on asthma control, treatment adherence, reduction in exacerbations, improvement of pulmonary function, symptom-free days, and HRQoL (O)?
2. Materials and Methods
2.1. Study Protocol
We conducted a systematic review and meta-analysis, adhering to the recommendations of the Cochrane handbook and the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement [,,]. To promote transparency and allow for methodological auditing, the study protocol was registered a priori in the PROSPERO international database (Registration: CRD420251040601).
2.2. Eligibility Criteria
2.2.1. Inclusion Criteria
Studies were deemed eligible based on the subsequent criteria: (i) The study design was a randomized controlled trial (RCT), irrespective of follow-up duration or specific RCT design. (ii) The publication window was between January 2010 and January 2025. To ensure a comprehensive analysis and capture all available evidence, no language restrictions were imposed. (iii) The study population included both adult and pediatric patients with an asthma diagnosis, where the intervention involved a technology-based tool for self-management as an adjunct to standard care. (iv) The trial reported at least one key outcome: asthma control, treatment adherence, exacerbations, pulmonary function (forced expiratory volume in 1 s [FEV1], FEV1/forced vital capacity [FVC], and peak expiratory flow [PEF]), symptom-free days, or HRQoL.
2.2.2. Exclusion Criteria
We excluded studies if they met the following criteria: (i) The cohort included patients with significant comorbidities that could confound symptom control. (ii) The publication type was a pilot study, conference abstract, protocol, or letter to the editor. (iii) The document was a preprint. (iv) A full-text version was not accessible for evaluation. (v) The publication represented redundant data from a cohort already included in another selected study.
2.3. Data Sources and Search Strategy
Three researchers (S.M.R., L.D.C., M.P.P.G.) independently formulated the search strategy between March and April 2025. A systematic consultation was performed across six major databases: PubMed, Cochrane Central Register of Controlled Trials (CENTRAL), Scopus, Web of Science, LILACS, and Science Direct. These databases were selected for their extensive coverage of pertinent scientific literature, international recognition, and the quality of their indexed studies, which facilitates access to reliable evidence and minimizes publication bias.
The search utilized Boolean combinations of terms related to the disease (“Asthma” OR “bronchial asthma”) and the intervention (“telemedicine” OR “mobile applications” OR “mHealth” OR “mobile health” OR “smartphone” OR “mobile phone” OR “eHealth”), combined with RCT filters (“randomized controlled trial” OR “controlled clinical trial” OR “randomized”). This core strategy was modified as needed for the syntax of each database.
To locate additional works, a manual review of the reference lists of included articles was conducted, supplemented by exploring other web sources. When necessary, original documents were consulted for missing data. Bibliographic records were managed, organized, and de-duplicated using the Rayyan—Intelligent Systematic Review platform (https://www.rayyan.ai/, accessed on 1 April 2025).
2.4. Selection and Extraction of Information
Article screening proceeded in two phases. Initially, two researchers (Y.L. and F.E.C.M.) independently screened all titles and abstracts to remove irrelevant publications. Studies proceeding from this stage underwent a full-text evaluation. If a full manuscript could not be obtained, the corresponding authors were contacted.
Any disagreements regarding inclusion were settled through discussion and consensus; a third reviewer was available to arbitrate unresolved conflicts. We quantified inter-rater agreement using Cohen’s Kappa.
Following final selection, three reviewers (S.M.R., L.D.C., and M.P.P.G.) performed data extraction, collecting information on:
- Study characteristics: Main author, publication year, country.
- Participant details: Sample size per group, age distribution, proportion of men.
- Intervention specifics: Type of technological resource, application context, duration.
- Outcomes: All reported clinical outcomes of interest. This extracted information was subsequently verified for accuracy and completeness by two other researchers (C.L.P. and F.E.C.M.).
2.5. Risk of Bias Assessment
Two reviewers (Y.L.M. and C.L.P.) independently evaluated the methodological quality of the included RCTs. We utilized the Cochrane Risk of Bias 1 (RoB 1) tool, implemented via Review Manager (RevMan) software (version 5.4®). The assessment focused on key domains: (i) random sequence generation, (ii) allocation concealment, (iii) blinding of participants and personnel, (iv) blinding of outcome assessors, (v) management of incomplete outcome data, and (vi) selective reporting. Each domain was judged as representing a low, high, or uncertain risk of bias. As with the selection process, any discrepancies in assessment were resolved through consensus discussion.
2.6. Statistical Analysis
We performed the statistical analysis using RevMan (version 5.4®) [,]. For all outcomes, we calculated the effect size and corresponding 95% confidence intervals (CI). A quantitative synthesis (meta-analysis) was conducted only for outcomes reported by two or more studies, including asthma control score, pulmonary function, symptom-free days, and HRQoL.
For continuous data, we extracted means and standard deviations (SD). When studies reported medians and interquartile ranges, we applied established methods for conversion to means and SDs. If outcomes were assessed at multiple time points, we prioritized the mean difference or, alternatively, the most recent available measurement.
We addressed heterogeneity in measurement scales. For scales with opposite interpretive directions (e.g., AQLQ-M, where lower is better, and ACQ, where lower is better), we applied transformations to unify the effect direction. For AQLQ-M, we used the formula: (maximum score + 1)—obtained score. For ACQ, we inverted the signs of the within-group mean differences. No data imputation was necessary as no significant missing data were identified in the primary studies.
The choice of effect measure depended on the data type. We used relative risk (RR) for dichotomous variables and mean difference (MD) for continuous outcomes. When studies used different scales for the same continuous outcome, we employed the standardized mean difference (SMD) to ensure comparability. A qualitative, narrative summary was provided for outcomes like treatment adherence and exacerbations, as high variability or insufficient data precluded their quantitative pooling.
We planned a priori subgroup analyses based on: tool type (web platform, mobile App, etc.), supervisor profile (supervised by a health professional vs. not), supervision mode (in-person vs. remote), intervention duration (up to 3 months, 3–6 months, >6 months), and population age (adult vs. pediatric). Sensitivity analyses were also planned by removing individual studies.
To account for heterogeneity, we used a random-effects model (inverse variance method) when the I2 statistic was >50%, and a fixed-effects model when I2 was ≤50%. Statistical significance was set at p < 0.05.
Publication bias was assessed by visual inspection of funnel plots and formally with Egger’s test in Jamovi® software (version 2.3). Finally, we assessed the overall certainty of the evidence using the GRADE (Grading of Recommendations, Assessment, Development and Evaluations) framework. Each outcome was rated as high, moderate, low, or very low based on risk of bias, inconsistency, imprecision, indirectness, and publication bias. These findings were compiled in a “Summary of Findings” (SoF) table.
3. Results
3.1. Studies Identified for the Review
A total of 1186 records were identified through search strategies in electronic databases; additionally, 32 records were retrieved through secondary search. After the removal of 186 duplicate records, 1000 studies were evaluated. Of these, 944 were excluded after reading titles and abstracts (Cohen’s Kappa index = 94%). Fifty-six documents were selected for full-text evaluation. Ten of these could not be retrieved. Among the 46 studies evaluated, 22 were excluded for the following reasons: they were not randomized clinical trials (n = 13), they were study protocols (n = 4), they did not include the target population (n = 2), or they did not evaluate pre-established outcomes (n = 3). The inter-rater agreement rate in this phase was 91%. In parallel, 32 additional records obtained by complementary methods were evaluated. Of these, 10 were not retrieved, and 20 were excluded after full-text evaluation: four were not randomized clinical trials and 16 did not report the outcomes of interest. In total, 26 studies met the inclusion criteria and were incorporated into the qualitative and quantitative synthesis. The detailed process of identification, selection, and exclusion of studies is presented in Figure 1.
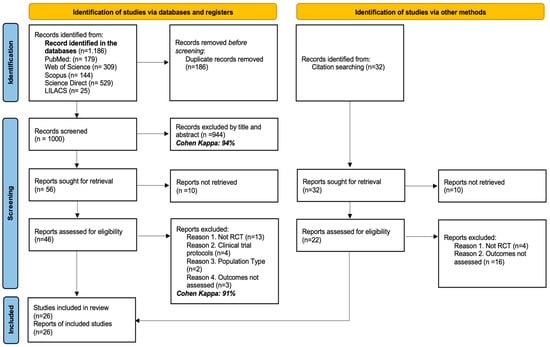
Figure 1.
PRISMA flow diagram with the search and study selection strategy.
3.2. Characteristics of the Studies Included in the Review
Twenty-six RCTs, published between 2011 and 2024, were included, with a higher concentration in the last five years, reflecting a sustained interest in evaluating interventions for asthma management. The studies were conducted in 15 countries, with the United States being the most represented (n = 8), followed by the Netherlands (n = 3); the other countries were represented by one or two investigations. The included populations covered a wide age range, from three-year-old children to 77-year-old adults, with studies specifically focused on pediatric and adolescent stages. In most cases, the participants had persistent, poorly controlled, or moderate to severe asthma, managed with conventional or specialized therapy. The most frequently evaluated outcomes were asthma control, HRQoL, treatment adherence, and pulmonary function; to a lesser extent, symptom-free days and exacerbations were measured. Several studies reported multiple outcomes simultaneously, although heterogeneity was identified in the methods and scales used. See Table 1.

Table 1.
Characteristics of the studies included in the review.
3.3. Characteristics of the Population and the Intervention Applied
All included studies evaluated digital interventions for asthma management using tools classified as mobile applications (n = 16), web platforms (n = 9), and telephone follow-up (n = 1). The number of participants ranged from 19 to 899, with sample sizes generally balanced between the intervention and control groups. The proportion of male participants was heterogeneous, with values ranging from 22% to 71%, without a uniform pattern among studies. Regarding the supervision modality, remote follow-up was the most frequently used (62%), while in ten studies, in-person strategies were employed. The follow-up time was variable, being most frequent between 3 and 6 months (38%), followed by those with a duration of more than six months (34%). See Table 2.

Table 2.
Characteristics of the participants and the intervention implemented.
3.4. Findings from the Bias Risk Analysis
The risk of bias analysis was conducted considering different methodological domains, as shown in Figure 2. This assessment was performed in the RevMan 5.4® package (accessed: June 2025), which allowed for the identification of both strengths and limitations in the design and execution of the clinical trials.
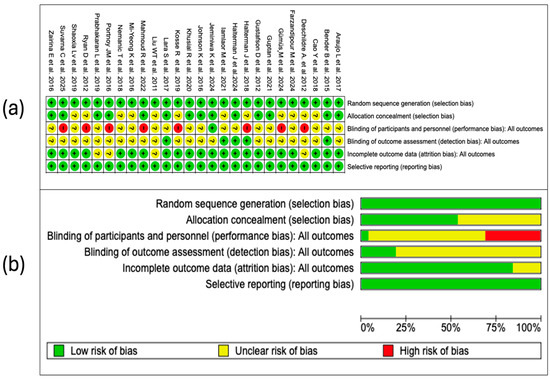
Figure 2.
An assessment of the potential risk of bias was conducted across all studies included in the review. Each item was categorized using a three-level system: (a) “+” symbol designates low risk of bias, “?” indicates uncertainty, and “–” denotes high risk. These categories are visually represented through a color-coded scale (green for low, yellow for unclear, and red for high risk). (b) Furthermore, a summary figure displays the overall distribution of studies within each category, allowing a comprehensive overview [,,,,,,,,,,,,,,,,,,,,,,,,,].
3.4.1. Random Sequence Generation
In all of the studies incorporated in this systematic review, the generation of the random sequence was categorized as low risk of bias. This evaluation indicates that the randomization methods were described in sufficient detail and executed according to robust methodological standards. The absence of judgments reporting uncertain or high risk in this domain strengthens the internal validity of the included trials and supports the reliability of the estimated effects.
3.4.2. Allocation Concealment
Of the 26 included studies, 12 (46%) were assessed as having a low risk of bias in this domain, while 14 (54%) had an uncertain risk. This rating was mainly due to the lack of sufficient information about the methods used to ensure that the allocation sequence remained concealed at the time of recruiting and assigning participants. Although allocation concealment is an essential methodological procedure to prevent the introduction of bias in the allocation of groups, its proper implementation does not depend on the type of intervention evaluated. Therefore, the identified uncertain risk reflects deficiencies in the methodological reporting, rather than inherent limitations of the study design.
3.4.3. Blinding of Participants and Personnel
In this domain, 8 studies (31%) were assessed as having a high risk of bias, 17 (65%) with an uncertain risk, and only 1 (4%) with a low risk. The high proportion of studies with high or uncertain risk is related to the nature of the interventions analyzed, focused on the use of digital tools and telemedicine strategies, in which the blinding of participants and personnel is often difficult to implement or not feasible. In these contexts, awareness of the allocation can influence the expectation of response or the subjective perception of the outcomes. Additionally, in many studies, the procedures adopted to mitigate this bias were not clearly described, which limited the possibility of making a more precise judgment.
3.4.4. Blinding in the Assessment of Outcomes
In this domain, in most cases (81%), the authors did not specify whether the outcome assessors were blinded to the allocation of the participants, which limited the possibility of making an accurate judgment about the presence or absence of detection bias. Although in digital interventions some outcomes are obtained through self-reporting, the lack of methodological information regarding the masking of those who collect or analyze the data introduces uncertainty about the objectivity in the evaluation of the results.
3.4.5. Incomplete Outcomes
Most of the studies were assessed as having a low risk of bias in this domain. Only five studies presented an uncertain risk, due to the lack of clarity in the description of the management of losses during follow-up or in the methods applied to handle missing data. In general terms, these results reflect an adequate methodological quality in the management of incomplete data in the included studies.
3.4.6. Selective Reporting
All included studies were assessed as having a low risk of bias in this domain. The coherence between the reported outcomes and those pre-specified in available registries or protocols, as well as the absence of apparent omissions, indicates adequate compliance with the principles of transparency in the communication of results. This methodological consistency reinforces the credibility of the findings and supports the integrity of the body of evidence analyzed (Figure 2a).
3.4.7. Summary of Risk of Bias
The overall assessment of the risk of bias, summarized in Figure 2b, shows an acceptable methodological quality in most of the evaluated domains. A predominance of low risk is observed in the generation of the random sequence, incomplete outcome data, and selective reporting, which indicates an adequate application of key procedures to ensure the internal validity of the included trials. However, relevant limitations are identified in sensitive domains. In particular, the blinding of participants and personnel presents the highest proportion of studies with uncertain risk, followed by a considerable number of studies with high risk in allocation concealment and in the blinding of outcome assessors. These methodological deficiencies, although expected in the context of non-pharmacological interventions such as telemedicine, should be taken into account when interpreting the estimated effects, especially when the outcomes depend on the patient’s perception. Overall, the risk of bias profile supports the robustness of the evidence for several domains, but also highlights critical areas that could affect confidence in the results of some primary studies.
3.5. Qualitative Synthesis of Scientific Evidence
3.5.1. Treatment Adherence
In total, 9 of the 26 studies included in the review evaluated adherence in the included asthmatic patients. In the trial by Bender B et al. [], an automated telephone call system with voice recognition, integrated with the electronic medical record, significantly improved adherence to inhaled corticosteroids in children with persistent asthma, with an increase of 25.4% in the intervention group compared to the control group according to the intention-to-treat analysis. Similarly, Kosse R et al. [] reported a significant increase in adherence in adolescents with low baseline adherence, especially in those with uncontrolled asthma, by implementing a mobile application with educational functions, reminders, and pharmaceutical contact.
In the study by Shaoxia L et al. [], a nurse-led intervention assisted by a mobile application increased treatment adherence in children (experimental group 94.4% versus control group: 92.6%, p < 0.05). Likewise, Mi-Yeong K et al. [] observed a statistically significant improvement in adherence scores after eight weeks of using a monitoring application based on action plans (p = 0.017). On the contrary, Gustafson et al. [], although they evaluated a combined intervention of eHealth and monthly nursing calls, did not find significant differences in adherence between groups, which the authors attribute to limitations in the measurement methods used. Although additional studies were considered, overall, the evidence suggests that digital interventions with interactive components and professional support can improve adherence, especially in populations with low baseline adherence, although better measurement methods are required to robustly validate these effects.
3.5.2. Disease Exacerbations
Four studies included in the review evaluated the frequency of exacerbations, although with heterogeneous definitions, follow-up durations, and measurement methods [,,,]. In the randomized trial by Shaoxia L et al. [], which included 152 children with asthma, a more pronounced decrease in the frequency of exacerbations was observed in the intervention group (nurse-led model assisted by a mobile application) compared to the control group. In contrast, the study by Deschildre A et al. conducted in 50 children with severe uncontrolled asthma, showed no statistically significant differences in the median of severe exacerbations between the groups: 2.0 (IQR 1.0–4.0) in the group with daily telemonitoring of FEV1 and 3.0 (IQR 1.0–4.0) in the conventional treatment group (p = 0.38). Nemanic T et al. [] documented a significant decrease in the proportion of patients who experienced exacerbations in both groups during the follow-up year (intervention: 47.1%; control: 49.0%). Although a trend towards a greater decrease was observed in the intervention group (median: 1.0 [IQR: 0–2]) compared to the control group (median: 0 [0, 1]), this difference did not reach statistical significance; furthermore, 13.7% of the patients in the intervention group reported a shorter duration of exacerbations. Finally, Ryan D et al. [], in a multicenter trial with 288 adolescents and adults, found no differences between the electronic and paper monitoring groups in terms of exacerbations, systemic steroid use, or unscheduled consultations, concluding that the intervention did not offer additional clinical benefits when both groups received structured care according to clinical guidelines.
3.6. Meta-Analysis
A meta-analysis was conducted encompassing four primary outcome measures (asthma control, pulmonary function, HRQoL, and symptom-free days) with a total of 5152 patients, from the studies of Araujo L et al. []., Bender B et al. [], Cao Y et al. [], Deschildre A et al. [], Farzandipour M et al. [], Gümüs M et al. [], Gupta R et al. [], Gustafson D et al. [], Halterman J et al. [], Halterman J et al. [], Iamlaor U et al. [], Jeminiwa K et al. [], Johnson K et al. [], Khusial R et al. [], Kosse R et al. [], Lara S et al. [], Liu W et al. [], Mahmoud R et al. [], Mi-Yeong K et al. [], Nemanic T et al. [], Portnoy M et al. [], Prabhakaran L et al. [], Ryan D et al. [], Shaoxia L et al. [], Suvarna C et al. [] and Zairina E et al. [].
3.6.1. Asthma Control
A total of 17 studies, including 2496 participants, evaluated the effect of digital health tools on asthma control. The meta-analysis, performed under a random-effects model, did not show a statistically significant difference between the groups (SMD: 0.90; 95% CI: −0.04 to 1.85; p = 0.06). However, the analysis revealed substantial heterogeneity among the studies (I2 = 98%, p < 0.00001), suggesting significant variability in the estimated effects. See Figure 3.
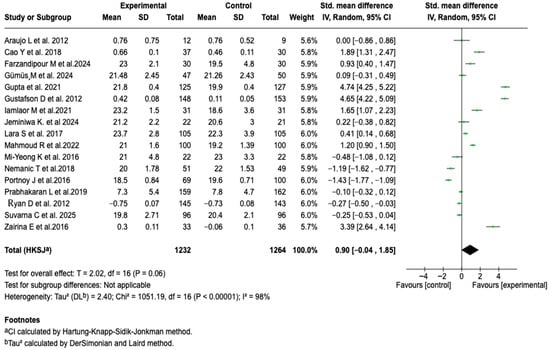
Figure 3.
Forest plot of the effect of digital health tools on asthma control in patients with a confirmed diagnosis of the disease [,,,,,,,,,,,,,,,,].
Given the high heterogeneity observed in the overall analysis, possible sources of variability were explored through subgroup analyses. No significant differences were identified associated with the type of tool used, the supervisor’s profile, the follow-up time, or the age group. However, a relevant difference was evidenced based on the type of supervision (See Figures S1–S5 of the Supplementary Material). Studies that implemented remote supervision showed a significant effect in favor of the intervention (SMD: 1.82; 95% CI: 0.28 to 3.37; p = 0.03), while those with in-person supervision showed no difference between groups. The comparison between subgroups was statistically significant (Chi2 = 6.73; p = 0.009), suggesting a possible modulating effect of the type of supervision on the efficacy of digital interventions. See Figure 4. The sensitivity analyses, modifying the meta-analysis model and gradually excluding individual studies, yielded consistent results, without a significant change in the size or direction of the effect.
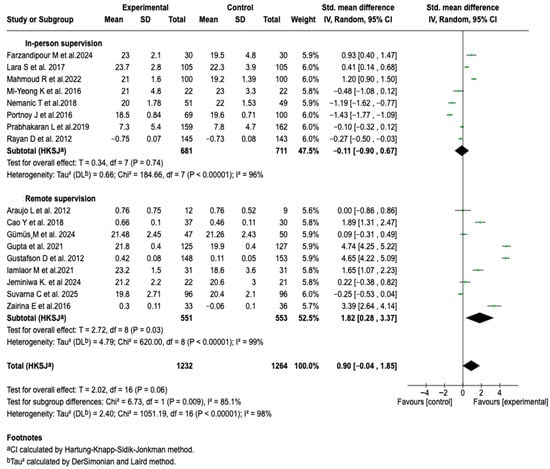
Figure 4.
Subgroup analysis of the effect of supervision mode on the efficacy of digital health tools for asthma control [,,,,,,,,,,,,,,,,].
3.6.2. Pulmonary Function
Forced Expiratory Volume in the First Second (FEV1)
Ten studies (n = 906) analyzed the impact of digital interventions on FEV1. The meta-analysis showed a significant difference in favor of the intervention group (SMD: 1.53; 95% CI: 0.54 to 2.51; p = 0.007), with considerable heterogeneity (I2 = 96%). This suggests that the incorporation of digital tools in the management of patients with asthma can translate into benefits in pulmonary function, particularly in the evaluated parameter (See Figure 5).
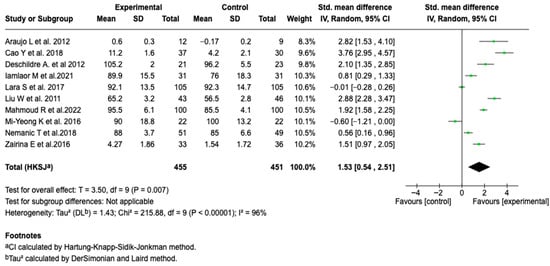
Figure 5.
Forest plot of the effect of digital health tools on forced expiratory volume in one second in patients with asthma [,,,,,,,,,].
To explore the heterogeneity observed in the FEV1 analysis, subgroup analyses were performed. No differences were identified based on the type of tool, supervisor profile, age group, or duration of the intervention (See Figures S6–S10 of the Supplementary Material). However, a differential effect was evidenced according to the mode of supervision. Remote supervision was associated with greater efficacy (SMD: 2.18; 95% CI: 1.09 to 3.28; p = 0.004) and less heterogeneity, although it remained high (See Figure 6). The sensitivity analyses performed by modifying the model and removing individual studies did not alter the behavior of the results, which confirms the consistency of the findings.
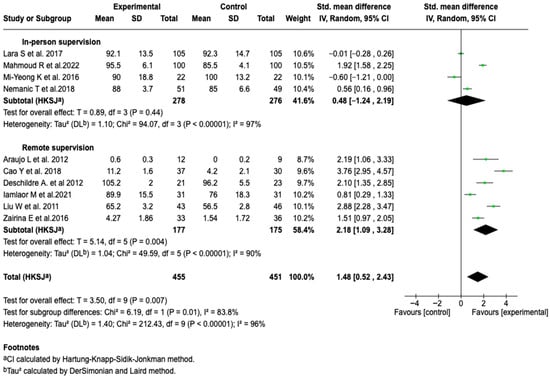
Figure 6.
Subgroup analysis of the effect of supervision mode on the efficacy of digital health tools in improving FEV1 in patients with asthma [,,,,,,,,,].
FEV1/FVC
The pooled analysis of four studies (n = 298) showed that digital tools were associated with a significant improvement in the FEV1/FVC ratio (SMD: 1.20; 95% CI: 0.29 to 2.10; p = 0.02). The heterogeneity was moderate (I2 = 78%). Due to the limited number of studies, it was not possible to perform formal analyses to explore heterogeneity. This result indicates a possible effect of digital interventions on the degree of bronchial obstruction, a central parameter in the functional monitoring of asthma. The sensitivity analyses showed no changes in the magnitude or direction of the effect, which supports the consistency of the result. See Figure 7.
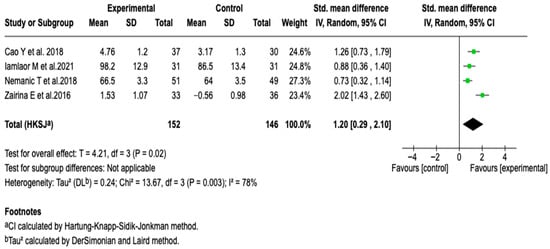
Figure 7.
Forest plot of the effect of digital health tools on the FEV1/FVC ratio in patients with asthma [,,,].
Peak Expiratory Flow
Five studies with a total of 472 patients evaluated the effect of digital tools on PEF. The meta-analysis did not show a statistically significant difference between the groups (SMD: 1.43; 95% CI: −1.19 to 4.05; p = 0.20), and reported considerable heterogeneity (I2 = 97%). As seen in Figure 8, none of the subgroup or sensitivity analyses modified the direction or magnitude of the effect, nor did they show consistent associations (See Figures S11–S13 of the Supplementary Material). These results suggest that, unlike other spirometric parameters, the impact of digital interventions on PEF is limited.
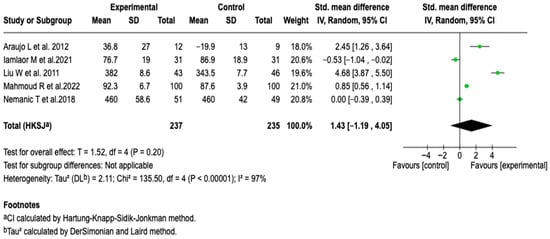
Figure 8.
Forest plot analysis of the effect of supervision mode on the efficacy of digital health tools in improving PEF in patients with asthma [,,,,].
3.6.3. Symptom-Free Days
Five studies (n = 1376) evaluated the impact of digital interventions on symptom-free days. The analysis did not show that the intervention was related to a greater number of symptom-free days (MD: 0.70; 95% CI: −0.80 to 2.20; p = 0.27), with a high heterogeneity of 84% (See Figure 9). The subgroup analyses according to the type of tool, profile of the person responsible for supervision, and follow-up modality did not reveal consistent associations (See Figures S14–S16 of the Supplementary Material). No relevant changes were identified in the sensitivity analyses either.
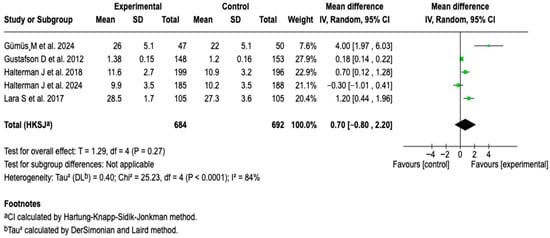
Figure 9.
Forest plot of the effect of digital health tools on symptom-free days in patients with asthma [,,,,].
3.6.4. Health-Related Quality of Life
The integrated analysis of twelve studies (n = 1706) did not establish a clear superiority of digital tools over standard management in improving HRQoL in patients with asthma (SMD: 0.86; 95% CI: −0.12 to 1.84; p = 0.08). See Figure 10.
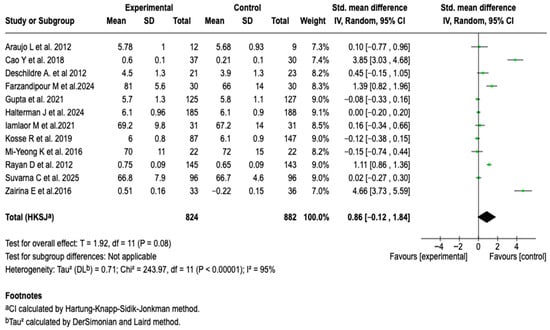
Figure 10.
Forest plot of the effect of digital health tools on HRQoL in patients with asthma [,,,,,,,,,,,].
Given the high degree of heterogeneity observed in the overall analysis, a subgroup analysis was performed in order to explore possible sources of variability. When stratifying the studies according to the type of digital tool used, a significant difference was found between subgroups (p = 0.001). In the subgroup of interventions based on mobile applications, a significant association was found in favor of the experimental group (SMD = 1.19; 95% CI: 0.47 to 1.90; p = 0.001), see Figure 11. No differences were observed in the rest of the subgroup analyses (See Figures S17–S21 of the Supplementary Material).
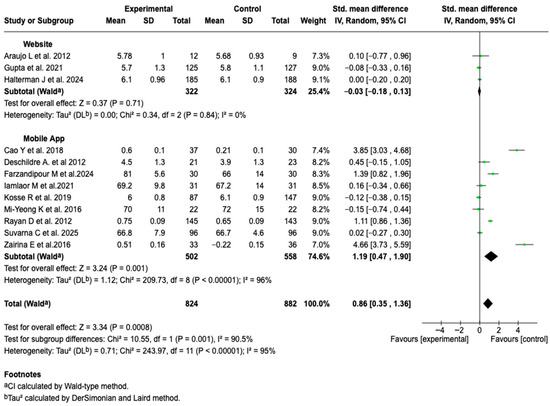
Figure 11.
Subgroup analysis of the effect of digital health tool type on HRQoL outcomes in patients with asthma [,,,,,,,,,,,].
3.7. Results of Publication Bias Assessment
We evaluated publication bias using funnel plots for asthma control (Figure 12a), FEV1 (Figure 12b), and HRQoL (Figure 12c). The asthma control and FEV1 plots showed symmetrical distribution, with no relevant signs of asymmetry. In contrast, HRQoL presented visual asymmetry. Egger’s regression confirmed the absence of bias for asthma control (p = 0.29) and FEV1 (p = 0.05), but showed significant bias in HRQoL (p = 0.008). See Figure 12.
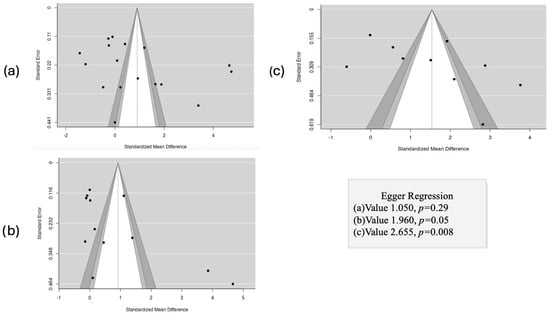
Figure 12.
Evaluation of publication bias using funnel plots and Egger’s regression test for the outcomes asthma control (a), FEV1 (b), and HRQoL (c).
3.8. Results of the GRADE Certainty of Evidence Assessment
The certainty of the evidence for the evaluated outcomes was mostly very low, mainly due to serious imprecision, wide confidence intervals that in several cases crossed the line of no effect, and to the marked inconsistency reflected in the high heterogeneity between studies. Some outcomes, such as FEV1/FVC, reached a low level of certainty, while the rest were rated as very low, which limits confidence in the estimated effects and suggests that the results should be interpreted with caution. See Table 3.

Table 3.
Findings from the assessment of the certainty of evidence using the GRADE approach.
4. Discussion
4.1. Main Findings of the Review
The present meta-analysis aimed to assess the effectiveness of digital health interventions in supporting asthma self-management among pediatric and adult populations. Twenty-six randomized controlled trials, comprising more than 5000 participants across multiple clinical settings, were included. The pooled analysis revealed that digital interventions were associated with statistically significant improvements in pulmonary function parameters, underscoring their potential as an evidence-based adjunct in asthma management. Specifically, a favorable effect was observed on FEV1 (SMD: 1.53; 95% CI: 0.54 to 2.51; p = 0.007) and on the FEV1/FVC ratio (SMD: 1.20; 95% CI: 0.29 to 2.10; p = 0.02), which suggests that these tools can contribute to reducing airway obstruction. In contrast to the above, the pooled analysis did not show a statistically significant effect of digital interventions on asthma control, PEF, symptom-free days, or HRQoL. However, it is imperative to note that most of these outcomes presented considerable statistical heterogeneity (I2 > 80%), which limits the certainty of the overall estimates and suggests that the effectiveness of these tools is context-dependent. The subgroup analyses allowed for the exploration of this variability, revealing patterns of interest. It was identified that the mode of supervision significantly modulates the effectiveness of the interventions; those with remote supervision showed a superior benefit both in asthma control and in the improvement of FEV1. Similarly, when analyzing HRQoL, it was observed that interventions based specifically on mobile applications were associated with a significant improvement, unlike other technological modalities. In addition to the quantitative findings, the qualitative synthesis suggests that digital tools can improve treatment adherence, although the evidence on their impact on reducing exacerbations is not conclusive.
4.2. Comparison with Pior Research
The findings of this meta-analysis, which reveal a significant improvement in pulmonary function but heterogeneous results for subjective outcomes, are consistent with previous literature, which describes a promising though complex landscape for digital health in asthma. Our conclusion that digital tools robustly improve FEV1 and the FEV1/FVC ratio aligns with systematic reviews such as that of Ramsey et al. [], who found that, in the pediatric population, 87% of digital interventions improved adherence and 53% were associated with better health outcomes. This effect on pulmonary function can be explained in light of the finding by Biblowitz et al. [], who observed that electronic monitoring devices (EMDs), especially those with reminders, were the most effective for improving objective treatment adherence. Consequently, it is plausible that digital monitoring and reminders promote a more consistent use of control medications, which translates directly into measurable physiological improvements. However, it is crucial to contextualize this improvement.
Although the present meta-analysis found high standardized mean differences for the pulmonary function parameters FEV1 and FEV1/FVC, these values must be interpreted with caution. While they are statistically significant, their magnitude may not be entirely plausible from a clinical standpoint and may reflect the influence of studies with small sample sizes and greater variability [,]. Furthermore, there was notable heterogeneity among the interventions, measurement methods, and follow-up durations. This variability could have amplified the combined effect. Therefore, these results likely represent an upper limit of the possible benefit, rather than the actual average clinical impact of digital self-management interventions on pulmonary function.
A systematic review with meta-analysis by Miller et al. [] concluded that, while mobile technology interventions were superior to standard treatment, they did not show additional efficacy compared to traditional paper-based monitoring. This important nuance suggests that the key component may be the act of self-monitoring itself, rather than the technological modality, which helps to explain the high heterogeneity and the sometimes modest effects found in our own analysis and in the literature in general. Perhaps the most significant result of our review is the identification of the moderators of efficacy. The absence of an overall effect on asthma control and quality of life, but the presence of a clear benefit in specific subgroups, refines current knowledge. Our finding that remote supervision is a key factor for improving both asthma control and FEV1 is strongly supported by the comprehensive scoping review by Mosnaim et al. []. In their analysis, they classified interventions into three levels and determined that only interactive and bidirectional interventions, those that involve two-way communication between the patient and the system or professional, demonstrated consistent improvements in both adherence and the reduction of asthma symptoms. Passive or unidirectional interventions (e.g., simple reminders or data collection without feedback) did not achieve this double impact. This suggests that technology is more potent not as a passive tool, but as a channel to facilitate continuous and responsive care. Likewise, our finding that mobile applications significantly improve HRQoL, despite the overall effect not being significant, also finds echo in the literature. Reviews such as that of Snoswell et al. [] reported an overall positive but small effect of telehealth interventions on HRQoL. The superiority of mobile applications in our analysis could be because more modern platforms better integrate the principles of behavior change. An analysis by Tinschert et al. [] of publicly available applications revealed that, although many lacked clinical validation, the highest quality ones tended to incorporate a greater number of behavior change techniques and gamification components. This could explain why applications, being more engaging and better designed to foster user commitment, have a more noticeable impact on an outcome as subjective and multifactorial as HRQoL.
These observations underscore the nuanced nature of the interventions’ effectiveness based on the supervision modality and strategy used, highlighting that the impact of digital tools depends not only on their content but also on how they are delivered and utilized. In particular, remote supervision and interactive interventions appear to enhance clinical outcomes. Furthermore, the choice of tool (e.g., mobile applications versus web platforms) may influence the degree of user engagement and, consequently, the effect on the measured outcomes. This differential approach reinforces the need to consider the specific characteristics of each subgroup when assessing the effectiveness of digital interventions. The different digital tools used for asthma self-management are shown in Figure 13.
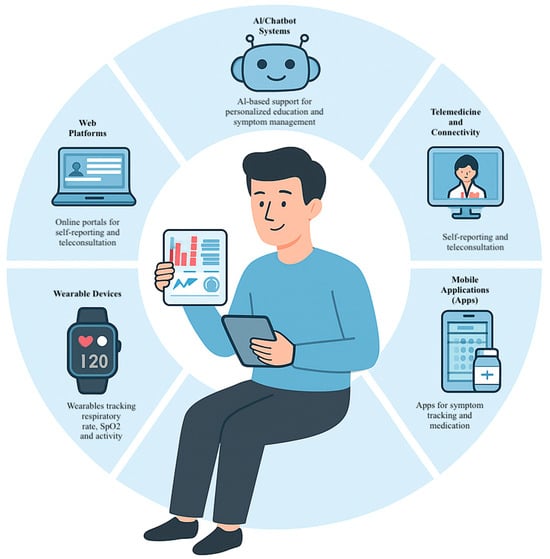
Figure 13.
Technological tools for asthma self-management: platforms, devices, and digital support systems used in the clinical trials included in the review. These tools encompass artificial intelligence systems, which provide personalized support in education and symptom management; web platforms for self-reporting and teleconsultation; wearable devices that monitor respiratory parameters and physical activity; mobile applications for symptom and medication tracking; and telemedicine systems that facilitate connectivity between patients and health professionals. The integration of these technologies promotes a more proactive, continuous, and patient-centered management, which can contribute to optimizing self-management and clinical decision-making in asthma.
A point that deserves special attention is the high heterogeneity (I2 > 80%) that characterizes our results, a constant in this field of research Mosnaim et al. []. This variability is a reflection of the diversity of technologies, populations, follow-up durations, and outcomes evaluated. It is in this context of dispersion that our work takes on special relevance, as it is not limited to confirming the general potential of digital health, but advances in the dissection of this heterogeneity, demonstrating that the mode of supervision and the type of tool are determining factors that must be considered for the design of future, more effective and personalized interventions.
Regarding treatment adherence and exacerbation frequency, the analyzed evidence was limited and heterogeneous, which precluded a quantitative synthesis. In general, the included studies showed disparate results, likely attributable to variability in measurement methods (ranging from electronic records to self-reported questionnaires) and the relatively short duration of the interventions. Similar to findings reported by previous reviews, such as those by Kosse et al. [] and Ramsey et al. [], programs that integrated interactive components and professional support tended to show more consistent improvements, especially in patients with low baseline adherence.
Regarding exacerbations, the available studies presented heterogeneous definitions and follow-up periods. Although some reported a reduction in the frequency or duration of episodes, with evidence suggesting low certainty, the majority did not reach statistical significance—a pattern also described in the review by Chan et al. [] It is likely that factors such as small sample sizes, population diversity, and short follow-up durations contributed to the lack of conclusive results. These findings, consistent with the previous literature, suggest that digital interventions show promising potential for optimizing adherence and reducing exacerbations.
Beyond the quantitative assessment, the possible mechanisms that could contribute to the observed effects of digital tools on asthma self-management can be interpreted from three complementary dimensions: behavioral, clinical, and organizational. On the behavioral level, reminders and automated feedback could foster better treatment adherence and more consistent symptom tracking []. In the clinical dimension, continuous monitoring via wearable devices or mobile applications generally facilitates the early detection of variations in respiratory function or symptom control, which could allow for a more timely response from the patient or healthcare team []. At the organizational level, interactive platforms and telemedicine systems have the potential to improve communication and care coordination, promoting more integrated follow-up [].
Taken together, these potential mechanisms suggest that the impact of digital interventions may depend not only on the technology used, but also on the degree to which they manage to support patient behavior, facilitate early clinical response, and strengthen care continuity—aspects that broaden the understanding of digital health’s role in asthma management.
4.3. Limitations of the Included Studies
Numerous methodological limitations were identified among the included studies, each of which merits detailed examination. Firstly, a considerable proportion of the trials had small sample sizes, which compromises the statistical power of the analyses and limits the ability to detect clinically significant effects, especially in specific subgroups. Secondly, there was marked heterogeneity in the design of the interventions. The studies evaluated a wide spectrum of digital tools (mobile applications, web platforms, telephone follow-up), with highly variable functionalities, duration, and intensity. This diversity makes direct comparison between studies and the identification of the “active ingredients” responsible for the potential benefits difficult. A recurring methodological limitation, identified through the risk of bias assessment, was the high or uncertain risk of blinding bias, for participants, personnel, and assessors. While this weakness is inherent in behavioral interventions where masking is unfeasible, it can introduce performance and detection biases, especially in subjective outcomes such as HRQoL or self-reported asthma control. Finally, the variability in populations (pediatric vs. adult, different degrees of asthma severity) and the diversity of measurement scales used for the same outcome contributed to the inconsistency of the results.
4.4. Limitations of the Review
The current meta-analysis revealed substantial statistical heterogeneity across most evaluated outcomes, thereby decreasing confidence in the pooled effect estimates and indicating that the efficacy of digital health interventions may vary according to contextual or population-specific factors. Although subgroup analyses were performed to explore this variability, significant residual heterogeneity persisted, indicating the influence of other unmeasured factors. On the other hand, outcomes of great relevance such as treatment adherence and the frequency of exacerbations could not be meta-analyzed due to the scarcity of studies that reported them in a standardized manner, limiting the synthesis to a qualitative analysis. Additionally, a significant publication bias was detected for the quality of life outcome, which suggests that studies with unfavorable results may have a lower probability of being published, leading to a possible overestimation of the effect.
The GRADE analysis revealed that the certainty of the evidence for most of the outcomes was low or very low. This rating reflects the recurrent methodological limitations in the primary studies, the imprecision in the results, and the marked inconsistency, which restricts the formulation of firm clinical recommendations. On the other hand, the search strategy was designed to identify randomized controlled trials on digital interventions oriented toward asthma self-management, using specific digital health descriptors such as telemedicine, mHealth, eHealth, and mobile applications. These terms broadly cover the technological tools used to promote self-care in asthma. Nonetheless, we recognize that future reviews could explore complementary strategies that include other descriptors related to self-management, in order to further broaden the sensitivity of the search.
4.5. Strengths of the Review
This meta-analysis makes a significant contribution by updating the available evidence with randomized clinical trials published up to the year 2024, which allows for a faithful reflection of the current state of knowledge. The strict adherence to the PRISMA guidelines and the prior registration of the protocol in PROSPERO reinforce the methodological transparency and minimize the risk of reporting bias. Likewise, this review is distinguished by the application of a rigorous methodological approach, through the combined use of the RoB I tool for the assessment of risk of bias, the Jadad scale for quality, and the GRADE system to rate the certainty of the evidence. The methodological approach adopted in this review enhances analytical robustness by facilitating a clearer understanding of both the strengths and potential weaknesses within the pooled evidence. Moreover, conducting an extensive literature search across diverse databases—without imposing language constraints—and implementing a parallel, independent procedure for study selection and data extraction provide additional assurance regarding the internal validity of the results.
4.6. Clinical and Public Health Implications
From a clinical perspective, the findings of this review suggest that digital health tools can be a useful complement in the management of asthma, particularly for improving objective parameters of pulmonary function. Health professionals could consider recommending these tools as a coadjuvant strategy to reinforce self-management. However, implementation should not be indiscriminate. The results indicate that the simple use of a digital tool does not guarantee success; the effect seems to be mediated by key factors []. The superiority of interventions with remote supervision suggests that technology is more effective when integrated into a care model that maintains a connection with the health professional, allowing for active follow-up and personalized feedback []. Similarly, mobile applications appear to be more effective for improving quality of life, possibly due to their greater accessibility and ability to integrate into the patient’s daily life []. Given the low certainty of the evidence, digital tools should not be considered a replacement for standard care, but a complement. The decision to incorporate a digital tool should be individualized, considering the patient’s preferences, their digital competence, and the availability of interventions that have been shown to be effective [].
From a public health standpoint, the implications are broader and more complex. If deployed equitably, digital tools have the potential to extend the reach of asthma care to geographically remote or underserved populations, possibly helping to reduce health disparities. However, this potential is countered by the significant risk of deepening the digital divide, where populations with lower digital literacy, socioeconomic status, or access to technology are left behind. Public health initiatives must therefore focus on inclusive implementation strategies to prevent exacerbating existing inequalities. Furthermore, these technologies could enhance health system efficiency by enabling population-level monitoring and optimizing the use of clinical resources. This also presents a new challenge for public health governance: establishing clear regulatory standards for data privacy, security, and clinical validation is crucial to ensure these digital tools are safe, effective, and trustworthy for widespread use.
4.7. Future Recommendations
Future research should focus on conducting high-quality randomized clinical trials, with larger samples and longer follow-up periods to evaluate the sustainability of the effects and long-term user engagement. It is essential that future studies describe the components of the intervention in detail, preferably using standardized frameworks, to facilitate replication and the identification of the most effective features. Direct comparative (head-to-head) studies are needed to evaluate different types of tools or specific functionalities. Likewise, it is crucial to standardize outcome variables and measurement instruments to allow for comparison between studies and the development of more robust meta-analyses. Research should also explore which patient profiles benefit most from these interventions, as well as evaluate important outcomes such as cost-effectiveness and the challenges of implementation in real-world clinical settings.
5. Conclusions
This systematic review and meta-analysis suggests a possible association between digital health interventions and improvement in pulmonary function in patients with asthma. However, these results must be interpreted with due caution. The overall certainty of the evidence was low to very low, and the substantial methodological heterogeneity among the included studies introduces a significant degree of uncertainty. The high variability observed among the studies limits the interpretability of the combined estimates and their clinical applicability; therefore, the pooled results should be considered exploratory approximations rather than definitive estimates of the effect. Regarding other outcomes of interest, such as asthma control, HRQoL, and symptom-free days, the available evidence was heterogeneous and inconsistent, failing to demonstrate uniform benefits in the combined analyses. Nonetheless, exploratory subgroup analyses suggested that elements such as remote monitoring and the use of mobile applications could modulate the effectiveness of the interventions, which provides valuable information on potential contextual factors for success. In this regard, the most relevant and clinically guiding findings of this review lie in the identification of these potential moderators of efficacy and in the qualitative synthesis, rather than in the overall combined values.
Given these considerations, prudence is required when extrapolating the results. Digital tools represent a promising field as a potential supplement to standard asthma care, but their definitive role and their clinical impact have yet to be clarified. Future studies, with rigorous designs, large samples, and adequate standardization of outcomes, will be essential to confirm these findings and specify the contexts in which these technologies may offer a significant clinical benefit.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app152312471/s1. Figure S1. Subgroup analysis of the effect of digital health tools on asthma control according to the type of tool used. Figure S2. Subgroup analysis of the effect of digital health tools on asthma control according to the supervisor’s profile. Figure S3. Subgroup analysis of the effect of digital health tools on asthma control according to the follow-up time. Figure S4. Subgroup analysis of the effect of digital health tools on asthma control according to the age group. Figure S5. Subgroup analysis of the effect of digital health tools on asthma control according to the type of supervision. Figure S6. Subgroup analysis of the effect of digital health tools on FEV1 according to the type of tool used. Figure S7. Subgroup analysis of the effect of digital health tools on FEV1 according to the supervisor’s profile. Figure S8. Subgroup analysis of the effect of digital health tools on FEV1 according to the follow-up time. Figure S9. Subgroup analysis of the effect of digital health tools on FEV1 according to the age group. Figure S10. Subgroup analysis of the effect of digital health tools on FEV1 according to the type of supervision. Figure S11. Subgroup analysis of the effect of digital health tools on PEF according to the type of tool used. Figure S12. Subgroup analysis of the effect of digital health tools on PEF according to the supervisor’s profile. Figure S13. Subgroup analysis of the effect of digital health tools on PEF according to the type of supervision. Figure S14. Subgroup analysis of the effect of digital health tools on symptom-free days according to the type of tool used. Figure S15. Subgroup analysis of the effect of digital health tools on symptom-free days according to the supervisor’s profile. Figure S16. Subgroup analysis of the effect of digital health tools on symptom-free days according to the type of supervision. Figure S17. Subgroup analysis of the effect of digital health tools on HRQoL according to the type of tool used. Figure S18. Subgroup analysis of the effect of digital health tools on HRQoL according to the supervisor’s profile. Figure S19. Subgroup analysis of the effect of digital health tools on HRQoL according to the follow-up time. Figure S20. Subgroup analysis of the effect of digital health tools on HRQoL according to the age group. Figure S21. Subgroup analysis of the effect of digital health tools on HRQoL according to the type of supervision.
Author Contributions
Conceptualization, C.L.P., F.E.C.M. and Y.L.; methodology, C.L.P., S.M.R. and Y.L.; validation, S.M.R., L.D.C. and M.P.P.G.; formal analysis, F.E.C.M. and L.D.C.; investigation, C.L.P., L.D.C., M.P.P.G. and F.E.C.M.; resources, Y.L.; data curation, M.P.P.G. and F.E.C.M.; writing—original draft preparation, C.L.P., S.M.R. and L.D.C.; writing—review and editing, C.L.P., S.M.R., L.D.C., M.P.P.G., F.E.C.M. and Y.L.; visualization, L.D.C. and M.P.P.G.; supervision, Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been funded by the Direccion General de Investigaciones of Universidad Santiago de Cali, under Call No. DGI-01-2025.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article are available in the published studies included in the meta-analysis and can be accessed through the respective publications.
Acknowledgments
This research has been funded by the Direccion General de Investigaciones of Universidad Santiago de Cali, under Call No. DGI-01-2025.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wenzel, S.E. Asthma Phenotypes: The Evolution from Clinical to Molecular Approaches. Nat. Med. 2012, 18, 716–725. [Google Scholar] [CrossRef]
- Soriano, J.B.; Abajobir, A.A.; Abate, K.H.; Abera, S.F.; Agrawal, A.; Ahmed, M.B.; Aichour, A.N.; Aichour, I.; Aichour, M.T.E.; Alam, K.; et al. Global, Regional, and National Deaths, Prevalence, Disability-Adjusted Life Years, and Years Lived with Disability for Chronic Obstructive Pulmonary Disease and Asthma, 1990–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet Respir. Respir. Med. 2017, 5, 691–706. [Google Scholar] [CrossRef]
- Porsbjerg, C.; Melén, E.; Lehtimäki, L.; Shaw, D. Asthma. Lancet 2023, 401, 858–873. [Google Scholar] [CrossRef]
- Marcano Belisario, J.S.; Huckvale, K.; Greenfield, G.; Car, J.; Gunn, L.H. Smartphone and Tablet Self Management Apps for Asthma. Cochrane Database Syst. Rev. 2013, 2013, CD010013. [Google Scholar] [CrossRef]
- Morrison, D.; Wyke, S.; Agur, K.; Cameron, E.J.; Docking, R.I.; MacKenzie, A.M.; McConnachie, A.; Raghuvir, V.; Thomson, N.C.; Mair, F.S. Digital Asthma Self-Management Interventions: A Systematic Review. J. Med. Internet Res. 2014, 16, e51. [Google Scholar] [CrossRef] [PubMed]
- Reddel, H.K.; FitzGerald, J.M.; Bateman, E.D.; Bacharier, L.B.; Becker, A.; Brusselle, G.; Buhl, R.; Cruz, A.A.; Fleming, L.; Inoue, H.; et al. GINA 2019: A Fundamental Change in Asthma Management: Treatment of Asthma with Short-Acting Bronchodilators Alone Is No Longer Recommended for Adults and Adolescents. Eur. Respir. J. 2019, 53, 1901046. [Google Scholar] [CrossRef] [PubMed]
- Gatheral, T.L.; Rushton, A.; Evans, D.J.; Mulvaney, C.A.; Halcovitch, N.R.; Whiteley, G.; Eccles, F.J.; Spencer, S. Personalised Asthma Action Plans for Adults with Asthma. Cochrane Database Syst. Rev. 2017, 2017, CD011859. [Google Scholar] [CrossRef] [PubMed]
- Holley, S.; Morris, R.; Knibb, R.; Latter, S.; Liossi, C.; Mitchell, F.; Roberts, G. Barriers and Facilitators to Asthma Self-management in Adolescents: A Systematic Review of Qualitative and Quantitative Studies. Pediatr. Pulmonol. 2017, 52, 430–442. [Google Scholar] [CrossRef]
- Miller, L.; Schüz, B.; Walters, J.; Walters, E.H. Mobile Technology Interventions for Asthma Self-Management: Systematic Review and Meta-Analysis. JMIR Mhealth Uhealth 2017, 5, e57. [Google Scholar] [CrossRef]
- Xiao, Q.; Wang, J.; Chiang, V.; Choi, T.; Wang, Y.; Sun, L.; Wu, Y. Effectiveness of mHealth Interventions for Asthma Self-Management: A Systematic Review and Meta-Analysis. Stud. Health Technol. Inf. 2018, 250, 144–145. [Google Scholar]
- Chongmelaxme, B.; Lee, S.; Dhippayom, T.; Saokaew, S.; Chaiyakunapruk, N.; Dilokthornsakul, P. The Effects of Telemedicine on Asthma Control and Patients’ Quality of Life in Adults: A Systematic Review and Meta-Analysis. J. Allergy Clin. Immunol. Pract. 2019, 7, 199–216.e11. [Google Scholar] [CrossRef] [PubMed]
- Exarchos, K.P.; Beltsiou, M.; Votti, C.-A.; Kostikas, K. Artificial Intelligence Techniques in Asthma: A Systematic Review and Critical Appraisal of the Existing Literature. Eur. Respir. J. 2020, 56, 2000521. [Google Scholar] [CrossRef]
- Chan, A.; De Simoni, A.; Wileman, V.; Holliday, L.; Newby, C.J.; Chisari, C.; Ali, S.; Zhu, N.; Padakanti, P.; Pinprachanan, V.; et al. Digital Interventions to Improve Adherence to Maintenance Medication in Asthma. Cochrane Database Syst. Rev. 2022, 2022, CD013030. [Google Scholar] [CrossRef]
- Liscano, Y.; Anillo Arrieta, L.A.; Montenegro, J.F.; Prieto-Alvarado, D.; Ordoñez, J. Early Warning of Infectious Disease Outbreaks Using Social Media and Digital Data: A Scoping Review. Int. J. Environ. Res. Public Health 2025, 22, 1104. [Google Scholar] [CrossRef]
- Liscano, Y.; Bernal, L.M.; Díaz Vallejo, J.A. Effectiveness of AI-Assisted Digital Therapies for Post-Stroke Aphasia Rehabilitation: A Systematic Review. Brain Sci. 2025, 15, 1007. [Google Scholar] [CrossRef]
- Kandola, A.; Edwards, K.; Straatman, J.; Dührkoop, B.; Hein, B.; Hayes, J. Digital Self-Management Platform for Adult Asthma: Randomized Attention-Placebo Controlled Trial. J. Med. Internet Res. 2024, 26, e50855. [Google Scholar] [CrossRef]
- Ljungberg, H.; Carleborg, A.; Gerber, H.; Öfverström, C.; Wolodarski, J.; Menshi, F.; Engdahl, M.; Eduards, M.; Nordlund, B. Clinical Effect on Uncontrolled Asthma Using a Novel Digital Automated Self-Management Solution: A Physician-Blinded Randomised Controlled Crossover Trial. Eur. Respir. J. 2019, 54, 1900983. [Google Scholar] [CrossRef]
- Anawade, P.A.; Sharma, D.; Gahane, S. A Comprehensive Review on Exploring the Impact of Telemedicine on Healthcare Accessibility. Cureus 2024, 16, e55996. [Google Scholar] [CrossRef] [PubMed]
- Herrero Martín, S.; Hueto Pérez De Heredia, J.; Cuesta Remón, A.; Gómez Fernández, M.; Antón, M.M.; Cabasés, J.; García Rey, R.; Cebollero Rivas, P. Is a Mobile Application Useful for Patients with Moderate-Severe Asthma? Arch. Bronconeumol. 2021, 57, 556–558. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Aparicio, M.; Almonacid, C.; Calvín Lamas, M.; Delgado, J.; Gandolfo-Cano, M.; López-Carrasco, V.; Vega, J.M.; Díaz-Pérez, D.; Villamañán, E. Telemedicina y asma grave en nuestro entorno: Reflexiones sobre la experiencia de los profesionales y propuestas para hacerla realidad. Open Respir. Arch. 2023, 5, 100239. [Google Scholar] [CrossRef]
- Hui, C.Y.; Walton, R.; McKinstry, B.; Jackson, T.; Parker, R.; Pinnock, H. The Use of Mobile Applications to Support Self-Management for People with Asthma: A Systematic Review of Controlled Studies to Identify Features Associated with Clinical Effectiveness and Adherence. J. Am. Med. Inform. Assoc. 2017, 24, 619–632. [Google Scholar] [CrossRef] [PubMed]
- McLean, G.; Murray, E.; Band, R.; Moffat, K.R.; Hanlon, P.; Bruton, A.; Thomas, M.; Yardley, L.; Mair, F.S. Interactive Digital Interventions to Promote Self-Management in Adults with Asthma: Systematic Review and Meta-Analysis. BMC Pulm. Med. 2016, 16, 83. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Prieto-Alvarado, D.E.; Parada-Gereda, H.M.; Molano, D.; Martinez, Y.L.; Tafurt, G.P.R.; Masclans, J.-R. Risk Factors and Outcomes of Ventilator-Associated Pneumonia in Patients with Traumatic Brain Injury: A Systematic Review and Meta-Analysis. J. Crit. Care 2025, 85, 154922. [Google Scholar] [CrossRef]
- Cruz Mosquera, F.E.; Perlaza, C.L.; Naranjo Rojas, A.; Murillo Rios, S.; Carrero Gallego, A.; Fischersworring, S.I.; Rodríguez, J.S.; Liscano, Y. Effectiveness of Probiotics, Prebiotics, and Symbiotic Supplementation in Cystic Fibrosis Patients: A Systematic Review and Meta-Analysis of Clinical Trials. Medicina 2025, 61, 489. [Google Scholar] [CrossRef] [PubMed]
- Cruz Mosquera, F.E.; Murillo, S.R.; Naranjo Rojas, A.; Perlaza, C.L.; Castro Osorio, D.; Liscano, Y. Effect of Exercise and Pulmonary Rehabilitation in Pre- and Post-Surgical Patients with Lung Cancer: Systematic Review and Meta-Analysis. Medicina 2024, 60, 1725. [Google Scholar] [CrossRef] [PubMed]
- Mosquera, F.E.C.; De La Rosa Caldas, M.; Naranjo Rojas, A.; Perlaza, C.L.; Liscano, Y. Probiotic, Prebiotic, and Synbiotic Supplementation for the Prevention and Treatment of Acute Otitis Media: A Systematic Review and Meta-Analysis. Children 2025, 12, 591. [Google Scholar] [CrossRef]
- Zairina, E.; Abramson, M.J.; McDonald, C.F.; Li, J.; Dharmasiri, T.; Stewart, K.; Walker, S.P.; Paul, E.; George, J. Telehealth to Improve Asthma Control in Pregnancy: A Randomized Controlled Trial. Respirology 2016, 21, 867–874. [Google Scholar] [CrossRef]
- Farzandipour, M.; Heidarzadeh Arani, M.; Sharif, R.; Nabovati, E.; Akbari, H.; Anvari, S. Improving Asthma Control and Quality of Life via a Smartphone Self-Management App: A Randomized Controlled Trial. Respir. Med. 2024, 223, 107539. [Google Scholar] [CrossRef]
- Suvarna, K.C.; Kumar, P.; Singh, K.; Kumar, J.; Goyal, J.P. Comparison of Telemedicine versus In-Person Visit for Control of Asthma in Children Aged 7–17 Years: A Randomized Controlled Trial. Indian J. Pediatr. 2025, 92, 467–473. [Google Scholar] [CrossRef]
- Jeminiwa, R.; Garza, K.B.; Chou, C.; Franco-Watkins, A.; Fox, B.I. Effects of Framed Mobile Messages on Beliefs, Intentions, Adherence, and Asthma Control: A Randomized Trial. Pharmacy 2024, 12, 10. [Google Scholar] [CrossRef]
- Halterman, J.S.; Fagnano, M.; Tremblay, P.; Butz, A.; Perry, T.T.; Wang, H. Effect of the Telemedicine Enhanced Asthma Management Through the Emergency Department (TEAM-ED) Program on Asthma Morbidity: A Randomized Controlled Trial. J. Pediatr. 2024, 266, 113867. [Google Scholar] [CrossRef]
- Gümüş, M.; Yardimci, F.; Duman Şenol, H.; Demir, E. Virtual Care for Paediatric Asthma: A Randomized Controlled Trial. Int. J. Nurs. Pract. 2024, 30, e13290. [Google Scholar] [CrossRef]
- Mahmoud, R.A.A.; Boshra, M.S.; Saeed, H.; Abdelrahim, M.E.A. The Impact of the Clip-Tone Training Device and Its Smartphone Application to Pressurized Metered-Dose Inhaler in Adult Asthmatics. J. Asthma 2023, 60, 227–234. [Google Scholar] [CrossRef]
- Gupta, R.S.; Fierstein, J.L.; Boon, K.L.; Kanaley, M.K.; Bozen, A.; Kan, K.; Vojta, D.; Warren, C.M. Sensor-Based Electronic Monitoring for Asthma: A Randomized Controlled Trial. Pediatrics 2021, 147, e20201330. [Google Scholar] [CrossRef]
- Iamlaor, U.; Taneepanichskul, S. Effectiveness of Asthma Self-Care Program Through Mobile Line Application (SALA) on Lung Function among Asthma Patients in Angthong Hospital: A Randomized Control Trial. J. Med. Assoc. Thai 2021, 104, 264–270. [Google Scholar] [CrossRef]
- Khusial, R.J.; Honkoop, P.J.; Usmani, O.; Soares, M.; Simpson, A.; Biddiscombe, M.; Meah, S.; Bonini, M.; Lalas, A.; Polychronidou, E.; et al. Effectiveness of myAirCoach: A mHealth Self-Management System in Asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 1972–1979.e8. [Google Scholar] [CrossRef]
- Prabhakaran, L.; Chun Wei, Y. Effectiveness of the eCARE Programme: A Short Message Service for Asthma Monitoring. BMJ Health Care Inf. 2019, 26, e100007. [Google Scholar] [CrossRef]
- Shaoxia, L.; Ye, X.; Wang, Z.; Xia, W.; Qi, Y.; Wang, W.; Chen, Y.; Cai, X.; Qian, X. A Randomized Controlled Trial of a Mobile Application-assisted Nurse-led Model Used to Improve Treatment Outcomes in Children with Asthma. J. Adv. Nurs. 2019, 75, 3058–3067. [Google Scholar] [CrossRef]
- Kosse, R.C.; Bouvy, M.L.; De Vries, T.W.; Koster, E.S. Effect of a mHealth Intervention on Adherence in Adolescents with Asthma: A Randomized Controlled Trial. Respir. Med. 2019, 149, 45–51. [Google Scholar] [CrossRef]
- Nemanic, T.; Sarc, I.; Skrgat, S.; Flezar, M.; Cukjati, I.; Marc Malovrh, M. Telemonitoring in Asthma Control: A Randomized Controlled Trial. J. Asthma 2019, 56, 782–790. [Google Scholar] [CrossRef]
- Halterman, J.S.; Fagnano, M.; Tajon, R.S.; Tremblay, P.; Wang, H.; Butz, A.; Perry, T.T.; McConnochie, K.M. Effect of the School-Based Telemedicine Enhanced Asthma Management (SB-TEAM) Program on Asthma Morbidity: A Randomized Clinical Trial. JAMA Pediatr. 2018, 172, e174938. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lin, S.-H.; Zhu, D.; Xu, F.; Chen, Z.-H.; Shen, H.-H.; Li, W. WeChat Public Account Use Improves Clinical Control of Cough-Variant Asthma: A Randomized Controlled Trial. Med. Sci. Monit. 2018, 24, 1524–1532. [Google Scholar] [CrossRef]
- Lara, S.; Roukema, J.; Boehmer, A.L.M.; Brouwer, M.L.; Hugen, C.A.C.; Niers, L.E.M.; Sprij, A.J.; Rikkers-Mutsaerts, E.R.V.M.; Rottier, B.L.; Donders, A.R.T.; et al. A Virtual Asthma Clinic for Children: Fewer Routine Outpatient Visits, Same Asthma Control. Eur. Respir. J. 2017, 50, 1700471. [Google Scholar] [CrossRef]
- Portnoy, J.M.; Waller, M.; De Lurgio, S.; Dinakar, C. Telemedicine Is as Effective as In-Person Visits for Patients with Asthma. Ann. Allergy Asthma Immunol. 2016, 117, 241–245. [Google Scholar] [CrossRef]
- Kim, M.-Y.; Lee, S.-Y.; Jo, E.-J.; Lee, S.-E.; Kang, M.-G.; Song, W.-J.; Kim, S.-H.; Cho, S.-H.; Min, K.-U.; Ahn, K.-H.; et al. Feasibility of a Smartphone Application Based Action Plan and Monitoring in Asthma. Asia Pac. Allergy 2016, 6, 174–180. [Google Scholar] [CrossRef]
- Johnson, K.B.; Patterson, B.L.; Ho, Y.-X.; Chen, Q.; Nian, H.; Davison, C.L.; Slagle, J.; Mulvaney, S.A. The Feasibility of Text Reminders to Improve Medication Adherence in Adolescents with Asthma. J. Am. Med. Inform. Assoc. 2016, 23, 449–455. [Google Scholar] [CrossRef]
- Bender, B.G.; Cvietusa, P.J.; Goodrich, G.K.; Lowe, R.; Nuanes, H.A.; Rand, C.; Shetterly, S.; Tacinas, C.; Vollmer, W.M.; Wagner, N.; et al. Pragmatic Trial of Health Care Technologies to Improve Adherence to Pediatric Asthma Treatment: A Randomized Clinical Trial. JAMA Pediatr. 2015, 169, 317. [Google Scholar] [CrossRef]
- Ryan, D.; Price, D.; Musgrave, S.D.; Malhotra, S.; Lee, A.J.; Ayansina, D.; Sheikh, A.; Tarassenko, L.; Pagliari, C.; Pinnock, H. Clinical and Cost Effectiveness of Mobile Phone Supported Self Monitoring of Asthma: Multicentre Randomised Controlled Trial. BMJ 2012, 344, e1756. [Google Scholar] [CrossRef]
- Gustafson, D.; Wise, M.; Bhattacharya, A.; Pulvermacher, A.; Shanovich, K.; Phillips, B.; Lehman, E.; Chinchilli, V.; Hawkins, R.; Kim, J.-S. The Effects of Combining Web-Based eHealth With Telephone Nurse Case Management for Pediatric Asthma Control: A Randomized Controlled Trial. J. Med. Internet Res. 2012, 14, e101. [Google Scholar] [CrossRef]
- Araújo, L.; Jacinto, T.; Moreira, A.; Castel-Branco, M.; Delgado, L.; Costa-Pereira, A.; Fonseca, J. Clinical Efficacy of Web-Based Versus Standard Asthma Self-Management. J. Investig. Allergol. Clin. Immunol. 2012, 22, 28–34. [Google Scholar]
- Deschildre, A.; Béghin, L.; Salleron, J.; Iliescu, C.; Thumerelle, C.; Santos, C.; Hoorelbeke, A.; Scalbert, M.; Pouessel, G.; Gnansounou, M.; et al. Home Telemonitoring (Forced Expiratory Volume in 1 s) in Children with Severe Asthma Does Not Reduce Exacerbations. Eur. Respir. J. 2012, 39, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-T.; Huang, C.-D.; Wang, C.-H.; Lee, K.-Y.; Lin, S.-M.; Kuo, H.-P. A Mobile Telephone-Based Interactive Self-Care System Improves Asthma Control. Eur. Respir. J. 2011, 37, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, R.R.; Plevinsky, J.M.; Kollin, S.R.; Gibler, R.C.; Guilbert, T.W.; Hommel, K.A. Systematic Review of Digital Interventions for Pediatric Asthma Management. J. Allergy Clin. Immunol. Pract. 2020, 8, 1284–1293. [Google Scholar] [CrossRef]
- Biblowitz, K.; Bellam, S.; Mosnaim, G. Improving Asthma Outcomes in the Digital Era: A Systematic Review. Pharm. Med. 2018, 32, 173–187. [Google Scholar] [CrossRef]
- Mosnaim, G.; Safioti, G.; Brown, R.; DePietro, M.; Szefler, S.J.; Lang, D.M.; Portnoy, J.M.; Bukstein, D.A.; Bacharier, L.B.; Merchant, R.K. Digital Health Technology in Asthma: A Comprehensive Scoping Review. J. Allergy Clin. Immunol. Pract. 2021, 9, 2377–2398. [Google Scholar] [CrossRef]
- Snoswell, C.L.; Rahja, M.; Lalor, A.F. A Systematic Review and Meta-Analysis of Change in Health-Related Quality of Life for Interactive Telehealth Interventions for Patients With Asthma. Value Health 2021, 24, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Tinschert, P.; Jakob, R.; Barata, F.; Kramer, J.-N.; Kowatsch, T. The Potential of Mobile Apps for Improving Asthma Self-Management: A Review of Publicly Available and Well-Adopted Asthma Apps. JMIR Mhealth Uhealth 2017, 5, e113. [Google Scholar] [CrossRef]
- Kosse, R.C.; Bouvy, M.L.; Belitser, S.V.; de Vries, T.W.; van der Wal, P.S.; Koster, E.S. Effective Engagement of Adolescent Asthma Patients With Mobile Health-Supporting Medication Adherence. JMIR Mhealth Uhealth 2019, 7, e12411. [Google Scholar] [CrossRef]
- Merchant, R.K.; Inamdar, R.; Quade, R.C. Effectiveness of Population Health Management Using the Propeller Health Asthma Platform: A Randomized Clinical Trial. J. Allergy Clin. Immunol. Pract. 2016, 4, 455–463. [Google Scholar] [CrossRef]
- Garin, N.; Zarate-Tamames, B.; Gras-Martin, L.; Milà, R.; Crespo-Lessmann, A.; Curto, E.; Hernandez, M.; Mestres, C.; Plaza, V. Clinical Impact of Electronic Monitoring Devices of Inhalers in Adults with Asthma or COPD: A Systematic Review and Meta-Analysis. Pharmaceuticals 2023, 16, 414. [Google Scholar] [CrossRef]
- Madanian, S.; Nakarada-Kordic, I.; Reay, S.D.; Chetty, T. Patients’ Perspectives on Digital Health Tools. PEC Innov. 2023, 2, 100171. [Google Scholar] [CrossRef]
- Marques, A.; Bosch, P.; De Thurah, A.; Meissner, Y.; Falzon, L.; Mukhtyar, C.; Bijlsma, J.W.; Dejaco, C.; Stamm, T.A. Effectiveness of Remote Care Interventions: A Systematic Review Informing the 2022 EULAR Points to Consider for Remote Care in Rheumatic and Musculoskeletal Diseases. RMD Open 2022, 8, e002290. [Google Scholar] [CrossRef] [PubMed]
- Naranjo-Rojas, A.; Perula-de Torres, L.Á.; Cruz-Mosquera, F.E.; Molina-Recio, G. Efficacy and Acceptability of a Mobile App for Monitoring the Clinical Status of Patients With Chronic Obstructive Pulmonary Disease Receiving Home Oxygen Therapy: Randomized Controlled Trial. J. Med. Internet Res. 2025, 27, e65888. [Google Scholar] [CrossRef]
- Shickh, S.; Rafferty, S.A.; Clausen, M.; Kodida, R.; Mighton, C.; Panchal, S.; Lorentz, J.; Ward, T.; Watkins, N.; Elser, C.; et al. The Role of Digital Tools in the Delivery of Genomic Medicine: Enhancing Patient-Centered Care. Genet. Med. 2021, 23, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).