Abstract
Hydrogen-rich water (HRW) has attracted significant attention for its physiological and therapeutic potential, driving efforts to develop a green and direct production approach. In particular, if solar energy could be utilized to power the process and the power-generation and water-production modules could be integrated into a single device, it would greatly enhance portability and user convenience, making it an ideal solution for personalized healthcare and outdoor applications. We demonstrate solar-assisted proton exchange membrane (PEM) electrolysis using symmetric IrO2 electrodes at both cathode and anode to directly generate HRW. The symmetric design simplifies manufacturing, mitigates lifetime mismatch and metal-ion cross-contamination. IrO2 films were electrodeposited on stainless steel substrates and annealed at 400–700 °C. When coupled with a 100 cm2 Si solar cell, the electrode annealed at 550 °C—featuring ~6 nm IrO2 nanocrystals embedded in an amorphous matrix—exhibited the highest hydrogen production rate. At an applied voltage of 4 V, this 550 °C-annealed IrO2 electrode produced approximately 1800 μmol h−1 of H2, corresponding to about 44 mL h−1 of H2 at 25 °C and 1 atm. Corrosion tests show the HRW is less aggressive to iron than DI, RO, and tap water, suggesting better compatibility with metallic components. During water splitting, the oxidation–reduction potential (ORP) rapidly decreases to <−300 mV within 0–10 min and then stabilizes, with the 550 °C–annealed electrode exhibiting the lowest ORP. Upon air exposure, the ORP increases by ~200 mV over 45–70 min yet remains reductive for >120 min, indicating persistent dissolved H2 and sustained performance. Overall, the symmetric IrO2 architecture provides a green, stable, and direct route to HRW production.
1. Introduction
Hydrogen is concurrently recognized as a carbon-free, clean energy carrier with high gravimetric energy density. When H2 dissolves in water, it forms hydrogen-rich water (HRW), which has attracted increasing attention for its potential antioxidative and health-promoting effects [,,,,]. Various water electrolyzer technologies have been studied and comprehensively reviewed in the literature; however, most of these investigations have focused on hydrogen as a fuel or energy-storage medium rather than as a dissolved therapeutic agent [,].
Alkaline electrolysis, anion exchange membrane (AEM) electrolysis, proton exchange membrane (PEM) electrolysis, and solid oxide electrolysis (SOE) are all widely studied for H2 production. These four technologies differ mainly in their electrolytes, operating temperature regimes, catalyst requirements, and overall system behavior. Alkaline electrolysis is commonly used to produce hydrogen in large-scale (commercial) applications. It uses liquid alkaline electrolytes such as KOH or NaOH and does not require noble-metal electrocatalysts. However, some degree of gas crossover between half-cells is inevitable and may reduce H2 purity. Hybrid heterojunction electrocatalysts, nano-heterostructured electrocatalysts, nanointerface electrocatalysts, and porous structures have been shown to enhance the performance of the oxygen evolution reaction (OER) and/or hydrogen evolution reaction (HER) [,,,]. AEM electrolysis is similar to conventional alkaline electrolysis and uses a solid AEM as the separator and ion-conducting medium, typically paired with a dilute alkaline or near-neutral electrolyte. Noble-metal-free catalysts are feasible for AEM; nevertheless, membrane and catholyte stability and durability are still under development. In both alkaline and AEM electrolysis, H2 and hydroxide ions (OH-) are produced at the cathode. The OH- traverses the diaphragm/membrane to the anode, where it participates in forming O2 and regenerating H2O. Performance improvements, including increased current density and enhanced stability, have been demonstrated through electrode-material optimization [,]. PEM electrolysis uses a proton-conducting membrane with pure-water feed at low temperatures. It delivers high current density, fast response, low gas crossover, and high-purity H2, but depends on scarce noble metals (Pt, Ir) and costly membranes. At the anode, water is oxidized to generate O2, protons (H+), and electrons (e−). The protons traverse the proton-conducting membrane to the cathode, while the electrons travel through the external circuit. At the cathode, protons and electrons recombine to produce H2. Improvements achieved by altering materials and structures have been widely discussed [,,]. SOE operates at high temperatures using ceramic oxygen-ion (or protonic) conductors. At the cathode, steam is reduced to H2 and oxide ions (O2−). The O2− ions traverse the solid electrolyte to the anode, where they are oxidized to form O2 and release electrons. The oxygen is discharged at the anode surface, while the electrons flow through the external circuit to the cathode. The use of novel electrode materials for performance enhancement has been explored by different groups [,].
Among these electrolysis technologies, PEM is typically faster due to the high activity and large electrochemically active area of noble-metal electrodes (usually Pt), and it is safer because it avoids liquid caustic electrolytes. The PEM water electrolyzer (PEMWE) is therefore an important research focus. In studies of PEMWE, IrO2 has been demonstrated to be a highly stable OER catalyst [,]. The influences of porosity, crystallinity, and nanostructure on the performance of IrO2 oxygen evolution catalysts have been extensively analyzed [,]. Cherevko et al. investigated the electrocatalytic performance of thermally oxidized Ir (IrO2) toward the OER and the HER, and compared its performance with that of Ir, Ru, and RuO2 []. After a potential sweep to the OER potential for 5 mA cm−2, Ir dissolution was <0.1 ng cm−2, indicating exceptional stability. However, despite its high stability, IrO2 shows relatively low activity, requiring an overpotential >400 mV to reach a current density of 5 mA cm−2, a level that may only be acceptable for PEMWEs when powered by low-cost renewable electricity. They concluded that IrO2 remains the catalyst of choice for the OER and may be considered for the HER when Pt contamination is a concern. IrO2 is stable and thus suitable for medical and healthcare applications. For example, activated iridium oxide (AIROF) microelectrodes have been widely studied to maximize charge-injection capacity and have been investigated both in vitro and in living organisms [,].
Solar-assisted PEMWE offers several advantages over a grid- or fossil-powered system without solar input. By coupling photovoltaics either directly or indirectly, it lowers operating electricity costs and carbon intensity, enabling genuinely green hydrogen with near-zero upstream emissions [,]. Direct-coupled systems are simple, reliable, and efficient, but face system-level constraints, especially in PV array configuration and tight voltage/current matching under variable irradiance. Indirect coupling, often integrated with an inverter and optionally batteries, stabilizes output, smooths irradiance swings, and reduces grid reliance, but increases system cost, complexity, and maintenance needs. Theoretical and experimental enhancements in solar-assisted PEMWE have been reported, including optimizing the number of PV cells in series and parallel, energy management strategies, and relative sizing [,,]. These studies demonstrate green hydrogen production via solar-assisted PEMWE and indicate that further investigation is warranted.
A potential by-product of water electrolysis is HRW, which has recently been widely studied for its possible human health benefits [,,,,]. In PEMWE, protons (H+) travel to the cathode of the membrane electrode assembly (MEA) water electrolyzer and combine with electrons to generate high-purity hydrogen gas (H2), which is typically collected by the water-displacement method. A portion of this hydrogen can dissolve into residual water along its escape path, yielding HRW. Vaishnavi et al. reported that HRW exhibits superior antimicrobial activity to chlorhexidine mouthwash and may be of potential relevance to preventing dental disease []. Kuzmanovic et al. found that a six-week HRW intervention improved muscle performance and elevated serum free testosterone and cortisol []. Their findings support the potential health benefits of HRW and highlight the need for further research, especially on environmentally friendly, straightforward, and cost-effective HRW production. Systematic studies on HRW produced by water electrolyzers are still lacking. The simultaneous generation of HRW during water electrolysis represents an emerging direction that offers both added value and cross-disciplinary potential. Using IrO2 on both electrodes in PEM water electrolysis is unconventional, since Pt-based cathodes normally minimize precious-metal demand. Nevertheless, a symmetric IrO2 MEA can unify catalyst fabrication, avoid Pt crossover, reduce heterogeneous metal contamination, and promote a more uniform corrosion pattern.
In this work, we investigate a solar-assisted PEMWE system that employs IrO2 at both electrodes, emphasizing its potential for portable and off-grid applications without reliance on conventional electricity. We further examine how growth conditions influence electrochemical performance and characterize the HRW generated by this approach.
2. Materials and Methods
2.1. Electrodeposition
IrO2 thin film was deposited onto stainless steel to fabricate electrodes for water electrolysis (Figure 1a). The deposition solution was prepared following a modified Yamanaka’s method []. In brief, 3 mM hydrogen hexachloroiridate hydrate (H2IrCl6·6H2O) was dissolved in deionized water under magnetic stirring, and 100 mM oxalic acid (H2C2O4·2H2O) was added to form a stable iridium–oxalate complex. The pH of the solution was adjusted to 10.5 by the gradual addition of K2CO3. The resulting solution was aged in the dark at room temperature for one week until it turned dark blue, indicating stabilization of the precursor. Electrochemical deposition was subsequently performed in the stabilized solution at room temperature using a Model 600E Series Electrochemical Analyzer (CH Instruments, Inc., Austin, TX, USA). The working electrode was polarized at a constant potential of 0.52 V (vs. Ag/AgCl) for 60 min, which enabled the continuous formation and growth of nano-porous iridium oxide (IrO2) on the stainless-steel electrode substrate.
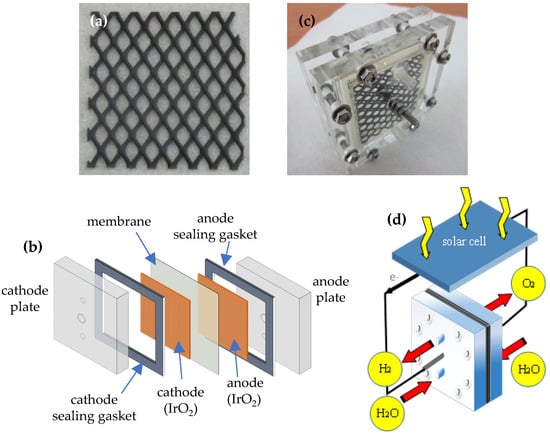
Figure 1.
(a) IrO2-coated stainless-steel electrode. (b) Schematic illustration of MEA. (c) MEA using symmetric IrO2 electrodes at both cathode and anode. (d) Schematic illustration of solar-assisted PEM water electrolyzer.
2.2. Fabrication of MEA and Water Electrolysis Cell
After electrodeposition, the nano-porous IrO2-coated stainless steel electrodes were annealed in a furnace at 400–700 °C for 0.5 h. As shown in Figure 1b,c, IrO2 electrodes were used as the anode and cathode, positioned on both sides of a polymer electrolyte membrane (PEM; Nafion 117, DuPont, Wilmington, DE, USA), forming an anode/PEM/cathode module that served as the MEA for water electrolysis. The electrolysis cell was subsequently operated in a solar cell-assisted configuration for hydrogen generation (Figure 1c). The additional bias required for water splitting was supplied by a 100 cm2 Si-based solar cell (0.48–1.26 W) illuminated by a tungsten lamp. Commercial Pt-Nb electrodes were also used as a Pt-Nb/PEM/Pt-Nb module in the MEA for comparison. Deionized (DI), reverse osmosis (RO), and tap water were used, and each had a pH near 7 during electrolysis. The I-V curve of the solar-assisted water electrolyzer was recorded at ambient conditions. Cyclic voltammetry (CV) was performed in 1.0 M H2SO4 using a glassy carbon working electrode, a platinum counter electrode, and an Ag/AgCl reference electrode. The oxidation-reduction potential (ORP) of the HRW was measured using an ORP meter. All experiments were conducted at room temperature under atmospheric pressure. A transmission electron microscope (FEI Tecnai G2 F20 S-TWIN; FEI, Hillsboro, OR, USA) was used to study the structure of the IrO2 coatings.
3. Results and Discussion
TEM images and electron diffraction patterns of IrO2 coatings are presented in Figure 2. The structure of the as-grown IrO2 coating remains amorphous, as no lattice or diffraction pattern is observed. After annealing at 400 °C, the presence of a diffraction pattern indicates that the IrO2 coating exhibits a mixed crystalline and amorphous structure. When the annealing temperature is increased to 550 °C, IrO2 nanocrystals and a diffraction pattern are both observed. The lattice spacing of these nanocrystals, approximately 6 nm in size, is about 0.315 nm, corresponding to the IrO2(110) phase. As the annealing temperature is further increased to 700 °C, large IrO2 nanocrystals, ~20 nm in size, are present across almost the entire surface of the TEM image, suggesting a highly crystallized structure of IrO2 coating. Notably, electrodeposition has yielded stable IrO2 films when cyclic voltammetry (0.05–0.55 V vs. SCE, 50 mV s−1, 50 cycles) is immediately followed by potential pulsing over the same potential window for 400–1000 pulses []. However, for PEMWE electrodes, a porous, high-surface-area IrO2 is desirable to increase the electrochemically active surface area and maintain high OER performance while reducing the overall Ir loading compared with dense films. Therefore, the working electrode was polarized at a constant potential of 0.52 V (vs. Ag/AgCl) for 60 min.
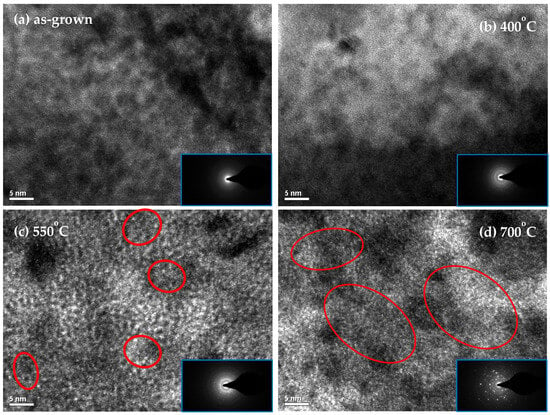
Figure 2.
TEM images of (a) as-grown, (b) 400 °C annealed, (c) 550 °C annealed, and (d) 700 °C annealed IrO2. IrO2 nanocrystals are highlighted with red circles.
Figure 3 shows the I-V curve of the MEA using Pt-Nb, as-grown IrO2 and annealed IrO2 electrodes for solar-assisted water electrolysis. The initial potential follows the order: 550 °C annealed IrO2 < 400 °C annealed IrO2 < Pt-Nb < as-grown IrO2 < 700 °C annealed IrO2. The electrode containing small IrO2 nanocrystals (~6 nm) shows the lowest initial potential, indicating the best performance for water electrolysis among our samples. At a fixed potential of 2.5 V, the order of current density is as follows: 700 °C annealed IrO2 < as-grown IrO2 < 400 °C annealed IrO2 < Pt-Nb < 550 °C annealed IrO2. A higher current density indicates a greater flow of current per unit area, generally reflecting a higher rate of electrochemical reactions. The 550 °C annealed IrO2 shows the highest current density (~103 mA/cm2), surpassing even that of Pt-Nb (~80 mA/cm2). Siracusano et al. studied nanosized IrO2 anode electrocatalysts and suggested that crystalline IrO2 particles of appropriate size embedded within an amorphous matrix likely reduce sintering, thereby potentially improving current density []. In our case, the 550 °C annealed IrO2, composed of small IrO2 nanocrystals (~6 nm), may exhibit comparable behavior, potentially resulting in the highest current density among our samples. When the potential exceeds 3 V, the Pt-Nb sample exhibits the highest current density, while the 550 °C annealed IrO2 still shows a higher current density compared to the other IrO2 samples. The current density of the sample containing ~6 nm IrO2 nanocrystals embedded within an amorphous matrix is 52 mA/cm2 at 2 V and 285 mA/cm2 at 4 V, respectively.
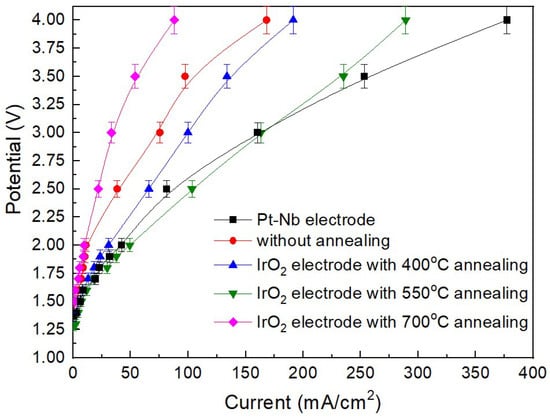
Figure 3.
PEMWE performance by using different electrodes.
Ngo et al. studied the influence of amorphous/crystalline IrO2 interfaces and reported that grain boundaries affect the current density; accordingly, the reduced current density observed for the sample containing larger IrO2 crystals likely stems from a combined effect of diminished surface area and a lower fraction of the more active amorphous phase in the catalyst []. In our study, the IrO2 electrode annealed at 550 °C exhibited superior electrocatalytic performance, primarily attributed to its nanocrystalline structure and grain-boundary effects. First, the nanocrystalline domains introduce numerous grain boundaries, which are regions of lower activation energy. In IrO2, both electrons and ions can migrate along these boundaries via vacancy-assisted hopping with lower energy barriers, facilitating charge transport and improving overall electrolysis efficiency. In contrast, the sample annealed at 700 °C, characterized by enlarged grains and fewer boundaries as confirmed by TEM selected-area diffraction (SAD; Figure 2), showed reduced catalytic activity due to diminished grain-boundary conduction. Moreover, grain-boundary defects and structural irregularities serve as electrochemically active sites, whose kinetics critically influence reaction rates. The 550 °C IrO2 electrode, maintaining a mixed amorphous–nanocrystalline architecture, provides both abundant active sites and efficient charge-transfer pathways, resulting in higher current density and enhanced electrocatalytic activity. Compared with fully amorphous or excessively crystallized samples, this nanocrystalline configuration represents an optimal balance between reaction kinetics and structural stability.
Figure 4a,b show the H2 and O2 production rates as a function of the applied voltage. For all samples, the H2 production rate is approximately twice that of O2, indicating the complete decomposition of H2O into hydrogen and oxygen. As shown in Figure 3, the gas production rate depends almost linearly on the current density. When the applied voltage is below 2.5 V, the gas production rate of the Pt-Nb sample is comparable to that of the 550 °C annealed IrO2, indicating similar performance between these two samples. However, when the voltage exceeds 3 V, the Pt-Nb sample exhibits the highest H2 and O2 production rates, while the 550 °C annealed IrO2 sample remains the best performer among the IrO2 samples. At 4 V, the 550 °C-annealed IrO2 sample produced approximately ~1800 μmol H2 and ~1000 μmol O2 per hour. This corresponds to about 44 mL h−1 of H2 and 22.5 mL h−1 of O2 at 25 °C and 1 atm. The low H2 production rate is likely attributable to suboptimal electrode pairing, wherein IrO2 is employed at both electrodes, thereby increasing the HER overpotential relative to Pt-based cathodes.
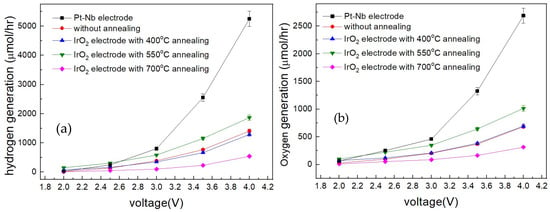
Figure 4.
(a) Hydrogen and (b) oxygen production rate as a function of applied cell potential.
In the context of PV-coupled outdoor operation with low and fluctuating irradiance, the rationale for adopting a symmetric IrO2|IrO2 MEA is to optimize robustness at moderate, dynamic potentials rather than to prioritize peak performance at sustained high voltages (>3 V) characteristic of an asymmetric Pt–Nb configuration. A symmetric IrO2 MEA provides three system-level advantages: (i) IrO2 has been evaluated both in vitro and in vivo and is used in healthcare-related applications [,], so employing IrO2 for hydrogen-rich water does not introduce new human-safety concerns, unlike the uncertainty surrounding Pt–Nb; (ii) our IrO2 coating on stainless steel enables very low Ir loading, yielding a more cost-effective electrode than Pt–Nb and insulating the system from the high cost and price volatility of Pt-based materials; and (iii) process symmetry standardizes catalyst-layer fabrication and assembly on both electrodes, eliminating Pt crossover, reducing heterogeneous-metal contamination, and mitigating asymmetric, metal-specific degradation for more uniform and predictable corrosion behavior. Consistent with this rationale, Cherevko et al. identified IrO2 as the catalyst of choice for the OER and as a viable option for the HER when Pt contamination is a concern []. Importantly, because the device is powered by coupled photovoltaic modules under outdoor conditions where sustained high voltages cannot be guaranteed, symmetric IrO2 operates robustly at moderate potentials with dynamic voltage swings, enabling effective hydrogen-rich water production while lowering requirements on the PV source—smaller-area modules and even lower-voltage amorphous-silicon panels become feasible. In contrast, although Pt–Nb can exhibit higher instantaneous performance under ideal, sustained high-voltage conditions, the heterogeneous-bipolar stack is more prone to cross-electrode metal migration and differential degradation, adding long-term reliability and maintenance uncertainty. Collectively, these advantages simplify process control, mitigate durability risks inherent to dissimilar electrodes, and enhance long-term stability—benefits that are more consequential for PV-coupled, fluctuating-power operation than maximizing performance only at >3 V.
Figure 5 shows the average cyclic voltammetry over 20 cycles at room temperature for the 550 °C annealed IrO2 sample. The C1, C2, and C3 peaks have been reported to be related to the hydrogen adsorption reaction, redox couples Ir(IV)/Ir(VI) and Ir(III)/Ir(IV), respectively [,]. The authors have suggested that these peaks observed for IrO2/C depend on the IrO2 structure, with amorphous iridium oxide typically contributing to featureless peaks. In our sample, the C2 and C3 peaks are clearly observed, whereas the C1 peak is not well-defined. This observation is most likely a result of the crystalline IrO2 particles (~6 nm) embedded within an amorphous matrix, as shown in Figure 2. The coexistence of crystalline and amorphous structures contributes to the simultaneous presence of both featureless and well-defined characteristics.
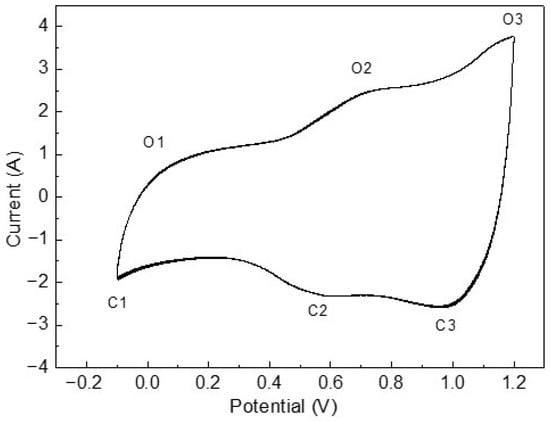
Figure 5.
Average cyclic voltammetry over 20 cycles at room temperature for the 550 °C annealed IrO2 sample.
To characterize the HRW, we performed ORP measurements. The ORP values of HRW, deionized (DI) water, reverse osmosis (RO) water, and tap water are listed in Table 1. The substantially negative ORP measured for the HRW (−332 mV at the start of data collection, after drifting from an initial value of about −400 mV) reflects residual dissolved molecular hydrogen generated using the 500 °C annealed IrO2 electrode. Control waters lacking measurable H2 all displayed positive ORP values (>+150 mV). These findings establish that the preparation successfully generated HRW with a strong reducing redox environment. To further assess the influence of redox potential differences, we immersed a 304 stainless-steel nail (model Y-03; Yun-Chang Hardware Tools Co., Ltd., Changhua, Taiwan) in each water sample for three months under ambient conditions. As shown in Figure 6, visible surface corrosion was observed on the nails in all water samples, with the extent following the order: tap > RO ≈ DI > HRW, consistent with the initial ORP values. The results suggest that dissolved molecular hydrogen may mitigate oxidative corrosion processes. This observation can be rationalized by established mechanisms through which dissolved hydrogen suppresses oxidation. Molecular hydrogen is a reductant and can consume dissolved oxygen. This lowers the concentration of oxidizing species. Recent work (e.g., Badley et al. []) shows that H2 can scavenge reactive oxygen species such as hydroxyl radicals, which may slow surface oxidation. Their findings suggest that H2 may slow metal corrosion in HRW. Dissolved hydrogen also lowers the ORP, shifting the solution toward more reducing conditions and decreasing the thermodynamic driving force for metal oxidation. The synergistic action of these effects makes HRW a less oxidizing environment and is consistent with the reduced corrosion observed in this study.

Table 1.
ORP value of different waters.

Figure 6.
Surface corrosion of nails in different water samples. Red circles highlight corrosion areas.
Figure 7a shows the ORP evolution during electrolysis using different IrO2 electrodes. The PEMWEs were integrated into a commercial water dispenser. In addition to the ORP meter, a dissolved hydrogen meter (ENH-1000, Trustlex Inc., Osaka, Japan) was used to monitor the H2 concentration in HRW, as shown in the inset of Figure 7a. Because the ORP signal often exhibited an initial transient with a highly variable peak during the first ≈ 30 s, the ORP reading was recorded only after approximately 30 s, once the value had stabilized. The most negative ORP observed was −515 mV using the IrO2 electrode annealed at 500 °C, at a dissolved H2 concentration of ~965 ppb; however, the value subsequently decreased substantially. According to the Nernst equation, a dissolved H2 level of ~965 ppb corresponds to an ORP in the negative 500 mV range, consistent with the measured value. For all samples, during the first 10 min of electrolysis, the ORP decreases sharply, followed by only a slight additional decline over the subsequent 50 min. After 1 h of electrolysis, the ORP values for the as-grown and 400, 550, and 700 °C-annealed IrO2 samples were −310, −366, −388, and −315 mV. The 550 °C-annealed IrO2 sample shows the highest current density (Figure 3), producing more H2; consequently, the dissolved H2 concentration increases and the ORP becomes more negative. Because dissolution of H2 in water is mildly exothermic, its equilibrium solubility decreases as temperature rises; warmer conditions therefore retain less dissolved hydrogen than cooler ones. After preparation, the dissolved H2 concentration decays as the liquid re-equilibrates with air’s extremely low hydrogen partial pressure, and the loss rate is accelerated by higher temperature, larger gas–liquid interfacial area, agitation, and permeable packaging. These temperature- and time-dependent losses of dissolved hydrogen shorten the practical shelf life of HRW.
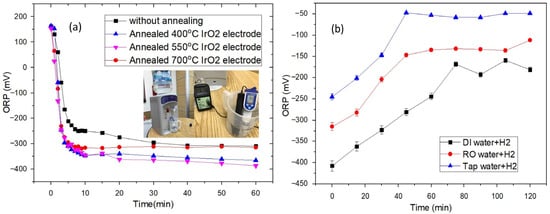
Figure 7.
(a) ORP evolution during electrolysis using different IrO2 electrodes. The embedded photograph shows the hydrogen-rich water generator. (b) Comparative ORP decline in H2-enriched DI, RO, and tap water as a function of open-air exposure time.
Figure 7b presents the ORP in H2-enriched DI, RO, and tap water as a function of open-air exposure time. Upon ambient, open-air exposure, all three HRW samples display a time-dependent loss of reducing potential (increase in ORP toward more positive values). During the first 70 min of open-air exposure, the ORP of the DI water sample increased almost linearly from below −400 mV to approximately −190 mV, after which it remained essentially stable near −190 mV with further exposure. For the RO water sample, a near-linear climb from −320 mV to ~−140 mV occurred in the first 45 min, with the ORP then remaining near −140 mV. The tap water sample, initially the least negative (~−250 mV), also exhibited an almost linear increase to ~−50 mV over 45 min and thereafter remained essentially stable at that value. ORP increased by approximately 200 mV in all samples; only the DI water sample showed a delayed plateau at about 70 min, whereas the others stabilized by roughly 45 min. Our data show that the HRW prepared in this work is still effective after 120 min of open-air exposure. The more negative value for DI water is likely due to the near absence of residual oxidants (free chlorine, chloramine, higher-valent metal ions) that would otherwise consume H2 or keep the mixed potential more positive.
4. Conclusions
In this study, electrochemically deposited IrO2 coatings on stainless steel were thermally tuned (400–700 °C) to optimize a solar-assisted water electrolyzer, in which identical IrO2 electrodes functioned as both anode and cathode for simultaneous hydrogen production and generation of HRW. Microstructural evolution from amorphous (as-grown) to mixed amorphous/crystalline (400 °C) and finally to highly crystalline IrO2 with enlarged grains (700 °C) was confirmed by TEM. Annealing at 550 °C produced ~6 nm IrO2 (110) nanocrystals embedded in an amorphous matrix, delivering the lowest initial cell potential and the highest current density (~103 mA/cm2 at 2.5 V), outperforming the commercial Pt-Nb benchmark in that voltage window. Stoichiometric H2:O2 (≈2:1) production verified efficient water splitting. HRW produced herein exhibited reduced corrosion of iron surfaces relative to deionized (DI), reverse-osmosis (RO), and tap water. The 550 °C electrode yielded the most negative ORP (−388 mV after 1 h of electrolysis), consistent with increased dissolved H2 levels. ORP time-course monitoring revealed a sharp initial drop (first 10 min), followed by a slower approach to a plateau. The superior performance is most likely attributable to the synergy between nanocrystalline and amorphous domains. These findings highlight a pathway toward green, stable, solar-assisted PEMWE with reduced noble-metal reliance for distributed hydrogen and HRW production.
Author Contributions
Conceptualization, C.-C.K. and Y.-H.P.; methodology, C.-C.K.; software, Z.-Y.L. and C.-K.T.; validation, C.-C.K. and Y.-H.P.; formal analysis, Z.-Y.L. and C.-K.T.; investigation, Z.-Y.L. and C.-K.T.; resources, Y.-H.P.; data curation, C.-C.K. and Y.-H.P.; writing—original draft preparation, C.-C.K.; writing—review and editing, C.-C.K. and Y.-H.P.; visualization, C.-C.K.; supervision, C.-C.K. and Y.-H.P.; project administration, C.-C.K. and Y.-H.P.; funding acquisition, Y.-H.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
This study was supported by the National Science and Technology Council (NSTC), Republic of China (Taiwan), under Grant No. NSTC 112-2221-E-259-01.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sim, M.; Kim, C.S.; Shon, W.J.; Lee, Y.K.; Choi, E.Y.; Shin, D.M. Hydrogen-rich water reduces inflammatory responses and prevents apoptosis of peripheral blood cells in healthy adults: A randomized, double-blind, controlled trial. Sci. Rep. 2020, 10, 12130. [Google Scholar] [CrossRef]
- Timón, R.; Olcina, G.; González-Custodio, A.; Camacho-Cardenosa, M.; Camacho-Cardenosa, A.; Martínez Guardado, I. Effects of 7-day intake of hydrogen-rich water on physical performance of trained and untrained subjects. Biol. Sport 2021, 38, 269–275. [Google Scholar] [CrossRef]
- LeBaron, T.W.; Singh, R.B.; Fatima, G.; Kartikey, K.; Sharma, J.P.; Ostojic, S.M.; Gvozdjakova, A.; Kura, B.; Noda, M.; Mojto, V.; et al. The Effects of 24-Week, High-concentration hydrogen-rich water on body composition, blood lipid profiles and inflammation biomarkers in men and women with Metabolic Syndrome: A Randomized Controlled Trial. Diabetes Metab. Syndr. Obes. 2020, 13, 889–896. [Google Scholar] [CrossRef]
- Vaishnavi, C.; Elangovan, G.P.; Thirumal, M.; Prashanth, S.V.; Deepika, D.; Pragathi, T.G. Comparison of the Antimicrobial Effect of Hydrogen Water and Chlorhexidine Mouth rinse in Toothbrush Disinfection Among Dental Students. J. Pharm. Bioallied Sci. 2025, 17, S528–S530. [Google Scholar] [CrossRef]
- Kuzmanovic, J.; Todorovic, N.; Ranisavljev, M.; Javorac, D.; Korovljev, D.; Tarnava, A.; Stajer, V.; Ostojica, S.M. The effects of drinking hydrogen-rich water for six weeks on exercise-related biomarkers in exercise-naïve men and women over 50 years following resistance training program: A randomized controlled pilot trial. Res. Sports Med. 2025, 33, 711–721. [Google Scholar] [CrossRef] [PubMed]
- El-Shafie, M. Hydrogen production by water electrolysis technologies: A review. Results Eng. 2023, 20, 101426. [Google Scholar] [CrossRef]
- Kumara, S.; Lim, H. An over view of water electrolysis technologies for green hydrogen production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Liu, S.; Li, B.; Mohite, S.V.; Devaraji, P.; Mao, L.; Xing, R. Ultrathin MoS2 nanosheets in situ grown on rich defective Ni0.96S as heterojunction bifunctional electrocatalysts for alkaline water electrolysis. Int. J. Hydrog. Energy 2020, 45, 29929–29937. [Google Scholar] [CrossRef]
- Qazi, U.Y.; Javaid, R.; Zahid, M.; Tahir, N.; Afzal, A.; Lin, X.-M. Bimetallic NiCo–NiCoO2 nano-heterostructures embedded on copper foam as a self-supported bifunctional electrode for water oxidation and hydrogen production in alkaline media. Int. J. Hydrog. Energy 2021, 46, 18936–18948. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, L.; Chen, T.; Liang, J.; Zhang, Q.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. Transition Metal/Metal Oxide Interface (Ni–Mo–O/Ni4Mo) Stabilized on N-Doped Carbon Paper for Enhanced Hydrogen Evolution Reaction in Alkaline Conditions. Ind. Eng. Chem. Res. 2021, 60, 5145–5150. [Google Scholar] [CrossRef]
- Lv, Z.; Ma, W.; Wang, M.; Dang, J.; Jian, K.; Liu, D.; Huang, D. Co-Constructing Interfaces of Multiheterostructure on MXene (Ti3C2Tx)-Modified 3D Self-Supporting Electrode for Ultraefficient Electrocatalytic HER in Alkaline Media. Adv. Funct. Mater. 2021, 31, 2102576. [Google Scholar] [CrossRef]
- Jang, M.J.; Yang, J.; Lee, J.; Park, Y.S.; Jeong, J.; Park, S.M.; Jeong, J.-Y.; Yin, Y.; Seo, M.-Y.; Choi, S.M.; et al. Superior performance and stability of anion exchange membrane water electrolysis: pH-controlled copper cobalt oxide nanoparticles for the oxygen evolution reaction. J. Mater. Chem. A 2022, 8, 4290. [Google Scholar] [CrossRef]
- Chen, N.; Paek, S.Y.; Lee, J.Y.; Park, J.H.; Lee, S.Y.; Lee, Y.M. High-performance anion exchange membrane water electrolyzers with a current density of 7.68 A cm−2 and a durability of 1000 hours. Energy Environ. Sci. 2021, 14, 6338–6348. [Google Scholar] [CrossRef]
- Xie, Z.; Yu, S.; Ma, X.; Li, K.; Ding, L.; Wang, W.; Cullen, D.A.; Meyer, H.M.; Yu, H.; Tong, J.; et al. MoS2 nanosheet integrated electrodes with engineered 1T-2H phases and defects for efficient hydrogen production in practical PEM electrolysis. Appl. Catal. B 2022, 313, 121458. [Google Scholar] [CrossRef]
- Jang, I.; Im, K.; Shin, H.; Lee, K.-S.; Kim, H.; Kim, J.; Yoo, S.J. Electron-deficient titanium single-atom electrocatalyst for stable and efficient hydrogen production. Nano Energy 2020, 78, 105151. [Google Scholar] [CrossRef]
- Jiang, G.; Yu, H.; Yao, D.; Li, Y.; Chi, J.; Zhang, H.; Shao, Z. Boosting the oxygen evolution stability and activity of a heterogeneous IrRu bimetallic coating on a WO3 nano-array electrode for PEM water electrolysis. J. Mater. Chem. A 2022, 10, 11893–11903. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiang, H.; Wang, S.; Qian, B.; Li, Q.; Ge, L.; Chen, H. Mn-doped Ruddlesden-Popper oxide La1.5Sr0.5NiO4+δ as a novel air electrode material for solid oxide electrolysis cells. Ceram. Int. 2021, 47, 1208–1217. [Google Scholar] [CrossRef]
- Vibhu, V.; Vinke, I.C.; Eichel, R.-A.; de Haart, L.G.J. Cobalt substituted Pr2Ni1−xCoxO4+δ (x = 0, 0.1, 0.2) oxygen electrodes: Impact on electrochemical performance and durability of solid oxide electrolysis cells. J. Power Sources 2021, 482, 228909. [Google Scholar] [CrossRef]
- Song, S.; Zhang, H.; Ma, X.; Shao, Z.; Baker, R.T.; Yi, B. Electrochemical investigation of electrocatalysts for the oxygen evolution reaction in PEM water electrolyzers. Int. J. Hydrog. Energy 2008, 33, 4955–4961. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, X.; Chen, H.; Gao, R.; Shi, L.; Yang, L.; Zou, X. Iridium-containing water-oxidation catalysts in acidic electrolyte. Chin. J. Catal. 2021, 42, 1054–1077. [Google Scholar] [CrossRef]
- Kim, J.-D.; Ohira, A. Water electrolysis using a porous IrO2/Ti/IrO2 catalyst electrode and Nafion membranes at elevated temperatures. Membranes 2021, 11, 330. [Google Scholar] [CrossRef]
- Bernicke, M.; Ortel, E.; Reier, T.; Bergmann, A.; Ferreira de Araujo, J.; Strasser, P.; Kraehnert, R. Iridium oxide coatings with templated porosity as highly active oxygen evolution catalysts: Structure-Activity relationships. ChemSusChem 2015, 8, 1908–1915. [Google Scholar] [CrossRef]
- Cherevko, S.; Geiger, S.; Kasian, O.; Kulyk, N.; Grote, J.; Savan, A.; Shrestha, B.R.; Merzlikin, S.; Breitbach, B.; Ludwig, A.; et al. Oxygen and hydrogen evolution reactions on Ru, RuO2, Ir, and IrO2 thin film electrodes in acidic and alkaline electrolytes: A comparative study on activity and stability. Catal. Today 2016, 262, 170–180. [Google Scholar] [CrossRef]
- Yang, Z.; Lin, J.; Zhang, H.; Lin, B.; Lin, G. A new direct coupling method for photovoltaic module-PEM electrolyzer stack for hydrogen production. Fuel Cells 2018, 18, 543–550. [Google Scholar] [CrossRef]
- Arunachalam, M.; Han, D.S. Efficient solar-powered PEM electrolysis for sustainable hydrogen production: An integrated approach. Emerg. Mater. 2024, 7, 1401–1415. [Google Scholar] [CrossRef]
- García-Valverde, R.; Espinosa, N.; Urbina, A. Optimized method for photovoltaic-water electrolyser direct coupling. Int. J. Hydrog. Energy 2011, 36, 10574–10586. [Google Scholar] [CrossRef]
- Cogan, S.F.; Troyk, P.R.; Ehrlich, J.; Plante, T.D.; Detlefsen, D.E. Potential-biased, asymmetric waveforms for charge-injection with activated iridium oxide (AIROF) neural stimulation electrodes. IEEE Trans. Biomed. Eng. 2006, 53, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Cogan, S.F.; Guzelian, A.A.; Agnew, W.F.; Yuen, T.G.H.; McCreery, D.B. Over-pulsing degrades activated iridium oxide films used for intracortical neural stimulation. J. Neurosci. Methods 2004, 137, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K. Anodically electrodeposited iridium oxide films (AEIROF) from alkaline solutions for electrochromic display devices. Jpn. J. Appl. Phys. 1989, 28, 632–637. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, T.; Cai, Z.; Cao, Y.; Yang, H.; Duan, Y.Y. Anodically electrodeposited iridium oxide films microelectrodes for neural microstimulation and recording. Sen. Actuator B-Chem. 2009, 137, 334–339. [Google Scholar] [CrossRef]
- Siracusano, S.; Baglio, V.; Blasi, A.D.; Briguglio, N.; Stassi, A.; Ornelas, R.; Trifoni, E.; Antonucci, V.; Arico, A.S. Electrochemical characterization of single cell and short stack PEM electrolyzers based on a nanosized IrO2 anode electrocatalyst. Int. J. Hydrogen Energy 2010, 35, 5558–5568. [Google Scholar] [CrossRef]
- Ngo, T.H.N.; Love, J.; O’Mullane, A.P. Investigating the influence of amorphous/crystalline interfaces on the stability of IrO2 for the oxygen evolution reaction in acidic electrolyte. ChemElectroChem 2023, 10, e202300438. [Google Scholar] [CrossRef]
- Jang, I.; Hwang, I.; Tak, Y. Attenuated degradation of a PEMFC cathode during fuel starvation by using carbon-supported IrO2. Electrochim. Acta 2013, 90, 148–156. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, H.; Ma, H.; Zhong, H.; Zou, Y. Study of carbon-supported IrO2 and RuO2 for use in the hydrogen evolution reaction in a solid polymer electrolyte electrolyzer. Electrochim. Acta 2010, 55, 1855–1861. [Google Scholar] [CrossRef]
- Badley, M.D.M.; Shoesmith, D.W.; Noёl, J.J. Effect of hydrogen on the dissolution of uranium dioxide in peroxide-containing environments. J. Electrochem. Soc. 2023, 170, 096506. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).