Abstract
1,2,4-Triazoles, as heterocyclic compounds, represent an attractive class of ligands in the design of metal–organic frameworks (MOFs) due to their donor–acceptor character, thermal and chemical stability, and ability to form extended coordination networks. This review summarizes the literature published between 2019 and 2025 on luminescent MOFs incorporating 1,2,4-triazole scaffold. The analysis covers synthetic conditions and provides a detailed discussion of the luminescent properties of these materials. Particular emphasis is placed on their applicability as luminescent probes for environmental monitoring and medical diagnostics, as well as on their potential use in light-emitting diode construction. The collected data highlight promising results, including strong correlations between analyte concentration and luminescent response, enabling sensitive detection with low limits of detection (LOD) for selected analytes. This article may serve as a valuable resource for chemists, physicists, and engineers involved in the design of functional materials and the development of detection and optoelectronic technologies.
1. Introduction
Triazoles belong to the class of five-membered heterocyclic compounds composed of three nitrogen atoms and two sp2-hybridized carbon atoms. Depending on the arrangement of the heteroatoms within the cyclic system, two structural isomers can be distinguished, 1,2,3-triazole and 1,2,4-triazole (Figure 1), the latter being the subject of this article. For each structural isomer, three (A–C) and two tautomeric forms (D–E), respectively, can be identified (Figure 1) [1].
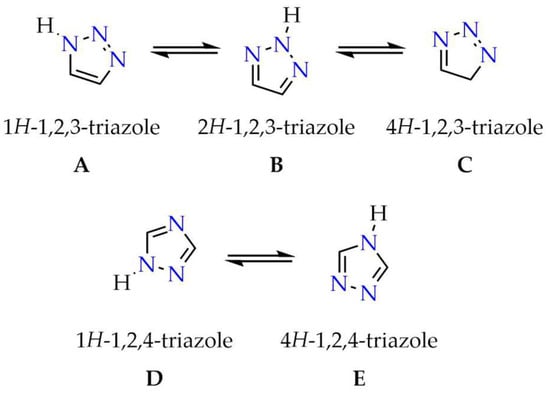
Figure 1.
Structural isomers of triazole and their tautomeric forms.
Derivatives of 1,2,4-triazole have attracted increasing scientific interest over the years in the search for new solutions in fields such as medicine, agriculture, metallurgy, optoelectronics, and specialty materials chemistry. They are renowned for their exceptional biological activity, which arises from their ability to form hydrogen bonds and complex with metals, thereby enabling interactions with various biological targets such as enzymes [2,3]. Compounds containing the 1,2,4-triazole ring exhibit antibacterial [4], antiviral [5], antioxidant [6], antidepressant [7], anticancer [8] and antidiabetic properties [9]. Representative drugs of this class include Letrozole, used in breast cancer treatment [10], the antiviral Ribavirin [11], and Rizatriptan with antimigraine activity [12] (Figure 2). The 1,2,4-triazole ring is also present in agrochemical formulations marketed as fungicides and plant growth regulators, with examples including Paclobutrazol [13], Uniconazole [14], and Myclobutanil [15] (Figure 2).
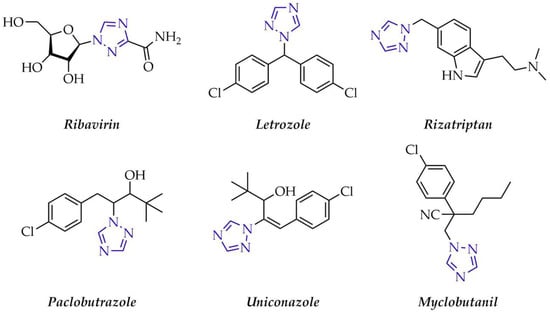
Figure 2.
Representative drugs and pesticides that contain 1,2,4-triazole scaffold.
Triazoles also represent valuable molecular cores in the design of materials for optoelectronic devices such as organic light-emitting diodes (OLEDs). This can be achieved through the preparation of donor–acceptor compounds [16,17], or transition metal complexes [18,19]. Luminescent properties have also been reported for coordination polymers [20] and metal–organic frameworks (MOFs) based on 1,2,4-triazole. These systems may serve as luminescent thermometers or sensors sensitive to environmental pollutants such as metallic cations and anions, pharmaceuticals, pesticides, and nitroaromatic compounds (NACs). Furthermore, they may find application in medical diagnostics by indicating the presence of key biomarkers in human body fluids. Functionalization of the triazole ring leads to compounds with diverse applications. An example is 1,2,4-triazole-3-thiol derivatives, successfully employed as electrolytes in dye-sensitized solar cells (DSSCs) [21]. Tang and coworkers utilized a 3-amino-5-mercapto-1,2,4-triazole derivative to fabricate an electrode capable of detecting 3-caffeoylquinic acid—a phenolic acid responsible for preventing type 2 diabetes—in food and blood samples under physiological conditions [22]. Bakov and coworkers developed a 1,2,4-triazole conjugate with a 1,8-naphthalimide fluorophore, which functions as a fluorescent probe sensitive to toxic volatile amines, particularly ammonia [23]. Due to their high nitrogen content, triazole-based compounds are widely applied in energetic materials [24], for instance, in the form of energetic ionic liquids (EILs) [25]. Owing to their ability to form weak interactions with steel surfaces, certain 1,2,4-triazoles generate protective films against corrosive agents, which is of particular importance in metallurgy [26,27]. The structures of the triazole units used in the described examples are shown in Figure 3.
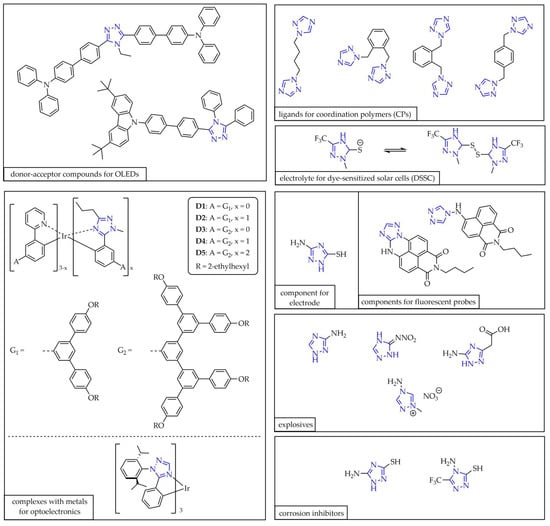
Figure 3.
Selected 1,2,4-triazoles for various purposes: optoelectronic applications, materials science, high-energy-density materials, medical diagnostics, and metallurgy.
In this review, we highlight selected synthetic approaches to the 1,2,4-triazole scaffold, taking into account its two tautomeric forms: 1H-1,2,4-triazole and 4H-1,2,4-triazole. The first literature reports on the synthesis of metal–organic frameworks incorporating a 1,2,4-triazole moiety within their structure appeared in 2004. Since then, and particularly in recent years, interest in such derivatives has been steadily increasing, as reflected by the growing number of related publications. The main focus of this review is to provide a systematic summary of scientific reports from recent years (2019–2025) concerning the preparation of metal–organic frameworks (MOFs) incorporating ligands based on this heterocyclic motif. Particular attention is paid to the luminescent properties of these materials and potential possibilities for their practical application. The subject matter has an interdisciplinary character, as it combines achievements from many scientific fields, including organic chemistry, inorganic chemistry, coordination chemistry, physics, biotechnology, and materials engineering. By presenting the most recent advances, this article aims to provide comprehensive knowledge to researchers working across different disciplines, highlighting the exceptional application potential of luminescent metal–organic frameworks (LMOFs).
2. Selected Methods for Obtaining 1,2,4-Triazoles
2.1. Synthesis of 1H-1,2,4-Triazoles
One of the tautomeric forms, 1H-1,2,4-triazole, can be obtained through a number of different methodologies, some of which are presented in Figure 4 (methods A–H). As described by Chen and coworkers (method A) [28], the substrates in the transformation leading to the 1,2,4-triazole ring may include hydrazone derivatives and aliphatic amines. The authors employed aerobic oxidation conditions in the presence of molecular iodine and tert-butyl hydroperoxide (TBHP) as oxidant, without the participation of transition metals. This cascade reaction proceeded through a sequence of subsequent transformations involving C–H functionalization, C=N double bond formation, and oxidative aromatization. Lee and coworkers developed a method for the synthesis of trisubstituted 1,2,4-triazole derivatives from N-acylated amides and various hydrazine hydrochlorides using microwave irradiation (method B) [29]. This approach enabled the desired products to be obtained within a few minutes, with good yields up to 88%. They also described a rapid and efficient one-pot method tested with various benzamide derivatives as starting materials. Method C presents the approach developed by Castanedo and coworkers, in which carboxylic acids and primary amidines were used as substrates [30]. These were subjected to the coupling reagent hexafluorophosphate azabenzotriazole tetramethyl uronium (HATU) and N,N-diisopropylethylamine (DIPEA) as base, yielding an acylamidine intermediate. In the subsequent step, it reacted with a monosubstituted hydrazine derivative, and within 1–3 h, the cyclic main product was formed. 1H-1,2,4-Triazoles can also be synthesized electrochemically from hydrazone and amidine derivatives in the presence of potassium iodide, as reported by Jiang and coworkers (method D) [31]. This strategy afforded a series of products bearing both alkyl and aryl substituents, with moderate to good yields, without the need for chemical oxidants. Huang and coworkers obtained 1,3-disubstituted 1,2,4-triazole derivatives via catalytic C(sp3)–H functionalization of amidines. They used a catalytic system consisting of copper(I) iodide (CuI), atmospheric oxygen as oxidant, and tripotassium phosphate (K3PO4) as base (method E) [32]. The authors described the synthesis of a series of triazole derivatives in good yields ranging from 72% to 92%. The 1,2,4-triazole ring may also be obtained via [3+2] cycloaddition of isocyanides and diazonium salts (method F). Liu and coworkers investigated this method and reported the influence of the catalyst on the regioselectivity of the reaction [33]. Formation of 1,3-disubstituted 1,2,4-triazole was observed when silver(I) carbonate (Ag2CO3) was used as catalyst (also acting as a base), whereas 1,5-disubstituted 1,2,4-triazole was obtained in the presence of copper(II) acetate (Cu(OAc)2) and lithium acetate (LiOAc) as base. Method G describes the synthesis of 1,3,5-trisubstituted 1,2,4-triazoles via base-promoted [3+2] dipolar cycloaddition of trifluoroacetohydrazonoyl chlorides with imides [34]. This procedure, reported by Zhang and coworkers, led to the desired trifluoromethyl-substituted triazole derivatives in excellent yields (91–99%) and high regioselectivity. Another approach (method H) involves a two-step heterocyclization comprising amination and cyclization of hydrazonamide with an acid chloride in the presence of triethylamine (TEA) [35]. This procedure, employed by Li and coworkers, was used to synthesize bioactive 1-aryl-1H-1,2,4-triazole-3-carboxylate derivatives, which were subsequently evaluated for their ability to inhibit nitric oxide (NO) production and cyclooxygenase COX-1/COX-2 activity.
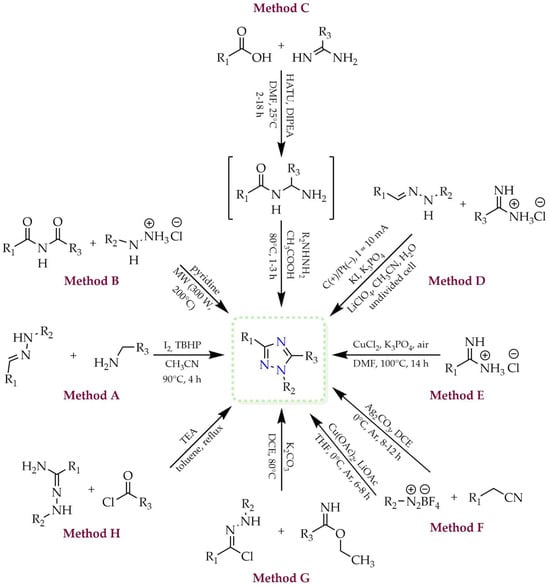
Figure 4.
Various methods for the preparation of 1H-1,2,4-triazole (R1, R2, R3 = H, alkyl, aryl, NH2, CF3, COOCH3, etc.).
2.2. Synthesis of 4H-1,2,4-Triazoles
The preparation of 4H-1,2,4-triazole derivatives requires the use of different starting materials and procedures compared to the 1H-1,2,4-triazole tautomer. Possible approaches to their synthesis are presented in Figure 5 (methods I–P). By selecting the appropriate method, it is possible to control the formation of one of these tautomeric structures. Method I, described by Olesiejuk and coworkers, involves the reaction of a diimidoyl dichloride derivative with an amine in boiling toluene, leading to cyclization and the formation of the 4H-1,2,4-triazole ring [36]. Using this procedure, precursor compounds were obtained, which were then subjected to Suzuki cross-coupling, resulting in derivatives exhibiting luminescent properties. Another synthetic approach involves the addition of hydrazides to secondary amides (method J) [37]. Bechara and coworkers developed an efficient one-pot method for this transformation, applying an amide activation strategy using trifluoromethanesulfonic anhydride (Tf2O) in the presence of 2-fluoropyridine (2-FPyr) as a base. In the subsequent step, the cyclodehydration process was induced by microwave irradiation. Method K relies on oxidative cyclization of various trifluoroacetimidoyl hydrazide derivatives and N,N-dimethylformamide (DMF) mediated by iodine (I2) [38]. Using this method, Lu and coworkers obtained a series of derivatives with yields ranging from 42 to 62% and demonstrated their application potential by synthesizing a key structural element of an anticoagulant drug acting as factor IXa inhibitor. The reaction between dimethylcyanamide and anhydrous hydrazine also allows for the construction of the 1,2,4-triazole ring, as reported by Yocca and coworkers (method L) [39]. Method M involves the intramolecular cyclization of hydrazine-1-carbothioamide derivative, which proceeds in an aqueous sodium hydroxide solution [40]. As reported by Rahim and coworkers, after neutralization of the medium with dilute hydrochloric acid, the desired 1,2,4-triazole derivative was successfully isolated. Method N, described by Hutchinson and coworkers, consists of the reaction of an amine with N′-((dimethylamino)methylene)-N,N-dimethylformohydrazonamide in an acidic medium generated by the addition of p-toluenesulfonic acid, resulting in substituted 1,2,4-triazole derivatives [41]. Such a moiety was also obtained, as reported by Gogoi and coworkers, from arylidenearylthiosemicarbazides in a Cu(II)-catalyzed reaction carried out in DMSO at 80 °C (method O) [42]. Using this procedure, two groups of heterocycles were obtained: 4,5-disubstituted 1,2,4-triazole-3-thiones, which upon extended reaction time underwent desulfurization to the corresponding 4,5-disubstituted 1,2,4-triazole derivatives. Oxidative cyclization of amidrazones and aldehydes also leads to the formation of the 4H-1,2,4-triazole system (method P). This was reported by Nakka and coworkers, who used ceric ammonium nitrate (CAN) as the oxidizing agent and polyethylene glycol as the reaction medium [43]. This way a series of 3,4,5-trisubstituted 1,2,4-triazole derivatives was successfully obtained in high yields (87–97%).
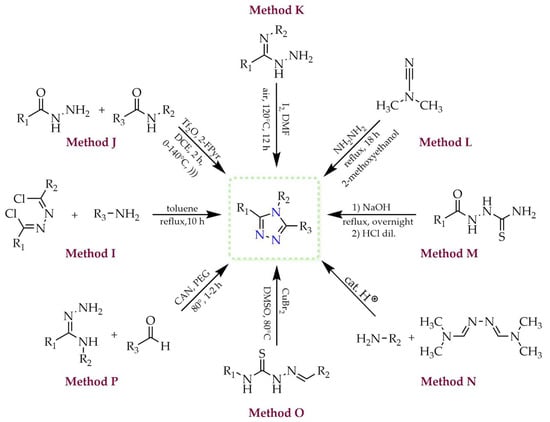
Figure 5.
Various methods for the preparation of 4H-1,2,4-triazole (R1, R2, R3 = H, alkyl, aryl, CF3, SH, etc.).
3. Triazole-Based Luminescent Metal—Organic Frameworks (LMOFs)
3.1. Metal—Organic Frameworks (MOFs)
Metal–organic frameworks (MOFs) represent a class of solid polymeric materials at the intersection between materials science and coordination chemistry. They are composed of metal ions or metal clusters, which act as nodes in the structure. Organic ligands connect these coordination centers, resulting in the formation of one-, two- or three-dimensional networks. Proper selection of linkers and nodes enables the preparation of functional materials with desired properties [44]. The first literature report on the application of a 1,2,4-triazole derivative for the construction of metal–organic frameworks dates back to 2004. Su and coworkers published an article in which they obtained a MOF with the formula [ZnF(3-NH2-5-COOH-TZ)]·solvents (1), using a solvothermal approach. The synthesis employed heating of 3-amino-1,2,4-triazole-5-carboxylic acid hemihydrate ([3-NH2-5-COOH-TZ], 1a), zinc(II) tetrafluoroborate hydrate, and zinc(II) fluoride tetrahydrate in aqueous ethanol for 30 h (Scheme 1) [45].
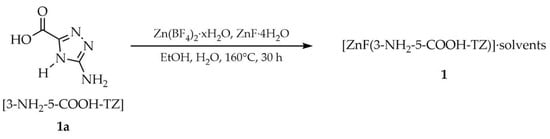
Scheme 1.
Synthesis of the first 1,2,4-triazole-based MOF described in the literature.
Ligands based on a triazole core are increasingly used for MOF construction. Important characteristics of linkers that influence the physicochemical properties of the material, apart from their size and shape, include the number of potential coordination sites. The greater the number of such sites, the greater the diversity of networks that can be obtained. This is achieved either by introducing coordination-capable functional groups, such as carboxyl or amino groups, or by designing the ligand structure around heterocycles such as pyridine, imidazole, etc. For this reason, the triazole ring represents an attractive structural motif as it contains three nitrogen atoms that can act as potential binding sites in coordination networks with metals, as illustrated in Figure 6 and Figure 7.
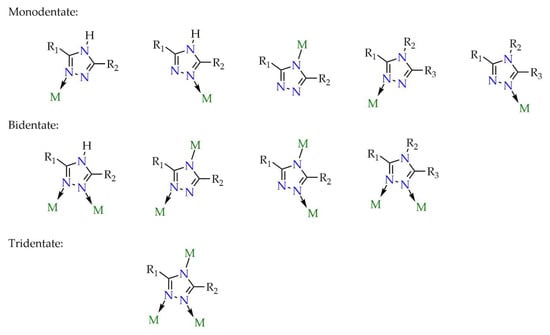
Figure 6.
Possible coordination modes for 4H-1,2,4-triazole derivatives (R1, R2, R3 = alkyl, aryl, pyridyl, isophthalic acid, etc.).
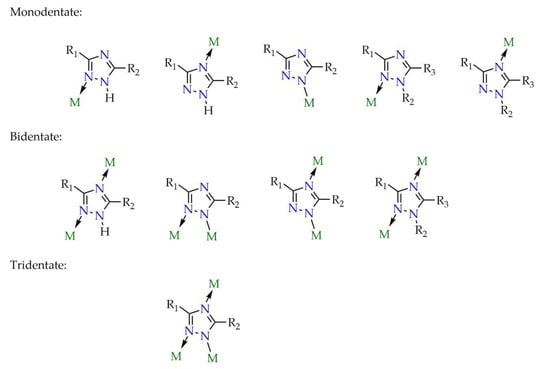
Figure 7.
Possible coordination modes for 1H-1,2,4-triazole derivatives (R1, R2, R3 = alkyl, aryl, pyridyl, isophthalic acid, etc.).
The construction of networks containing organic ligands and metal nodes requires suitable conditions that enable the formation of coordination bonds. The literature describes numerous methodologies for MOF’s preparation, including microwave-assisted, sonochemical, electrochemical, and mechanochemical methods [46]. Well-known MOFs such as MOF-5, MOF-74, MOF-177, and MOF-199 have been obtained by mixing starting materials at room temperature [47]. The most important methods for obtaining luminescent 1,2,4-triazole-based MOFs are solvothermal and hydrothermal approaches. These are defined as reactions carried out in sealed vessels under pressure (greater than 1 atm) at a temperature above the boiling point of the solvent [48]. In the hydrothermal process, the reaction takes place in the presence of water, while in the solvothermal process an additional organic solvent (protic or aprotic) is used [49,50].
For example, Sun and coworkers described a solvothermal approach to the synthesis of lanthanide-based MOFs [51]. Europium(III) and terbium(III) nitrate pentahydrates (Ln(NO3)3·6H2O, Ln = Eu, Tb) and 5-(1H-1,2,4-triazol-1-yl)isophthalic acid ([1-L1-TZ, L1 = 3,5-dicarboxylic acid)], 2a, Scheme 2) were employed as starting materials. Substrates (0.1 mmol) were placed in a Teflon-lined stainless-steel container together with 4 mL of water and DMF. The vessel was tightly sealed and heated at 85 °C for 72 h, then cooled to room temperature under ambient conditions. Colorless crystals of [Eu2(1-L1-TZ)3(H2O)2]·2H2O·2DMF (2) and [Tb2(1-L1-TZ)3(H2O)2]·2H2O·2DMF (3) were filtered off, washed with DMF, and air-dried. The products were obtained in yields of 47% and 42%, respectively.
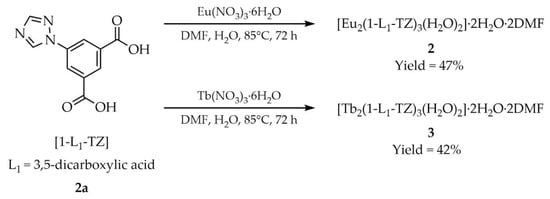
Scheme 2.
Synthesis of lanthanide-based MOFs by solvothermal approach.
In turn, Liu and coworkers employed hydrothermal conditions to synthesize a zinc-based MOF with the formula Zn(CH3COO)2(L) (4, Scheme 3) [52]. For this synthesis, zinc(II) acetate dihydrate, 3-amino-1,2,4-triazole ([3-NH2-TZ], 4a) and water were used. The reaction was carried out in a sealed vessel at 180 °C for 72 h. Pure crystals were obtained in 90% yield.
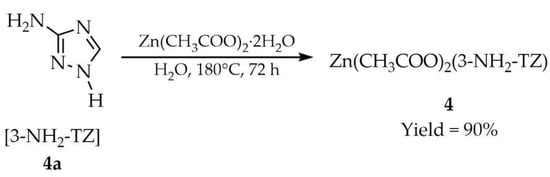
Scheme 3.
Synthesis of a Zn-based MOF by hydrothermal approach.
An important issue after isolating a MOF product from the reaction mixture is the confirmation of its composition [53], which is typically performed by means of elemental analysis and infrared (IR) spectroscopy. In addition, determining its crystal structure is essential, which is achieved through X-ray crystallography. The percentage content of metallic components is determined using inductively coupled plasma mass spectrometry (ICP-MS). Scanning electron microscopy (SEM) analysis enables high-resolution imaging of the material’s surface. For MOFs with optoelectronic applications, the required properties include high thermal and chemical stability [54]. For this purpose, thermogravimetric analysis (TG) is employed, belonging to the family of thermal analysis methods, allowing observation of changes in physical properties as a function of temperature or time. Structural stability during aging processes and after exposure to different solvents is assessed by powder X-ray diffraction (PXRD). Porosity and specific surface area are determined based on nitrogen sorption isotherms and using the Brunauer–Emmett–Teller (BET) model [55]. In the preparation of luminescent probes, this method is used to assess the possibility of regeneration and reusability of the probe. Analyses employed to determine luminescent properties include UV–Vis spectroscopy and photoluminescence (PL) spectroscopy [56].
The working principle of luminescent probes constructed on the basis of MOFs lies in the fact that the analyte can induce an “turn-on” effect or luminescence enhancement, which is quantitatively described by the enhancement constant (KEC). However, the most commonly observed phenomenon is luminescence “turn-off” or quenching, for which the Stern–Volmer constant (KSV) is determined. An important parameter describing the sensitivity of the probe is the limit of detection (LOD), defined based on the obtained constant, which indicates the lowest concentration of analyte that can be detected using the material. Numerous mechanisms of analyte detection by coordination porous materials have been reported in the literature, where the response is reflected in changes in the luminescence spectrum. These include photoelectron transfer (PET), resonance energy transfer (RET), competition absorption (CA), chemical conversion (CC), and structural transformation (ST) [57].
Metal–organic frameworks exhibit many other properties attractive for applications, among which their porosity deserves special attention, as it enables storage and transport of guest molecules. They also feature high thermal stability [44,58,59]. The appropriate pore size and developed surface area allow adsorption and storage of gases such as hydrogen (H2) [60], methane (CH4) [61], and acetylene (C2H2) [62]. Morphological aspects also enable selective gas separation, e.g., methane from nitrogen (CH4/N2) [63], ethylene (C2H4) from CO2 or C2H2 [62], as well as in ternary mixtures [64], and CO2 from N2, H2, and CH4 [65]. Promising results have also been obtained for selective capture of toxic gases such as sulfur dioxide (SO2) [66], nitrogen dioxide (NO2) [66], hydrogen sulfide (H2S) [67] and ammonia (NH3) [68]. This phenomenon has also been exploited for water capture from air in the development of water sorption-driven devices [69]. The structures of metal–organic frameworks contain metallic nodes that can act as catalytic sites, making them applicable in heterogeneous catalysis [70,71]. Their porosity also allows encapsulation of guest molecules that serve as additional catalytic centers, such as metallic nanoparticles or enzymes [72]. They can be used not only in thermal catalysis but also in reactions where they act as electro- [73] or photocatalysts [74]. Metal–organic frameworks represent a promising class of materials for advanced biomedical applications, such as targeted drug delivery systems, diagnostic imaging techniques, and radio- and phototherapy. MOFs have been studied as contrast agents for magnetic resonance imaging (MRI) [75] and computed tomography (CT) [76]. The possibility of exchanging metal nodes in structural networks enables the preparation of MOFs containing positron-emitting radioisotopes, which can be successfully detected by positron emission tomography (PET) [77]. Frameworks incorporating components capable of absorbing exogenous energy in the form of light or X-rays can convert it to trigger localized therapeutic effects in photodynamic therapy (PDT) [78] and radiotherapy–radiodynamic therapy (RT-RDT) [79]. High porosity and suitable adsorption/release profiles of active substances allow the development of efficient drug delivery systems, which are particularly important for targeted therapy [80]. MOFs can also be designed as optical sensors, whose operation is based on solvatochromic [81] or luminescent [82] effects. Furthermore, they have been applied in the development of electromechanical sensors [83], chemiresistive sensors [84], electrochemical sensors [85], and chemicapacitors [86]. Their potential use in chemically sensitive field-effect transistors (ChemFETs) has also been demonstrated [87]. An interesting approach involves creating cross-reactive arrays, enabling the discrimination of multiple analytes in multicomponent mixtures [88]. Finally, MOFs are a promising class of materials for energy-related applications, such as batteries and supercapacitors [89].
In the following subsections, 1,2,4-triazole-based LMOFs constructed with metals such as zinc, cadmium, and lanthanides including europium, gadolinium, terbium, lanthanum, holmium, and dysprosium, as well as metal clusters and frameworks containing cobalt, copper, and lead, are described. Fundamental data on their luminescent properties, including absorption, excitation, and emission wavelengths, are summarized in Table 1, Table 2, Table 3, Table 4 and Table 5. Particular attention is given to their potential for luminescent sensing of various analytes, with quenching or enhancement constants and detection limits provided. Table 6 summarizes these parameters for the detection of cations and anions, while Table 7 presents the corresponding data for organic compounds, whose structures are shown in Figure 8. For some of the obtained MOFs, the quantum yield and luminescence lifetime were determined, and these results are summarized in Table 8.
3.1.1. Zinc-Based Metal–Organic Frameworks Containing 1,2,4-Triazole Organic Linker
One of the most widely employed metal ions for the construction of 1,2,4-triazole-based MOFs is zinc. In 2019, two studies were reported on this subject. He and coworkers described the synthesis of a three-dimensional porous framework, {[Zn2(TZ)2(DBTDC-O2)]·DMAc}n (5, Scheme 4), obtained from two organic ligands: 4H-1,2,4-triazole (HTZ, 5a) and S,S-dioxodibenzothiophene-3,7-dicarboxylic acid (H2DBTDC-O2, 5b) in a dimethylacetamide (DMAc) medium [90]. Compound 5 exhibits high water stability across a broad pH range (3–12) and, through luminescence quenching or enhancement, functions as a versatile sensor for detecting metal cations (Fe3+, Al3+), anions (SiF62−, Cr2O72−) (Table 6, Entry 1), and even antibiotics such as nitrofurantoin (NFT) and nitrofurazone (NFZ) (Table 7, Entry 1). In order to reveal the porosity of the material, a nitrogen sorption isotherm was also measured at 77 K.
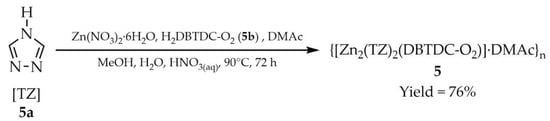
Scheme 4.
Synthesis of a zinc-based MOF (5) from 4H-1,2,4-triazole (5a) and S,S-dioxodibenzothiophen-3,7-dicarboxylic acid (5b).
Zou and coworkers reported the solvothermal synthesis of {[Zn4(TZ)6(HCOO)2]}n (6, Scheme 5), based on the tautomeric 1H-1,2,4-triazole (TZ, 6a) [91]. Here, 2,2′-biquinoline-4,4′-dicarboxylic acid did not coordinate Zn2+ ions directly but facilitated framework formation, while formate anions incorporated into the MOF skeleton originated from the decomposition of N,N-dimethylformamide (DMF). The resulting material displayed both thermal and aqueous stability. Owing to its luminescent properties (Table 1), framework 6 was demonstrated to act as a luminescent sensor sensitive to Cr2O72− ions (Table 6, Entry 2).

Scheme 5.
Preparation of a Zn-based MOF (6) from 1H-1,2,4-triazole (6a) and 2,2′-biquionoline-4,4′-dicarboxylic acid.
In 2020, Qin and coworkers synthesized another Zn-based MOF, [(CH3)2NH2]6[Zn6(OBA)6(4-NH2-3,5-(L1)2-TZ)3(SO4)2]·SO4·x(solvent) (7, Scheme 6), employing 4-amino-3,5-bis(4-pyridyl)-1,2,4-triazole ([4-NH2-3,5-(L1)2-TZ, L1 = 4-pyridyl, 7a), 4,4′-oxybis(benzoic acid) (H2OBA, 7b), and hydrated zinc sulfate [92]. This compound, due to the correlation of luminescence intensity and temperature, has the potential to be used as a thermal sensor. Temperature-dependent emission spectra were recorded between 87 and 277 K, revealing a gradual decrease in luminescence intensity with increasing temperature. Furthermore, compound 7 demonstrated the ability to detect and quantify Ni2+ and PO43− ions in aqueous solution (Table 6, Entry 3).
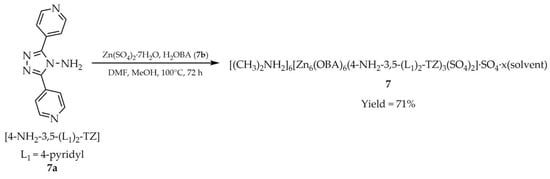
Scheme 6.
Synthesis of a zinc-based MOF (7) from 4-amino-3,5-bis(4-pyridyl)-1,2,4-triazole (7a) and H2OBA = 4,4′-oxybis(benzoic acid) (7b).
Cao and coworkers reported a novel three-dimensional Zn-based framework constructed from the ligand 5,5′-di(pyridin-2-yl)-3,3′-bi(1,2,4-triazole) ([5,5′-(L1)2-3,3′-TZ2, L1 = 2-pyridyl], 8a, Scheme 7) [93]. The material, formulated as {[Zn6Cl6([5,5′-(L1)2-3,3′-TZ2)3]·4,5H2O}n (8) was obtained under solvothermal conditions in a water–acetone medium. Both thermal and chemical stability were confirmed. Solid-state luminescence tests in the presence of various cations and anions revealed that compound 8 functions as a sensitive and efficient fluorescent sensor for Cu2+, Ag+, MnO4−, and Cr(VI) species (Cr2O72− and CrO42−) (Table 6, Entry 4). Importantly, the sensor could be rapidly regenerated and reused for at least four detection cycles.
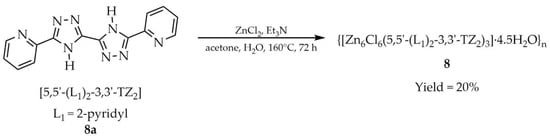
Scheme 7.
Preparation of a Zn-based MOF (8) using 5,5′-di(pyridyn-2-yl)-3,3′-bi(1,2,4-triazole) (8a).
Another Zn-MOF reported by Liu and co-workers displayed luminescent properties (Table 1) and also served as an efficient heterogeneous catalyst for the Knoevenagel condensation between benzaldehyde derivatives and malononitrile [94]. Framework {[Zn(H4-L1-TZ)2]}n (9, Scheme 8) was obtained hydrothermally from 5-(4H-1,2,4-triazol-4-yl)isophthalic acid ([4-L1-TZ, L1 = 3,5-dicarboxyphenyl], 9a), hydrated zinc perchlorate, lithium hydroxide, and water. Anion-sensing studies in aqueous media revealed that compound 9 exhibited the strongest luminescence response toward CN− ions, compared with F−, Cl−, Br−, CH3COO−, NO3−, H2PO4−, and HSO4−. Notably, luminescence enhancement was observed in the presence of cyanide, although the enhancement constant (KEC) and limit of detection (LOD) were not reported.
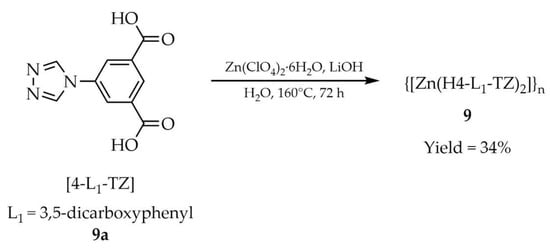
Scheme 8.
Synthesis of a zinc-based MOF (9) employing 5-(4H-1,2,4-triazol-4-yl)isophthalic acid (9a).
In another study, Li and coworkers employed 1-(4-(1H-imidazole-5-yl)phenyl)-1H-1,2,4-triazole ([1-L1-TZ, L1 = (4-(1H-imidazole-5-yl)phenyl], 10a, Scheme 9), 1,2,3-benzenetricarboxylic acid (H3btc, 10b), and sodium 5-sulfoisophthalate (NaH2SIP, 10c) as ligands for the synthesis of four MOFs based on zinc, manganese, and cadmium [95]. Two of the zinc-containing products displayed luminescent properties: [Zn2(1-L1-TZ)(1-L1-TZ)(SIP)(H2O)]·4H2O (10) and Zn3(1-L1-TZ)2(btc)2(H2O)2]·H2O (11) (Table 1). The reactions were performed in aqueous media with sodium hydroxide as base, and hydrated zinc sulfate as the metal source. Interestingly, the same reagents afforded different framework compositions depending on the reaction temperature: 180 °C for 10 and 140 °C for 11. Among the obtained MOFs, compound 10 exhibited the highest thermal stability and the most intense luminescence, with a quantum yield of 5.24% and the longest emission lifetime (29.56 ns). For compound 11, the quantum yield and emission lifetime were determined to be 3.18% and 18.53 ns, respectively.
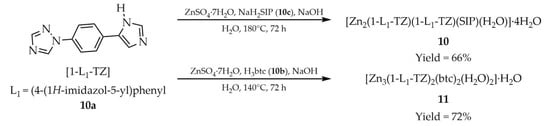
Scheme 9.
Preparation of two zinc-based MOFs (10, 11) using 1-(4-(1H-imidazole-5-yl)phenyl)-1H-1,2,4-triazole (10a), 1,2,3-benzenetricarboxylic acid (10b) and sodium 5-sulfoisophthalate (10c).
Liu and coworkers reported the synthesis and characterization of a novel two-dimensional zinc-based MOF, Zn(CH3COO)2(3-NH2-TZ) (4, Scheme 10), constructed from 3-amino-1,2,4-triazole ([3-NH2-TZ] 4a) [52]. The material was obtained via hydrothermal treatment of zinc acetate hydrate with 3-amino-1,2,4-triazole at 180 °C. Luminescence studies revealed strong blue emission at 433 nm upon excitation at 382 nm (Table 1). A weaker green emission (487–600 nm) corresponded to long-persistent luminescence (LPL), with an afterglow lasting up to ~3 s.
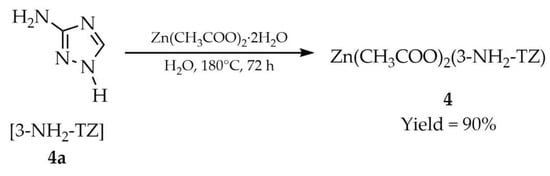
Scheme 10.
Synthesis of a zinc-based MOF (4) employing 3-amino-1,2,4-triazole (4a).
Rui-Ying Yu and coworkers described the synthesis and characterization of a Zn-MOF with the formula Zn6(ABTC)2(TZ)4(H2O)4(DMSO)2 (12, Scheme 11), employing unsubstituted 1,2,4-triazole ([TZ], 12a), and 3,3′,5,5′-azobenzenetetracarboxylic acid (H4ABTC, 12b) as ligands [96]. The synthesis was carried out in polar solvents such as dimethylformamide (DMF) and dimethylsulfoxide (DMSO). UV-Vis analysis showed that compound 12 exhibited an absorption band at 337 nm, which is a slight red shift compared to the free ligand 12b (Table 1). It shows strong blue emission with chromaticity coordinates (CIE) of (0.1363, 0.2079), suggesting its potential for LED applications.
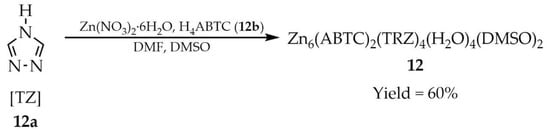
Scheme 11.
Preparation of a zinc-based MOF (12) from 1,2,4-triazole (12a) and 3,3′,5,5′-azobenzenetetracarboxylic acid (12b).
In the same year, Fang and coworkers reported the synthesis and properties of a luminescent zinc-based MOF, [Zn(3-L1-TZ)2]·DMA (13, Scheme 12), derived from 4-(1H-1,2,4-triazol-1-yl)benzoic acid ([3-L1-TZ, L1 = 4-benzoic acid, 13a) and zinc acetate dihydrate in medium composed of N,N-dimethylacetamide (DMA) and toluene [97]. The authors investigated the use of product 13 as a bifunctional luminescent sensor for nitrobenzene (NB) (Table 7, Entry 2) and Fe3+ ions (Table 6, Entry 5). Compound 13 displayed blue-violet luminescence with a quantum yield of 7.4%. Gas sorption analysis revealed selective adsorption of CO2 at 195 K, with a type I isotherm typical for microporous materials.
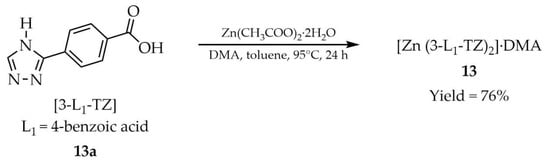
Scheme 12.
Synthesis of a zinc-based MOF (13) from 4-(1H-1,2,4-triazol-1-yl)benzoic acid (13a).
Another Zn-MOF constructed by Liu and coworkers, [Zn3(3-NH2-TZ)2(4-NH2-TZ)2(SO4)2]n (14, Scheme 13), was synthesized solvothermally from ZnSO4·7H2O and two different aminotriazole ligands: 3-amino-1,2,4-triazole ([3-NH2-TZ], 14a) and 4-amino-1,2,4-triazole ([4-NH2-TZ], 14b) [98]. In addition to luminescent properties (Table 1), compound 14 exhibited high sensitivity and selectivity toward Fe3+ ions (Table 6, Entry 6).
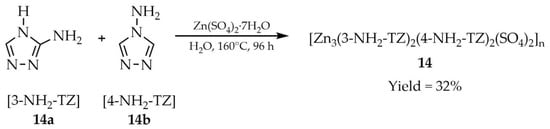
Scheme 13.
Preparation of a Zn–MOF (14) using 3-amino-1,2,4-triazole (14a) and 4-amino-1,2,4-triazole (14b).
Chen and coworkers described the synthesis of a Zn-MOF formulated as [Zn(1-L1-TZ)(NO2 mbda)]·H2O (15, Scheme 14), using 1-(4-(1H-imidazol-5-yl)phenyl)-1H-1,2,4-triazole ([1-L1-TZ, L1 = (4-1H-imidazol-5-yl)phenyl)], 15a) and 5-nitroisophthalic acid (H2NO2 mbda, 15b) as ligands [99]. The reaction was carried out in a mixture of solvents DMF/EtOH/H2O using hydrated zinc sulfate. Luminescence properties were investigated (Table 1), and a luminescence lifetime of 8 ns was determined, with a quantum yield of 2.15%. Beyond zinc-based frameworks, the same review also discussed MOFs with magnetic properties based on other metals such as copper, cobalt, and manganese.

Scheme 14.
Synthesis of a zinc-based MOF (15) from 1-(4-(1H-imidazol-5-yl)phenyl)-1H-1,2,4-triazole (15a) and 5-nitroisophthalic acid (15b).
In 2022, Qin and coworkers used 4-amino-3,5-bis(4-pyridyl)-1,2,4-triazole ([4-NH2-3,5-(L1)2-TZ, L1 = 4-pyridyl, 16a, Scheme 15) and 4,4′-oxybis(benzoic acid) (H2OBA, 16b) as ligands, resulting in the MOF [Zn2(OBA)2(4-NH2-3,5-(L1)2-TZ)·3DMF·4H2O]n (16) [100]. The framework, obtained from zinc nitrate hexahydrate in DMF/EtOH solution, displayed an emission quantum yield of 18% and a luminescence lifetime of 6.27 ns. Product 16 functioned as a sensitive and selective sensor for Fe3+ and Cr2O72− ions (Table 6, Entry 7). Furthermore, it could act as a host for lanthanide ion encapsulation (Eu3+, Tb3+), enabling tuning of luminescent properties. Immersion of compound 16 in DMF containing Eu3+/Tb3+ (25 mM, 2:3 molar ratio) yielded Eu3+/Tb3+@compound 16 (2:3) (16c), which exhibited white-light emission with excellent CIE coordinates (0.32, 0.33), a moderate correlated color temperature (CCT) of 6180 K, and an absolute quantum yield of 25.3%. The corresponding luminescence lifetimes were 484.5 μs and 1260.7 μs. These findings highlight the potential of compound 16 for white light emitting devices. Detection studies with Eu3+/Tb3+@compound 16 (3:2) (16d) confirmed its applicability as a probe for Fe3+ and Cr2O72− ions (Table 6, Entry 8).
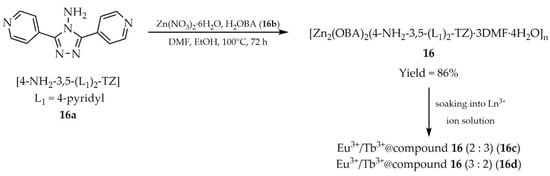
Scheme 15.
Preparation of zinc-based frameworks (16, 16c, 16d) from 4-amino-3,5-bis(4-pirydylo)-1,2,4-triazole (16a).
In a subsequent report, Xian and coworkers described the synthesis and characterization of {[Zn(L)0.5(3-L1-5-L2-TZ)]·H2O·DMF}n (17, Scheme 16), obtained from 3-(4′-imidazolobenzene)-5-(pyridine-4′-yl)-1,2,4-triazole ([3-L1-5-L2-TZ, L1 = 4-imidazolobenzene, L2 = 4-pyridyl], 17a) and 1,3-bis(3,5-dicarboxyphenoxy)benzene (H4L, 17b) as ligands [101]. The reaction was carried out using zinc nitrate hexahydrate in an aqueous DMF solution. The luminescent properties of compound 17 (Table 1) were investigated, and its potential application as a multifunctional sensor was evaluated. Luminescence quenching was observed for nitrofurazone (NZF) (Table 7, Entry 3), Fe3+, CrO42– and Cr2O72− ions (Table 6, Entry 9) in aqueous solutions. A comparable response was also obtained in the presence of p-aminophenol (PAP) (Table 7, Entry 3) in simulated urine, a known biomarker of phenamine, which highlights its significance for medical diagnostics.
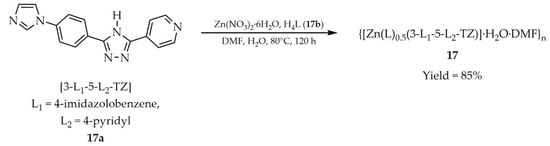
Scheme 16.
Synthesis of a Zn–MOF (17) using 3-(4′-imidazolobenzene)-5-(pyridine-4′-yl)-1,2,4-triazole (17a) and 1,3-bis(3,5-dicarboxyphenoxy)benzene (17b).
Yu and coworkers reported the synthesis of two-dimensional (2D) metal–organic framework (MOF) nanosheets via a facile top-down strategy, derived from a three-dimensional (3D) layered MOF {[Zn(3,5-(L1)2-TZ)(H2O)2]·H2O}n (18, Scheme 17), and explored their luminescence-based applications (Table 1) [102]. The reaction employed 3,5-bis(3′,5′-dicarboxyphenyl)-1H-1,2,4-triazole ([3,5-(L1)2-TZ, L1 = 3,5-dicarboxyphenyl], 18a) as ligand and zinc salt (Zn(SO4)2·7H2O) as the metal source, performed in an aqueous ethanol solution. The study focused on the luminescent sensing of selected anti-inflammatory drugs and pesticides. Both the bulk MOF (18) and the exfoliated nanosheets (18b) were examined, with the latter exhibiting enhanced quenching efficiency due to their larger surface area. A total of eight commercial pharmaceuticals were tested, including anti-inflammatory agents: salicylic acid (SA), naproxen (NP), ibuprofen (BF), and diclofenac sodium (DCF), as well as antibiotics: penicillin (PC), nalidixic acid (NA), neomycin sulfate (NS), and erythromycin (EC). Colloidal suspensions of nanosheets-MOF (18b) containing these drugs were probed for luminescence quenching. The strongest response was observed for DCF (93.20%, Table 7, Entry 4), while significantly lower values were recorded for EC, NS, and NA at 24.86%, 22.10%, and 19.78%, respectively. The remaining analytes (SA, NP, BF, and PC) showed negligible quenching effects. In addition, seven pesticides: tebuconazole (TZ), cyanazine (CZ), amitrole (AR), permethrin (PT), atrazine (AZ), diuron (DR), and tetramethylthiuram disulfide (TMTD) were screened. High selectivity was demonstrated towards TMTD, for which luminescence quenching reached 94.79% (Table 7, Entry 4), whereas the other pesticides induced only minor effects.
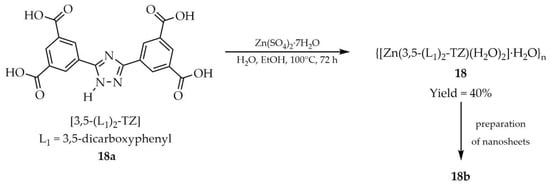
Scheme 17.
Synthesis of a zinc–framework (18) from 3,5-bis(3′,5′-dicarboxyphenyl)-1H-1,2,4-triazole (18a) and the subsequent preparation of nanosheets (18b).
In 2024, Chen and coworkers reported the synthesis of a three-dimensional dinuclear zinc-based metal–organic framework {[Zn2·(3-NH2-TZ)2·(pta)]·3H2O}n (19, Scheme 18), constructed from 3-amino-1,2,4-triazole (3-NH2-TZ], 19a) and terephthalic acid (H2pta, 19b), under solvothermal conditions using Zn(NO3)2 [103]. A crystalline nanomaterial (19c) with particle sizes of ca. 100 nm was prepared by means of a cell fragmentation apparatus and subjected to further analyses. The fluorescence lifetime of 19c was determined to be 2.7241 ns, with a quantum yield of 3.02%. In addition to its luminescent properties (Table 1), attention was directed toward the capacity of 19c to adsorb hexavalent chromium. The effects of dosage, pH, and temperature on adsorption efficiency were systematically evaluated. The maximum experimental adsorption capacity of 19c reached 125.52 mg/g. Reusability studies revealed that, after five adsorption–desorption cycles, the material retained ~98% of its adsorption efficiency.


Table 1.
Luminescence properties of Zn-MOFs based on 1,2,4-triazole.
Table 1.
Luminescence properties of Zn-MOFs based on 1,2,4-triazole.
| Zn-MOFs | ||||
|---|---|---|---|---|
| MOF | λabs [nm] | λex [nm] | λem [nm] | Ref. |
| 5 a | - | 327 | 392 | [90] |
| 5a a | - | 350 | 421 | |
| 5b a | - | 345 | 394 | |
| 6 a | ~290 | 270 | 295 | [91] |
| 6a a | - | 270 | 274 | |
| 7 a | - | 310 | 375; 474 | [92] |
| 7a a | - | 290 | 354 | |
| 7b a | - | 346 | 563 | |
| 8 b | 420 | 326 | 420 | [93] |
| 8a a | - | 380 | ~482 | |
| 9 c | 310 | 310 | 410 | [94] |
| 10 a | 280–340 | 335 | 396 | [95] |
| 10a a | ~315 | - | - | |
| 11 a | 280–340 | 330 | 384 | |
| 4 a | - | 382 | 433, 487–600 | [52] |
| 12 a | 337, 450 | 280 | 392 | [96] |
| 12b a | 331, 450 | 280 | 389 | |
| 13 a | - | 322 a, 302 d | 408 a, 333 d | [97] |
| 13a a | - | 299 a, 291 d | 357 a, 333 d | |
| 14 e | - | 340 | 459 | [98] |
| 14a e | - | 340 | 366, 423 | |
| 14b e | - | 340 | 524 | |
| 15 a | - | 328 | 418 | [99] |
| 15a a | - | 330 | 410 | |
| 16 a | - | 359 a, 290 f | 415 a, 370 f | [100] |
| 16a a | - | 290 | 354 | |
| 16b a | - | 346 | 563 | |
| 17 a | - | 335 | 395 | [101] |
| 17a a | - | 278 | 429 | |
| 17b a | - | 320 | 398 | |
| 18 g | - | 285 | 370 | [102] |
| 18a g | - | 285 | 348 | |
| 18b h | - | 285 | 359 | |
| 19a a | - | 340 | 400 | [103] |
| 19b a | - | 350 | 430 | |
| 19c a | - | 340 | 451 | |
a solid state; b ground sample immersed in 1 mL DMF: H2O (v/v = 1:1); c suspension in water; d dispersed in DMF; e powder; f suspension in DMF; g indefinite; h suspension in MeOH/isopropanol (v/v = 4:1).
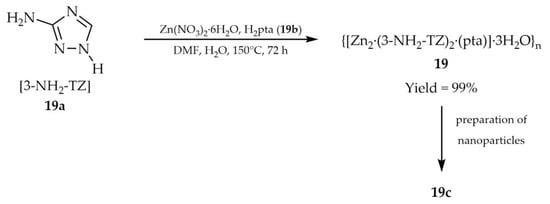
Scheme 18.
Synthesis of a zinc-based MOF (19) from 3-amino-1,2,4-triazole (19a) and terephthalic acid (19b) followed by the preparation of nanoparticles (19c).
3.1.2. Cadmium-Based Metal–Organic Frameworks Containing 1,2,4-Triazole Linker
Another frequently employed ion in the synthesis of 1,2,4-triazole-based MOFs is cadmium cation Cd2+. In 2019, three research articles addressing this topic were published. Cui et al. reported the solvothermal synthesis of [Cd4(H23,5-(L1)2-TZ)2(3,5-(L1)-TZ)·H2O]·3H2O (20, Scheme 19) using 5,5′-(1H-1,2,4-triazole-3,5-diyl)diisophthalic acid ([3,5-(L1)-TZ, L1 = 3,5-dicarboxyphenyl], 20a), cadmium nitrate tetrahydrate in a mixed solvent system of acetylacetone (ACAC), ethanol, and DMF [104]. The obtained MOF 20 was post-synthetically functionalized with Tb3+ cations, resulting in a new material (20b) exhibiting enhanced luminescent properties (Table 2), characterized by green emission under UV irradiation. Moreover, it proved to be a highly selective and sensitive fluorescent probe for detecting arginine in urine at low concentrations of 0.69 ppm in water (Table 7, Entry 5). Arginine is a biomarker of cystinuria, an autosomal recessive genetic disease that leads to impaired absorption of dibasic amino acids such as cystine, lysine, ornithine, and arginine. Importantly, other components contained in urine had a negligible effect on the degree of luminescence quenching. A paper fluorescence assay was developed by applying compound 20b to filter paper, demonstrating the application potential of the presented research.
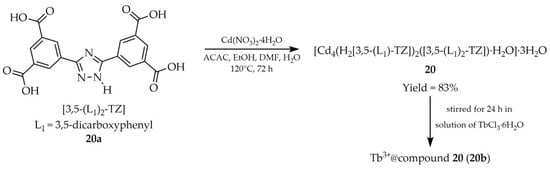
Scheme 19.
Synthesis of a cadmium-based MOF (20) using 5,5′-(1H-1,2,4-triazole-3,5-diyl)diisophthalic acid (20a) and its subsequent modification into a terbium-containing compound.
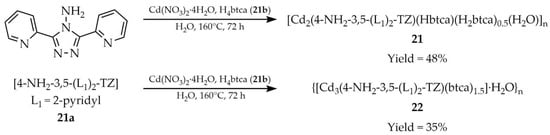
Another article from 2019 by Zhang et al. described the synthesis and characterization of two cadmium-based luminescent MOFs: [Cd2(4-NH2-3,5-(L1)2-TZ)(Hbtca)(H2btca)0.5(H2O)]n (21, Scheme 20) and {[Cd3(4-NH2-3,5-(L1)2-TZ)(btca)1.5]·H2O}n (22, Scheme 20) [105]. The frameworks were constructed using two ligands: 3,5-di(2-pyridyl)-4-amino-1,2,4-triazole ([4-NH2-3,5-(L1)2-TZ, L1 = 2-pyridyl], 21a) and 1,2,4,5-benzenetetracarboxylic acid (H4btca, 21b), which were used in various molar ratios, and an aqueous solution of Cd(NO3)2·4H2O. Compound 21 exhibited luminescence quenching in the presence of 2,4,6-trinitrophenol (TNP, Table 7, Entry 6 and 7), and a simple paper-based test was devised for detection by immersion in the solution. This is an example of the practical application of the presented research. Quenching was also observed in the presence of Cu2+ cation (Table 6, Entry 10).
Scheme 20.
Preparation of cadmium-based MOFs (21, 22) from 3,5-di(2-pyridyl)-4-amino-1,2,4-triazole (21a) and 1,2,4,5-benzenetetracarboxylic acid (21b).
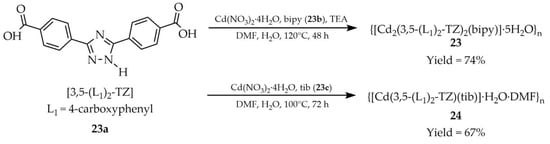
Zhang and coworkers reported the synthesis of two cadmium-based MOFs named {[Cd2(3,5-(L1)2-TZ)2(bipy)]·5H2O}n (23, Scheme 21) and {[Cd(3,5-(L1)2-TZ)(tib)]·H2O·DMF}n (24) using three ligands: 3,5-bis(4′-carboxyphenyl)-1,2,4-triazole ([3,5-(L1)2-TZ, L1 = 4-carboxyphenyl], (23a), 4,4′-bipyridine (bipy, 23b), and 1,3,5-tris(1-imidazolyl)benzene (tib, 23c) [106]. Both products 23 and 24 displayed luminescent properties (Table 2). Additionally, compound 24 was tested for solvent detection and showed pronounced luminescence quenching in aqueous acetonitrile solutions (Table 7, Entry 8).
Scheme 21.
Synthesis of cadmium-based frameworks (23, 24) from 3,5-bis(4′-carboxyphenyl)-1,2,4-triazole (23a), 4,4′-bipyridine (23b), and 1,3,5-tris(1-imidazolyl)benzene (23c).
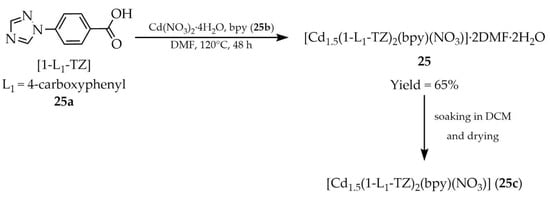
In 2020, Singh and co-workers synthesized [Cd1.5(1-L1-TZ)2(bpy)(NO3)]·2DMF·2H2O (25, Scheme 22) employing two heterocyclic ligands: 1-(4-carboxyphenyl)-1,2,4-triazole ([1-L1-TZ, L1 = 4-carboxyphenyl], 25a) and 4,4′-bipyridine (bpy, 25b) [107]. The activated MOF with the formula [Cd1.5(1-L1-TZ)2(bpy)(NO3)] (25c) exhibited high CO2 adsorption selectivity over N2 and CH4, rendering it a promising candidate for gas separation applications. Moreover, due to the luminescence quenching phenomenon (Table 6, Entry 11) compound 25c enables the detection of Fe3+ cations and anions containing chromium(VI) (CrO42– and Cr2O72–).
Scheme 22.
Preparation of cadmium-based frameworks (25, 26) employing 1-(4-carboxyphenyl)-1,2,4-triazole (25a) and 4,4′-bipyridine (25b).
In the previously mentioned work by Li et al., the cadmium-based MOF [Cd(H21-L1-TZ)(SIP)(H2O)]·4H2O (26, Scheme 23) was synthesized from 1-(4-(1H-imidazole-5-yl)phenyl)-1H-1,2,4-triazole ([1-L1-TZ, L1 = (4-(1H-imidazole-5-yl)phenyl), 10a), sodium 5-sulfoisophthalate (NaH2SIP), and cadmium salt hydrate in aqueous medium [95]. The luminescent properties of 26 were evaluated (Table 2) and its quantum efficiency was determined to be 2.62%, and the luminescence lifetime was 14.62 ns.
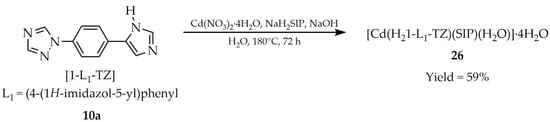
Scheme 23.
Synthesis of a cadmium-based MOF (26) from 1-(4-(1H-imidazole-5-yl)phenyl)-1H-1,2,4-triazole (10a).
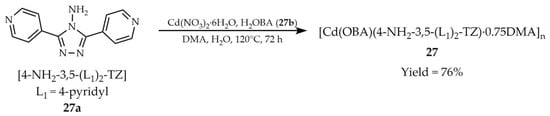
Qin and coworkers reported the solvothermal synthesis of a luminescent material with the formula [Cd(OBA)(4-NH2-3,5-(L1)2-TZ).0.75DMA]n (27, Scheme 24), obtained from 4-amino-3,5-bis(4-pyridyl)-1,2,4-triazole ([4-NH2-3,5-(L1)2-TZ, L1 = 4-pyridyl], 27a) and 4,4′-oxybis(benzoic acid) (H2OBA, 27b) [108]. Upon activation, compound 27 exhibited sorption properties towards H2 and CO2. Moreover, it was found to act as a sensitive and selective sensor for Cr3+, CrO42−and Cr2O72− ions (Table 6, Entry 12). A luminescent response was also obtained for a paper test prepared by the researchers, which was obtained by applying substance 27 to the blotting paper, highlighting the potential practical application of these studies.
Scheme 24.
Formation of a cadmium-based framework (27) from 4-amino-3,5-bis(4-pyridyl)-1,2,4-triazole (27a) and 4,4′-oxybis(benzoic acid) (27b).
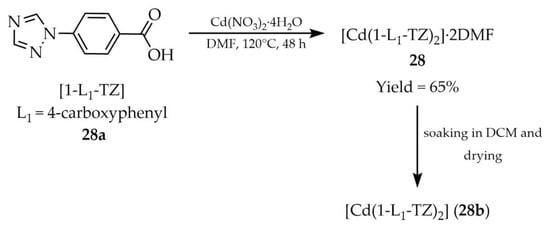
In 2021, Singh and coworkers described the synthesis of a MOF with the formula [Cd(1-L1-TZ)2]·2DMF (28, Scheme 25), prepared from 4-(1,2,4-triazol-1-yl)benzoic acid ([1-L1-TZ, L1 = 4-carboxyphenyl], 28a), cadmium salt such as Cd(NO3)2·4H2O and DMF as a solvent [109]. The material was activated by removal of DMF molecules from the framework (28b), and its sorption capacity revealed CO2 uptake at 298K of 29.23 cm3g−1 (1.30 mmolg−1). Furthermore, high selectivity for CO2 over N2 and CH4 was demonstrated. Compound 28b was also shown to act as a luminescent sensor exhibiting a “turn-on” luminescence response for the detection of toxic compounds such as 3-(diethylamino)phenol (DEP) and the pesticide isoproturon (IPT) (Table 7, Entry 9). In addition, a “turn-off” luminescence response was observed for the detection of the pesticide dichloran (2,6-dichloro-4-nitroaniline) (Table 7, Entry 9) with high selectivity and a very low detection limit (124 nM), accompanied by distinct colorimetric changes under UV light.
Scheme 25.
Synthesis of a cadmium-based framework (28) using 4-(1,2,4-triazol-1-yl)benzoic acid (28a) and its subsequent modification into 28b.
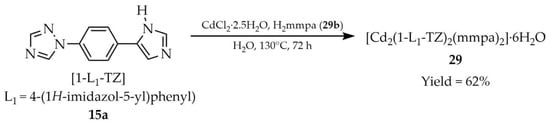
Chen and coworkers reported the synthesis of a MOF with the formula [Cd2(1-L1-TZ)2(mmpa)2]·6H2O (29, Scheme 26), obtained from 1-(4-(1H-imidazol-5-yl)phenyl)-1H-1,2,4-triazole ([1-L1-TZ, L1 = (4-1H-imidazol-5-yl)phenyl)], 15a) and 5-methylisophthalic acid (H2mmpa, 29b) [99]. The luminescent properties of compound 29 were studied (Table 2), revealing a luminescence lifetime of 16 ns and a quantum yield of ~5% (5.32%). Through luminescence quenching, compound 29 was able to detect 4-nitrophenol (NP) (Table 7, Entry 10) and Ag+ cations (Table 6, Entry 13). Sorption tests revealed that the microporous material 29 exhibited selective adsorption of CO2 over N2, as well as water vapor uptake.
Scheme 26.
Preparation of a Cd-based MOF (29) from 1-(4-(1H-imidazol-5-yl)phenyl)-1H-1,2,4-triazole (15a) and 5-methylisophthalic acid (29b).
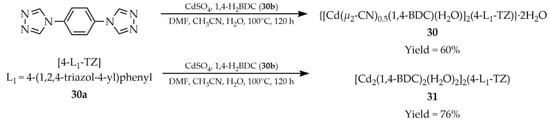
In 2022, Hong et al. published a study describing the solvothermal synthesis of two luminescent MOFs: {[Cd(μ2-CN)0.5(1,4-BDC)(H2O)]2(4-L1-TZ)}∙2H2O (30, Scheme 27) and [Cd2(1,4-BDC)2(H2O)2]2(4-L1-TZ) (31) [110]. For their preparation, two ligands were used: 1,4-phenylene-4,4′-bis(1,2,4-triazole) ([4-L1-TZ, L1 = 4-(1,2,4-triazol-4-yl)phenyl], 30a), 1,4-dicarboxybenzene (1,4-H2BDC, 30b) and cadmium sulfate. The same reagents were employed for both syntheses, with the difference being that a solvent mixture of DMF/CH3CN/H2O (v/v/v; 1:3:2) was used for compound 30, while for compound 31 the ratio was 1:4:1, respectively. Luminescence studies were also performed (Table 2). Interestingly, for compound 30 the emission intensity decreased with increasing temperature, whereas for compound 31 the emission intensity initially increased with temperature between 80 and 130 K, but decreased at temperatures above 130 K. Good linearity and reversibility were demonstrated for these responses, indicating the potential of the obtained materials as thermometric sensors.


Table 2.
Luminescence properties of Cd-MOFs based on 1,2,4-triazole.
Table 2.
Luminescence properties of Cd-MOFs based on 1,2,4-triazole.
| Cd-MOFs | ||||
|---|---|---|---|---|
| MOF | λabs [nm] | λex [nm] | λem [nm] | Ref. |
| 20 a | - | 323 | 458 | [104] |
| 20a a | - | 323 | 470 | |
| 20b a | - | 285 | 490, 546, 585, 620 | |
| 21 a | - | 357 | 427 | [105] |
| 21a a | - | 357 | 404 | |
| 22 a | - | 357 | 420 | |
| 23 a | - | 425 | 369 | [106] |
| 23a a | - | 273 | 376 | |
| 23b a | - | 344 | 415 | |
| 23c a | - | 360 | 407 | |
| 24 a | - | 450 | 368 | |
| 25c b | - | 265 | 406 | [107] |
| 26 a | 280–340 | 336 | 428 | [95] |
| 10a a | 315 | - | - | |
| 27 a | - | 368 | 420 | [108] |
| 27a a | - | 290 | 354 | |
| 27b a | - | 346 | 563 | |
| 28b c | - | 272 | 329 | [109] |
| 29 a | - | 323 | 375 | [99] |
| 15a a | - | 330 | 410 | |
| 29b a | - | 280 | ~380 | |
| 30 a | - | 330 | 428 | [110] |
| 30a a | - | 330 | 440 | |
| 31 a | - | 330 | 434 | |
a solid state; b suspension in water; c suspension in DMF.
Scheme 27.
Synthesis of cadmium-based frameworks (30, 31) starting from 1,4-phenylene-4,4′-bis(1,2,4-triazole) (30a) and 1,4-dicarboxybenzene (30b).
3.1.3. Lanthanide-Based Metal–Organic Frameworks Containing 1,2,4-Triazole Linker
From the lanthanide family, MOFs in which 1,2,4-triazole was coordinated with europium, gadolinium, terbium, lanthanum, holmium, and dysprosium have been primarily investigated. In 2019, Yu and coworkers reported the synthesis of four lanthanide derivatives: [Eu(3-L1-5-L2-TZ)(H2O)]n (32, Scheme 28), [Gd(3-L1-5-L2-TZ)(H2O)]n (33), [Tb(3-L1-5-L2-TZ)(H2O)]n (34) and [Eu0.41Gd0.42Tb0.17(3-L1-5-L2-TZ)(H2O)]n (35), obtained using the ligand (3-(3,5-dicarboxylphenyl)-5-(4-carboxylphenyl)-1H-1,2,4-triazole ([3-L1-5-L2-TZ, L1 = 3,5-dicarboxyphenyl, L2 = 4-carboxyphenyl], 32a) [111]. The reactions were carried out with hydrated salts EuCl3·6H2O, GdCl3·6H2O, and TbCl3·6H2O in a mixture of DMF, water, and HNO3. One of the products, 35, containing europium, gadolinium, and terbium, exhibited white-light emission with CIE coordinates (0.330, 0.338) upon excitation at 320 nm, both in the solid state and in a well-dispersed 0.01 M aqueous suspension. Its sensing properties toward twenty selected antibiotics from six classes were studied, including β-lactams: penicillin (PCL), amoxicillin (ACL), cefixime (CFX), cefradine (CFD); aminoglycosides: gentamycin (GTM), kanamycin (KNM); macrolides: roxithromycin (ROX), azithromycin (AZM); quinolones: ciprofloxacin (CPFX), norfloxacin (NFX); nitrofurans: nitrofurazone (NZF), nitrofurantoin (NFT); nitroimidazoles: metronidazole (MNZ), dimetridazole (DTZ), ornidazole (ODZ); and others: tetracycline hydrochloride (TC), vancomycin (VCC), sulfadiazine (SDZ), lincomycin (LCC), and carbamazepine (CBZ) in aqueous solution. Eight compounds provided a selective luminescence response (Table 7, Enty 11). A color change in the luminescence was observed upon addition of tetracycline hydrochloride (TC) and nitrofurans (NZF and NFT), shifting to yellow and orange, respectively. For sulfadiazine (SDZ) and carbamazepine (CBZ), a blue shift was noted, while nitroimidazole antibiotics (MNZ, DTZ, and ODZ) induced pronounced luminescence quenching.
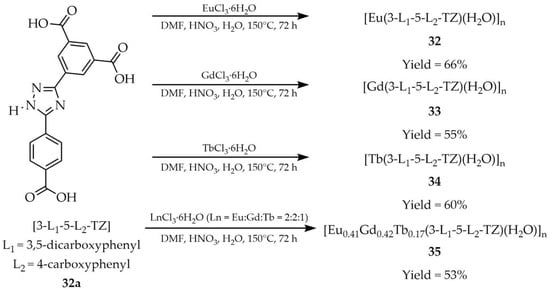
Scheme 28.
Synthesis of four MOFs (32, 33, 34, 35) from (3-(3,5-dicarboxylphenyl)-5-(4-carboxylphenyl)-1H-1,2,4-triazole (32a) and hexahydrate lanthanide chlorides.
Li and coworkers reported the synthesis of a metal–organic framework {[Eu(3,5-(L1)2-TZ)(HCOO)]·0,5H2O}n (36, Scheme 29), obtained from europium salt Eu(NO3)3·6H2O under solvothermal conditions using the ligand 3,5-bis(4′-carboxyphenyl)-1,2,4-triazole ([3,5-(L1)2-TZ, L1 = 4-carboxyphenyl], 36a) [112]. Studies demonstrated that this compound could serve as a luminescent sensor for the detection of PO43− anion (Table 6, Entry 14) and acetylacetone (Table 7, Entry 12) in aqueous solution.
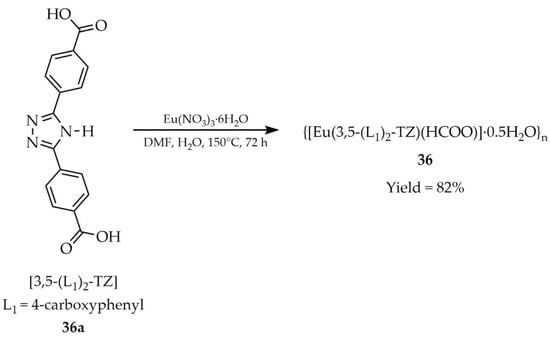
Scheme 29.
Preparation of a europium-based MOF (36) using 3,5-bis(4′-carboxyphenyl)-1,2,4-triazole (36a).
In 2021, Zhan and coworkers described the synthesis of four MOFs: {[Me2NH2][Ln3(3-L1-5-L2-TZ)3(HCOO)]·7DMF}n (Ln = Gd (37), Ln = Tb (38), Ln = Ho (39), Ln = Dy (40) Scheme 30), obtained from solvothermal reactions of the hydrated nitrates of gadolinium, terbium, holmium, and dysprosium with the tricarboxylate ligand 3-(3,5-dicarboxylphenyl)-5-(4-carboxylphenyl)-1,2,4-triazole ([3-L1-5-L2-TZ, L1 = 3,5-dicarboxyphenyl, L2 = 4-carboxyphenyl), 37a) [113]. The luminescence properties of compound 38 (Table 3) were investigated and demonstrated potential for the detection of nitroaromatic compounds through luminescence quenching. Compound 37 containing Gd(III), after desolvation, exhibited high selectivity for adsorption of C2H2 and CO2 over CH4, indicating potential for use in industrial gas separation.
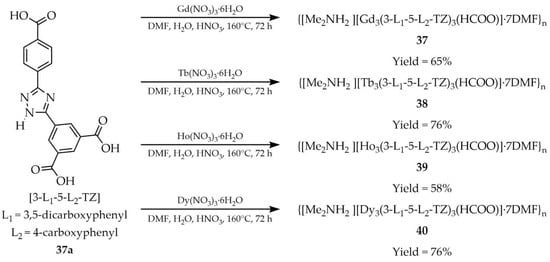
Scheme 30.
Synthesis of lanthanide-based MOFs (37, 38, 39, 40) from 3-(3,5-dicarboxylphenyl)-5-(4-carboxylphenyl)-1,2,4-triazole (37a).
The Zhang group reported one metal–organic framework based on terbium(III) with the formula Tb2(3,5-(L1)2-TZ)(μ2-H2O)(μ3-OH)2 (41, Scheme 31), synthesized from the dicarboxylate ligand 3,5-bis(4′-carboxyphenyl)-1,2,4-triazole) ([3,5-(L1)2-TZ, L1 = 4-carboxyphenyl], 41a), hydrated terbium nitrate, and a water–DMF solvent mixture [114]. The product exhibited stability in various organic solvents as well as in aqueous media over a wide pH range (0–11). Luminescence studies revealed that compound 41 selectively quenched luminescence in the presence of MnO4− ions (Table 6, Entry 15) and could also detect aromatic nitro-compounds in DMF solution, particularly 4-nitrophenol (Table 7, Entry 13). Its quantum yield was estimated at 9.5%, with a luminescence lifetime of 143 μs observed at the emission wavelength of 548 nm.
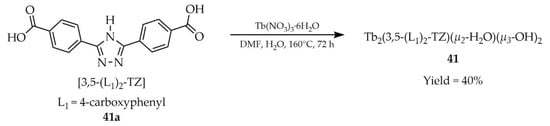
Scheme 31.
Formation of a terbium-based framework (41) from 3,5-bis(4′-carboxyphenyl)-1,2,4-triazole) (41a).
In 2022, Luo and coworkers presented the synthesis of {[Tb(3,5-(L1)2-TZ)(CH3COO)(H2O)]·0.5DMF}n (42, Scheme 32) using 3,5-bis(4′-carboxyphenyl)-1,2,4-triazole ([3,5-(L1)2-TZ, L1 = 4-carboxyphenyl, 42a) as an organic ligand with the participation of Tb(NO3)3·6H2O [115]. The reaction was conducted in a three-component solvent mixture of water, DMF, and acetic acid. Product 42 functioned as a luminescent sensor for creatinine detection in aqueous solution (Table 7, Entry 14), an important biomarker in medical diagnostics with key relevance for kidney function monitoring. Moreover, the sensor retained its activity in diluted serum samples and could be recycled and reused at least five times without significant loss of sensitivity.
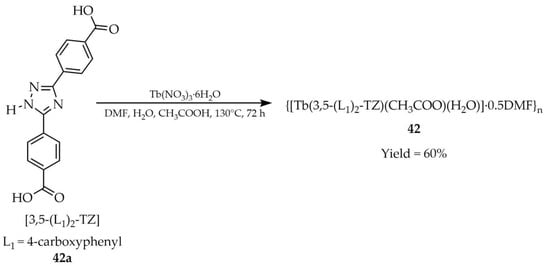
Scheme 32.
Synthesis of a terbium-based MOF (42) using 3,5-bis(4′-carboxyphenyl)-1,2,4-triazole (42a).
Wang and coworkers described the synthesis of MOFs based on the salt: hydrated europium(III) nitrate and ligands: 3,5-bis(3′-carboxyphenyl)-1,2,4-triazole ([3,5-(L1)2-TZ, L1 = 3-carboxyphenyl], 43a, Scheme 33), oxalate (ox, 43b) and 1,10-phenanthroline (phen, 43c) [116]. The obtained compound, [Eu2(3,5-(L1)2-TZ)(phen)2(ox)2(H2O)]·H2O (43), exhibited a quantum yield of 52.51%. It demonstrated a highly selective luminescence quenching response to colchicine (Table 7, Entry 15), even in the presence of interfering substances. Colchicine is used clinically in the treatment of inflammatory diseases, gout, and cancers. As a water-soluble compound, its presence in wastewater may exert long-term environmental impacts. In this study, a composite film was fabricated by embedding compound 43 in polytetrafluoroethylene, which exhibited red luminescence that was immediately quenched upon exposure to colchicine.
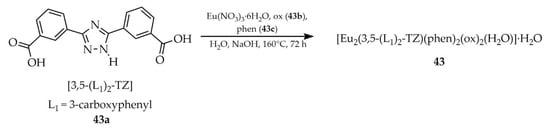
Scheme 33.
Preparation of a europium-based framework (43) from 3,5-bis(3′-carboxyphenyl)-1,2,4-triazole (43a), oxalate (43b) and 1,10-phenanthroline (43c).
In 2023, Sun and coworkers reported the synthesis of two MOFs with the formula [Ln2(1-L1-TZ)3(H2O)2]·2H2O·2DMF containing europium(III) (2, Scheme 34) or terbium(III) (3) in place of the lanthanide (Ln), and the ligand function was performed by 5-(1H-1,2,4-triazol-1-yl)isophthalic acid ([1-L1-TZ, L1 = 3,5-dicarboxylic acid], 2a). The reactions were carried out using hydrated europium and terbium nitrates in a DMF/water solvent mixture. Compound 2 exhibited red emission, while compound 3 displayed green emission. Both materials demonstrated the ability to detect nitroaromatic compounds (NACs) (Table 7, Entry 16 and 17) as well as Fe3+ cations (Table 6, Entry 16 and 17).
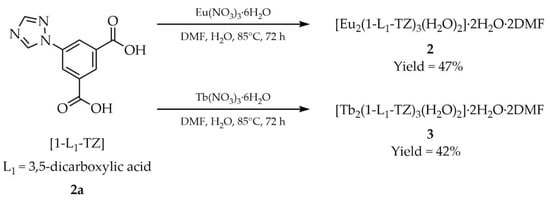
Scheme 34.
Synthesis of lanthanide-based MOFs (2, 3) from 5-(1H-1,2,4-triazol-1-yl)isophthalic acid (2a).
In a 2024 an article by Wang and coworkers, described the synthesis of a series of metal–organic frameworks based on europium(III) and terbium(III) [117]. Among them, the best bimetallic material, Eu0.096Tb0.904-MOF (44, Scheme 35), was identified. This compound was obtained from europium and terbium chlorides with a dicarboxylate ligand bearing a 1,2,4-triazole ring, namely 5-(4-(triazol-1-yl)phenyl)isophthalic acid ([1-L1-TZ, L1 = [(1,1′-biphenyl)-3,5-dicarboxylic acid], 44a), under DMF/water conditions. The resulting product 44 exhibited the highest quantum yield of 42%, with a luminescence lifetime of 544 μs. It was shown to act as a detector for hydroxyflutamide, a drug used in the treatment of castration-resistant prostate cancer (CRPC) (Table 7, Entry 18). Excessive use of this drug can lead to serious side effects, which may be mitigated by monitoring its blood concentration. The studies revealed a linear correlation between hydroxyflutamide concentration in human serum and the luminescence response of compound 44, indicating its potential as a ratiometric luminescent sensor. In a subsequent step, hydroxyflutamide was incorporated into this material (44b) to form a composite platform capable of detecting CRPC biomarkers such as the androgen receptor (AR) and prostate-specific antigen (PSA) (Table 7, Entry 19).
Scheme 35.
Preparation of bimetallic europium–terbium-based frameworks (44, 44b) employing 5-(4-(triazol-1-yl)phenyl)isophthalic acid (44a).
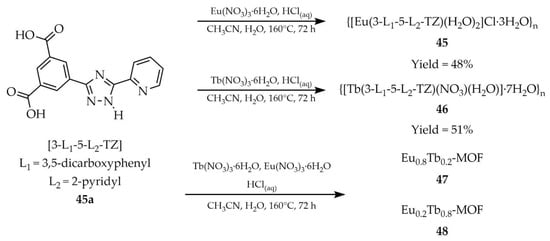
In 2025, lanthanide-based derivatives functioning as both thermometers and luminescent sensors were described. Zhang and coworkers reported two isostructural MOFs with the formulas {[Eu(3-L1-5-L2-TZ)(H2O)2]Cl·3H2O}n (45, Scheme 36) and {[Tb(3-L1-5-L2-TZ)(NO3)(H2O)]·7H2O}n (46) [118]. The syntheses employed 3-(3,5-dicarboxylophenyl)-5-(pyrid-2-yl)-1H-1,2,4-triazole ([3-L1-5-L2-TZ, L1 = 3,5-dicarboxyphenyl, L2 = 2-pyridyl], 45a as the ligand, hydrated europium and terbium nitrates, and a mixed acetonitrile/water solvent system. The quantum yields of compounds 45 and 46 were determined to be 9.8% and 10.1%, respectively, with corresponding luminescence lifetimes of 1.15 ms and 0.94 ms. Both compounds showed luminescent sensing capability toward Fe3+ cations, Cr2O72− anions (Table 6, Entry 18 and 19), and 2,4,6-trinitrophenol (TNP) (Table 7, Entry 20 and 21). In addition, a series of EuxTb1–x-MOF was obtained by tuning the molar ratio of Eu3+ and Tb3+ ions. Among them, Eu0.8Tb0.2-MOF (47) emitted nearly ideal white light with CIE coordinates (0.316, 0.332), making it a promising candidate for diode applications. Conversely, Eu0.2Tb0.8-MOF (48) exhibited temperature sensitivity, giving a linear luminescent response in the range of 303–373 K.
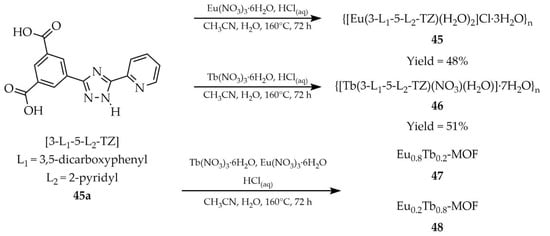
Scheme 36.
Synthesis of lanthanide-based MOFs (45, 46, 47, 48) using 3-(3,5-dicarboxylphenyl)-5-(pyrid-2-yl)-1H-1,2,4-triazole (45a).
Li et al. reported the synthesis of [Eu3(3,5-(TZ)2-3′,5′-(L1)2-1,1′-biphenyl)4(CH3COO) (H2O)7]·(CH3CN)5 (49, Scheme 37), employing (3′,5′-di(1H-1,2,4-triazole-1-yl)-[1,1′-biphenyl]-3,5-dicarboxylic acid ([3,5-(TZ)2-3′,5′-(L1)2-1,1′-biphenyl, L1 = carboxyl], 49a) as the ligand, europium nitrate hexahydrate, and an acetonitrile/water solvent mixture [119]. This compound exhibited a luminescence “turn-off” response in the presence of caffeic acid (CA) (Table 7, Entry 22). While CA exerts beneficial biological effects such as antioxidant and anti-inflammatory activity at low concentrations, at higher levels it may contribute to carcinogenesis. Therefore, developing methodologies for the determination of its concentration is significant.


Table 3.
Luminescence properties of lanthanide metal–organic frameworks based on 1,2,4-triazole.
Table 3.
Luminescence properties of lanthanide metal–organic frameworks based on 1,2,4-triazole.
| Ln-MOFs | ||||
|---|---|---|---|---|
| MOF | λabs [nm] | λex [nm] | λem [nm] | Ref. |
| 32 | 204 b | 350 a | 485, 591, 614, 651, 699 a | [111] |
| 33 | 204 b | 390 a | 479 a | |
| 34 | 204 b | 347 | 491, 545, 584, 619 a | |
| 35 | 204 b | 320 a | 439, 490, 545, 590, 613 a | |
| 32a | - | 372 a | 420–550 a | |
| 36 | - | - | 594, 617, 652, 699 c | [112] |
| 38 a | - | 314 | 543, 584, 621 | [113] |
| 37a a | - | 351 | 408, 468, 520 | |
| 41 a | 280 | 493, 548, 589, 623 | [114] | |
| 42 a | - | 250 | 491, 547, 586, 623 | [115] |
| 42 c | - | 250 | 380, 491, 547, 586, 623 | |
| 42a c | - | 250 | ~380 | |
| 43 a | - | 622 | 336, 395, 466, 536 | [116] |
| 43 a | - | 336 | 583, 597, 622, 655, 698 | |
| 2 a | - | 350 | 593, 613, 651, 701 | [51] |
| 3 a | - | 350 | 489, 545, 588, 621 | |
| 44 c | - | 250 | ~350, 492, 543, 583, 617 | [117] |
| 45 a | - | 300 | 580, 593, 617, 651, 700 | [118] |
| 46 a | - | 337 | 490, 545, 584, 622 | |
| 45a a | - | 280 | 460 | |
| 49 a | - | 327 | 589, 614, 648, 696 | [119] |
| 49a a | - | 321 | 350 | |
a solid state; b in methanol; c suspension in water.
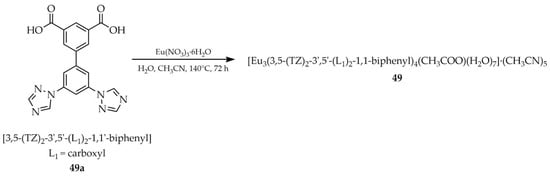
Scheme 37.
Synthesis of a europium-based framework (49) from (3′,5′-di(1H-1,2,4-triazole-1-yl)-[1,1′-biphenyl]-3,5-dicarboxylic acid (49a).
3.1.4. Cluster-Based Metal–Organic Frameworks Containing 1,2,4-Triazole Linker
Metal clusters represent a distinct field of inorganic and organometallic chemistry, significantly expanding the diversity of metal–organic frameworks (MOFs) due to their unique architectures. They are defined as molecular compounds containing two or more metal atoms connected by metal–metal bonds or by bridging ligands without direct metal–metal bonding [120].
In 2019, an article of Zhang et al. reported a metal–organic framework based on the heterothiometallic cluster [W2(μ3-S)6(μ4-S)2Cu8]4+ and 4-amino-3,5-bis(3-pyridyl)-1,2,4-triazole ([(4-NH2)(3,5-(L1)2-TZ, L1 = 3-pyridyl], 50a, Scheme 38) with the formula {[W2(μ3-S)6(μ4-S)2Cu8(CN)4((4-NH2)(3,5-(L1)2-TZ)]·CH3CN·H2O}n (50) [121]. For the synthesis, in addition to the indicated ligand, (NH4)2WS4, CuCN and NH4SCN were used along with DMF and acetonitrile as solvents. Compound 50 exhibited luminescence quenching in the presence of nitroaromatic compounds (NACs): nitrobenzene (NB), 2,4-dinitrotoluene (2,4-DNT), trinitrotoluene (TNT), and trinitrophenol (TNP) in acetonitrile (Table 7, Entry 23). The best detection results were obtained for TNP, which is a contaminant found in aquatic environments; therefore, tests with compound 50 as a suspension in water were also conducted, giving low detection limits (0.66 μM).

Scheme 38.
Preparation of a bimetallic tungsten–copper framework (50) from 4-amino-3,5-bis(3-pyridyl)-1,2,4-triazole) (50a).
In 2021, Wang and coworkers described the synthesis of a three-dimensional metal–organic framework [Cu(4-TZ-L1-4-TZ)Cl0.33](ClO4)0.67}n (51, Scheme 39) constructed from copper clusters and 4,4′-di(4H-1,2,4-triazol-4-yl)methyldiphenyl ([4-TZ-L1-4-TZ, L1 = 4,4′-methyldiphenyl], 51a) [122]. For the synthesis, Cu(ClO4)2·6H2O was used as the metal source and a water/methanol solvent mixture. Luminescence studies of the triazole ligand and compound 51 were performed in DMF and in the solid state (Table 4). The obtained MOF 51 showed a quantum yield of 4.81% and a luminescence lifetime of 1.46 ns. Furthermore, its potential for detection of Dy3+ cations, through luminescence enhancement, and CN− anions, through luminescence quenching, was demonstrated; however, no data on detection limits or enhancement/quenching constants were provided.
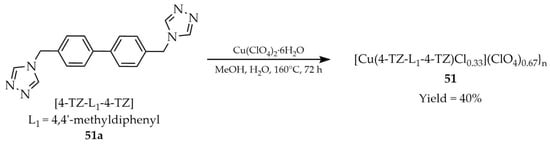
Scheme 39.
Synthesis of a copper-based MOF (51) using 4,4′-di(4H-1,2,4-triazol-4-yl)methyldiphenyl (51a).
In the same year, An and coworkers reported the metal–organic framework of the following formula {[Cd(1-L1-TZ)2(H2O)2]·(ClO4)2·H2O}n (52, Scheme 40), obtained by a sonochemical method from cadmium(II) clusters and 1-(4-aminobenzyl)-1H-1,2,4-triazole ([1-L1-TZ, L1 = 4-aminobenzyl], 52a) [123]. The synthesis was performed using Cd(ClO4)2·6H2O in aqueous solution. Optimal results were achieved with a 1:2 ratio of salt to ligand, an ultrasonic power of 100 W, and a reaction time of 30 min. Compound 52 exhibited good chemical stability in various solvents and aqueous solutions across a wide pH range (2–12). Luminescence studies (Table 7, Entry 24) revealed a luminescence “turn-on” effect in the presence of 5-hydroxytryptamine (5-HT), also known as serotonin. Serotonin is an important neurotransmitter in the human body responsible for regulating many physiological processes such as body temperature, sleep, and appetite. Further luminescence studies of compound 52 (Table 7, Entry 24) showed luminescence quenching in the presence of 2,6-pyridinedicarboxylic acid (DPA), enabling its use as a sensor for this analyte. DPA is a known chelating agent capable of forming coordination compounds with metals.
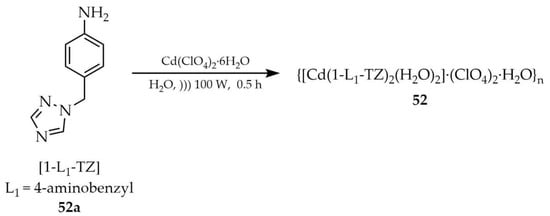
Scheme 40.
Formation of 52 from 1-(4-aminobenzyl)-1H-1,2,4-triazole (52a) and cadmium(II) clusters.
Wang and coworkers described the synthesis and characterization of a three-dimensional europium-based MOF. The material {[Eu4(5-(1-TZ)-L1)4(ox)(OH)2(H2O)4]·3H2O}n (53, Scheme 41) was obtained under solvothermal conditions using 5-(1,2,4-triazol-1-yl)isophthalic acid ([5-(1-TZ)-L1, L1 = 3,5-dicarboxyphenyl], 53a), oxalic acid (H2ox, 53b), europium salt Eu(NO3)3·6H2O, and a methanol/water solvent mixture [124]. Compound 53, containing basic triazole Lewis centers, exhibited luminescence (Table 4), which was quenched in the presence of organic amines (Table 7, Entry 25), such as diethylamine (DEA), trimethylamine (TMA), triethylamine (TEA), ethylenediamine (EDA), and aniline. The luminescence quenching mechanism was attributed to differences in the lowest unoccupied molecular orbital (LUMO) energies between the triazole ligand and the tested amines, which hinder energy transfer to the emissive Eu3+ state. These findings suggest the potential use of this MOF as a sensor for organic amines, relevant for environmental protection and human health.
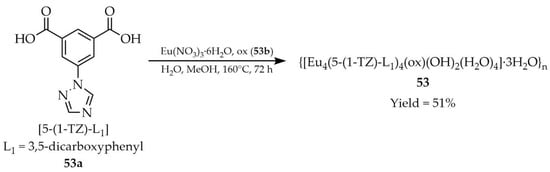
Scheme 41.
Synthesis of a europium-based MOF (53) employing 5-(1,2,4-triazol-1-yl) isophthalic acid (53a) and oxalic acid (53b).
In the same year, Wang and coworkers presented the structure and properties of a nanoporous zinc-containing MOF {[Zn(μ6-(1-L1-TZ)]·(DMAC)2}n (54, Scheme 42) (DMAC = dimethylacetamide), synthesized from 4,4′-((1H-1,2,4-triazol-1-yl)methylene)dibenzoic acid ([1-L1-TZ, L1 = 4,4′-methylenedibenzoic acid, 54a) [125]. The transformation was carried out using ZnCl2 and methanol and dimethylacetamide as solvents. The developed material 54 could be applied as a luminescent probe for dichromate anions (Cr2O72−) (Table 6, Entry 20), showing a correlated luminescence quenching response. Furthermore, compound 54 was sensitive to dipicolinic acid (DPA) (Table 7, Entry 26), characteristic of anthrax spores, which may occur in raw milk, making it a useful luminescent probe for their detection. However, the authors did not provide detailed luminescence data for compound 54.
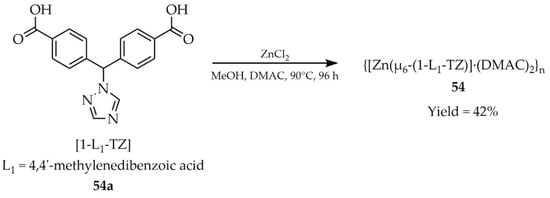
Scheme 42.
Preparation of a nanoporous zinc-based MOF (54) from 4,4′-((1H-1,2,4-triazol-1-yl)methylene)dibenzoic acid (54a).
In 2022, Liu and coworkers investigated a luminescent MOF based on a {Zn5} cluster with the formula [Zn5(μ3-OH)2(TDA)4(3,5-(L1)2-TZ)2]n (55, Scheme 43), constructed from 1H-3,5-bis(4-pyridyl)-1,2,4-triazole ([3,5-(L1)2-TZ, L1 = 4-pyridyl], 55a) and thiophene-2,5-dicarboxylic acid (H2TDA, 55b) [126]. The synthesis used Zn(NO3)3·6H2O as the metal source and was carried out in DMF. Compound 55 could be employed as a multifunctional luminescent probe sensitive to Ag+ cations (Table 6, Entry 21), Cr2O72− anions (Table 6, Entry 21), and trinitrophenol (TNP) (Table 7, Entry 27). The detection mechanism was based on luminescence quenching of compound 55 suspensions in DMF in the presence of the analytes. An exception was the cation-detection studies, which were carried out in aqueous suspensions of compound 55 activated by guest-exchange treatment, replacing DMF with dichloromethane (DCM).


Table 4.
Luminescence properties of cluster-based MOFs containing 1,2,4-triazole.
Table 4.
Luminescence properties of cluster-based MOFs containing 1,2,4-triazole.
| Cluster-Based Metal–Organic Frameworks | ||||
|---|---|---|---|---|
| MOF | λabs [nm] | λex [nm] | λem [nm] | Ref. |
| 50 | - | 284 a, 334 b | 339 a, 382 b | [121] |
| 50a | - | 284 a, 334 b | 355 a, 390 b | |
| 51 | - | 320 c, 320 ± 5 e | 422 c, 450 e | [122] |
| 51a | - | 320 d, 320 ± 5 e | 388 d, 402 e | |
| 52 e | - | 284 | 347 | [123] |
| 53 e | - | 357 | 595, 620, 650, 700 | [124] |
| 53a e | - | 370 | 504 | |
| 55 e | - | 366 | 428 | [126] |
| 55a e | - | 442 | 493 | |
| 55b e | - | 349 | 402 | |
a suspension in acetonitrile; b suspension in water; c suspension in DMF; d solution in DMF; e solid state.
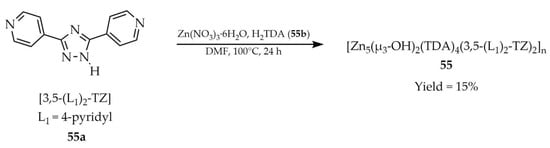
Scheme 43.
Formation of a zinc-based framework (55) using 1H-3,5-bis(4-pyridyl)-1,2,4-triazole (55a) and thiophene-2,5-dicarboxylic acid (55b).
3.1.5. Lead-Based Metal–Organic Frameworks Containing 1,2,4-Triazole Linker
In 2019, Sun and coworkers described metal–organic frameworks based on lead(II), [Pb1.5(3-L1-5-L2-TZ)]2·(DMA)3(H2O)4 (56, Scheme 44), synthesized using the polycarboxylate ligand 3-(3,5-dicarboxylphenyl)-5-(4-carboxylphenyl)-1H-1,2,4-triazole ([3-L1-5-L2-TZ, L1 = 3,5-dicarboxylphenyl, L2 = 4-carboxylphenyl], 56a). The reaction was carried out with Pb(NO3)2, N,N-dimethylacetamide (DMA) and water with the addition of HNO3 as the reaction medium [127]. The developed material 56 exhibited two luminescence emission maxima at 380 nm (λex = 280 nm) and 540 nm (λex = 380 nm) (Table 5). Tests performed in a series of solvents (H2O, EtOH, MeOH, DMF, DMA) revealed luminescence quenching by dimethylaniline (DMA), observed for the emission peak at 380 nm (λex = 280 nm), while the opposite effect, i.e., luminescence enhancement, was observed at 540 nm (λex = 380 nm). Metal cation detection experiments were also carried out, and among the twenty-three tested cations four ions: Fe3+, In3+, Zr4+ and Al3+ induced a pronounced decrease in luminescence intensity (Table 6, Entry 22) in aqueous suspensions at an excitation wavelength of 280 nm. Compound 56 was also investigated for detection of selected drugs, aromatic, and nitroaromatic compounds (Table 7, Entry 28). As a result, five substances were identified with the best detection performance: metronidazole (MNZ), dimetridazole (DTZ), 2,4,6-trinitrophenol (TNP), 4-nitroaniline (4-NA), and nitrobenzene (NB).
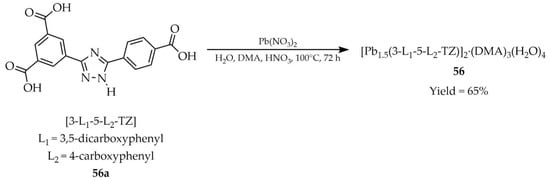
Scheme 44.
Synthesis of a lead-based MOF (56) from 3-(3,5-dicarboxylphenyl)-5-(4-carboxylphenyl)-1-H-1,2,4-triazole (56a).
3.1.6. Cobalt-Based Metal–Organic Frameworks Containing 1,2,4-Triazole Linker
In 2021, Chand and coworkers described an anionic MOF {[Me2NH2]0.5[Co(3,5-NH2-TZ)0.5(NH2BDC)] ⋅ xG}n (G = guest molecule) (57, Scheme 45), synthesized from 3,5-diamino-1,2,4-triazole ([3,5-NH2-TZ], 57a) and 2-amino-1,4-benzenedicarboxylic acid (H2NH2-BDC, 57b) [128]. The reaction was carried out with hydrated cobalt salt CoCl2·4H2O in DMF. The selected components contain free amino groups, which, together with Me2NH2+ cations, enable the binding of guest molecules within the pores. Material 57 exhibited blue luminescence (Table 5) and showed increased luminescence intensity in the presence of Al3+ cations (Table 6, Entry 23). This allowed detection at very low concentrations down to 17.5 ppb, without interference from other metal cations present in the sample, indicating sensitive and selective detection. A practical aspect of the study was also demonstrated by applying compound 57 onto filter paper, enabling its use as a simple test for Al3+ cations, with a bright luminescent signal under UV light (365 nm) visible to the naked eye in real time. An enhancement of luminescence was also observed in the presence of the Lewis acid boron trifluoride (BF3).
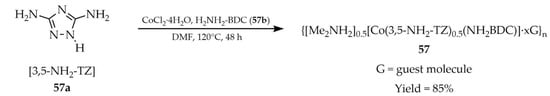
Scheme 45.
Synthesis of a cobalt-based framework (57) using 3,5-diamino-1,2,4-triazole (57a) and 2-amino-1,4-benzenedicarboxylic acid (57b).
3.1.7. Copper-Based Metal–Organic Frameworks Containing 1,2,4-Triazole Linker
In 2019, Qian and coworkers described the synthesis of MOF based on copper(II) {[Cu((1-L1-TZ)3N)(pim)]·H2O}n (58, Scheme 46) using tris(4-(1,2,4-triazol-1-yl)phenyl)amine ([(1-L1-TZ)3N, L1 = 4-phenyl], 58a) and pimelate (pim, 1,5-pentanedicarboxylate, 58b) [129]. The reaction was performed with Cu(NO3)·3H2O in the presence of NaOH using a solvent mixture of water, methanol, and DMF. The obtained material 58 exhibited luminescent properties (Table 5) but the studies particularly focused on its photocatalytic ability to degrade selected organic dyes. The degradation efficiency of methylene blue under visible light irradiation reached 90.3% after 75 min, while for rhodamine B (RhB), 90.1% degradation was achieved after 150 min. Compared to commercially available TiO2, better results were obtained. The photocatalytic mechanism was thoroughly discussed and confirmed by experiments using different scavengers such as mannitol, terephthalic acid, ammonium oxalate, and benzoquinone. These results highlight the potential application of this MOF for the removal of dye pollutants from water under visible light.




Table 5.
Luminescence properties of other metal-based MOFs containing 1,2,4-triazole.
Table 5.
Luminescence properties of other metal-based MOFs containing 1,2,4-triazole.
| Other M-Based Metal–Organic Frameworks | |||||
|---|---|---|---|---|---|
| M | MOF | λabs [nm] | λex [nm] | λem [nm] | Ref. |
| Pb | 56 a | - | 280 | 380 | [127] |
| 56 a | - | 380 | 540 | ||
| 56a a | - | 280 | ~400 | ||
| Co | 57 a | - | 340 | 430 | [128] |
| Cu | 58 a | 512 | 330 | 412 | [129] |
| 58a a | - | 310 | 411 | ||
a solid state.

Figure 8.
Examples of various organic compounds that can be detected using luminescent 1,2,4-triazole-based MOF sensors.

Table 6.
1,2,4-Triazole-based MOFs as luminescent sensors for metal cations and anions.
Table 6.
1,2,4-Triazole-based MOFs as luminescent sensors for metal cations and anions.
| Entry | MOF | Sensing of Cations | Sensing of Anions | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Cation | Ksv [L/mol] | Detection Limit [mol/L] or ppm | Anion | Ksv [L/mol] | Detection Limit [mol/L] or ppm | |||
| 1 | 5 a | Fe3+ | 1.01 × 104 | 4.61 × 10−6 | Cr2O72− | 1.24 × 104 | 2.55 × 10−6 | [90] |
| Al3+ | - | 4.43 × 10−6 | SiF62− | - | 1.35 × 10−5 | |||
| 2 | 6 a | - | - | - | Cr2O72− | 9.72 × 103 | 5.82 × 10−4 | [91] |
| 3 | 7 b | Ni2+ | 9.81 × 104 | 0.46 × 10−6 | PO43− | 6.91 × 103 | 6.5 × 10−6 | [92] |
| 4 | 8 c | Cu2+ | 5.96 × 104 | 0.76 × 10−6 | MnO4− | 2.00 × 105 | 6.14 × 10−6 | [93] |
| Ag+ | 1,67 × 104 | 6.40 × 10−6 | Cr2O72− | 1.85 × 105 | 13.64 × 10−6 | |||
| - | - | - | CrO42− | 5.89 × 104 | 12.33 × 10−6 | |||
| 5 | 13 d | Fe3+ | 4.85 × 104 | - | - | - | - | [97] |
| 6 | 14 e | Fe3+ | 4.22 × 103 | 0.92 × 10−9 | - | - | - | [98] |
| 7 | 16 f | Fe3+ | 7.69 × 104 | 0.58 × 10−6 | Cr2O72− | 3.28 × 104 | 1.37 × 10−6 | [100] |
| 8 | 16d f | Fe3+ | 4.61 × 104 | 0.98 × 10−6 | Cr2O72− | 2.16 × 104 | 2.08 × 10−6 | |
| 9 | 17 a | Fe3+ | 2.21 × 104 | 1.85 × 10−6 | Cr2O72− | 1.02 × 104 | 2.21 × 10−6 | [101] |
| - | - | - | CrO42− | 1.26 × 104 | 3.78 × 10−6 | |||
| 10 | 21 a | Cu2+ | 3.83 × 104 | 1.39 × 10−6 | - | - | - | [105] |
| 11 | 25c a | Fe3+ | 1.13 × 104 | 0.098 | Cr2O72− | 5.42 × 104 | 0.32 | [107] |
| - | - | - | CrO42− | 1.73 × 104 | 0.28 | |||
| 12 | 27 e | Cr3+ | 4.39 × 103 | 10.25× 10−6 | Cr2O72− | 1.49 × 105 | 9.18 × 10−6 | [108] |
| - | - | - | CrO42− | 4.90 × 103 | 0.3 × 10−6 | |||
| 13 | 29 a | Ag+ | 8.16 × 103 | 1.4 × 10−6 | - | - | - | [99] |
| 14 | 36 a | - | - | - | PO43− | 1.27 × 104 | 2.74 × 10−4 | [112] |
| 15 | 41 e | - | - | - | MnO4− | 1.69 × 104 | 1.6 × 10−4 | [114] |
| 16 | 2 f,g | Fe3+ | 1.03 × 104 | 8.78 × 10−3 | - | - | - | [51] |
| 17 | 3 f,h | Fe3+ | 1.49 × 104 | 8.60 × 10−3 | - | - | - | |
| 18 | 45 a | Fe3+ | 7.36 × 103 | 0.57 × 10−6 | Cr2O72− | 4.85 × 103 | 2.29 × 10−6 | [118] |
| 19 | 46 a | Fe3+ | 1.18 × 104 | 0.93 × 10−6 | Cr2O72− | 1.37 × 104 | 1.54 × 10−6 | |
| 20 | 54 a | - | - | - | Cr2O72− | 1.22 × 104 | 0.023 × 10−6 | [125] |
| 21 | 55 | Ag+ a | 1.751 × 105 | 5.96 × 10−6 | Cr2O72− f | 7.397 × 104 | 4.34 × 10−6 | [126] |
| 22 | 56 a | Fe3+ | 1.2 × 105 i | 2.5 i | - | - | - | [127] |
| 4.2 × 103 j | 71 j | - | - | - | ||||
| 56 a | In3+ | 1.6 × 105 i | - | - | - | - | ||
| 56 a | Zr4+ | 1.6 × 105 i | - | - | - | - | ||
| 56 a | Al3+ | 4.3 × 104 i | - | - | - | - | ||
| 23 | 57 f | Al3+ | - | 0.0175 | - | - | - | [128] |
a suspension in aqueous solution; b soaked in different M(NO3)x solution; c ground sample immersed in 1 mL DMF: H2O (v/v = 1:1); d immersed in DMF; e immersed in aqueous solution; f suspension in DMF; g based on the luminescence intensity at 613 nm; h based on the luminescence intensity at 545 nm; i λex = 280 nm; j λex = 380 nm.

Table 7.
1,2,4-Triazole-based MOFs as luminescent sensors for different organic compounds (drugs, antibiotics, pesticides, NACs, etc.).
Table 7.
1,2,4-Triazole-based MOFs as luminescent sensors for different organic compounds (drugs, antibiotics, pesticides, NACs, etc.).
| Entry | MOF | Detected Compound | KSV or KEC [L/mol] | Detection Limit [mol/L] or ppm | Ref. |
|---|---|---|---|---|---|
| 1 | 5 a | NZF | 5.2 × 104 | 4.04 × 10−7 | [90] |
| NFT | 1.8 × 105 | 3.53 × 10−7 | |||
| 2 | 13 b | NB | 8.58 × 103 | - | [97] |
| 3 | 17 a | PAP | 1.95 × 104 | 3.53 × 10−6 | [101] |
| NZF | 5.50 × 104 | 0.46 × 10−6 | |||
| 4 | 18b c | DCF | 4.32 × 104 | 0.62 × 10−6 | [102] |
| TMTD | 3.10 × 104 | 0.76 × 10−6 | |||
| 5 | 21b a | arginine | 2.76 × 104 | 0.69 | [104] |
| 6 | 18 a | TNP | 1.63 × 104 | 3.11 × 10−7 | [105] |
| 7 | 18 d | TNP | 2.99 × 104 | 5.95 × 10−7 | |
| 4-NP | 3.84 × 103 | 2.53 × 10−6 | |||
| 2,4-DNT | 3.76 × 103 | 3.47 × 10−6 | |||
| NB | 5.49 × 103 | 2.61 × 10−6 | |||
| 4-NT | 4.54 × 103 | 3.31 × 10−6 | |||
| 1,3-DNB | 5.21 × 103 | 2.58 × 10−6 | |||
| 8 | 24 a | acetone | 12.89 | - | [106] |
| 9 | 28b d | DEP | 3.74 × 105 | 79.8 × 10−9 | [109] |
| IPT | 1.82 × 103 | 2.26 × 10−7 | |||
| dichloran | 3.88 × 104 | 1.24 × 10−7 | |||
| 10 | 29 d | 4-NP | 4.37 × 104 | 3.29 × 10−7 | [99] |
| 11 | 35 a | TC e | 4.06 × 104 | 0.887 | [111] |
| NZF e | 1.03 × 104 | 2.770 | |||
| NFT e | 1.90 × 105 | 0.189 | |||
| SDZ e | 1.90 × 104 | 1.890 | |||
| CBZ e | 9.64 × 104 | 0.373 | |||
| MNZ f | 1.66 × 105 | 0.217 | |||
| DTZ f | 1.64 × 105 | 0.219 | |||
| ODZ f | 2.54 × 105 | 0.142 | |||
| 12 | 36 a | acetylacetone | 3.22 × 102 | 1.21 × 10−4 | [112] |
| 13 | 41 d | m-DNB | 3.08 × 102 | 0.23 × 10−3 | [114] |
| NB | 2.26 × 103 | 0.19 × 10−3 | |||
| 4-NT | 4.01 × 103 | 0.10 × 10−3 | |||
| 2,4-DNT | 4.45 × 103 | 0.22 × 10−3 | |||
| TNP | 9.10 × 103 | 0.17× 10−3 | |||
| 4-NP | 3.38 × 104 | 0.20 × 10−3 | |||
| 14 | 42 a | creatinine | 5.1 × 10−3 | 1.7 × 10−6 | [115] |
| 15 | 43 a | colchicine | 57,963 | 2.43 × 10−5 | [116] |
| 16 | 2 d,g | 2-NT | 9.01 × 103 | 4.58 × 10−5 | [51] |
| 3-NT | 28.43 × 103 | 1.53 × 10−5 | |||
| 4-NT | 27.87 × 103 | 2.01 × 10−5 | |||
| 4-NP | 10.76 × 103 | 7.68 × 10−6 | |||
| 17 | 3 d,e | 2-NT | 8.01 × 103 | 1.44 × 10−5 | |
| 3-NT | 40.87 × 103 | 5.60 × 10−6 | |||
| 4-NT | 30.59 × 103 | 8.30 × 10−6 | |||
| 4-NP | 11.81 × 103 | 2.85 × 10−6 | |||
| 18 | 44 h | hydroxyflutamide | 4.98 × 1012 | 8.37 × 10−15 | [117] |
| 19 | 44b i | PSA | 7.9 × 1010 | 4.99 × 10−13 | |
| AR | 2.9 × 108 | 0.14 × 10−9 | |||
| 20 | 45 a | TNP | 6.93 × 103 | 1.63 × 10−6 | [118] |
| 21 | 46 a | TNP | 1.21 × 104 | 1.35 × 10−6 | |
| 22 | 49 d | CA | 2.49 × 105 | 36.4 × 10−9 | [119] |
| 23 | 50 | TNP | 7.66 × 104 j 6.36 × 104 a | 0.81 × 10−6 j 0.66 × 10−6 a | [121] |
| NB | 3.94 × 104 j | 1.00 × 10−6 j | |||
| 2,4-DNT | 3.10 × 104 j | 1.11 × 10−6 j | |||
| TNT | 1.18 × 104 j | 2.15 × 10−6 j | |||
| 24 | 52 a | 5-HT | 6.00 × 104 | 0.040 × 10−3 | [123] |
| DPA | 6.30 × 104 | 0.078 × 10−3 | |||
| 25 | 53 k | EDA | 8.08 × 103 | 4.29 × 10−7 | [124] |
| DEA | 6.15 × 103 | 4.94 × 10−7 | |||
| TMA | 4.83 × 103 | 5.89 × 10−7 | |||
| TEA | 2.92 × 103 | 9.84 × 10−7 | |||
| aniline | 2.46 ×103 | 1.012 × 10−6 | |||
| 26 | 54 a | DPA | 1.48 × 105 | 0.298 × 10−6 | [125] |
| 27 | 55 d | TNP | 1.856 × 105 | 1.59 × 10−6 | [126] |
| 28 | 56 a | TNP | 3.3 × 104 k | 9 | [127] |
| 3.6 × 104 l | 8 | ||||
| 4-NA | 2.3 × 104 k | 13 | |||
| 2.4 × 104 l | 12.5 | ||||
| NB | 1.5 × 104 k | 20 | |||
| 0.7 × 104 l | 43 | ||||
| MNZ | 1.3 × 104 k | 23 | |||
| 1.5 × 104 l | 20 | ||||
| DTZ | 0.9 × 104 k | 33 | |||
| 1.5 × 104 l | 20 |
a suspension in aqueous solution; b immersed in DMF; c suspension in MeOH/isopropanol (v/v = 4:1); d suspension in DMF; e based on the luminescence intensity at 545 nm; f based on Tb3+ component; g based on the luminescence intensity at 613 nm; h DMSO solution; i human serum aqueous solution 10%; j suspension in acetonitrile; j ethanol emulsion; k λex = 280 nm; l λex = 380 nm.
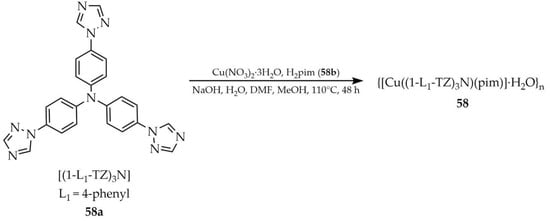
Scheme 46.
Preparation of a copper-based MOF (58) from tris(4-(1,2,4-triazol-1-yl)phenyl)amine (58a) and pimelate (58b).
4. General Remarks on Structure-Property Relationships and Current Challenges for 1,2,4-Triazole-Based LMOFs
The diversity of methodologies available for constructing the 1,2,4-triazole heterocyclic ring enables the synthesis of numerous derivatives exhibiting valuable properties for a wide range of applications. Among these are luminescent metal–organic frameworks (LMOFs), which can serve as functional materials for OLED fabrication and luminescent sensing, offering an attractive alternative to existing material solutions. Table 8 presents the influence of the metal center, ligand structure, and reaction medium on luminescence efficiency. In general, the 1,2,4-triazole core is characterized by high physical and chemical stability, as well as the ability to form coordination bonds with metals due to the presence of nitrogen atoms in the ring. The coordination potential can be further enhanced by incorporating additional heterocycles, such as pyridine or imidazole, or functional groups such as amino and carboxyl groups into the ligand structure. The most important feature of these ligands determining the luminescent properties of the resulting material is the presence of an extended conjugated system, provided mainly by aromatic rings, which enables excitation and subsequent emission of radiation. For this reason, alkyl substituents are generally not employed in the design of such structures, and the triazole ring alone is rarely used. In the case of lanthanide-based MOFs, an antenna effect is observed, in which the organic ligand sensitizes the metal center, and the resulting emission spectrum contains only the characteristic bands of the metal ion. Consequently, as shown in Table 8, these materials exhibit exceptionally high quantum yields (9.5–52.51%) and long luminescence lifetimes (143,000–1,150,000 ns). A crucial factor in the successful commercialization of these technologies is the ability to scale the process from laboratory to industrial level. The synthesis of 1,2,4-triazole-based ligands typically involves a multistep sequence of transformations, which reduces the overall yield, increases costs, and may impose an environmental burden. The study indicates that the solvothermal method is the most frequently employed approach for synthesizing MOFs based on this organic ligand, using solvents such as DMF, water, methanol, DMSO, DMA, ethanol, acetonitrile, and DMAC. The advantages of this method include moderate-to-excellent product yields (15–99%), control over structural features such as morphology and porosity, and the use of inexpensive, readily available, and environmentally benign inorganic salts. However, this is a high-pressure process, which poses a challenge for large-scale manufacturing. Furthermore, it necessitates the use of organic solvents of varying toxicity; therefore, more sustainable routes compliant with current environmental regulations include hydrothermal synthesis or the use of regenerable ionic liquids. An equally promising alternative involves electrochemical methods, which enable reactions to proceed under ambient pressure and at room temperature. The current research efforts on triazole-based LMOFs should be directed toward developing more efficient and environmentally friendly synthetic strategies for ligand preparation and evaluating the effectiveness of the proposed methodologies for producing such porous materials.


Table 8.
The influence of the metal center and ligand structure on luminescence efficiency.
Table 8.
The influence of the metal center and ligand structure on luminescence efficiency.
| Metal | Ligand | Medium | MOF | Quantum Yield [%] | Luminescence Lifetime [ns] | Ref. |
|---|---|---|---|---|---|---|
| Zn | 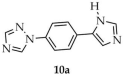 | H2O | 10 | 5.24 | 29.56 | [95] |
| 11 | 3.18 | 18.53 | ||||
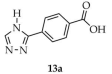 | DMA/ toluene | 13 | 7.4 | - | [97] | |
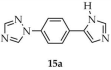 | DMF/ EtOH/ H2O | 15 | 2.15 | 8 | [99] | |
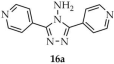 | DMF/ EtOH | 16 | 18 | 6.27 | [100] | |
| 16c | 25.3 | 484,500, 1,260,700 | ||||
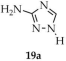 | DMF/ H2O | 19c | 3.02 | 2.7241 | [103] | |
| Cd | 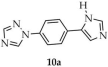 | H2O | 26 | 2.62 | 14.62 | [95] |
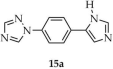 | H2O | 29 | 5.32 | 16 | [99] | |
| Tb | 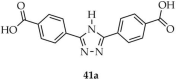 | DMF/ H2O | 41 | 9.5 | 143,000 | [114] |
| Eu | 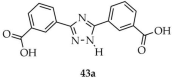 | H2O | 43 | 52.51 | 726,000 | [116] |
| Eu, Tb | 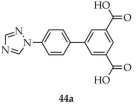 | DMF/ H2O | 44 | 42 | 544,000 | [117] |
| Eu | 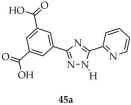 | CH3CN/H2O | 45 | 9.8 | 1,150,000 | [118] |
| Tb | 46 | 10.1 | 940,000 | |||
| Cu | 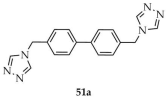 | MeOH/ H2O | 51 | 4.81 | 1.46 | [122] |
5. Conclusions
Metal–organic frameworks (MOFs) represent a broad class of materials with diverse application potential, ranging from energy storage systems and catalysis to gas separation. The specific field of application depends on their physicochemical properties, which can be tuned by the appropriate selection of metal nodes forming the structure and the organic ligands connecting them. A special class of organic compounds used as ligands in the construction of such materials consists of five-membered 1,2,4-triazoles conjugated with other aromatic or heteroaromatic rings and substituted with amino or carboxylic groups. Studies conducted between 2019 and 2025 have highlighted their usefulness in the construction of LMOFs incorporating d-block transition metals such as zinc, cadmium, cobalt, and copper; f-block lanthanides including europium, gadolinium, terbium, lanthanum, holmium, and dysprosium; as well as lead and metal clusters. In addition to their luminescent properties, many of the reported MOFs exhibit sensitive and selective detection capabilities toward metal cations and anions, pharmaceuticals, pesticides, and nitroaromatic compounds (NACs), which are important environmental pollutants. Promising results have also demonstrated the effectiveness of 1,2,4-triazole-based MOFs as luminescent probes for various biomolecules, indicating their high potential in medical diagnostics. Moreover, some of these materials display high color purity of emitted light, making them attractive candidates for integration into OLED devices. Future perspectives for 1,2,4-triazole-based LMOFs include their incorporation with optical sensors and electronic components to construct integrated devices for the detection of harmful substances in food and drinking water. The development of such technologies could enable the creation of a new generation of biosensors synchronized with smartphone applications, providing an innovative approach to health monitoring.
Author Contributions
K.K. and A.K. contributed their ideas related to concept design, data collection, manuscript preparation, language editing, and revision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Balabin, R.M. Tautomeric Equilibrium and Hydrogen Shifts in Tetrazole and Triazoles: Focal-Point Analysis and Ab Initio Limit. J. Chem. Phys. 2009, 131, 154307. [Google Scholar] [CrossRef]
- Lei, Y.; Peng, Z.; He, F.; Wang, G. Structure-Activity Relationships and Mechanism Studies of Novel 1,2,4-Triazole-Schiff Base Derivatives as Urease Inhibitors: Synthesis, Enzyme Kinetics, Spectroscopy, Thermodynamics, and Molecular Docking. Bioorg. Med. Chem. 2025, 128, 118289. [Google Scholar] [CrossRef] [PubMed]
- Rajesab, P.; Mathada, B.S.; Niranjan, V.; Sinha, L.; Setlur, A.S.; Maurya, A.; Chandrashekar, K.; Prasad, O. Unveiling a Novel Thiazolo[2,3-c][1,2,4]Triazole Scaffolds as a Dual Cyclooxygenase and Cholinesterase Inhibitors: Spectral Analysis, DFT Calculations, in Vitro and in Silico Studies. J. Mol. Struct. 2025, 1345, 143166. [Google Scholar] [CrossRef]
- Gao, F.; Wang, T.; Xiao, J.; Huang, G. Antibacterial Activity Study of 1,2,4-Triazole Derivatives. Eur. J. Med. Chem. 2019, 173, 274–281. [Google Scholar] [CrossRef] [PubMed]
- El-Sebaey, S.A. Recent Advances in 1,2,4-Triazole Scaffolds as Antiviral Agents. ChemistrySelect 2020, 5, 11654–11680. [Google Scholar] [CrossRef]
- Koparir, P. Synthesis, Antioxidant and Antitumor Activities of Some of New Cyclobutane Containing Triazoles Derivatives. Phosphorus Sulfur. Silicon Relat. Elem. 2019, 194, 1028–1034. [Google Scholar] [CrossRef]
- Khaliullin, F.A.; Klen, E.E.; Nikitina, I.L.; Pavlov, V.N.; Rozit, G.A.; Gaisina, G.G.; Samorodov, A.V. Synthesis and Antidepressant Activity of Thietane-Containing 4-(2-Oxo-2-Phenylethyl)-1H-1,2,4-Triazol-4-Ium Bromides. Pharm. Chem. J. 2023, 56, 1596–1603. [Google Scholar] [CrossRef]
- Manjunath, R.; Hakkimane, S.S.; Shashikala, B.S.; Gaonkar, S.L. Design, Synthesis and Evaluation of Benzodioxole and Bromofuran Tethered 1,2,4-Triazole Hybrids as Potential Anti Breast Cancer Agents with Computational Insights. Sci. Rep. 2025, 15, 25680. [Google Scholar] [CrossRef]
- Marzouk, M.A.; Elsayed, M.M.; Shehab, W.S.; Fawzy, S.M.; Mohammed, S.M.; Abdel-Razek, M.A.; Khedr, G.E.; Elsayed, D.A. Dual α-Amylase and α-Glucosidase Inhibition by 1,2,4-Triazole Derivatives for Diabetes Treatment. Sci. Rep. 2025, 15, 27172. [Google Scholar] [CrossRef]
- Lamb, H.M.; Adkins, J.C. Letrozole: A Review of Use in Postmenopausal Women with Advanced Breast Cancer. Drugs 1998, 56, 1125–1140. [Google Scholar] [CrossRef]
- Parker, W.B. Metabolism and Antiviral Activity of Ribavirin. Virus Res. 2005, 107, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Wellington, K.; Plosker, G.L. Rizatriptan: An Update of Its Use in the Management of Migraine. Drugs 2002, 62, 1539–1574. [Google Scholar] [CrossRef] [PubMed]
- Desta, B.; Amare, G. Paclobutrazol as a Plant Growth Regulator. Chem. Biol. Technol. Agric. 2021, 8, 1. [Google Scholar] [CrossRef]
- Saito, S.; Okamoto, M.; Shinoda, S.; Kushiro, T.; Koshiba, T.; Kamiya, Y.; Hirai, N.; Todoroki, Y.; Sakata, K.; Nambara, E.; et al. A Plant Growth Retardant, Uniconazole, is a Potent Inhibitor of ABA Catabolism in Arabidopsis. Biosci. Biotechnol. Biochem. 2006, 70, 1731–1739. [Google Scholar] [CrossRef]
- Quinn, J.A.; Fujimoto, T.T.; Egan, A.R.; Shaber, S.H. The Properties of RH 3866, a New Triazole Fungicide. Pestic. Sci. 1986, 17, 357–362. [Google Scholar] [CrossRef]
- Minaev, B.; Stakhira, P.; Panchenko, O.; Minaeva, V.; Kutsiy, S.; Melnykov, S.; Danyliv, I.; Volyniuk, D.; Grazulevicius, J.V.; Kudelko, A.; et al. Theoretical and Experimental Investigation of Exciplex-Forming and Electroluminescent Properties of 4-Ethyl-3,5-Bis[4-(N,N-Diphenylamine)Biphenyl-4-yl]-4H-1,2,4-Triazole. Opt. Mater. 2024, 147, 114606. [Google Scholar] [CrossRef]
- Xu, L.; Sun, M.; Zhou, Y.; Lou, J.; Xie, M.; Li, Z.; Sun, Q.; Pan, Y.; Xue, S.; Yang, W. A New Multifunctional Fluorescent Molecule for Highly Efficient Non-Doped Deep-Blue Electro-Fluorescence with High Color-Purity and Efficient Phosphorescent OLEDs. Org. Chem. Front. 2023, 10, 490–498. [Google Scholar] [CrossRef]
- Koodalingam, M.; Gao, M.; Jang, J.; Burn, P.L.; Kistemaker, J.C.M.; Puttock, E.V.; Shaw, P.E. Effect of Emissive Ligand Number on the Optoelectronic Properties of Dendronised Heteroleptic Green Emitting Iridium(III) Complexes. Org. Electron. 2025, 138, 107192. [Google Scholar] [CrossRef]
- Ryu, C.H.; Lim, J.; Kim, M.B.; Lee, J.H.; Hwang, H.; Lee, J.Y.; Lee, K.M. Tris(5-Phenyl-1H-1,2,4-Triazolyl)Iridium(III) Complex and Its Use in Blue Phosphorescent Organic Light-Emitting Diodes to Provide an External Quantum Efficiency of up to 27.8%. Adv. Opt. Mater. 2021, 9, 2001957. [Google Scholar] [CrossRef]
- Erer, H.; Demir, S.; Karaman, B.; Arıcı, M.; Yeşilel, O.Z. Synthesis and Characterization of Cobalt(II) and Zinc(II) Coordination Polymers Constructed from Bis(Isophthalic Acid) and Bis(1,2,4-Triazole) Linkers. J. Inorg. Organomet. Polym. Mater. 2023, 33, 2386–2398. [Google Scholar] [CrossRef]
- Hilmi, A.; Shoker, T.A.; Ghaddar, T.H. Universal Low-Temperature MWCNT-COOH-Based Counter Electrode and a New Thiolate/Disulfide Electrolyte System for Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2014, 6, 8744–8753. [Google Scholar] [CrossRef]
- Tang, H.; Shen, J.; Dai, J.; Rajalakshmi, K.; Muthusamy, S.; Kannan, P.; Zhu, D.; You, B.; Liu, X. Sensitive Determination of Caffeoylquinic Acid in Food and Blood Samples Using Nanostructured Conducting Polymer Composite Modified Electrode. Food Chem. 2025, 492, 145337. [Google Scholar] [CrossRef]
- Bakov, V.V.; Georgiev, N.I.; Donkova, N.G.; Bojinov, V.B. Ratiometric 1,8-Naphthalimide/1,2,4-Triazole Conjugates as Highly Sensitive and Self-Regenerating Solid-State “Naked Eye” Probes for Ammonia and Low Molecular Weight Volatile Amines. J. Photochem. Photobiol. A Chem. 2025, 469, 116590. [Google Scholar] [CrossRef]
- Venugopal, S.; Saha, S.; Kumbhakarna, N.; Vargeese, A.A. Insights into Triazole-Based Energetic Material Design from Decomposition Pathways of Triazole Derivatives. Phys. Chem. Chem. Phys. 2025, 27, 4269–4277. [Google Scholar] [CrossRef] [PubMed]
- Santhosh, G.; Supriya, N.; Vijayalakshmi, K.P.; Deepthi, T. Synthesis, Characterization, Pyrolysis and Thermokinetic Studies of an Energetic Ionic Liquid: 4-Amino-1-Methyl-1,2,4-Triazolium Nitrate. J. Ion. Liq. 2025, 5, 100161. [Google Scholar] [CrossRef]
- Özkan, C.; Armaki, A.M.; Rahimi, E.; Anusuyadevi, P.R.; Nie, H.Y.; Hedberg, Y.; Taheri, P.; Mol, A. Quasi-Stable Adsorption as a Stepping Stone to Stable Corrosion Inhibition. Appl. Surf. Sci. 2025, 712, 164060. [Google Scholar] [CrossRef]
- Al-Ahmed, Z.A.M.; Kamel, M.M.; Mahmoud, M.A.A.; Ali, S.A.M.; Ibrahim, A.Z.; Alshehri, B.M. 4-Amino-5-(Trifluoromethyl)-4H-1,2,4-Triazole-3-Thiol as an Effective Corrosion Inhibitor for Low Carbon Steel in HCl Environment: Experimental and Theoretical Studies. BMC Chem. 2025, 19, 205. [Google Scholar] [CrossRef]
- Chen, Z.; Li, H.; Dong, W.; Miao, M.; Ren, H. I2-Catalyzed Oxidative Coupling Reactions of Hydrazones and Amines and the Application in the Synthesis of 1,3,5-Trisubstituted 1,2,4-Triazoles. Org. Lett. 2016, 18, 1334–1337. [Google Scholar] [CrossRef]
- Lee, J.; Hong, M.; Jung, Y.; Cho, E.J.; Rhee, H. Synthesis of 1,3,5-Trisubstituted-1,2,4-Triazoles by Microwave-Assisted N-Acylation of Amide Derivatives and the Consecutive Reaction with Hydrazine Hydrochlorides. Tetrahedron 2012, 68, 2045–2051. [Google Scholar] [CrossRef]
- Castanedo, G.M.; Seng, P.S.; Blaquiere, N.; Trapp, S.; Staben, S.T. Rapid Synthesis of 1,3,5-Substituted 1,2,4-Triazoles from Carboxylic Acids, Amidines, and Hydrazines. J. Org. Chem. 2011, 76, 1177–1179. [Google Scholar] [CrossRef]
- Jiang, R.; Mu, Y.; Hou, J.; Wan, Y.; Hong, Y.; Yang, Z.; Tang, D. Electrochemical Cyclization of Hydrazones and Amidines to Access Trisubstituted 1,2,4-Triazoles. Synlett 2023, 34, 1804–1808. [Google Scholar] [CrossRef]
- Huang, H.; Guo, W.; Wu, W.; Li, C.J.; Jiang, H. Copper-Catalyzed Oxidative C(sp3)–H Functionalization for Facile Synthesis of 1,2,4-Triazoles and 1,3,5-Triazines from Amidines. Org. Lett. 2015, 17, 2894–2897. [Google Scholar] [CrossRef]
- Liu, J.Q.; Shen, X.; Wang, Y.; Wang, X.S.; Bi, X. Cycloaddition of Isocyanides with Aryl Diazonium Salts: Catalyst-Dependent Regioselective Synthesis of 1,3- and 1,5-Disubstituted 1,2,4-Triazoles. Org. Lett. 2018, 20, 6930–6933. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, J.L.; Chen, Z.; Wang, R. Base-Promoted (3+2) Cycloaddition of Trifluoroacetohydrazonoyl Chlorides with Imidates En Route to Trifluoromethyl-1,2,4-Triazoles. J. Org. Chem. 2022, 87, 14514–14522. [Google Scholar] [CrossRef]
- Li, S.M.; Tsai, S.E.; Chiang, C.Y.; Chung, C.Y.; Chuang, T.J.; Tseng, C.C.; Jiang, W.P.; Huang, G.J.; Lin, C.Y.; Yang, Y.C.; et al. New Methyl 5-(Halomethyl)-1-Aryl-1H-1,2,4-Triazole-3-Carboxylates as Selective COX-2 Inhibitors and Anti-Inflammatory Agents: Design, Synthesis, Biological Evaluation, and Docking Study. Bioorg. Chem. 2020, 104, 104333. [Google Scholar] [CrossRef]
- Olesiejuk, M.; Kudelko, A.; Swiatkowski, M.; Kruszynski, R. Synthesis of 4-Alkyl-4H-1,2,4-Triazole Derivatives by Suzuki Cross-Coupling Reactions and Their Luminescence Properties. Molecules 2019, 24, 652. [Google Scholar] [CrossRef]
- Bechara, W.S.; Khazhieva, I.S.; Rodriguez, E.; Charette, A.B. One-Pot Synthesis of 3,4,5-Trisubstituted 1,2,4-Triazoles via the Addition of Hydrazides to Activated Secondary Amides. Org. Lett. 2015, 17, 1184–1187. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.N.; Zhang, J.; Li, J.; Chen, Z.; Wu, X.F. Metal-Free Synthesis of 3-Trifluoromethyl-1,2,4-Triazoles via Oxidative Cyclization of Trifluoroacetimidohydrazides with N,N-Dimethylformamide as Carbon Synthons. Green Synth. Catal. 2022, 3, 385–388. [Google Scholar] [CrossRef]
- Yocca, S.R.; Yount, J.; Zeller, M.; Byrd, E.F.C.; Piercey, D.G. 1,3,4,5-Tetraamino-1,2,4-Triazolium Cation: An Energetic Moiety. Inorg. Chem. 2021, 60, 9645–9652. [Google Scholar] [CrossRef] [PubMed]
- Rahim, F.; Ullah, H.; Taha, M.; Hussain, R.; Sarfraz, M.; Iqbal, R.; Iqbal, N.; Khan, S.; Ali Shah, S.A.; Albalawi, M.A.; et al. Synthesis of New Triazole-Based Thiosemicarbazone Derivatives as Anti-Alzheimer’s Disease Candidates: Evidence-Based In Vitro Study. Molecules 2023, 28, 21. [Google Scholar] [CrossRef]
- Hutchinson, S.M.; Ardón-Muñoz, L.G.; Ratliff, M.L.; Bolliger, J.L. Catalytic Preparation of 1-Aryl-Substituted 1,2,4-Triazolium Salts. ACS Omega 2019, 4, 17923–17933. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, A.; Guin, S.; Rajamanickam, S.; Rout, S.K.; Patel, B.K. Synthesis of 1,2,4-Triazoles via Oxidative Heterocyclization: Selective C–N Bond Over C–S Bond Formation. J. Org. Chem. 2015, 80, 9016–9027. [Google Scholar] [CrossRef] [PubMed]
- Nakka, M.; Tadikonda, R.; Rayavarapu, S.; Sarakula, P.; Vidavalur, S. A Simple and Efficient Synthesis of 3,4,5-Trisubstituted/N-Fused 1,2,4-Triazoles via Ceric Ammonium Nitrate Catalyzed Oxidative Cyclization of Amidrazones with Aldehydes Using Polyethylene Glycol as a Recyclable Reaction Medium. Synthesis 2015, 47, 517–525. [Google Scholar] [CrossRef]
- James, S.L. Metal-Organic Frameworks. Chem. Soc. Rev. 2003, 32, 276–288. [Google Scholar] [CrossRef]
- Su, C.Y.; Goforth, A.M.; Smith, M.D.; Pellechia, P.J.; Zur Loye, H.C. Exceptionally Stable, Hollow Tubular Metal-Orgarnic Architectures: Synthesis, Characterization, and Solid-State Transformation Study. J. Am. Chem. Soc. 2004, 126, 3576–3586. [Google Scholar] [CrossRef]
- Dutt, S.; Kumar, A.; Singh, S. Synthesis of Metal Organic Frameworks (MOFs) and Their Derived Materials for Energy Storage Applications. Clean Technol. 2023, 5, 140–166. [Google Scholar] [CrossRef]
- Tranchemontagne, D.J.; Hunt, J.R.; Yaghi, O.M. Room Temperature Synthesis of Metal-Organic Frameworks: MOF-5, MOF-74, MOF-177, MOF-199, and IRMOF-0. Tetrahedron 2008, 64, 8553–8557. [Google Scholar] [CrossRef]
- Rabenau, A. The Role of Hydrothermal Synthesis in Preparative Chemistry. Angew. Chem. Int. Ed. Engl. 1985, 24, 1026–1040. [Google Scholar] [CrossRef]
- Yoshimura, M.; Byrappa, K. Hydrothermal Processing of Materials: Past, Present and Future. J. Mater. Sci. 2008, 43, 2085–2103. [Google Scholar] [CrossRef]
- Férey, G. Metal-Organic Frameworks: The Young Child of the Porous Solids Family. Stud. Surf. Sci. Catal. 2007, 170, 66–84. [Google Scholar] [CrossRef]
- Sun, J.; Xi, Y.; Gao, L.; Hu, M.; Liu, W.; Ma, E.; Huang, R.; Qin, W.; Wu, G. Two Isostructural Ln-MOFs Containing Triazole Groups as Luminescent Probes for Efficient Sensing of NACs and Fe3+. Inorganica Chim. Acta 2023, 547, 121376. [Google Scholar] [CrossRef]
- Liu, J.; Yang, L.Y.; Luo, F. A New Zn-Triazole MOF Showing Very Long-Lived Luminescence up to 3 s. J. Solid State Chem. 2021, 301, 122369. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Kalmutzki, M.J.; Diercks, C.S. Introduction to Reticular Chemistry: Metal-Organic Frameworks and Covalent Organic Frameworks; Wiley: Hoboken, NJ, USA, 2019; pp. 1–509. [Google Scholar] [CrossRef]
- Abid, H.R.; Azhar, M.R.; Iglauer, S.; Rada, Z.H.; Al-Yaseri, A.; Keshavarz, A. Physicochemical Characterization of Metal Organic Framework Materials: A Mini Review. Heliyon 2024, 10, e23840. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.G.; Salinger, J.L.; Bingel, L.W.; Walton, K.S. Determining Surface Areas and Pore Volumes of Metal-Organic Frameworks. J. Vis. Exp. 2024, 7, e65716. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.L.; Razavi, S.A.A.; Piroozzadeh, M.; Morsali, A. Sensing Organic Analytes by Metal–Organic Frameworks: A New Way of Considering the Topic. Inorg. Chem. Front. 2020, 7, 1598–1632. [Google Scholar] [CrossRef]
- Zhao, Y.; Zeng, H.; Zhu, X.W.; Lu, W.; Li, D. Metal–Organic Frameworks as Photoluminescent Biosensing Platforms: Mechanisms and Applications. Chem. Soc. Rev. 2021, 50, 4484–4513. [Google Scholar] [CrossRef]
- Raptopoulou, C.P. Metal-Organic Frameworks: Synthetic Methods and Potential Applications. Materials 2021, 14, 310. [Google Scholar] [CrossRef]
- Baumann, A.E.; Burns, D.A.; Liu, B.; Thoi, V.S. Metal-Organic Framework Functionalization and Design Strategies for Advanced Electrochemical Energy Storage Devices. Commun. Chem. 2019, 2, 86. [Google Scholar] [CrossRef]
- Pham, T.D.; Sengupta, D.; Farha, O.K.; Snurr, R.Q. Investigation of Anionic Metal-Organic Frameworks with Extra-Framework Cations for Room Temperature Hydrogen Storage. Chem. Mater. 2024, 36, 3794–3802. [Google Scholar] [CrossRef]
- Xie, W.; Fu, Q.; Yang, L.-Z.; Yan, L.; Zhang, J.; Zhao, X. Methane Storage and Purification of Natural Gas in Metal-Organic Frameworks. ChemSusChem 2025, 18, e202401382. [Google Scholar] [CrossRef]
- Wang, J.W.; Fan, S.C.; Li, H.P.; Bu, X.; Xue, Y.Y.; Zhai, Q.G. De-Linker-Enabled Exceptional Volumetric Acetylene Storage Capacity and Benchmark C2H2/C2H4 and C2H2/CO2 Separations in Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2023, 62, e202217839. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, X.; Liu, S.; Liu, X.; Liu, G.; Liu, R.; Niu, J.; Yuan, Z.; Zhang, J.; Yi, X.; et al. Melamine-Derived Nitrogen-Doped Carbon Foam Supported Bimetallic NiZn-MOFs as an Efficient Adsorbent for CH4 Storage. J. Porous Mater. 2025, 32, 1309–1319. [Google Scholar] [CrossRef]
- Li, Y.Z.; Wang, G.D.; Xu, F.; Yin, Q.; Zhao, D.; Qi, J.; Sui, Y.; Hou, L.; Wang, Y.Y. A Robust Indium-Organic Framework with Open Tubular Channels for Efficient Separation of Acetylene. Nano Res. 2024, 17, 3139–3146. [Google Scholar] [CrossRef]
- Hou, W.; Cheng, J.; Hu, L.; Wu, Y.; Zhou, J. Mixed Matrix Membranes Based on NbOF52− Anion-Pillared Porous MOFs for Efficient CO2 Separation. J. Memb. Sci. 2024, 693, 122323. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, S.; Ye, P.; Zhang, L.; Shen, X.; Huang, Q.; Xu, H.; Liu, H.; Li, S. In Situ Synthesis of Hierarchical Porous Zr-MOFs on Columnar Activated Carbon and Application in Toxic Gas Adsorption. Inorg. Chem. 2022, 61, 18355–18364. [Google Scholar] [CrossRef]
- Obeso, J.L.; Amaro, D.R.; Flores, C.V.; Gutiérrez-Alejandre, A.; Peralta, R.A.; Leyva, C.; Ibarra, I.A. Chemical Transformations of Highly Toxic H2S to Promising Clean Energy in MOFs. Coord. Chem. Rev. 2023, 485, 215135. [Google Scholar] [CrossRef]
- Kim, D.W.; Kang, D.W.; Kang, M.; Lee, J.H.; Choe, J.H.; Chae, Y.S.; Choi, D.S.; Yun, H.; Hong, C.S. High Ammonia Uptake of a Metal–Organic Framework Adsorbent in a Wide Pressure Range. Angew. Chem. Int. Ed. 2020, 59, 22531–22536. [Google Scholar] [CrossRef]
- Cheng, L.; Dang, Y.; Wang, Y.; Chen, K.J. Recent Advances in Metal–Organic Frameworks for Water Absorption and Their Applications. Mater. Chem. Front. 2024, 8, 1171–1194. [Google Scholar] [CrossRef]
- Chen, W.; Cai, P.; Zhou, H.C.; Madrahimov, S.T. Bridging Homogeneous and Heterogeneous Catalysis: Phosphine-Functionalized Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2024, 63, e202315075. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, R.; Pramanik, B.; Mondal, S.; Das, M.C. A Highly Chemically Robust 3D Interpenetrated MOF Heterogeneous Catalyst for the Synthesis of Hantzsch 1,4-Dihydropyridines and Drug Molecules. Small 2024, 20, 2309281. [Google Scholar] [CrossRef] [PubMed]
- Bavykina, A.; Kolobov, N.; Khan, I.S.; Bau, J.A.; Ramirez, A.; Gascon, J. Metal-Organic Frameworks in Heterogeneous Catalysis: Recent Progress, New Trends, and Future Perspectives. Chem. Rev. 2020, 120, 8468–8535. [Google Scholar] [CrossRef]
- Sun, N.; Shah, S.S.A.; Lin, Z.; Zheng, Y.Z.; Jiao, L.; Jiang, H.L. MOF-Based Electrocatalysts: An Overview from the Perspective of Structural Design. Chem. Rev. 2025, 125, 2703–2792. [Google Scholar] [CrossRef] [PubMed]
- Juma, A.K.; Merican, Z.M.A.; Haruna, A. Recent Progress of MOF-Based Photocatalysts for Environmental Application and Sustainability Considerations. Chem. Eng. Res. Des. 2024, 208, 391–435. [Google Scholar] [CrossRef]
- Bunzen, H.; Jirák, D. Recent Advances in Metal-Organic Frameworks for Applications in Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2022, 14, 50445–50462. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Gao, J.; Sun, Y.; Duan, F.; Chen, B.; Lv, G.; Li, H.; Jiang, X.; Wu, Y.; Zhang, J.; et al. Fusing Positive and Negative CT Contrast Nanoagent for the Sensitive Detection of Hepatoma. Adv. Sci. 2023, 10, e2304668. [Google Scholar] [CrossRef]
- Duan, D.; Liu, H.; Xu, M.; Chen, M.; Han, Y.; Shi, Y.; Liu, Z. Size-Controlled Synthesis of Drug-Loaded Zeolitic Imidazolate Framework in Aqueous Solution and Size Effect on Their Cancer Theranostics in Vivo. ACS Appl. Mater. Interfaces 2018, 10, 42165–42174. [Google Scholar] [CrossRef]
- Yu, J.; Li, Q.; Wei, Z.; Fan, G.; Wan, F.; Tian, L. Ultra-Stable MOF@MOF Nanoplatform for Photodynamic Therapy Sensitized by Relieved Hypoxia Due to Mitochondrial Respiration Inhibition. Acta Biomater. 2023, 170, 330–343. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, C.; Mei, Z.; Yang, H.; Wang, B.; Xie, C.; Xu, Y.; Tian, J. Oxygen-Enriched MOF-Hemoglobin X-Ray Nanosensitizer for Enhanced Cancer Radio-Radiodynamic Therapy. ACS Mater. Lett. 2023, 5, 3237–3247. [Google Scholar] [CrossRef]
- Yadav, P.; Kumari, S.; Yadav, A.; Bhardwaj, P.; Maruthi, M.; Chakraborty, A.; Kanoo, P. Biocompatible Drug Delivery System Based on a MOF Platform for a Sustained and Controlled Release of the Poorly Soluble Drug Norfloxacin. ACS Omega 2023, 8, 28367–28375. [Google Scholar] [CrossRef]
- Timofeeva, M.; Kenzhebayeva, Y.; Burzak, N.; Bazhenova, A.; Lunev, A.; Novikov, A.S.; Bondarenko, A.B.; Shipilovskikh, S.A.; Dyachuk, V.A.; Milichko, V.A. A Light-Driven Ultrafast Sensor Based on Biocompatible Solvatochromic Metal-Organic Frameworks. Mater. Horizons 2024, 12, 1255–1261. [Google Scholar] [CrossRef]
- Panda, S.K.; Mishra, S.; Singh, A.K. Recent Progress in the Development of MOF-Based Optical Sensors for Fe3+. Dalt. Trans. 2021, 50, 7139–7155. [Google Scholar] [CrossRef]
- Ghommem, M.; Hemid, M.; Alattar, B.; Sabouni, R.; Elhady, A.; Shama, Y.S.; Arabi, M.; Abdel-Rahman, E.M. Development of MEMS Gas Sensors Equipped with Metal Organic Frameworks. Sens. Actuators A Phys. 2024, 371, 115296. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, H.; Song, B.; Yuan, K.; Xiao, H.; Cao, Y.; Cao, Q. Metal–Organic Framework (MOF) Derivatives as Promising Chemiresistive Gas Sensing Materials: A Review. Int. J. Environ. Res. Public Health 2023, 20, 4388. [Google Scholar] [CrossRef]
- Daniel, M.; Mathew, G.; Anpo, M.; Neppolian, B. MOF Based Electrochemical Sensors for the Detection of Physiologically Relevant Biomolecules: An Overview. Coord. Chem. Rev. 2022, 468, 214627. [Google Scholar] [CrossRef]
- Yuan, H.; Cui, J.; Li, N.; Li, M.; Yu, X.; Fan, W.; Karmakar, A.; Dong, J.; Pennycook, S.J.; Cai, H.; et al. On-Chip Template-Directed Conversion of Metal Hydroxides to Metal-Organic Framework Films with Enhanced Adhesion. ACS Appl. Mater. Interfaces 2020, 12, 36715–36722. [Google Scholar] [CrossRef]
- Banning, D.H.; Davenport, A.M.; Lakanen, N.M.; Huang, J.; Brozek, C.K.; Johnson, D.W. ChemFET Anion Sensor Based on MOF Nanoparticles. Chempluschem 2024, 90, e202400622. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, H.; Yan, S.; Zhou, Y.; Guo, X.; An, D.; Zhong, W.; Zhang, Y. Fabrication of MOF-Based Nanozyme Sensor Arrays and Their Application in Disease Diagnosis. Coord. Chem. Rev. 2025, 532, 216506. [Google Scholar] [CrossRef]
- Peng, Y.; Xu, J.; Xu, J.; Ma, J.; Bai, Y.; Cao, S.; Zhang, S.; Pang, H. Metal-Organic Framework (MOF) Composites as Promising Materials for Energy Storage Applications. Adv. Colloid Interface Sci. 2022, 307, 102732. [Google Scholar] [CrossRef]
- He, H.; Zhu, Q.Q.; Li, C.P.; Du, M. Design of a Highly-Stable Pillar-Layer Zinc(II) Porous Framework for Rapid, Reversible, and Multi-Responsive Luminescent Sensor in Water. Cryst. Growth Des. 2019, 19, 694–703. [Google Scholar] [CrossRef]
- Zou, J.Y.; Li, L.; You, S.Y.; Zhang, S.W. A Zinc(II) Triazolate Framework with Luminescence Response toward Dichromate Anion in Aqueous Solution. Inorganica Chim. Acta 2019, 498, 119126. [Google Scholar] [CrossRef]
- Qin, B.; Zhang, X.; Zhang, J. A New Multifunctional Zinc-Organic Framework with Rare Interpenetrated Tripillared Bilayers as a Luminescent Probe for Detecting Ni2+ and PO43−in Water. Cryst. Growth Des. 2020, 20, 5120–5128. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, Y.; Gu, L.W.; Qin, X.M.; Li, H.Y.; Bian, H.D.; Huang, F.P. A Zinc2+-Dpbt Framework: Luminescence Sensing of Cu2+, Ag+, MnO4− and Cr(VI) (Cr2O72−and CrO42−) Ions. New J. Chem. 2020, 44, 10681–10688. [Google Scholar] [CrossRef]
- Liu, F.; Kumar, S.; Li, S.; You, H.; Ren, P.; Zhao, L. Bifunctional Design of Stable Metal-Organic Framework Bearing Triazole–Carboxylate Mixed Ligand: Highly Efficient Heterogeneous Catalyst for Knoevenagel Condensation Reaction under Mild Conditions. Catal. Commun. 2020, 142, 106032. [Google Scholar] [CrossRef]
- Li, W.D.; Chen, S.S.; Han, S.S.; Zhao, Y. The Syntheses, Structures, and Properties of Metal-Organic Frameworks Based on Mixed Multi-N Donor and Carboxylate Ligands. J. Solid State Chem. 2020, 283, 121133. [Google Scholar] [CrossRef]
- Yu, R.Y.; Zhang, J.W.; Qu, P.; Liu, D.S.; Wang, R. Rational Design of a Rare Zn-MOF Material Based on Mixed Carboxylate-Azolate Ligands and Its Strong Blue Luminescence. Inorg. Nano-Met. Chem. 2021, 54, 30–34. [Google Scholar] [CrossRef]
- Fang, X.D.; Yao, J.; Fan, R.; Bai, X.F.; Liu, Y.E.; Hou, C.F.; Xu, Q.Q.; Zhu, A.X.; Huang, B. A Luminescent Zinc-Organic Framework as Bifunctional Chemosensors for Detection of Nitrobenzene and Fe3+. J. Solid State Chem. 2021, 294, 121854. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, P.; Dong, Y. New Luminescent Zn(II) Compound as a Highly Selective and Sensitive Luminescence Probe for the Detection of Fe3+ in Aqueous Solution. Inorg. Nano-Met. Chem. 2021, 51, 218–223. [Google Scholar] [CrossRef]
- Chen, S.S.; Han, S.S.; Ma, C.B.; Li, W.D.; Zhao, Y. A Series of Metal-Organic Frameworks: Syntheses, Structures and Luminescent Detection, Gas Adsorption, Magnetic Properties. Cryst. Growth Des. 2021, 21, 869–885. [Google Scholar] [CrossRef]
- Qin, B.; Zhang, X.; Dang, J.; Yue, D.; Zhang, B.; Li, W.; Gahungu, G.; Wang, Z.; Zhang, J. A 2-Fold Interpenetrated Zinc-Organic Framework with Lewis Basic Triazole Sites: Luminescence Sensing of Fe3+ and Cr2O72−, and Warm White-Light Emission by Encapsulated Ln3+ Ions. CrystEngComm 2022, 24, 7058–7065. [Google Scholar] [CrossRef]
- Xian, G.; Wang, L.; Wan, X.; Yan, H.; Cheng, J.; Chen, Y.; Lu, J.; Li, Y.; Li, D.; Dou, J.; et al. Two Multiresponsive Luminescent Zn-MOFs for the Detection of Different Chemicals in Simulated Urine and Antibiotics/Cations/Anions in Aqueous Media. Inorg. Chem. 2022, 61, 7238–7250. [Google Scholar] [CrossRef]
- Yu, C.X.; Jiang, W.; Wang, K.Z.; Liang, A.P.; Song, J.G.; Zhou, Y.L.; Sun, X.Q.; Liu, L.L. Luminescent Two-Dimensional Metal-Organic Framework Nanosheets with Large π-Conjugated System: Design, Synthesis, and Detection of Anti-Inflammatory Drugs and Pesticides. Inorg. Chem. 2022, 61, 982–991. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Liu, Y.; Shi, P.; Xu, S.; Bin, Y. A Bifunctional Three-Dimensional Zn(II) Metal-Organic Framework with Strong Luminescence and Adsorption Cr(VI) Properties. ACS Omega 2024, 9, 18429–18437. [Google Scholar] [CrossRef]
- Cui, R.; Wan, Y.; Ji, G.; Liu, Z. A Highly Selective and Sensitive Fluorescent Sensor Based on Tb3+-Functionalized MOFs to Determine Arginine in Urine: A Potential Application for the Diagnosis of Cystinuria. Analyst 2019, 144, 5875–5881. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, W.; Wu, J.; Tang, G.; Zhang, C. Two Mixed-Ligand Cd(II)-Organic Frameworks with Unique Topologies: Selective Luminescence Sensing of TNP and Cu2+ Ions with Recyclable Performances. New J. Chem. 2019, 43, 16078–16088. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Lin, H.; Wen, Y.; Zhu, Q.L. Metal-Organic Frameworks Based on a Bent Triazole Dicarboxylic Acid: Magnetic Behaviors and Selective Luminescence Sensing Properties. Cryst. Growth Des. 2019, 19, 1057–1063. [Google Scholar] [CrossRef]
- Singh, M.; Senthilkumar, S.; Rajput, S.; Neogi, S. Pore-Functionalized and Hydrolytically Robust Cd(II)-Metal-Organic Framework for Highly Selective, Multicyclic CO2 Adsorption and Fast-Responsive Luminescent Monitoring of Fe(III) and Cr(VI) Ions with Notable Sensitivity and Reusability. Inorg. Chem. 2020, 59, 3012–3025. [Google Scholar] [CrossRef]
- Qin, B.W.; Zhang, X.Y.; Zhang, J.P. A Stable Multifunctional Cadmium-Organic Framework Based on 2D Stacked Layers: Effective Gas Adsorption, and Excellent Detection of Cr3+, CrO42−, and Cr2O72−. Dye. Pigment. 2020, 174, 108011. [Google Scholar] [CrossRef]
- Singh, M.; Palakkal, A.S.; Pillai, R.S.; Neogi, S. N-Functionality Actuated Improved CO2 adsorption and Turn-on Detection of Organo-Toxins with Guest-Induced Fluorescence Modulation in Isostructural Diamondoid MOFs. J. Mater. Chem. C 2021, 9, 7142–7153. [Google Scholar] [CrossRef]
- Hong, B.Q.; Qi, Y.J.; Lai, R.D.; Ge, R.; Zheng, S.T.; Li, X.X. Two Luminescent Metal-Organic Frameworks with Temperature-Dependent Emission. J. Solid State Chem. 2022, 309, 122967. [Google Scholar] [CrossRef]
- Yu, M.; Yao, X.; Wang, X.; Li, Y.; Li, G. White-Light-Emitting Decoding Sensing for Eight Frequently-Used Antibiotics Based on a Lanthanide Metal-Organic Framework. Polymers 2019, 11, 99. [Google Scholar] [CrossRef]
- Li, L.; Zou, J.Y.; You, S.Y.; Liu, Y.W.; Cui, H.M.; Zhang, S.W. A Dual Luminescent Chemosensor Derived from a Europium(III) Metal-Organic Framework for Quantitative Detection of Phosphate Anions and Acetylacetone in Aqueous Solution. Dye. Pigment. 2020, 173, 108004. [Google Scholar] [CrossRef]
- Zhan, C.H.; Huang, D.P.; Wang, Y.; Mao, W.T.; Wang, X.J.; Jiang, Z.G.; Feng, Y.L. Four Anionic Ln-MOFs for Remarkable Separation of C2H2-CH4/CO2-CH4 and Highly Sensitive Sensing of Nitrobenzene. CrystEngComm 2021, 23, 2788–2792. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, G.; Zhang, H.; Wang, G.; Han, H. A Stable Terbium(III) Metal-Organic Framework as a Dual Luminescent Sensor for MnO4− Ions and Nitroaromatic Explosives. J. Solid State Chem. 2021, 295, 121924. [Google Scholar] [CrossRef]
- Luo, L.; Xie, Y.; Hou, S.L.; Ma, Y.; Zhao, B. Recyclable Luminescent Sensor for Detecting Creatinine Based on a Lanthanide-Organic Framework. Inorg. Chem. 2022, 61, 9990–9996. [Google Scholar] [CrossRef]
- Wang, H.; Liu, D.; Wei, M.; Qi, W.; Li, X.; Niu, Y. A Stable and Highly Luminescent 3D Eu(III)-Organic Framework for the Detection of Colchicine in Aqueous Environment. Environ. Res. 2022, 208, 112652. [Google Scholar] [CrossRef]
- Wang, X.; Gopalsamy, K.; Clavier, G.; Maurin, G.; Ding, B.; Tissot, A.; Serre, C. Lanthanide MOF-Based Luminescent Sensor Arrays for the Detection of Castration-Resistant Prostate Cancer Curing Drugs and Biomarkers. Chem. Sci. 2024, 15, 6488–6499. [Google Scholar] [CrossRef]
- Zhang, S.R.; Zhang, W.T.; Li, X.; Xu, G.J.; Xie, W.; Xu, Y.H.; Xu, N.; Su, Z.M. Multifunctional Lanthanide Metal-Organic Frameworks Act as Fluorescent Probes for the Detection of Cr2O72−, Fe3+, and TNP, White Light-Emitting Diodes, and Luminescence Thermometers. Inorg. Chem. 2025, 64, 2990–2999. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Tan, Z.; Liu, L.; Wang, Q.; Yang, T.; Zhou, X.; Niu, Z. A Luminescent Lanthanide MOF for the Selective and Ultra-High Sensitivity Detection of Caffeic Acid. Appl. Organomet. Chem. 2025, 39, e8002. [Google Scholar] [CrossRef]
- Keister, J.B. Organometallic Clusters. In Comprehensive Organometallic Chemistry III; Elsevier: Amsterdam, The Netherlands, 2007; pp. 755–780. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Feng, J.; Gong, L.; Humphrey, M.G.; Zhang, C. Decanuclear Cluster-Based Metal-Organic Framework with a (3,11)-Connected Topology and Highly Sensitive 2,4,6-Trinitrophenol Detection. Inorg. Chem. 2019, 58, 9749–9755. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.N.; Liang, Y.; Cheng, L.; Fang, Y. Chiral Fluorescent Metal-Organic Framework with a Pentanuclear Copper Cluster as an Efficient Luminescent Probe for Dy3+Ion and Cyano Compounds. Inorg. Chem. 2021, 60, 15085–15090. [Google Scholar] [CrossRef]
- An, J.D.; Wang, T.T.; Shi, Y.F.; Huo, J.Z.; Wu, X.X.; Liu, Y.Y.; Ding, B. Convenient Ultrasonic Preparation of a Water Stable Cluster-Based Cadmium(II) Coordination Material and Highly Sensitive Fluorescent Sensing for Biomarkers DPA and 5-HT. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 247, 119092. [Google Scholar] [CrossRef]
- Wang, J.R.; Fu, J.; Zhang, Y.J.; Liang, J.C.; Zhou, R.S.; Gong, S.M.; Song, J.F. A Butterfly Shaped Eu4(OH)2 Cluster-Based Luminescent Metal-Organic Framework with Lewis Basic Triazole Sites Demonstrating Turn off Sensing in the Presence of Organic Amines. Dalt. Trans. 2022, 52, 136–146. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, J.Y.; Wang, T.T.; Liu, Y.Y.; Zhang, L.X.; Huo, J.Z.; Ding, B. Solvo-Thermal Synthesis of a Unique Cluster-Based Nano-Porous Zinc(II) Luminescent Metal-Organic Framework for Highly Sensitive Detection of Anthrax Biomarker and Dichromate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 274, 121132. [Google Scholar] [CrossRef]
- Liu, H.F.; Tao, Y.; Wu, T.X.; Li, H.Y.; Zhang, X.Q.; Huang, F.P.; Bian, H.D. A {Zn5} Cluster-Based Metal–Organic Framework: Multifunctional Detection of Ag+, Cr2O72−, and 2,4,6-Trinitrophenol (TNP). Appl. Organomet. Chem. 2022, 36, e6456. [Google Scholar] [CrossRef]
- Sun, Y.; Dong, B.X.; Liu, W.L. An Adjustable Dual-Emission Fluorescent Metal-Organic Framework: Effective Detection of Multiple Metal Ions, Nitro-Based Molecules and DMA. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 223, 117283. [Google Scholar] [CrossRef]
- Chand, S.; Verma, G.; Pal, A.; Pal, S.C.; Ma, S.; Das, M.C. Porous Anionic Co(II) Metal-Organic Framework, with a High Density of Amino Groups, as a Superior Luminescent Sensor for Turn-on Al(III) Detection. Chem. A Eur. J. 2021, 27, 11804–11810. [Google Scholar] [CrossRef]
- Qian, L.L.; Wang, Z.X.; Zhu, L.M.; Li, K.; Li, B.L.; Wu, B. Synthesis, Structure, Spectral Characteristic and Photocatalytic Degradation of Organic Dyes of a Copper Metal-Organic Framework Based on Tri(Triazole) and Pimelate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 214, 372–377. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).