Abstract
Grapevine leafroll disease (GLD), caused by a complex of grapevine leafroll-associated viruses (GLRaVs), is among the most widespread and economically damaging viral diseases of grapevine. While its physiological and yield impacts are well recognized, the broader ecological implications for vineyard ecosystems remain poorly understood. This review integrates traditional literature analysis with bibliometric approaches to synthesize current knowledge on GLRaV occurrence, diversity, host responses, epidemiology, diagnostics, and management. Data from 729 peer-reviewed articles were categorized into six research clusters: global occurrence and first reports, viral diversity and characterization, host–pathogen interactions, epidemiology and vector dynamics, effects on vine physiology and fruit composition, and diagnostic and management strategies. Our findings highlight GLRaVs as dynamic pathogens shaped by genetic variability, human-mediated plant trade, and ecological interactions with vectors and vineyard biodiversity. Knowledge gaps persist regarding mixed infections, underexplored viticultural regions, ecological impacts, and sustainable management. Future work should prioritize high-resolution genomics, multi-omics approaches, improved diagnostics, ecological studies, and innovative management tools. By framing GLD not only as an agronomic but also as an ecological challenge, this review provides a foundation for more holistic strategies to safeguard vineyard health and productivity.
1. Introduction
Grapevine leafroll disease (GLD), caused by a complex of viruses known as Grapevine leafroll-associated viruses (GLRaVs), has a long history, the characteristic symptoms being identified in retrospectively observed grapevine specimens, preserved in herbarium from Sicily, dating back to the middle of the 19th century. In the beginning, it was mistaken for a physiological disorder of reddening of the grapevine leaves, referred to as “rugeau” or rossore” [,]. The disease is widespread in all grape-growing countries [], now being recognized as a major viral threat to grapevine plant development, grapes yield and quality, and vineyard longevity, causing significant economic damage [,,]. The behavior of the grapevine in the presence of GLRaVs was influenced by different factors such as location, stress factors, age, cultivar, and rootstock [,,,,]. Being a perennial and vegetatively propagated plant, the grapevine is subject to the attack of many diseases and pathogens, of which intracellular pathogens (viruses, viroids, phloem- and xylem limited prokariotes) are difficult to control and can cause serious economic damage []. Grapevine (Vitis spp.) is the crop affected by the most viruses. Of the 102 viruses known in grapevine, 6 are GLRaVs. These viruses belong to the family Closteroviridae: 4 viruses of genus Ampelovirus (GLRaV-1, GLRaV-3, GLRaV-4, and GLRaV-13), 1 virus of genus Closterovirus (GLRaV-4), and 1 virus of genus Velarvirus (GLRaV-7) []. Recently, a new Ampelovirus, grapevine leafroll-associated virus S (GLRaV-S), was discovered by high-throughput sequencing []. The disease can be produced by single or mixed infections of GLRaVs [] or by GLRaVs with other viruses [,,].
The importance of the field of grapevine virology led to the organization of the International Council for the Study of Viruses and Viral-like Diseases of the Grapevine (ICVG), a body that deals strictly with viruses of this crop plant, in 1962. The ICVG meets every three years and is now in its twentieth session. In the Proceedings of the 20th Congress of ICVG, held in Thessaloniki, Greece (25–29 September 2023) 97 scientific papers (including oral, posters, and keynote presentations) were published, of which 13 (13.4%) had leafroll-associated viruses as their topic.
Some review-type articles have also been published about the grapevine leafroll disease [,,], and on related ecology and ecosystems [,,,,,,,]. However, no review has been written so far about the ecological consequences of grapevine leafroll disease in vineyard ecosystems. In order to address this, a review of ISI-indexed articles was conducted, employing both a traditional and bibliometric review. The obtained results highlight current knowledge and trends, forming a valuable foundation for future studies intended to analyze the link between grapevine leafroll disease in vineyards and their consequences.
The present paper deals with grapevine leafroll disease with regard to the key research conducted on the following areas: the occurrence of leafroll-associated viruses in different viticultural areas of the world, including their diversity and characterization; virus distribution in grapevine; the plant response to the infection as effects on vine physiology and yield; and the biological and epidemiological properties of the involved viruses.
2. Materials and Methods
This study began with a bibliometric analysis intended to evaluate the global scientific research about GLD/GLRaVs, including the influence of climate on disease symptoms, virus transmission and seasonal detection, and vectors activity between 1984 and 2025. The Science Citation Index Expanded (SCI-Expanded) from the Web of Science database, complemented by Scopus, were used. This allowed the identification of relevant publications and the testing of different search strategies. After testing different keywords, the phrase “grapevine leafroll associated virus” was selected as our main search term.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed []. The selection process of the papers included in this review is illustrated in Figure 1. A total of 672 publications was retrieved from Scopus and 694 from Web of Science, accounting for 1366 publications. Duplicates (558) were then removed and the titles and abstracts of the remaining 808 papers were checked in order to select those studies that fitted the following inclusion criteria: studies published in English and articles whose title and/or abstract referred to the specific topic. The following exclusion criteria were adopted; articles without abstract, non-scientific articles, non-published materials, and letters to editors were excluded. After this manually performed screening, 5 bibliographic sources were excluded, and 6 full papers were not retrieved. The remaining 797 full-text records were deeply inspected. A total of 61 articles were excluded because they were irrelevant to the topic, and another 7 were excluded because they had no abstracts. Finally, 729 papers were included in this systematic review (Figure 1).
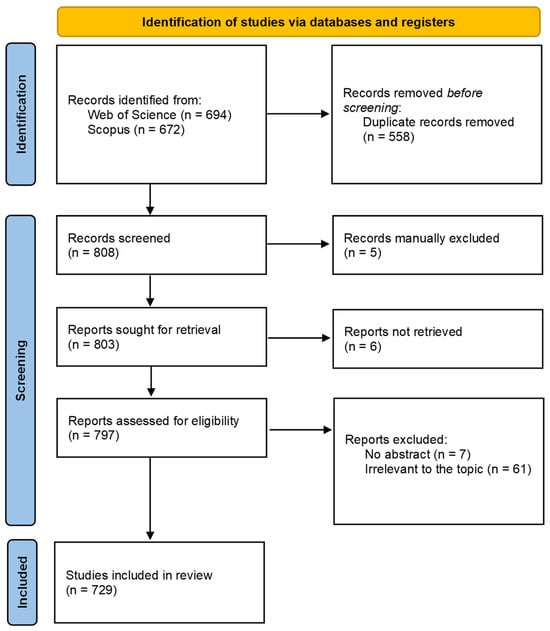
Figure 1.
Selection process of the eligible reports based on the PRISMA 2020 flow diagram.
Data analysis and visualization were performed using Web of Science Core Collection (version 5.35, Clarivate) [], Scopus [], Microsoft Excel (version 2024) [], and Geochart []. To further explore bibliometric relationships, VOSviewer (version 1.6.20) [] was applied for mapping co-authorship networks, co-citation patterns, and keyword co-occurrence clusters.
This generated a literature review that allowed us to conduct a more detailed analysis of the 729 selected articles. Our findings were then grouped into six research clusters: (1) first reports of grapevine leafroll-associated virus occurrence worldwide, (2) global occurrence and characterization of grapevine leafroll-associated viruses, (3) factors influencing grapevine leafroll disease dynamics and host responses, (4) biological and epidemiological properties of grapevine leafroll-associated viruses, (5) genomic diversity and molecular diagnostics of grapevine leafroll-associated viruses, (6) effects of grapevine leafroll disease on vine physiology, yield, and fruit composition. An overview of the complete methodological framework is illustrated in Figure 2.
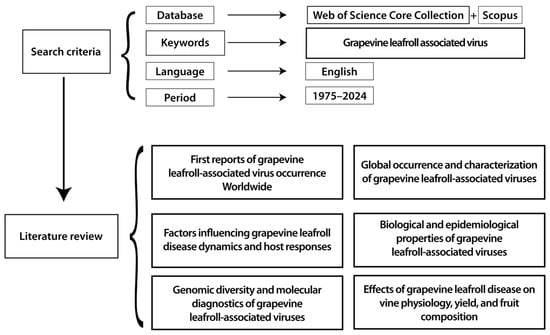
Figure 2.
Schematic presentation of the workflow used in our research.
3. Results
3.1. First Reports of Grapevine Leafroll-Associated Virus Occurrence Worldwide
The results of the survey of first documented occurrences of GLRaVs in different countries, including host varieties and geographic origins, are presented in Table 1

Table 1.
First documented country-level occurrences of grapevine leafroll-associated viruses (GLRaVs).
The first documented occurrences of various GLRaVs have been reported across multiple continents, encompassing both major wine-producing and emerging viticulture regions. GLRaV-1 has been detected for the first time in countries including India, Nigeria, Turkey, the United Kingdom, and others, affecting both local cultivars and international varieties. GLRaV-2 records span diverse geographical zones from Bulgaria and Morocco to multiple U.S. states, often involving both V. vinifera and hybrid grape species. GLRaV-3, the most widely distributed, shows initial detections in countries from China and Jordan to Tunisia, Ukraine, and several U.S. viticultural areas. Reports of GLRaV-4, GLRaV-5, GLRaV-7, GLRaV-9, and others also illustrate a broadening geographic range, with new records emerging in South America, Europe, Africa, Asia, and Oceania. These first reports highlight the expanding global footprint of leafroll disease and suggest both historical underdetection and recent spread.
3.2. Global Occurrence and Characterization of Grapevine Leafroll-Associated Viruses
Extensive surveys and molecular studies have been conducted worldwide to assess the prevalence, distribution, and genetic diversity of GLRaVs, with a particular focus on their ecological and epidemiological implications in vineyard ecosystems. Below is a synthesis of reported findings from various countries.
Greece: A broad ELISA-based survey across major grape-growing regions, including commercial vineyards and varietal collections, detected GLRaV-7 both in symptomless and symptomatic vines. The virus was often associated with GLRaV-1 and GLRaV-3. Although ELISA proved suitable for detection, the study emphasized the need for improved reagents for GLRaV-7 [].
Croatia: In the key indigenous red cultivar Plavac Mali, screening for 16 viruses revealed mixed infections as common, with GLRaV-3 being the most prevalent (85.71%). Phylogenetic analysis confirmed the presence of groups I and II, highlighting the cultivar’s high infection rate and the current genetic heterogeneity of GLRaV-3 [].
Slovenia: Sequencing of the coat protein (CP) and p23 proteins from the Slovenian GLRaV-4 variant (055-SI) showed 88% and 85% amino acid identity, respectively, to reference isolate LR106. Phylogenetic clustering confirmed its grouping with other GLRaV-4 strains. The variable N-terminal CP region was deemed unsuitable for molecular detection [].
Montenegro: Surveys in the Skadar Lake basin detected Grapevine fanleaf virus (GFLV), GLRaV-1, GLRaV-2, and GLRaV-3, with GLRaV-3 being the most frequent (54.5%), followed by GFLV (23%), GLRaV-1 (20%), and GLRaV-2 (0.6%). GLRaV-6 and GLRaV-7 were absent [].
Bosnia and Herzegovina: Eighty-eight percent of vines tested positive for at least one virus. GLRaV-3 was most common (84%), followed by GFLV (43%), GLRaV-1 (14%), Grapevine fleck virus (GFkV) (10%), and Arabis mosaic virus (ArMV) (0.2%). GLRaV-3 isolates displayed 75–100% nucleotide identity with reference sequences, clustering into the major phylogenetic group [].
Albani: Infections by GFLV, GFkV, Grapevine virus A (GVA), GLRaV-1, and GLRaV-3 were detected. ELISA revealed virus presence in 83.5% of 530 V. vinifera vines and in 46% of 24 American rootstocks [].
Hungary: Four GLRaV-3 positive samples from the northwest region were confirmed by RT-PCR. Phylogenetic analysis placed isolates in groups II and IV, the latter clustering with South African, Austrian, and Syrah isolates, indicating two variant groups in the country [].
Czech Republic: ELISA testing of phloem scrapings revealed GLRaV-1, GFkV, and GVA as the most prevalent viruses, with incidences of 14–25% [].
Germany: Long-term monitoring in southwestern vineyards found GLRaV-1 in 2.1% of samples, while GLRaV-3 was nearly absent (<0.1%). Virus-infected planting material showed no overall decline over 12 years, suggesting ongoing vector-mediated spread [].
Poland: RT-PCR surveys in 23 vineyards detected GLRaV-1 (2.2%), GLRaV-2 (1.9%), and GLRaV-3 (1.5%) in both single and mixed infections. The overall infection rate reached 82.6% [].
Italy: In Emilia-Romagna, immunoelectron microscopy revealed GLRaV-1, GLRaV-3, and GVA in 60.6% of 150 clone selections from 18 cultivars. GLRaV-2 and GLRaV-5 were absent. Viruses occurred individually or in combinations [].
Portugal: GLRaV-5 CP gene phylogeny revealed eight lineages, four present in this study, with high diversity but no evidence of vector-mediated spread. Results suggest that divergence is driven primarily by vegetative propagation [].
Romania: GLRaV-3 was extracted from leaves by PEG precipitation and purified by differential centrifugation. The concentration of the preparations was estimated by ELISA and the virus particles were visualized by electron microscopy []. DAS-ELISA of 199 samples detected Grapevine Pinot gris virus (GPGV) (53.76%) as the most frequent virus, followed by GFkV and GLRaV-1+3. Older cultivars showed the highest infection rates. GPGV was occasionally found in mixed infections and is recommended for inclusion in certification schemes [].
Ukraine: Surveys in Odesa region vineyards found GLRaV-3 as the cause of observed leafroll symptoms. GLRaV-1 was absent. Phylogenetic analysis linked Ukrainian GLRaV-3 isolates closely to Russian counterparts [].
China: GLRaV-1 was detected in 36.4% of samples, clustering into eight CP groups and seven HSP70 groups, with evidence of natural selection and recombination events. GLRaV-3 occurred in 100% of surveyed samples, segregating into five phylogenetic groups with notable genetic diversity [,].
Georgia: First confirmed in 2008, GLRaV-3, along with GLRaV-1, -2, -4, Grapevine virus B (GVB), Grapevine rupestris stem pitting-associated virus (GRSPaV), and Grapevine red blotch virus (GRBV), was widespread. Multiple virus infections were common, correlating with yield and quality losses [].
Iran: GLRaV-1+3 predominated in most regions, with GLRaV-3 considered the main causal agent of GLD [].
India: GLRaV-4 isolates showed close phylogenetic relationships with those from Israel, Turkey, and the USA. Recombination events were identified in the CP and p23 genes [].
Pakistan: Detected viruses included GLRaV-2 (37.3%), GLRaV-1 (2.3%), GLRaV-2RG (5%), GLRaV-3 (7%), and GLRaV-4 strains (16.6%), and GLRaV-7 (4.2%) [].
Turkey: Nationwide surveys found GLRaV-1 (8.36%) to be most common, followed by GLRaV-3 (5.78%), GLRaV-7 (3.86%), and GLRaV-2 (2.41%) [].
Syria: ELISA testing revealed infections in 71% of V. vinifera plants, most commonly GVA (54.7%), GLRaV-1 (47.3%), GFkV (29.7%), and GLRaV-3 (23.9%) [].
Tunisia: In the cultivar Sakasly, GLRaV-3 detection varied between ELISA and RT-PCR. The absence of known vectors in the Tunisian Sahara suggests a naturally occurring heat therapy effect [].
Algeria: GLRaV-1 was found in 5.4% of samples from central and western regions, clustered into phylogenetic groups I, II, and XVI [].
South Africa: Five GLRaV-3 genetic variant groups (I, II, III, VI, VII) have been identified, with management strategies relying on vector control and the removal of symptomatic vines [,].
Canada (Nova Scotia): GLRaV-1 (3.4%) and GLRaV-3 (22.8%) were present; GLRaV-2 and GLRaV-4 were absent [].
Chile (Atacama region): In a survey conducted from 2007 to 2009, the most prevalent virus was GLRaV-2: GLRaV-1 (8.8%), GLRaV-2 (46.8%), GLRaV-3 (9.1%), GVA (12.3%), 30.7% GFkV (307%), GFLV (9.6%) [].
USA: In Washington State, GLRaV-3 was more common than GRBV. In New York’s Finger Lakes region, 68% of vineyard blocks were virus-positive, with GLRaV-3 the most prevalent. Findings highlight the need for certified planting material [,].
Mexico: Over the past 50 years, nine grapevine viruses have been reported, including GLRaV-3 and GRBV [].
Argentina: In Mendoza, Planococcus ficus was identified as the sole mealybug species transmitting GLRaV-3, with evidence of natural spread in infested vineyards [].
Australia: GLRaV-3 isolates showed 99.5% sequence identity, with a unique ORF encoding a 20.4 kDa protein of unknown function located near the 3′-end of the genome [].
3.3. Factors Influencing Grapevine Leafroll Disease Dynamics and Host Responses
In this context, “disease dynamics” refers to the temporal and spatial variation in infection prevalence within and between vineyards, driven by interactions among the virus, host genotype, vector populations, and environmental factors.
3.3.1. Interaction Between GLRaV-3 Infection and Abiotic Stress Tolerance
The impact of abiotic and biotic stresses on plants is often interconnected, covering drought, salinity, temperature changes, or viral infections. For example, the combination of NaCl-induced salt stress and the infection with GLRaV-3 has effects on the entire physiology. This is proved by the V. vinifera cv. ‘Cabernet Sauvignon’, a GLRaV-3 infection that changed the salinity tolerance by improving osmotic and antioxidant processes. The salt stress led to higher levels of soluble sugars and proline. This led to an increased activity of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT), indicating an activation of detoxification pathways [].
Other plant–virus systems have also reported similar virus–stress interactions. In these cases, the virus, especially its genotype and strain, caused either hormonal and redox signaling growths or a reduced tolerance. The GLRaV-3 infection causes a salt tolerance related to crosstalk between abscisic acid (ABA) and stress–response signaling. This includes the SOS (Salt Overly Sensitive) pathway and MAPK cascades that regulate ion homeostasis and reactive oxygen species detoxification [,,,,,,,,]. In addition, proteomic studies support the hypothesis that GLRaV-3 alters stress networks. This is proved by the presence of proteins such as small heat shock protein class I (18.1 kDa) and MAP3K epsilon kinase [].
These findings show that the GLRaV-3 infection can reprogram stress pathways and influence the performance of vines, a fact that is important under the present environmental context. However, it is important to note that these effects depend on the genotype and further studies are needed to consolidate these findings.
3.3.2. Effectiveness of Spatial Roguing in Virus Incidence Reduction
Spatial roguing implies the removal of infected vines, as well as their neighboring rows. The impact of this spatial roguing was studied over 5 years in a field experiment from the V. vinifera cv. ‘Cabernet franc’ vineyard in the Finger Lakes region, New York. The study showed a decrease in virus incidence from 5% in 2016 to <1% by 2020–2021. This emphasizes the need to integrate roguing in the management of leafroll diseases [].
Similar results were obtained in other viticultural areas. For example, roguing strategies from California have reduced the prevalence of GLRaV-3. The impact was even higher when combined with vector suppression and using certified planting material [,]. When implementing roguing programs at early disease stages, studies from New Zealand and South Africa showed a lower infection rate and an overall extended vineyard lifespan [,]. However, roguing alone can be insufficient, especially in areas known for mealybug abundance or delayed interventions. In these cases, roguing could not halt the progression of the epidemic, highlighting once again the need to integrate more practices.
All these studies show that roguing is effective, although it depends on incidence, timing, and local considerations. The reintroduction of viruses is minimized when overwintering and early-stage mealybugs are targeted, while periodic surveys ensure a low infection.
3.3.3. Seasonal Patterns of Virus Detection and Distribution
GLRaV detection from U.S. vineyards was shown to vary seasonally, a fact that impacts both its surveillance as well as its diagnostic accuracy. As such, GLRaV-2 was detected all year-round, while GLRaV-1, GLRaV-3, GLRaV-4, and GLRaV-2RG were detected during November–February. On the other hand, viral concentrations were the lowest during early spring (April–May), a fact that can be correlated with lower metabolic activity and carbohydrate translocation []. A persistent infection that can be easily identified is GLRaV-3, which remained relatively stable across all seasons. However, the virus distribution was uneven across grapevines; GLRaV-2 was the most uniform, followed by GLRaV-1 and GLRaV-3. GLRaV-4 showed the most irregular distribution.
Similar seasonal trends were also highlighted in other studies. For example, some studies from California and Washington confirmed that late-season sampling improves the detection of GLRaV-3 and GLRaV-1, while spring samples are prone to false negatives because of low viral loads [,]. In New Zealand, Bell et al. [] showed that virus detection is most reliable in mature basal leaves in autumn. Other studies from South Africa reported GLRaV-3 concentrations being influenced by canopy position and sampling time and highlighted the need for standardized protocols [,].
The spatial heterogeneity of virus distribution is also influenced by environmental and physiological factors. A study realized by Komar et al. [] and Maree et al. [] showed that GLRaV-3 prefers the trunk and basal portions of shoots, while younger tissues show delayed infection. Furthermore, GLRaV species show different viral movements, related to their molecular interactions and the host’s vascular architecture [,].
All these studies show the importance of sampling time and tissue type in detecting viruses and making the most advantageous decisions. Early detection is improved by integrating temporal dynamics into diagnostic protocols, reducing false negatives and supporting helpful interventions like roguing. Only by understanding seasonal and spatial patterns can these monitoring strategies be integrated.
3.4. Biological and Epidemiological Properties of Grapevine Leafroll-Associated Viruses
3.4.1. Characterization of GLRaV-2-H4 Isolate
V.
The mechanical inoculation of a V. rupestris host led to a novel GLRaV-2 isolate (GLRaV-2-H4). Unlike the GLRaV-2 Semillon isolate, H4 induced necrotic local lesions in Nicotiana clevelandii and caused severe systemic symptoms in N. occidentalis. This highlights a different variant, with different host reactions and molecular features [].
GLRaV-2 is known for its genetic and biological diversity, with distinct groups (A–E) that show different host range, pathogenicity, and transmissibility [,]. Some isolates, such as GLRaV-2-Semillon and GLRaV-2-93/955, are associated with graft incompatibility syndromes, while others remain latent []. This complexity complicates diagnosis and impacts certain programs.
As such, GLRaV-2-H4 is just further proof that GLRaV is a complex species with distinct lineages and highlights the need to monitor it and supply appropriate management strategies.
3.4.2. Use of Foliar Symptoms for Field Identification of GLRaV-3
Leafroll disease is named for the distinct foliar symptoms that can be observed on sensitive grapevine cultivars from late summer and through autumn (i.e., reddening or yellowing leaves on red or white cultivars, respectively, green veins, leaves downrolling). Leafroll symptoms are expected to be evident in indicator cultivars, regardless of the GLRaV (s) involved.
In a study conducted in New Zealand, no leafroll symptoms were seen on white-berry GLRaV-3-infected cultivars, but a high variability of the leaves’ symptoms was noted in the beginning of the growing season, depending on regional vineyards, cultivars, and virus genotypes []. Earlier symptoms of the GLRaV-3-infected Pinot noir cultivar were closely correlated with reduced grapevine performance []. Unlike other GLRaVs, GLRaV-3 infects vines, which often have no symptoms or have mild symptoms []. In three vineyards established with certified grapevine material in two geographic locations in eastern Washington State, symptomatic plants were positive for GLRaV-3 only [].
The presence of symptoms and the damage induced by GLRaVs and vitiviruses are influenced by climate and they are more significant in Mediterranean climate than in cooler regions. The risk of climate change can be found in the fact that the transmission of these viruses is influenced by the increasing abundance and vector activity of these viruses [].
Leafroll-affected grapevines of red cultivars showed symptoms in the field earlier when they were stressed by deficit irrigation. In conditions of in vitro culture, the GLRaV-3 symptoms on microshoots appeared after 4–8 weeks by the addition of 4% sorbitol to induce water stress (water deficit) []. This deficit could intensify in the coming years due to climate change affecting water availability []. Efforts are necessary to ensure the sustainable management of water resources and their long-term availability [,,].
GLRaV-7 infects only grapevine and it is an asymptomatic virus, transmitted by propagation material [].
3.4.3. Seasonal Progress and Tissue Distribution of GLRaV-3 in Different Cultivars
The distribution of GLRaV-3 depends on temporal, spatial, and environmental conditions. In the case of Chardonnay, Cabernet Sauvignon, Italia, and Thompson Seedless vines, it was found that the virus moves from perennial tissues to new shoots and leaves during the early growth season. The levels remain consistent until late summer and autumn. However, the different conditions between cultivars are important to note, as Thompson Seedless showed a less uniform distribution [].
Other studies have also shown similar patterns, regardless of the climate (temperate or continental). The most common situation was the peak of GLRaV-3 between veraison and harvest, and its decline during winter. These seasonal changes impact the detection of the virus. For example, the samples collected during mid-summer (leaves, petioles) showed a consistent diagnostic, while early-spring samples showed false negatives as the viral tiers were lower [,].
The virus is also impacted by vine vigor, pruning or other environmental causes. For example, vineyards in cold climates may display limited virus translocation because of reduced sap flows. On the other hand, high temperatures can suppress viral replication in certain tissues. All these factors showcase the need to consider seasons and patterns when designing sampling strategies.
As such, it can be concluded that the diagnosis and spread of diseases in vineyard ecosystems is impacted by the vine distribution and temporal progression of GLRaV-3.
3.4.4. Transmission of Leafroll-Associated Viruses
GLRaV-1, -3, -4, and -13 are naturally transmitted to grapevine by soft scale insects (Coccidae) and mealybugs (Pseudococcidae) [,,,]. Leafroll management involves reducing vector populations, targeting the mealybugs that survived the winter and those in the second stage of development in early periods of vine development, as well as the summer generation from later in mid-summer [].
No vectors are known for GLRaV-2, which is the only leafroll virus mechanically transmissible to some herbaceous hosts, but not to grapevine []. Also, no vectors are known for GLRaV-7. This virus has been proposed as nonpathogenic for grapevine [], which is the first reported virus of Closteroviridae transmitted from grapevine to some herbaceous plant by dodder []. Of the six viruses associated with leafroll, GLRaV-3 is the most widespread virus in vineyards worldwide, and together with GLRaV-1, has the highest prevalence in Europe [].
GLRaV-2 and GPGV were detected in seedlings derived from seeds of infected grapevines [].
Vegetative propagation of grapevine is the transmission path of all viruses infecting the stock plants, and the grafting encounters viruses from both the scion and the rootstock in the newly obtained plant.
3.5. Genomic Diversity and Molecular Diagnostics of Grapevine Leafroll-Associated Viruses
Comprehensive studies on GLRaVs, members of the family Closteroviridae, reveal high genomic diversity, unique molecular features, and significant advances in detection methods. These findings have implications for taxonomy, epidemiology, and disease management in vineyard ecosystems.
GLRaV-1: The complete genome sequences of two GLRaV-1 isolates, WA-CH and WA-PN, were determined to be 18,731 nt and 18,946 nt, respectively. Both encode nine putative open reading frames (ORFs) arranged similarly to Australian and Canadian isolates. In addition to two divergent CP copies, both genomes contain CP-homologous domains in four genes, a feature unique among Closteroviridae []. Analysis of ten ORFs revealed unusually high sequence variation in ORFs 3, 6, and 7, encoding a heat shock protein 70 homolog (HSP70h) and two divergent CPs (CPd1, CPd2) [].
A sensitive immunocapture reverse transcription PCR (IC-RT-PCR) assay was developed using degenerate primers targeting the conserved HSP70h region. This method amplified a 511 bp fragment of the GLRaV-1 HSP70 gene, enabling detection with ~125-fold greater sensitivity than ELISA, and with high specificity against GLRaV-2, -3, and -4 [].
GLRaV-2: Genetic variability among GLRaV-2 isolates was investigated using CP gene phylogenetics, identifying five clades: PN, H4, RG, BD, and PV20. The RG isolate appeared more virulent, reducing rooted graft production in nurseries (especially with certain rootstocks) but not inducing leafroll symptoms. PN group variants, the most widespread, caused both graft incompatibility and leafroll symptoms []. In the Pacific Northwest (PNW), GLRaV-2 isolates were assigned to six lineages, with local populations clustering in PN, H4, and RG groups. CP amino acid sequences showed lineage-specific differences, and selection pressure analyses indicated distinct evolutionary constraints on different genomic regions []. The genetic variability of 19 isolates of GLRaV-2 from Portuguese grapevine cultivars was pointed out, showing a segregation into three major phylogroups (PN, 93/955, and H4) [].
GLRaV-3: GLRaV-3 (Ampelovirus trivitis) has one of the largest positive-sense RNA genomes among plant viruses (~18.5 kb), surpassed only by citrus tristeza virus and GLRaV-1. Distinctive features include long non-coding regions and replication associated with the outer mitochondrial membrane. Research progress has been limited by the absence of infectious cDNA clones and efficient experimental infection systems [].
Molecular interaction studies in three cultivars revealed cultivar-specific small RNA and gene expression responses, with Chenin blanc showing distinct expression patterns. Genes consistently responsive across cultivars may underlie GLRaV-3 symptom development []. Diagnostic improvements include universal primers targeting the divergent HSP70h gene (ORF4) for broad detection, alongside variant-specific primers for groups 1, 2, 6 (NZ-1), and NZ2 for multiplex RT-PCR assays []. The Chinese LN isolate genome (18,563 nt) clustered in group 3, sharing 87.99–98.15% identity with other isolates []. In Slovakia, the SK04 isolate (group 1) showed 89.4–99.5% identity with global isolates and high genetic stability after vegetative propagation []. By single-strand conformation polymorphism (SSCP), cloning, and sequencing, two major groups of divergent molecular variants of GLRaV-3 were revealed, suggesting a low molecular variability of the virus [].
In a survey performed in South Africa, five GLRaV-3 variants were detected, the variant groups II and VI being the most prominent as single infections and in combination with each other and other variants []. Then, three genetic variants of GLRaV-3 (I, II, III) were identified by single-strand conformation polymorphism (SSCP) profiles generated from a region amplified in ORF5 [,].
GLRaV-4 and related viruses: Complete sequences of GLRaV-4 and GLRaV-6 resembled subgroup I Ampelovirus members. Findings challenge current taxonomy, suggesting that several GLRaVs may be divergent GLRaV-4 isolates rather than distinct species []. Washington State isolates of GLRaV-4 strains 4, 5, and 9 had genome sizes of 13,824 nt, 13,820 nt, and 13,850 nt, respectively, with conserved 5′ and 3′ non-translated regions. Recombination events between distinct strains produced stable, epidemiologically successful chimeric viruses, indicating an important role for recombination in GLRaV-4 evolution [].
GLRaV-5, -7, and -13: Partial sequencing of GLRaV-5 revealed four ORFs (A–D) encoding an HSP70h, a 51 kDa protein similar to GLRaV-3 p55, and two CPs (one divergent). GLRaV-5, a member of the GLRaV-4LV group, shares conserved genomic features such as a highly conserved p5 gene, with phylogenies (except for p23) showing monophyly [,]. The GLRaV-7-Alb isolate genome (16,404 nt) contains nine ORFs, with ORF8 (25 kDa) and ORF9 (27 kDa) lacking homology to known viral proteins [Jelkmann]. GLRaV-13, a novel putative Ampelovirus, has a genome of 17,608 nt with eleven ORFs, showing the closest but still distant relatedness to GLRaV-1 within subgroup I [].
Advances in Molecular Diagnostics.
Improvements in RNA extraction, with quality assessment via 18S rRNA TaqMan® assays, enhanced virus detection. Real-time TaqMan® RT-PCR outperformed conventional RT-PCR for detecting GLRaVs from both purified RNA and crude extracts [].
A GLRaV-1 TaqMan® qRT-PCR assay targeting the CP region, validated to EPPO standards, demonstrated high sensitivity and specificity []. For GLRaV-2, universal primers targeting the 3′ genome end enabled broad-variant quantitative detection [].
GLRaV-3 detection was improved via IC-RT-PCR applicable to both infected tissue and viruliferous mealybugs []. High-resolution melting (HRM) analysis following real-time RT-PCR allowed simultaneous identification of GLRaV-3 variant groups I, II, III, and VI [].
A multiplex RT-PCR method for major grapevine RNA viruses (GFLV, ArMV, GLRaV-1, GLRaV-3, GFkV) was at least 10,000 times more sensitive than ELISA [].
3.6. Effects of Grapevine Leafroll Disease on Vine Physiology, Yield, and Fruit Composition
Grapevine leafroll disease (GLD) significantly alters grapevine physiology, yield, and fruit composition. The disease reduces yields, delays fruit ripening, lowers soluble solids, and increases titratable acidity in fruit juice []. Infected vines often show reduced foliar sugar accumulation correlated with decreased photosynthesis, likely due to feedback inhibition, while cold acclimation of dormant tissues remains unaffected, suggesting minimal impact on plant cold hardiness []. However, no quantitative relationship between viral load and berry composition of Crimson Seedless with mixed infections (GLRaV-3, GLRaV-4 strain 9, and grapevine virus A) was found [].
Infections with grapevine leafroll-associated virus 1 (GLRaV-1) markedly decrease leaf net photosynthetic rate (Pn), stomatal conductance (gs), and transpiration rate (E), as well as pigments, soluble proteins, ribulose-1,5-bisphosphate carboxylase (RuBPC), and nitrate reductase activity. In isolated thylakoids, GLRaV-1 strongly inhibits whole-chain and photosystem II (PSII) activity, while photosystem I (PSI) activity is only marginally affected [].
Symptom severity is cultivar-dependent; in white grape varieties, symptoms tend to be milder than in red ones, and the disease is often not considered economically significant if yields are stable. However, in Rías Baixas (Spain), Albariño grapes infected with GLRaV-3 exhibited a mean potential alcoholic degree (PAD) 1° lower than virus-free counterparts. Similarly, in a young experimental vineyard, GLRaV-3-infected plants had must sugar contents 1° Brix lower than healthy vines, along with higher titratable acidity and lower pH, especially under adverse weather. Although early vine development appeared unaffected, symptomatic leaves exhibited reduced net photosynthesis, potentially leading to long-term yield declines [].
When GLRaV-3 infection coincided with moderate water deficit, photosynthesis (AN) decreased in both studied cultivars. Water stress also caused stomatal closure, reduced mesophyll conductance (gm), and lowered maximum carboxylation velocity (Vcmax) and electron transport rate (Jmax) []. GLRaV-3 alone reduced leaf net photosynthesis, stomatal conductance, and transpiration before and after symptom onset. Reductions in PSII quantum efficiency and pigment content appeared only after symptoms developed. Infection inhibited maximum carboxylation efficiency, electron transport rate, and triose phosphate use by ~30%. Yield and soluble solids per vine declined by 40% and 43%, respectively, alongside reductions in shoot number, shoot growth, leaf area, internode length, vine size, and cane lignification [].
In Sauvignon blanc from New Zealand, GLRaV-3 slowed ripening but had minimal effects on juice and wine chemistry at equivalent ripeness. Pre-2008 infections reduced titratable acidity relative to uninfected controls, while amino acid changes did not alter basic wine parameters. Physiological effects included reduced CO2 assimilation (AN) and electron transport rate (Jflux), driven by lower CO2 diffusion from anatomical leaf changes and reduced Rubisco activity. Even asymptomatic plants showed compromised physiological processes, underscoring the importance of early detection [].
Mixed infections with GLRaV-1 and GLRaV-3 in ‘Touriga Nacional’ reduced light-saturated CO2 fixation and carboxylation efficiency, while paradoxically increasing apparent quantum yield. Yield losses were primarily due to reduced cluster weight. In Cabernet Sauvignon, GLRaV-3 altered berry gene expression patterns, delaying and incompletely achieving maturation. Infected berries showed reduced transcription of sugar and anthocyanin biosynthesis genes, consistent with lower sugar levels and markedly reduced anthocyanin accumulation—hallmarks of GLD [].
Figure 3 presents a schematic review of the results from the literature review.
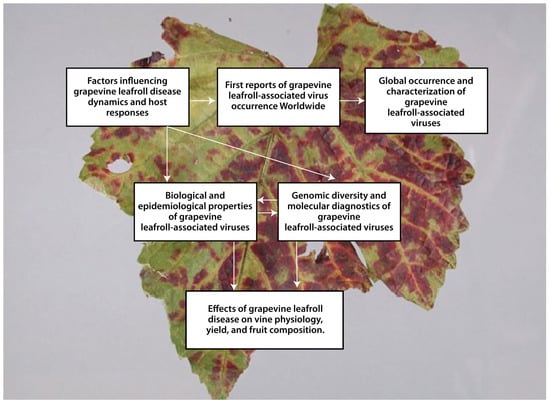
Figure 3.
Schematic overview of our results.
4. Discussion
4.1. First Reports of Grapevine Leafroll-Associated Virus Occurrence Worldwide
The first documented records of GLRaVs across diverse viticultural regions underscore both the complexity and global scale of leafroll disease emergence. The spatial pattern of detection—from major viticulture areas such as Italy, Chile, and California to less traditionally associated regions including Nigeria, India, and Morocco—suggests that GLRaVs are not constrained by geography, climate, or level of viticultural development [,,]. Instead, their occurrence appears closely tied to the movement of infected propagation material and the presence of efficient vector species [,].
The high frequency of GLRaV-3 in initial detection reports is consistent with its well-established global prevalence, broad host range within V. vinifera, and its capacity for efficient transmission by multiple mealybug and scale insect vectors [,,]. GLRaV-3 remains the most widely distributed species, with confirmed presence across Europe, Asia, Africa, Oceania, and the Americas [,,]. The appearance of GLRaV-1 and GLRaV-2 in both established winegrowing nations and in emerging markets indicates that these viruses may have been historically overlooked due to diagnostic limitations, particularly in regions where vineyard virus testing has only recently become systematic [,]. This raises the possibility that the currently termed “first reports” in some countries may represent the first molecular confirmations of infections that have been present for decades.
The detection of GLRaVs in non-vinifera grape species, such as V. rotundifolia, V. aestivalis, and V. labruscana, is of particular epidemiological concern [,]. These wild and hybrid grape species may serve as long-term reservoirs, facilitating viral persistence in ecosystems even in the absence of commercial vineyards. Such reservoirs may contribute to recurrent outbreaks despite vineyard-level sanitation measures, especially if native vector species are capable of transmitting the viruses between cultivated and wild hosts [,].
The broad geographical scope of these first reports also raises questions about viral strain diversity and possible adaptation to local vector species or grapevine genotypes. Introductions of GLRaVs into geographically isolated viticultural areas could lead to unique evolutionary trajectories, potentially altering virulence, transmission efficiency, or host range []. This underlines the importance of integrating molecular epidemiology and phylogenetic tools into global monitoring efforts to detect such evolutionary shifts [].
It was observed that the pattern of detections across multiple continents within relatively short timeframes strongly suggests that the globalization of plant material trade has outpaced the implementation of harmonized phytosanitary protocols. The spread of these viruses across national and continental boundaries highlights an urgent need for stricter certification programs, traceability of nursery stock, and international cooperation in disease surveillance [,,].
It can be said that these first reports are not merely historical records but early-warning indicators of the potential for further viral spread. The establishment of GLRaVs in new viticultural regions carries ecological implications for vineyard biodiversity, pest–pathogen dynamics, and long-term grapevine productivity. Without coordinated, science-based interventions, the current pattern of detections may presage a more widespread and entrenched global presence of grapevine leafroll disease.
4.2. Global Occurrence and Characterization of Grapevine Leafroll-Associated Viruses
The worldwide picture that emerges from recent surveys and molecular analyses of GLRaVs is anything but uniform. Patterns of prevalence, genetic diversity, and management responses vary markedly between viticultural regions, shaped by local histories of grape cultivation, plant material exchange, and vector dynamics.
4.2.1. Regional Patterns and Epidemiological Contexts
In parts of the Mediterranean and Balkans—such as Croatia, Bosnia and Herzegovina, and Montenegro—GLRaV-3 often reaches alarming incidence levels, sometimes infecting over 80% of vines [,]. Mixed infections with GLRaV-1, GFLV, or GFkV are common, reflecting the combined effects of uncertified propagation material and the persistence of mealybug and scale insect vectors over decades. Comparably high levels have been reported from Georgia [], Iran [], and South Africa [], where vector populations are abundant and long-established.
By contrast, some regions appear to have kept the disease largely in check. In southwestern Germany, for example, GLRaV-3 remains rare (<0.1% prevalence), even after more than a decade of monitoring []. Similarly, certain Canadian vineyards report only sporadic infections, suggesting that colder climates, combined with effective vector management and sanitation, can slow virus spread. Tunisia’s Saharan vineyards are a particularly interesting case: the absence of known vectors and extreme summer heat may act as a natural form of “heat therapy” against GLRaVs [].
Other countries exhibit patterns shaped by more recent introductions or restricted virus diversity. In Ukraine’s Odesa region, only GLRaV-3 has been detected so far [], while China presents an entirely different picture—GLRaV-3 is ubiquitous, and GLRaV-1, -2, and -4 occur at lower but still significant levels, with multiple phylogenetic groups coexisting [,]. In the United States, regional contrasts are stark; high infection rates in New York’s Finger Lakes [] stand in sharp contrast to more moderate levels in Washington State [], pointing to the role of local management practices and certification standards.
Synthesizing across regions, several key factors appear to drive these geographic differences in GLRaV occurrence and transmission:
- (1)
- Vector ecology plays a dominant role. The abundance and species composition of mealybugs and soft scales strongly correlate with disease prevalence, with warmer climates favoring rapid population growth and year-round transmission cycles.
- (2)
- Propagation practices are a second determinant. Regions relying heavily on uncertified or locally propagated plant material tend to accumulate mixed infections over time.
- (3)
- Climatic constraints, particularly cold winters or high summer temperatures, can suppress both vectors and virus survival, as seen in Canada and Tunisia.
- (4)
- Regulatory and management frameworks, including certification schemes, monitoring programs, and coordinated vine removal, further modulate transmission dynamics, as illustrated by the contrasting outcomes between New York and Washington State.
These insights imply that effective prevention and control strategies must be region-specific. In vector-dense, warm-climate viticultural zones, integrated pest management targeting mealybugs and scales remains crucial, whereas in cooler or arid regions, maintaining strict certification and sanitation standards can prevent reintroduction. Tailoring control approaches to the interplay between environmental conditions, vector biology, and propagation pathways is therefore essential to interrupt the ecological feedback loops that sustain grapevine leafroll disease at regional scales.
The geographical distribution of the studies conducted is closely related to the distribution of the number of articles per year. Our analysis shows that this distribution, examined in five-year intervals over the period 1970–2025, displays a statistically significant variation (Wald statistical test, p < 0.005). Furthermore, when assessing the variability in the number of articles across these five-year intervals during 1970–2025 using ANOVA and Likelihood Type 1 Test methods, the results indicate rejection of the null hypothesis (chi-square = 129.7421, p < 0.005). The trend in the number of documents published over this period (1970–2025) is clearly increasing, as confirmed by the results of the SS Whole Model vs. SS Residual test, which yielded a multiple R-square value of 0.697426 and a p-value of 0.011692. These findings once again demonstrate the growing interest of researchers in this topic.
4.2.2. Genetic Diversity and Its Implications
On a global scale, GLRaV-3 stands out not only for its ubiquity but for the breadth of its genetic variation. In South Africa alone, at least five genetic groups (I, II, III, VI, VII) have been documented (Allsopp et al.), while in China and Croatia, multiple groups are found side by side in the same vineyard [,]. This diversity is likely driven by repeated introductions via plant material, vegetative propagation of infected stock, and active vector transmission.
Other GLRaVs reveal equally intriguing patterns. GLRaV-1 in China forms eight distinct CP gene groups, with recombination events shaping their evolution []. GLRaV-4 isolates from India display evidence of both inter- and intraspecies recombination in CP and p23 genes [], while GLRaV-5 in Portugal clusters into eight lineages that appear to have diversified without vector involvement, likely through clonal propagation []. In Slovenia, the N-terminal CP region of a GLRaV-4 variant proved so variable that it was unsuitable for routine molecular detection [], underscoring the risk of false negatives if diagnostic assays are not regularly updated.
4.2.3. Management Challenges and Strategies
The uneven distribution of GLRaVs and their genetic complexity directly shape management strategies. In high-incidence areas with active vectors—such as South Africa, Georgia, and parts of Argentina—integrated programs combining vector suppression, removal of symptomatic vines, and strict use of certified planting material are indispensable [,,]. Conversely, in environments where vectors are scarce or absent, as in parts of Tunisia, vigilance is still needed to prevent accidental introductions, especially given the mobility of nursery stock [].
However, the effectiveness and sustainability of these measures vary markedly among viticultural regions. Studies assessing integrated control programs in South Africa and New Zealand have shown that vector suppression and roguing can substantially reduce incidence over several years, yet long-term success depends on continued monitoring and replanting with virus-free stock—both of which are resource-intensive and often constrained by limited funding and labor availability []. In lower-income wine-producing regions, such as parts of Eastern Europe and South America, the cost of large-scale vine replacement and certification compliance can hinder sustained implementation, highlighting the need for cost-sharing mechanisms or policy incentives to support growers [,].
Long-term persistence of low-level infections in regions like Germany shows that even modest vector activity and occasional planting of infected vines can maintain the disease []. Areas with high viral diversity face additional hurdles; variant-specific detection failures may allow certain strains to circulate undetected. Deploying broad-spectrum molecular assays and maintaining updated sequence reference databases that capture the diversity present in each viticultural zone can mitigate this issue, but such approaches require continual investment in diagnostic infrastructure and skilled personnel.
Alo, virus elimination techniques—such as thermotherapy or meristem culture—are effective at generating virus-free material, yet their practical adoption depends on technical capacity and economic feasibility. In countries with strong certification systems (e.g., France, the U.S.), these methods are routinely integrated into propagation schemes, while in other regions, limited infrastructure or weak regulatory enforcement reduces their impact [,].
Perhaps most crucially, the absence or weakness of certification schemes in parts of Eastern Europe, Asia, and the Americas continues to drive virus persistence. As several authors have argued [,], coordinated global action is needed: harmonized certification protocols, routine surveillance, and integration of molecular epidemiology into vineyard health policies. Such harmonization should also account for economic and regional feasibility to ensure long-term sustainability. Only with these measures—supported by policy frameworks that encourage equitable participation—can control efforts keep pace with the biological and ecological adaptability of GLRaVs.
4.3. Factors Influencing Grapevine Leafroll Disease Dynamics and Host Responses
Given the global impact and distribution of leafroll disease, efforts have been made to manage the spread of GLRaV, characterize its effects, and understand the interaction between grapevine and GLRaV.
4.3.1. Interaction Between GLRaV-3 Infection and Salt Stress Tolerance
Plants grown in the field are simultaneously subjected to different abiotic and biotic stress factors (drought, climatic changes, salinity, cold, pathogen infection). The response of plants to several combined stress factors is much more complex than that to a single stress factor; the signaling pathways, being different, can interact and inhibit each other, resulting in increased tolerance or weakening of plant defenses [,,,,,,].
The transduction of salt and drought stress signals consists of ionic and osmotic homeostasis signaling pathways and detoxification. The ionic aspect of salt stress is signaled via SOS pathway, where a protein kinase complex controls the expression and activity of ion transporters such as SOS1. Osmotic stress activates several protein kinases as mitogen-activated kinases, which can mediate the osmotic homeostasis and/or the responses of detoxification. []. In a study on protein–protein interactions between GLRaV-3 and the host plant, two proteins (a small heat shock protein class I of 18.1 kDa and the MAP3K epsilon protein kinase 1) involved in plant responses to various stress factors, including pathogen infections, were identified [].
4.3.2. Effectiveness of Spatial Roguing in Virus Incidence Reduction
This spatial roguing method involves removing the visibly infected grapevine along with its immediate neighbors within the same row, even if they do not show symptoms, to prevent the virus spreading.
In red-berried cultivars, leaf discoloration associated with GLRaV-3 infection proved to be a reliable indicator for visual identification of infected vines. For these cultivars, visual symptom assessment is recommended as a cost-effective alternative to ELISA tests during roguing programs aimed at GLRaV-3 management [].
The damages produced by GLRaV-3 were reduced when the growers used certified planting material and also then rogued and replanted with certified stock [].
4.3.3. Seasonal Patterns of Virus Detection and Distribution
GLRaVs are detectable throughout the year, despite the fact that the symptoms on the leaves are visible starting from the onset of berry maturation or veraison and show symptoms until they fall []. GLRaVs are phloem-limited viruses and, as a result, they lead to the symptoms affecting frequently phloem tissue, veins, and cambium, resembling nutritional deficiencies. Also, their association with the phloem may be responsible for the low concentration of the closteroviruses in infected plants, making their detection difficult []. In order to obtain virus-free planting material, reliable virus diagnostic methods are needed [].
4.4. Biological and Epidemiological Properties of Grapevine Leafroll-Associated Viruses
The role of GLRaVs in disease etiology is not yet completely clarified [,].
4.4.1. Characterization of GLRaV-2-H4 Isolate
GLRaV-2-H4 is a biological variant of GLRa V-2. Having a high molecular heterogeneity and a broad range of biological properties, the evaluation of the genetic variability in a specific vine-growing region is necessary for a reliable detection of all the variants of GLRaV-2 []. Two strains of GLRaV-2 (named 94/970 and 93/955) were obtained by mechanical transmission from grapevines to Nicotiana benthamiana. They were different with regard to the development of symptoms, but indistinguishable from the point of view of the molecular weight of their capsid proteins or serologically. However, a difference in the pattern of minor dsRNA bands was observed []. A strain of GLRaV-2, consistently associated with Kober 5BB incompatibility in Europe, seems to be involved in the decline of young grapevines in California, and it has been detected in affected grapevines in Chile and Argentina. Other molecular variants of GLRaV-2 have been reported in New Zealand, Chile, and Australia in association with conditions of young grapevine decline. Based on the differential responses of a group of 18 rootstocks, up to 5 different graft-transmitted incompatibility-inducing agents have been detected in California [].
4.4.2. Use of Foliar Symptoms for Field Identification of GLRaV-3
Visual observation of the grapevine was the first method of detecting leafroll disease. In general, V. vinifera grapevine cultivars are susceptible to GLRaVs infections and present disease symptoms, whereas some rootstocks and certain white V. vinifera cultivar are symptomless or have latent infections.
In recent years, hyperspectral imaging has begun to be used in order to improve optical-based diagnosis. The latent phase, when GLRaV-3-infected grapevine plants do not show symptoms, offers the possibility to evaluate the scalability of imaging spectroscopy-based disease detection []. Ground-based hyperspectral imaging was used to identify white and red grapevine plants infected with GLRaV-1 or -3. The models of disease detection have been developed for greenhouse and field grapevine of white and red cultivars. Also, the differentiation of asymptomatic plants from healthy ones was successful [].
4.4.3. Seasonal Progress and Distribution of GLRaV-3 in Different Cultivars
The first report regarding the seasonal dynamics and distribution in grapevine tissues of GLRaV-2 and GLRaV-3 in cold climates has been realized by symptom monitoring, Western blotting, and RT-qPCR, on Cabernet Franc and Chardonnay varieties over two consecutive years (2015 and 2016), during May–October. For a reliable diagnostic of these GLRaVs, the recommendation at the end of the study was the use of young berries early in the season and leaves and cambial scrapings from late July to harvest [].
4.5. Genomic Diversity and Molecular Diagnostics of Grapevine Leafroll-Associated Viruses
The genomic and diagnostic datasets available for the various GLRaVs confirm just how heterogeneous this group of closteroviruses really is, both at the sequence level and in their biological behavior. Over the past two decades, complete or near-complete genome sequences have been published for several representatives, including GLRaV-1 [], GLRaV-2 [], GLRaV-3 [], GLRaV-5 [], and GLRaV-9 []. These datasets reveal notable differences in genome size, gene complement, and ORF arrangement. GLRaV-1 and GLRaV-3, for instance, both fall within the upper size range for plant RNA viruses (~18.5–18.9 kb), yet each carries a suite of unusual features—among them multiple, divergent CP genes and CP-homologous domains embedded within non-structural proteins. Such redundancy in structural protein coding regions is rare and may reflect adaptive advantages in vector transmission or in navigating the diverse cellular environments of different V. genotypes.
A study regarding the isolates of GLRaV-3 from Galicia (Spain) genetically characterized by the function of the various factors (age, origin, location, variety) suggested that the uncontrolled exchange of infected grapevine has been a major agent of virus spread, and it did not support isolation of the GLRaV-3 isolates [].
Within species, the degree of variation is equally striking. GLRaV-1 shows pronounced polymorphism in ORFs 3, 6, and 7, whereas GLRaV-2 isolates segregate into multiple well-supported clades with distinct phenotypic profiles. The discovery of intra-species recombination within the GLRaV-4 complex, producing stable hybrid genomes that persist in the field [], reinforces the view that recombination is not an occasional accident but an active driver of closterovirus evolution. Interestingly, the apparent genomic stability of certain GLRaV-3 genotypes, such as the Slovak SK04 variant [], contrasts with the more fluid recombination landscape of GLRaV-4, hinting at species- or lineage-specific evolutionary constraints.
From a taxonomic standpoint, these findings are problematic. The close genetic relationships between GLRaV-4, GLRaV-6, and other “species” currently treated as discrete challenge the robustness of existing demarcation criteria. If such taxa are re-delimited, there will be downstream consequences for naming conventions, diagnostic assay targets, and even phytosanitary regulation.
Host responses add another layer of complexity. Transcriptomic studies with GLRaV-3 show that symptom expression is not simply a matter of virus presence; it is strongly modulated by the host genotype. Chenin blanc, for example, mounts a gene expression profile distinct from other cultivars under the same infection pressure []. Similarly, the GLRaV-2 RG clade can cause severe graft incompatibility in nurseries while remaining symptomless in the field [], making visual inspections an unreliable indicator of impact.
The diagnostic implications are obvious. Sequence divergence in key regions such as CP and HSP70h can easily lead to false negatives when primer design is based on a narrow set of reference sequences. Advances such as universal primers for GLRaV-3 [] and GLRaV-2 [], highly sensitive TaqMan® qRT-PCR for GLRaV-1 [], and multiplex RT-PCR capable of detecting multiple viruses in a single reaction [] represent important steps forward. That said, assay sensitivity is only as good as the RNA template fed into the reaction. The use of improved extraction protocols and internal controls (e.g., 18S rRNA quantification; Osman et al., [] is an underappreciated but critical part of the workflow. The extension of these assays to insect vectors, as demonstrated with immunocapture RT-PCR in mealybugs [], opens the door to integrated plant–vector surveillance.
From a management perspective, the take-home message is that genomic surveillance and diagnostic sensitivity must evolve in parallel. Assays need regular updating to track a moving genetic target, and disease control strategies should not rely solely on visible symptomatology. The recognition of “silent” but economically damaging infections—such as those caused by GLRaV-2 RG variants—makes a strong case for mandatory molecular testing in propagation material. Moreover, the taxonomic ambiguities in the GLRaV-4 complex are not merely academic; they will affect quarantine lists, certification schemes, and the design of monitoring programs.
In sum, the GLRaV landscape is highly dynamic, shaped by mutation, recombination, host–virus interplay, and human-mediated movement of plant material, and has many methods and outcomes (Table 2). Continued investment in high-resolution genomics, coupled with adaptable and well-validated diagnostics, will be essential if pace is to be kept with this moving target and productive, healthy vineyard ecosystems are to be maintained.

Table 2.
Methods used and outcomes for genomic diversity assessments of various GLRaVs.
4.6. Effects of Grapevine Leafroll Disease on Vine Physiology, Yield, and Fruit Composition
The studies reviewed here converge on a consistent picture: grapevine leafroll disease (GLD), particularly when caused by GLRaV-1, GLRaV-3, is not merely a cosmetic problem of reddened leaves but a physiological disruptor with both immediate and potentially long-term consequences for vineyard productivity.
Leafroll negatively affects rooting ability, graft attachment, plant vigor, photosynthesis, and the modulation of host genes involved in a variety of biological functions [].
The most robustly documented effect is a reduction in photosynthetic capacity, which appears early in infection and persists throughout the growing season. Reductions in net photosynthesis (Pn or AN), stomatal conductance, and transpiration have been reported across cultivars and growing conditions [,,,]. The mechanisms are multifaceted: direct impacts on photosynthetic electron transport, declines in pigments and Rubisco activity, and anatomical changes in leaves that restrict CO2 diffusion [,]. Interestingly, inhibition of photosystem II (PSII) activity is consistently more pronounced than that of photosystem I [], and feedback inhibition from reduced sink demand—manifested as foliar sugar accumulation—may further depress photosynthesis [].
Physiological stress is not limited to photosynthesis. GLRaV-3 also affects enzymatic activities central to nitrogen assimilation [] and alters the balance between growth and maturation processes. Reduced shoot number, internode length, and cane lignification [] suggest that infections may have carry-over effects on perennial structure and thus future productivity, even if early vine vigor appears unaffected []. This cumulative perspective is important; in young vineyards, yield loss may be negligible at first, but physiological constraints can compound over seasons.
From a viticultural standpoint, the most visible impacts for growers and winemakers are delayed ripening, reduced sugar accumulation, and altered acidity profiles [,]. These effects, although variable by cultivar and environment, can be economically relevant. In Albariño and other cultivars [], a 1° Brix or PAD reduction is common, and titratable acidity tends to be elevated. These changes directly affect harvest timing decisions and, for some styles of wine, may compromise target alcohol levels or flavor balance.
The variability in economic impact between red and white cultivars deserves attention. While symptoms are typically milder in white varieties, this does not necessarily mean the absence of significant physiological effects. For example, in Sauvignon blanc, GLRaV-3 slowed ripening, yet at equivalent ripeness, the juice and wine chemistry differences were minimal []. This suggests that in some white cultivars, disease management decisions may hinge more on vineyard scheduling and harvest logistics than on direct quality loss—although asymptomatic infections still compromise carbon assimilation and warrant early detection [].
The interaction between GLRaV-3 infection and water deficit is particularly revealing. Moderate drought intensified reductions in photosynthetic parameters such as Vcmax and Jmax [], pointing to additive or even synergistic stress effects. In regions where water scarcity is becoming more common, such combined stressors may accelerate the decline in vine performance.
At the molecular level, changes in gene expression underpin the visible delays in berry maturation. In Cabernet Sauvignon, GLRaV-3 downregulated key genes for sugar and anthocyanin biosynthesis [], providing a mechanistic explanation for both the reduced sweetness and diminished pigmentation characteristic of infected fruit. This is consistent with earlier observations of altered sink strength and carbohydrate partitioning.
Mixed infections add another layer of complexity. The combined presence of GLRaV-1 and GLRaV-3 in ‘Touriga Nacional’ not only depressed photosynthetic performance but also reduced yield primarily through smaller cluster weights []. The increase in apparent quantum yield observed in this study is intriguing, possibly reflecting compensatory adjustments in photosynthetic efficiency under chronic stress, but this warrants further investigation.
GLRaV-3-infected grapevine showed reduced symptoms of late downy mildew (Plasmopara viticola fungus) in the field, this low susceptibility to fungus disease being partially explained by some modifications induced by the presence of the virus [].
Overall, these data highlight that GLD is a physiologically active disease with broad consequences—reducing carbon assimilation, altering vine architecture, shifting fruit composition, and, in some contexts, diminishing wine quality. The degree of impact depends on cultivar, virus strain (s), environmental conditions, and the presence of other stresses. These nuances reinforce the need for site- and cultivar-specific management strategies. While some vineyards may tolerate infection without immediate yield loss, the underlying physiological changes suggest that long-term productivity and fruit quality are at risk.
4.6.1. Gaps and Limitations
Despite significant advances in understanding GLD and its associated viruses (GLRaVs), several knowledge gaps and limitations remain:
- Incomplete understanding of disease etiology: The exact role of individual GLRaVs in symptom development and yield loss is not fully elucidated, particularly in cases of mixed infections or latent infections where symptoms are absent or mild.
- Regional biases in research: Most studies have been conducted in well-established viticulture regions (Europe, North America, South Africa, China), while data from emerging wine-growing regions (e.g., Africa beyond South Africa, Central Asia, parts of South America) remain limited.
- Variability in detection methods: Although molecular diagnostics have advanced, inconsistencies remain in assay sensitivity and specificity, especially for genetically diverse or newly emerging GLRaV variants.
- Limited ecological perspective: While physiological and yield impacts are documented, the ecological consequences of GLRaVs on vineyard ecosystems—such as interactions with microbial communities, insect vectors, or wild V. species—are underexplored.
- Management challenges: Existing control strategies (use of certified material, vector control, roguing) are not universally effective, and the long-term sustainability of these measures under changing climate and globalized plant trade is uncertain.
4.6.2. Future Research Directions
To address the above gaps, future research should prioritize the following:
- Clarifying virus–host interactions: Multi-omics approaches (genomics, transcriptomics, metabolomics) should be used to dissect host responses to single and mixed GLRaV infections, linking molecular changes to physiological and agronomic outcomes.
- Expanding geographical coverage: Systematic surveys and molecular characterization of GLRaVs in underrepresented viticultural areas are needed to capture global diversity and track viral spread.
- Improving diagnostics: Development of broad-spectrum, multiplex, and portable diagnostic tools (e.g., CRISPR-based assays, nanopore sequencing) could enhance early and field-level detection, including in vectors.
- Understanding ecological impacts: Studies should investigate how GLRaV infections affect vineyard biodiversity, soil microbiota, vector dynamics, and potential virus reservoirs in wild grapevine populations.
- Innovative management strategies: Exploration of novel approaches such as RNA interference (RNAi), biological control of vectors, or breeding for virus-resistant/tolerant grapevine cultivars could provide more sustainable solutions.
- Socioeconomic assessments: Economic studies should quantify the cost–benefit of different control strategies across diverse production systems, helping growers adopt regionally adapted management approaches.
5. Conclusions
Grapevine leafroll disease continues to represent a complex challenge for viticulture worldwide. The accumulated evidence confirms that GLRaVs impair photosynthesis, reduce vine vigor, alter fruit composition, and compromise long-term vineyard sustainability. However, beyond these physiological and yield impacts, their ecological effects on vineyard biodiversity, soil microbial communities, insect vector dynamics, and potential reservoirs in wild V. species are insufficiently studied. Current management practices—certified propagation material, vector control, and roguing—remain only partially effective, particularly under the pressures of climate change and globalized plant movement. Advancing sustainable solutions will require integrating molecular epidemiology, ecological monitoring, and socioeconomic assessments, alongside the development of novel approaches such as RNA interference, resistant cultivars, and biological vector control. Ultimately, a coordinated, multidisciplinary framework is needed to balance production goals with ecological resilience.
In the short term, research should prioritize mapping virus–vector–host interactions under diverse vineyard ecosystems to identify key transmission pathways, characterizing the ecological impacts of GLD on soil microbiota and beneficial insect communities through metagenomic and field-based approaches, and assessing the effectiveness of existing management practices under varying climatic conditions to refine region-specific control strategies.
In the medium term, research efforts should focus on developing and field-testing virus-resistant or tolerant grapevine cultivars through advanced breeding and biotechnological approaches, evaluating the potential of RNA interference and biological control agents for vector suppression in integrated pest management frameworks, and establishing long-term ecological monitoring networks to assess the broader ecosystem consequences of GLD and management interventions.
By setting these research priorities, the field can move toward a more holistic and evidence-based understanding of GLD as both a viticultural and ecological challenge.
Author Contributions
Conceptualization, E.-C.B. and L.D.; methodology, E.-C.B. and L.D.; software, L.D. and G.M.; validation, I.-C.G. and D.-E.V.; formal analysis, I.-C.G. and D.-E.V.; investigation, I.-C.G. and D.-E.V.; resources, I.-C.G. and D.-E.V.; data curation, I.-C.G. and D.-E.V.; writing—original draft preparation, E.-C.B. and L.D.; writing—review and editing, E.-C.B. and L.D.; visualization, L.D. and G.M.; supervision: L.D. and G.M.; project administration, E.-C.B. and L.D.; funding acquisition, L.D. All authors have read and agreed to the published version of the manuscript.
Funding
The work of Gabriel Murariu was supported by a grant from the Ministry of Research, Innovation and Digitization, CNCS/CCCDI-UEFISCDI, project number PN-IV-P8-8.1-PRE-HE-ORG-2024-0212, within PNCDI IV.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Martelli, G.P.; Boudon-Padieu, E. (Eds.) Options Méditerranéennes. Serie B: Studies and Research; CIHEAM (Centre International de Hautes Etudes Agronomiques Méditerranéen): Bari, Italy, 2006; Volume 55, ISSN 1016-1228. [Google Scholar]
- Boudon-Padieu, É.; Maixner, M. Potential effects of climate change on distribution and activity of insect vectors of grapevine pathogens. In Proceedings of the International and Multi-Disciplinary “Global Warming, Which Potential Impacts on the Vineyards?”, Beaune, France, 28–30 March 2007; p. 23. [Google Scholar]
- Fuchs, M.; Martinson, T.E.; Loeb, G.M.; Hoch, H.C. Survey for the three major leafroll disease-associated viruses in Finger Lakes vineyards in New York. Plant Dis. 2009, 93, 395–401. [Google Scholar] [CrossRef]
- Atallah, S.S.; Gómez, M.I.; Conrad, J.M.; Nyrop, J.P. A plant-level, spatial, bioeconomic model of plant disease diffusion and control: Grapevine leafroll disease. Am. J. Agric. Econ. 2015, 97, 199–218. [Google Scholar] [CrossRef]
- Mannini, F.; Digiaro, M. The effects of viruses and viral diseases on grapes and wine. In Grapevine Viruses: Molecular Biology, Diagnostics and Management; Meng, B., Martelli, G., Golino, D., Fuchs, M., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Alabi, O.J.; Casassa, L.F.; Gutha, L.R.; Larsen, R.C.; Henick-Kling, T.; Harbertson, J.F.; Naidu, R.A. Impacts of Grapevine leafroll disease on fruit yield and grape and wine chemistry in a wine grape (Vitis vinifera L.) cultivar. PLoS ONE 2016, 11, e0149666. [Google Scholar] [CrossRef] [PubMed]
- Naidu, R.A.; Rowhani, A.; Fuchs, M.; Golino, D.; Martelli, G.P. Grapevine leafroll: A complex viral disease affecting a high-value fruit crop. Plant Dis. 2014, 98, 1172–1185. [Google Scholar] [CrossRef]
- Constable, F.E.; Connellan, J.; Nicholas, P.; Rodoni, B.C. The reliability of woody indexing for detection of grapevine virus-associated diseases in three different climatic conditions in Australia. Aust. J. Grape Wine Res. 2012, 19, 74–80. [Google Scholar] [CrossRef]
- Cabaleiro, C.; Segura, A.; García-Berrios, J.J. Effects of Grapevine leafroll-associated virus 3 on the physiology and must of Vitis vinifera L. cv. Albariño following contamination in the field. Am. J. Enol. Vitic. 1999, 50, 40–44. [Google Scholar] [CrossRef]
- Reynard, J.S.; Brodard, J.; Zufferey, V.; Rienth, M.; Gugerli, P.; Schumpp, O.; Blouin, A.G. Nuances of Responses to Two Sources of Grapevine Leafroll Disease on Pinot Noir Grown in the Field for 17 Years. Viruses 2022, 14, 1333. [Google Scholar] [CrossRef]
- Cui, Z.-H.; Bi, W.-L.; Pan, C.; Xu, Y.; Wang, Q.-C. Abiotic stress improves in vitro biological indexing of Grapevine leafroll-associated virus-3 in red grapevine cultivars. Aust. J. Grape Wine Res. 2015, 21, 490–495. [Google Scholar] [CrossRef]
- Mannini, F.; Mollo, A.; Credi, R. Field performance and wine quality modification in a clone of Vitis vinifera cv. Dolcetto after GLRaV-3 elimination. Am. J. Enol. Vitic. 2012, 63, 144–147. [Google Scholar] [CrossRef]
- Fuchs, M. Grapevine viruses: A multitude of diverse species with simple but overall poorly adopted management solutions in the vineyard. J. Plant Pathol. 2020, 102, 643–653. [Google Scholar] [CrossRef]
- Prajapati, M.R.; Gupta, N.; Shimray, M.; Gehlot, J.; Tiwari, A.; Thapa, P.; Diksha, D.; Holkar, S.K.; Mahajan, P.J.; Saha, S.; et al. Genome characterization of a newly discovered grapevine leafroll-associated virus S, in the genus ampelovirus by high-throughput sequencing. J. Genet. Eng. Biotechnol. 2025, 23, 100494. [Google Scholar] [CrossRef]
- Sharma, A.M.; Wang, J.; Duffy, S.; Zhang, S.; Wong, M.K.; Rashed, A.; Cooper, M.L.; Daane, K.M.; Almeida, R.P. Occurrence of grapevine leafroll-associated virus complex in Napa Valley. PLoS ONE 2011, 6, e26227. [Google Scholar] [CrossRef]
- Sultanova, N.; Rastgou, M.; Huseynova, I. Occurrence of Single and Mixed Viral Infections of Grapevine (Vitis Spp.) in Azerbaijan. Pol. J. Environ. Stud. 2024, 33, 4345–4353. [Google Scholar] [CrossRef]
- Schoelz, J.; Volenberg, D.; Adhab, M.; Fang, Z.; Klassen, V.; Cpinka, V.; Al Rwahnih, M. A Survey of Viruses Found in Grapevine Cultivars Grown in Missouri. Am. J. Enol. Vitic. 2021, 72, 73–84. [Google Scholar] [CrossRef]
- Hančević, K.; Čarija, M.; Radić Brkanac, S.; Gaši, E.; Likar, M.; Zdunić, G.; Regvar, M.; Radić, T. Grapevine Leafroll-Associated Virus 3 in Single and Mixed Infections Triggers Changes in the Oxidative Balance of Four Grapevine Varieties. Int. J. Mol. Sci. 2022, 24, 8. [Google Scholar] [CrossRef] [PubMed]
- Naidu, R.A.; Maree, H.J.; Burger, J.T. Grapevine leafroll disease and associated viruses: A unique pathosystem. Annu. Rev. Phytopathol. 2015, 53, 613–634. [Google Scholar] [CrossRef]
- Charles, J.G.; Cohen, D.; Walker, J.T.S.; Forgie, S.A.; Bell, V.A.; Breen, K.C. A review of the ecology of Grapevine leafroll-associated virus type 3 (GLRaV-3). N. Z. Plant Prot. 2006, 59, 330–337. [Google Scholar]
- Almeida, R.P.; Daane, K.M.; Bell, V.A.; Blaisdell, G.K.; Cooper, M.L.; Herrbach, E.; Pietersen, G. Ecology and management of grapevine leafroll disease. Front. Microbiol. 2013, 4, 94. [Google Scholar] [CrossRef]
- Dincă, L.; Crisan, V.; Ienaşoiu, G.; Murariu, G.; Drăşovean, R. Environmental Indicator Plants in Mountain Forests: A Review. Plants 2024, 13, 3358. [Google Scholar] [CrossRef]
- Bratu, I.; Dinca, L.; Constandache, C.; Murariu, G. Resilience and decline: The impact of climatic variability on temperate oak forests. Climate 2025, 13, 119. [Google Scholar] [CrossRef]
- Dincă, L.; Constandache, C.; Postolache, R.; Murariu, G.; Tupu, E. Timber Harvesting in Mountainous Regions: A Comprehensive Review. Forests 2025, 16, 495. [Google Scholar] [CrossRef]
- Budău, R.; Timofte, C.S.C.; Mirisan, L.V.; Bei, M.; Dincă, L.; Murariu, G.; Racz, K.A. Living landmarks: A review of monumental trees and their role in ecosystems. Plants 2025, 14, 2075. [Google Scholar] [CrossRef]
- Dinca, L.; Murariu, G.; Lupoae, M. Understanding the ecosystem services of riparian forests: Patterns, gaps, and global trends. Forests 2025, 16, 947. [Google Scholar] [CrossRef]
- Enescu, C.M.; Mihalache, M.; Ilie, L.; Dinca, L.; Constandache, C.; Murariu, G. Agricultural benefits of shelterbelts and windbreaks: A bibliometric analysis. Agriculture 2025, 15, 1204. [Google Scholar] [CrossRef]
- Dinca, L.; Coca, A.; Tudose, N.C.; Marin, M.; Murariu, G.; Munteanu, D. The Role of Trees in Sand Dune Rehabilitation: Insights from Global Experiences. Appl. Sci. 2025, 15, 7358. [Google Scholar] [CrossRef]
- Murariu, G.; Dinca, L.; Munteanu, D. Trends and applications of principal component analysis in forestry research: A literature and bibliometric review. Forests 2025, 16, 1155. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Clarivate. Web of Science Core Collection. Available online: https://clarivate.com/academia-government/lp/pivot-rp/?campaignname=UL_PivotRP_LeadGen_AG_Global&campaignid=701QO00000BvPD5YAN&utm_campaign=UL_PivotRP_LeadGen_AG_Global&utm_source=Google&utm_medium=Paid_Search&utm_content=&utm_term=&gad_source=1&gad_campaignid=22932413470&gbraid=0AAAAACv9_uM80D8RkAoKAvA66B-nwF5dA&gclid=CjwKCAiAzrbIBhA3EiwAUBaUdcYek4E9ZI2G8vm6Q6B1NvmDgkQ1cExBwVa15FjHNVq8QdS5kvMxshoCo1AQAvD_BwE (accessed on 10 June 2025).
- Elsevier; Scopus. Available online: https://www.elsevier.com/products/scopus (accessed on 11 June 2025).
- Microsoft Corporation. Microsoft Excel. Available online: https://www.microsoft.com/en-us/microsoft-365/excel?legRedir=true&CorrelationId=3bb60ab0-fe13-41a4-812b-2627667cf346 (accessed on 16 July 2025).
- Geochart. Available online: https://developers.google.com/chart/interactive/docs/gallery/geochart (accessed on 23 July 2025).
- VOSviewer. Available online: https://www.vosviewer.com/ (accessed on 5 July 2025).
- Kumar, S.; Sawant, S.D.; Sawant, I.S.; Prabha, K.; Jain, R.K.; Baranwal, V.K. First report of Grapevine leafroll-associated virus 1 infecting grapevines in India. Plant Dis. 2012, 96, 1828. [Google Scholar] [CrossRef]
- Zongoma, A.M.; Dangora, D.B.; Al Rwahnih, M.; Bako, S.P.; Alegbejo, M.D.; Alabi, O.J. First report of Grapevine leafroll-associated virus 1 infecting grapevines (Vitis spp.) in Nigeria. Plant Dis. 2018, 102, 258. [Google Scholar] [CrossRef]
- Gazel, M.; Caglayan, K.; Elçi, E.; Öztürk, L. First report of Grapevine Pinot gris virus in grapevine in Turkey. Plant Dis. 2016, 100, 657. [Google Scholar] [CrossRef]
- Immanuel, T.M.; Delmiglio, C.; Ward, L.I.; Denton, J.O.; Clover, G.R.G. First Reports of Grapevine virus A, Grapevine fleck virus, and Grapevine leafroll-associated virus 1 in the United Kingdom. Plant Dis. 2015, 99, 898. [Google Scholar] [CrossRef]
- Crnogorac, A.; Panno, S.; Mandić, A.; Gašpar, M.; Caruso, A.G.; Noris, E.; Davino, S.; Matić, S. Survey of five major grapevine viruses infecting Blatina and Žilavka cultivars in Bosnia and Herzegovina. PLoS ONE 2021, 16, e0245959. [Google Scholar] [CrossRef]
- Elbeaino, T.; Kontra, L.; Demian, E.; Jaksa-Czotter, N.; Slimen, A.B.; Fabian, R.; Lazar, J.; Tamisier, L.; Digiaro, M.; Massart, S.; et al. Complete sequence, genome organization and molecular detection of grapevine line pattern virus, a new putative anulavirus infecting grapevine. Viruses 2020, 12, 602. [Google Scholar] [CrossRef]
- Pop, I.; Gugerli, P.; Banu, E.; Tomoioaga, L. Results regarding the identification of closteroviruses associated with the leafroll disease of some grapevine varieties grown in Romania. In Proceedings of the Extended Abstracts 11th ICVG Meeting, Montreux, Switzerland, 5–10 September 1993; pp. 123–124. [Google Scholar]
- Bertazzon, N.; Angelini, E.; Signorotto, M.; Genov, N. First report of grapevine Pinot gris virus and grapevine leafroll-associated virus 2 in Bulgarian vineyards. J. Plant Dis. Prot. 2021, 128, 597–599. [Google Scholar] [CrossRef]
- Afechtal, M.; Bibi, I.; Aarabe, A.; Sbaghi, M.; Ouantar, M.; Faddoul, Z. First report of grapevine leafroll-associated virus 2 in Moroccan vineyards. J. Plant Pathol. 2017, 29, 533–543. [Google Scholar]
- Porotikova, E.V.; Dmitrenko, U.D.; Yurchenko, E.G.; Vinogradova, S.V. First Report of Grapevine leafroll-associated virus 2 in Russian Grapevines (Vitis vinifera). Plant Dis. 2019, 103, 164. [Google Scholar] [CrossRef]
- Aboughanem-Sabanadzovic, N.; Sabanadzovic, S. First report of Grapevine leafroll-associated virus 2 infecting muscadine (Vitis rotundifolia) and summer grape (Vitis aestivalis) in the United States. Plant Dis. 2015, 99, 163. [Google Scholar] [CrossRef]
- Jones, T.J.; Westover, F.; Nita, M. First Report of Grapevine leafroll-associated virus-2 and -3 in Texas Vineyards. Plant Dis. 2014, 98, 1592. [Google Scholar] [CrossRef]
- Lunden, S.; Qiu, W. First report of Grapevine leafroll-associated virus 2 in a hybrid grape ‘Vidal Blanc’ in Missouri. Plant Dis. 2012, 96, 462. [Google Scholar] [CrossRef]
- Gugerli, P.; Ramel, M.E. Grapevine leafroll associated virus II analyzed by monoclonal antibodies. In Proceedings of the 11th Congress of ICVG, Montreux, Switzerland, 5–10 September 1993; pp. 23–24. [Google Scholar]
- Ribeiro, G.P.; Saldarelli, P.; Hong, N.; Xiang, B.C.; Zhang, X.L.; Wang, G.P.; Martelli, G.P. First record of three grapevine viruses in the Chinese Province of Sinkiang. J. Plant Pathol. 2004, 86, 152. [Google Scholar]
- Anfoka, G.H.; Shahrour, W.; Nakhla, M.K. Detection and molecular characterization of Grapevine fanleaf virus and Grapevine leafroll-associated virus 3 in Jordan. J. Plant Pathol. 2004, 86, 203–207. [Google Scholar]
- Kostadinovska, E.; Mitrev, S.; Bianco, P.A.; Casati, P.; Bulgari, D. First report of Grapevine virus A and Grapevine fleck virus in the Former Yugoslav Republic of Macedonia. Plant Dis. 2014, 98, 1747. [Google Scholar] [CrossRef]
- Mahfoudhi, N.; Selmi, I.; Elair, M.; Garfi, G.; Pasta, S.; Carimi, F.; Pacifico, D. First report of grapevine leafroll-associated virus 3 in wild vines (Vitis vinifera subsp. sylvestris) in Tunisia. J. Plant Pathol. 2021, 103, 1039. [Google Scholar] [CrossRef]
- Mishchenko, L.T.; Konup, L.O.; Dunich, A.A.; Gorobets, V.F.; Konup, A.I.; Zaimenko, N.V.; Kozub, N.O.; Dashchenko, A.V.; Chistyakova, V.L.; Shcherbakova, T.O.; et al. First report of grapevine leafroll-associated virus-3 on peony plants in Ukraine. J. Plant Dis. Prot. 2023, 130, 189–198. [Google Scholar] [CrossRef]
- Hoffmann, M.; Talton, W.; Nita, M.; Jones, T.; Al Rwahnih, M.; Sudarshana, M.; Almeyda, C. First report of grapevine leafroll-associated virus 3 in Vitis vinifera in North Carolina. J. Plant Pathol. 2021, 103, 385–386. [Google Scholar] [CrossRef]
- Mekuria, T.A.; Karasev, A.V.; Martin, R.R.; Naidu, R.A. First report of Grapevine leafroll-associated virus-3 in six wine grape cultivars in Idaho. Plant Dis. 2009, 93, 1218. [Google Scholar] [CrossRef]
- Soule, M.J.; Eastwell, K.C.; Naidu, R.A. First report of Grapevine leafroll associated virus-3 in American Vitis spp. Grapevines in Washington State. Plant Dis. 2006, 90, 1461. [Google Scholar] [CrossRef]
- Pietersen, G.; Spreeth, N.; Oosthuizen, T.; van Rensburg, A.; van Rensburg, M.; Lottering, D.; Rossouw, N.; Tooth, D. Control of grapevine leafroll disease spread at a commercial wine estate in South Africa: A case study. Am. J. Enol. Vitic. 2013, 64, 296–305. [Google Scholar] [CrossRef]
- Martelli, G.P. (Ed.) Directory of Infectious Diseases of Grapevines and Viroses and Virus-like Diseases of the Grapevine: Bibliographic Report 1998–2004; Ciheam: Paris, France, 2006. [Google Scholar]
- Gugerli, P.; Brugger, J.J.; Bovey, R. L’enroulement de la vigne: Mise en evidence de particules virales et developpement d’une methode immuno-enzymatique pour le diagnostic rapide. Rev. Suisse Vitic. Arboric. Hortic. 1984, 16, 299–304. [Google Scholar]
- Kuhn, G.B. Identificação, incidencia e controle do virus do enrolamento da folha da videira no Estado do Rio Grande do Sul. Fitopatol. Bras. 1989, 14, 220–226. [Google Scholar]
- Minafra, A.; Hadidi, A. Sensitive detection of grapevine virus A, B, or leafroll-associated III from viruliferous mealybugs and infected tissue by cDNA amplification. J. Virol. Methods 1994, 47, 175–187. [Google Scholar] [CrossRef]
- Escobar, P.F.; Fiore, N.; Valenzuela, P.D.T.; Engel, E.A. First Detection of Grapevine leafroll-associated virus 4 in Chilean Grapevines. Plant Dis. 2008, 92, 1474. [Google Scholar] [CrossRef]
- Pei, G.Q.; Dong, Y.F.; Zhang, Z.P.; Fan, X.D. First Report of Grapevine leafroll-associated virus 4 and 5 in Grapevines in China. Plant Dis. 2010, 94, 130. [Google Scholar] [CrossRef] [PubMed]
- Olah, R.; Turcsan, M.; Jaksa-Czotter, N.; Galbacs, Z.N.; Olah, K.; Sardi, D.N.; Plesko, I.M.; Varallyay, E. First report of Grapevine leafroll-associated virus 4 infecting grapevine in Hungary. Plant Dis. 2024, 108, 2245. [Google Scholar] [CrossRef]
- Rizzo, D.; Luvisi, A.; Stefani, L.; Paoli, M.; Marchi, G.; Panattoni, A.; Materazzi, A. First report of Grapevine leafroll associated virus-4 strain 5 in Italy. J. Plant Pathol. 2014, 96, 129. [Google Scholar]
- Choueiri, E.; Jreijiri, F.; Habib, W.; Abou Kubaa, R.; Elbeaino, T. First report on the occurrence of Grapevine leafroll-associated virus-4 strain 6 in Lebanon. Plant Dis. 2017, 101, 1066. [Google Scholar] [CrossRef]
- Štrukelj, M.; Pleško, I.M.; Urek, G. Molecular characterization of a grapevine leafroll-associated virus 4 from Slovenian vineyards. Acta Virol. 2016, 60, 174–180. [Google Scholar] [CrossRef]
- Kaya, A.; Erilmez, S.; Paylan, I.C.; Erkan, S. First report of grapevine leafroll-associated virus 4 in vineyards of Turkey. Plant Dis. 2012, 96, 1230. [Google Scholar] [CrossRef]
- Padilla, C.V.; Cretazzo, E.; Hita, I.; López, N.; Padilla, V.; Velasco, L. First report of Grapevine leafroll-associated virus 5 in Spain. Plant Dis. 2010, 94, 1507. [Google Scholar] [CrossRef]
- Gómez Talquenca, S.; Muñoz, C.; Grau, O.; Gracia, O. First description of Grapevine leafroll-associated virus 5 in Argentina and partial genome sequence. Virus Genes 2009, 38, 184–186. [Google Scholar] [CrossRef]
- Engel, E.A.; Escobar, P.; Montt, C.; Gómez-Talquenca, S.; Valenzuela, P.D.T. First report on the occurrence of Grapevine leafroll-associated virus 7 and 9 in Chilean grapevines. Plant Dis. 2008, 92, 1252. [Google Scholar] [CrossRef]
- Padilla, V.; Cretazzo, E.; Alcalá, M.J.; Hita, I.; Lopez, N.; Padilla, V.; Velasco, L. First report of Grapevine leafroll-associated virus 9 in Spain. J. Plant Pathol. 2013, 95, 662. [Google Scholar] [CrossRef]
- Buzkan, N.; Karadağ, S.; Kaya, A.; Baloğlu, S.; Minafra, A.; Ben-Dov, Y. First report of the occurrence of Grapevine leafroll-associated virus-5 in Turkish vineyards. J. Phytopathol. 2010, 158, 448–449. [Google Scholar] [CrossRef]
- Lyu, M.D.; Li, M.J.; Li, J.; Li, X.M.; Cheng, Y.Q. First report of Grapevine leafroll-associated virus 7 in two native grape varieties in China. Plant Dis. 2013, 97, 150. [Google Scholar] [CrossRef]
- Morales, R.Z.; Monis, J. First detection of Grapevine leafroll-associated virus-7 in California vineyards. Plant Dis. 2007, 91, 465. [Google Scholar] [CrossRef]
- Peake, B.K.; Mackie, A.E.; Sivasithamparam, K.; Habili, N.; McKirdy, S.J. First report of grapevine leafroll associated virus 9 (GLRaV-9) in Western Australia. Australas. Plant Pathol. 2004, 33, 445–446. [Google Scholar] [CrossRef]
- Giampetruzzi, A.; Chiumenti, M.; Roberto, R.; Pirolo, C.; Minafra, A.; Saldarelli, P. First detection of Grapevine leafroll-associated virus 9 in Italy. J. Plant Pathol. 2011, 93, S4. [Google Scholar]
- Padilla, C.V.; Cretazzo, E.; López, N.; de Rosa, B.G.; Padilla, V.; Velasco, L. First report of Grapevine leafroll-associated virus 4 (GLRaV-4) in Spain. New Dis. Rep. 2010, 21, 21. [Google Scholar] [CrossRef]
- Jarugula, S.; Soule, M.J.; Rowhani, A.; Naidu, R.A. First report of Grapevine leafroll-associated virus-9 in Washington state vineyards. Plant Dis. 2008, 92, 485. [Google Scholar] [CrossRef]
- Mahfoudhi, N.; Digiaro, M.; Dhouibi, M.H. Transmission of Grapevine leafroll viruses by Planococcus ficus (Hemiptera: Pseudococcidae) and Ceroplastes rusci (Hemiptera: Coccidae). Plant Dis. 2009, 93, 999–1002. [Google Scholar] [CrossRef]
- Avgelis, A.; Boscia, D. Grapevine leafroll-associated closterovirus 7 in Greece. Phytopathol. Mediterr. 2001, 40, 289–292. [Google Scholar]
- Čarija, M.; Radić, T.; Černi, S.; Mucalo, A.; Zdunić, G.; Vončina, D.; Jagunić, M.; Hančević, K. Prevalence of virus infections and GLRaV-3 genetic diversity in selected clones of Croatian indigenous grapevine cultivar Plavac Mali. Pathogens 2022, 11, 176. [Google Scholar] [CrossRef]
- Zindović, J.; Viršček Marn, M.; Mavrič Pleško, I. Phytosanitary status of grapevine in Montenegro. EPPO Bull. 2014, 44, 60–64. [Google Scholar] [CrossRef]
- Tomić, L.; Štajner, N.; Jovanović-Cvetković, T.; Cvetković, M.; Javornik, B. Identity and genetic relatedness of Bosnia and Herzegovina grapevine germplasm. Sci. Hortic. 2012, 143, 122–126. [Google Scholar] [CrossRef]
- Merkuri, J.; Martelli, G.P.; Boscia, D.; Savino, V. Viruses of grapevine in Albania. EPPO Bull. 1994, 24, 215–220. [Google Scholar] [CrossRef]
- Cseh, E.; Palkovics, L.; Apró, M.; Gáborjányi, R.; Kocsis, L.; Takács, A.P. Occurrence and evolutionary relationships of Grapevine leafroll-associated virus 3 isolates in Hungary. J. Plant Pathol. 2013, 95, S1.51. [Google Scholar]
- Komínek, P. Distribution of grapevine viruses in vineyards of the Czech Republic. J. Plant Pathol. 2008, 90, 357–358. [Google Scholar]
- Messmer, N.; Bohnert, P.; Schumacher, S.; Fuchs, R. Studies on the occurrence of viruses in planting material of grapevines in southwestern Germany. Viruses 2021, 13, 248. [Google Scholar] [CrossRef]
- Komorowska, B.; Berniak, H.; Golis, T. Detection of grapevine viruses in Poland. J. Phytopathol. 2014, 162, 326–331. [Google Scholar] [CrossRef]
- Credi, R.; Giunchedi, L. Grapevine leafroll-associated viruses and Grapevine virus A in selected Vitis vinifera cultivars in northern Italy. Plant Pathol. 1996, 45, 1110–1116. [Google Scholar] [CrossRef]
- Esteves, F.; Teixeira Santos, M.; Eiras-Dias, J.E.; Fonseca, F. Occurrence of grapevine leafroll-associated virus 5 in Portugal: Genetic variability and population structure in field-grown grapevines. Arch. Virol. 2012, 157, 1747–1765. [Google Scholar] [CrossRef]
- Grecu, C.; Buciumeanu, E.; Dumitraşcu, M. Purification du clostérovirus du type III associé à l’enroulement de la vigne [Purification of closterovirus type III associated with grapevine leafroll disease]. Rev. Roum. Virol. 1994, 45, 19–23. [Google Scholar]
- Guță, I.C.; Buciumeanu, E.C. Grapevine Pinot gris virus infecting grapevine in Romania. Hort. Sci. 2021, 48, 47–50. [Google Scholar] [CrossRef]
- Kyrychenko, A.M.; Hrynchuk, K.V.; Antipov, I.O.; Konup, A.I. A survey of grapevine leafroll-associated virus 1 and 3 in the south of Ukraine and development of primers for GLRaV-3 identification. Mikrobiol. Zhurnal 2022, 84, 82–91. [Google Scholar] [CrossRef]
- Fan, X.; Hong, N.; Dong, Y.; Ma, Y.; Zhang, Z.P.; Ren, F.; Wang, G. Genetic diversity and recombination analysis of grapevine leafroll-associated virus 1 from China. Arch. Virol. 2015, 160, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Li, M.J.; Qi, H.H.; Guo, R.; Liu, X.M.; Wang, Q.; Cheng, Y.Q. Occurrence of grapevine leafroll-associated viruses in China. Plant Dis. 2013, 97, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Brannen, P.M.; Deom, C.M.; Alabi, O.J.; Naidu, R.A. Prevalence of viruses in commercial wine grape vineyards in Georgia. Plant Health Prog. 2018, 19, 342–346. [Google Scholar] [CrossRef]
- Naderpour, M.; Shahbazi, R.; Alizadeh, A.; Karimi, S.; Tabei, M.; Hajizadeh, M.; Hosseinibay, K. The status of Grapevine leafroll-associated viruses in Iran. Acta Hortic. 2020, 1269, 113–118. [Google Scholar] [CrossRef]
- Rai, R.; Khurana, S.P.; Kumar, S.; Sharma, S.K.; Watpade, S.; Baranwal, V.K. Characterization of Grapevine leafroll-associated virus 4 from Indian vineyards. J. Plant Pathol. 2017, 99, 255–259. [Google Scholar]
- Rasool, S.; Naz, S.; Rowhani, A.; Diaz-Lara, A.; Golino, D.A.; Farrar, K.D.; Al Rwahnih, M. Survey of grapevine pathogens in Pakistan. J. Plant Pathol. 2019, 101, 725–732. [Google Scholar] [CrossRef]
- Akbaş, B.; Kunter, B.; Ilhan, D. Occurrence and distribution of grapevine leafroll-associated viruses 1, 2, 3 and 7 in Turkey. J. Phytopathol. 2007, 155, 122–124. [Google Scholar] [CrossRef]
- Mslmanieh, T.; Digiaro, M.; Elbeaino, T.; Boscia, D.; Martelli, G.P. Viruses of grapevine in Syria. EPPO Bull. 2006, 36, 523–528. [Google Scholar] [CrossRef]
- Ben Salem-Fnayou, A.; Gugerli, P.; Zemni, H.; Mliki, A.; Ghorbel, A. Decreased detectability of Grapevine leafroll-associated virus 3 in Sakasly grapevines cultivated under the Sahara conditions. J. Phytopathol. 2006, 154, 528–533. [Google Scholar] [CrossRef]
- Lehad, A.; Selmi, I.; Louanchi, M.; Aitouada, M.; Mahfoudhi, N. Occurrence and diversity of Grapevine leafroll-associated virus 1 in Algeria. Phytopathol. Mediterr. 2019, 58, 277–282. [Google Scholar]
- Aldrich, D.J.; Bester, R.; Burger, J.; Maree, H.J. Characterisation of different GLRaV-3 variant infections by determining virus concentration ratios and miRNA expression profiles. Vitis 2019, 58, 79–86. [Google Scholar]
- Allsopp, E. Transmission of grapevine leafroll-associated virus 3 by vine mealybug, Planococcus ficus (Signoret), to grapevines treated with imidacloprid. S. Afr. J. Enol. Vitic. 2015, 36, 252–255. [Google Scholar] [CrossRef]
- Poojari, S.; Moreau, D.L.; Kahl, D.; Ritchie, M.; Ali, S.; Úrbez-Torres, J.R. Disease incidence and genetic variability of economically important grapevine viruses in Nova Scotia. Can. J. Plant Pathol. 2020, 42, 584–594. [Google Scholar] [CrossRef]
- Fiore, N.; Zamorano, A.; Rivera, L.; González, F.; Aballay, E.; Montealegre, J.; Pino, A.M. Grapevine Viruses in the Atacama Region of Chile. J. Phytopathol. 2011, 159, 743–750. [Google Scholar] [CrossRef]
- Adiputra, J.; Kesoju, S.R.; Naidu, R.A. The relative occurrence of Grapevine leafroll-associated virus 3 and Grapevine red blotch virus in Washington State vineyards. Plant Dis. 2018, 102, 2129–2135. [Google Scholar] [CrossRef]
- Diaz-Lara, A.; Aguilar-Molina, V.H.; Monjarás-Barrera, J.I.; Vončina, D.; Erickson, T.M.; Al Rwahnih, M. Potential implications and management of grapevine viruses in Mexico: A review. Int. J. Plant Biol. 2023, 14, 177–189. [Google Scholar] [CrossRef]
- de Borbón, C.M.; Gracia, O.; Talquenca, G.S.G. Mealybugs and grapevine leafroll-associated virus 3 in vineyards of Mendoza, Argentina. Am. J. Enol. Vitic. 2004, 55, 283–285. [Google Scholar] [CrossRef]
- Habili, N.; Fazeli, C.F.; Ewart, A.; Hamilton, R.; Cirami, R.; Saldarelli, P.; Rezaian, M.A. Natural spread and molecular analysis of grapevine leafroll-associated virus 3 in Australia. Phytopathology 1995, 85, 1418–1422. [Google Scholar] [CrossRef]
- Hao, X.; Jiao, B.; Liu, Z.; Wang, X.; Wang, J.; Zhang, J.; Wang, Q.C. Crosstalk between grapevine leafroll-associated virus-3 (GLRaV-3) and NaCl-induced salt stress in in vitro cultures of the red grape ‘Cabernet Sauvignon’. Plant Cell Tissue Organ Cult. (PCTOC) 2021, 144, 649–660. [Google Scholar] [CrossRef]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef]
- Pandey, P.; Irulappan, V.; Bagavathiannan, M.V.; Senthil-Kumar, M. Impact of combined abiotic and biotic stresses on plant growth and avenues for crop improvement by exploiting physio-morphological traits. Front. Plant Sci. 2017, 8, 537. [Google Scholar] [CrossRef]
- Apostol, E.N.; Stuparu, E.; Scarlatescu, V.; Budeanu, M. Testing Hungarian oak (Quercus frainetto Ten.) provenances in Romania. iForest 2020, 13, 9–15. [Google Scholar] [CrossRef]
- Besliu, E.; Curtu, A.L.; Apostol, E.N.; Budeanu, M. Using adapted and productive European beech (Fagus sylvatica L.) provenances as future solutions for sustainable forest management in Romania. Land 2024, 13, 183. [Google Scholar] [CrossRef]
- Budeanu, M.; Şofletea, N.; Petriţan, I.C. Among-population variation in quality traits in two Romanian provenance trials with Picea abies L. Baltic For. 2014, 20, 37–47. [Google Scholar]
- Budeanu, M.; Popescu, F.; Beşliu, E.; Apostol, N.E. Diallel crossing (10x10) in Swiss stone pine. Juvenile-adult correlations and genetic gain for predicting forward selection. Ann. For. Res. 2024, 67, 109–120. [Google Scholar] [CrossRef]
- Budeanu, M.; Beşliu, E.; Pepelea, D. Testing the radial increment and climate–growth relationship between Swiss stone pine European provenances in the Romanian Carpathians. Forests 2025, 16, 391. [Google Scholar] [CrossRef]
- Zhu, J. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef]
- Mostert, I.; Bester, R.; Burger, J.T.; Maree, H.J. Investigating protein–protein interactions between Grapevine leafroll-associated virus 3 and Vitis vinifera. Phytopathology 2023, 113, 1994–2005. [Google Scholar] [CrossRef]
- Hesler, S.; Cox, R.; Bhandari, R.; Loeb, G.; Martinson, T.; Fuchs, M. Spatial roguing reduces the incidence of leafroll disease and curtails its spread in a Finger Lakes Cabernet franc vineyard. Am. J. Enol. Vitic. 2022, 73, 227–236. [Google Scholar] [CrossRef]
- Wallingford, A.K.; Fuchs, M.F.; Martinson, T.; Hesler, S.; Loeb, G.M. Slowing the spread of grapevine leafroll-associated viruses in commercial vineyards with insecticide control of the vector, Pseudococcus maritimus (Hemiptera: Pseudococcidae). J. Insect Sci. 2015, 15, 112. [Google Scholar] [CrossRef] [PubMed]
- Blaisdell, G.K.; Cooper, M.L.; Kuhn, E.J.; Taylor, K.A.; Daane, K.M.; Almeida, R.P. Disease progression of vector-mediated Grapevine leafroll-associated virus 3 infection of mature plants under commercial vineyard conditions. Eur. J. Plant Pathol. 2016, 146, 105–116. [Google Scholar] [CrossRef]
- Bell, V.A.; Hedderley, D.I.; Pietersen, G.; Lester, P.J. Vineyard-wide control of grapevine leafroll-associated virus 3 requires an integrated response. J. Plant Pathol. 2018, 100, 399–408. [Google Scholar] [CrossRef]
- Abou-Ghanem, N.; Sabanadzovic, S.; Castellano, M.A.; Boscia, D.; Martelli, G.P. Properties of a new isolate of grapevine leafroll-associated virus 2. Vitis 2000, 39, 119–121. [Google Scholar]
- Jooste, A.E.C.; Maree, H.J.; Bellstedt, D.U.; Goszczynski, D.E.; Pietersen, G.; Burger, J.T. Three genetic grapevine leafroll-associated virus 3 variants identified from South African vineyards show high variability in their 5′ UTR. Arch. Virol. 2010, 155, 1997–2006. [Google Scholar] [CrossRef]
- Komar, V.; Vigne, E.; Demangeat, G.; Fuchs, M. Beneficial effect of selective virus elimination on the performance of Vitis vinifera cv. Chardonnay. Am. J. Enol. Vitic. 2007, 58, 202–210. [Google Scholar] [CrossRef]
- Maree, H.J.; Almeida, R.P.; Bester, R.; Chooi, K.M.; Cohen, D.; Dolja, V.V.; Fuchs, M.F.; Golino, D.A.; Jooste, A.E.; Martelli, G.P.; et al. Grapevine leafroll-associated virus 3. Front. Microbiol. 2013, 4, 82. [Google Scholar] [CrossRef]
- Osman, F.; Leutenegger, C.; Golino, D.; Rowhani, A. Real-time RT-PCR (TaqMan®) assays for the detection of Grapevine leafroll-associated viruses 1–5 and 9. J. Virol. Methods 2007, 141, 22–29. [Google Scholar] [CrossRef]
- Alabi, O.J.; Al Rwahnih, M.; Karthikeyan, G.; Poojari, S.; Fuchs, M.; Rowhani, A.; Naidu, R.A. Grapevine leafroll-associated virus 1 occurs as genetically diverse populations. Phytopathology 2011, 101, 1446–1456. [Google Scholar] [CrossRef]
- Maree, H.J.; Pirie, M.D.; Oosthuizen, K.; Bester, R.; Rees, D.J.G.; Burger, J.T. Phylogenomic analysis reveals deep divergence and recombination in an economically important grapevine virus. PLoS ONE 2015, 10, e0126819. [Google Scholar] [CrossRef]
- Turturo, C.; Saldarelli, P.; Yafeng, D.; Digiaro, M.; Minafra, A.; Savino, V.; Martelli, G.P. Genetic variability and population structure of Grapevine leafroll-associated virus 3 isolates. J. Gen. Virol. 2005, 86, 217–224. [Google Scholar] [CrossRef]
- Chooi, K.M.; Bell, V.A.; Blouin, A.G.; Cohen, D.; Mundy, D.; Henshall, W.; MacDiarmid, R.M. Grapevine leafroll-associated virus 3 genotype influences foliar symptom development in New Zealand vineyards. Viruses 2022, 14, 1348. [Google Scholar] [CrossRef] [PubMed]
- Cabaleiro, C.; Pesqueira, A.M.; García-Berrios, J.J. Assessment of symptoms of grapevine leafroll disease and relationship with yield and quality of Pinot Noir grape must in a 10-year study period. Plants 2023, 12, 2127. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Hanner, R.H.; Meng, B. Probing into the Effects of Grapevine Leafroll-Associated Viruses on the Physiology, Fruit Quality and Gene Expression of Grapes. Viruses 2021, 13, 593. [Google Scholar] [CrossRef]
- Donda, B.P.; Kesoju, S.R.; Arnold, K.; McRoberts, N.; Naidu, R.A. Spatio-Temporal Spread of Grapevine Leafroll Disease in Washington State Vineyards. Plant Dis. 2023, 107, 1471–1480. [Google Scholar] [CrossRef]
- Tanne, E.; Spiegel-Roy, P.; Shlamovitz, N. Rapid in vitro indexing of grapevine viral diseases: The effect of stress-inducing agents on the diagnosis of leafroll. Plant Dis. 1996, 80, 972–974. [Google Scholar] [CrossRef]
- Marin, M.; Clinciu, I.; Tudose, N.C.; Ungurean, C.; Mihalache, A.L.; Mărțoiu, N.E.; Tudose, O.N. Assessment of seasonal surface runoff under climate and land use change scenarios for a small forested watershed: Upper Tarlung Watershed (Romania). Water 2022, 14, 2860. [Google Scholar] [CrossRef]
- Davidescu, S.O.; Clinciu, I.; Tudose, N.C.; Ungurean, C. An evaluating methodology for hydrotechnical torrent-control structures condition. Ann. For. Res. 2012, 55, 125–143. [Google Scholar] [CrossRef]
- Mihalache, A.L.; Marin, M.; Davidescu, Ș.O.; Ungurean, C.; Adorjani, A.; Tudose, N.C.; Davidescu, A.A.; Clinciu, I. Physical status of torrent control structures in Romania. Environ. Eng. Manag. J. 2020, 19, 861–872. [Google Scholar] [CrossRef]
- Marin, M.; Tudose, N.C.; Ungurean, C.; Mihalache, A.L. Application of life cycle assessment for torrent control structures: A review. Land 2024, 13, 1956. [Google Scholar] [CrossRef]
- Al Rwahnih, M.; Saldarelli, P.; Rowhani, A. Grapevine leafroll-associated virus 7. In Grapevine Viruses: Molecular Biology, Diagnostics and Management; Meng, B., Martelli, G.P., Golino, D.A., Fuchs, M., Eds.; Springer: Cham, Switzerland, 2017; pp. 221–228. [Google Scholar]
- Tsai, C.W.; Daugherty, M.P.; Almeida, R.P.P. Seasonal dynamics and virus translocation of Grapevine leafroll-associated virus 3 in grapevine cultivars. Plant Pathol. 2012, 61, 977–985. [Google Scholar] [CrossRef]
- Douglas, N.; Krüger, K. Transmission efficiency of Grapevine leafroll-associated virus 3 (GLRaV-3) by the mealybugs Planococcus ficus and Pseudococcus longispinus (Hemiptera: Pseudococcidae). Eur. J. Plant Pathol. 2008, 122, 207–212. [Google Scholar] [CrossRef]
- Herrbach, E.; Alliaume, A.; Prator, C.A.; Daane, K.M.; Cooper, M.L.; Almeida, R.P.P. Vector Transmission of Grapevine Leafroll-Associated Viruses. In Grapevine Viruses: Molecular Biology, Diagnostics and Management; Meng, B., Martelli, G., Golino, D., Fuchs, M., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Mahfoudhi, N.; Habili, N.; Masri, S.A.; Dhouibi, M.H. First report on the occurrence of Grapevine leafroll-associated viruses 5 and 9 in Tunisian grapevines. Plant Dis. 2007, 91, 1359. [Google Scholar] [CrossRef]
- Hommay, G.; Beuve, M.; Herrbach, E. Transmission of Grapevine Leafroll-Associated Viruses and Grapevine Virus A by Vineyard-Sampled Soft Scales (Parthenolecanium corni, Hemiptera: Coccidae). Viruses 2022, 14, 2679. [Google Scholar] [CrossRef]
- Cabaleiro, C.; Segura, A. Some characteristics of the transmission of Grapevine leafroll-associated virus 3 by Planococcus citri Risso. Eur. J. Plant Pathol. 1997, 103, 373–378. [Google Scholar] [CrossRef]
- Chooi, K.M.; Bell, V.A.; Blouin, A.G.; Sandanayaka, M.; Gough, R.; Chhagan, A.; MacDiarmid, R.M. The New Zealand perspective of an ecosystem biology response to grapevine leafroll disease. Adv. Virus Res. 2024, 118, 213–272. [Google Scholar]
- Habili, N.; Wu, Q.; Rinaldo, A.; Constable, F. A Chronological Study on Grapevine Leafroll-Associated Virus 2 in Australia. Viruses 2023, 15, 1105. [Google Scholar] [CrossRef]
- Mikona, C.; Jelkmann, W. Replication of Grapevine leafroll-associated virus-7 (GLRaV-7) by Cuscuta species and its transmission to herbaceous plants. Plant Dis. 2010, 94, 471–476. [Google Scholar] [CrossRef]
- Sforza, R.; Boudon-Padieu, E.; Greif, C. New Mealybug Species Vectoring Grapevine Leafroll-Associated Viruses-1 and -3 (GLRaV-1 and -3). Eur. J. Plant Pathol. 2003, 109, 975–981. [Google Scholar] [CrossRef]
- Zhang, C.W.; Huang, H.Q.; Huang, W.T.; Li, H.W.; Chi, H.; Cheng, Y.Q. Grapevine leafroll-associated virus 2 and grapevine ‘Pinot gris’ virus are present in seedlings developed from seeds of infected grapevine plants. Vitis 2022, 61, 21–25. [Google Scholar]
- Donda, B.P.; Jarugula, S.; Naidu, R.A. An analysis of the complete genome sequence and subgenomic RNAs reveals unique features of the Ampelovirus, Grapevine leafroll-associated virus 1. Phytopathology 2017, 107, 1069–1079. [Google Scholar] [CrossRef]
- Little, A.; Fazeli, C.F.; Rezaian, M.A. Hypervariable genes in Grapevine leafroll associated virus 1. Virus Res. 2001, 80, 109–116. [Google Scholar] [CrossRef]
- Sefc, K.M.; Leonhardt, W.; Steinkellner, H. Partial sequence identification of grapevine-leafroll-associated virus-1 and development of a highly sensitive IC-RT-PCR detection method. J. Virol. Methods 2000, 86, 101–106. [Google Scholar] [CrossRef]
- Bertazzon, N.; Borgo, M.; Vanin, S.; Angelini, E. Genetic variability and pathological properties of Grapevine leafroll-associated virus 2 isolates. Eur. J. Plant Pathol. 2010, 127, 185–197. [Google Scholar] [CrossRef]
- Jarugula, S.; Alabi, O.J.; Martin, R.R.; Naidu, R.A. Genetic variability of natural populations of Grapevine leafroll-associated virus 2 in Pacific Northwest vineyards. Phytopathology 2010, 100, 698–707. [Google Scholar] [CrossRef]
- Fonseca, F.; Esteves, F.; Teixeira Santos, M.; Brazão, J.; Eiras-Dias, J.E. Genetic variants of Grapevine leafroll-associated virus 2 infecting Portuguese grapevine cultivars. Phytopathol. Mediterr. 2016, 55, 73–88. [Google Scholar]
- Fust, C.; Lameront, P.; Shabanian, M.; Song, Y.; Abou Kubaa, R.; Bester, R.; Meng, B. Grapevine leafroll-associated virus 3: A global threat to grapevine and wine industries but a gold mine for scientific discovery. J. Exp. Bot. 2025, 76, eraf039. [Google Scholar] [CrossRef]
- Bester, R.; Jooste, A.E.; Maree, H.J.; Burger, J.T. Real-time RT-PCR high-resolution melting curve analysis and multiplex RT-PCR to detect and differentiate grapevine leafroll-associated virus 3 variant groups I., II, III and VI. Virol. J. 2012, 9, 219. [Google Scholar] [CrossRef]
- Chooi, K.M.; Cohen, D.; Pearson, M.N. Generic and sequence-variant specific molecular assays for the detection of the highly variable Grapevine leafroll-associated virus 3. J. Virol. Methods 2013, 189, 20–29. [Google Scholar] [CrossRef]
- Fei, F.; Lyu, M.D.; Li, J.; Fan, Z.F.; Cheng, Y.Q. Complete nucleotide sequence of a Chinese isolate of Grapevine leafroll-associated virus 3 reveals a 5′ UTR of 802 nucleotides. Virus Genes 2013, 46, 182–185. [Google Scholar] [CrossRef]
- Glasa, M.; Predajna, L. Partial sequence analysis of a Grapevine leafroll-associated virus 3 isolate from Slovakia. J. Plant Pathol. 2012, 94, 675–679. [Google Scholar]
- Jooste, A.E.; Pietersen, G.; Burger, J.T. Distribution of grapevine leafroll associated virus-3 variants in South African vineyards. Eur. J. Plant Pathol. 2011, 131, 371–381. [Google Scholar] [CrossRef]
- Jooste, A.E.C.; Goszczynski, D.E. Single-strand conformation polymorphism (SSCP), cloning and sequencing reveals two major groups of divergent molecular variants of grapevine leafroll-associated virus 3 (GLRaV-3). Vitis 2005, 44, 39–43. [Google Scholar]
- Jooste, A.E.C.; Molenaar, N.; Burger, J.T.; De Koker, W.C.; Bester, R.; Morey, L.; Maree, H.J. Identification and distribution of multiple virus infections in Grapevine leafroll diseased vineyards. Eur. J. Plant Pathol. 2015, 142, 363–375. [Google Scholar] [CrossRef]
- Jooste, A.E.C.; Pietersen, G.; Burger, J.T. Distribution of grapevine leafroll associated virus-3 variants in South African vineyards. Eur. J. Plant Pathol. 2011, 131, 371–381. [Google Scholar] [CrossRef]
- Abou-Ghanem, N.; Sabanadzovic, S.; Minafra, A.; Saldarelli, P.; Martelli, G.P. Some properties of Grapevine leafroll-associated virus 2 and molecular organization of the 3′ region of the viral genome. J. Plant Pathol. 1998, 80, 37–46. [Google Scholar]
- Adiputra, J.; Jarugula, S.; Naidu, R.A. Intra-species recombination among strains of the ampelovirus Grapevine leafroll-associated virus 4. Virol. J. 2019, 16, 139. [Google Scholar] [CrossRef]
- Good, X.; Monis, J. Partial genome organization, identification of the coat protein gene, and detection of Grapevine leafroll-associated virus-5. Phytopathology 2001, 91, 274–281. [Google Scholar] [CrossRef]
- Thompson, J.R.; Fuchs, M.; Perry, K.L. Genomic analysis of Grapevine leafroll associated virus-5 and related viruses. Virus Res. 2012, 163, 19–27. [Google Scholar] [CrossRef]
- Ito, T.; Nakaune, R. Molecular characterization of a novel putative ampelovirus tentatively named grapevine leafroll-associated virus 13. Arch. Virol. 2016, 161, 2555–2559. [Google Scholar] [CrossRef]
- Osman, F.; Golino, D.; Hodzic, E.; Rowhani, A. Virus distribution and seasonal changes of grapevine leafroll-associated viruses. Am. J. Enol. Vitic. 2018, 69, 70–76. [Google Scholar] [CrossRef]
- Morán, F.; Olmos, A.; Glasa, M.; Silva, M.B.D.; Maliogka, V.; Wetzel, T.; Ruiz-García, A.B. A novel and highly inclusive quantitative real-time rt-pcr method for the broad and efficient detection of grapevine leafroll-associated virus 1. Plants 2023, 12, 876. [Google Scholar] [CrossRef]
- Beuve, M.; Sempé, L.; Lemaire, O. A sensitive one-step real-time RT-PCR method for detecting Grapevine leafroll-associated virus 2 variants in grapevine. J. Virol. Methods 2007, 141, 117–124. [Google Scholar] [CrossRef]
- Acheche, H.; Fattouch, S.; M’hirsi, S.; Marzouki, N.; Marrakchi, M. Use of optimised PCR methods for the detection of GLRaV-3: A closterovirus associated with grapevine leafroll in Tunisian grapevine plants. Plant Mol. Biol. Rep. 1999, 17, 31–42. [Google Scholar] [CrossRef]
- Bester, R.; Burger, J.T.; Maree, H.J. Transcriptome analysis reveals differentially expressed small RNAs and genes associated with grapevine leafroll-associated virus 3 infections. Physiol. Mol. Plant Pathol. 2017, 100, 220–236. [Google Scholar] [CrossRef]
- López-Fabuel, I.; Wetzel, T.; Bertolini, E.; Bassler, A.; Vidal, E.; Torres, L.B.; Yuste, A.; Olmos, A. Real-time multiplex RT-PCR for the simultaneous detection of the five main grapevine viruses. J. Virol. Methods 2013, 188, 21–24. [Google Scholar] [CrossRef]
- Atallah, S.S.; Gomez, M.I.; Fuchs, M.F.; Martinson, T.E. Economic impact of Grapevine leafroll disease on Vitis vinifera cv. Cabernet Franc in Finger Lakes vineyards of New York. In Working Papers 2011; Cornell Univ., Dept. of Applied Economics and Management, No. 126597: Ithaca, NY, USA, 2011. [Google Scholar]
- Martínez, L.; Miranda, C.; Royo, J.B.; Urrestarazu, J.; Martínez de Toda, F.; Balda, P.; Santesteban, L.G. Direct and indirect effects of three virus infections on yield and berry composition in grapevine (Vitis vinifera L.) cv. ‘Tempranillo’. Sci. Hortic. 2016, 212, 20–28. [Google Scholar] [CrossRef]
- Salo, W.; Considine, J.A.; Considine, M.J. Influence of mixed and single infection of grapevine leafroll-associated viruses and viral load on berry quality. Tree Physiol. 2024, 44, tpae035. [Google Scholar] [CrossRef]
- Bertamini, M.; Malossini, U.; Muthuchelian, K.; Nedunchezhian, N. Physiological response of field grown grapevine (Vitis vinifera L. cv. Marzemino) to grapevine leafroll-associated virus (GLRaV-1). Phytopathol. Mediterr. 2005, 44, 256–265. [Google Scholar]
- Cabaleiro, C.; Pesqueira, A.M.; Barrasa, M.; García-Berrios, J.J. Analysis of the losses due to grapevine leafroll disease in Albariño vineyards in Rias Baixas (Spain). Ciência Téc. Vitiv. 2013, 28, 43–50. [Google Scholar]
- El Aou-ouad, H.; Montero, R.; Medrano, H.; Bota, J. Interactive effects of grapevine leafroll-associated virus 3 (GLRaV-3) and water stress on the physiology of Vitis vinifera L. cv. Malvasia de Banyalbufar and Giro-Ros. J. Plant Physiol. 2016, 196, 106–115. [Google Scholar] [CrossRef]
- Endeshaw, S.T.; Sabbatini, P.; Romanazzi, G.; Schilder, A.C.; Neri, D. Effects of grapevine leafroll associated virus 3 infection on growth, leaf gas exchange, yield and basic fruit chemistry of Vitis vinifera L. cv. Cabernet Franc. Sci. Hortic. 2014, 170, 228–236. [Google Scholar] [CrossRef]
- Montero, R.; Mundy, D.; Albright, A.; Grose, C.; Trought, M.C.T.; Cohen, D.; Chooi, K.M.; MacDiarmid, R.; Flexas, J.; Bota, J. Effects of Grapevine leafroll-associated virus 3 (GLRaV-3) and duration of infection on fruit composition and wine chemical profile of Vitis vinifera L. cv. Sauvignon blanc. Food Chem. 2016, 197, 1177–1183. [Google Scholar] [CrossRef]
- Vega, A.; Gutiérrez, R.A.; Pena-Neira, A.; Cramer, G.R.; Arce-Johnson, P. Compatible GLRaV-3 viral infections affect berry ripening decreasing sugar accumulation and anthocyanin biosynthesis in Vitis vinifera. Plant Mol. Biol. 2011, 77, 261–274. [Google Scholar] [CrossRef]
- Tsai, C.W.; Rowhani, A.; Golino, D.A.; Daane, K.M.; Almeida, R.P. Mealybug transmission of grapevine leafroll viruses: An analysis of virus–vector specificity. Phytopathology 2010, 100, 830–834. [Google Scholar] [CrossRef]
- Al Rwahnih, M.; Daubert, S.; Urbez-Torres, J.R.; Cordero, F.; Rowhani, A. Deep sequencing evidence from single grapevine plants reveals a virome dominated by mycoviruses. Arch. Virol. 2011, 156, 397–403. [Google Scholar] [CrossRef]
- Petter, F.; Giovani, B.; Trontin, C. International Cooperation to Support the Diagnosis of Forestry Pests: The Role of EPPO and Euphresco. Forests 2023, 14, 1461. [Google Scholar] [CrossRef]
- Rubio, R.O.; Rubio, E.O.; Pérez, C.O.; Izquierdo, M.A.P.; Rustioni, L.; Failla, O.; Chipashvili, R.; Maghradze, D. Ecological and sanitary characteristics of the Eurasian wild grapevine (Vitis vinifera L. ssp. sylvestris (Gmelin) Hegi) in Georgia (Caucasian region). Plant Genet. Resour. 2012, 10, 155–162. [Google Scholar]
- Pietersen, G.; Bell, V.A.; Krüger, K. Management of grapevine leafroll disease and associated vectors in vineyards. In Grapevine viruses: Molecular Biology, Diagnostics and Management; Springer International Publishing: Cham, Switzerland, 2017; pp. 531–560. [Google Scholar]
- Jung, Y. Re-creating economic and cultural values in Bulgaria’s wine industry: From an economy of quantity to an economy of quality? Econ. Anthropol. 2016, 3, 280–292. [Google Scholar] [CrossRef]
- Overton, J.; Murray, W.E. Playing the scales: Regional transformations and the differentiation of rural space in the Chilean wine industry. J. Rural. Stud. 2011, 27, 63–72. [Google Scholar] [CrossRef]
- Laimer, M.; Barba, M. Elimination of systemic pathogens by thermotherapy, tissue culture, or in vitro micrografting. In Virus and Virus-like Diseases of Pome and Stone Fruits; American Phytopathological Society: St. Paul, MN, USA, 2011; pp. 389–393. [Google Scholar]
- Bhat, A.I.; Rao, G.P. Virus elimination by meristem-tip culture. In Characterization of Plant Viruses: Methods and Protocols; Springer: New York, NY, USA, 2020; pp. 465–477. [Google Scholar]
- Bell, V.A.; Blouin, A.G.; Cohen, D.; Hedderley, D.I.; Oosthuizen, T.; Spreeth, N.; Lester, P.J.; Pietersen, G. Visual symptom identification of grapevine leafroll-associated virus 3 in red berry cultivars supports virus management by roguing. J. Plant Pathol. 2017, 99, 477–482. [Google Scholar]
- Fuller, K.B.; Alston, J.M.; Golino, D.A. The benefits from certified virus-free nursery stock: A case study of Grapevine leafroll-3. In The North Coast Region of California; Robert Mondavi Institute-Center for Wine Economics Working Paper Number 1306; UC-Davis: Davis, CA, USA, 2013; p. 35. [Google Scholar]
- Sun, Y.-D.; Folimonova, S.Y. Location matters: From changing a presumption about the citrus tristeza virus tissue tropism to understanding the stem pitting disease. New Phytol. 2022, 233, 631–638. [Google Scholar] [CrossRef]
- Olmos, A.; Bertolini, E.; Ruiz-García, A.B.; Martínez, C.; Peiró, R.; Vidal, E. Modeling the accuracy of three detection methods of Grapevine leafroll-associated virus 3 during the dormant period using a Bayesian approach. Phytopathology 2016, 106, 510–518. [Google Scholar] [CrossRef]
- Martelli, G.P. Directory of virus and virus-like diseases of the grapevine and their agents. J. Plant Pathol. 2014, 96, 1–136. [Google Scholar]
- Burger, J.T.; Maree, H.J.; Gouveia, P.; Naidu, R.A. Grapevine leafroll-associated virus 3. In Grapevine Viruses: Molecular Biology, Diagnostics and Management; Springer International Publishing: Cham, Switzerland, 2017; pp. 167–195. [Google Scholar]
- Bertazzon, N.; Angelini, E. Advances in the detection of Grapevine leafroll-associated virus 2 variants. J. Plant Pathol. 2004, 86, 283–290. [Google Scholar]
- Goszczynski, D.E.; Kasdorf, G.G.F.; Pietersen, G.; van Tonder, H. Detection of two strains of grapevine leafroll-associated virus 2. Vitis 1996, 35, 133–135. [Google Scholar]
- Martelli, G.P.; Gallitelli, D. Emerging and re-emerging viruses of plants. In Encyclopedia of Virology; Bamford, D.H., Zuckerman, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 86–92. [Google Scholar]
- Galvan, F.E.R.; Pavlick, R.; Trolley, G.; Aggarwal, S.; Sousa, D.; Starr, C.; Forrestel, E.; Bolton, S.; Alsina, M.D.M.; Dokoozlian, N.; et al. Scalable Early Detection of Grapevine Viral Infection with Airborne Imaging Spectroscopy. Phytopathology 2023, 113, 1439–1446. [Google Scholar] [CrossRef]
- Bendel, N.; Kicherer, A.; Backhaus, A.; Köckerling, J.; Maixner, M.; Bleser, E.; Klück, H.-C.; Seiffert, U.; Voegele, R.T.; Töpfer, R. Detection of Grapevine leafroll-associated virus 1 and 3 in white and red grapevine cultivars using hyperspectral imaging. Remote Sens. 2020, 12, 693. [Google Scholar] [CrossRef]
- Shabanian, M.; Xiao, H.; Meng, B. Seasonal dynamics and tissue distribution of two major viruses associated with grapevine Leafroll under cool climate condition. Eur. J. Plant Pathol. 2020, 158, 1017–1031. [Google Scholar] [CrossRef]
- Fazeli, C.F.; Habili, N.; Rezaian, M.A. Efficient cloning of cDNA from grapevine leafroll-associated virus 4 and demonstration of probe specificity by the viral antibody. J. Virol. Methods 1998, 70, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Abou Ghanem-Sabanadzovic, N.; Sabanadzovic, S.; Gugerli, P.; Rowhani, A. Genome organization, serology and phylogeny of Grapevine leafroll-associated viruses 4 and 6: Taxonomic implications. Virus Res. 2012, 163, 120–128. [Google Scholar] [CrossRef]
- Ling, K.S.; Zhu, H.Y.; Drong, R.F.; Slightom, J.L.; McFerson, J.R.; Gonsalves, D. Nucleotide sequence of the 3′-terminal two-thirds of the grapevine leafroll-associated virus-3 genome reveals a typical monopartite closterovirus. J. Gen. Virol. 1998, 79, 1299–1307. [Google Scholar] [CrossRef]
- Alkowni, R.; Rowhani, A.; Daubert, S.; Golino, D. Partial characterization of a new ampelovirus associated with grapevine leafroll disease. J. Plant Pathol. 2004, 86, 123–133. [Google Scholar]
- Pesqueira, A.M.; Cabaleiro, C.; Velasco, L. Genetic analysis of Grapevine leafroll-associated virus 3 population from Galicia, Spain. Plant Pathol. 2015, 65, 310–321. [Google Scholar] [CrossRef]
- Vondras, A.M.; Lerno, L.; Massonnet, M.; Minio, A.; Rowhani, A.; Liang, D.; Garcia, J.; Quiroz, D.; Figueroa-Balderas, R.; Golino, D.A.; et al. Rootstock influences the effect of grapevine leafroll-associated viruses on berry development and metabolism via abscisic acid signalling. Mol. Plant Pathol. 2021, 22, 984–1005. [Google Scholar] [CrossRef]
- Halldorson, M.M.; Keller, M. Grapevine leafroll disease alters leaf physiology but has little effect on plant cold hardiness. Planta 2018, 248, 1201–1211. [Google Scholar] [CrossRef]
- Cabaleiro, C.; Segura, A. Temporal analysis of Grapevine leafroll-associated virus 3 epidemics. Eur. J. Plant Pathol. 2006, 114, 441–446. [Google Scholar] [CrossRef]
- Cabaleiro, C.; Pesqueira, A.; García-Berrios, J. Influence of Grapevine leafroll-associated virus 3 in mature plants of Vitis vinifera L. cv Albariño on 110R and 196.17C rootstocks. S. Afr. J. Enol. Vitic. 2021, 42, 165–174. [Google Scholar] [CrossRef]
- Montero, R.; El Aou Ouad, H.; Pacifico, D.; Marzachì, C.; Castillo, N.; García, E.; Del Saz, N.F.; Florez-Sarasa, I.; Flexas, J.; Bota, J. Effects of Grapevine leafroll-associated virus 3 on physiology in asymptomatic plants of Vitis vinifera. Ann. Appl. Biol. 2017, 171, 155–171. [Google Scholar] [CrossRef]
- Moutinho-Pereira, J.; Correia, C.M.; Gonçalves, B.; Bacelar, E.A.; Coutinho, J.F.; Ferreira, H.F.; Lousada, J.L.; Cortez, M.I. Impacts of leafroll-associated viruses (GLRaV-1 and -3) on the physiology of the Portuguese grapevine cultivar ‘Touriga Nacional’ growing under field conditions. Ann. Appl. Biol. 2012, 160, 237–249. [Google Scholar] [CrossRef]
- Repetto, O.; Bertazzon, N.; De Rosso, M.; Miotti, L.; Flamini, R.; Angelini, E.; Borgo, M. Low susceptibility of grapevine infected by GLRaV-3 to late Plasmopara viticola infections: Towards understanding the phenomenon. Physiol. Mol. Plant Pathol. 2012, 79, 55–63. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).