Abstract
Objectives: Periapical periodontitis, which is a periodontal dysfunction, is a current clinical problem. Due to the frequency of occurrence and the adverse effects they cause, they are considered a social disease. They require detailed diagnostics to implement appropriate treatment. Mathematical calculations based on data obtained from radiological images used in routine clinical practice may help differentiate the forms of periodontitis. This study aimed to evaluate the areas affected by periodontitis in comparison to the healthy tissues of the periapical area. Methods: The study analyzed texture components using the gray-level co-occurrence matrix (GLCM) and the gray-level run-length matrix (GRLM) on an orthopantomogram (OPG) from 50 patients with clinically confirmed periodontitis treated at the Department of Maxillofacial and Plastic Surgery, University of Bialystok. Texture analysis was performed on defined regions of interest (ROIs) to distinguish diseased from healthy tissues. We employed four classification algorithms to assess model performance. Results: The data set included 50 patients, with 76 cases of periodontitis and 50 healthy ROIs. The reference standard was clinical diagnosis confirmed by two specialist doctors. The best-performing algorithm achieved an AUC of 98%. Conclusions: The obtained results showed significant statistical differences in the inflamed regions compared to the control, which may aid in diagnosing and selecting the treatment method for periodontitis.
1. Introduction
Diseases of the periapical tissues of the tooth are referred to as periapical periodontal diseases and include pathological processes affecting the periodontium, root cementum, and alveolar bone [1,2]. Periapical periodontitis, also known as apical periodontitis, is an inflammatory condition of the tissues surrounding the apex of a tooth root, most often resulting from untreated or advanced pulpitis or pulp necrosis. These conditions are primarily inflammatory in nature. They remain a significant and clinically relevant issue, affecting not only dentists but also clinicians in other specialties due to the close relationship between dental and systemic diseases. Healthy periapical tissues serve as the last barrier preventing the spread of infection from infected root canals to other, sometimes very distant, organs [2]. When inflamed, however, periapical tissues lose their defensive function and may themselves become a cause of serious systematic diseases, affecting the heart, kidneys, joints, and other organs [3,4,5,6]. The spread of inflammation from the tooth canal system to the periodontal tissue provokes an inflammatory reaction of varying severity, which may gradually extend to adjacent tissues. Periodontitis is also the most common cause of infections, abscesses, and osteitis of the face and neck, conditions that can progress rapidly and pose a significant threat to the patient’s health and even life [7].
The pathogenesis of periapical tissue inflammation is a complex and ongoing subject of discussion [8,9]. It is now well-established that such conditions arise through immunological reactions, both humoral and cellular, triggered primarily by infectious (bacterial) as well as non-infectious factors, including mechanical, chemical, thermal, trauma, and autoallergic mechanisms [2]. Because of the wide range and complexity of etiological factors, periapical inflammation can manifest in various clinical forms. Broadly, it follows a twofold course: acute inflammation, typically associated with pronounced pain, or chronic inflammation, which often progresses without symptoms. Acute inflammatory symptoms may also occur with exacerbation of long-standing chronic lesions [2,7]. Importantly, the majority of periapical inflammations are non-exacerbated and therefore asymptomatic. This creates the risk of latent alveolar infection foci within the oral cavity. Detecting and treating such silent infections represent a significant responsibility for the dentist. Their successful management requires not only extensive knowledge and diligence but also systematic clinical and radiological follow-up, even after treatment has been completed [2].
Radiological diagnosis of periodontitis typically involves periapical radiographs (PR) and orthopantomogram (OPG) of the maxilla and mandible, which reveal pathological changes of the periodontium [10,11]. PRs demonstrate high diagnostic accuracy, exceeding 90%, making them the most frequently used method for detecting dental pathologies [12]. In contrast, OPGs provides a panoramic image of both the mandible and the maxilla in a single exposure, enabling assessment of the entire oral cavity [13,14]. Unfortunately, OPGs have lower effectiveness, reaching only about 48.8% [15]. Both PR and OPG have inherent limitations [16], including the superimposition of anatomical structures [16], their two-dimensional nature [16], and the requirement that a lesion must cause 30–50% bone mineral loss before become visible radiographically [17]. Despite these limitations, they remain fundamental diagnostic tools due to their accessibility and widespread use in clinical practice. Increasingly, cone-beam computed tomography (CBCT) has become increasingly important in endodontic diagnosis [16,18,19]. CBCT offers detailed three-dimensional visualization of dental and bone structures, providing more precise information than traditional radiographs [20]. It has proven superior to PR in detecting periapical lesions [21]. A recent study by Mostafapoor et al. [22] demonstrated that CBCT achieved 95% sensitivity and 90% specificity in diagnosing apical periodontitis. However, some studies present conflicting results, highlighting a risk of overdiagnosis, as confirmed by discrepancies with histological findings [23]. Chang et al. [24] investigated the detection of periapical lesions in cancellous bone using radiographic methods. Two independent specialists identified potential lesions, which were subsequently confirmed histopathologically, achieving a classification accuracy of 97.6%. Similar studies [25] have evaluated PR, OPG, and CBCT, concluding that OPG has notable limitations and should not be the sole diagnostic method, particularly when assessing the periodontium after root canal treatment. For more accurate diagnosis, supplementary imaging, such as PR or CBCT, is often necessary. These studies emphasize that the human eye’s ability to detect periapical changes depends on numerous factors. Medical imaging provides much more information than visual inspection, as a standard grayscale image has a range of 0 to 255 levels. However, human vision cannot reliably distinguish between many levels of gray. Image perception is also influenced by factors like lighting conditions and individual variability in visual interpretation. In a study by Kazimierczak et al. [26], periapical lesions were evaluated using OPG and CBCT in combination with artificial intelligence (AI). The AI achieved a sensitivity of 33.33% for OPG images and 77.78% for CBCT images. Nevertheless, there remains a lack of studies capable of characterizing structural changes in bone around tooth apices. Consequently, efforts are being made to extract quantitative information from images. One such approach is texture analysis, which quantifies the spatial distribution of pixel intensity in an image. This method enables the assessment of tissue characteristics, such as smoothness, graininess, roughness, and homogeneity [27].
In recent years, medical image analysis based on texture analysis has gained increasing attention [27,28,29]. Methods like the gray-level co-occurrence matrix (GLCM) and the gray-level run-length matrix (GRLM) allow for precise and objective assessment of image texture. The texture parameters derived from these methods provide a basis for recognizing texture type, shape features, and characteristic structural elements. They also enable the accurate evaluation of changes occurring within the tissues by analyzing texture features in specific directions. Texture analysis has been applied in several dental and medical imaging contexts. For example, it has been used to detect periodontal inflammation in CBCT images [27], to compare gingival inflammation with healthy oral tissue in optical coherence tomography (OCT) images [28], and to differentiate periapical granulomas from periapical cysts in magnetic resonance (MR) images [29].
This study aimed to examine different texture features of inflammatory changes in the periodontium of the lower teeth in OPG images extracted from GRLM and GLCM and to perform a classification of the obtained texture features.
2. Materials and Methods
2.1. Data Collection
The study included 50 patients (24 women, aged 19–56 years, with a mean age of 42, and 26 men, aged 26–62 years, with a mean age of 48 years) who were treated at the Department of Maxillofacial and Plastic Surgery, University of Bialystok, between 1 March 2024, and 1 March 2025. Inflammatory changes in the periodontium of the lower teeth were evaluated on OPG images in patients treated for mandibular fractures. The study included selected teeth with inflammatory periodontal changes located on the side opposite to the fracture, as we intended to use the OPG of these patients for further research related to the assessment of periodontal inflammation. The ROI was evaluated in the mandible, in the 3rd or 4th quadrant. Teeth with vital pulp and normal periapical tissues were excluded. Patients with lesions exceeding 1 cm of bone destruction around the root apices, extensive bone loss, root displacement, suspected metabolic, genetic, neoplastic diseases, or bone dysplasia were also excluded. Additionally, individuals using bone substitute materials were not included in the study. Inclusion criteria: presence of periapical periodontitis in the area of the premolar and molar teeth on the side opposite to the fracture. Exclusion criteria: past mandibular fractures, previous surgical procedures involving the jawbones and teeth, genetic disorders affecting the structure of bones and teeth, history of radiotherapy, chemotherapy, or bisphosphonate treatment, specific jaw infections (tuberculosis, actinomycosis, syphilis), and systemic diseases (e.g., diabetes, osteoporosis, rheumatoid arthritis, hormonal disorders, kidney diseases). The criteria for diagnosing periapical periodontitis included data from the medical history (history of mild tooth pain in the past or absence of tooth pain symptoms), clinical examination (carious lesion on the tooth, lack of pulp response to vitality tests, filling, prosthetic crown), and OPG (uneven widening of the periodontal space, destruction of the alveolar bone plate, thinning of the bone structure around the tooth roots, processes of destruction and regeneration of the tooth and periapical tissues). The diagnosis was based on the combination of these symptoms and OPG images. The OPG imaging was performed using a panoramic and cephalometric extraoral dental radiograph (Orthopantomograph OP100 and Orthoceph OC100 dental X-ray system, Instrumentarium Imaging Inc., Tuusula, Finland).
2.2. Image Processing
The study was designed to utilize OPG input images to assess the structure of periodontal inflammatory changes based on texture analysis. The study workflow is presented in Figure 1 and includes the following steps performed on the input images: mask annotation, texture feature extraction, statistical analysis, and image classification.
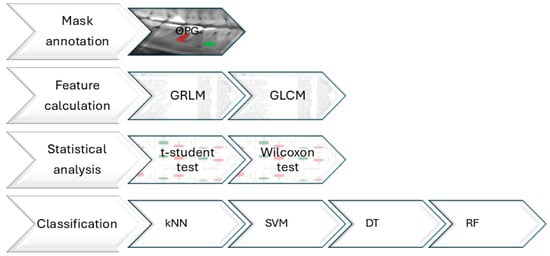
Figure 1.
The study workflow for orthopantomographic (OPG) images of healthy (green color) and periodontally inflamed tissues (red color). Images were pre-processed to segment regions of interests (ROIs). From given ROIs, texture features were extracted using the gray-level co-occurrence matrix (GLCM) and the gray-level run-length matrix (GRLM). Significant texture features were selected. Based on the selected texture features, images were classified using four classifiers: support vector machine (SVM), k-nearest neighbors (kNN), random forest (RF), and decision tree (DT) algorithms.
2.2.1. Mask Annotation
The masks representing healthy and periodontal inflammatory changes in tissues were manually annotated (Figure 2). An expert clinician with specialized training performed the annotation. The masks segmented each image into ROIs representing the area of study. The masks were annotated using qMaZda software version 4.6 [30,31].
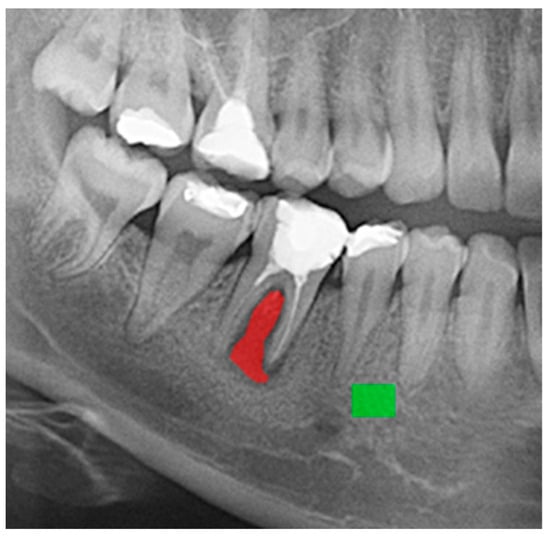
Figure 2.
Example of region of interest (ROI) outlines: red for the periodontal inflammatory changes in tissues and green for the healthy tissues.
2.2.2. Extraction of Image Texture Features
Texture features were computed from the determined ROIs of monochrome images with gray levels ranging from 0 (meaning black color pixel) to 255 (meaning white color pixel) using dedicated qMaZda software version 4.6 [30,31]. The color image RGB (R—red, G—green, and B—blue components) was converted to a monochrome color model YUV, where the Y (brightness) component is a weighted sum of the R, G, and B components. The Y component was calculated by means of the formula
The image analysis was performed without normalization and quantized with five (5) bits per pixel, as the images were recorded on the same device by the same radiologist.
The following texture features were selected to extract qualitative information from images:
- (i)
- Gray-level run-length matrix (GRLM), where contains elements representing the number of occurrences of pixel sequences with grayscale level and length [32]:where is the maximum run length. Based on the matrix, the following seven features can be calculated for four image directions (i.e., [33]:
- Short-run emphasis (ShrtREmph), second-order inversion moment examining the impact of short runs,
- Long-run emphasis (LngREmph), second-order inversion moment examining the impact of long runs,
- Gray-level nonuniformity (GLevNonUni), coefficient of heterogeneity of gray-level distribution,
- Run-length nonuniformity (RLNonUni), coefficient of heterogeneity of run-length distribution,
- A fraction of the image in runs (Fraction), the proportion of runs in an image,
- Run-length nonuniformity moment (MRLNonUni), variability of the distribution of run lengths in the image,
- Gray-level nonuniformity moment (MGLNonUni), variability of the distribution of gray level in the image,
- (ii)
- The gray-level co-occurrence matrix (GLCM), contains the probabilities of occurrence of pairs of pixels with grayscale levels and for a specified distance (5) between pixels and direction of analysis:Based on the matrix, the following eleven features can be calculated for different positions determined by the distance between pixels (d = 5 in this study) and four image directions (i.e., [34]:
- Angular second moment (AngScMom) measures homogeneity, and a high value indicates constant or repeatable pixel grayscale,
- Contrast (Contrast) measures the difference between the darkest and lightest pixels in an image, and a low value indicates no difference between pixels,
- Correlation (Correlat) describes the degree of correlation between pixels in the image, and a high value means that the pixels have similar values,
- Sum of squares (SumOfSqs) measures the range of pixel values around the mean,
- Inverse difference moment (InvDfMom) describes local homogeneity, and a high value means smaller differences between pixels and greater homogeneity,
- Sum average (SumAverg) determines the average value of the sum of pixel intensity pairs, indicating the general brightness of the image,
- Sum variance (SumVarnc) represents the range of pixel intensity values,
- Sum entropy (SumEntrp) measures disorder in the distribution of intensity sums, and a high value indicates greater unpredictability,
- Entropy (Entropy) measures the disorder and complexity of texture and indicates the degree of randomness,
- Difference variance (DifVarnc) describes the spread of pixel value differences,
- Difference entropy (DifEntrp) measures the texture complexity of the differences between pixelswhere
Input data containing features that differed significantly from one another were standardized using the StandardScaler from the Scikit–learn library in Python version 3.11 (https://scikit-learn.org/stable/index.html, accessed on 2 June 2025) [35].
2.2.3. Image Classification
Data classification is one of the most commonly used machine learning tasks. It focuses on assigning appropriate classes to new objects. Various techniques and learning algorithms, known as classifiers, can be used to solve classification tasks. Among them, we can distinguish classifiers:
- The support vector machine (SVM) classifier is a machine learning algorithm that finds a hyperplane in space ( —number of features) that classifies data points. The SVM classifier works by finding the optimal hyperplane that maximizes the distance between the closest points of different classes, called support vectors. This allows the classifier to effectively separate data even when the classes are non-linearly separable in the original space [36]. The SVM classification steps were executed with predefined hyperparameters: a cost parameter C = 10 and a radial basis function (RBF) kernel with gamma = 0.01; numerical tolerance was set to 0.001 (https://scikit-learn.org, accessed on 2 June 2025).
- The k-nearest neighbors classifier (kNN) is a simple classifier that assigns a new point in feature space to a specific class based on the distance from the nearest neighbors. This distance is interpreted as the inverse of a similarity measure. Using a distance metric, such as Euclidean, kNN calculates the distance between the new point and other points in the training set. It then assigns the new point to the class to which most of its nearest neighbors belong. The number determines the number of neighbors considered in the classification [36]. The kNN classification steps were executed with predefined hyperparameters, k = 5, using the Euclidean distance metric and uniform weighting (https://scikit-learn.org, accessed on 2 June 2025).
- The decision tree classifier (DT) is a popular classification algorithm used in models that combine multiple classifiers [36]. The basic idea is to recursively divide a data set into smaller subsets based on how this division will affect the final class selection. The DT classification steps were executed with predefined hyperparameters; the maximum depth of the tree = 4, using the Gini function to measure the quality of a split (https://scikit-learn.org, accessed on 2 June 2025).
- The random forest classifier (RF) is a set of decision trees. The idea of its construction is to train a group of single decision tree classifiers, each for a separate subset of data [36]. Predictions from individual trees are used in majority voting, which aims to select the class that received the most votes. The RF classification steps were executed with predefined hyperparameters; the number of trees in the forest = 100 (https://scikit-learn.org, accessed on 2 June 2025).
Images were classified as 0 (healthy tissue) or 1 (periodontally inflamed tissue) based solely on the selected texture features (the selection was made based on statistical analysis, choosing those that showed significant statistical differences) extracted from OPG images using four classifiers: SVM, kNN, RF, and DT. The performance of the classifier in texture analysis methods was assessed using the error rate, which is described in terms of true and false positives and true and false negatives as follows. True Positive (TP): correct identification of abnormal cases; True Negative (TN): correct identification of normal cases; False Positive (FP): normal cases incorrectly labeled as abnormal; and False Negative (FN): abnormal cases incorrectly labeled as normal. These terms are crucial for evaluating the clinical effectiveness of the classification algorithms. The key metrics were calculated as Recall (Sensitivity) = TP/(TP + FN) × 100), the ability to correctly detect abnormal cases; Precision = TN/(TN + FP) × 100), the ability to accurately identify normal cases; F1 score = 2 × (Precision × Recall)/(Precision + Recall), combines precision and recall as the harmonic mean; Accuracy = (TP + TN)/(TP + TN + FP + FN) × 100, the overall correctness of the diagnoses; and the area under the curve (AUC) of the receiver operating characteristic (ROC). Classification metrics were calculated relative to the healthy and periodontitis tissues. Classification performance was evaluated using 5-fold stratified cross-validation accuracy (CV accuracy). Image classification and metrics were implemented using the Scikit–learn library in Python version 3.11 (https://scikit-learn.org/stable/index.html, accessed on 2 June 2025) [35].
2.3. Statistical Analysis
Data from 50 OPG images included separately annotated ROIs of the control group representing the healthy tissues (healthy group, n = 50) and the periodontal inflammatory changes in tissues (periodontitis group, n = 76). Each ROI was characterized by 72 image texture features (11 GLCM features and 7 GRLM features in four directions). Data series were tested independently for univariate distributions using a Shapiro–Wilk normality test. The paired t-test (for Gaussian distributed data) or the Wilcoxon signed-rank test (for non-Gaussian distributed data) was used to distinguish the ROIs obtained from healthy and periodontally inflamed tissues. Multiple comparison corrections, such as the Bonferroni correction, were applied to adjust p-values for the multiple tests performed. The sample size was determined through a power analysis with power (1 − β) set to 0.8 and an alpha (α) level of 0.05 to ensure adequate sensitivity for detecting a significant effect. The significantly difference features between groups in terms of mean ± standard deviation (SD) were reported.
All statistical analyses were performed using the SciPy library in Python (https://scipy.org/, accessed on 2 June 2025) [37], with a significance level set at p < 0.05.
3. Results
Among the 72 texture features extracted from different directions (H, V, N, and Z) using GRLM and GLCM, 66 features showed significant differences between healthy and periodontitis tissues. The values of all examined texture features with a marked significant level are presented in Figure 3 and Figure 4.
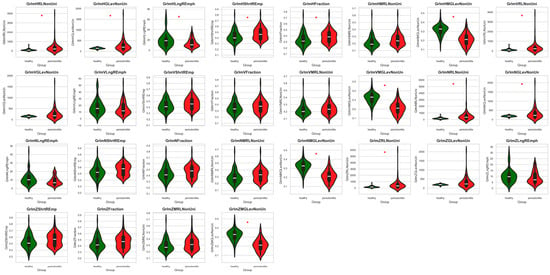
Figure 3.
Features of gray-level run-length matrix (GRLM) comparing between the healthy tissue and periodontitis tissue groups. Differences between groups are indicated with a red star when p < 0.05.
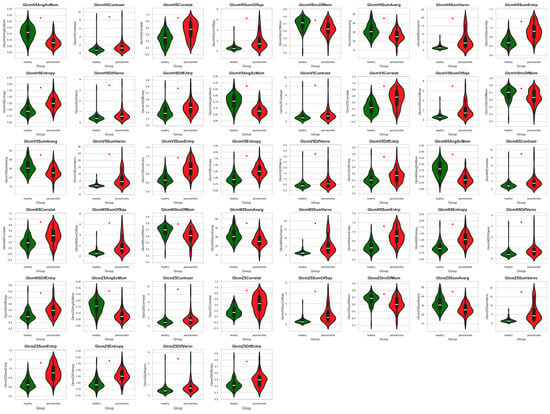
Figure 4.
Features of the gray-level co-occurrence matrix (GLCM) comparing between the healthy tissue and periodontitis tissue groups. Differences between groups are indicated with a red star when p < 0.05.
For the GRLM features, 20 out of 28 (71.4%) differed between the healthy tissue and periodontitis tissue groups. ShrtREmph, GLevNonUni (H direction), RLNonUni, Fraction (H and N directions), and MRLNonUni (H and N directions) were higher in periodontitis images than in healthy images. In contrast, LngREmph (H direction) and MGLNonUni were lower in periodontitis images than in healthy images (Figure 3, Table 1).

Table 1.
Values (mean ± SD) of gray-level run-length matrix (GRLM) significant features of orthopantomographic (OPG) images comparing the healthy tissue and periodontitis tissue groups. Statistical significance was set at p < 0.05 ( after the Bonferroni correction).
Regarding the features extracted using the GLCM, 44 out of 44 (100%) differed between the healthy tissue and periodontitis tissue groups. Contrast, Correlat, SumOfSqs, SumVarnc, SumEntrp, Entropy, DifVarnc, and DifEntrp in all directions were higher in periodontitis images, while AngScMom, InvDfMom, and SumAverg were lower compared to healthy images (Figure 4, Table 2).

Table 2.
Values (mean ± SD) of the gray-level co-occurrence matrix (GLCM) significant features of orthopantomographic (OPG) images comparing between the healthy tissue and periodontitis tissue groups. Statistical significance was set at p < 0.05 ( after the Bonferroni correction).
Choosing the direction of analysis is not a simple matter; however, the number of calculated parameters makes further analysis even more challenging. Therefore, instead of selecting a specific direction, averaging was used to obtain a more stable and representative result.
For the GRLM features, all of them differed between the healthy tissue and the periodontitis tissue groups after averaging across directions. ShrtREmph, GLevNonUni, RLNonUni, Fraction, and MRLNonUni were higher in periodontitis images than in healthy images. In contrast, LngREmph and MGLNonUni were lower in periodontitis images than in healthy images (Figure 5, Table 3).

Figure 5.
Features of gray-level run-length matrix (GRLM) averaged across analysis directions comparing between the healthy tissue and periodontitis tissue groups. Differences between groups are indicated with a red star when p < 0.05.

Table 3.
Values (mean ± SD) of gray-level run-length matrix (GRLM) significant features averaged across analysis directions of orthopantomographic (OPG) images comparing between the healthy tissue and periodontitis tissue groups. Statistical significance was set at p < 0.05 ( after the Bonferroni correction).
For the GLCM features, all of them also differed between the healthy tissue and the periodontitis tissue groups. Contrast, Correlat, SumOfSqs, SumVarnc, SumEntrp, Entropy, DifVarnc, and DifEntrp were higher in periodontitis images, while AngScMom, InvDfMom, and SumAverg were lower compared to healthy images (Figure 6, Table 4).

Figure 6.
Features of the gray-level co-occurrence matrix (GLCM) averaged across analysis directions comparing between the healthy tissue and periodontitis tissue groups. Differences between groups are indicated with a red star when p < 0.05.

Table 4.
Values (mean ± SD) of the gray-level co-occurrence matrix (GLCM) significant features averaged across analysis directions of orthopantomographic (OPG) images comparing between the healthy tissue and periodontitis tissue groups. Statistical significance was set at p < 0.05 ( after the Bonferroni correction).
The ROC analysis of the algorithms for classifying OPG images demonstrated a high AUC of the RF and DT algorithms (0.99) when using texture features from the features in directions that differed significantly between the healthy and periodontitis tissue images; an even higher AUC of the SVM, RF, and DT algorithms (0.96) was achieved with the mean feature set (Figure 7).
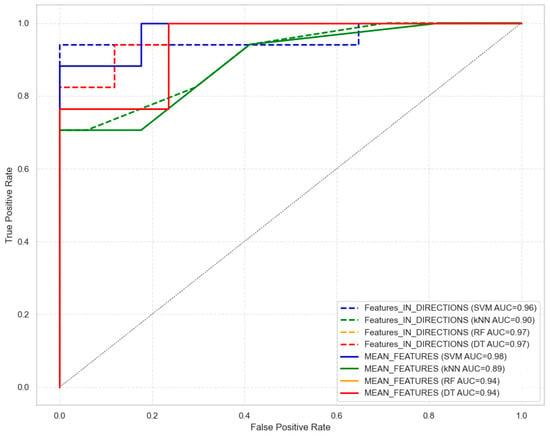
Figure 7.
Receiver operating characteristic (ROC) and area under the curve (AUC) of the support vector machine (SVM), the k-nearest neighbors (kNN), the random forest (RF), and the decision tree (DT) algorithms, comparing classification efficiency between significantly different texture-feature-based identification of the healthy and periodontitis tissues analyzed in directions and averaging across directions. The results of classification using RF and DT are the same.
These ROC findings were supported by classification metrics, all of which exceeded 0.76 in accuracy. The highest classification performance was achieved by the SVM algorithm using the feature set in directions, with an accuracy of 0.94, precision of 1.00, and an F1 score of 0.94 (Table 5). Slightly lower performance was observed for the RF algorithm (accuracy: 0.88; precision: 0.84; F1 score: 0.89) and the DT algorithm (accuracy: 0.88; precision: 0.84; F1 score: 0.89), and the lowest was observed for the kNN algorithm (accuracy: 0.76; precision: 0.80; F1 score: 0.89) (Table 5). The highest classification performance was also achieved by the SVM algorithm using the mean feature set, with an accuracy of 0.88, precision of 0.88, and an F1 score of 0.89 (Table 5). Slightly lower performance was observed for the kNN algorithm (accuracy: 0.85; precision: 1.00; F1 score: 0.88), and the lowest was observed for the RF and DT algorithms (accuracy: 0.82; precision: 0.82; F1 score: 0.82) (Table 5). CV accuracy was also highest for the feature set in directions of the SVM algorithm (0.86), and it was lowest for the DT algorithm (0.77) (Table 5). Slightly lower CV accuracy was observed for the mean feature set of the SVM algorithm (0.84).

Table 5.
Classification metrics of 5-fold stratified cross-validation (mean, [min; max]: accuracy, precision, recall, F1 score) of the support vector machine (SVM), the k-nearest neighbors (kNN), the random forest (RF), and the decision tree (DT) algorithms, comparing classification efficiency between significantly different texture-feature-based identification of the healthy and periodontitis tissues analyzed in directions and averaging across directions.
4. Discussion
The evaluation of pantomographic image texture enables the identification of inflammatory changes in the periodontium. Periodontitis can be assessed using various imaging techniques. Fleiner et al. utilized CBCT, which allowed for accurate evaluation of bone structures, particularly bone defects and furcation lesions [38]. The applied approach provided a more precise determination of radiological density, facilitating the detection of subtle changes in bone tissue. Gonçalves et al. also applied CBCT in patients with grade C periodontitis and identified significant texture features that may accelerate clinical decision making in the treatment process [27]. Similarly, CBCT combined with texture analysis has been shown to support accuracy and quantification of tumor lesions’ heterogeneity [39]. Pociaski et al. evaluated the usefulness of texture analysis in differentiating between two common types of lytic lesions visible on radiographic images [40].
In this study, panoramic images were used, as they are routinely examined and enable the simultaneous visualization of multiple anatomical structures. Segmented ROIs, including areas affected by periodontitis and healthy oral tissue, were crucial for conducting texture analysis. Our texture analysis results demonstrated statistically significant differences in textural features between the healthy and periodontitis tissue groups. In GLCM analysis, values of Contrast, Correlat, SumOfSqs, SumVarnc, SumEntrp, Entropy, DifVarnc, and DifEntrp in all directions increased with periodontitis changes, whereas values of AngScMom, InvDfMom, and SumAverg decreased. The increase in Contrast indicates greater differences in gray levels between neighboring pixels, indicating potential pathological changes, such as tissue destruction. Higher Correlation values indicate stronger dependencies between gray levels in neighboring pixels, pointing to altered pathological texture. Increased values of SumOfSqs, SumVarnc, SumEntrp, Entropy, DifVarnc, and DifEntrp suggest greater textural complexity, heterogeneity, and irregularity, which are characteristic of pathological tissues. Similarly, in GLRLM analysis, values of ShrtREmph, GLevNonUni (H direction), RLNonUni, Fraction (H and N directions), and MRLNonUni (H and N directions) increased with periodontitis changes, while LngREmph (H direction) and MGLNonUni decreased. An increase in ShrtREmph indicates greater textural irregularity and complexity, while higher GLevNonUni and RLNonUni values reflect increased gray level diversity. Conversely, decreases in LngREmph (in the H direction) and MGLNonUni values suggest that the texture becomes more fragmented and less uniform due to pathological alternations.
The results of the texture analysis of panoramic images suggest a strong potential for detecting inflammatory changes in the oral cavity. These findings may provide a foundation for further research focused on periodontitis identification. Moreover, AI-based classification methods proved to be effective diagnostic tools, achieving high accuracy in correctly identifying periodontitis cases based on panoramic images. It is worth highlighting that the analysis of statistically significant differences in texture features between healthy tissue and periodontitis was further validated using classifiers. Classification based on all significant texture features in the identified directions of the GLCM and GLRLM analyses achieved an accuracy of up to 94% with SVM, while generalization of the calculated features through averaging across directions reached 88% with SVM.
However, several limitations of this study should be acknowledged. First, all images were acquired using a single device, which may limit the generalization of the findings across different imaging systems. Second, the study was conducted on a relatively small patient group, reducing the ability to extrapolate the results to larger or more diverse populations, particularly those with comorbidities. Third, the diagnoses were not confirmed histopathologically, and therefore the results should be interpreted with caution. Finally, OPG images are known to have inherent limitations, including low spatial resolution and potential distortion. Further studies should aim to validate these findings using more precise imaging modalities, such as CBCT, intraoral PR, or OCT.
5. Conclusions
Texture analysis of OPG images, utilizing various feature extraction methods based on GRLM and GLCM, enables the identification of significant differences in the bone tissue surrounding titanium fixations in the maxilla and in the mandible compared to healthy bone. GLCM and GRLM features provide the most valuable information for the quantitative assessment of oral tissue, reflecting changes in pixel intensity distribution, heterogeneity, and texture asymmetry. Additionally, the application of classification confirmed the potential of using textural features as a diagnostic support for regions affected by periodontitis.
Author Contributions
Conceptualization, B.A., M.B. and J.B.; methodology, B.A., M.B., E.M., K.Ł. and J.B.; software, M.B., E.M. and Ł.W.; validation, J.B.; formal analysis, B.A., E.M. and M.B.; investigation, B.A., M.B., Ł.W., K.Ł. and J.B.; resources, B.A. and M.B.; data curation, B.A.; writing—original draft preparation, B.A. and M.B.; writing—review and editing, B.A., M.B., E.M., K.Ł., Ł.W. and J.B.; visualization, M.B. and Ł.W.; supervision, J.B.; project administration, B.A., M.B. and J.B.; funding acquisition, B.A. and J.B. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the Polish Ministry of Science and Higher Education as a part of the project WZ/WM–IIB/2/2024.
Institutional Review Board Statement
All patients were informed about the nature of the study and the purpose of analyzing orthopantomographic images. Each of them provided written informed consent to participate in the study. The experiment was conducted in accordance with the Declaration of Helsinki-1964 (with modifications from October 2013, Fortaleza, Brazil). All procedures included in this study were approved by the Bioethics Committee of the Medical University of Bialystok (APK.002.102.2024), issued on 22 February 2024, for the period from 22 February 2024, to 30 December 2030.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Raja, S.V.; Emmadi, P.; Namasivayam, A.; Thyegarajan, R.; Rajaraman, V. The periodontal–endodontic continuum: A review. J. Conserv. Dent. Endod. 2008, 11, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Hegde, J.; Prakash, V.; Srirekha, A. Concise Conservative Dentistry and Endodontics-E Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Bahekar, A.A.; Singh, S.; Saha, S.; Molnar, J.; Arora, R. The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: A meta-analysis. Am. Heart J. 2007, 154, 830–837. [Google Scholar] [CrossRef]
- Rahimi, A.; Afshari, Z. Periodontitis and cardiovascular disease: A literature review. ARYA Atheroscler. 2021, 17, 1. [Google Scholar]
- Chapple, I.L.; Hirschfeld, J.; Cockwell, P.; Dietrich, T.; Sharma, P. Interplay between periodontitis and chronic kidney disease. Nat. Rev. Nephrol. 2025, 21, 226–240. [Google Scholar] [CrossRef]
- Araújo, V.M.A.; Melo, I.M.; Lima, V. Relationship between periodontitis and rheumatoid arthritis: Review of the literature. Mediat. Inflamm. 2015, 2015, 259074. [Google Scholar] [CrossRef]
- Orstavik, D. Essential Endodontology: Prevention and Treatment of Apical Periodontitis; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Graunaite, I.; Lodiene, G.; Maciulskiene, V. Pathogenesis of apical periodontitis: A literature review. J. Oral Maxillofac. Res. 2012, 2, e1. [Google Scholar] [CrossRef]
- Braz-Silva, P.H.; Bergamini, M.L.; Mardegan, A.P.; De Rosa, C.S.; Hasseus, B.; Jonasson, P. Inflammatory profile of chronic apical periodontitis: A literature review. Acta Odontol. Scand. 2019, 77, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Boreak, N.; Hakami, A.; Hakami, S.; Nahari, H.; Alhakami, M.; Al Dira, M.; Mahdali, H.; Kariri, S.F. A systematic review of traditional radiology: Conservative therapy in endodontics. Saudi J. Oral. Dent. Res. 2022, 7, 317–323. [Google Scholar] [CrossRef]
- Rotstein, I.; Simon, J.H. Diagnosis, prognosis and decision-making in the treatment of combined periodontal-endodontic lesions. Periodontology 2000 2004, 34, 165–203. [Google Scholar] [CrossRef]
- Karamifar, K.; Tondari, A.; Saghiri, M.A. Endodontic periapical lesion: An overview on the etiology, diagnosis and current treatment modalities. Eur. Endod. J. 2020, 5, 54. [Google Scholar] [CrossRef]
- Nardi, C.; Calistri, L.; Pradella, S.; Desideri, I.; Lorini, C.; Colagrande, S. Accuracy of orthopantomography for apical periodontitis without endodontic treatment. J. Endod. 2017, 43, 1640–1646. [Google Scholar] [CrossRef]
- Estrela, C.; Bueno, M.R.; Leles, C.R.; Azevedo, B.; Azevedo, J.R. Accuracy of cone beam computed tomography and panoramic and periapical radiography for detection of apical periodontitis. J. Endod. 2008, 34, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Nardi, C.; Calistri, L.; Pietragalla, M.; Vignoli, C.; Lorini, C.; Berti, V.; Mungai, F.; Colagrande, S. Electronic processing of digital panoramic radiography for the detection of apical periodontitis. La Radiol. Medica 2020, 125, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Dawood, A.; Mannocci, F.; Wilson, R.; Pitt Ford, T. Detection of periapical bone defects in human jaws using cone beam computed tomography and intraoral radiography. Int. Endod. J. 2009, 42, 507–515. [Google Scholar] [CrossRef]
- Bender, I.; Seltzer, S. Roentgenographic and direct observation of experimental lesions in bone: I. J. Endod. 2003, 29, 702–706. [Google Scholar] [CrossRef]
- Arias, E.; Huang, Y.-H.; Zhao, L.; Seelaus, R.; Patel, P.; Cohen, M. Virtual surgical planning and three-dimensional printed guide for soft tissue correction in facial asymmetry. J. Craniofacial Surg. 2019, 30, 846–850. [Google Scholar] [CrossRef]
- Garlapati, K.; DB, G.B.; Chaitanya, N.C.; Guduru, H.; Rembers, A.; Soni, P. Evaluation of preference and purpose of utilisation of cone beam computed tomography (CBCT) compared to orthopantomogram (OPG) by dental practitioners—A cross-sectional study. Pol. J. Radiol. 2017, 82, 248–251. [Google Scholar] [CrossRef]
- Kaasalainen, T.; Ekholm, M.; Siiskonen, T.; Kortesniemi, M. Dental cone beam CT: An updated review. Phys. Medica 2021, 88, 193–217. [Google Scholar] [CrossRef]
- Davies, A.; Mannocci, F.; Mitchell, P.; Andiappan, M.; Patel, S. The detection of periapical pathoses in root filled teeth using single and parallax periapical radiographs versus cone beam computed tomography—A clinical study. Int. Endod. J. 2015, 48, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Mostafapoor, M.; Hemmatian, S. Evaluation of the accuracy values of cone-beam CT regarding apical periodontitis: A systematic review and meta-analysis. Oral Radiol. 2022, 38, 309–314. [Google Scholar] [CrossRef]
- Kruse, C.; Spin-Neto, R.; Reibel, J.; Wenzel, A.; Kirkevang, L.-L. Diagnostic validity of periapical radiography and CBCT for assessing periapical lesions that persist after endodontic surgery. In Dentomaxillofacial Radiology; Oxford University Press: Oxford, UK, 2017; Volume 46. [Google Scholar] [CrossRef]
- Chang, L.; Umorin, M.; Augsburger, R.A.; Glickman, G.N.; Jalali, P. Periradicular lesions in cancellous bone can be detected radiographically. J. Endod. 2020, 46, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Nardi, C.; Calistri, L.; Grazzini, G.; Desideri, I.; Lorini, C.; Occhipinti, M.; Mungai, F.; Colagrande, S. Is panoramic radiography an accurate imaging technique for the detection of endodontically treated asymptomatic apical periodontitis? J. Endod. 2018, 44, 1500–1508. [Google Scholar] [CrossRef]
- Kazimierczak, W.; Wajer, R.; Wajer, A.; Kiian, V.; Kloska, A.; Kazimierczak, N.; Janiszewska-Olszowska, J.; Serafin, Z. Periapical lesions in panoramic radiography and CBCT imaging-assessment of AI’s diagnostic accuracy. J. Clin. Med. 2024, 13, 2709. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, B.C.; de Araújo, E.C.; Nussi, A.D.; Bechara, N.; Sarmento, D.; Oliveira, M.S.; Santamaria, M.P.; Costa, A.L.F.; Lopes, S. Texture analysis of cone-beam computed tomography images assists the detection of furcal lesion. J. Periodontol. 2020, 91, 1159–1166. [Google Scholar] [CrossRef]
- Won, J.; Huang, P.-C.; Spillman Jr, D.R.; Chaney, E.J.; Adam, R.; Klukowska, M.; Barkalifa, R.; Boppart, S.A. Handheld optical coherence tomography for clinical assessment of dental plaque and gingiva. J. Biomed. Opt. 2020, 25, 116011. [Google Scholar] [CrossRef]
- Juerchott, A.; Pfefferle, T.; Flechtenmacher, C.; Mente, J.; Bendszus, M.; Heiland, S.; Hilgenfeld, T. Differentiation of periapical granulomas and cysts by using dental MRI: A pilot study. Int. J. Oral Sci. 2018, 10, 17. [Google Scholar] [CrossRef]
- Szczypiński, P.M.; Klepaczko, A. MaZda—A framework for biomedical image texture analysis and data exploration. In Biomedical Texture Analysis; Elsevier: Amsterdam, The Netherlands, 2017; pp. 315–347. [Google Scholar]
- Szczypiński, P.M.; Strzelecki, M.; Materka, A.; Klepaczko, A. MaZda-a software package for image texture analysis. Comput. Methods Programs Biomed. 2009, 94, 66–76. [Google Scholar] [CrossRef]
- Galloway, M.M. Texture analysis using gray level run lengths. Comput. Graph. Image Process. 1975, 4, 172–179. [Google Scholar] [CrossRef]
- Tang, X. Texture information in run-length matrices. IEEE Trans. Image Process. 1998, 7, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Haralick, R.M. Statistical and structural approaches to texture. Proc. IEEE 1979, 67, 786–804. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Bębas, E.; Borowska, M.; Derlatka, M.; Oczeretko, E.; Hładuński, M.; Szumowski, P.; Mojsak, M. Machine-learning-based classification of the histological subtype of non-small-cell lung cancer using MRI texture analysis. Biomed. Signal Process. Control 2021, 66, 102446. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Fleiner, J.; Hannig, C.; Schulze, D.; Stricker, A.; Jacobs, R. Digital method for quantification of circumferential periodontal bone level using cone beam CT. Clin. Oral Investig. 2013, 17, 389–396. [Google Scholar] [CrossRef]
- Lubner, M.G.; Smith, A.D.; Sandrasegaran, K.; Sahani, D.V.; Pickhardt, P.J. CT texture analysis: Definitions, applications, biologic correlates, and challenges. Radiographics 2017, 37, 1483–1503. [Google Scholar] [CrossRef]
- Pociask, E.; Nurzynska, K.; Obuchowicz, R.; Bałon, P.; Uryga, D.; Strzelecki, M.; Izworski, A.; Piórkowski, A. Differential diagnosis of cysts and granulomas supported by texture analysis of intraoral radiographs. Sensors 2021, 21, 7481. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).