Abstract
Despite intense interest in the catalytic potential of transition metal oxide heterostructures, originating from their large surface area and tunable chemistry, the fabrication of well-defined multicomponent oxide coatings with controlled architectures remains challenging. Here, we demonstrate a simple and effective swelling-assisted sequential infiltration synthesis (SIS) strategy to fabricate hierarchically porous multicomponent metal-oxide electrocatalysts with tunable bimetallic composition. A combination of solution-based infiltration (SBI) of transition metals, iron (Fe), nickel (Ni), and cobalt (Co), into a block copolymer (PS73-b-P4VP28) template, followed by vapor-phase infiltration of alumina using sequential infiltration synthesis (SIS), was employed to synthesize porous, robust, conformal and transparent multicomponent metal-oxide coatings like Fe/AlOx, Fe+Ni/AlOx, and Fe+Co/AlOx. Electrochemical assessments for the oxygen evolution reaction (OER) in a 0.1 M KOH electrolyte demonstrated that the Fe+Ni/AlOx composite exhibited markedly superior catalytic activity, achieving an impressive onset potential of 1.41 V and a peak current density of 3.29 mA/cm2. This superior activity reflects the well-known synergistic effect of alloying transition metals with a trace of Fe, which facilitates OER kinetics. Overall, our approach offers a versatile and scalable path towards the design of stable and efficient catalysts with tunable nanostructures, opening new possibilities for a wide range of electrochemical energy applications.
1. Introduction
Multicomponent metal oxides, especially transition metal oxides (TMOs), hold a pivotal role in the advancement of numerous technological fields [1,2,3,4], including catalysis [5,6], energy storage [7], and environmental remediation [8,9], primarily due to their distinctive electronic and structural characteristics. The inherent versatility of transition metal oxides, stemming from their ability to exhibit multiple oxidation states [10,11], makes them indispensable for addressing contemporary technological challenges. An effective approach to improving the performance of TMOs is the development of multicomponent systems, in which the nanoscale integration of two distinct transition metals can give rise to synergistic properties that exceed those of the individual components [12,13]. For the creation of novel active sites at the interface between these metals, such as iron (Fe) with nickel (Ni) and iron (Fe) with cobalt (Co), aluminum oxide (Al2O3) support plays a vital role by providing a high surface area for nanoparticle dispersion, preventing agglomeration, which can further modulate the electronic properties of the active metals [14,15]. Beyond compositional strategies, other methods like modifying surfaces or interfaces to improve electron transfer, surface amorphization [16], doping [16,17], engineering defects [18], and active surface area engineering [19] by designing porous thin films are being investigated to further enhance the efficiency of transition metal oxides.
Porous films are highly versatile materials with immense potential, particularly in electrochemical applications [20,21,22]. The introduction of porosity into the film enhances active surface area, which not only improves the diffusion of molecules through the structure but also boosts charge transfer and enhances the selectivity of electrochemical reactions [23,24]. This leads to greater efficiency, sensitivity, and long-term stability of the materials [24,25,26,27]. The incorporation of transition metals like Fe, Ni, and Co into the porous thin-film matrix can further enhance their electronic, magnetic, and catalytic properties [28,29,30]. For example, Fe-Ni nanoparticles anchored on Al2O3 exhibit outstanding electrocatalytic performance toward both the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER) [29,30,31]. Studies have shown that incorporating iron into nickel oxide can overcome the limitations of pure nickel inert sites, with some Fe-Ni catalysts even outperforming IrO2 in OER [32,33]. This enhancement is attributed to iron substitution, which strengthens the binding of OH* and reduces the energy barrier for O–O bond formation, making O2 evolution thermodynamically favorable [32,33,34]. Fe-Ni/N-doped carbon composites, synthesized using aluminum compounds, show promise as anode materials for lithium-ion batteries, offering improved capacity and stability [35]. Fe-Ni and Fe-Co oxides show their effectiveness in removing various pollutants and pharmaceutical byproducts from water through catalytic degradation [36,37].
Atomic layer deposition (ALD) has attracted considerable interest in recent years for its ability to deposit highly uniform, conformal thin films with porous architectures [38,39,40]. Although ALD provides exceptional conformality, precise thickness control, pore size manipulation, and high compositional homogeneity, the slow deposition rate presents as a major challenge for producing thick films in less time [41,42]. Sequential infiltration synthesis (SIS), a modification to conventional ALD which utilizes a sacrificial polymer template for the synthesis of conformal coatings, presents itself as a promising candidate to address this slow deposition rate bottleneck of traditional ALD [43]. Block copolymers self-assemble into periodic nanoscale domains that can be selectively infiltrated to produce high-surface-area frameworks for catalyst integration. For instance, PS-b-P4VP has been employed to template porous Ni films exhibiting improved hydrogen evolution activity, as well as to direct the formation of Pt alloy nanoparticle arrays with markedly enhanced fuel oxidation performance [44,45].
Our prior works demonstrate the use of the sequential infiltration synthesis (SIS) method to synthesize porous conformal coatings for various applications such as anti-reflective coatings, self-cleaning surfaces, gas sensors, and other opto-electronics applications [43,46,47,48]. SIS is a vapor-phase infiltration technique derived from ALD which involves a polymeric template infiltration with inorganic precursors via alternating exposures to a metal–organic vapor and an oxidant [43,49,50,51], which permeate the polymer and react within its nanochannels [52]. This process entraps inorganic material throughout the polymer’s free volume, yielding an organic–inorganic hybrid that can be converted into a porous inorganic network after removing the polymer [52,53]. However, a known limitation of SIS is the incomplete precursor penetration in thicker polymer films, which can lead to inhomogeneous loading with depth [49]. This depth-of-infiltration limitation can be addressed through a swelling-based infiltration (SBI) step prior to SIS [54,55,56], where a polymer template is soaked in a polar solvent to expand its domains and generate additional porosity that serves to create pathways for the precursors. Swelling the polymer templates prior to infiltration significantly enhances the infiltration process, consistently yielding thicker films compared to those synthesized using non-swelled templates, a finding supported by our previous research [43,54,55,56,57,58]. Therefore, this swelling-assisted SIS presents itself as a promising technique for the synthesis of scalable, porous, and conformal coatings.
Herein, we report on a fabrication of hierarchically structured, transparent, yet mechanically robust nanoporous thin film consisting of multicomponent Fe-Co and Fe-Ni oxide catalysts embedded in an AlOx matrix, synthesized by a swelling-based infiltration technique (SBI) of the metal Acetylacetonates and Sequential Infiltration Synthesis (SIS) of the AlOx matrix. This approach is designed to harness the synergistic activity of the Fe, Ni, and Co mix to create conformal coatings that are mechanically and chemically durable. The Fe–Ni/AlOx composite produced by our synthesis method exhibited an impressive OER onset potential of 1.41 V (vs RHE, in 0.1 M KOH). At the same time, the AlOx matrix serves to anchor the active metal-oxide nanodomains, which is expected to suppress catalyst dissolution and aggregation during long-term electrolysis. By uniting highly active 3D transition-metal centers with a resilient oxide architecture, our synthesis strategy addresses the key limitations of conventional Ni–Fe catalysts. The results demonstrate a new paradigm for designing a conformal, nanoscale framework that maximizes both activity and durability through sequential vapor-phase infiltration and template swelling.
2. Materials and Methods
2.1. Materials
Iron (III) acetylacetonate (99.99%), cobalt (II) acetylacetonate (97%), and nickel (II) acetylacetonate (98%) were procured from Sigma-Aldrich (St. Louis, MO, USA) and utilized without additional purification. The block copolymer polystyrene-b-poly(4-vinylpyridine) (PS 73-b-P4VP 28 was obtained from Polymer Source Inc. (Dorval, QC, Canada). Indium tin oxide (ITO)-coated glass slides (25 mm × 25 mm × 1.1 mm; resistivity: 8–12 Ω/sq) were sourced from Sigma-Aldrich. Pre-cleaned blank microscope slides (6 × 1 × 4.5 inches) were acquired from AmScope. Silicon substrates were purchased from Silicon Valley Microelectronics, Inc. (Santa Clara, CA, USA) Trimethylaluminum (TMA), used as a metal–organic precursor for atomic layer deposition (ALD), was also obtained from Sigma-Aldrich.
2.2. Polymer Sample Preparation
A 20 mg/mL solution of the block copolymer in toluene was prepared and filtered through 0.4 µm and subsequently 0.2 µm polytetrafluoroethylene (PTFE) filters (Fisher Scientific, Hampton, VA, USA). Substrates, including glass slides, silicon wafers, ITO-coated glass slides, and quartz crystal microbalance (QCM) crystals, were sequentially sonicated for 10 min each in deionized water, acetone, and isopropanol to remove surface contaminants. The filtered solution was spin-coated onto the cleaned substrates at 2000 rpm for 50 s. The resulting polymer films were annealed on a hot plate in air at 70 °C for 30 min to ensure solvent evaporation and film stabilization.
2.3. Chemical Infiltration: Swelling-Based Infiltration (SBI)
For the infiltration of transition metal oxides, a swelling-based infiltration (SBI) technique was employed. Solutions of 0.5 wt% metal acetylacetonates, such as Fe(acac)3, Co(acac)2, Ni(acac)2, and their mixtures, were prepared in pure ethanol and stirred at 125 rpm for 2 h. This concentration was determined to be optimal based on QCM analysis and electrochemical performance evaluations. Polymer-coated substrates (QCM crystals, ITO glass slides, glass slides, and silicon wafers) were immersed in the prepared solutions and heated to 75 °C for 1 h to facilitate infiltration into the ethanol-swollen polar domains of the block copolymer. The swelling temperature and time were chosen to maximize infiltration of metal acetylacetonate while minimizing mass loss of the block copolymer, which likely arises from partial dissolution during micelle opening [53,59]. Post-infiltration, samples were removed and dried in a fume hood at ambient temperature for a minimum of 3 h to prevent collapse of the swollen polymer matrix.
2.4. Vapor Phase Infiltration: Sequential Infiltration Synthesis (SIS)
Following SBI, alumina was deposited via sequential infiltration synthesis (SIS) using a Veeco Savannah S100 ALD system (Plainview, NY, USA). Samples were placed in the reactor chamber at 90 °C under a base pressure of 450 mTorr. Five cycles of TMA and H2O at a pulse rate of 0.5 s and 1 s exposure, respectively, were conducted to deposit alumina, with each cycle followed by a 300 s purge using clean dry air (CDA) to remove unreacted precursors and byproducts.
2.5. Polymer Removal
To remove the polymer template post-infiltration, samples were subjected to thermal annealing at 450 °C in a flowing oxygen atmosphere using a ThermoFisher Lindberg Blue M tube furnace (Hampton, VA, USA). Removal of the polymer template yielded nanoporous metal oxide films with a porosity of ~75%, as verified by ellipsometry measurements.
2.6. Sample Characterization
Quantitative analysis of the infiltration process was performed using a quartz crystal microbalance (QCM). An SRS QCM200 system was employed with AT-cut QCM crystals (Inficon Inc., East Syracuse, NY, USA) featuring a resonant frequency of approximately 5 MHz and 1-inch diameter Ti/Au electrodes. Frequency shifts recorded by the system were used to determine mass changes in polymer films, allowing for an estimation of the amount of infiltrated material.
High-resolution structural and elemental analyses were conducted using electron microscopy and spectroscopy. Changes in the polymer during the adsorption of the precursor were evaluated using a Nicolet 6700 (Thermo Scientific, Hampton, VA, USA) Fourier transform infrared spectrometer (FTIR) at a spectral range of 600–1800 cm−1. High-resolution transmission electron microscopy (HR-TEM) images were obtained using a JEOL JEM-2100F field-emission gun TEM (Tokyo, Japan). Energy-dispersive X-ray (EDX) mapping was performed using an FEI Talos F200i scanning/transmission electron microscope (ThermoFisher Scientific, Waltham, MA, USA) to analyze elemental distribution. Surface chemical states were investigated using X-ray photoelectron spectroscopy (XPS) measurements, carried out on a PHI 5000 VersaProbe system (Chanhassen, MN, USA) with monochromatic Al Kα radiation (1486.6 eV).
Optical properties were assessed using UV-Visible transmittance spectroscopy. Spectra were recorded using a Shimadzu UV-1800 spectrophotometer (Kyoto, Japan).
Thermal properties were evaluated using thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC). These measurements were performed simultaneously using a TA Instruments SDT 650 system (DE, USA). Samples, contained in 6 mm outer diameter alumina crucibles with lids (MSE Supplies LLC, Tucson, AZ, USA), were heated to 500 °C at a rate of 30 °C/min under a nitrogen flow. An empty crucible served as the reference for these measurements.
Electrochemical characterization was performed using a three-electrode setup controlled by a CHI 6000E electrochemical analyzer (CH Instruments, Inc., Bee Cave, TX, USA). The synthesized heterostructures functioned as the working electrode. A graphite rod served as the counter electrode, and an Ag/AgCl electrode was used as the reference electrode.
Oxygen Evolution Reaction (OER) tests were conducted in a 0.1 M KOH electrolyte at 23 °C with a rotation speed of 500 rpm, performed at a scan rate of 5 mV/s over a potential range of 0 to 1 V. The Ag/AgCl reference electrode was calibrated against the reversible hydrogen electrode (RHE) by determining the potential at which the current crossed zero. The potential versus RHE was calculated using the following relation:
where E0 is the standard potential of the Ag/AgCl electrode. Current densities were normalized to the geometric surface area of the electrode.
ERHE = EAg/AgCl + E0 + 0.0591 × pH
3. Results and Discussion
Figure 1 illustrates a schematic representation of the swelling-assisted SIS process used for the fabrication of porous multicomponent thin-film structures. Specifically, the spin-coated amphiphilic block copolymer film is immersed in a metal acetyl acetonate-containing a solution of ethanol, which is used as a selective swelling agent of the polar domains (polyvinyl pyridine) of the BCP. This leads to the incorporation of salts of Fe, Ni, and Co into the polymer structure which infiltrate the polar domains of the BCP and remain there after the BCP film is removed from the solution and dried out. The resulting infiltrated BCP film is exposed to the second infiltration step, using vapor phase infiltration of alumina from the TMA precursor, leading to the formation of the multicomponent organic–inorganic film. During the final step, the organic component of the film, the actual BCP template, is removed by annealing in oxygen flow, resulting in a nanoporous Fe/Co/Ni-modified alumina coating.
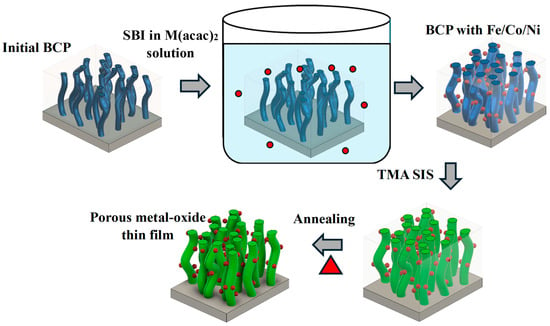
Figure 1.
Schematic of the swelling-assisted SIS method for the synthesis of multicomponent metal-oxide nanoporous thin films.
To track mass changes during the infiltration steps, specifically during SBI and SIS, quartz crystal microbalance (QCM) sensors were employed, as depicted in Figure 2. The QCM data revealed a discernible increase in the mass of the polymer templates post-infiltration from both SBI and SIS. Notably, the mass uptake observed during the SIS process was approximately twice that recorded during the SBI phase, indicating a more substantial incorporation of metal oxide precursors via vapor-phase infiltration compared to solution-based methods. FTIR analysis (Figure S1), used to track infiltration and identify reactive sites in the SBI and SIS stages, indicated that swelling in ethanol enhanced CH bending absorption bands of the CH3 and CH2 groups (1580–1410 cm−1) known to favor alumina uptake [60,61]. Overall, the spectra show that Fe, Ni, Co, and alumina precursors during the PS-b-P4VP template infiltration compete for the same reactive sites.
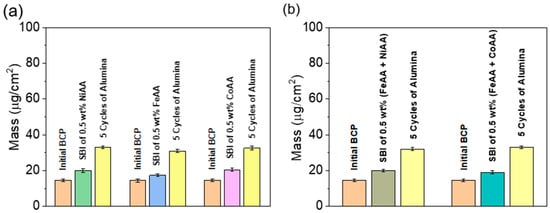
Figure 2.
QCM monitoring of precursor uptake during sequential infiltration. (a) Mass evolution during SBI of individual metal acetylacetonates (Ni(acac)2, Fe(acac)3, Co(acac)2) and subsequent SIS of alumina. (b) Mass evolution during SBI of mixed metal acetylacetonates (Fe(acac)3 + Ni(acac)2 and Fe(acac)3 + Co(acac)2), followed by alumina SIS.
The resulting nanoporous films (Figure S2) are further analyzed using high-resolution transmission electron microscopy (HRTEM) to identify the polycrystalline nature of the synthesized metal-oxide thin films, as shown in Figure 3. The high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) images, along with energy-dispersive X-ray spectroscopy (EDS) mapping show a uniform distribution of Fe and Al across all the porous samples. However, in the multicomponent systems, Fe+Co/AlOx and Fe+Ni/AlOx, distinct segregated regions were observed, possibly domains of mixed metal oxides. The presence of multiple randomly oriented crystallite domains and phases is further suggested by the lattice fringes in electron diffraction (ED) patterns. Additionally, Figure 3a,b(v,vi) reveal the co-existence of multiple metal oxides within a particular region of the AlOx matrix.
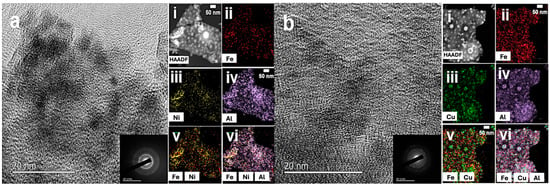
Figure 3.
HRTEM images of Fe+Ni/AlOx and Fe+Co/AlOx (a and b, respectively). The insets demonstrate the electron diffraction patterns. High-angle annular dark-field TEM images of (a(i)) Fe+Ni/AlOx, and (b(i)) Fe+Co/AlOx. EDXS elemental mapping of individual elements for (a(ii–vi)) Fe+Ni/AlOx, and (b(ii–vi)) Fe+Co/AlOx corresponding to (a,b(ii)) Fe (red), (a(iii)) Ni (yellow), and (b(iii)) Co (green), (a,b(iv)) Al (purple) elements and elemental mapping overlays of (a(v)) Fe and Ni, (a(vi)) Fe, Ni, and Al, (b(v)) Fe and Co, and (b(vi)) Fe, Co, and Al elements.
An XPS study was conducted for the elemental analysis of the synthesized metal-oxide thin films, as shown in Figure 4. For the Fe/AlOx sample (Figure 4a), a distinct Fe 2p1/2 peak at around 721 eV and Fe 2p3/2 peak at around 707 eV were observed, indicating 2+ and 3+ oxidation states of Fe [62]. The satellite peaks in the fitted Fe spectra further suggest various iron species within the AlOx matrix. A distinct, narrow Al 2p peak at about 71 eV confirmed a uniform chemical environment for aluminum in AlOx, primarily in a 3+ oxidation state. The multicomponent fitted O 1s curve with a main peak at around 528 eV suggests Al-O and Fe-O interactions. In the case of Fe+Ni/AlOx (Figure 4d–g), with the incorporation of Ni, significant changes in the iron peak positions and shapes were observed, highlighting nickel’s strong influence on iron’s oxidation state. A distinct Ni 2p3/2 peaks at around 853 eV and a 2p1/2 peak at around 872 eV with other fitted satellite peaks indicate the presence of Ni in a (2+) oxidation state, potentially forming compounds like NiO, NiOOH, and NiFe2O4 [63,64,65,66]. The Fe3+ in these Ni-based dopants enhances OER by inducing higher Ni valence states, active oxygen species formation, and intrinsic activity, resulting in the synergistic generation of reactive oxygen intermediates on the Ni sites [67,68]. In the case of the Fe+Co/AlOx sample, the addition of Co led to the diminishing of the Fe 2p1/2 peak (Figure 4h), indicating a significant interaction between Fe and Co, possibly forming intermetallic compounds or mixed oxides such as CoFe2O4 [69]. The fitted Co 2p XPS spectra exhibit a 2p3/2 peak at around 781 eV and a 2p1/2 peak at around 799 eV, revealing the existence of Co in both 2+ and 3+ oxidation states, likely as Co(OH)2 and Co3O4 compounds [70,71]. These mixed-valence states like Co (2+) and (3+) and even Fe (2+) and (3+) improve electrical conductivity and create a reservoir of lattice oxygen/hydroxide [68]. In the case of Al 2p spectra, with the incorporation of Fe and Ni, the deconvoluted Al 2p spectra exhibited two distinct components, likely associated with Al-O and Al-OH bonds, or possibly some minor intermetallic interactions. Although Al is redox-inactive, it helps stabilize higher oxidation states during OER by exerting an inductive effect on nearby Ni/Fe. Al inclusion in Fe-Ni/Al oxyhydroxides also encourages the formation of Ni3+/Fe3+ active sites, and most often leaches during the initial cycling, adding more porosity and oxygen vacancies that improve OER performance [72]. Similarly, the O 1s spectra also showed two separate components, which could correspond to similar interactions to those observed in the Al 2p spectra [73,74].
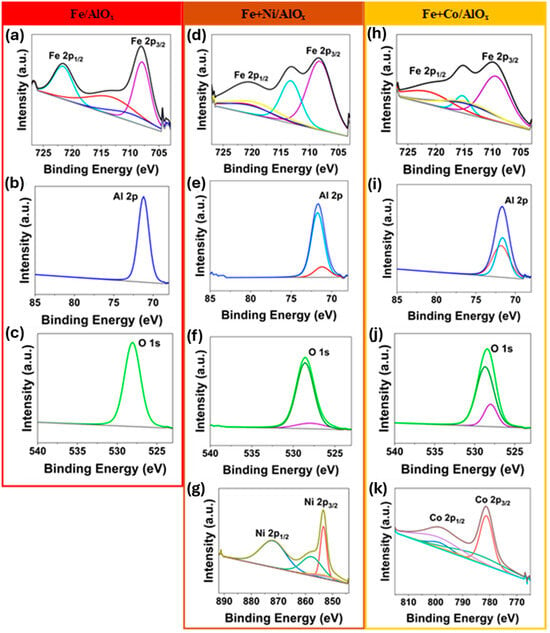
Figure 4.
XPS spectra of Fe/AlOx, (a–c), Fe+Ni/AlOx (d–g), and Fe+Co/AlOx (h–k) porous metal-oxide thin film structures. The spectra are encased in color-coded frames corresponding to each sample for the better visualization (red, brown and yellow frames correspond to Fe/AlOx, Fe+Ni/AlOx and Fe+Co/AlOx, respectively).
Electrochemical assessments in 0.1 M KOH solution were conducted using indium tin oxide (ITO)-coated glass electrodes to investigate the oxygen evolution reaction (OER) performance of various transition metal–alumina catalysts. Cyclic Voltammetry measurements, as depicted in Figure 5a,b, revealed that the Fe+Ni/AlOx catalyst achieved a peak current density of 3.29 mA/cm2, with an onset potential of 1.41 V. In comparison, the Fe+Co/AlOx catalyst exhibited a lower current density of 0.72 mA/cm2 and a higher onset potential of 1.46 V. These findings suggest that incorporating nickel into the iron–alumina matrix enhances catalytic activity, whereas cobalt incorporation appears to diminish performance. The superior activity of the Fe+Ni/AlOx catalyst is further corroborated by its mass-specific current density, reaching 7305 mA g−1, outperforming both its Fe/AlOx (3503 mA/g) and Fe+Co/AlOx (1682 mA/g) counterparts. Control experiments utilizing pre-annealed and post-annealed ITO substrates demonstrated negligible OER activity, indicating that the observed electrochemical performance predominantly arises from the catalytic properties of the transition metal–alumina composites and not the substrate. The Tafel slopes for the samples are summarized in Supplementary Figure S3.
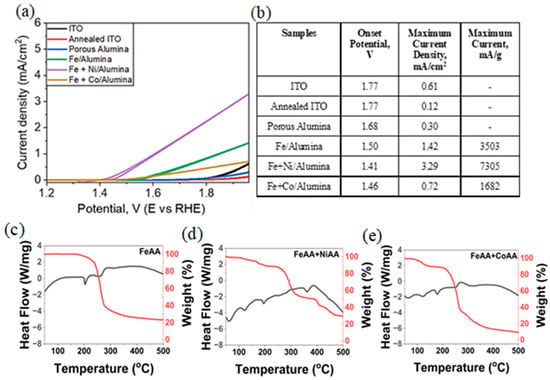
Figure 5.
(a) Current density vs. potential curves for ITO glass, annealed ITO glass, porous AlOx coated ITO, Fe/AlOx-coated ITO, Fe+Ni/AlOx-coated ITO, and Fe+Co/AlOx-coated ITO. (b) Table summarizing the onset potential and maximum current density for all samples. TGA (red)-DSC (black) curves of (c) Fe/AlOx, (d) Fe+Ni/AlOx, and (e) Fe+Co/AlOx.
To elucidate the origin of the very high electrochemical activity of the Fe + Ni system, consistent with earlier studies reporting similarly high activity in a zinc oxide matrix [75], we further analyzed the formation of the oxides. Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) of the metal acetylacetonate precursors reveal distinct thermal decomposition profiles, as shown in Figure 5c, which exhibits a sharp mass loss of approximately 70% centered around 300 °C, coinciding with a significant exothermic peak. This indicates a rapid thermal decomposition process leading to iron-based residues, likely iron oxide species [76]. The thermal behavior of mixed precursor systems, Fe(acac)3 + Ni(acac)2 (Figure 5d) and Fe(acac)3 + Co(acac)2 (Figure 5e), deviates significantly from that of the individual Fe component curve. In both mixtures, the onset of decomposition shifts to lower temperatures (around 250–300 °C), and the overall thermal events become broader. These changes suggest potential interactions between the metal complexes, possibly altering their decomposition kinetics or leading to synergistic thermal effects. Notably, the Fe + Co mixture shows a new exothermic peak around 250 °C and a smoother mass loss curve compared to Fe(acac)3 alone, indicating that cobalt may catalyze or modulate the decomposition of the iron precursor. Fe + Ni system decomposition is more thermally delayed and occurs more uniformly, which could promote a more homogeneous distribution of metal species within the polymer matrix, potentially enhancing oxide formation for superior OER performance, as previously discussed.
With a view to applying such coatings for electrochromic applications, we analyzed the optical performance of the resulting porous metal-oxide thin films by evaluating the changes in UV-Vis transmittance spectra, as shown in Figure 6. All the samples exhibited high transparency, with values over 80% transmittance. The lowest values were observed for Fe+Ni/AlOx, which exhibited a decrease in transmittance from 91% to around 81%. Furthermore, the visual transparency of the porous films can be observed in Figure 6b,c, which show a high order of transparency, indicating potential for transparent conformal optical coatings.
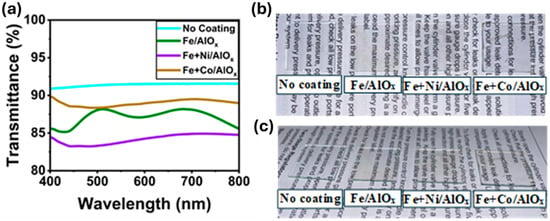
Figure 6.
(a) UV-Vis spectra and (b,c) photograph showing visual transparency of uncoated glass, Fe/AlOx-coated glass, Fe+Ni/AlOx-coated glass, and Fe+Co/AlOx-coated glass.
4. Conclusions
In conclusion, we demonstrated that a two-step polymer-infiltration method, combining solution-based infiltration (SBI) and sequential infiltration synthesis (SIS), can be effectively used to synthesize robust porous electrocatalysts using AlOx-supported Fe, Fe+Ni, or Fe+Co oxides. This approach enables the formation of conformal coatings with embedded metal-oxide nanodomains within a hierarchical AlOx matrix. Among the synthesized samples, the Fe+Ni/AlOx composite exhibited the best oxygen evolution reaction activity, attributable to the synergistic Ni–Fe effect that lowers the OER energy barriers. In contrast, the Fe–Co combination offered only limited improvement over Fe alone, underscoring how metal composition critically controls activity. Importantly, the interconnected AlOx scaffold not only increases porosity and accessibility but also stabilizes the nanoparticles against aggregation, thereby improving catalyst durability. These findings underscore the importance of alloying transition metals and employing oxide supports when designing versatile and stable electrocatalysts. Furthermore, the transparency and robustness of the porous coatings expand their potential use in integrated photoelectrochemical devices, as well as other advanced energy applications such as supercapacitors, batteries, and CO reduction systems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app151910473/s1, Figure S1: FTIR analysis of PS-b-P4VP BCP during infiltration processes; Figure S2: SEM Image of deposited metal-oxides thin film structures Porous Alumina(a), Fe/AlOx(b), Fe+Ni/AlOx(b) and Fe+Co/AlOx(c); Figure S3: Tafel slopes from linear sweep for ITO glass, annealed ITO glass, porous AlOx, Fe/AlOx, Fe+Ni/AlOx, Fe+Co/AlOx.
Author Contributions
Conceptualization, D.B. and E.V.S.; methodology, D.B.; validation, C.O., V.G. and K.D.O.; investigation, C.O., K.D.O. and V.G.; resources, D.B.; data curation, C.O., K.D.O. and D.B.; writing, C.O., V.G., D.B. and E.V.S.; supervision, D.B. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the support of this work by the National Science Foundation, Award No. 2045662. Work at the Center for Nanoscale Materials was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC0206CH-11357.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Acknowledgments
This work was performed in part at the University of North Texas’ Materials Research Facility.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kowalczyk, A.; Rutkowska, M.; Gnyla, S.; Pacia, M.; Chmielarz, L. Synergistic Effect of Co and Ni Co-Existence on Catalytic Decomposition of Ammonia to Hydrogen—Effect of Catalytic Support and Mg-Al Oxide Matrix. ChemEngineering 2024, 8, 55. [Google Scholar] [CrossRef]
- Arizapana, K.; Schossig, J.; Wildy, M.; Weber, D.; Gandotra, A.; Jayaraman, S.; Wei, W.; Xu, K.; Yu, L.; Mugweru, A.M.; et al. Harnessing the Synergy of Fe and Co with Carbon Nanofibers for Enhanced CO2 Hydrogenation Performance. ACS Sustain. Chem. Eng. 2024, 12, 1868–1883. [Google Scholar] [CrossRef] [PubMed]
- Satrughna, J.A.K.; Kanwade, A.R.; Shirage, P.M. Synergistic effect of multi-transition metal co-substitution in high cycle life performance of NaxCo0.5Fe0.25Mn0.25O2 cathode for sodium-ion batteries. Sustain. Energy Fuels 2025, 9, 3354–3373. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, X.; Zhang, Q.; Lv, D.; Zuo, S.; Li, J. Comprehensive Analysis of the Synergistic Effects of Bimetallic Oxides in CoM/γ-Al2O3 (M = Cu, Fe, or Ni) Catalysts for Enhancing Toluene Combustion Efficiency. Molecules 2025, 30, 1188. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Q.; Lin, Y.; Liu, J.; Pan, J.; Hu, J.; Xu, X. Boosting oxygen evolution reaction by FeNi hydroxide-organic framework electrocatalyst toward alkaline water electrolyzer. Nat. Commun. 2024, 15, 7278. [Google Scholar] [CrossRef]
- Lys, A.; Zabolotnii, V.; Čaplovičová, M.; Tepliakova, I.; Berzins, A.; Sahul, M.; Čaplovič, Ľ.; Pogrebnjak, A.; Iatsunskyi, I.; Viter, R. Core-shell nanofibers of ZnFe2O4/ZnO for enhanced visible-light photoelectrochemical performance. J. Alloys Compd. 2024, 984, 173885. [Google Scholar] [CrossRef]
- Lun, S.; Wang, H.; Deng, Y.; Cui, J.; Liang, P.; Wang, K.; Lv, L.; Wan, H.; Wang, H. FeNi decorated nitrogen-doped hollow carbon spheres as ultra-stable bifunctional oxygen electrocatalyst for rechargeable zinc–air battery with 2.7% decay after 300 hours cycling. RSC Adv. 2024, 14, 3857–3866. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Mahmoud, S.A.; Mohamed, A.A. Interfacially engineered metal oxide nanocomposites for enhanced photocatalytic degradation of pollutants and energy applications. RSC Adv. 2025, 15, 15561–15603. [Google Scholar] [CrossRef]
- Ahmad, I.; Aftab, A.; Fatima, A.; Mekkey, S.D.; Melhi, S.; Ikram, S. A comprehensive review on the advancement of transition metals incorporated on functional magnetic nanocomposites for the catalytic reduction and photocatalytic degradation of organic pollutants. Coord. Chem. Rev. 2024, 514, 215904. [Google Scholar] [CrossRef]
- Yuan, C.; Wu, H.; Bin Xie, Y.; Lou, X.W. Mixed Transition-Metal Oxides: Design, Synthesis, and Energy-Related Applications. Angew. Chem. Int. Ed. 2014, 53, 1488–1504. [Google Scholar] [CrossRef]
- Low, W.H.; Khiew, P.S.; Lim, S.S.; Siong, C.W.; Ezeigwe, E.R. Recent development of mixed transition metal oxide and graphene/mixed transition metal oxide based hybrid nanostructures for advanced supercapacitors. J. Alloys Compd. 2019, 775, 1324–1356. [Google Scholar] [CrossRef]
- Li, S.; Li, E.; An, X.; Hao, X.; Jiang, Z.; Guan, G. Transition metal-based catalysts for electrochemical water splitting at high current density: Current status and perspectives. Nanoscale 2021, 13, 12788–12817. [Google Scholar] [CrossRef]
- Wan, H.; Liu, X.; Wang, H.; Ma, R.; Sasaki, T. Recent advances in developing high-performance nanostructured electrocatalysts based on 3d transition metal elements. Nanoscale Horiz. 2019, 4, 789–808. [Google Scholar] [CrossRef]
- Park, J.-Y.; Lee, Y.-J.; Khanna, P.K.; Jun, K.-W.; Bae, J.W.; Kim, Y.H. Alumina-supported iron oxide nanoparticles as Fischer–Tropsch catalysts: Effect of particle size of iron oxide. J. Mol. Catal. A Chem. 2010, 323, 84–90. [Google Scholar] [CrossRef]
- Tijani, M.M.; Aqsha, A.; Mahinpey, N. Synthesis and study of metal-based oxygen carriers (Cu, Co, Fe, Ni) and their interaction with supported metal oxides (Al2O3, CeO2, TiO2, ZrO2) in a chemical looping combustion system. Energy 2017, 138, 873–882. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.; Kim, S. Recent advances in amorphous electrocatalysts for oxygen evolution reaction. Front. Chem. 2022, 10, 1030803. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Lin, X.-M.; Liu, Y.; Filatov, A.S.; Li, D.; Stamenkovic, V.R.; Yang, D.; Prakapenka, V.B.; Lei, A.; Shevchenko, E.V. Binary Transition-Metal Oxide Hollow Nanoparticles for Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2018, 10, 24715–24724. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, Q.; Song, B.; Xu, P. Regulation Strategy of Transition Metal Oxide-Based Electrocatalysts for Enhanced Oxygen Evolution Reaction. Acc. Mater. Res. 2022, 3, 1088–1100. [Google Scholar] [CrossRef]
- Zhu, Y.; Tang, Z.; Yuan, L.; Li, B.; Shao, Z.; Guo, W. Beyond conventional structures: Emerging complex metal oxides for efficient oxygen and hydrogen electrocatalysis. Chem. Soc. Rev. 2025, 54, 1027–1092. [Google Scholar] [CrossRef]
- Laušević, Z.; Apel, P.Y.; Krstić, J.B.; Blonskaya, I.V. Porous carbon thin films for electrochemical capacitors. Carbon 2013, 64, 456–463. [Google Scholar] [CrossRef]
- Sievers, B.; Granja, L.P.; Zelcer, A.; Juan, D.; Ferrari, V.; Passanante, S.; Lombardo, M.V.; Fuertes, M.C.; Fuentes, R.; Sacanell, J. Tuning Electrochemical Properties and Thermal Stability of YSZ Mesoporous Thin Films for SOFC Applications. ACS Appl. Energy Mater. 2025, 8, 894–902. [Google Scholar] [CrossRef]
- Luo, J.; Cao, M.; Naresh, N.; Borah, J.; Li, S.; Wang, T.; Sarma, B.K.; Yao, J.; Parkin, I.P.; Boruah, B.D. Chemically Processed Porous V2O5 Thin-Film Cathodes for High-Performance Thin-Film Zn-Ion Batteries. Adv. Funct. Mater. 2025, 35, 2417607. [Google Scholar] [CrossRef]
- Bose, R.; Goud, G.S.; Helal, M.I.; Barsoum, I.; Cho, S.O.; Alfantazi, A. Self-Ordered Anodic Porous Oxide Layers as a High Performance Electrocatalyst for Water Oxidation. ACS Appl. Energy Mater. 2024, 7, 4402–4411. [Google Scholar] [CrossRef]
- Sakthinathan, S.; Meenakshi, G.A.; Vinothini, S.; Yu, C.-L.; Chen, C.-L.; Chiu, T.-W.; Vittayakorn, N. A Review of Thin-Film Growth, Properties, Applications, and Future Prospects. Processes 2025, 13, 587. [Google Scholar] [CrossRef]
- Pan, Z.; Yang, J.; Kong, J.; Loh, X.J.; Wang, J.; Liu, Z. “Porous and Yet Dense” Electrodes for High-Volumetric-Performance Electrochemical Capacitors: Principles, Advances, and Challenges. Adv. Sci. 2022, 9, 2103953. [Google Scholar] [CrossRef]
- de Nooijer, N.; Plazaola, A.A.; Rey, J.M.; Fernandez, E.; Tanaka, D.A.P.; Annaland, M.v.S.; Gallucci, F. Long-Term Stability of Thin-Film Pd-Based Supported Membranes. Processes 2019, 7, 106. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, S.; Yu, M.; Li, P.; Zhang, X.; Wang, H.; Guo, T.; Liu, C. Fabrication and application of porous organic single-crystal films in highly sensitive gas sensors. Nano Res. 2025, 18, 94907299. [Google Scholar] [CrossRef]
- Omotosho, K.D.; Lyon, Z.; Shevchenko, E.V.; Berman, D. Accessibility and Mechanical Stability of Nanoporous Zinc Oxide and Aluminum Oxide Coatings Synthesized via Infiltration of Polymer Templates. Polymers 2023, 15, 4088. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, H.; Feng, L. A Review on Advanced FeNi-Based Catalysts for Water Splitting Reaction. Energy Fuels 2020, 34, 13491–13522. [Google Scholar] [CrossRef]
- Gu, X.; Liu, Z.; Li, M.; Tian, J.; Feng, L. Surface structure regulation and evaluation of FeNi-based nanoparticles for oxygen evolution reaction. Appl. Catal. B 2021, 297, 120462. [Google Scholar] [CrossRef]
- Akbari, N.; Nandy, S.; Chae, K.H.; Najafpour, M.M. Dynamic Changes of an Anodized FeNi Alloy during the Oxygen Evolution Reaction under Alkaline Conditions. Langmuir 2023, 39, 11807–11818. [Google Scholar] [CrossRef] [PubMed]
- Morales-Guio, C.G.; Liardet, L.; Hu, X. Oxidatively Electrodeposited Thin-Film Transition Metal (Oxy)hydroxides as Oxygen Evolution Catalysts. J. Am. Chem. Soc. 2016, 138, 8946–8957. [Google Scholar] [CrossRef]
- Ha, M.-A.; Alia, S.M.; Norman, A.G.; Miller, E.M. Fe-Doped Ni-Based Catalysts Surpass Ir-Baselines for Oxygen Evolution Due to Optimal Charge-Transfer Characteristics. ACS Catal. 2024, 14, 17347–17359. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; An, N.; Kang, Z.; Menezes, P.W.; Chen, Z. Understanding Advanced Transition Metal-Based Two Electron Oxygen Reduction Electrocatalysts from the Perspective of Phase Engineering. Adv. Mater. 2024, 36, e2400140. [Google Scholar] [CrossRef]
- Liu, C.; Li, Q.; Kang, W.; Lei, W.; Wang, X.; Lu, C.; Naebe, M. Structural design and mechanism analysis of hierarchical porous carbon fibers for advanced energy and environmental applications. J. Mater. Chem. A Mater. 2022, 10, 10–49. [Google Scholar] [CrossRef]
- Yan, W.; Liu, Y.; Bai, Y.; Chen, Y.; Zhou, H.; Ahmad, W. Intelligent MEMS Sensor Based on an Oxidized Medium-Entropy Alloy (FeCoNi) for H2 and CO Recognition. ACS Appl. Mater. Interfaces 2024, 16, 49474–49483. [Google Scholar] [CrossRef]
- Qi, Y.; Zou, M.; Ajarem, J.S.; Allam, A.A.; Wang, Z.; Qu, R.; Zhu, F.; Huo, Z. Catalytic degradation of pharmaceutical and personal care products in aqueous solution by persulfate activated with nanoscale FeCoNi-ternary mixed metal oxides. Sep. Purif. Technol. 2023, 314, 123585. [Google Scholar] [CrossRef]
- Tugrul, D.; Doganay, D.; Unalan, H.E.; Imer, B. ALD grown undoped ZnO and Al-doped-ZnO thin-film heaters. Vacuum 2025, 233, 113942. [Google Scholar] [CrossRef]
- Ghazy, A.; Zanders, D.; Devi, A.; Karppinen, M. Atomic and Molecular Layer Deposition of Functional Thin Films Based on Rare Earth Elements. Adv. Mater. Interfaces 2025, 12, 2400274. [Google Scholar] [CrossRef]
- Fang, M.; Ho, J.C. Area-Selective Atomic Layer Deposition: Conformal Coating, Subnanometer Thickness Control, and Smart Positioning. ACS Nano 2015, 9, 8651–8654. [Google Scholar] [CrossRef] [PubMed]
- Leskelä, M.; Ritala, M. Atomic Layer Deposition Chemistry: Recent Developments and Future Challenges. Angew. Chem. Int. Ed. 2003, 42, 5548–5554. [Google Scholar] [CrossRef]
- Johnson, R.W.; Hultqvist, A.; Bent, S.F. A brief review of atomic layer deposition: From fundamentals to applications. Mater. Today 2014, 17, 236–246. [Google Scholar] [CrossRef]
- Berman, D.; Shevchenko, E. Design of functional composite and all-inorganic nanostructured materials via infiltration of polymer templates with inorganic precursors. J. Mater. Chem. C Mater. 2020, 8, 10604–10627. [Google Scholar] [CrossRef]
- Clavijo, R.F.; Riba-Moliner, M.; González-Campo, A.; Sort, J.; Pellicer, E.; Eiler, K. PS-b-P4VP block copolymer micelles as a soft template to grow openly porous nickel films for alkaline hydrogen evolution. Catal. Today 2023, 423, 113916. [Google Scholar] [CrossRef]
- Kumar, L.; Singh, S.; Horechyy, A.; Fery, A.; Nandan, B. Block Copolymer Template-Directed Catalytic Systems: Recent Progress and Perspectives. Membranes 2021, 11, 318. [Google Scholar] [CrossRef] [PubMed]
- Pleshek, D.; Tran, J.; Li, Y.; Shirani, A.; Shevchenko, E.V.; Berman, D. Swelling-Assisted Sequential Infiltration Synthesis of Nanoporous ZnO Films with Highly Accessible Pores and Their Sensing Potential for Ethanol. ACS Appl. Mater. Interfaces 2021, 13, 35941–35948. [Google Scholar] [CrossRef]
- Omotosho, K.D.; Gurung, V.; Banerjee, P.; Shevchenko, E.V.; Berman, D. Self-Cleaning Highly Porous TiO2 Coating Designed by Swelling-Assisted Sequential Infiltration Synthesis (SIS) of a Block Copolymer Template. Polymers 2024, 16, 308. [Google Scholar] [CrossRef]
- Ji, C.; Zhang, Z.; Omotosho, K.D.; Berman, D.; Lee, B.; Divan, R.; Guha, S.; Shevchenko, E.V. Porous but Mechanically Robust All-Inorganic Antireflective Coatings Synthesized using Polymers of Intrinsic Microporosity. ACS Nano 2022, 16, 14754–14764. [Google Scholar] [CrossRef]
- Peng, Q.; Tseng, Y.; Darling, S.B.; Elam, J.W. Nanoscopic Patterned Materials with Tunable Dimensions via Atomic Layer Deposition on Block Copolymers. Adv. Mater. 2010, 22, 5129–5133. [Google Scholar] [CrossRef]
- Chen, Y.; Xiong, S. Directed self-assembly of block copolymers for sub-10 nm fabrication. Int. J. Extrem. Manuf. 2020, 2, 032006. [Google Scholar] [CrossRef]
- Yang, G.G.; Choi, H.J.; Han, K.H.; Kim, J.H.; Lee, C.W.; Jung, E.I.; Jin, H.M.; Kim, S.O. Block Copolymer Nanopatterning for Nonsemiconductor Device Applications. ACS Appl. Mater. Interfaces 2022, 14, 12011–12037. [Google Scholar] [CrossRef] [PubMed]
- Cara, E.; Murataj, I.; Milano, G.; De Leo, N.; Boarino, L.; Lupi, F.F. Recent Advances in Sequential Infiltration Synthesis (SIS) of Block Copolymers (BCPs). Nanomaterials 2021, 11, 994. [Google Scholar] [CrossRef] [PubMed]
- Berman, D.; Sha, Y.; Shevchenko, E.V. Effect of Polymer Removal on the Morphology and Phase of the Nanoparticles in All-Inorganic Heterostructures Synthesized via Two-Step Polymer Infiltration. Molecules 2021, 26, 679. [Google Scholar] [CrossRef]
- Ghoshal, T.; O’COnnell, J.; Sinturel, C.; Andreazza, P.; Holmes, J.D.; Morris, M.A. Solvent mediated inclusion of metal oxide into block copolymer nanopatterns: Mechanism of oxide formation under UV-Ozone treatment. Polymer 2019, 173, 197–204. [Google Scholar] [CrossRef]
- Yi, D.H.; Nam, C.-Y.; Doerk, G.; Black, C.T.; Grubbs, R.B. Infiltration Synthesis of Diverse Metal Oxide Nanostructures from Epoxidized Diene–Styrene Block Copolymer Templates. ACS Appl. Polym. Mater. 2019, 1, 672–683. [Google Scholar] [CrossRef]
- Singh, S.; Vasquez, J.F.B.; Perova, T.S.; Morris, M.A. Fabrication of metal-oxide arrays: Mechanism of solvent-mediated metal infiltration into block copolymer nanopatterns. Clean. Technol. Environ. Policy 2023, 25, 1361–1369. [Google Scholar] [CrossRef]
- Berman, D.; Guha, S.; Lee, B.; Elam, J.W.; Darling, S.B.; Shevchenko, E.V. Sequential Infiltration Synthesis for the Design of Low Refractive Index Surface Coatings with Controllable Thickness. ACS Nano 2017, 11, 2521–2530. [Google Scholar] [CrossRef]
- Omotosho, K.; Tran, J.; Shevchenko, E.V.; Berman, D. Polymer infiltration synthesis of inorganic nanoporous coatings: Does polymer template affect their properties? Surf. Coat Technol. 2023, 452, 129107. [Google Scholar] [CrossRef]
- She, Y.; Goodman, E.D.; Lee, J.; Diroll, B.T.; Cargnello, M.; Shevchenko, E.V.; Berman, D. Block-Co-polymer-Assisted Synthesis of All Inorganic Highly Porous Heterostructures with Highly Accessible Thermally Stable Functional Centers. ACS Appl. Mater. Interfaces 2019, 11, 30154–30162. [Google Scholar] [CrossRef]
- She, Y.; Lee, J.; Diroll, B.T.; Lee, B.; Aouadi, S.; Shevchenko, E.V.; Berman, D. Rapid Synthesis of Nanoporous Conformal Coatings via Plasma-Enhanced Sequential Infiltration of a Polymer Template. ACS Omega 2017, 2, 7812–7819. [Google Scholar] [CrossRef]
- Gurung, V.; Omotosho, K.D.; Obe, O.; Shevchenko, E.; Berman, D. Fabrication of nanoporous doped metal oxide coatings via selective infiltration of a block copolymer template: The case of Al-doped zinc oxide. Surf. Coat. Technol. 2025, 512, 132418. [Google Scholar] [CrossRef]
- Kazakova, M.A.; Morales, D.M.; Andronescu, C.; Elumeeva, K.; Selyutin, A.G.; Ishchenko, A.V.; Golubtsov, G.V.; Dieckhöfer, S.; Schuhmann, W.; Masa, J. Fe/Co/Ni mixed oxide nanoparticles supported on oxidized multi-walled carbon nanotubes as electrocatalysts for the oxygen reduction and the oxygen evolution reactions in alkaline media. Catal. Today 2020, 357, 259–268. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, H.; Humayun, M.; Fu, Y.; Xu, X.; Feng, C.; Wang, C. Constructing nanoporous crystalline/amorphous NiFe2O4/NiO electrocatalyst for high efficiency OER/UOR. J. Alloys Compd. 2023, 936, 168206. [Google Scholar] [CrossRef]
- Li, X.; Wang, M.; Fu, J.; Lu, F.; Li, Z.; Wang, G. Sulfurized NiFe2O4 Electrocatalyst with In Situ Formed Fe-NiOOH Nanoparticles to Realize Industrial-Level Oxygen Evolution. Small 2024, 20, e2310040. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Xie, J.; Sun, Y.; Liu, J.; Liu, B.; Wang, R.; Ma, F.; Liu, M.; Zou, J. Fe2O3/spinel NiFe2O4 heterojunctions in-situ wrapped by one-dimensional porous carbon nanofibers for boosting oxygen evolution/reduction reactions. Int. J. Hydrogen Energy 2022, 47, 21329–21343. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Lau, L.W.M.; Gerson, A.; Smart, R.S.C. X-ray photoelectron spectroscopic chemical state quantification of mixed nickel metal, oxide and hydroxide systems. Surf. Interface Anal. 2009, 41, 324–332. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, S.; Zhang, C.; Sheng, Z.; Zhang, H.; Feng, R.; Ni, Y.; Tang, X.; Gu, Y.; Zhou, X.; et al. Active oxygen species mediate the iron-promoting electrocatalysis of oxygen evolution reaction on metal oxyhydroxides. Nat. Commun. 2023, 14, 6826. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-X.; Mao, Y.; Zhou, Y.; Wang, Z.; Wei, S.; Cowie, B.C.; Marshall, A.T.; Wang, Z.; Waterhouse, G.I. Divalent site doping of NiFe-layered double hydroxide anode catalysts for enhanced anion-exchange membrane water electrolysis. Chem. Eng. J. 2025, 508, 160753. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, X.; Yang, F.; Liu, Z. Synergistic effect of Co and Fe bimetallic oxides/hydroxides composite structure as a bifunctional electrocatalyst for enhancing overall water splitting performance. J. Alloys. Compd. 2022, 895, 162614. [Google Scholar] [CrossRef]
- Laïk, B.; Richet, M.; Emery, N.; Bach, S.; Perrière, L.; Cotrebil, Y.; Russier, V.; Guillot, I.; Dubot, P. XPS Investigation of Co–Ni Oxidized Compounds Surface Using Peak-On-Satellite Ratio. Application to Co20Ni80 Passive Layer Structure and Composition. ACS Omega 2024, 9, 40707–40722. [Google Scholar] [CrossRef]
- Chen, Q.; Su, K.; Zhang, M.; Ma, Q. Fe3O4/Co3O4 core-shell nanocomposites modified structure and properties of heavy metal oxide diamagnetic glasses. J. Non Cryst. Solids 2019, 509, 10–22. [Google Scholar] [CrossRef]
- Baker, J.G.; Schneider, J.R.; Torres, J.A.G.; Singh, J.A.; Mackus, A.J.M.; Bajdich, M.; Bent, S.F. The Role of Aluminum in Promoting Ni–Fe–OOH Electrocatalysts for the Oxygen Evolution Reaction. ACS Appl. Energy Mater. 2019, 2, 3488–3499. [Google Scholar] [CrossRef]
- Sherwood, P.M.A. Introduction to Studies of Aluminum and its Compounds by XPS. Surf. Sci. Spectra 1998, 5, 1–3. [Google Scholar] [CrossRef]
- Fukumizu, H.; Sekine, M.; Hori, M.; McIntyre, P.C. Initial growth analysis of ALD Al2O3 film on hydrogen-terminated Si substrate via in situ XPS. Jpn. J. Appl. Phys. 2020, 59, 016504. [Google Scholar] [CrossRef]
- Omotosho, K.D.; Ozoude, C.; Gurung, V.; Banerjee, P.; Filatov, A.; Shevchenko, E.; Berman, D. Synthesis of multicomponent oxygen evolution reaction coatings via block copolymer templating with vapor- and solution-phase precursors. J. Colloid Interf. Sci. 2025, 703, 139078. [Google Scholar] [CrossRef]
- Haham, H.; Grinblat, J.; Sougrati, M.-T.; Stievano, L.; Margel, S. Engineering of Iron-Based Magnetic Activated Carbon Fabrics for Environmental Remediation. Materials 2015, 8, 4593–4607. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).