Abstract
Background/Objectives: Skin temperature has been considered a physiological variable associated with the risk of pressure injuries. This systematic review analyzed the available evidence regarding the relationship between skin temperature and the development, progression, or prevention of pressure injuries in humans. Methods: A systematic search was conducted in the PubMed, Scopus, and Dimensions databases, including studies published between 2013 and 2023 in English or Spanish. PRISMA 2020 guidelines and EQUATOR network checklists (CONSORT, STROBE, CARE) were applied to assess methodological quality. Risk of bias was evaluated using RoB 2, ROBINS-I, ROBINS-E, and JBI tools. Results: The reviewed studies reported thermal variations in tissues subjected to sustained pressure, some of which preceded the appearance of visible clinical signs of tissue damage. However, methodological heterogeneity, lack of standardized thermal thresholds, and variability in measurement conditions limited the generalizability of the findings. Conclusions: Skin temperature may be associated with relevant pathophysiological mechanisms in the development of pressure injuries. Its measurement could complement traditional clinical tools, such as the Braden scale, enhancing early risk identification. More robust, multicenter, and standardized studies are needed to validate its clinical applicability.
1. Introduction
Pressure injuries (PIs), currently classified as dependency-related skin lesions (DRSLs), represent a critical challenge for healthcare systems, significantly affecting individuals with reduced mobility or functional dependence [1]. These injuries result from the collapse of capillary flow due to sustained pressure, leading to tissue hypoxia, edema, progressive cellular damage, and ultimately necrosis [2,3]. Despite preventive efforts, the global prevalence of PIs in hospital settings ranges from 5% to 35%, causing considerable impacts on morbidity, healthcare costs, and patient quality of life [4,5]. Preventive measures combine risk assessment (e.g., Braden), skin inspection and moisture management, scheduled repositioning and early mobilization, and the use of pressure-redistributing support surfaces [6]. However, variable adherence, the moderate predictive capacity, and some subjectivity of risk scales, and the fact that these surfaces do not replace pressure relief, allow deep tissue damage to develop before visible signs appear, perpetuating the burden of PIs. This has driven the search for early detection methods that enable interventions before visible skin damage appears. In long-term care units, intensive care settings, and among patients with chronic comorbidities, the occurrence of pressure injuries is often underestimated due to the subtlety of early warning signs. Standard clinical monitoring tools rely heavily on visual inspection, which may not detect subclinical tissue alterations [6]. Therefore, advancing the understanding of underlying physiological changes is essential for timely and targeted interventions.
From a pathophysiological perspective, the development of PIs is mediated by ischemia-reperfusion phenomena that “disrupts” the microvascular homeostasis of the tissue [7,8]. Prolonged pressure reduces capillary perfusion, while pressure release leads to reperfusion flow, which can cause additional damage through oxidative stress, inflammation, and endothelial dysfunction [9,10]. These processes occur before the appearance of clinical signs such as non-blanchable erythema, limiting the effectiveness of traditional visual inspection methods for early detection. Considering this limitation, the evaluation of biophysical parameters has been proposed as a complementary strategy for identifying incipient tissue damage. Among these indicators, skin temperature has gained particular relevance as a dynamic and measurable signal that reflects local perfusion status. Alterations in superficial blood flow produce localized thermal fluctuations which, if properly interpreted, could serve as indirect markers of underlying vascular compromise [11].
In this context, evidence from cutaneous thermography indicates that interpretation prioritizes within-patient differentials (ΔT) over population ‘normal’ values. Under controlled conditions, reactive hyperemia typically raises skin temperature by ~0.5–1.0 °C; when acute tissue injury is present (e.g., a local wound), increases of ≈0.7 °C have been reported; in inflammatory processes, skin temperature can rise by up to ~2 °C relative to adjacent/contralateral skin; ischemia, in turn, tends to produce cooling of comparable magnitude Accordingly, ΔT ≥ 1–2 °C are generally considered clinically relevant under controlled conditions [12]. Nevertheless, in the sacral region, smaller thresholds with prognostic value have been described; for example, a cooling of ~−0.1 °C relative to adjacent skin predicted PIs with high sensitivity, outperforming Braden (Cai et al.) [13]. Clinically, this would allow for the identification of “silent zones” at risk before visible changes in the skin occur.
Recent literature has highlighted the clinical relevance of thermal markers across various contexts. For example, Rossel-Diago et al. (2024) conducted a systematic review on diabetic foot complications, identifying thermal asymmetries and pressure–heat interactions as early indicators of tissue stress [14]. Their findings support the integration of thermographic monitoring as a non-invasive tool to detect pressure-induced tissue compromise—even beyond the plantar region. In the context of pressure injuries, thermal variations resulting from changes in superficial blood flow reveal consistent patterns: initial hypoperfusion is commonly associated with localized cooling, followed by a temperature increase during reperfusion phases [15,16]. Studies by Lachenbruch et al. (2013) and Cai et al. (2021) have demonstrated that these thermal gradients—detectable through infrared thermography—can precede the clinical appearance of visible injuries in at-risk zones [13,17]. This growing body of evidence reinforces the role of skin temperature as a valuable clinical parameter for anticipating tissue vulnerability and optimizing prevention and monitoring strategies in high-risk patients.
Despite its promise, the clinical application of temperature-based assessments is currently limited by significant methodological variability [13,17]. Studies differ widely in terms of sensor technology (e.g., infrared thermography vs. contact thermistors), measurement protocols (distance, environmental control, thermal stabilization), and criteria for defining early-stage tissue injury. These inconsistencies reduce comparability and hinder the development of universal guidelines. In this context, critically reviewing and synthesizing the literature is essential to outline the methodological standards and clinical conditions under which thermal monitoring may offer the greatest benefit.
Particularly in high-risk populations—such as older adults, immobile patients, or those in intensive care—subtle viability changes often escape routine checks. Continuous thermography highlights within-patient ΔT patterns—persistent cooling at bony prominences, rebound warming after unloading, and spatial asymmetries—that precede visible change [12,13]. Detecting these motifs in real time enables rapid, individualized adjustments to repositioning cadence, off-loading, moisture control, and support-surface configuration [13].
Emerging evidence indicates that thermal signatures of early tissue stress—localized cooling with ischemia and reactive hyperthermia after unloading—often precede clinical signs such as non-blanchable erythema [13,17]. Incorporating skin-temperature monitoring into routine nursing assessments, ideally with automated alerts within electronic health records, could strengthen early-warning workflows. Nevertheless, clinical adoption remains constrained by the lack of standardized protocols and validated interpretation algorithms, limited consensus on optimal anatomical sites and clinically meaningful ΔT thresholds, and insufficient linkage between specific thermal changes and well-defined stages of pressure-injury evolution. Prospective studies addressing these gaps are needed to ensure reliability and applicability in practice.
To date, although the literature highlights the potential of skin temperature as a physiological marker of tissue stress, its broader clinical adoption is constrained by heterogeneity in methodological design and insufficient standardization. This systematic review aims to examine current scientific evidence on the relationship between skin temperature and the development, progression, or prevention of pressure injuries in humans. By analyzing the range of methodologies, clinical findings, and contextual factors reported across studies, we seek to identify key trends, limitations, and priorities for future research that can support the clinical integration of thermal monitoring in pressure injury management.
2. Materials and Methods
A systematic review of the literature was conducted following the PRISMA 2020 guidelines [18], aiming to critically analyze the available evidence on skin temperature measurement in relation to the development, progression, or prevention of pressure injuries (PIs) in humans. The methodological approach was based on the recommendations of the Cochrane Handbook for Systematic Reviews [19], and the EQUATOR Network guidelines (STROBE, CONSORT, and CARE) [20,21,22,23]. The search strategy, inclusion and exclusion criteria, quality assessment, and narrative synthesis plan were pre-defined in an internal working protocol.
The research question was formulated using the PICO framework [24]:
- Population (P): Humans at risk of pressure injuries or exposed to sustained external pressure at classical high-risk sites
- Intervention/Exposure (I): Skin temperature assessment using various thermal measurement methods.
- Comparison (C): Within-person comparators, including adjacent/contralateral skin, non-loaded sites, or pre- vs. post-unloading measures; between-person comparators when applicable.
- Outcome (O): Primary: presence, onset/incidence, progression, or staging of pressure injuries (PIs) and their relationship with skin temperature.Secondary: within-patient ΔT thresholds, reference ranges by anatomical region, studies on prevention and standardization, and analogous evidence on cutaneous lesions with shared mechanisms (mechanical loading, microclimate, and microvasculature).
Articles were searched in PubMed, Scopus, and Dimensions databases, including studies published from January 2013 to April 2023. The terms “pressure injury”, “pressure ulcer”, “skin temperature”, and “temperature” were used in combination with the Boolean operators AND and OR [23,24]. Filters for human studies in English or Spanish were applied. The detailed search equations and combinations are reported in the appendices (Table A1). The search was formally closed in April 2023.
We included observational studies, clinical trials, and methodological studies that assessed skin temperature by any thermal method, relating it to the risk, diagnosis, or progression of pressure injuries. Systematic reviews, animal studies, isolated case reports, research protocols, technical articles without clinical application, and studies without full-text access were excluded. Only studies meeting at least 80% of the methodological quality criteria were selected. The study selection process involved three phases: (1) title and abstract screening, (2) full-text review, and (3) methodological quality assessment. Two independent, blinded reviewers conducted the selection process. Discrepancies were resolved by consensus or, if necessary, by a third reviewer. The entire selection process was documented using a PRISMA 2020 flow diagram [18], presented in the results section.
For each included study, the following data were extracted: author names, year of publication, country, study type, population characteristics, thermal measurement method, main findings regarding skin temperature, and clinical outcomes. The article review was conducted independently by two researchers using a structured matrix developed ad hoc.
The methodological quality of the studies was assessed using specific tools: RoB 2 for randomized clinical trials [25], ROBINS-I for non-randomized studies [26], ROBINS-E for observational studies [26], and the Joanna Briggs Institute (JBI) Manual for Evidence Synthesis [27]. Two independent reviewers performed this evaluation, and the results are summarized in a risk of bias table [28]. Due to observed heterogeneity in study designs, thermal measurement methods, and populations, a narrative descriptive analysis was conducted [29,30,31].
3. Results
3.1. Study Selection
A total of 626 records were initially identified from PubMed (n = 165), Scopus (n = 20), and Dimensions (n = 441). After removing duplicates, 580 records were screened. Following title and abstract screening, 531 records were excluded, resulting in 37 articles selected for full-text review. Of these, 19 were excluded due to not meeting inclusion criteria or presenting low methodological quality (<80% compliance with EQUATOR guidelines) [20]. Ultimately, 18 studies were included in this systematic review. The selection process is illustrated in the PRISMA 2020 flow diagram (Figure 1) [18].
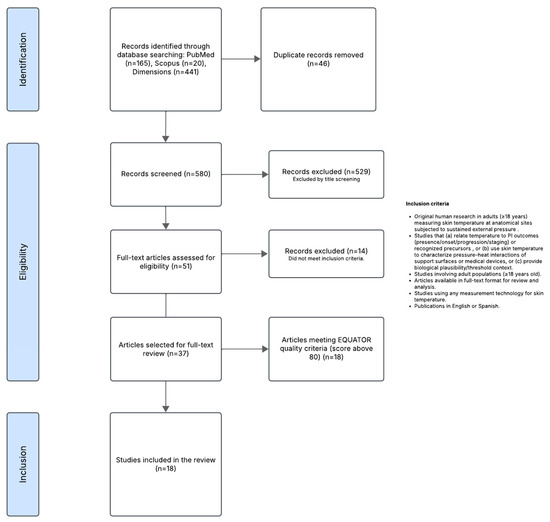
Figure 1.
PRISMA 2020 Flow.
General Context of the Included Studies
The 18 studies included were published between 2013 and 2022. Most studies originated from the United States (n = 5), followed by Spain (n = 2), Japan (n = 2), and China (n = 2). Individual studies were also included from Brazil, Poland, Taiwan, the Netherlands, Turkey, the Czech Republic, and Sweden (n = 1 each). Prospective and retrospective observational studies predominated (n = 12), followed by randomized clinical trials (n = 3) and pilot studies with experimental or exploratory designs (n = 3). This distribution reflects a growing interest in applying thermal measurement to the prevention and diagnosis of pressure injuries.
3.2. Methodological Quality
3.2.1. Study Design
The methodological quality assessment, based on STROBE, CONSORT, CARE, and CONSORT-Pilot standards [20,23], showed compliance ranging from 81.8% to 100% of the evaluated items. Ten studies reported compliance above 90%, indicating high methodological quality; five studies ranged between 85% and 90%, and the remaining five between 80% and 85%. The main deficiencies are related to the description of sampling methods, confounding control strategies, and the critical discussion of limitations. Individual study compliance percentages are detailed in Table 1.
3.2.2. Risk of Bias
A critical appraisal of the risk of bias was conducted using tools appropriate to each study’s methodological design. Three randomized clinical trials were evaluated using the RoB 2 tool [25], showing low risk of bias across all domains (Table S1). Two quasi-experimental studies were assessed using ROBINS-I [26] (Table S2), indicating moderate risk, particularly due to lack of blinding, limited confounder control, and selective reporting. Thirteen observational studies were assessed with ROBINS-E [26] (Table S3), all categorized as ‘some concerns’ mainly due to limitations in outcome measurement and reporting bias. Additionally, two non-comparative studies—a crossover experiment and a case report—were evaluated using JBI checklists [27] (Table S4), both showing low risk in most items, with some uncertainty regarding measurement standardization and clinical context description.
Despite these methodological limitations, the use of differentiated and rigorous tools ensured a solid critical appraisal process. The observed risk levels reflect common features in applied clinical research, especially in settings where randomized designs are not always feasible. Thus, the findings synthesized in this review should be interpreted with caution but also acknowledged as relevant evidence to guide future research and clinical decisions regarding the use of skin temperature as a marker in the context of pressure injuries. Table 1 summarizes the tools used per article.

Table 1.
Assessment of methodological reporting according to EQUATOR checklists and tools used for risk of bias evaluation.
Table 1.
Assessment of methodological reporting according to EQUATOR checklists and tools used for risk of bias evaluation.
| Author(s) | Year | Study Type | Reporting Guideline (EQUATOR) | Compliance (%) | Risk of Bias Tool |
|---|---|---|---|---|---|
| Lin et al. [32] | 2021 | Longitudinal observational | STROBE | 81.8% | ROBINS-E |
| García-Molina et al. [33] | 2021 | Randomized clinical trial | CONSORT | 94.0% | RoB 2 |
| Cai et al. [13] | 2021 | Prospective cohort observational | STROBE | 81.8% | ROBINS-E |
| Leenen et al. [34] | 2020 | Randomized clinical trial | CONSORT | 98.0% | RoB 2 |
| Kanazawa et al. [35] | 2016 | Cross-sectional observational | STROBE | 96.1% | ROBINS-E |
| Bilska et al. [36] | 2020 | Randomized trial | CONSORT | 98.0% | RoB 2 |
| Yilmaz et al. [37] | 2019 | Prospective observational | STROBE | 86.4% | ROBINS-E |
| Soares et al. [38] | 2019 | Cross-sectional descriptive-correlational | STROBE | 95.5% | ROBINS-E |
| Yavuz et al. [39] | 2018 | Retrospective observational | STROBE | 77.3% | ROBINS-E |
| Mayrovitz et al. [40] | 2018 | Prospective cohort observational | STROBE | 77.3% | ROBINS-E |
| Lachenbruch et al. [41] | 2015 | Crossover experimental pilot study | CONSORT-PILOT | 98.1% | JBI |
| Kanazawa et al. [42] | 2016 | Retrospective cohort observational | STROBE | 86.4% | ROBINS-E |
| Cox et al. [43] | 2016 | Prospective observational | STROBE | 90.9% | ROBINS-E |
| Staffa et al. [44] | 2016 | Case study | CARE | 96.1% | JBI |
| Källman et al. [45] | 2015 | Longitudinal descriptive observational | STROBE | 86.4% | ROBINS-E |
| Lachenbruch et al. [17] | 2013 | Experimental pilot study | CONSORT-PILOT | 100% | ROBINS-I |
| Jiang et al. [46] | 2020 | Prospective observational | STROBE | 86.4% | ROBINS-E |
| Lupiáñez-Pérez et al. [47] | 2021 | Quasi-experimental | STROBE | 95.5% | ROBINS-I |
3.2.3. Data Extraction and Synthesis
The selected articles were organized and analyzed according to their main methodological and clinical characteristics to facilitate comparison between studies. Table 2 below summarizes the most relevant aspects of each publication: author, year, objective, methodology, technology used, and anatomical site or region of interest. This synthesis enables the identification of trends, similarities, and differences in the approach to skin temperature as a physiological variable in the study of pressure injuries, as well as the different technological approaches applied for its measurement.

Table 2.
Summary of methodological and clinical characteristics of the selected studies on skin temperature in pressure injury research.
The included studies exhibit notable methodological heterogeneity, both in the study designs employed and the clinical settings and technologies addressed. Infrared thermography was the most frequently used technology, appearing in 11 out of the 18 articles, whether through portable infrared cameras or smartphone-attached devices. This predominance reflects the growing acceptance of thermography as a non-invasive tool for analyzing risk and progression of pressure injuries.
Regarding research objectives, five major thematic approaches were identified:
- Early detection and risk stratification (n = 4). Prospective cohort studies in ICU with infrared thermography that define ΔT thresholds and diagnostic performance, plus descriptive studies with spot IR thermometry/thermography that explore temporal sensitivity and intrapatient comparators.
- Follow-up of evolution and prognosis (n = 4). Observational studies were included in cohorts with established pressure injuries, periinjury-bed gradients, retrospective series that relate cold edge with scour, and longitudinal studies with heat maps during treatments.
- Prevention and standardization (n = 8). Laboratory studies with combined loads (pressure–shear–temperature) and hyperemia models, clinical studies of positioning/perfusion (30° supine/lateral), comparisons of support surfaces (cushions), tests with devices (cervical collars), feasibility/reference values of IR thermometry, and works proposing protocols/SOPs for thermal capture and visualization were included.
- Analogous evidence (n = 2) included studies on skin lesions with shared load-microvasculature mechanisms informing thermal interpretation in LP, which included cross-sectional cohort studies with elevated absolute plantar temperatures in diabetic neuropathy and prolonged thermographic follow-up of a case with pre-ulceration and post-revascularization changes.
- Predictive/analytical models (n = 5). Of the studies grouped into the previous categories, those that mentioned relative ROC thresholds of ΔT, multivariate models (Cox) that integrate hemodynamic/metabolic variables, thermal edge-bed-perilesion rules, and experimental regressions that quantified the weight of temperature vs. pressure; basis for AI and automated monitoring were reviewed.
The most frequently assessed anatomical site was the sacral region (n = 11), followed by the plantar area, heels, and scapular regions, highlighting a specific focus on high-risk areas for ulceration in bedridden patients. Sample sizes varied considerably, ranging from single-patient case studies to cohorts exceeding 400 participants, directly influencing the external validity of the findings. In terms of design, longitudinal observational and cohort studies predominated (n = 12), followed by randomized clinical trials (n = 3) and pilot studies with experimental approaches (n = 3), indicating a still-limited presence of high-evidence-level designs. Among clinical findings, more than half of the studies reported significant thermal gradients, either as skin cooling under sustained pressure or temperature elevation after unloading, supporting the hypothesis of a relationship between skin temperature and tissue perfusion. However, one study documented the absence of thermal sensitivity in lesions with advanced infection, underscoring the limitations of the method in certain scenarios.
Finally, the increasing use of computational approaches, such as machine learning algorithms, and the development of smart solutions for real-time thermal monitoring, suggest a transition toward the integration of these technologies in preventive clinical practice.
Final del formulario.
4. Discussion
The evidence synthesized in this review suggests that skin temperature is a useful physiological biomarker to anticipate alterations in tissue viability in areas subjected to mechanical load. In different clinical and experimental contexts, intrapatient thermal patterns were identified that preceded the visible signs: (i) decrease in temperature during the load exposure phase, compatible with hypoperfusion/ischemia, and (ii) increase in temperature in the postload phase, which could be related to reactive hyperemia/reperfusion. These thermal gradients and asymmetries, observed in bony prominences and support areas, are compatible with tissue perfusion patterns in ischemia-reperfusion processes; however, on their own, they do not demonstrate microvascular dysfunction and should be interpreted based on the clinical and hemodynamic context of the patients [17,41,45].
Below, we summarize the findings by analytical axes: a. Early detection, risk stratification; b. Prognosis and follow-up; c. Prevention and measurement standards; and d. Analogous evidence, i.e., studies on skin lesions that share loading mechanisms or associated with microvasculature, such as diabetic foot ulcers, integrated considering biological plausibility, although not combined with the clinical effects of pressure injuries.
Early detection and risk stratification.
Cai et al. identified that a sacral relative temperature ≤ −0.1 °C predicted the onset of LP 48 h in advance and showed superior performance to Braden (AUC ≈ 0.90); moreover, the risk was 13.67 times higher below that threshold, supporting the use of intrapatient temperature differentials as an objective warning signal in ICU patients [13]. Accordingly, Jiang et al. found optimal cut-off values for the relative temperature ≤ −0.1 °C; although it additionally explored blood pressure and glucose measurements, each variable was associated with increased risk, highlighting an HR of 6.36 for temperature differentials and a peak incidence around days 4–5 of hospitalization [46]. From the physiological point of view, thermal decrease during loading may be associated with hypoperfusion and postload temperature increase with reactive hyperemia, which is why these studies provide mechanistic plausibility to interpret gradients at risk sites. Taken together, these data support the use of relative temperature by temperature differentials per patient in regions exposed to mechanical load to stratify risk and promote early interventions (repositioning, unloading, and even microclimate management), with the caveat that multicenter validations and standardized protocols are required for routine implementation [13,46].
However, not all studies support thermal monitoring for risk stratification. Mayrovitz et al. found no significant association between sacral thermal differences and vascular disease status in critically ill patients, concluding that temperature differentials did not reliably identify high-risk patients [40]. Similarly, Yılmaz et al. failed to observe predictive associations using infrared thermometry in ICU patients [37]. These negative findings highlight critical methodological considerations: Mayrovitz et al. used thermal thresholds derived from healthy subjects (−1.5 °C) applied to critically ill patients with vascular disease, while allowing only 4 min for thermal stabilization. These limitations underscore the importance of population-specific threshold validation, adequate stabilization protocols, and appropriate patient selection criteria for successful thermal monitoring implementation.
Prognosis and follow-up of established injuries.
Cox et al. observed in discolored intact skin that relatively cooler centers than adjacent skin were associated with a higher probability of necrosis at day 7 in the multivariate analysis, while negative capillary filling emerged as a consistent prognostic cofactor. The authors emphasize that the thermal signal is phase-dependent (early cooling due to ischemia vs. increased reperfusion/inflammation), so serial measurements and intrapatient comparators are preferable to isolated absolute values [43]. In practical terms, this evidence supports close monitoring and early intervention when the discolored center is colder and capillary filling is negative, avoiding decisions based on a single reading.
Lin et al. [32], on the other hand, in the context of pressure ulcers in more advanced stages, showed that a higher perilesional temperature than that of the wound bed and “normal” skin is associated with better healing, while colder perilesions are associated with worse evolution. This perilesional gradient > bed is consistent with granulation/re-epithelialization activity and preserved peripheral perfusion; conversely, a “hypothermic” border may reflect perfusion involvement of marginal tissue. Clinically, these patterns suggest intrapatient operating thresholds (perilesion vs. bed) useful for stratifying prognosis and adjusting management, debridements, and follow-up frequency.
In the same vein as perilesional border analysis, Kanazawa et al. (2016) found that a colder edge than the bed/perilesion predicts the development of undermining (RR ≈ 4), anticipating a relevant complication [35]. This peripheral marker can be incorporated as a warning signal to intensify wound contour surveillance and plan debridement and positioning strategies prior to subepidermal extension of the lesion.
On the other hand, and from a longitudinal “heat map” perspective, Bilska et al. (2020) showed that segments with increased or elevated temperature accompany orderly healing with minimal granulation; in hypergranulation the pattern starts lower and reverses after debridement; In this study, superficial thermography reflected the local hemodynamic status rather than the type of treatment [36], which supports the use of this technique as a complementary prognostic tool rather than as a substitute for clinical assessment. This aims to integrate standardized thermal series with environmental control and capture protocols.
In summary, the findings mentioned above provide actionable information: (i) colder wound center in discolored intact skin + negative capillary filling → high risk, (ii) warmer perilesion than the wound bed → favorable healing, (iii) colder perilesional border → risk of undermining. Clinical adoption in this context requires acquisition/interpretation protocols (ambient control, thermal stabilization, distance/angle, contralateral or adjacent comparators) and serial measurements to capture phase changes. However, these studies do not present validation of operational thresholds or decision algorithms necessary for standardization.
Prevention and measurement standards.
The evidence converges on two messages: (i) simultaneously managing mechanical load and microclimate, and (ii) standardizing thermal measurement. Through laboratory tests, Lachenbruch et al. showed that skin temperature, combined with pressure, better predicts the magnitude of reactive hyperemia (ischemia index) than pressure alone; increases of just 1 °C have a physiological weight comparable to several additional mmHg of pressure, supporting control of skin T° as part of pressure risk management [17,41].
Regarding bed positioning, Källman et al. They found that supine at 30° offers greater tissue perfusion during loading than 0° supine, 30° lateral, or 90° lateral, even after adjusting for interface pressure, temperature, and time; temperature increased in all postures, with postload hyperemia and high interindividual variability, indicating that reducing pressure is not enough: postures that preserve perfusion should be chosen and the increase in skin temperature should be monitored [45]. In healthy volunteers, Lupiáñez-Pérez et al. observed that 2 h of loading in the sacrum/trochanter significantly raised the local temperature, while perfusion and SaO2 tended to increase without reaching significance; their proposal is oriented towards personalized repositioning by anatomical region considering loading time and T° [47]. These patterns are consistent with experimental evidence from Lachenbruch et al., who mention that when pressure and T° are combined and sustained, ischemia increases and that cooling the skin could attenuate it, which is a priority hypothesis for clinical validation [17,41].
In terms of devices and support surfaces, García-Molina et al. They compared five wheelchair cushions: they found similar thermal responses in 10 min but large differences in pressure redistribution (better performance with open-cell polyurethane and spring-type base), supporting multimodal evaluations (pressure + T° + comfort) and providing a thermographic capture protocol useful for standardization [33]. In their study of cervical collars, Leenen et al. showed that after 20 min, there was a high incidence of indentation marks (first step to LP associated with devices) and increased cutaneous T° (~0.5–1.8 °C; difference between models ~0.3 °C, no clinical relevance). Operational recommendations are derived: limiting the duration of immobilization, serial skin evaluation protocols, and design improvements that reduce pressure peaks and heat/humidity accumulation [34].
On standardization of thermal measurement, Soares et al. demonstrated the clinical feasibility of non-contact infrared thermometry (low cost, minimal contamination), establishing reference values by region and highlighting controlling environment and distance/angle to interpret variations [38]. With respect to the thermography technique, Kanazawa et al. They proposed a reproducible classification of bed-edge-perilesion: a colder edge than bed/perilesion predicted undermining at one week, which is useful for early interventions; they also recommend viewing ranges (32–40 °C) and combining with ultrasound to determine lesion depth [42]. In a cross-sectional way, Källman, Lupiáñez-Pérez, and Lachenbruch agree in prioritizing relative metrics (ΔT) between zones/times and standardized time windows of loading and postloading to improve comparability and clinical interpretation [17,41,45,47].
In summary, effective prevention integrates: (a) pressure management (30° postures, surfaces with better dispersion) + microclimate management (breathable materials, local ventilation/cooling where appropriate); (b) clear thermal SOPs (anatomical sites, fixed distance, environmental control, ΔT and pre/postload windows); and (c) temperature screening criteria with local validation before universal thresholds are adopted. With the contributions of García-Molina [33], Leenen [34], Källman [45], Kanazawa [42], Soares [38], Lupiáñez-Pérez [47], and Lachenbruch [17,41], a coherent basis is available to standardize measurement and operationalize temperature-centered prevention in daily practice.
Analogous evidence: diabetic foot and conceptual transfer to LP.
Yavuz et al. quantified absolute resting plantar temperatures (not asymmetries) and showed that, in people with diabetic neuropathy with and without a history of ulcer, all regions of the foot exceeded 30 °C, with differences of ~3–5 °C compared to controls without neuropathy [39]. They proposed an acute-chronic framework: acute increases due to friction/shear during walking and chronic elevation due to inflammation and autonomic dysfunction (anidrosis), both raising tissue metabolic demand; Under mechanical load, compensatory vasodilation may be insufficient, accelerating tissue failure. They connected these findings with thermal damage thresholds (~35 °C) and with the synergistic role of temperature + pressure/shear, reinforcing that microclimate and load act in combination and that thermal management (in addition to off-loading) is relevant for prevention [39].
Staffa et al. provided longitudinal thermographic follow-up at home (>1 year) in a patient with T2DM and vascular disease: they detected sustained contralateral ΔT (≈2 °C) and the appearance of a heel hot-spot (ΔT ≈ 4.7 °C) weeks before a heel pressure ulcer was confirmed. After revascularization, asymmetries decreased, suggesting a functional link between perfusion, skin temperature, and lesion appearance/evolution. The case underscores the clinical utility of ΔT (in addition to absolute temperature) as a warning signal and the need for capture protocols (acclimatization, environmental control, non-reflective background) for reliability and comparability; although it is single-case evidence, the sequence “ΔT growing → thermal focus → lesion” under loading zones is plausible and transferable to LP to guide focal off-loading and microclimate control [44].
Together, diabetic foot lesions provide an analogous pathophysiological model: the sustained elevated temperature in loaded tissue increases metabolic demands while perfusion may be compromised, creating an imbalance that accelerates tissue damage. Heat maps and ΔTs (contralateral or local) help locate at-risk areas before visible changes, supporting preventive interventions (afterload, more dissipative surfaces, exposure times) and the integration of thermal metrics into clinical surveillance [39,44].
Predictive models and analytics (thresholds, regression, and AI opportunities).
Several works advance from observation to quantitative prediction:
- Relative thresholds and operational classification. In ICU, Cai et al. established a sacral relative cut-off point of −0.1 °C (colder risk region than control) that anticipated LP ~48 h before visual inspection and with AUC ~0.90, surpassing Braden; the probability of LP was higher when the relative temperature ≤ −0.1 °C [13]. Kanazawa (2016b) showed that a colder wound edge (edge < bed/perilesional) quadrupled the risk of undermining at 1 week (high sensitivity/specificity and kappa), proposing a simple rule applicable to the patient’s bedside [42].
- Multivariable risk models. Jiang et al. used Cox to combine relative temperature, DBP, and glycemia, with optimal cut-off points (DBP 63.5 mmHg; glucose 9.9 mmol/L; relative temperature −0.1 °C); the group with relative temperature ≤ −0.1 °C had HR ~6.36, with increased risk around days 4–5 of hospitalization [46].
- Mechanistic modelling of ischemia. In the laboratory with healthy volunteers, Lachenbruch et al. used fixed-effect regressions to predict the magnitude of reactive hyperemia (ischemia index) from pressure and temperature (shear was not significant in superficial skin with the protocols used). They found that +1 °C contributes ~8–14× more to ischemia than +1 mmHg, and that the effect of temperature is more marked between 32–36 °C; they concluded that lowering skin temperature could mitigate ischemia and the risk of LP, and that managing pressure + temperature is a better predictor of risk than pressure alone [17,41].
- Towards AI and automation. In addition to thresholds and regressions, advanced thermal analysis approaches (e.g., machine learning on thermograms) for risk classification have been explored, opening the door to decision support systems that can be integrated into nursing routines. The validity of portable smartphone-type cameras for standardized relative reading also facilitates bedside flows and telemonitoring, although their use was more of validation than prediction in itself [42].
Regarding the models and metrics explored by these authors, some common patterns are observed. (i) Relative metrics (local/contralateral ΔT or edge-rules) are more robust than absolute values against environmental variability; (ii) multivariate models that integrate thermal signals with hemodynamic/metabolic signals improve the temporal stratification of risk; (iii) mechanistic models support thermal intervention (cooling/modulation of the microclimate) as a complement to pressure relief; and (iv) translating AI into practice requires standardized capture protocols, multicenter datasets, and external validation prior to clinical adoption.
Limitations.
This review presents several limitations. First, methodological heterogeneity across studies—including thermal devices, capture protocols, and controlled environmental conditions—hindered direct comparisons and precluded a quantitative meta-analysis. Although strict quality criteria were applied, some studies had small sample sizes, lacked control groups, and used observational designs that limited causal inference. Most studies were also conducted in hospital or controlled experimental settings, limiting the generalizability of findings to other clinical contexts such as home care or critical care units. Additionally, there was a lack of standardization in the clinical criteria used to define early tissue damage, introducing variability in interpreting thermal gradients.
Although validated tools were used to assess risk of bias (RoB 2, ROBINS-I, ROBINS-E, JBI), methodological variations were identified, especially in confounder control and clinical follow-up completeness. Nevertheless, the studies met acceptable quality standards and provided a sound basis for interpreting observed patterns. Still, caution is advised in extrapolating findings, and standardized research in clinical settings with patients at varying risk levels and larger sample sizes is strongly recommended for validation. The literature search was closed in April 2023; thus, studies published afterward were not included in this analysis.
Clinical implications
The results of this review reinforce the clinical potential of skin temperature as a non-invasive biomarker for the early detection and monitoring of pressure injuries. Its incorporation into routine assessment protocols could enhance the diagnostic capacity of healthcare teams, particularly for high-risk populations such as critically ill or bedridden patients. Thermographic assessment, especially when integrated into smart mattresses, wearable sensors, or digital platforms—could complement traditional risk scales, allowing for more objective and continuous evaluation. This strategy may help prevent advanced injuries and optimize care resources by enabling more timely and targeted interventions.
Physiopathological considerations
From a physiopathological perspective, the thermal variations reported reflect underlying perfusion disturbances that may precede or accompany pressure-related tissue damage. Decreased temperature in compressed areas may signal early hypoperfusion or capillary collapse, while post-decompression increases suggest reactive hyperemia due to reperfusion. These thermal responses may be modulated by factors such as microvascular autoregulation, local inflammation, or endothelial integrity, making thermography a valuable tool for exploring underlying pathophysiological dynamics. Understanding these mechanisms through thermal analysis provides clinical and scientific value and could inform the design of more targeted preventive interventions.
Future research directions
Based on the findings presented, future studies should focus on multicenter research using standardized protocols and representative clinical populations, including individuals with comorbidities and in non-hospital settings. In addition, research should explore the utility of thermography as a dynamic marker by incorporating serial measurements to analyze thermal trajectories during tissue damage or healing. Another promising avenue is the development and validation of predictive models using artificial intelligence that integrate thermal, clinical, and contextual variables to generate real-time alerts. These approaches could support the implementation of decision-support systems, particularly in settings with limited resources or high patient loads.
5. Conclusions
The evidence analyzed in this systematic review supports the role of skin temperature as a relevant physiological marker in the study of pressure injuries. Through various techniques and in multiple clinical contexts, it has been shown that thermal fluctuations in the skin, especially in areas subjected to continuous pressure, can anticipate tissue damage before perceptible signs appear, positioning temperature as a potential early warning signal. Moreover, the research findings suggest that skin temperature is useful not only for risk prediction but also as a dynamic monitoring tool for wound healing, clinical evolution, and therapeutic response. Techniques such as infrared thermography and thermal sensors have facilitated the recognition of these patterns. However, methodological diversity, lack of device standardization, inconsistent measurement conditions, and absence of validated clinical thresholds limit the extrapolation of findings.
Additionally, individual factors such as vascular condition, skin tone, and support surface type can affect the thermal responses observed. The adoption of emerging tools, along with the use of artificial intelligence and multivariate predictive models, creates new opportunities for automated analysis of thermal data and integration into preventive care programs. Ultimately, skin temperature emerges as a promising, accessible, and non-invasive biomarker that, if properly validated and standardized, could transform current paradigms in pressure injury prevention and care.
Supplementary Materials
Supplementary Materials can be downloaded at: https://www.mdpi.com/article/10.3390/app15179537/s1. Table S1. Full domain-by-domain risk of bias assessment for randomized controlled trials using the RoB 2 tool. Table S2. Risk of bias assessment for non-randomized interventional studies using ROBINS-I. Table S3. Risk of bias assessment for observational studies using ROBINS-E. Table S4. Critical appraisal of descriptive studies, case reports, and case series using the JBI checklist. Figure S1. Risk of bias plot for randomized controlled trials (RoB 2 tool), generated with robvis (Cochrane). Figure S2. Risk of bias plot for non-randomized interventional studies (ROBINS-I), generated with robvis. Figure S3. Risk of bias plot for observational studies (ROBINS-E), generated with robvis. Figure S4. Risk of bias plot for descriptive studies, case reports, and case series (JBI checklist), summarized with robvis.
Author Contributions
Conceptualization, C.J.C.; methodology, C.J.C., R.N.Z.B. and J.E.M.J.; software, C.J.C.; validation, R.N.Z.B. and J.E.M.J.; formal analysis, C.J.C. and R.N.Z.B.; investigation, C.J.C. and J.E.M.J.; data curation, C.J.C.; writing—original draft preparation, C.J.C.; writing—review and editing, R.N.Z.B. and J.E.M.J.; visualization, C.J.C.; supervision, R.N.Z.B. and J.E.M.J.; project administration, C.J.C.; funding acquisition, R.N.Z.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been funded by Dirección General de Investigaciones of Universidad Santiago de Cali under call No. DGI-01-2025 and project 441-621124-752.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Abbreviation | Full Term |
| °C | Degrees Celsius |
| CARE | Case Report Guidelines |
| CONSORT | Consolidated Standards of Reporting Trials |
| EQUATOR | Enhancing the QUAlity and Transparency Of health Research |
| AI | Artificial Intelligence |
| DRSL | Dependency-Related Skin Lesions |
| PI | Pressure Injuries |
| JBI | Joanna Briggs Institute |
| PICO | Patient, Intervention, Comparison, Outcome |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RoB 2 | Risk of Bias 2.0 |
| ROBINS-E | Risk of Bias in Non-randomized Studies—Exposure |
| ROBINS-I | Risk of Bias in Non-randomized Studies of Interventions |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| SOP | Standard Operative Procedures |
Appendix A

Table A1.
Search Strategy.
Table A1.
Search Strategy.
| Database | Search Strategy |
|---|---|
| PubMed | (“pressure injury” [Title] OR “pressure ulcer” [Title]) AND (“skin temperature” [Title] OR “temperature” [Title]) AND (english [lang] OR spanish [lang]) AND (“2013” [Date-Publication]: “2023” [Date-Publication]) AND (humans [MeSH Terms]) |
| Scopus | TITLE(“pressure injury” OR “pressure ulcer”) AND TITLE(“skin temperature” OR “temperature”) AND (LIMIT-TO(LANGUAGE, “English”) OR LIMIT-TO(LANGUAGE, “Spanish”)) AND (PUBYEAR > 2012 AND PUBYEAR < 2024) |
| Dimensions | search publications where title contains “pressure injury” or “pressure ulcer” and title contains “skin temperature” or “temperature” and language in [“en”, “es”] and year in [2013:2023] |
References
- European Pressure Ulcer Advisory Panel; National Pressure Injury Advisory Panel; Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers/Injuries: Clinical Practice Guideline. In The International Guideline; National Pressure Injury Advisory Panel: Schaumburg, IL, USA, 2019. [Google Scholar]
- Gefen, A.; Brienza, D.M.; Cuddigan, J.; Haesler, E.; Kottner, J. Our contemporary understanding of the aetiology of pressure ulcers/pressure injuries. Int. Wound J. 2022, 19, 692–704. [Google Scholar] [CrossRef]
- National Pressure Injury Advisory Panel. Pressure Injury Staging System: Updated; National Pressure Injury Advisory Panel: Schaumburg, IL, USA, 2016. [Google Scholar]
- Haesler, E. International Review: Pressure Injury Prevention: A Global Perspective; Wounds International: London, UK, 2019. [Google Scholar]
- Smith, D.M.; Guihan, M.; LaVela, S.L.; Garber, S.L. Factors predicting pressure ulcer occurrence in veterans with spinal cord injury. Am. J. Phys. Med. Rehabil. 2008, 87, 749–757. [Google Scholar] [CrossRef]
- Pancorbo-Hidalgo, P.L.; García-Fernández, F.P.; Pérez-López, C.; Soldevilla Agreda, J.J. Prevalencia de lesiones por presión y otras lesiones cutáneas relacionadas con la dependencia en población adulta en hospitales españoles: Resultados del 5º Estudio Nacional de 2017. Gerokomos 2019, 30, 76–86. Available online: http://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S1134-928x2019000200076&lng=es (accessed on 1 March 2024).
- Stekelenburg, A.; Oomens, C.W.J.; Strijkers, G.J.; Nicolay, K.; Bader, D.L.; Baaijens, F.P.T. Displacement-induced strain and strain rate distributions in skeletal muscle surrounding an ischial tuberosity under compressive load. J. Biomech. 2007, 40, 283–291. [Google Scholar]
- Salcido, R.; Donofrio, J.G.; Fisher, S.B.; LeGrand, E.K.; Dickey, K.; Carney, J.M.; Schosser, R.; Liang, R. Histopathology of pressure ulcers as a result of sequential computer-controlled pressure sessions in a fuzzy rat model. Adv. Wound Care 1994, 7, 23–28. [Google Scholar] [PubMed]
- Bouten, C.V.; Oomens, C.W.; Baaijens, F.P.; Bader, D.L. The etiology of pressure ulcers: Skin deep or muscle bound? Arch. Phys. Med. Rehabil. 2003, 84, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Shabarchin, A.A.; Barbeau, H.; Lamontagne, M.; Gagnon, D. Tissue ischemia and recovery from ischemia during sitting in individuals with paraplegia. J. Rehabil. Res. Dev. 2014, 51, 1051–1058. [Google Scholar]
- Mifsud, T.; Modestini, C.; Mizzi, A.; Falzón, O.; Cassar, K.; Mizzi, S. Los efectos de los cambios de temperatura de la piel sobre la integridad del tejido cutáneo: Una revisión sistemática. Adv. Skin Wound Care 2022, 35, 555–565. (In Spanish) [Google Scholar] [CrossRef]
- Wang, Y.P.; Cheng, R.H.; He, Y.; Mu, L.Z. Thermal Analysis of Blood Flow Alterations in Human Hand and Foot Based on Vascular-Porous Media Model. Front. Bioeng. Biotechnol. 2022, 9, 786615. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cai, F.; Jiang, X.; Hou, X.; Wang, D.; Wang, Y.; Deng, H.; Guo, H.; Wang, H.; Li, X. Application of infrared thermography in the early warning of pressure injury: A prospective observational study. J. Clin. Nurs. 2021, 30, 3257–3266. [Google Scholar] [CrossRef]
- Rosell-Diago, M.P.; Izquierdo-Renau, M.; Julian-Rochina, I.; Arrébola, M.; Miralles, M. Thermography, Temperature, Pressure Force Distribution and Physical Activity in Diabetic Foot: A Systematic Review. Appl. Sci. 2024, 14, 8726. [Google Scholar] [CrossRef]
- Brienza, D.M.; Karg, P. Seat cushion design for elderly wheelchair users based on minimization of soft tissue deformation using FE modeling. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 610–618. [Google Scholar]
- Luboz, V.; Promayon, E.; Payan, Y. Linear elastic finite element models for computer-based simulation of soft tissue compression. Comput. Methods Biomech. Biomed. Eng. 2014, 17, 1–10. [Google Scholar]
- Lachenbruch, C.; Tzen, Y.T.; Brienza, D.M.; Karg, P.E.; Lachenbruch, P.A. The relative contributions of interface pressure, shear stress, and temperature on tissue ischemia: A cross-sectional pilot study. J. Tissue Viability 2013, 22, 14–22. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- EQUATOR Network. Enhancing the Quality and Transparency of Health Research [Internet]. Available online: http://www.equator-network.org (accessed on 12 November 2024).
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med. 2007, 4, e296. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomized trials. Ann. Intern. Med. 2010, 152, 726–732. [Google Scholar] [CrossRef]
- Riley, D.S.; Barber, M.S.; Kienle, G.S.; Aronson, J.K.; von Schoen-Angerer, T.; Tugwell, P.; Kiene, H.; Helfand, M.; Altman, D.G.; Sox, H.; et al. CARE guidelines for case reports: Explanation and elaboration document. J. Clin. Epidemiol. 2017, 89, 218–235. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, version 6.2; Cochrane: London, UK, 2021. [Google Scholar]
- Centre for Evidence-Based Medicine. University of Oxford. PICO Framework [Internet]. Available online: https://www.cebm.net/2014/06/pico-framework/ (accessed on 18 February 2025).
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Chapter 7: Systematic reviews of etiology and risk. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI: Adelaide, Australia, 2020; Available online: https://synthesismanual.jbi.global (accessed on 18 February 2025).
- Wolff, R.F.; Moons, K.G.; Riley, R.D.; Whiting, P.F.; Westwood, M.; Collins, G.S.; Reitsma, J.B.; Kleijnen, J.; Mallett, S.; PROBAST Group. PROBAST: A Tool to Assess Risk of Bias and Applicability of Prediction Model Studies. Ann. Intern. Med. 2019, 170, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Haidich, A.B. Meta-analysis in medical research. Hippokratia 2010, 14 (Suppl. S1), 29–37. [Google Scholar] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0; Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Lin, Y.H.; Chen, Y.C.; Cheng, K.S.; Yu, P.J.; Wang, J.L.; Ko, N.Y. Higher Periwound Temperature Associated with Wound Healing of Pressure Ulcers Detected by Infrared Thermography. J. Clin. Med. 2021, 10, 2883. [Google Scholar] [CrossRef] [PubMed]
- García-Molina, P.; Casasus, S.R.; Sanchis-Sánchez, E.; Balaguer-López, E.; Ruescas-López, M.; Blasco, J.M. Evaluation of interface pressure and temperature management in five wheelchair seat cushions and their effects on user satisfaction. J. Tissue Viability 2021, 30, 337–344. [Google Scholar] [CrossRef]
- Leenen, J.P.L.; Ham, H.W.; Leenen, L.P.H. Indentation marks, skin temperature and comfort of two cervical collars: A single-blinded randomized controlled trial in healthy volunteers. PLoS ONE 2020, 15, e0227055. [Google Scholar] [CrossRef]
- Kanazawa, T.; Nakagami, G.; Goto, T.; Noguchi, H.; Oe, M.; Miyagaki, T.; Hayashi, A.; Sasaki, S.; Sanada, H. Use of smartphone attached mobile thermography assessing subclinical inflammation: A pilot study. J. Wound Care 2016, 25, S6–S14. [Google Scholar] [CrossRef]
- Bilska, A.; Stangret, A.; Pyzlak, M.; Wojdasiewicz, P.; Szukiewicz, D. Skin surface infrared thermography in pressure ulcer outcome prognosis. Adv. Clin. Exp. Med. 2020, 29, 871–876. [Google Scholar] [CrossRef]
- Yilmaz, İ.; Günes, Ü.Y. Sacral Skin Temperature and Pressure Ulcer Development: A Descriptive Study. Ostomy Wound Manag. 2019, 65, 22–28. [Google Scholar] [CrossRef]
- Soares, R.S.; Lima, S.B.; Eberhardt, T.D.; Rodrigues, L.R.; Martins, R.S.; Silveira, L.B.; Alves, P.J. Skin temperature as a clinical parameter for nursing care: A descriptive correlational study. Rev. Bras. Enferm. 2019, 72 (Suppl. S2), 193–199. [Google Scholar] [CrossRef]
- Yavuz, M.; Ersen, A.; Hartos, J.; Lavery, L.A.; Wukich, D.K.; Hirschman, G.B.; Armstrong, D.G.; Quiben, M.U.; Adams, L.S. Temperature as a Causative Factor in Diabetic Foot Ulcers: A Call to Revisit Ulceration Pathomechanics. J. Am.Podiatr. Med. Assoc. 2018, 108, 212–216. [Google Scholar] [CrossRef]
- Mayrovitz, H.N.; Spagna, P.E.; Taylor, M.C. Sacral Skin Temperature Assessed by Thermal Imaging. Adv. Skin Wound Care 2018, 31, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Lachenbruch, C.; Tzen, Y.T.; Brienza, D.M.; Karg, P.E.; Lachenbruch, P.A. Relative contributions of interface pressure, shear stress, and temperature on ischemic-induced, skin-reactive hyperemia in healthy volunteers: A repeated measures laboratory study. J. Tissue Viability 2015, 24, 95–101. [Google Scholar]
- Kanazawa, T.; Kitamura, A.; Nakagami, G.; Goto, T.; Miyagaki, T.; Hayashi, A.; Sasaki, S.; Mugita, Y.; Iizaka, S.; Sanada, H. Lower temperature at the wound edge detected by thermography predicts undermining development in pressure ulcers: A pilot study. Int. Wound J. 2016, 13, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Kaes, L.; Martinez, M.; Moles, D. A Prospective, Observational Study to Assess the Use of Thermography to Predict Progression of Discolored Intact Skin to Necrosis Among Patients in Skilled Nursing Facilities. Ostomy Wound Manag. 2016, 62, 14–26. [Google Scholar]
- Staffa, E.; Bernard, V.; Kubíček, L.; Vlachovský, R.; Vlk, D.; Mornstein, V.; Staffa, R. Using Noncontact Infrared Thermography for Long-term Monitoring of Foot Temperatures in a Patient with Diabetes Mellitus. J. Am. Podiatr. Med. Assoc. 2016, 106, 364–368. [Google Scholar]
- Källman, U.; Engström, M.; Bergstrand, S.; Ek, A.C.; Fredrikson, M.; Lindberg, L.G.; Lindgren, M. The effects of different lying positions on interface pressure, skin temperature, and tissue blood flow in nursing home residents. Clin. Biomech. 2015, 30, 737–742. [Google Scholar] [CrossRef]
- Jiang, X.; Hou, X.; Dong, N.; Deng, H.; Wang, Y.; Ling, X.; Guo, H.; Zhang, L.; Cai, F. Skin temperature and vascular attributes as early warning signs of pressure injury. J. Tissue Viability 2020, 29, 15–21. [Google Scholar] [CrossRef]
- Lupiáñez-Pérez, I.; Gómez-González, A.J.; Marfil-Gómez, R.M.; Morales-Asencio, J.M.; García-Mayor, S.; León-Campos, Á.; Kaknani-Uttumchandani, S.; Moya-Suárez, A.B.; Aranda-Gallardo, M.; Morilla-Herrera, J.C. Tissue temperature, flux and oxygen of sacral and trochanteric area under pressure of healthy subjects: A quasi-experimental study. Int. Wound J. 2021, 18, 461–471. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).