Abstract
The advancement of photocatalytic materials is critical for addressing environmental challenges such as water remediation, where efficient, robust, and reusable systems are in high demand. In this search, the development of hierarchically organized photocatalytic configurations with spatial control over active sites can significantly enhance performance. With this in mind, we present here a novel biomimetic approach for the patterned growth of TiO2-ZnO photocatalytic heterostructures using solid-binding peptides (SBPs) as molecular linkers. Specifically, using bi-functional SBPs with selective affinity for both oxides, we achieve site-specific, molecularly guided deposition of TiO2 nanoparticles onto pre-patterned ZnO-coated substrates. Leveraging the specific recognition capabilities and strong binding affinities of the engineered SBPs, the proposed biomimetic methodology allows for the fabrication of well-organized hybrid nanostructures under sustainable conditions. Photocatalytic degradation assays employing methyl orange as a model contaminant indicate that the patterned architecture enhances both the accessibility of the active photocatalytic sites and the recoverability of the material. This reusability is a critical parameter for the practical deployment of photocatalytic systems in water purification technologies. The obtained results underscore the potential of SBP-mediated molecular recognition as a versatile tool for green nanofabrication of functional materials with advanced architectural and catalytic properties.
1. Introduction
Nowadays, a large number of high-tech applications require the production of functional materials and hybrid structures with an increasing degree of organization and patterning. Patterned nanomaterials offer significant potential across multiple fields, the prime example being the fabrication of electronic circuits, where spatially controlled functionalization is critical (e.g., enabling targeted binding in designated regions while preventing unwanted interactions in others). A high degree of directional assembly is also demanded in emerging applications in the field of biocompatible sensors and actuators, high-capacity batteries or photocatalytic systems for air and water decontamination [,,]. In the latter case, it has been recently observed that ordered structures with a controlled local distribution of the photocatalyst material can lead to higher mass transfer and surface selectivity, resulting in higher photocatalytic efficiency [,]. In this regard, preparative nanotechnology is rapidly evolving towards the fabrication of ever more complex materials in the range of micro- and nanometers with precise structure and performance [,,]. The controlled fabrication of nanoscale components and hierarchical structures can be achieved through diverse manufacturing strategies; however, most of them involve physical or chemical methods under harsh and/or environmentally hazardous conditions. Biosynthetic methods then become an attractive, biological alternative to achieve the synthesis of crystalline nanomaterials in a more sustainable and greener way. For example, microorganisms such as microalgae, fungi and bacteria, as well as bacteriophages, can be used as biofactories to produce single-element and multi-element inorganic nanomaterials []. Some other studies have demonstrated that biological molecules such as proteins can operate as enzymes or templates for material synthesis in vitro []. In recent years, it has been shown that synthetic peptides can also operate as effective building blocks to direct the biomimetic assembly of well-ordered architectures []. Combining the tools of molecular biology and nanotechnology, this processing strategy is ground on the concept of molecular recognition. Its key premise is that genetically or chemically engineered poly-peptides specific to inorganic species can be used as linkers for the (self-)assembly of materials with a controlled organization []. These so-called solid-binding peptides (SBPs) display a short sequence of amino acids, typically 7 to 12, and their specific recognition capabilities arise from a combination of non-covalent interactions and structural conformations that endorse their binding to inorganic materials and surfaces [,]. These features result in moderate to high affinity for a target inorganic surface (metal, oxide), in such a way that the same peptide cannot be presumed to effectively bind other surface without re-screening. On this basis, several applications have benefited from the use of SBPs, including enzyme immobilization processes for biocatalysis [], regenerative medicine and drug delivery processes [], or the removal of harmful nanoparticles from aqueous media in a sort of water remediation strategy mediated by the formation of molecular linkers [].
With this in mind, in this contribution, we aim to use the specific recognition, selectivity and strong binding affinity characteristics of SBPs to build up a photocatalyst system following a predefined pattern. The photoactive material will be deposited on a substrate on which the pattern has been previously created and the overall assembly will be evaluated for the degradation of methyl orange, a standard analyte in the analysis of contaminated water. The use of the supporting substrate brings a loss of photocatalyst surface area which is partly offset by the aforementioned benefit of dealing with an ordered pattern (easier accessibility to the photocatalyst active sites); but in return, it adds the possibility of having an easily recoverable and reusable system once the wastewater has been treated (neat advantage compared to the use of loose nanoparticles or randomly powder packed beds) [,]. Moreover, with the SBP acting as a hierarchy director, we have also planned the photocatalyst block in the form of a two-semiconductor heterostructure. Although more complex to fabricate, heterostructured architectures can display significant advantages over single-phase configurations [,]. That is the case of semiconductor TiO2. Due to an ideal combination of optical and electrical properties, it is the photocatalyst of choice for most commercial applications, but it still faces efficiency problems related to the fast recombination of the photoexcited electron–hole pairs or a limited sensitivity to the visible spectrum [,]. Both issues can be improved when forming heterojunctions with another semiconductor such as ZnO, eventually inducing a higher photocatalytic reaction rate and a better overall performance [,]. In this regard, recent literature on photocatalytic ZnO–TiO2 heterostructures is extensive and cover a variety of fabrication strategies, but few studies have addressed the spatial and precise organization of the semiconductor constituents. As a sort of proof-of-concept investigation, our primary objective here is not so much an explicit improvement of the photocatalytic efficiency of the heterostructured system, but rather to demonstrate the viability of the proposed biomimetic approach as a sustainable method for assembling ZnO–TiO2 materials with well-defined patterning and retained functionality. The overall photocatalytic assembly will comprise a patterned ZnO-coated substrate to which TiO2 nanoparticles will be attached via the solid-binding peptide molecules; this scenario involves the use of bi-functional polypeptide molecules with affinity for both oxides, i.e., two-segment peptide molecules, and the sketch of an incubation methodology that will ultimately result in a consolidated heterostructure.
2. Materials and Methods
The key element in this biomimetic approach to materials growth is the use of bi-segmented SBPs consisting of two distinct amino acid sequences, each capable of recognizing and selectively binding to one of the two inorganic oxides of interest. In particular, the following two segments were joined together by a specialized company (Eurogentec): VRTRDDARTHRK with known affinity for ZnO [] and LNAAVPFTMAGS selective to TiO2 []. The fusion was effected by a cysteine-cysteine coupling through a S-S bridge. The engineered peptide is supplied as a lyophilized powder which, on reconstitution, should always be used under high humidity conditions to avoid denaturation of the organic molecule. The protocol for the preparation of the patterned photocatalytic heterostructures then comprises a four-step methodology which starts with the creation of a zinc oxide pattern on an iron foil substrate. This is followed by a first incubation stage whereby the solid-binding peptide is assembled on the ZnO patterned surface through its ZnO-selective segment. A second incubation step is next conducted in which the other segment of the peptide is used to immobilize the TiO2 counterpart (nanoparticles). Finally, a thermal treatment is applied that removes the SBP molecules and consolidates the ZnO–TiO2 heterostructure. Figure 1 illustrates the envisaged four-step procedure.
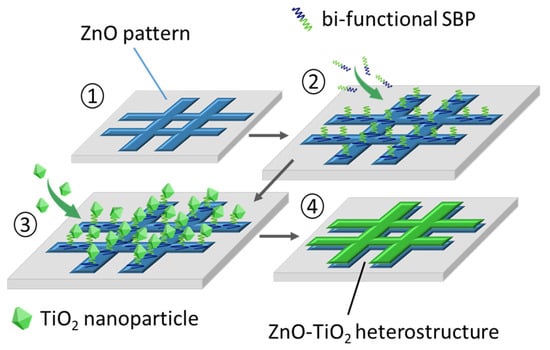
Figure 1.
A four-step protocol to produce the patterned ZnO–TiO2 photocatalytic heterostructure using SBP molecules with affinity for both oxides.
The ZnO pattern was created by shaping a square mesh design using Au grids as a template (Aname G150-Au grids, 125/40 μm nominal hole/bar); the grids were modified by galvanization (Zn coating) followed by oxidation (formation of ZnO), and finally glued onto high purity iron substrate foils (Merck, Darmstadt, Germany). On the other hand, anatase nanoparticles were synthesized following a solvothermal routine that allows to obtain (001) faceted TiO2 nanocrystals, this being a well-known aspect to achieve increased photoactivity, but also to favor adhesion with SBPs [,]. On the basis of previous work by the team, the standard procedure is carried out at 200 °C for 24 h in a Teflon-lined stainless steel autoclave, using Ti(OBut)4 as the TiO2 precursor and trifluoroacetic acid (TFAA) as a morphological control agent to promote crystal faceting [].
Thermogravimetric analyses in a TGA 951-2000 furnace (TA instruments, New Castle, DE, US) were used to elucidate the heating treatments leading to the formation of the ZnO layer and the closing removal of the linking peptide molecules. A Hitachi S-4700 cold FESEM microscope equipped with an EDS probe was used to investigate the microstructure of the samples and the generated heterostructures in the different steps of the processing protocol. Micro-Raman spectroscopy measurements were performed using a WiTec Confocal Raman system (alpha-300R model) to confirm the formation of the ZnO coating on the galvanized grids as well as to detect the adhesion of the peptide molecules onto the ZnO surface after the first incubation step. A 532 nm excitation laser was used to collect spectra from various points and areas with differing dimensions and integration times. The incident laser power was set to 0.5 mW. With a fixed Raman spectral resolution of 0.02 cm−1, the confocal microscope provided an optical resolution of 200 nm laterally and 500 nm vertically. Spectral data were analyzed and processed using WITec Project software (version 2.02). Photocatalytic tests were carried out by positioning the consolidated ZnO–TiO2 heterostructured samples in glass vials, each containing 2 mL of a 10−6 M methyl orange (MO) solution. A control vial with only the MO solution was included in every experiment for reference. Irradiation was performed using a System Photolab LED365-14/450-12c photoreactor (APRIA Systems S.L., Cantabria, Spain), featuring a 10 × 10 cm2 irradiation area, with all vials placed at a fixed distance of 10 cm from the light source. The UV–A LEDs (λ = 365–370 nm) were operated at 90% intensity, while the visible LEDs (λ = 400–700 nm) were set to 50% intensity. The degradation of MO was monitored by collecting solution aliquots at predetermined irradiation intervals and analyzing them via UV–Vis spectroscopy. Measurements were conducted using 1 cm quartz cuvettes in a double-beam spectrophotometer (Analytik Jena Specord 200 Plus, Analytik Jena, Jena, Germany), equipped with a deuterium lamp for the UV range (190–318 nm) and a tungsten lamp for the UV–Vis range (318–600 nm). MO concentration as a function of irradiation time was derived from the UV–Vis absorbance data. The cyclability of the produced heterostructures was also evaluated, rinsing the samples with distilled water between each cycle.
3. Results and Discussion
As indicated, the first step in the biomimetic preparation of the photocatalytic ZnO–TiO2 heterostructures involves the construction of a square-meshed ZnO pattern, for which gold grids like those used as sample holders in transmission electron microscopy were used as the base template, Figure 2. To that end the grids were first treated in a ZnSO4/H2O galvanization solution, placing them in an electrochemical cell equipped with a Pt counter electrode whose nominal area is comparable to the Au slices. For the electroplating, different conditions were tested and eventually after 15 min under a constant flux of 0.12 A, the grids were fully covered by a homogeneous deposition of micron-sized Zn metallic particles, with a characteristic plate-like morphology as revealed by the FESEM images, Figure 2a. The EDS elemental mapping further evidenced the uniform presence of Zn atoms across the entire surface of the grid bars, Figure 2b. The subsequent transformation of the metallic zinc particles to zinc oxide was induced by a thermal oxidation treatment at 200 °C (5 min); according to TGA analyses this temperature was enough to produce a net weight gain of the galvanized grids which can be ascribed to the formation of the oxide. When inspecting these treated samples in the FESEM, it is observed that, although the particles on the grid retain the platelet shape, their surface is much less sharp after the annealing, Figure 2c. As for the composition, the identification with EDS is now ambiguous so we resorted to confocal Raman spectroscopy to corroborate the presence of ZnO. In particular, by analyzing the wavenumber range between 400 and 650 cm−1 in which some of the leading vibrational modes of the hexagonal wurtzite-like structure of ZnO are usually observed [], we were able to confirm a widespread spatial distribution of ZnO over the grid bars (the color variations in the mapping being attributed to topographical differences), Figure 2d. Finally, the oxidized grids were glued onto flat iron substrate foils, thus resulting in the intended pattern for the differential assembly of the ZnO-selective segment of the peptides: the grid bars exposing the zinc oxide-rich regions and the voids between them revealing the ZnO-free iron surface, Figure 2e.
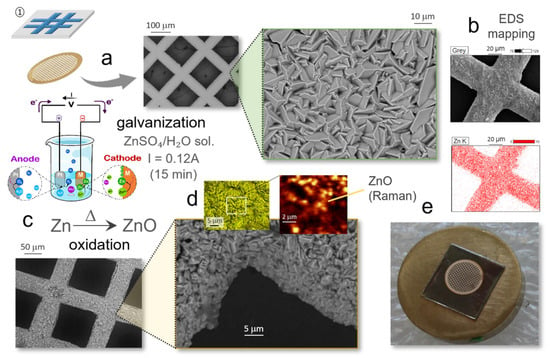
Figure 2.
Step 1 of the protocol: construction of square-meshed ZnO pattern on gold grids. (a) Galvanization of the gold grids and (b) FESEM-EDS elemental mapping showing the presence of Zn on the surface of the grid bars. (c) Thermal oxidation of the grid (200 °C/5 min). (d) Raman mapping confirming the presence of the as-formed ZnO. (e) Adhesion of the ZnO patterned grid on a flat iron substrate foil.
The second step of the protocol comprises the attachment of the solid-binding peptide to the patterned substrate, and this is where the recognition and selective affinity capabilities of the biomolecule first come into play, Figure 3. In particular, only the ZnO-selective segment of the peptide is expected to act at this stage, Figure 3a, enabling its binding to the ZnO-rich grid bars in the substrate and not to the Fe-rich cavities. The basic procedure consists of immersing the substrate-grid pack in an aqueous solution of the biomolecules (0.1 mg/mL) and then carrying out an incubation process. This is conditioned by the peptide’s own specifications, with operating temperature being a particularly critical parameter: temperatures above 40–45 °C may disrupt the SPB’s three-dimensional (secondary) structure, potentially compromising its binding functionality. Observing this circumstance, several tests were conducted and eventually the most efficient results were obtained for 1 h incubations at 37 °C, Figure 3b. This was in agreement with previous studies with this same ZnO-selective SBP [,], showing that both lower and higher incubation times are ineffective as they lead, respectively, to insufficient anchoring or to inhomogeneous accumulations in certain regions of the grids (corners in particular). With one-hour incubation, on the other hand, the peptide assembly is homogeneous and follows well the pattern created by the oxidized grids on the iron foil substrate. The FESEM images in Figure 3c,d confirm this scenario, showing how the underlying ZnO plates are uniformly covered by a sort of fuzzy layer after the incubation. Higher magnification images reveal the presence of elongated filamentous structures within the layer, which likely correspond to peptide strands that have collapsed or folded upon themselves during the drying process required for microscopy sample preparation; however, these features do not reflect the actual configuration during incubation, where SBP molecules remain in a hydrated state to preserve their native structure and functionality. As in the previous case, the presence of the attached peptide molecules was examined by confocal Raman spectroscopy. On this occasion, the spatial mapping was completed by recording the signals in the wavenumber range between 2800 and 3100 cm−1, Figure 3e, as in this region, where these biomolecules rendered their most intense bands (associated with the amine groups and the stretching of CH, CH2 and CH3) []. The obtained plots and spectra substantiate an effective linkage of the bi-functional peptide molecules to the ZnO-rich surfaces, and would be now leaving their TiO2-binding sequences exposed to the reaction medium. Such premise is to be confirmed by the subsequent third step of the protocol, which consists of running a new incubation stage to now immobilize the TiO2 nanoparticles.
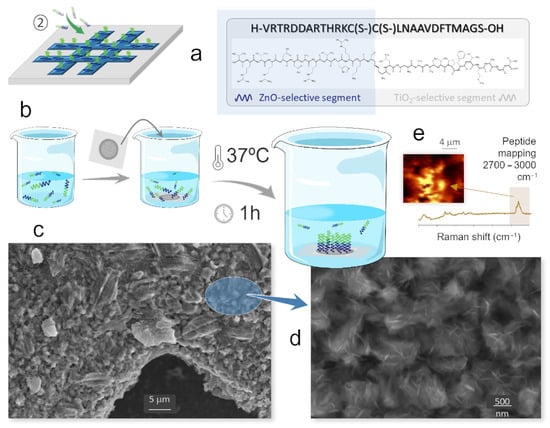
Figure 3.
Step 2 of the protocol: attachment of the SBP on the ZnO patterned substrate. (a) ZnO-selective segment of the SBP acting at this stage. (b) Incubation of the ZnO patterned substrate with the SBP solution. (c,d) FESEM images of the grid bars after the incubation. (e) Raman mapping confirming the adhesion of the peptide molecules to the ZnO-coated grid bars.
To that end, the as-incubated substrate-grid-ZnO-peptide pack is straight immersed in an aqueous suspension of the anatase nanoparticles (0.04 mg/mL), again keeping the assembly at 37 °C for 1 h, Figure 4a. If the assumption is fulfilled, it is now the TiO2-selective segment of the peptide that would come into action (the ZnO-selective segment already bound to the substrate), Figure 4b. At this point, however, the specific morphology of the anatase nanoparticles to be used is also a relevant feature to take into account. Firstly, because the exposure of the different facets of the TiO2 crystals will largely determine their photoreactivity response []. But in addition, it has been observed that peptides may not only be material specific, but can also show certain selectivity towards the morphology of the inorganic surfaces [,]. Morphology can be effectively tuned by adjusting synthesis parameters. In the particularly case of TiO2 nanoparticles, fluorinated surfactants are particularly effective, as fluorine anions can selectively adsorb on the surface of certain faces and thus contribute to their stabilization and morphological prominence []. In our case, Figure 4c shows that the practiced synthesis in the presence of TFAA results in a truncated tetragonal bipyramid morphology of the anatase nanoparticles which is enclosed by eight {101} family facets and two more {001} facets, all containing 6-fold (Ti6c) and 5-fold (Ti5c) coordinated Ti atoms in different proportions []. This being a favorable scenario for achieving a good photoreactivity, also enables the interaction with the peptides, the molecular recognition episode, and their subsequently assembly with the ZnO particles. All this was confirmed by FESEM analyses: the images in Figure 4d illustrate that the anatase nanoparticles are now broadly distributed across the underlying ZnO surface. This suggests that, driven by the peptide molecules, a successful linkage between the ZnO and the TiO2 semiconductor materials has been achieved. The higher magnification image even shows how in some regions, these truncated tetrahedrons are agglomerated, as if stacked on top of each other, which would indicate the anticipated selective bonding by the 001 plane.
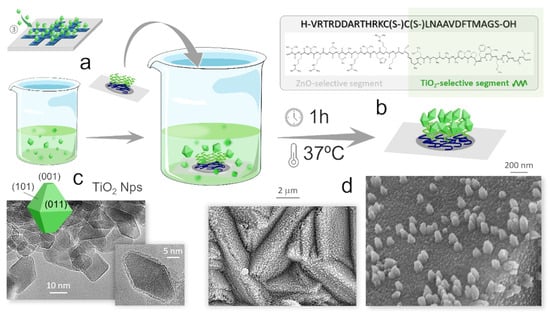
Figure 4.
Step 3 of the protocol: immobilization of the TiO2 nanoparticles. (a) Incubation of the substrate-grid-ZnO-peptide pack in an aqueous suspension of anatase nanoparticles. (b) TiO2-selective segment of the SBP acting at this stage. (c) TEM images showing the truncated bipyramid morphology of the (TiO2-TFAA) anatase nanoparticles. (d) FESEM images of the grid bars after this second incubation, showing the TiO2 nanoparticles covering the underlying ZnO surface.
The last step of the protocol then consists of consolidating the as-formed heterojunctions by applying a smooth heating treatment that will remove the SBP molecules. The specific treatment conditions were again determined by TGA studies which showed a slight weight loss until a plateau was reached at 400 °C, Figure 5a (the mass gain from 500 °C onwards is to be attributed to the oxidation of the iron foil). A rapid (5 min) calcination at this temperature fully disposes the SBP, but is still mild enough for the TiO2 particles to retain their nanometric dimensions, Figure 5b, and to form a consolidated layer above the ZnO pattern that eventually leads to the attempted ZnO–TiO2 composite as evidenced by EDX analyses, Figure 5c.
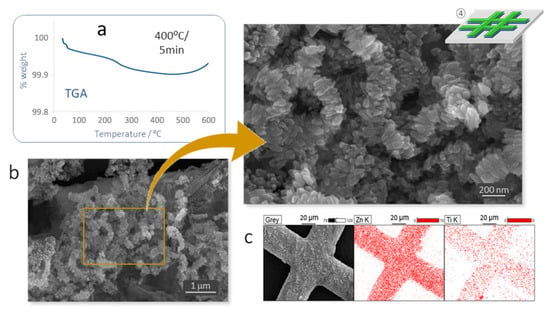
Figure 5.
Step 4 of the protocol: consolidation of the ZnO–TiO2 heterojunctions. (a) TG analysis to investigate the decomposition of the SBPs: 400 °C/5 min. (b) FESEM images and (c) EDS elemental confirming the consolidation of the ZnO–TiO2 heterostructure.
Photocatalytic Performance
Figure 6a illustrates the setup used to monitor the photocatalytic degradation of methyl orange (MO). As detailed in the experimental section, vials containing the consolidated ZnO–TiO2 heterostructures in a 10−6 M solution of the dye molecule are placed at a fixed distance of 10 cm from the LED array of the photoreactor, and irradiated until the presence of MO is no longer detectable by UV–Vis spectroscopy. Methyl orange typically exhibits a strong absorbance peak around 465 nm which is primarily associated with π-π* electronic transitions in the azo group (N=N), specifically when the compound is in its acidic form. Upon exposure to light in the presence of the photocatalyst, the azo group is broken down, leading to a decrease in the intensity of that peak at 465 nm. In our case, Figure 6b shows a progressive decomposition of the MO pollutant molecule, with degradation rates above 60% within the first 24 h and reaching almost complete mineralization after two days of light exposure (48 h and above). On the other hand, Figure 6c demonstrates that similar degradation efficiency is achieved during a second photocatalysis cycle and it is only after the third cycle that a slight reduction in performance is detected, particularly in achieving full mineralization. Figure 6d then shows the concentration ratio (C/C0) of dye degradation over time, calculated by monitoring the change in the absorbance maximum (λ = 465 nm) during irradiation across three consecutive cycles. To construct the kinetic curves, absorbance maxima from the recorded spectra were converted to concentration values using a previously established calibration curve. These concentrations were then normalized to the initial concentration (C0) and plotted as a function of irradiation time. As shown, all three cycles exhibit comparable degradation rates, and at no point does the reaction stop. This indicates that no intermediate compounds are formed that could poison or inhibit the catalyst’s surface activity (something which is a common impediment in commercial TiO2-based catalysts such as P90, PC100 or Degussa P25 []). The observed decline after the third cycle may then be attributed to carbonates or hydroxyl-type subspecies bound to the surface of the photocatalyst; these residues likely require more thorough water rinsing or even some soft temperature assistance to fully restore the photoactivity of the heterostructure for continued reuse. Additionally, the overall photocatalytic performance of the system is an area to optimize in order to, for example, reduce degradation times, and this is something we are currently working on by designing new pattern configurations (e.g., self-supporting 3D porous structures). But for now, these initial results clearly demonstrate the feasibility of the proposed peptide-mediated biomimetic approach to guide the targeted assembly of complex and highly organized composite structures at the nanoscale.
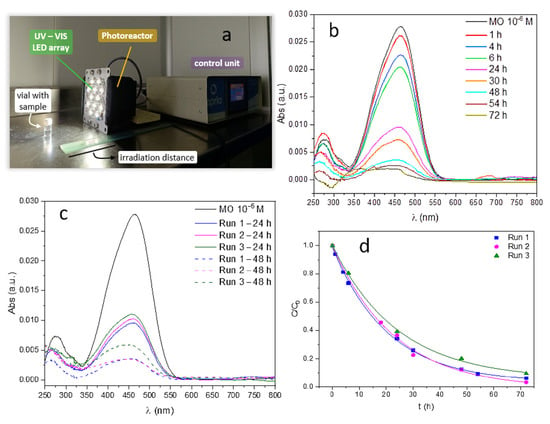
Figure 6.
Photocatalytic tests of the produced ZnO–TiO2 heterostructures (methyl orange degradation). (a) Experimental setup. (b) Reduction in MO absorbance intensity with irradiation time. (c) Variation in absorbance for three consecutive photocatalytic cycles. (d) Concentration ratio of dye degradation over time (using the absorbance maximum: λ = 465 nm) across three consecutive cycles.
4. Conclusions
This work establishes the efficacy of molecular recognition mechanisms inherent to solid-binding peptides (SBPs) in driving the controlled assembly of TiO2-ZnO heterostructures, highlighting the potential of this biomimetic approach as a sustainable and environmentally friendly fabrication tool for engineering functional nanoscale materials with tailored interfaces. The spatial organization in the obtained photocatalytic heterostructure leads to an operative degradation of methyl orange. Further, the peptide-mediated selective binding mechanism is highly specific and precise, enabling excellent reproducibility in the assembly process. Furthermore, by optimizing the consolidation stage of the heterostructures, their deposition onto polymeric and flexible substrates could be explored, including an evaluation of the peptide’s influence on the functional performance (e.g., role in improving charge separation), and even the necessity for a substrate might be entirely bypassed by developing fully self-supported porous 3D heterostructures. Taken together, the results obtained point to a great versatility of this biomimetic approach, which opens new avenues for the design of a wide range of hybrid nanomaterials and multifunctional (spatially localized/distributed) configurations for applications in photocatalysis, sensing, energy storage, and beyond.
Author Contributions
A.C.-A., investigation, methodology, validation, writing—original draft, writing—review and editing. L.S.-M., investigation, methodology, writing—review and editing. M.C., investigation and writing—review and editing. D.G.C., conceptualization, methodology, supervision, and writing—review and editing. A.C.C., conceptualization, methodology, and writing—review and editing. T.J., conceptualization, funding acquisition, methodology, supervision, and writing—review and editing. M.P., conceptualization, funding acquisition, supervision, writing—original draft, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the Projects TED2021-132779B-I00 and TED2021-129876B-I00 funded by MICIU/AEI/10.13039/501100011033 and by the European Union NextGenerationEU/PRTR. A. Castellanos-Aliaga acknowledges the Grant FPU21/03335 funded by MICIU/AEI/10.13039/501100011033 and by the FSE+. L. San-Miguel acknowledges the Grant PIPF Program (PIPF-2023/ECO-30217) funded by Comunidad de Madrid.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Acknowledgments
We also thank the CSIC Hub CONEXIÓN-FOTOCATÁLISIS (OASIS) for support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hasan, M.M.; Hossain, M.M. Nanomaterials-patterned flexible electrodes for wearable health monitoring: A review. J. Mater. Sci. 2021, 56, 14900–14942. [Google Scholar] [CrossRef] [PubMed]
- Mekuye, B.; Abera, B. Nanomaterials: An overview of synthesis, classification, characterization, and applications. Nano Select. 2023, 4, 486. [Google Scholar] [CrossRef]
- Greco, E.; De Spirt, A.; Miani, A.; Piscitelli, P.; Trombin, R.; Barbieri, P.; Marín, E. Nanomaterials in photocatalysis: An in-depth analysis of their role in enhancing indoor air quality. Appl. Sci. 2025, 15, 1629. [Google Scholar] [CrossRef]
- Iborra-Torres, A.; Hus, M.; Nguyen, K.; Vamvakeros, A.; Sajjad, M.T.; Dunn, S.; Mertens, M.; Jacques, S.; Beale, A.M.; Likozar, B.; et al. 3D printed SrNbO2N photocatalyst for degradation of organic pollutants in water. Mater. Adv. 2023, 4, 3461–3472. [Google Scholar] [CrossRef]
- Ma, J.; Cai, B.; Zhang, S.; Jian, T.; De Yoreo, T.J.; Chen, C.L.; Baneyx, F. Nanoparticle-mediated assembly of peptoid nanosheets functionalized with solid-binding proteins: Designing heterostructures for hierarchy. Nano Lett. 2021, 21, 1636–1642. [Google Scholar] [CrossRef]
- Bernardo, M.S.; Villanueva, P.G.; Jardiel, T.; Calatayud, D.G.; Peiteado, M.; Caballero, A.C. Ga-doped ZnO self-assembled nanostructures obtained by microwave-assisted hydrothermal synthesis: Effect on morphology and optical properties. J. Alloys Compd. 2017, 722, 920–927. [Google Scholar] [CrossRef]
- Yuan, X.; Sunyer-Pons, N.; Terrado, A.; León, J.L.; Hadziioannou, G.; Cloutet, E.; Villa, K. 3D-printed organic conjugated trimer for visible-light-driven photocatalytic applications. ChemSusChem 2023, 16, e202202228. [Google Scholar] [CrossRef]
- Gumiel, C.; Jardiel, T.; Calatayud, D.G.; Vranken, T.; Van Bael, M.K.; Hardy, A.; Calzada, M.L.; Jiménez, R.; García-Hernández, M.; Mompeán, F.J.; et al. Nanostructure stabilization by low-temperature dopant pinning in multiferroic BiFeO3-based thin films produced by aqueous chemical solution deposition. J. Mater. Chem. C 2020, 8, 4234–4245. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, S.Y. Biosynthesis of inorganic nanomaterials using microbial cells and bacteriophages. Nat. Rev. Chem. 2020, 4, 638–656. [Google Scholar] [CrossRef]
- Naik, R.R.; Stringer, S.J.; Agarwal, G.; Jones, S.E.; Stone, M.O. Biomimetic synthesis and patterning of silver nanoparticles. Nat. Mater. 2002, 1, 169–172. [Google Scholar] [CrossRef]
- Levin, A.; Hakala, T.A.; Schnaider, L.; Bernardes, G.J.L.; Gazit, E.; Knowles, T.P.J. Biomimetic peptide self-assembly for functional materials. Nat. Rev. Chem. 2022, 4, 615–634. [Google Scholar] [CrossRef]
- Sun, L.; Li, P.; Chen, C. Molecular recognition characteristics of co-assembled peptides on atomically flat graphite surfaces. J. Colloid Interf. Sci. 2025, 679, 435–445. [Google Scholar] [CrossRef]
- Bansal, R.; Care, A.; Lord, M.S.; Walsh, T.R.; Sunna, A. Experimental and theoretical tools to elucidate the binding mechanisms of solid-binding peptides. New Biotechnol. 2019, 52, 9–18. [Google Scholar] [CrossRef]
- Yucesoy, D.T.; Akkineni, S.; Tamereler, C.; Hinds, B.J.; Sarikaya, M. Solid-binding peptide-guided spatially directed immobilization of kinetically matched enzyme cascades in membrane nanoreactors. ACS Omega 2021, 6, 27129–27139. [Google Scholar] [CrossRef]
- Alvisi, N.; de Vries, R. Biomedical applications of solid-binding peptides and proteins. Mater. Today Bio 2023, 19, 100580. [Google Scholar] [CrossRef]
- Jardiel, T.; Peiteado, M.; Castellanos-Aliaga, A.; Caballero, A.C.; Calatayud, D.G. Peptide-driven bio-assisted removal of metal oxide nanoparticles from an aqueous suspension: A novel strategy for water remediation. J. Clean. Prod. 2021, 285, 124852. [Google Scholar] [CrossRef]
- Bielan, Z.; Dudziak, S.; Kubiak, A.; Kowalska, E. Application of spinel and hexagonal ferrites in heterogeneous photocatalysis. Appl. Sci. 2021, 11, 10160. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, Z.; Liu, H.; Zhang, S.; Wang, P.; Lu, J.; Tong, Y. Heterojunction architecture of N-Doped WO3 nanobundles with Ce2S3 nanodots hybridized on a carbon textile enables a highly efficient flexible photocatalyst. Adv. Funct. Mater. 2019, 29, 1903490. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, Y.; Jiang, Z.; Xu, F.; Xian, Q.; Sun, C.; Tong, Q.; Zou, W.; Duan, X.; Wang, S. CeO2 nanocrystal-modified layered MoS2/g-C3N4 as 0D/2D ternary composite for visible-light photocatalytic hydrogen evolution: Interfacial consecutive multi-step electron transfer and enhanced H2O reactant adsorption. Appl. Catal. B-Environ. 2019, 259, 118072. [Google Scholar] [CrossRef]
- Calatayud, D.G.; Flores, R.M.; Castellanos-Aliaga, A.; Peiteado, M.; Palomares, F.J.; Caballero, A.C.; Jardiel, T. Tailoring the visible light photoactivity of un-doped defective TiO2 anatase nanoparticles through a simple two-step solvothermal process. Nanotechnology 2020, 31, 045603. [Google Scholar] [CrossRef]
- Alcázar-Medina, T.; Chairez-Hernández, I.; Lemus-Santana, A.A.; Núñez-Núñez, C.M.; Proal-Nájera, J.B. Amoxicillin degradation by TiO2 P25 solar heterogeneous photocatalysis: Influence of pH and oxidizing agent H2O2 addition. Appl. Sci. 2023, 13, 7857. [Google Scholar] [CrossRef]
- Das, A.; Liu, D.; Wu, Y.; Abzakh, B.A.; Madhumitha, R.; Preethi, M.; Kazakova, E.A.; Vasenko, A.S.; Prezhado, O.V. Origin of the improved photoelectrochemical and photocatalytic activity in a ZnO–TiO2 nanohybrid revealed by experimental and density functional theory studies. J. Phys. Chem. Lett. 2024, 15, 7524–7532. [Google Scholar] [CrossRef] [PubMed]
- Andronic, L.; Isac, L.; Cazan, C.; Anesca, A. Simultaneous adsorption and photocatalysis processes based on ternary TiO2–CuxS–fly ash hetero-structures. Appl. Sci. 2020, 10, 8070. [Google Scholar] [CrossRef]
- Vreuls, C.; Zocchi, G.; Genin, A.; Archambeau, C.; Martial, J.; Van de Weerdt, C. Inorganic-binding peptides as tools for surface quality control. J. Inorg. Biochem. 2010, 104, 1013–1021. [Google Scholar] [CrossRef]
- Whaley, S.R.; English, D.S.; Hu, E.L.; Barbara, P.F.; Belcher, A.M. Selection of peptides with semiconductor binding specificity for directed nanocrystal assembly. Nature 2000, 405, 665–668. [Google Scholar] [CrossRef]
- Heinz, H. Understanding Molecular Recognition on Metallic and Oxidic Nanostructures from a Perspective of Computer Simulation and Theory. In Bio-Inspired Nanotechnology; Knecht, M., Walsh, T., Eds.; Springer: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Calatayud, D.G.; Jardiel, T.; Peiteado, M.; Illas, F.; Giamello, E.; Palomares, F.J.; Fernández-Hevia, D.; Caballero, A.C. Synthesis and characterization of blue faceted anatase nanoparticles through extensive fluorine lattice doping. J. Phys. Chem. C 2015, 119, 21243–21250. [Google Scholar] [CrossRef]
- Lima, L.; Caldas, L.S.; Ali, A.; Barreto, J.; Freitas, R.; Mazzarella, A.; Felix, G.; Carozo, V.; Stavale, F. Growth and Raman spectroscopy of ultrathin ZnO(0001) films on Ag(001). Surf. Sci. 2021, 704, 121748. [Google Scholar] [CrossRef]
- Freire, P.T.C.; Barboza, F.M.; Lima, J.A.; Melo, F.E.A.; Filho, J.M. Raman Spectroscopy of Amino Acid Crystals; Raman Spectroscopy and Applications; Maaz, K., Ed.; IntechOpen: London, UK, 2017; pp. 202–224. [Google Scholar]
- Calatayud, D.G.; Jardiel, T.; Rodríguez, M.; Peiteado, M.; Fernández-Hevia, D.; Caballero, A.C. Soft solution fluorine-free synthesis of anatase nanoparticles with tailored morphology. Ceram. Int. 2013, 39, 1195–1202. [Google Scholar] [CrossRef]
- Liu, X.; Du, G.; Li, M. True photoreactivity origin of Ti3+-doped anatase TiO2 crystals with respectively dominated exposed {001}, {101}, and {100} facets. ACS Omega 2019, 4, 14902–14912. [Google Scholar] [CrossRef]
- Calatayud, D.G.; Jardiel, T.; Peiteado, M.; Fernández-Rodríguez, C.; Espino Estévez, R.; Doña Rodríguez, J.M.; Palomares, F.J.; Rubio, F.; Fernández-Hevia, D.; Caballero, A.C. Highly photoactive anatase nanoparticles obtained using trifluoroacetic acid as an electron scavenger and morphological control agent. J. Mater. Chem. A 2013, 1, 14358–14367. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).