Featured Application
This study presents a novel sample preparation strategy combining modified QuEChERS with graphene oxide clean-up. This strategy can be applied to routine LC-MS/MS-based monitoring of pharmaceutical residues in wastewater to mitigate the impact of pharmaceuticals on aquatic ecosystems.
Abstract
Detecting pharmaceuticals in environmental matrices, particularly in wastewater, is crucial due to their potential environmental occurrence and unpredictable ecological and health-related consequences. These substances, often present in trace amounts, require highly sensitive and selective analytical methods for effective monitoring. A modified version of the QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) method was evaluated to evaluate 18 pharmaceuticals and 2 metabolites in wastewater samples using liquid chromatography with tandem mass spectrometry (LC-MS/MS). The method’s performance was assessed using linearity, recovery, precision, limits of quantification (LOQ) and detection (LOD), and the matrix effect (ME). The final method was based on acetonitrile, Na2EDTA, citrate buffer, and graphene oxide (GO). Finally, the calibration curves prepared in acetonitrile and the matrix extract showed a correlation coefficient of 0.99. Most of the compounds had LOQ values lower than 0.5 μg⋅mL−1. Recoveries were achieved in the 70–98% range, with RSD lower than 13%. GO allowed the elimination of the ME, which occurred in the range of −11% to 15%. The results indicate that a low-cost and straightforward method is suitable for routinely monitoring pharmaceuticals in wastewater, which is crucial for minimizing the impact of pollutants on aquatic ecosystems.
1. Introduction
The presence of pharmaceuticals in the environment is an alarming problem, yet their widespread use is essential for treating diseases in humans and animals [1]. Changes in clinical practice, a continuing increase in population, and higher incidences of chronic and age-related diseases are leading to a raised need and consumption of pharmaceuticals. This trend is evident in Organisation for Economic Co-operation and Development (OECD) countries—between 2000 and 2019, the consumption of antihypertensive drugs increased by an average of 65%, antidiabetic and antidepressant drugs doubled, while the increase in the uptake of lipid-modifying medicines was as much as 400% [2]. Furthermore, the expanding proportion of people aged 65 and older globally leads to aging populations in most countries, a key driver for the pharmaceutical market. The market has experienced significant growth since the beginning of the century, with linear growth and revenues of USD1.61 trillion worldwide in 2023. It is expected to grow further, with a compound annual growth rate (CAGR) of 7.6% from 2023 to 2030 [3].
Increased pharmaceutical production entails the risk of their release into the environment, both at the production and distribution stage and due to inappropriate use, disposal of unused or expired medicines, and inefficient treatment of wastewater and landfill leachate. As a result, active substances contained in medicines can accumulate in the environment, posing a potential threat to ecosystems and public health [4].
Furthermore, pharmaceuticals are processed in the body once they are taken in, and then between 30% and 90% of the dose taken is excreted unchanged or metabolized, mainly in feces or urine [5,6]. They end up in treatment plants and domestic wastewater treatment systems in these forms and subsequently enter ecosystems. Pharmaceuticals resist degradation, persist for a long time in the environment, and become transported along food chains. Recent work constantly indicates the presence of pharmaceuticals in soil [3,7,8,9,10,11], surface water [10,12,13], groundwater [13,14], and even drinking water [15,16,17,18,19,20]. Pharmaceuticals are also taken up by soil organisms [21] and plants [22,23]. Their ubiquity raises concerns about possible environmental and human health risks [24], highlighting the need to monitor wastewater, the overriding source of pharmaceuticals in the environment.
Wastewater treatment is a crucial component in protecting the environment and humans from the presence of pharmaceuticals in ecosystems and the following consequences. Nevertheless, conventional treatment methods do not entirely remove some drug residues, potentially leading to their presence in wastewater leaving treatment plants [1]. Hence, there is an urgent need for reliable and detailed methodologies to determine concentrations of pharmaceuticals in wastewater before they enter the environment and to realistically determine the effectiveness of the technology used at the treatment plant.
Consequently, developing efficient analytical methods, including precise sample preparation, is becoming essential for effectively monitoring pharmaceutical residues in wastewater and evaluating treatment efficiency. Sample preparation is a critical step, affecting the quality of the results obtained by removing interferents from the matrix, stabilizing analytes, and ensuring compatibility with downstream analytical techniques. Many procedures have been developed for the preparation of aqueous samples, including wastewater, such as solid-phase extraction (SPE) [25,26,27], as well as microextraction techniques: dispersive liquid–liquid microextraction (DLLME) [28,29,30,31], liquid microextraction using hollow fiber (HF–LPME) [32,33,34], ultrasound-assisted emulsification microextraction (USAEME) [35,36,37], and single-drop microextraction (SDME) [38,39,40] have been reported. An alternative, less researched strategy for determining pharmaceuticals in environmental samples is using QuEChERS-based extraction methods (fast, easy, cheap, effective, robust, and safe) [41].
The QuEChERS method was initially designed to extract pesticide residues from fruits and vegetables [42]. The method was subsequently applied to the analysis of other food matrices, such as cereals [43,44]; legumes [44]; oils [45]; and environmental samples, e.g., soil [1,46,47,48]. Due to its simplicity and “low bias” toward specific groups of compounds, the technique was also developed to study residues of alkylphenols, bisphenol A [49], mycotoxins [50,51], pyrrolizidine alkaloids [52], polycyclic aromatic hydrocarbon (PAHs) [53], and surfactants [54], including per- and polyfluoroalkyl substances (PFASs) [55] in a variety of matrices. Due to the possibility of overcoming the complexity of the wastewater matrix and the great diversity of pharmaceuticals in physicochemical properties, modifications of the QuEChERS method appear to be a promising technique for extracting these compounds from wastewater. The technique avoids several drawbacks of traditional SPE, such as high solvent consumption, lengthy procedures, and poor selectivity in complex wastewater matrices. When combined with graphene oxide (GO) as a clean-up sorbent, the method enhances overall extraction efficiency and limits matrix effects, making it a more effective tool for detecting pharmaceutical residues in wastewater.
This work aims to improve the QuEChERS technique for simultaneously determining a range of pharmaceuticals in wastewater by improving sample extraction and clean-up procedures. Improving the accuracy and efficiency of the analysis for the detection of drugs in wastewater will support the monitoring of their levels in wastewater treatment plants and, therefore, contribute to the development of effective methods to control the efficiency of these facilities. Effective surveillance is crucial for adequate water quality management, helping to improve wastewater treatment methods and minimizing pollutants’ impact on aquatic ecosystems. In addition, it will provide a low-cost and straightforward tool to track the type and quantity of substances collectively consumed by the populations served by the treatment plants.
2. Materials and Methods
2.1. Materials and Reagents
Acetonitrile, acetone, methanol, ethyl acetate, formic acid (all LC-MS grade), ammonium formate (NH4HCO2; ≥99%), and ethylenediaminetetraacetic acid disodium salt dehydrate (Na2EDTA; ≥99%) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ultrapure water (LC grade 18 Ω cm) was obtained from a MilliQ water purification system (Millipore Ltd., Bedford, MA, USA).
Pre-weighed QuEChERS kits containing analytical-grade reagents—magnesium sulfate, sodium chloride (NaCl), trisodium citrate dihydrate (Na3C6H5O7⋅2H2O), and disodium hydrogen citrate sesquehydrate (Na2HC6H5O7⋅1.5H2O) salt—were procured from Agilent Technologies (Santa Clara, CA, USA); pre-weighed kits containing magnesium sulfate (MgSO4) and sodium acetate (CH3COONa) were obtained from Supelco (Bellefonte, PA, USA).
For clean-up methods, pre-weighted sorbent mixtures PSA/C18/MgSO4 (primary secondary amine/octadecylsilane/anhydrous magnesium sulfate) and PSA/GCB/MgSO4 (primary secondary amine/graphitized carbon black/anhydrous magnesium sulfate) kits were purchased from Perlan Technologies (Santa Clara, CA, USA). Chitosan from shrimp shells, Fe3O4 magnetic nanoparticles (Fe3O4MNPs), and graphene oxide dispersion in H2O (GO) were obtained from Sigma-Aldrich (Steinheim, Germany). The GO dispersion, at a concentration of 2 mg/mL, is chloride-free (purified by dialysis), consists of monolayer sheets, and has an average sheet diameter of less than 10 µm.
For the preparation of the matrix, i.e., the model wastewater according to PN-72/C-04550.09, the following components were used (all with purity ≥ 98%): casein peptone, enriched dry broth, ammonium chloride (NH4Cl), calcium chloride (CaCl2), monobasic potassium (KH2PO4), dibasic potassium phosphate (K2HPO4), and sodium acetate (CH3COONa) from Sigma-Aldrich (Steinheim, Germany).
2.2. Standards
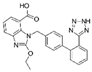
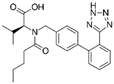
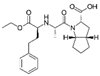
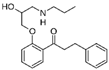
Analytical standards (purity ≥ 95%): atenolol, bisoprolol, candesartan cilexetil, ciprofloxacin, duloxetine hydrochloride, doxycycline hydrochloride, escitalopram oxalate, fluoxetine hydrochloride, indapamide, levonorgestrel, metformin hydrochloride, metoprolol, propafenone hydrochloride, ramipril, sertraline hydrochloride, torasemide anhydrous, valsartan, and venlafaxine hydrochloride and its metabolites—venlafaxine O-desmethyl and venlafaxine N-oxide—were purchased from Sigma-Aldrich (Steinheim, Germany). The following substances were used as internal standards (IS): atenolol-d7 (≥97%) and metformin-d6 (≥95%), purchased from Sigma Aldrich (Steinheim, Germany). An overview of the physicochemical properties of the studied target drug active substances is given in Table 1.

Table 1.
Physicochemical profiles of target drug active substances.
The stock standard solutions of pharmaceuticals (at around 1000 μg⋅mL−1) were prepared by dissolving an accurate weighted amount of reference standards in an appropriate solvent (acetone, acetonitrile, methanol, or an appropriate mixture). The working standard solutions of multiple compounds, in concentrations of 0.001, 0.005, 0.010, 0.050, 0.100, 0.500, and 1.000 μg⋅mL−1, were prepared via serial dilution of the stock solution of individual pharmaceuticals in acetonitrile. They were used for spiking and preparing solvent and matrix-matched calibration curves for the pharmaceuticals in the solvent and matrix. A solution of the internal standards (atenolol-d7 and metformin-d6) was prepared in acetonitrile (0.5 μg mL−1) and used to prepare working calibration solutions and during the sample spiking procedure. All stock and working standard solutions were protected from direct light and stored in dark glass bottles at 4 °C until analysis.
2.3. Model Wastewater
The procedure for optimizing pharmaceutical extraction and extract clean-up was carried out on model (synthetic) wastewater. They were prepared to reflect the qualitative composition of actual municipal wastewater. To this end, enriched broth (152 mg⋅L−1) and casein peptone (226 mg⋅L−1) were used to reflect the source of carbon compounds and organic nitrogen; NH4Cl (20 mg⋅L−1) was used as a source of ammonium nitrogen, and KH2PO4 (16 mg⋅L−1) and K2HPO4 (40 mg⋅L−1) were used as a source of phosphorus. CH3COONa (212 mg⋅L−1) was added to increase COD. The model wastewater was prepared using tap water filtered on an activated carbon column to preserve macro- and micronutrients and remove chlorine and organic pollutants [56]. Their BOD and COD values were approximately 300 mg⋅L−1 and 500 mg⋅L−1, respectively. The model wastewater was stored at −20 °C until analysis.
2.4. Sample Preparation Extraction and Clean-Up
2.4.1. QuEChERS-Based Extraction
The extraction of pharmaceuticals from wastewater was based on modified QuEChERS protocols. It was carried out in ten variants, each variant in three repetitions. The experimental details of the QuEChERS protocols tested are summarized in Table 2.

Table 2.
Details of the procedures tested for the QuEChERS-based extraction of pharmaceuticals from model wastewater.
The final procedure is outlined below. In a 50 mL PTFE centrifuge tube, 10 mL of wastewater was placed. Subsequently, 10 mL 0.2 M Na2EDTA in H2O and 50 µL internal standard (0.5⋅μg mL−1) were added and shaken vigorously. Then, 10 mL of acetonitrile was added and mixed for 1 min by hand, followed by 1 min on a Vortex shaker. The sample was placed in a freezer (−20 °C) for 15 min, and after that time, the pre-weighed citrate buffer kit was added. The sample was again shaken for 1 min by hand and 1 min on a Vortex shaker. The tube was then centrifuged for 5 min at 4500 rpm. A total of 5 mL of supernatant was transferred to a 15 mL PTFE tube and frozen for 30 min (−60 °C). After defrosting, the extract was filtered through a 0.45 µm PTFE filter, transferred into the glass autosampler vial, and analyzed via LC-MS/MS.
2.4.2. Clean-Up
The clean-up process was optimized for the most favorable extraction variant regarding recovery and matrix effect (i.e., the variant described in the previous point).
To the defrosted extract, as described in Section 2.4.1, one of five sorbents was added: pre-weighted PSA/C18/MgSO4, PSA/GCB/MgSO4, 200 mg of chitosan, 1 mL of GO, or 1 mL of Fe3O4MNPs, then vortexed for 1 min. Then, the extract was centrifuged for 10 min at 4500 rpm, filtered through a 0.45 mm PTFE filter, transferred into the glass autosampler vial, and analyzed via LC-MS/MS.
2.5. LC-MS/MS Conditions
An Eksigent Ultra LC-100 (Eksigent Technologies, Dublin, CA, USA) liquid chromatography system was operated at a flow rate of 0.4 mL min−1 without split using a Kinetex C18 100 mm × 2.1 mm, 2.6 µm (Phenomenex, Torrance, CA, USA) analytical column, maintained at 40 °C during the experiments. The volume injected into the LC-MS/MS system was 10 μL. The binary mobile phase consisted of water with 0.2% formic acid and 5 mM ammonium formate (phase A) and methanol with 0.2% formic acid and 5 mM ammonium formate (phase B). The gradient elution starts at 99% A and 9% B, rising linearly to 40% A and 60% B in 1.5 min. In the next step, it rose linearly to 5% A and 95% B in 4.5 min and was held for 2.0 min. After ramping, the mobile phase composition was returned to the initial condition in 1 min, and this was held for 2.5 min for re-equilibration.
System MS/MS 6500 QTRAP (AB Sciex Instruments, Foster City, CA, USA) was used for mass spectrometric analysis, equipped with an electrospray ionization source (ESI). The capillary voltage was maintained at 5000 V for the positive ion mode, and the temperature of the turbo heaters was set at 400 °C. The nebulizer gas (GS1), auxiliary gas (GS2), and curtain gas (CUR) (nitrogen) were used at a pressure of 50, 40, and 35 psi, respectively. Nitrogen was also used as the nebulizer and collision gas. All pharmaceuticals were detected in the multiple reaction monitoring mode (MRM). For each compound, the precursor ion and two product ions were determined. One product ion was used for quantification, and one was used for qualification. The MRM transitions for the pharmaceuticals are given in Table 3.

Table 3.
MRM parameters for selected pharmaceuticals analyzed using LC-MS/MS.
2.6. Method Validation and Matrix Effect
The developed method was validated using model wastewater to assess its linearity, recovery, precision, limit of quantification (LOQ), limit of detection (LOD), matrix effects (ME), and uncertainty (U).
The method’s linearity was assessed by analyzing a series of standard samples with six different concentrations, ranging from LOQ to 1.0 μg⋅mL−1, in both pure solvent and matrix extracts of model wastewater over three consecutive days. The limit of quantification (LOQ) was determined as the lowest spiking level, validated by satisfactory recovery values (70–120%) and RSD (≤20%). The limits of detection (LOD) were calculated based on a signal-to-noise ratio of 3.
During recovery testing, model wastewater samples free of pharmaceuticals were fortified with appropriate volumes of standard pharmaceutical solutions at concentrations of 0.001, 0.01, and 0.1 μg⋅mL−1. After a one-hour equilibration period, the samples were subjected to the previously described procedure. For each concentration level, five replicates were analyzed. Precision was determined as the relative standard deviation (RSD%) for each spiking level. Recoveries were deemed acceptable when falling within the range of 70–120%, with an RSD of no more than 20%.
The matrix effect (ME) was estimated by comparing the slopes of six points on the matrix-fitted calibration curves and the solvent-fitted calibration curves according to the following formula:
Depending on the percentage of ME, different matrix effects could be observed: negative—high signal suppression (≥50%) and moderate signal suppression (20 to 50%); no matrix effect (−20 < ME < 20%); positive—moderate signal enhancement (20–50%) and high signal enhancement (≥50%) [57,58].
The measurement uncertainty was estimated basing on the data obtained in the validation study. The relative expanded uncertainty was calculated by using the coverage factor k = 2 at the confidence level of 95% [59].
3. Results and Discussion
3.1. LC–MS/MS Optimisation
A review of relevant literature and the authors’ own experience indicated that liquid chromatography coupled with tandem quadrupole mass spectrometry (LC-MS/MS) in the multiple reaction monitoring (MRM) mode is well-suited for the determination of pharmaceutical residues with diverse physicochemical properties [60,61,62,63,64,65,66].
In the initial phase of the study, detection parameters were selected and optimized. For this purpose, standard solutions of individual pharmaceuticals at a concentration of 0.1 µg⋅mL−1 were introduced into the mass spectrometer to establish appropriate operating conditions. The first aspect evaluated was the optimal ionization technique—electrospray ionization (ESI) or atmospheric pressure chemical ionization (APCI). Across all tested compounds, APCI produced a significantly lower signal intensity (in terms of peak height and area), which led to the selection of ESI due to its superior signal response.
The ionization mode (positive or negative) was also examined. The positive mode consistently yielded better signal intensity for all analytes, although negative ionization was found to be effective for acidic compounds such as candesartan, valsartan, and ramipril. The addition of ammonium formate to the standard solutions proved to be a critical factor, significantly enhancing signal intensity by facilitating ion formation.
The full-scan mode was initially used to identify the most intense precursor-to-product ion transitions for each compound. These transitions were then employed in the MRM mode, with two transitions selected per analyte to ensure reliable qualitative identification. In each case, protonated molecules ([M+H]+) were chosen as the precursor ions.
Subsequently, fragmentation of the selected ions was optimized by adjusting detector parameters specific to each analyte, including the declustering potential, collision energy, and ion exit potential from the collision cell. Fragmentation spectra were recorded at varying collision energies. Lower energy values favored the formation of higher-mass fragments, while increased energy led to more abundant low-mass ions and the disappearance of heavier ones. For each MRM transition, the collision energy that provided the highest sensitivity was selected. Specific values of tested parameters are presented in Table 3.
Determining the optimal parameters of ion sources, including curtain gas pressure, spray support pressure, auxiliary pressure, temperature, and voltage applied to the electrode, was an important part of the work of selecting MS/MS detection conditions. It was particularly challenging to select the aforementioned parameters in the case of the analysis of a mixture of 20 pharmaceuticals.
Compounds with diverse physicochemical features (diverse lipophilicity, acid–base nature, and different types and quantities of functional groups in molecule), deciding their retentive properties, were included in the composition of the separated mixtures.
The mixtures subjected to separation included compounds with varying physicochemical properties—such as differences in lipophilicity, acid–base character, and the presence of diverse functional groups—all influencing their chromatographic retention behavior.
Optimal chromatographic conditions were established based on key analyte characteristics, including molecular weight, logP, and pKa values. During the optimization process, several variables were evaluated: the type of column packing, its temperature, the mobile phase composition and pH, the flow rate, and the elution program. The primary objective was to achieve high separation efficiency, sensitivity, and reproducibility within a short analysis time (under 10 min). Although full separation of co-eluting analytes is not strictly required in MRM mode, improving the resolution reduces matrix interferences and enhances both sensitivity and selectivity. Additionally, the differences in retention times helped confirm analyte identity.
Choosing the appropriate stationary phase was a crucial step in developing the reversed-phase separation method. For this purpose, tests were performed on a series of columns with diverse parameters (length, diameter, grain size, and polarity) specifically designed for ultra-efficient liquid chromatography. The best results were obtained with a core–shell Kinetex C18 column (100 mm × 2.1 mm, 2.6 µm). A fixed, non-porous silicone core is the packing of this column, on which a porous silica layer is deposited, and a monolayer of dimethyl-n-octadecyl silane is immobilized on its surface. Such a phase is “endcapped” by means of the appropriate reagent to deactivate the silica as much as possible. This configuration ensured high efficiency and a sharp peak resolution.
The composition of the mobile phase also had a significant impact on chromatographic performance. Water served as the main component, while methanol and acetonitrile were tested as organic modifiers. Both solvents provided good retention, but methanol was chosen due to its lower cost. Adding acidic modifiers—acetic or formic acid—enhanced analyte retention, with formic acid proving more effective and thus being selected for final use. Lowering the mobile phase pH was found to improve peak shape, increase selectivity, and enhance sensitivity.
Gradient elution was necessary to effectively separate the broad range of pharmaceuticals. Adjustments to the gradient profile—such as modifying the initial proportion of the organic solvent—helped improve resolution. In early elution stages, weakly retained compounds (e.g., atenolol and metoprolol) migrated rapidly due to their low logP values. Shortening the gradient duration for the organic phase allowed better peak shapes for hydrophobic compounds and reduced the analysis time without compromising efficiency.
The developed chromatographic method was characterized by satisfactory reproducibility of retention times regardless of the number of determined pharmaceuticals or their physicochemical properties. Moreover, a short time of analysis, and thus low cost and solvent consumption, are fundamental advantages of the developed methodology.
3.2. Extraction and Clean-Up Optimisation
A significant step in this research was to develop a method for isolating and purifying pharmaceuticals from wastewater samples. Wastewater represents a complex matrix for sample preparation in assessing organic pollutants due to the synergy of chemical, physical, and biological factors. On the other hand, pharmaceuticals, as a group of pollutants, show great structural diversity. Different functional groups, such as esters, carboxylic acids, ketones, and amides, affect many of their characteristics, including stability, reactivity, and water solubility, significantly complicating the sample preparation process [67]. The main objective of this work was to develop a QuEChERS procedure that would shorten the complex and time-consuming extraction and clean-up processes and simultaneously provide good analyte recoveries and low ME. Model wastewater was enriched with known concentrations of pharmaceuticals to develop the method. The composition of the extraction medium was optimized by selecting organic solvents, buffer mixtures, and additives in formic acid and 0.2 M Na2EDTA. The clean-up process was then optimized due to the complexity of the matrix and the consequent need for proper purification from various co-extracted interfering substances.
3.2.1. Extraction
Various modifications of the QuEChERS technique were used to isolate pharmaceuticals from wastewater. Depending on the variant, 10–106% recoveries were obtained for all compounds tested (Figure 1). Recovery values above 100% may result from matrix effects that cause signal enhancement, as well as inherent analytical variability. Such occurrences are common in complex matrices like wastewater and are accounted for in the method validation. The original procedure involves using acetonitrile as the extraction solvent (Figure 1). The original procedure involves the use of acetonitrile as the extraction solvent [42,68,69]. This variant (trial 1) yielded 25–88% recoveries, with satisfactory recoveries (above 70%) obtained for only seven compounds. The average recovery for this variant was 60%.
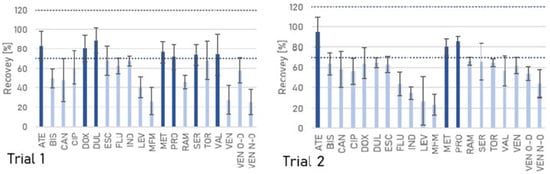
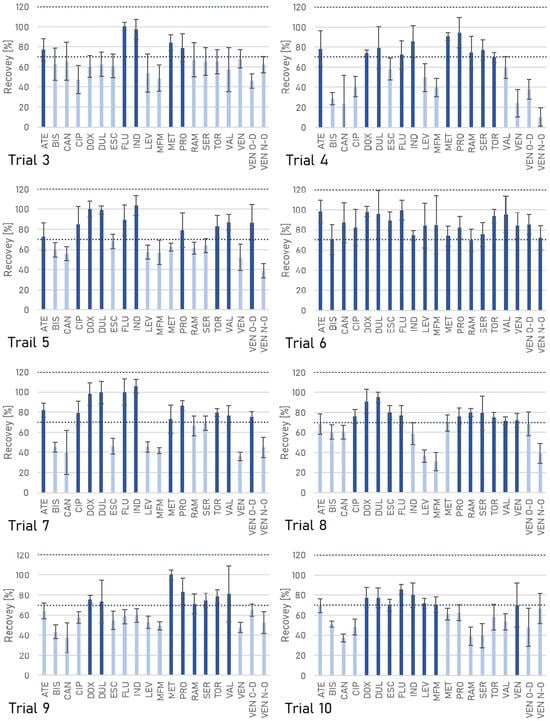
Figure 1.
The recoveries of the pharmaceuticals obtained in different extraction trials.
Acidic conditions can accelerate the desalting process and increase the stability of compounds in acetonitrile, as demonstrated for pesticides [70]. Furthermore, at acidic pH, the anionic sites of the matrix and analytes become protonated, leading to electrostatic repulsion between them, increasing the extraction efficiency [71]. Acidification of acetonitrile with formic acid (trial 4) significantly improved the recoveries of fluoxetine (from 62% to 72%), indapamide (from 67% to 85%), and ramipril (from 46% to 75%). However, it negatively affected the recovery of valsartan (from 74% to 59%). The use of ethyl acetate instead of acetonitrile (trial 7) improved the recoveries of ciprofloxacin (from 61% to 79%), fluoxetine (from 62% to 100%), indapamide (from 67% to 107%), torasemide (from 68% to 80%), and venlafaxine-O-desmethyl (from 58% to 75%).
The choice of buffers directly impacts the recovery rates of pharmaceuticals during extraction—it allows pH adjustment to ensure that as many compounds as possible are obtained in the neutral state. This procedure minimizes the presence of charged forms that could increase their affinity for water during drug extraction [72]. In the QuEChERS technique, there are two different standards regarding the type of buffer [68,73]: the US standard AOAC Official Method 2007.01 [74], which includes the use of an acetate buffer (pH about 4.8), and the European Committee for Standardisation (CEN) Standard Method EN 15662 [75], which includes the use of a citrate buffer (pH about 5–5.5), weaker in terms of ionic strength. For their comparison, an extraction with acetonitrile was performed. The acetate buffer-based method (trial 2) yielded satisfactory recoveries for only two beta-blockers (atenolol and metoprolol) and an antiarrhythmic drug (propafenone). The use of citrate (trial 3) yielded about twice the recovery of levonorgestrel (but still less than 70%) and fluoxetine, and nearly three times better for indapamide compared to the acetate buffer version (trial 2). No significant difference in recoveries (greater than 5%) was observed for bisoprolol, doxycycline, escitalopram, duloxetine, metoprolol, ramipril, sertraline, torasemide, and valsartan. However, the mean recovery rate for acetonitrile extraction (trial 3) with citrate buffer was higher compared to the version without buffer (trial 1) (67 and 60%, respectively).
The use of EDTA in the extraction of pharmaceuticals allows higher recoveries to be achieved by increasing the ionic strength, potentially increasing their solubility in the organic solvent [1]. Due to their strong chelating properties, EDTA and its salts can interact more strongly with metals in the matrix and thus exchange with analytes at active sites or mask adsorption sites [71]. Therefore, adding EDTA or its salts in the extraction process prevents the complexation of analytes with cations such as Mg2+ or Ca2+. Improvements in extraction efficiency by preventing chelate formation have been observed for fluoroquinolone and tetracycline compounds—for example, [76,77]. On the other hand, using EDTA may reduce the efficiency of the clean-up step [68].
In some cases, the presence of Na2EDTA adversely affected pharmaceutical recoveries. Ethyl acetate extraction in the presence of Na2EDTA (trial 9) yielded significantly lower recoveries of atenolol, ciprofloxacin, doxycycline, duloxetine, fluoxetine, and indapamide compared to the procedure without the chelating agent (trial 7) (mean recoveries of 64 and 70%, respectively). In the case of acetonitrile extraction, an adverse effect of the presence of Na2EDTA (trial 5) was observed for only two of the three beta-blockers tested—atenolol and metoprolol—and sertraline, an SSRI antidepressant. A possible rationale for this is that when Na2EDTA is in excess, it chelates metals and organic compounds [78].
Using the acetonitrile—citrate buffer—Na2EDTA combination (trial 6) resulted in the best recoveries, 70–99% (average of 85%). The recoveries of bisoprolol, candesartan, and venlafaxine N-oxide in this variant were satisfactory (above 70%). Nevertheless, this variant had relatively high RSD values, as high as 22% for levonorgestrel, 24% for duloxetine, and 29% for metformin (mean of 14%).
The matrix effect, directly related to the use of the MS/MS technique, is a significant factor affecting the quality of the results. The presence of co-extracted substances in the sample can interfere with the ionization process at the spectrometer interface, leading to increased or decreased signal intensities of the analytes. Consequently, this can result in falsification of quantitative results [79]. The use of the described QuEChERS protocols was associated with the presence of substances in the extract, causing strong attenuation or amplification of the analyte signals of pharmaceuticals (ME between −89 and 79%). ME values for trial 6 were the most promising (ME between −28 and 37%); for 65% of the compounds, a satisfactory ME value (between −20 and 20%) was achieved (Figure 2). An additional clean-up step based on using different sorbents was introduced to eliminate or reduce ME.
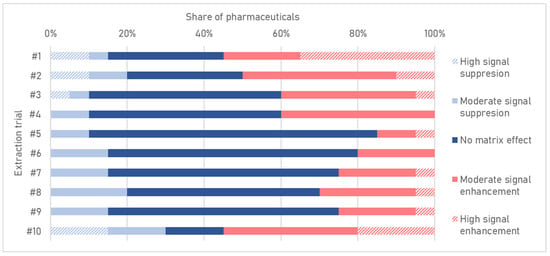
Figure 2.
Matrix effects of pharmaceuticals for the modified QuEChERS procedures.
3.2.2. Clean-Up
The clean-up step is necessary to reduce the ME, which strongly influences recoveries [80], but can also result in loss of analytes [68]. As indicated earlier, samples were previously frozen before the actual purification, which is the most common method for direct clean-up using the QuEChERS technique. This procedure mainly allows the removal of fats from the sample [81], which in the effluent can be up to 2000–5000 mg⋅L−1, depending on the origin [82,83,84]. Compared to the melting point of fats (usually below 40 °C), the melting point of pharmaceuticals is generally high (Table 1)—it is estimated that for 78.9% of pharmaceuticals, it is in the range of 80–240 °C. In comparison, for more than 90%, it is higher than 50 °C [85,86]. Due to the differences in melting point, the extracted pharmaceuticals are still dissolved in the organic solvent. At the same time, the frozen co-extracted fat can be easily separated from the extracts via centrifugation [81].
To minimize ME during the analysis of pharmaceuticals in wastewater, the effectiveness of different sorption materials used to purify extracts—PSA/C18/MgSO4, PSA/GCB/MgSO4, chitosan, GO, and Fe3O4MNPs—was assessed. Without a clean-up step, 35% of pharmaceuticals showed signal suppression or enhancement, confirming the need for appropriate sample purification.
GO had a negligible effect on the recovery values (Figure 3); they were in the 70–98% range when used. It also reduced RSD values to 3–13%, and provided satisfactory ME values, ranging from −11% to 15%. The usefulness of GO has previously been demonstrated in determining 52 veterinary drugs in mutton, using a reduced graphene oxide-coated melamine sponge. The material enabled ME values ≤ ±13% to be obtained in the 10–500 μg·kg−1 range [87]. GO has been applied in the clean-up of guttation liquid samples to analyze insecticides, providing negligible ME and analyte recoveries between 70% and 120% with relative standard deviations < 20% for most compounds tested [88]. Yadav et al. [89] described using GO to extract organophosphorus pesticides from water samples. Reduced graphene oxide–titanium dioxide (rGO-TiO2) nanomaterial has been successfully applied as a dispersive solid phase extraction (d-SPE) adsorbent to extract, enrich, and purify aflatoxins in poultry feed [90].
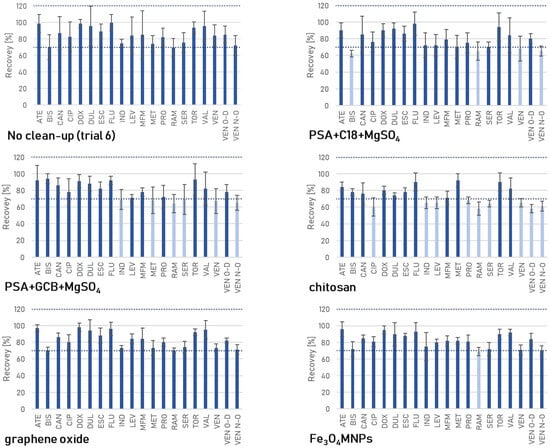
Figure 3.
The recoveries of the pharmaceuticals obtained using different clean-up methods.
Fe3O4MNPs generally yielded comparable recovery values to the GO clean-up option but slightly reduced ramipril recovery below the acceptable value. Significantly, the ME for some pharmaceuticals was not reduced to the desired degree with either Fe3O4MNPs or PSA/C18/MgSO4 and was −25% and −23%, 32% and 38%, 25% and 22%, −24% and −21%, 23% and 24%, and 25% and 26%, respectively. Clean-up with classical sorbents—PSA/C18/MgSO4 and PSA/GCB/MgSO4—negatively affected the recoveries of some compounds, including ramipril and venlafaxine. Like GO, chitosan significantly reduced the ME and recovery values below the acceptable limit for almost half of the compounds (Figure 4). The application of PSA/C18/MgSO4 and Fe3O4MNPs resulted in moderate signal enhancement for indapamide, levonorgestrel, and two metabolites of venlafaxine, while suppression was observed for doxycycline and ramipril.
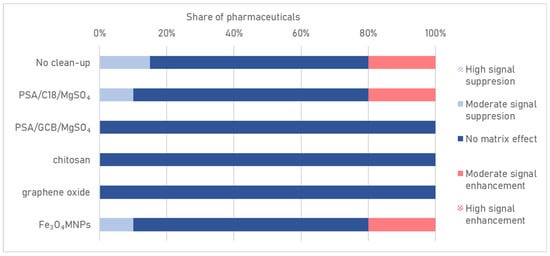
Figure 4.
Matrix effects of pharmaceuticals for different clean-up methods.
3.3. Validation Study
In the conducted study, linearity, recovery, precision, limit of detection (LOD), limit of quantification (LOQ), and uncertainty (U) were assessed to validate the method. The validation was performed under optimized extraction and clean-up conditions using model wastewater.
The linearity of the calibration curves was assessed using calibration solutions at concentrations of 0.005, 0.01, 0.05, 0.1, 0.5, and 1.0 μg⋅mL−1 in solvent and wastewater extract on three consecutive days using LC-MS/MS. The evaluation results show that all compounds’ coefficient of determination (R2) was 0.99 or higher.
The recovery was determined in five replicates at three fortification levels: 0.001, 0.01, and 0.1 μg⋅mL−1. All compounds showed satisfactory recoveries in the 71–97% range at 0.001 μg⋅mL−1, 70–98% at 0.01 μg⋅mL−1, and 73–99% at 0.1 μg mL−1. All pharmaceuticals yielded RSD not exceeding 16%. The RSD of twelve compounds at each level was less than 10%.
The limit of quantification (LOQ) was determined as the lowest enrichment level confirmed by satisfactory recovery (70–120%) and RSD (≤20%) values. The LOQ was in the range of 0.15–0.70 μg⋅mL−1 for all compounds. The limit of detection (LOD) values of individual pharmaceuticals were calculated using the chromatogram when the signal-to-noise (S/N) ratio was higher than 3 [91].
The data obtained from the validation study were utilized to estimate the measurement uncertainty (U) associated with the analytical results. The expanded measurement uncertainties were determined using a “top-down” empirical model [92], with values ranging from 9% to 28% (coverage factor k = 2, confidence level 95%). Precision was identified as the primary source of uncertainty. Additionally, the uncertainty associated with recovery, calculated based on a rectangular distribution, was incorporated into the overall uncertainty budget to prevent an underestimation of the total uncertainty.
The results are presented in Table 4, demonstrating the proposed method’s suitability.

Table 4.
Average recoveries, LODs, LOQs, and expanded uncertainties U, % (k = 2, confidence level 95%) of optimized procedure.
4. Conclusions
A robust and sensitive analytical method for determining trace-level pharmaceuticals in wastewater was successfully developed and validated. The approach combines a modified QuEChERS extraction procedure with graphene oxide-based clean-up and LC-MS/MS detection, resulting in high recovery rates, acceptable precision, low limits of quantification, and minimal matrix effects. Incorporating GO significantly improved clean-up efficiency without compromising analyte recovery, enhancing the overall reliability of the method.
Due to its simplicity, low cost, and analytical performance, the method is suitable for routinely monitoring pharmaceutical residues in complex environmental matrices such as wastewater. Beyond wastewater, this strategy holds potential for adaptation to other sample types, such as surface waters, sediments, or sludge; nevertheless, it requires further development and validation. Further development may focus on expanding the scope to additional emerging contaminants, miniaturizing the sample preparation process, or integrating the method into on-site or automated monitoring systems. These improvements could expand its applicability in environmental surveillance and regulatory frameworks aimed at reducing pharmaceutical pollution.
Author Contributions
Conceptualization, W.R. and P.K.; methodology, P.K.; validation, W.R. and P.K.; formal analysis, W.R. and P.K.; investigation, W.R. and P.K.; resources, W.R.; data curation, P.K.; writing—original draft preparation, W.R.; writing—review and editing, W.R. and P.K.; visualization, W.R.; supervision, P.K.; funding acquisition, W.R. All authors have read and agreed to the published version of the manuscript.
Funding
This project was financed in the framework of the Bialystok University of Technology program “Projekt PhD” (No: WI/WB-IIŚ/12/2023).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in this article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mravcová, L.; Amrichová, A.; Navrkalová, J.; Hamplová, M.; Sedlář, M.; Gargošová, H.Z.; Fučík, J. Optimization and Validation of Multiresidual Extraction Methods for Pharmaceuticals in Soil, Lettuce, and Earthworms. Environ. Sci. Pollut. Res. 2024, 31, 33120–33140. [Google Scholar] [CrossRef]
- OECD Publishing. Health at a Glance 2021: OECD Indicators; OECD Publishing: Paris, France, 2021; Volume 2. [Google Scholar]
- Pérez-Lucas, G.; Navarro, S. How Pharmaceutical Residues Occur, Behave, and Affect the Soil Environment. J. Xenobiotics 2024, 14, 1343–1377. [Google Scholar] [CrossRef]
- Godfrey, A.R.; Dunscombe, J.; Gravell, A.; Hunter, A.; Barrow, M.P.; van Keulen, G.; Desbrow, C.; Townsend, R. Use of QuEChERS as a Manual and Automated High-Throughput Protocol for Investigating Environmental Matrices. Chemosphere 2022, 308, 136313. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Chu, L.M. Fate of Antibiotics in Soil and Their Uptake by Edible Crops. Sci. Total Environ. 2017, 599–600, 500–512. [Google Scholar] [CrossRef]
- Solliec, M.; Roy-Lachapelle, A.; Gasser, M.O.; Coté, C.; Généreux, M.; Sauvé, S. Fractionation and Analysis of Veterinary Antibiotics and Their Related Degradation Products in Agricultural Soils and Drainage Waters Following Swine Manure Amendment. Sci. Total Environ. 2016, 543, 524–535. [Google Scholar] [CrossRef]
- Wang, L.; Deng, Q.; Hu, H.; Liu, M.; Gong, Z.; Zhang, S.; Xu-Monette, Z.Y.; Lu, Z.; Young, K.H.; Ma, X.; et al. Glyphosate Induces Benign Monoclonal Gammopathy and Promotes Multiple Myeloma Progression in Mice. J. Hematol. Oncol. 2019, 12, 70. [Google Scholar] [CrossRef]
- Choudhury, M.; Adhikari, M.D.; Agarwal, S.; Samanta, P.; Sharma, A.; Kundu, D.; Kumar, S. Pharmaceuticals and Personal Care Products in Soil: Sources, Impacts and Myco-Remediation Strategies. Emerg. Contam. 2025, 11, 100488. [Google Scholar] [CrossRef]
- Zhang, C.; Barron, L.P.; Stürzenbaum, S.R. Pollution of Soil by Pharmaceuticals: Implications for Metazoan and Environmental Health. Annu. Rev. Pharmacol. Toxicol. 2024, 65, 547–565. [Google Scholar] [CrossRef] [PubMed]
- Saeed, H.; Padmesh, S.; Singh, A.; Nandy, A.; Singh, S.P.; Deshwal, R.K. Impact of Veterinary Pharmaceuticals on Environment and Their Mitigation through Microbial Bioremediation. Front. Microbiol. 2024, 15, 1396116. [Google Scholar] [CrossRef] [PubMed]
- Grenni, P.; Visca, A.; Caracciolo, A.B. Antibiotics as Emerging Pollutants of Soil Ecosystems. In Frontier Studies in Soil Science; Springer: Cham, Switzerland, 2024; pp. 21–41. ISBN 978-3-031-50503-4. [Google Scholar]
- Kachhawaha, A.S.; Nagarnaik, P.M.; Jadhav, M.; Pudale, A.; Labhasetwar, P.K.; Banerjee, K. Optimization of a Modified QuEChERS Method for Multiresidue Analysis of Pharmaceuticals and Personal Care Products in Sewage and Surface Water by LC-MS/MS. J. AOAC Int. 2017, 100, 592–597. [Google Scholar] [CrossRef]
- Simarro-Gimeno, C.; Pitarch, E.; Albarrán, F.; Rico, A.; Hernández, F. Ten Years of Monitoring Pharmaceuticals and Pesticides in the Aquatic Environment Nearby a Solid-Waste Treatment Plant: Historical Data, Trends and Risk Assessment. Environ. Pollut. 2025, 366, 125496. [Google Scholar] [CrossRef]
- Li, Z.; Yu, X.; Yu, F.; Huang, X. Occurrence, Sources and Fate of Pharmaceuticals and Personal Care Products and Artificial Sweeteners in Groundwater. Environ. Sci. Pollut. Res. 2021, 28, 20903–20920. [Google Scholar] [CrossRef]
- Peng, Y.; Gautam, L.; Hall, S.W. The Detection of Drugs of Abuse and Pharmaceuticals in Drinking Water Using Solid-Phase Extraction and Liquid Chromatography-Mass Spectrometry. Chemosphere 2019, 223, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Ebele, A.J.; Oluseyi, T.; Drage, D.S.; Harrad, S.; Abou-Elwafa Abdallah, M. Occurrence, Seasonal Variation and Human Exposure to Pharmaceuticals and Personal Care Products in Surface Water, Groundwater and Drinking Water in Lagos State, Nigeria. Emerg. Contam. 2020, 6, 124–132. [Google Scholar] [CrossRef]
- Jiang, X.; Qu, Y.; Zhong, M.; Li, W.; Huang, J.; Yang, H.; Yu, G. Seasonal and Spatial Variations of Pharmaceuticals and Personal Care Products Occurrence and Human Health Risk in Drinking Water—A Case Study of China. Sci. Total Environ. 2019, 694, 133711. [Google Scholar] [CrossRef]
- Muambo, K.E.; Kim, M.G.; Kim, D.H.; Park, S.; Oh, J.E. Pharmaceuticals in Raw and Treated Water from Drinking Water Treatment Plants Nationwide: Insights into Their Sources and Exposure Risk Assessment. Water Res. X 2024, 24, 100256. [Google Scholar] [CrossRef]
- Mazhandu, Z.; Mashifana, T. Active Pharmaceutical Contaminants in Drinking Water: Myth or Fact? DARU J. Pharm. Sci. 2024, 32, 925–945. [Google Scholar] [CrossRef]
- Zanni, S.; Cammalleri, V.; D’Agostino, L.; Protano, C.; Vitali, M. Occurrence of Pharmaceutical Residues in Drinking Water: A Systematic Review. Environ. Sci. Pollut. Res. 2024, 32, 10436–10463. [Google Scholar] [CrossRef]
- Carter, L.J.; Garman, C.D.; Ryan, J.; Dowle, A.; Bergström, E.; Thomas-Oates, J.; Boxall, A.B.A. Fate and Uptake of Pharmaceuticals in Soil-Earthworm Systems. Environ. Sci. Technol. 2014, 48, 5955–5963. [Google Scholar] [CrossRef] [PubMed]
- Kodešová, R.; Klement, A.; Golovko, O.; Fér, M.; Nikodem, A.; Kočárek, M.; Grabic, R. Root Uptake of Atenolol, Sulfamethoxazole and Carbamazepine, and Their Transformation in Three Soils and Four Plants. Environ. Sci. Pollut. Res. 2019, 26, 9876–9891. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Tang, M.; Xu, X. Mechanism of Uptake, Accumulation, Transport, Metabolism and Phytotoxic Effects of Pharmaceuticals and Personal Care Products within Plants: A Review. Sci. Total Environ. 2023, 892, 164413. [Google Scholar] [CrossRef]
- Gao, Q.; Dong, Q.; Wu, L.; Yang, Y.; Hale, L.; Qin, Z.; Xie, C.; Zhang, Q.; Van Nostrand, J.D.; Zhou, J. Environmental Antibiotics Drives the Genetic Functions of Resistome Dynamics. Environ. Int. 2020, 135, 105398. [Google Scholar] [CrossRef]
- Belay, M.H.; Precht, U.; Mortensen, P.; Marengo, E.; Robotti, E. A Fully Automated Online SPE-LC-MS/MS Method for the Determination of 10 Pharmaceuticals in Wastewater Samples. Toxics 2022, 10, 103. [Google Scholar] [CrossRef]
- Mostafa, A.; Shaaban, H.; Alqarni, A.; Al-Ansari, R.; Alrashidi, A.; Al-Sultan, F.; Alsulaiman, M.; Alsaif, F.; Aga, O. Multi-Class Determination of Pharmaceuticals as Emerging Contaminants in Wastewater from Eastern Province, Saudi Arabia Using Eco-Friendly SPE-UHPLC-MS/MS: Occurrence, Removal and Environmental Risk Assessment. Microchem. J. 2023, 187, 108453. [Google Scholar] [CrossRef]
- de la Serna Calleja, M.Á.; Bolado, S.; Jiménez, J.J.; López-Serna, R. Performance Critical Comparison of Offline SPE, Online SPE, and Direct Injection for the Determination of CECs in Complex Liquid Environmental Matrices. Microchem. J. 2023, 187, 108395. [Google Scholar] [CrossRef]
- Guan, J.; Zhang, C.; Wang, Y.; Guo, Y.; Huang, P.; Zhao, L. Simultaneous Determination of 12 Pharmaceuticals in Water Samples by Ultrasound-Assisted Dispersive Liquid–Liquid Microextraction Coupled with Ultra-High Performance Liquid Chromatography with Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2016, 408, 8099–8109. [Google Scholar] [CrossRef]
- Herrera-Herrera, A.V.; Hernández-Borges, J.; Borges-Miquel, T.M.; Rodríguez-Delgado, M. ángel Dispersive Liquid-Liquid Microextraction Combined with Ultra-High Performance Liquid Chromatography for the Simultaneous Determination of 25 Sulfonamide and Quinolone Antibiotics in Water Samples. J. Pharm. Biomed. Anal. 2013, 75, 130–137. [Google Scholar] [CrossRef]
- Lemos, V.A.; Barreto, J.A.; Santos, L.B.; dos Santos de Assis, R.; Novaes, C.G.; Cassella, R.J. In-Syringe Dispersive Liquid-Liquid Microextraction. Talanta 2022, 238, 123002. [Google Scholar] [CrossRef] [PubMed]
- Kamal El-Deen, A.; Elmansi, H.; Belal, F.; Magdy, G. Recent Advances in Dispersion Strategies for Dispersive Liquid–Liquid Microextraction from Green Chemistry Perspectives. Microchem. J. 2023, 191, 108807. [Google Scholar] [CrossRef]
- Díaz-Álvarez, M.; Martín-Esteban, A. Preparation and Further Evaluation of L-Menthol-Based Natural Deep Eutectic Solvents as Supported Liquid Membrane for the Hollow Fiber Liquid-Phase Microextraction of Sulfonamides from Environmental Waters. Adv. Sample Prep. 2022, 4, 100047. [Google Scholar] [CrossRef]
- Es’haghi, Z. Determination of Widely Used Non-Steroidal Anti-Inflammatory Drugs in Water Samples by in Situ Derivatization, Continuous Hollow Fiber Liquid-Phase Microextraction and Gas Chromatography-Flame Ionization Detector. Anal. Chim. Acta 2009, 641, 83–88. [Google Scholar] [CrossRef]
- Mlunguza, N.Y.; Ncube, S.; Mahlambi, P.N.; Chimuka, L.; Madikizela, L.M. Determination of Selected Antiretroviral Drugs in Wastewater, Surface Water and Aquatic Plants Using Hollow Fibre Liquid Phase Microextraction and Liquid Chromatography—Tandem Mass Spectrometry. J. Hazard. Mater. 2020, 382, 121067. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Saldaña, V.; Castro-García, C.; Rodríguez-Maese, R.; Leal-Quezada, L.O. Advances in the Extraction Methods for the Environmental Analysis of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs). TrAC Trends Anal. Chem. 2023, 169, 117409. [Google Scholar] [CrossRef]
- Avino, P.; Notardonato, I.; Passarella, S.; Vincenzo Russo, M. Determination of Non-Steroidal Anti-Inflammatory Drugs in Animal Urine Samples by Ultrasound Vortex-Assisted Dispersive Liquid-Liquid Microextraction and Gas Chromatography Coupled to Ion Trap-Mass Spectrometry. Appl. Sci. 2020, 10, 5441. [Google Scholar] [CrossRef]
- Arghavani-Beydokhti, S.; Rajabi, M.; Asghari, A. Coupling of Two Centrifugeless Ultrasound-Assisted Dispersive Solid/Liquid Phase Microextractions as a Highly Selective, Clean, and Efficient Method for Determination of Ultra-Trace Amounts of Non-Steroidal Anti-Inflammatory Drugs in Complicated Matrices. Anal. Chim. Acta 2018, 997, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Nciri, N. A Swift, Straightforward, and Innovative Approach for Detecting Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) in Aquatic Matrices Using Direct Immersion (DI) Three-Phase Single-Drop Microextraction (SDME) Coupled In-Line with Capillary Electrophoresis CE. In Trends in Environmental Sustainability and Green Energy—CGEEE 2023: Springer Proceedings in Earth and Environmental Sciences; Kim, J., Chen, Z., Eds.; Springer: Cham, Switzerland, 2024; pp. 27–40. ISBN 978-3-031-52330-4. [Google Scholar]
- Kiszkiel-Taudul, I.; Starczewska, B. Single Drop Microextraction Coupled with Liquid Chromatography-Tandem Mass Spectrometry (SDME-LC-MS/MS) for Determination of Ranitidine in Water Samples. Microchem. J. 2019, 145, 936–941. [Google Scholar] [CrossRef]
- Yıldırım, S.; Cocovi-Solberg, D.J.; Uslu, B.; Solich, P.; Horstkotte, B. Lab-In-Syringe Automation of Deep Eutectic Solvent-Based Direct Immersion Single Drop Microextraction Coupled Online to High-Performance Liquid Chromatography for the Determination of Fluoroquinolones. Talanta 2022, 246, 123476. [Google Scholar] [CrossRef]
- Szarka, A.; Vnuková, L.; Keršňáková, Z.; Viktoryová, N.; Hrouzková, S. Contamination with Pharmaceuticals in Aquatic Environment: Focus on Analytical Methodologies. Appl. Sci. 2024, 14, 8645. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction” for the Determination of Pesticide Residues in Produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, S.H.; Eun, H.R.; Kim, S.M.; Jeong, M.J.; Baek, J.W.; Lee, Y.H.; Noh, H.H.; Shin, Y. Enhancement of Tricyclazole Analysis Efficiency in Rice Samples Using an Improved QuEChERS and Its Application in Residue: A Study from Unmanned Arial Spraying. Appl. Sci. 2024, 14, 5607. [Google Scholar] [CrossRef]
- Nardelli, V.; D’amico, V.; Ingegno, M.; Della Rovere, I.; Iammarino, M.; Casamassima, F.; Calitri, A.; Nardiello, D.; Li, D.; Quinto, M. Pesticides Contamination of Cereals and Legumes: Monitoring of Samples Marketed in Italy as a Contribution to Risk Assessment. Appl. Sci. 2021, 11, 7283. [Google Scholar] [CrossRef]
- Wenio, I.; Derewiaka, D.; Majewska, E.; Bartosiewicz, I.; Ryszkowska, E. Development of a Rapid Method for Simultaneous Determination of Pesticides in Plant Oil Using GC-MS/MS. Appl. Sci. 2024, 14, 4923. [Google Scholar] [CrossRef]
- Jo, H.W.; Park, M.G.; Jeon, H.J.; Moon, J.K.; Lee, S.E. Analysis of Multiresidue Pesticides in Agricultural Paddy Soils near Industrial Areas in Korea by GC–MS/MS and LC–MS/MS Using QuEChERS Extraction with DSPE Clean-up. Appl. Sci. 2021, 11, 8415. [Google Scholar] [CrossRef]
- Mahdavi, V.; Heris, M.E.S.; Dastranj, M.; Farimani, M.M.; Eslami, Z.; Aboul-Enein, H.Y. Assessment of Pesticide Residues in Soils Using a QuEChERS Extraction Procedure and LC-MS/MS. Water. Air. Soil Pollut. 2021, 232, 1–10. [Google Scholar] [CrossRef]
- Acosta-Dacal, A.; Rial-Berriel, C.; Díaz-Díaz, D.; Bernal-Suárez, M.; Luzardo, O.P. Optimization and Validation of a QuEChERS-Based Method for the Simultaneous Environmental Monitoring of 218 Pesticide Residues in Clay Loam Soil. Sci. Total Environ. 2021, 753, 142015. [Google Scholar] [CrossRef]
- Boti, V.; Kobothekra, V.; Albanis, T.; Konstantinou, I. Quechers-Based Methodology for the Screening of Alkylphenols and Bisphenol a in Dairy Products Using LC-LTQ/Orbitrap Ms. Appl. Sci. 2021, 11, 9358. [Google Scholar] [CrossRef]
- Areo, O.M.; Abafe, O.A.; Gbashi, S.; Njobeh, P.B. Detection of Multi-Mycotoxins in Rooibos and Other Consumed Teas in South Africa by a Modified QuEChERS Method and Ultra-High Performance Liquid Chromatography Tandem Mass Spectrometry. Food Control 2023, 143, 109255. [Google Scholar] [CrossRef]
- González-Jartín, J.M.; Rodríguez-Cañás, I.; Alfonso, A.; Sainz, M.J.; Vieytes, M.R.; Gomes, A.; Ramos, I.; Botana, L.M. Multianalyte Method for the Determination of Regulated, Emerging and Modified Mycotoxins in Milk: QuEChERS Extraction Followed by UHPLC–MS/MS Analysis. Food Chem. 2021, 356, 129647. [Google Scholar] [CrossRef] [PubMed]
- Casado, N.; Morante-Zarcero, S.; Sierra, I. Application of the QuEChERS Strategy as a Useful Sample Preparation Tool for the Multiresidue Determination of Pyrrolizidine Alkaloids in Food and Feed Samples: A Critical Overview. Appl. Sci. 2022, 12, 4325. [Google Scholar] [CrossRef]
- Temerdashev, Z.A.; Ovsepyan, S.K.; Musorina, T.N.; Vasileva, L.V.; Vasilev, A.M.; Korpakova, I.G. QuEChERS Extraction of PAHs from Various Soils and Sediments Followed by Chromatographic Determination. J. Anal. Chem. 2023, 78, 1159–1173. [Google Scholar] [CrossRef]
- Alexandre, B.; Barbara, G.; Laure, W.; Bruno, D.; Adriana, G.O.; Emmanuelle, V. Development of a Multiple-Class Analytical Method Based on the Use of Synthetic Matrices for the Simultaneous Determination of Commonly Used Commercial Surfactants in Wastewater by Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2016, 1450, 64–75. [Google Scholar] [CrossRef]
- Surma, M.; Sznajder-Katarzyńska, K.; Wiczkowski, W.; Zieliński, H. Assessment of Bioactive Surfactant Levels in Selected Cereal Products. Appl. Sci. 2022, 12, 5242. [Google Scholar] [CrossRef]
- Ofman, P.; Skoczko, I. PAH Removal Effectiveness Comparison from Hydraulic Fracturing Model Wastewater in SBR Reactors with Granular and Flocked Activated Sludge. Desalin. Water Treat. 2018, 134, 41–51. [Google Scholar] [CrossRef]
- Kaczynski, P.; Hrynko, I.; Lozowicka, B. Evolution of Novel Sorbents for Effective Clean-up of Honeybee Matrix in Highly Toxic Insecticide LC/MS/MS Analysis. Ecotoxicol. Environ. Saf. 2017, 139, 124–131. [Google Scholar] [CrossRef]
- Anagnostopoulos, C.J.; Aplada Sarli, P.; Liapis, K.; Haroutounian, S.A.; Miliadis, G.E. Validation of Two Variations of the QuEChERS Method for the Determination of Multiclass Pesticide Residues in Cereal-Based Infant Foods by LC-MS/MS. Food Anal. Methods 2012, 5, 664–683. [Google Scholar] [CrossRef]
- Walorczyk, S. Validation and Use of a QuEChERS-Based Gas Chromatographic-Tandem Mass Spectrometric Method for Multiresidue Pesticide Analysis in Blackcurrants Including Studies of Matrix Effects and Estimation of Measurement Uncertainty. Talanta 2014, 120, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Yum, H.; Jeong, S.; Jang, M.; Moon, S.; Kang, M.; Kim, B.; Kim, D.; Choe, S.; Yang, W.; Kim, J.; et al. Fast and Reliable Analysis of Veterinary Metomidate and Etomidate in Human Blood Samples by Liquid Chromatography–Tandem Mass Spectrometry (LC–MS/MS) in a Postmortem Case. J. Forensic Sci. 2021, 66, 2532–2538. [Google Scholar] [CrossRef] [PubMed]
- Christophoridis, C.; Veloutsou, S.; Mitsika, E.; Zacharis, C.K.; Christia, C.; Raikos, N.; Fytianos, K. Determination of Illicit Drugs and Psychoactive Pharmaceuticals in Wastewater from the Area of Thessaloniki (Greece) Using LC–MS/MS: Estimation of Drug Consumption. Environ. Monit. Assess. 2021, 193, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Canela, C.; Edo, S.; Rodríguez, N.; Gotor, G.; Lacorte, S. Comprehensive Characterization of 76 Pharmaceuticals and Metabolites in Wastewater by Lc-Ms/Ms. Chemosensors 2021, 9, 273. [Google Scholar] [CrossRef]
- Morsy, M.I.; Nouman, E.G.; Abdallah, Y.M.; Zainelabdeen, M.A.; Darwish, M.M.; Hassan, A.Y.; Gouda, A.S.; Rezk, M.R.; Abdel-Megied, A.M.; Marzouk, H.M. A Novel LC-MS/MS Method for Determination of the Potential Antiviral Candidate Favipiravir for the Emergency Treatment of SARS-CoV-2 Virus in Human Plasma: Application to a Bioequivalence Study in Egyptian Human Volunteers. J. Pharm. Biomed. Anal. 2021, 199, 114057. [Google Scholar] [CrossRef]
- Turković, L.; Bočkor, L.; Ekpenyong, O.; Silovski, T.; Lovrić, M.; Crnković, S.; Nigović, B.; Sertić, M. Development and Validation of a Novel LC-MS/MS Method for the Simultaneous Determination of Abemaciclib, Palbociclib, Ribociclib, Anastrozole, Letrozole, and Fulvestrant in Plasma Samples: A Prerequisite for Personalized Breast Cancer Treatment. Pharmaceuticals 2022, 15, 614. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Yu, S.; Chai, G.; Liu, J.; Zhou, Q. (Tony) An LC-MS/MS Method for Simultaneous Analysis of the Cystic Fibrosis Therapeutic Drugs Colistin, Ivacaftor and Ciprofloxacin. J. Pharm. Anal. 2021, 11, 732–738. [Google Scholar] [CrossRef]
- Habler, K.; Brügel, M.; Teupser, D.; Liebchen, U.; Scharf, C.; Schönermarck, U.; Vogeser, M.; Paal, M. Simultaneous Quantification of Seven Repurposed COVID-19 Drugs Remdesivir (plus Metabolite GS-441524), Chloroquine, Hydroxychloroquine, Lopinavir, Ritonavir, Favipiravir and Azithromycin by a Two-Dimensional Isotope Dilution LC–MS/MS Method in Human Serum. J. Pharm. Biomed. Anal. 2021, 196, 113935. [Google Scholar] [CrossRef]
- Sadutto, D.; Picó, Y. Sample Preparation to Determine Pharmaceutical and Personal Care Products in an All-Water Matrix: Solid Phase Extraction. Molecules 2020, 25, 5204. [Google Scholar] [CrossRef]
- Salvia, M.V.; Vulliet, E.; Wiest, L.; Baudot, R.; Cren-Olivé, C. Development of a Multi-Residue Method Using Acetonitrile-Based Extraction Followed by Liquid Chromatography-Tandem Mass Spectrometry for the Analysis of Steroids and Veterinary and Human Drugs at Trace Levels in Soil. J. Chromatogr. A 2012, 1245, 122–133. [Google Scholar] [CrossRef]
- Rutkowska, E.; Kaczyński, P.; Iwaniuk, P.; Łozowicka, B.; Hrynko, I.; Jankowska, M.; Konecki, R.; Rogowska, W.; Rusiłowska, J.; Pietkun, M.; et al. An Extensive Pesticide Residue Study in Minor Polish Vegetables Based on Critical Consumer Diets. Food Control 2025, 176, 111383. [Google Scholar] [CrossRef]
- Zaidon, S.Z.; Ho, Y.B.; Hamsan, H.; Hashim, Z.; Saari, N.; Praveena, S.M. Improved QuEChERS and Solid Phase Extraction for Multi-Residue Analysis of Pesticides in Paddy Soil and Water Using Ultra-High Performance Liquid Chromatography Tandem Mass Spectrometry. Microchem. J. 2019, 145, 614–621. [Google Scholar] [CrossRef]
- Lafay, F.; Daniele, G.; Fieu, M.; Pelosi, C.; Fritsch, C.; Vulliet, E. Ultrasound-Assisted QuEChERS-Based Extraction Using EDTA for Determination of Currently-Used Pesticides at Trace Levels in Soil. Environ. Sci. Pollut. Res. 2022, 1–15. [Google Scholar] [CrossRef]
- Rodríguez-Llorente, D.; Hernández, E.; Gutiérrez-Sánchez, P.; Navarro, P.; Ismael Águeda, V.; Álvarez-Torrellas, S.; García, J.; Larriba, M. Extraction of Pharmaceuticals from Hospital Wastewater with Eutectic Solvents and Terpenoids: Computational, Experimental, and Simulation Studies. Chem. Eng. J. 2023, 451, 138544. [Google Scholar] [CrossRef]
- Lehotay, S.J.; Son, K.A.; Kwon, H.; Koesukwiwat, U.; Fu, W.; Mastovska, K.; Hoh, E.; Leepipatpiboon, N. Comparison of QuEChERS Sample Preparation Methods for the Analysis of Pesticide Residues in Fruits and Vegetables. J. Chromatogr. A 2010, 1217, 2548–2560. [Google Scholar] [CrossRef] [PubMed]
- Lehotay, S.J.; O’Neil, M.; Tully, J.; García, A.V.; Contreras, M.; Mol, H.; Heinke, V.; Anspach, T.; Lach, G.; Fussell, R.; et al. Determination of Pesticide Residues in Foods by Acetonitrile Extraction and Partitioning with Magnesium Sulfate: Collaborative Study. J. AOAC Int. 2007, 90, 485–520. [Google Scholar] [CrossRef]
- CEN-CENELEC. Available online: https://www.cencenelec.eu/ (accessed on 12 April 2025).
- Pailler, J.Y.; Krein, A.; Pfister, L.; Hoffmann, L.; Guignard, C. Solid Phase Extraction Coupled to Liquid Chromatography-Tandem Mass Spectrometry Analysis of Sulfonamides, Tetracyclines, Analgesics and Hormones in Surface Water and Wastewater in Luxembourg. Sci. Total Environ. 2009, 407, 4736–4743. [Google Scholar] [CrossRef]
- Lindsey, M.E.; Meyer, M.; Thurman, E.M. Analysis of Trace Levels of Sulfonamide and Tetracycline Antimicrobials in Groundwater and Surface Water Using Solid-Phase Extraction and Liquid Chromatography/Mass Spectrometry. Anal. Chem. 2001, 73, 4640–4646. [Google Scholar] [CrossRef]
- Paíga, P.; Santos, L.H.M.L.M.; Delerue-Matos, C. Development of a Multi-Residue Method for the Determination of Human and Veterinary Pharmaceuticals and Some of Their Metabolites in Aqueous Environmental Matrices by SPE-UHPLC–MS/MS. J. Pharm. Biomed. Anal. 2017, 135, 75–86. [Google Scholar] [CrossRef]
- Kaczyński, P.; Łozowicka, B. A Novel Approach for Fast and Simple Determination Pyrrolizidine Alkaloids in Herbs by Ultrasound-Assisted Dispersive Solid Phase Extraction Method Coupled to Liquid Chromatography–Tandem Mass Spectrometry. J. Pharm. Biomed. Anal. 2020, 187, 113351. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, C.; Hong, Y.; Lee, W.; Lee, S.; Chung, H.; Kim, H.; Jeong, D.H. Determination of Pharmaceuticals in Solid Samples in Municipal Wastewater Treatment Plants by Online SPE LC–MS/MS Using QuEChERS Extraction. Environ. Monit. Assess. 2021, 193, 1–14. [Google Scholar] [CrossRef]
- Kaczyński, P. Large-Scale Multi-Class Herbicides Analysis in Oilseeds by Rapid One-Step QuEChERS-Based Extraction and Cleanup Method Using Liquid Chromatography–Tandem Mass Spectrometry. Food Chem. 2017, 230, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Adulkar, T.V.; Rathod, V.K. Pre-Treatment of High Fat Content Dairy Wastewater Using Different Commercial Lipases. Desalin. Water Treat. 2015, 53, 2450–2455. [Google Scholar] [CrossRef]
- Adulkar, T.V.; Rathod, V.K. Ultrasound Assisted Enzymatic Pre-Treatment of High Fat Content Dairy Wastewater. Ultrason. Sonochem. 2014, 21, 1083–1089. [Google Scholar] [CrossRef]
- Ekka, B.; Mieriņa, I.; Juhna, T.; Turks, M.; Kokina, K. Quantification of Different Fatty Acids in Raw Dairy Wastewater. Clean. Eng. Technol. 2022, 7, 100430. [Google Scholar] [CrossRef]
- Mao, F.; Kong, Q.; Ni, W.; Xu, X.; Ling, D.; Lu, Z.; Li, J. Melting Point Distribution Analysis of Globally Approved and Discontinued Drugs: A Research for Improving the Chance of Success of Drug Design and Discovery. ChemistryOpen 2016, 5, 357–368. [Google Scholar] [CrossRef]
- Tetko, I.V.; Sushko, Y.; Novotarskyi, S.; Patiny, L.; Kondratov, I.; Petrenko, A.E.; Charochkina, L.; Asiri, A.M. How Accurately Can We Predict the Melting Points of Drug-like Compounds? J. Chem. Inf. Model. 2014, 54, 3320–3329. [Google Scholar] [CrossRef]
- Ji, B.; Yang, L.; Ren, C.; Xu, X.; Zhao, W.; Yang, Y.; Xu, G.; Zhao, D.; Bai, Y. A Modified QuEChERS Method Based on a Reduced Graphene Oxide-Coated Melamine Sponge for Multiresidue Analysis of Veterinary Drugs in Mutton by UPLC–MS/MS. Food Chem. 2024, 433, 137376. [Google Scholar] [CrossRef]
- Hrynko, I.; Łozowicka, B.; Kaczyński, P. Development of Precise Micro Analytical Tool to Identify Potential Insecticide Hazards to Bees in Guttation Fluid Using LC–ESI–MS/MS. Chemosphere 2021, 263, 128143. [Google Scholar] [CrossRef]
- Yadav, S.; Goel, N.; Kumar, V.; Singhal, S. Graphene Oxide as Proficient Adsorbent for the Removal of Harmful Pesticides: Comprehensive Experimental Cum DFT Investigations. Anal. Chem. Lett. 2019, 9, 291–310. [Google Scholar] [CrossRef]
- Tanveer, Z.I.; Ahmad, K.; Dong, Z.; Chen, Y.; Liu, X.; Wu, Y.; Xu, T. Evaluation of Reduced Graphene Oxide-Based Nanomaterial as Dispersive Solid Phase Extraction Sorbent for Isolation and Purification of Aflatoxins from Poultry Feed, Combined with UHPLC–MS/MS Analysis. Food Addit. Contam. Part A 2023, 40, 1035–1048. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Gupta, V. Methods for the Determination of Limit of Detection and Limit of Quantitation of the Analytical Methods. In Proceedings of the Chronicles of Young Scientists; Medknow Publications and Media Pvt. Ltd.: Mumbai, India, 2011; Volume 2, p. 21. [Google Scholar]
- Medina-Pastor, P.; Valverde, A.; Pihlström, T.; Masselter, S.; Gamon, M.; Mezcua, M.; Rodríguez-Torreblanca, C.; Fernández-Alba, A.R. Comparative Study of the Main Top-down Approaches for the Estimation of Measurement Uncertainty in Multiresidue Analysis of Pesticides in Fruits and Vegetables. J. Agric. Food Chem. 2011, 59, 7609–7619. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).