Dual Production of Full-Fat Soy and Expanded Soybean Cake from Non-GMO Soybeans: Agronomic and Nutritional Insights Under Semi-Organic Cultivation
Abstract
Featured Application
Abstract
1. Introduction
- (i)
- Compare the agronomic performance and seed composition under semi-organic (P1) and conventional (P2) weed control systems.
- (ii)
- Evaluate the nutritional, functional, and anti-nutritional properties of full-fat soy (FFS) and expanded soybean cake (ESC) produced via barothermal processing.
- (iii)
- Assess the storage stability, oxidative safety, and microbiological quality of both products during shelf-life under ambient conditions.
2. Materials and Methods
2.1. Field Experiment and Sample Collection
2.1.1. Design and Location
2.1.2. Climatic Conditions
2.1.3. Plant Material and Agronomic Practices
- P1 (semi-organic): post-emergence weed control was carried out mechanically twice per growing season using a rotary harrow. Herbicides were used only in pre-emergence stages and limited post-emergence applications, aligned with semi-organic practice standards.
- P2 (conventional): Weed control relied exclusively on chemical herbicides, with both pre-emergence and multiple post-emergence applications. No mechanical interventions were applied in this system.
2.1.4. Sampling and Biometric Data Collection
2.2. Laboratory Analysis of Soybean Seeds
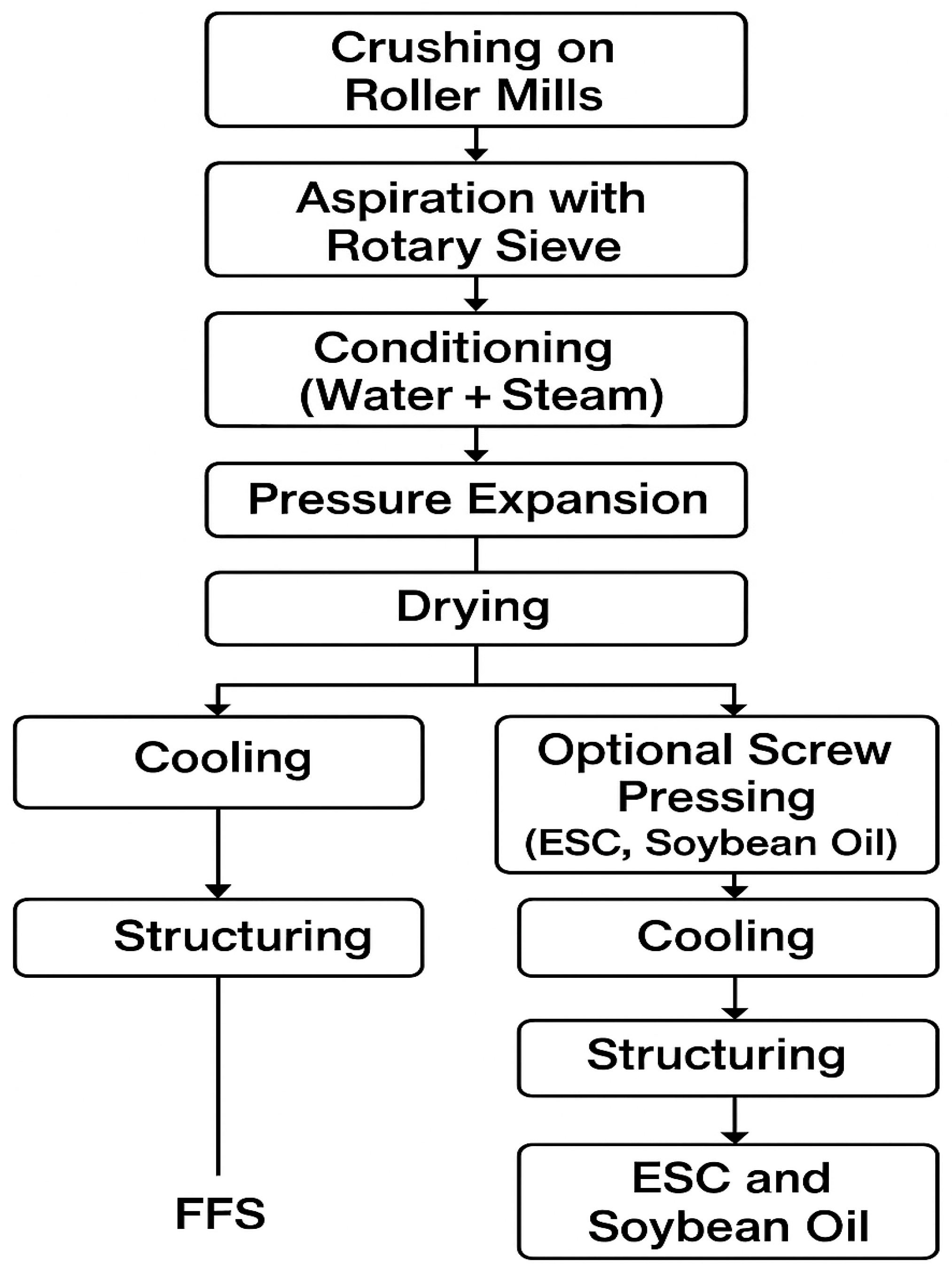
2.2.1. Processing of Soybean Seeds
2.2.2. Chemical Composition and Analytical Procedures
- Crude Protein (CP): Kjeldahl method (PN-EN ISO 20483:2007).
- Oil Content (OC): Soxhlet extraction (PN-EN ISO 734-1:2007).
- Crude Fiber (CF): PN-EN ISO 6865.
- Moisture and Ash: Gravimetric methods.
- Fatty Acid Profile (SFA, MUFA, PUFA, n-3, n-6): PN-EN ISO 12966-1:2015 + AC:2015 + ISO 12966-2:2017.
- Total and Digestible Carbohydrates: Subtraction method according to Regulation (EU) No 1169/2011 and EC No 152/2009.
- Dietary Fiber (DF): AOAC 985.29.
- Non-Starch Polysaccharides (NSP): GC per Englyst & Cummings, AOAC 994.13.
- Klason Lignin (KL): AACC 32-25.
- Uronic Acid (UA): Colorimetric method.
- Raffinose Family Oligosaccharides (RFO): GC method.
- Phytic Acid (PA): Haug & Lantzsch method.
- Total Phenolic Content (TPC): Folin–Ciocalteu assay.
2.2.3. Storage Stability Assessment
2.3. Statistical Analysis
3. Results and Discussion
3.1. Agronomic Performance Under Semi-Organic and Conventional Systems
3.2. Nutritional Composition of Soybean Seeds
3.3. Processing Outcomes and Product Differentiation (FFS vs. ESC)
3.4. Functional and Anti-Nutritional Properties of Soy Products
3.5. Fatty Acid Profile and Lipid Composition
3.6. Oxidative Stability and Microbiological Safety During Storage
3.7. Integration into Circular Protein Systems
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rotundo, J.L.; Marshall, R.; McCormick, R.; Truong, S.K.; Styles, D.; Gerde, J.A.; Gonzalez-Escobar, E.; Carmo-Silva, E.; Janes-Bassett, V.; Logue, J.; et al. European Soybean to Benefit People and the Environment. Sci. Rep. 2024, 14, 7612. [Google Scholar] [CrossRef] [PubMed]
- Feed Protein: Overview of EU Production and Options to Diversify Sources. Available online: https://agriculture.ec.europa.eu/media/news/feed-protein-overview-eu-production-and-options-diversify-sources-2024-05-24_en?prefLang=pl&etrans=pl (accessed on 25 May 2025).
- Debaeke, P.; Forslund, A.; Guyomard, H.; Schmitt, B.; Tibi, A. Could Domestic Soybean Production Avoid Europe’s Protein Imports in 2050? OCL 2022, 29, 38. [Google Scholar] [CrossRef]
- European Parliament; Directorate General for Internal Policies of the Union; Areté; S&P Global Commodity Insights; Natural Resources Institute Finland. The Dependency of the EU’s Food System on Inputs and Their Sources; Publications Office: Luxembourg, 2024. [Google Scholar]
- Vugt, T.; Nadeu, E. European Protein Diversification: Growing Opportunities for Farmers. 2025. Available online: https://ieep.eu/wp-content/uploads/2025/03/European-Protein-Diversification-Growing-opportunities-for-farmers-IEEP-2025.pdf (accessed on 9 July 2025).
- Islam, M.S.; Muhyidiyn, I.; Islam, M.R.; Hasan, M.K.; Hafeez, A.G.; Hosen, M.M.; Saneoka, H.; Ueda, A.; Liu, L.; Naz, M.; et al. Soybean and Sustainable Agriculture for Food Security. In Soybean—Recent Advances in Research and Applications; Ohyama, T., Takahashi, Y., Ohtake, N., Sato, T., Tanabata, S., Eds.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Purnhagen, K.P.; Clemens, S.; Eriksson, D.; Fresco, L.O.; Tosun, J.; Qaim, M.; Visser, R.G.F.; Weber, A.P.M.; Wesseler, J.H.H.; Zilberman, D. Europe’s Farm to Fork Strategy and Its Commitment to Biotechnology and Organic Farming: Conflicting or Complementary Goals? Trends Plant Sci. 2021, 26, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Karges, K.; Bellingrath-Kimura, S.D.; Watson, C.A.; Stoddard, F.L.; Halwani, M.; Reckling, M. Agro-Economic Prospects for Expanding Soybean Production beyond Its Current Northerly Limit in Europe. Eur. J. Agron. 2022, 133, 126415. [Google Scholar] [CrossRef]
- Klaiss, M.; Schmid, N.; Betrix, C.-A.; Baux, A.; Charles, R.; Messmer, M.M. Organic Soybean Production in Switzerland. OCL 2020, 27, 64. [Google Scholar] [CrossRef]
- Tataridas, A.; Kanatas, P.; Chatzigeorgiou, A.; Zannopoulos, S.; Travlos, I. Sustainable Crop and Weed Management in the Era of the EU Green Deal: A Survival Guide. Agronomy 2022, 12, 589. [Google Scholar] [CrossRef]
- Winkler, J.; Dvořák, J.; Hosa, J.; Martínez Barroso, P.; Vaverková, M.D. Impact of Conservation Tillage Technologies on the Biological Relevance of Weeds. Land 2022, 12, 121. [Google Scholar] [CrossRef]
- Weed Management Alternatives to the Use of Glyphosate, 3rd ed.; Pesticide Action Network Europe (PAN Europe): Brussels, Belgium, 2023; Available online: https://www.pan-europe.info/resources/reports/2023/03/weed-management-alternatives-use-glyphosate (accessed on 1 June 2025).
- Sims, B.; Corsi, S.; Gbehounou, G.; Kienzle, J.; Taguchi, M.; Friedrich, T. Sustainable Weed Management for Conservation Agriculture: Options for Smallholder Farmers. Agriculture 2018, 8, 118. [Google Scholar] [CrossRef]
- PN-EN ISO 12099:2017; Animal Feeding Stuffs—Guidelines for the Application of near Infrared Spectrometry (NIR). Polish Committee for Standardization: Warsaw, Poland, 2017.
- Ambroziak, K.; Wenda-Piesik, A. Comparative Characterization of Hemp Seed Cakes from Dehulled and Hulled Cannabis sativa, L. Var. Oleifera Cv. ‘Henola’: Nutritional, Functional, and Storage Stability Insights. Foods 2025, 14, 1605. [Google Scholar] [CrossRef] [PubMed]
- Toleikiene, M.; Slepetys, J.; Sarunaite, L.; Lazauskas, S.; Deveikyte, I.; Kadziuliene, Z. Soybean Development and Productivity in Response to Organic Management above the Northern Boundary of Soybean Distribution in Europe. Agronomy 2021, 11, 214. [Google Scholar] [CrossRef]
- He, Z.; Xiong, J.; Kent, A.D.; Deng, Y.; Xue, K.; Wang, G.; Wu, L.; Van Nostrand, J.D.; Zhou, J. Distinct Responses of Soil Microbial Communities to Elevated CO2 and O3 in a Soybean Agro-Ecosystem. ISME J. 2014, 8, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S. Response of Rhizobium to Environmental Stress. Agric. Food E-Newsl. 2020, 2, 117–119. [Google Scholar]
- Kurosaki, H.; Yumoto, S. Effects of Low Temperature and Shading during Flowering on the Yield Components in Soybeans. Plant Prod. Sci. 2003, 6, 17–23. [Google Scholar] [CrossRef]
- Setiyono, T.D.; Weiss, A.; Specht, J.; Bastidas, A.M.; Cassman, K.G.; Dobermann, A. Understanding and Modeling the Effect of Temperature and Daylength on Soybean Phenology under High-Yield Conditions. Field Crops Res. 2007, 100, 257–271. [Google Scholar] [CrossRef]
- Matthews, M.L.; Marshall-Colón, A.; McGrath, J.M.; Lochocki, E.B.; Long, S.P. Soybean-BioCro: A Semi-Mechanistic Model of Soybean Growth. Silico Plants 2022, 4, diab032. [Google Scholar] [CrossRef]
- Sun, T.; Cui, L.; Zong, L.; Zhang, S.; Jiao, Y.; Xue, X.; Jin, Y. Weed Recognition at Soybean Seedling Stage Based on YOLOV8nGP + NExG Algorithm. Agronomy 2024, 14, 657. [Google Scholar] [CrossRef]
- Wenda-Piesik, A.; Ambroziak, K. The Choice of Soybean Cultivar Alters the Underyielding of Protein and Oil under Drought Conditions in Central Poland. Appl. Sci. 2022, 12, 7830. [Google Scholar] [CrossRef]
- Jin, J.; Liu, X.; Wang, G.; Mi, L.; Shen, Z.; Chen, X.; Herbert, S.J. Agronomic and Physiological Contributions to the Yield Improvement of Soybean Cultivars Released from 1950 to 2006 in Northeast China. Field Crops Res. 2010, 115, 116–123. [Google Scholar] [CrossRef]
- Koebernick, J.; Gillen, A.M.; Fett, R.; Patel, S.; Fallen, B.; Pantalone, V.; Shannon, G.; Li, Z.; Scaboo, A.; Schapaugh, W.; et al. Soybean Test Weight in Relation to Genotype, Environment, and Genotype × Environment Interaction in the Southern United States. Agron. J. 2024, 116, 1265–1274. [Google Scholar] [CrossRef]
- FAO; WHO. Codex Alimentarius Commission Procedural Manual; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy; The World Health Organization (WHO): Geneva, Switzerland, 2023. [CrossRef]
- Mudgil, D.; Barak, S. Composition, Properties and Health Benefits of Indigestible Carbohydrate Polymers as Dietary Fiber: A Review. Int. J. Biol. Macromol. 2013, 61, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, M.; Jensen, M.G. Dietary Fibres in the Regulation of Appetite and Food Intake. Importance of Viscosity. Appetite 2011, 56, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Baird, P.; Davis, R.H., Jr.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health Benefits of Dietary Fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef] [PubMed]
- Carr, T.P.; Cornelison, R.M.; Illston, B.J.; Stuefer-Powell, C.L.; Gallaher, D.D. Plant Sterols Alter Bile Acid Metabolism and Reduce Cholesterol Absorption in Hamsters Fed a Beef-Based Diet. Nutr. Res. 2002, 22, 745–754. [Google Scholar] [CrossRef]
- Lunn, J.; Buttriss, J.L. Carbohydrates and Dietary Fibre. Nutr. Bull. 2007, 32, 21–64. [Google Scholar] [CrossRef]
- Jha, R.; Berrocoso, J.D. Review: Dietary Fiber Utilization and Its Effects on Physiological Functions and Gut Health of Swine. Animal 2015, 9, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Szulc, J.; Błaszak, B.; Wenda-Piesik, A.; Gozdecka, G.; Żary-Sikorska, E.; Bąk, M.; Bauza-Kaszewska, J. Zero Waste Technology of Soybeans Processing. Sustainability 2023, 15, 14873. [Google Scholar] [CrossRef]
- Schulp, C.J.E.; Ulug, C.; Elise Stratton, A.; Williams, T.G.; Verburg, P.H. Linking Production, Processing, and Consumption of Plant-Based Protein Alternatives in Europe. Glob. Environ. Change 2024, 89, 102940. [Google Scholar] [CrossRef]
- Hou, L.; Wang, Y.; Wang, Z.; Gao, R.; Zhou, X.; Yang, S.; Luo, X.; Jiang, Z.; Liu, Z. Effects of Biochar on Soil Quality in a Maize Soybean Rotation on Mollisols. Agronomy 2025, 15, 1226. [Google Scholar] [CrossRef]
- Ordóñez, R.A.; Archontoulis, S.V.; Martinez-Feria, R.; Hatfield, J.L.; Wright, E.E.; Castellano, M.J. Root to Shoot and Carbon to Nitrogen Ratios of Maize and Soybean Crops in the US Midwest. Eur. J. Agron. 2020, 120, 126130. [Google Scholar] [CrossRef]
- Qiu, L.-J.; Xing, L.-L.; Guo, Y.; Wang, J.; Jackson, S.A.; Chang, R.-Z. A Platform for Soybean Molecular Breeding: The Utilization of Core Collections for Food Security. Plant Mol. Biol. 2013, 83, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Alfaro-Wisaquillo, M.C.; Ali, M.; Patiño, D.; Oviedo-Rondon, E.O.; Vann, R.; Joseph, M. Variations in Soybean Nutritional and Anti-Nutritional Quality Based on Location and Planting Dates. Int. J. Food Sci. Technol. 2024, 59, 5411–5419. [Google Scholar] [CrossRef]
- Jeon, Y.-H.; Gu, B.-J.; Ryu, G.-H. Investigating the Potential of Full-Fat Soy as an Alternative Ingredient in the Manufacture of Low- and High-Moisture Meat Analogs. Foods 2023, 12, 1011. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Triphati, M.; Triphathi, N.; Singh, J.; Tiwari, S. Nutritional and Anti-Nutritional Factors in Soybean. Acta Sci. Agric. 2025, 8, 46–63. [Google Scholar] [CrossRef]
- Rebello, C.J.; Boué, S.; Levy, R.J.; Puyau, R.; Beyl, R.A.; Greenway, F.L.; Heiman, M.L.; Keller, J.N.; Reynolds, C.F.; Kirwan, J.P. Safety and Tolerability of Whole Soybean Products: A Dose-Escalating Clinical Trial in Older Adults with Obesity. Nutrients 2023, 15, 1920. [Google Scholar] [CrossRef] [PubMed]
- Bales, A.M.; Lock, A.L. Effects of Raw and Roasted High Oleic Soybeans on Milk Production of High-Producing Dairy Cows. J. Dairy Sci. 2024, 107, 10869–10881. [Google Scholar] [CrossRef] [PubMed]
- Soja w Uprawie i Żywieniu Zwierząt; Staniak, M., Świątkiewicz, M., Instytut Uprawy, Nawożenia i Gleboznawstwa-Państwowy Instytut Badawczy, Instytut Zootechniki, Eds.; Instrukcja Upowszechnieniowa/[Instytut Uprawy, Nawożenia i Gleboznawstwa]; Dział Upowszechniania i Wydawnictw IUNG-PIB w Puławach: Puławy, Poland, 2024. [Google Scholar]
- FAO. Fats and Fatty Acids in Human Nutrition: Report of an Expert Consultation (Food and Nutrition Paper No. 91). 2010. Report of an Expert Consultation. Available online: https://www.fao.org/fileadmin/user_upload/nutrition/docs/requirements/fatsandfattacidsreport.pdf (accessed on 30 May 2025).
- Lemke, S.L.; Vicini, J.L.; Su, H.; Goldstein, D.A.; Nemeth, M.A.; Krul, E.S.; Harris, W.S. Dietary Intake of Stearidonic Acid–Enriched Soybean Oil Increases the Omega-3 Index: Randomized, Double-Blind Clinical Study of Efficacy and Safety. Am. J. Clin. Nutr. 2010, 92, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, B.; Brothersen, C.; McMahon, D.J. Fortification of Foods with Omega-3 Polyunsaturated Fatty Acids. Crit. Rev. Food Sci. Nutr. 2014, 54, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Kondratowicz-Pietruszka, E.; Ostasz, L. Dynamics of Oxidation of Heated Vegetable Oils; Cracow University of Economics: Kraków, Poland, 2010. [Google Scholar]
- Qin, P.; Wang, T.; Luo, Y. A Review on Plant-Based Proteins from Soybean: Health Benefits and Soy Product Development. J. Agric. Food Res. 2022, 7, 100265. [Google Scholar] [CrossRef]
- Fukuzumi, A.; Tokumasu, N.; Matsuo, A.; Yano, E.; Zaima, N.; Moriyama, T. Detection and Characterization of the Soybean Allergen Gly m 7 in Soybeans and Processed Soybean Foods. Allergies 2021, 1, 233–246. [Google Scholar] [CrossRef]
- Sarwar Gilani, G.; Xiao, C.W.; Cockell, K.A. Impact of Antinutritional Factors in Food Proteins on the Digestibility of Protein and the Bioavailability of Amino Acids and on Protein Quality. Br. J. Nutr. 2012, 108, S315–S332. [Google Scholar] [CrossRef] [PubMed]
- Asioli, D.; Aschemann-Witzel, J.; Caputo, V.; Vecchio, R.; Annunziata, A.; Næs, T.; Varela, P. Making Sense of the “Clean Label” Trends: A Review of Consumer Food Choice Behavior and Discussion of Industry Implications. Food Res. Int. 2017, 99, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Caporgno, M.P.; Mathys, A. Trends in Microalgae Incorporation Into Innovative Food Products with Potential Health Benefits. Front. Nutr. 2018, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Bali, A.; Zabulionė, A.; Kumar, S.P.; Liudvinavičiūtė, D.; Pečiulytė, L.; Rutkaitė, R.; Ertbjerg, P.; Šalaševičienė, A. Effects of Extrusion Conditions on the Morphological, Functional, and Sensory Properties of Soy Press Cake Extrudates. Heliyon 2024, 10, e32614. [Google Scholar] [CrossRef] [PubMed]
- Świątkiewicz, M.; Szczepanik, K.; Gala, Ł.; Grela, E.R.; Witaszek, K.; Barszcz, M.; Tuśnio, A.; Taciak, M. Determination of the Impact of Extruded Soybean Press Cake on Rearing and Health Indices of Piglets. Agriculture 2024, 14, 1899. [Google Scholar] [CrossRef]
- Wang, Z.; Peters, B.A.; Yu, B.; Grove, M.L.; Wang, T.; Xue, X.; Thyagarajan, B.; Daviglus, M.L.; Boerwinkle, E.; Hu, G.; et al. Gut Microbiota and Blood Metabolites Related to Fiber Intake and Type 2 Diabetes. Circ. Res. 2024, 134, 842–854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Effects of Soybean–Corn Rotation on Crop Yield, Economic Benefits, and Water Productivity in the Corn Belt of Northeast China. Sustainability 2023, 15, 11362. [Google Scholar] [CrossRef]
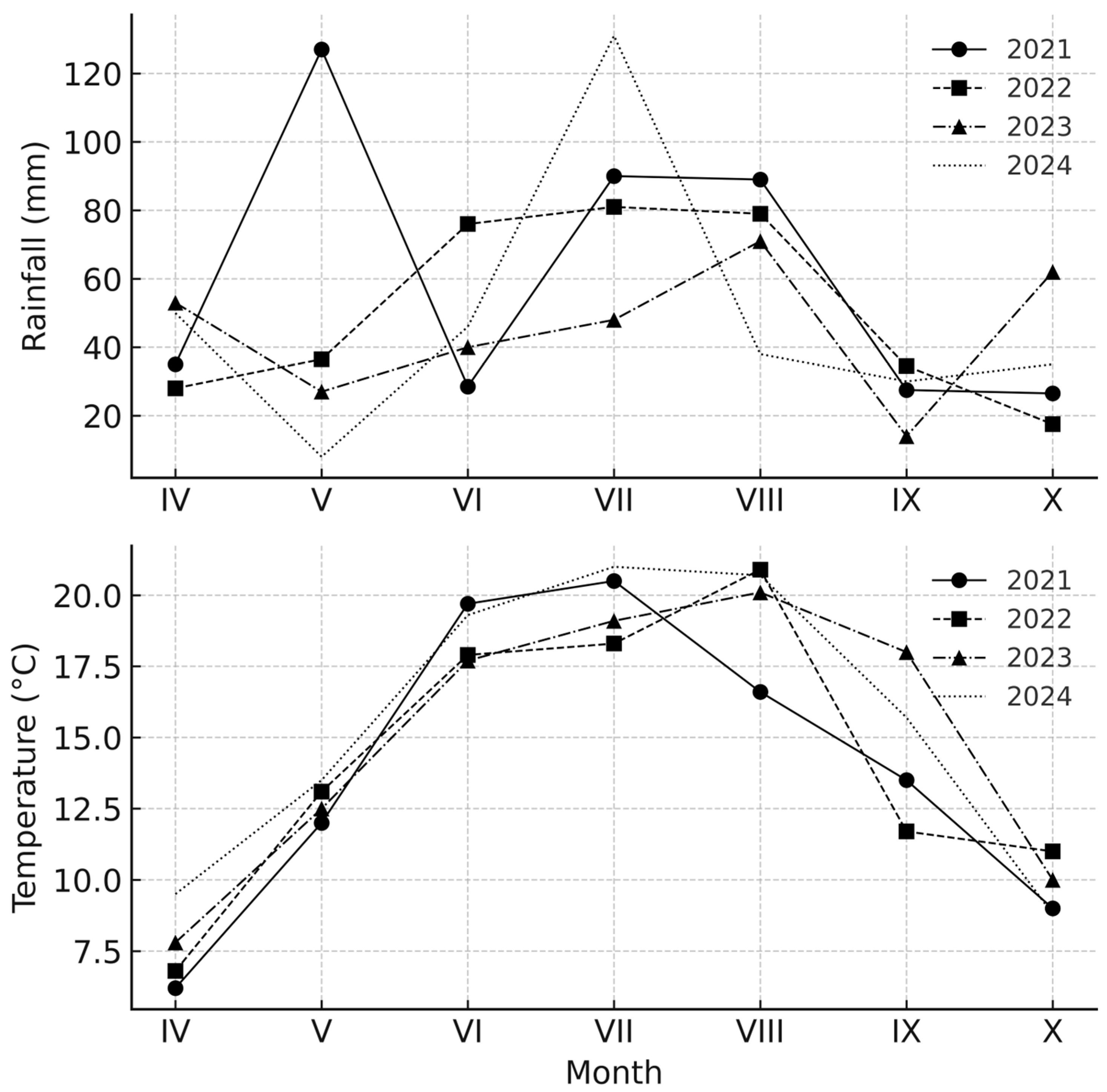
| Year | Treatment | Pre-Emergence Herbicides (g ha−1) | Post-Emergence Herbicides (g ha−1) | Mechanical Weeding | Sowing Date | Harvest Date |
|---|---|---|---|---|---|---|
| 2021 | P1 | Metribuzin 200 + S-metolachlor 960 | None | 2× | 11 May | 30 October |
| P2 | Metribuzin 200 + S-metolachlor 960 | None | None | 11 May | 30 October | |
| 2022 | P1 | Metribuzin 200 + S-metolachlor 960 | 1× Bentazon 600 + Imazamox 28 | 2× | 05 May | 1 October |
| P2 | Metribuzin 200 + S-metolachlor 960 | 1× Bentazon 600 + Imazamox 28 | None | 05 May | 1 October | |
| 2023 | P1 | Metribuzin 200 + S-metolachlor 960 | 2× Bentazon 300 + Imazamox 14 | 2× | 22 April | 22 September |
| P2 | Metribuzin 200 + S-metolachlor 960 | 2× Bentazon 300 + Imazamox 14 | None | 22 April | 22 September | |
| 2024 | P1 | Metribuzin 240 + Clomazone 48 + Petoxamide 800 | 4× Bentazon 150 + Imazamox 7 | 2× | 27 April | 9 September |
| P2 | Metribuzin 240 + Clomazone 48 + Petoxamide 800 | 4× Bentazon 150 + Imazamox 7 | None | 27 April | 9 September |
| Stage | Duration (min) | Temperature (°C) | Physico-Chemical Transformation |
|---|---|---|---|
| Crushing on roller mills | 0.1 | 15–20 | Particle size reduction (4–6 fragments per seed) |
| Aspiration with rotary sieve | 0.7 | 15–20 | Husk removal |
| Conditioning (water + steam) | 0.3 | 80–85 | Moisture increase and mixing |
| Steam buffering (saturation) | 13.3 | 95–98 | Inactivation of trypsin inhibitors |
| Pressure expansion | 0.5 | 120–135 | Gelatinization, cell rupture |
| Drying | 16 | 120 → 60 | Moisture reduction to ~10% |
| Optional screw pressing | 1.5 | ~80–120 | Fat removal # |
| Cooling | 8.0 | 60 → 35 | Protein stabilization, microbiological control |
| Mechanical structuring | 0.2 | 35 → 30 | Compaction and fraction uniformity |
| Year | Treatment | Post-Emergence Density (Plants Per m2) | Pre Harvest Density (Plants Per m2) | Number of Pods Per Plant | Plant Height at Maturity Stage(cm) | Seed Yield Converted to 13% Moisture (dt ha−1) | TSW (g) |
|---|---|---|---|---|---|---|---|
| 2021 | P1 z | 55.10 ± 2.20 b,* | 50.20 ± 2.00 a | 29.90 ± 3.05 a | 74.90 ± 11.30 a | 30.20 ± 0.20 a | 223.20 ± 5.12 a |
| P2 | 62.30 ± 2.49 a | 48.80 ± 1.96 a | 31.00 ± 3.20 a | 70.50 ± 12.02 b | 28.60 ± 0.20 b | 201.30 ± 5.40 b | |
| 2022 | P1 | 49.60 ± 1.98 a | 48.60 ± 1.94 a | 30.30 ± 2.85 a | 60.90 ± 8.80 a | 21.20 ± 0.14 a | 192.60 ± 4.80 a |
| P2 | 50.20 ± 2.00 a | 43.80 ± 1.75 b | 27.10 ± 3.10 b | 60.30 ± 7.60 a | 20.40 ± 0.15 a | 180.70 ± 4.30 b | |
| 2023 | P1 | 52.40 ± 2.09 a | 50.20 ± 2.00 a | 30.50 ± 4.12 a | 74.90 ± 7.19 a | 30.40 ± 0.22 a | 215.10 ± 4.80 a |
| P2 | 53.60 ± 2.14 a | 49.30 ± 1.97 a | 30.60 ± 4.00 a | 71.90 ± 8.30 b | 27.20 ± 0.21 b | 205.30 ± 4.60 b | |
| 2024 | P1 | 58.20 ± 2.32 b | 57.40 ± 2.30 a | 36.50 ± 3.60 a | 87.70 ± 12.40 a | 35.60 ± 0.20 a | 220.70 ± 5.30 a |
| P2 | 62.40 ± 2.49 a | 57.70 ± 2.30 a | 34.40 ± 3.18 b | 81.90 ± 13.10 b | 33.40 ± 0.20 b | 215.40 ± 5.10 a |
| Year | Treatment | Crude Protein (%) | Oil Content (%) | Water (%) | Bulk Density (kg hL−1) | Admixture (%) |
|---|---|---|---|---|---|---|
| 2021 | P1 z | 33.14 ± 1.12 a,* | 18.97 ± 0.67 a | 13.23 ± 0.42 a | 69.70 ± 4.70 a | 1.01 ± 0.02 a |
| P2 | 33.32 ± 1.30 a | 18.65 ± 0.72 a | 13.37 ± 0.37 a | 65.52 ± 5.10 b | 0.66 ± 0.04 b | |
| 2022 | P1 | 32.00 ± 0.96 a | 20.11 ± 0.55 a | 13.31 ± 0.34 a | 69.85 ± 6.11 a | 1.15 ± 0.04 a |
| P2 | 32.64 ± 1.06 a | 19.50 ± 0.60 a | 13.05 ± 0.27 a | 69.52 ± 5.80 a | 1.12 ± 0.03 a | |
| 2023 | P1 | 33.51 ± 1.07 a | 19.28 ± 0.64 a | 12.97 ± 0.30 a | 70.86 ± 6.95 a | 1.10 ± 0.02 a |
| P2 | 34.12 ± 1.32 a | 18.90 ± 0.69 a | 12.60 ± 0.32 a | 70.48 ± 7.14 a | 1.13 ± 0.02 a | |
| 2024 | P1 | 32.52 ± 1.14 a | 20.02 ± 0.58 a | 12.46 ± 0.33 a | 71.13 ± 8.02 a | 1.09 ± 0.04 a |
| P2 | 32.53 ± 1.17 a | 20.05 ± 0.62 a | 11.78 ± 0.33 b | 70.03 ± 7.80 a | 1.06 ± 0.04 a |
| Characteristic | 2022 | 2023 | 2024 | |||
|---|---|---|---|---|---|---|
| FFS | ESC | FFS | ESC | FFS | ESC | |
| Oil content (%) | 20.79 ± 0.35 a,* | 11.56 ± 0.56 b | 21.35 ± 0.25 a | 13.33 ± 0.51 b | 22.40 ± 0.54 a | 12.05 ± 0.25 b |
| Crude protein (%) | 34.48 ± 0.58 b | 38.98 ± 0.68 a | 34.30 ± 0.90 b | 37.57 ± 0.22 a | 32.40 ± 0.67 b | 37.40 ± 0.40 a |
| Ash (%) | 4.82 ± 0.10 a | 4.99 ± 0.11 a | 5.05 ± 0.45 a | 5.50 ± 0.13 a | 5.10 ± 0.35 a | 5.05 ± 0.15 a |
| Sugar (%) | 9.23 ± 0.24 b | 10.98 ± 0.15 a | 8.75 ± 0.05 b | 10.83 ± 0.23 a | 7.80 ± 0.16 b | 10.75 ± 0.25 a |
| Crude fiber (%) | 5.57 ± 0.42 b | 6.49 ± 0.11 a | 5.65 ± 0.35 a | 5.88 ± 0.28 a | 5.40 ± 0.31 b | 6.10 ± 0.50 a |
| Dietary fiber (%) | 13.63 ± 1.36 b | 16.19 ± 0.37 a | 14.30 ± 0.30 b | 15.84 ± 0.54 a | 14.00 ± 0.65 b | 16.30 ± 0.90 a |
| TOT Carb (%) | 43.70 ± 1.08 a | 31.48 ± 0.91 b | 37.80 ± 0.20 a | 31.64 ± 0.90 b | 43.00 ± 0.67 a | 31.10 ± 2.10 b |
| Dig Carb (%) | 12.81 ± 0.25 b | 22.84 ± 1.90 a | 13.35 ± 0.25 b | 19.03 ± 1.67 a | 14.20 ± 0.23 b | 19.35 ± 0.95 a |
| EV (kcal 100 g−1) | 410.2 ± 5.41 a | 379.4 ± 5.02 b | 416.5 ± 13.50 a | 380.5 ± 6.70 b | 432.0 ± 9.04 a | 389.5 ± 0.50 b |
| Water (%) | 11.93 ± 0.42 a | 12.13 ± 0.16 a | 12.35 ± 0.05 a | 10.70 ± 0.40 a | 12.50 ± 0.05 a | 10.20 ± 0.10 a |
| Compound | Product | ||||
|---|---|---|---|---|---|
| FFS | ESC | DSMB x | SC y | RS z | |
| CO (%) | 21.51 ± 0.28 d,* | 12.31 ± 0.27 c | 1.78 ± 0.10 a | 6.10 ± 0.10 b | 20.68 ± 0.49 d |
| CP (%) | 33.73 ± 0.44 a | 37.98 ± 0.29 a,b | 45.28 ± 1.44 b | 42.05 ± 0.05 a,b | 34.28 ± 0.64 a |
| Ash (%) | 4.99 ± 0.10 a | 5.18 ± 0.06 a,b | 6.30 ± 0.13 b | 5.95 ± 0.05 b | 4.24 ± 0.08 a |
| CF (%) | 5.54 ± 0.24 b | 6.16 ± 0.12 c | 4.20 ± 0.05 a | 6.50 ± 0.05 c | 5.58 ± 0.19 b |
| PDI (%) | 35.01 ± 3.09 c | 26.46 ± 2.34 b | 14.31 ± 1.31 a | 18.20 ± 0.05 a | 85.30 ± 2.10 d |
| KOH-SP (% CP) | 80.79 ± 4.11 a | 90.98 ± 1.54 b | 94.30 ± 1.58 b | 78.20 ± 0.10 a | 96.00 ± 0.00 b |
| DM (%) | 87.74 ± 0.24 a | 88.99 ± 0.20 a | 88.53 ± 0.33 a | 87.80 ± 0.50 a | 88.24 ± 0.34 a |
| TIA (mg/g) | 2.86 ± 0.17 a | 3.33 ± 0.38 a | 2.15 ± 0.38 a | 8.60 ± 0.05 b | 19.48 ± 0.29 c |
| Compound Content (% Dry Matter) | 2022 | 2023 | 2024 | |||
|---|---|---|---|---|---|---|
| FFS | ESC | FFS | ESC | FFS | ESC | |
| CP | 36.12 ± 0.39 b,* | 41.99 ± 0.25 a | 36.10 ± 0.95 b | 39.82 ± 0.24 a | 34.16 ± 0.71 b | 39.42 ± 0.43 a |
| CO | 24.13 ± 0.04 a | 14.78 ± 0.05 b | 24.90 ± 0.30 a | 16.77 ± 0.64 b | 26.16 ± 0.63 a | 15.07 ± 0.31 b |
| Ash | 6.31 ± 0.00 a | 6.24 ± 0.00 a | 6.64 ± 0.59 b | 6.77 ± 0.16 a | 6.73 ± 0.46 a | 6.17 ± 0.19 b |
| T-NSP | 10.34 ± 0.01 b | 12.90 ± 0.08 a | 10.89 ± 0.01 b | 12.43 ± 0.08 a | 10.69 ± 0.01 b | 12.71 ± 0.08 a |
| UA | 2.53 ± 0.01 a | 2.52 ± 0.04 a | 2.67 ± 0.01 a | 2.43 ± 0.04 b | 2.61 ± 0.01 a | 2.48 ± 0.04 b |
| KL | 1.20 ± 0.01 a | 1.33 ± 0.03 a | 1.26 ± 0.01 a | 1.28 ± 0.02 a | 1.24 ± 0.01 b | 1.31 ± 0.02 a |
| RFO | 3.93 ± 0.04 a | 4.11 ± 0.03 a | 4.14 ± 0.04 a | 3.96 ± 0.04 b | 4.06 ± 0.03 a | 4.05 ± 0.04 b |
| DF | 17.98 ± 0.00 b | 20.99 ± 0.00 a | 18.94 ± 0.39 b | 20.22 ± 0.68 a | 18.58 ± 0.86 b | 20.68 ± 1.14 a |
| PA | 1.40 ± 0.01 a | 1.43 ± 0.01 a | 1.47 ± 0.01 a | 1.38 ± 0.01 b | 1.45 ± 0.01 a | 1.41 ± 0.01 b |
| TPC | 2.49 ± 0.03 a | 2.43 ± 0.02 a | 2.62 ± 0.03 a | 2.34 ± 0.02 b | 2.57 ± 0.03 a | 2.39 ± 0.03 b |
| Heat Treatment Method | Storage Duration (Months) | SFA (%) | MUFA (%) | PUFA (%) | n-3 (%) | n-6 (%) |
|---|---|---|---|---|---|---|
| FFS | 1 | 34.55 ± 0.01 a,* | 23.85 ± 0.46 a | 65.25 ± 0.79 a | 7.60 ± 0.23 a | 49.30 ± 1.49 a |
| 3 | 34.40 ± 0.40 a | 22.40 ± 0.60 a | 64.00 ± 1.00 a | 8.00 ± 0.40 a | 52.60 ± 2.40 a | |
| 6 | 33.70 ± 0.00 a | 24.20 ± 0.00 a | 62.30 ± 0.00 a | 6.60 ± 0.00 a | 47.00 ± 0.00 a | |
| ESC | 1 | 15.88 ± 0.74 c | 22.93 ± 1.77 b | 69.58 ± 5.14 b | 7.93 ± 0.77 b | 57.44 ± 4.90 b |
| 3 | 18.60 ± 1.83 b | 23.18 ± 2.22 b | 70.75 ± 6.23 b | 8.83 ± 0.96 b | 58.58 ± 6.20 b | |
| 6 | 22.00 ± 0.00 a | 28.00 ± 0.00 a | 86.00 ± 1.00 a | 11.00 ± 0.00 a | 75.00 ± 1.00 a |
| Characteristic | FFS | ESC | Oil | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 6 | 1 | 3 | 6 | 1 | 3 | 6 | |
| AV (mg g−1 KOH) | 2.03 ± 0.01 a,b,* | 3.10 ± 0.02 b | 4.40 ± 0.03 c | 1.37 ± 0.01 a | 1.83 ± 0.01 a | 3.25 ± 0.02 b | 0.64 ± 0.00 a | 2.10 ± 0.01 a | 13.8 ± 0.90 b |
| FFA (%) | 1.33 ± 0.01 a | 2.67 ± 0.02 b | 3.46 ± 0.02 c | 0.89 ± 0.01 a | 1.48 ± 0.01 b | 2.01 ± 0.01 c | 0.95 ± 0.01 a | 1.15 ± 0.01 a,b | 1.50 ± 0.01 b |
| PV (meq O2 kg−1) | 2.62 ± 0.02 a,b | 3.25 ± 0.02 b | 5.70 ± 0.04 c | 1.13 ± 0.01 a | 1.48 ± 0.01 a | 2.15 ± 0.01 a,b | 4.20 ± 0.03 a | 4.50 ± 0.03 a | 4.80 ± 0.03 a |
| ANV | 0.61 ± 0.00 a | 0.95 ± 0.01 a,b | 1.30 ± 0.01 b | 0.38 ± 0.00 a | 1.30 ± 0.01 b | 3.61 ± 0.02 c | 0.40 ± 0.00 a | 0.60 ± 0.00 a | 0.90 ± 0.01 a |
| Totox index | 5.84 ± 0.04 a,b | 7.45 ± 0.05 a,b | 12.7 ± 08 b | 2.65 ± 0.02 a | 4.25 ± 0.03 a | 7.91 ± 0.05 a,b | 8.80 ± 0.06 a | 9.60 ± 0.06 a | 10.5 ± 0.07 a |
| Soy Product | Month | MAM at 30 °C | Coli | Y & M | Enterobacteriaceae | CPS | Salmonella |
|---|---|---|---|---|---|---|---|
| FFS | 1 | <100 | <10 | <10 | <10 | <10 | nd 1 |
| 3 | 129 | <10 | <100 | <10 | <10 | nd | |
| 6 | 1624 | <100 | 113 | <10 | <10 | nd | |
| ESC | 1 | <100 | <10 | <10 | <10 | <10 | nd |
| 3 | <100 | <10 | <100 | <10 | <10 | nd | |
| 6 | 1000 | <10 | <100 | <10 | <10 | nd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambroziak, K.; Wenda-Piesik, A. Dual Production of Full-Fat Soy and Expanded Soybean Cake from Non-GMO Soybeans: Agronomic and Nutritional Insights Under Semi-Organic Cultivation. Appl. Sci. 2025, 15, 8154. https://doi.org/10.3390/app15158154
Ambroziak K, Wenda-Piesik A. Dual Production of Full-Fat Soy and Expanded Soybean Cake from Non-GMO Soybeans: Agronomic and Nutritional Insights Under Semi-Organic Cultivation. Applied Sciences. 2025; 15(15):8154. https://doi.org/10.3390/app15158154
Chicago/Turabian StyleAmbroziak, Krystian, and Anna Wenda-Piesik. 2025. "Dual Production of Full-Fat Soy and Expanded Soybean Cake from Non-GMO Soybeans: Agronomic and Nutritional Insights Under Semi-Organic Cultivation" Applied Sciences 15, no. 15: 8154. https://doi.org/10.3390/app15158154
APA StyleAmbroziak, K., & Wenda-Piesik, A. (2025). Dual Production of Full-Fat Soy and Expanded Soybean Cake from Non-GMO Soybeans: Agronomic and Nutritional Insights Under Semi-Organic Cultivation. Applied Sciences, 15(15), 8154. https://doi.org/10.3390/app15158154






