Abstract
The effects of different levels of microbial transglutaminase (MTGase) at 0% (control), 0.25%, 0.50% and 1% on the physicochemical properties, volatile compounds and free amino acid composition of pastırma, a Turkish dry-cured meat product, were investigated. The MTGase treatment had no significant effect on the aw, L* and b* values of pastırma. The thiobarbituric acid reactive substances value decreased as the MTGase level increased. The maximum cutting force was found to be higher in enzyme-treated pastırma groups compared with the control. Enzyme treatment increased the maximum stress–relaxation force, but no statistical difference was observed between the 0.50% and 1% enzyme treatments. No significant differences were observed between groups in the volatile compound profile. However, in the correlation analysis, the control group showed a close correlation with the 0.25% MTGase group. This was also the case for the 0.5% and 1% MTGase groups. In the samples, glutamic acid, arginine, alanine, cystine and valine were determined to be the dominant free amino acids, and glutamic acid showed a close correlation with valine; lysine with arginine; and cystine with serine. MTGase had no significant effect on the total free amino acid content.
1. Introduction
Microbial transglutaminase (MTGase), used as a protein modifier enzyme in the food industry, and in mediated bioconjugation for its extraordinary cross-linking ability in industrial applications [], is obtained commercially from Streptoverticillium mobaraense by fermentation. MTGase exhibits optimum activity at pH 5–8 and at 50 °C. It can also maintain its activity at some temperatures slightly above freezing point [,,]. Furthermore, MTGase does not need any cofactor to show activity. The Food and Drugs Administration (FDA) has included this enzyme on the GRAS (Generally Recognized as Safe) list since 1998 []. Some studies have been conducted on the use of microbial transglutaminase in meat products such as restructured/formed dry-cured ham [,,,], restructured cooked ham [,], cooked ham [], cooked/restructured pork shoulder [], chicken döner kebab [], frankfurter [], dry-cured lamb ham [] and pastırma [].
Pastırma (a traditional Turkish dry-cured meat product), classified as an intermediate moisture food product, is produced from the whole muscle of certain parts of beef or water buffalo carcasses. The two important groups of microorganisms in pastırma production are lactic acid bacteria and Gram (+)-catalase (+) cocci (mainly coagulase negative staphylococci). In pastırma, the pH and water activity values usually range from 5.50 to 6.00 and 0.85 to 0.90, respectively. Pastırma production consists of the following processes: first curing, then drying, after that pressing and finally covering the meat with a pasta called çemen [,]. Pastırma has a distinctive characteristic regarding its production conditions, production time and raw material when considering similarly processed meat products such as Rohschinken, jambon de Savoie, dry-cured ham, lacon, prosciutto di Parma, Lachschinken, country-style ham and dry-cured pork shoulder. Moreover, the smoking process is not applied in the production process of pastırma []. In the dry-curing process, which is the first stage of pastırma production, nitrate and/or nitrite are used together with salt. Before this stage, a process called incision is applied in order for the curing components to diffuse well in the meat [,]. In the incision, the blade is held parallel to the muscle fibers and at an angle of 45°, and cuts are made in a series in the meat []. This application may cause textural defects in the final product, and this situation affects consumer preference negatively. The effects of using transglutaminase on both these textural defects and the quality characteristics of traditional pastırma types (sırt, kuşgömü, şekerpare, kürek and bohça) were investigated during cold storage under modified atmosphere conditions. However, in this study, only the effects of a 0.5% level of MTGase were investigated []. On the other hand, the effects of different levels (0.05–0.8%) of MTGase in restructured dry-cured hams were investigated [,,]. In a study conducted on dry-cured ham, 1% MTGase was also used []. The effects of different transglutaminase levels on the quality characteristics of pastırma have not yet been investigated. The aim of this study was to determine the effects of different MTGase levels (0%, 0.25%, 0.50% and 1%) on pH, aw, instrumental color, thiobarbituric acid reactive substances (TBARS), volatile compound profile, free amino acid composition and some textural parameters (stress–relaxation and cutting tests) of pastırma (sırt type) produced under controlled conditions.
2. Materials and Methods
2.1. Material
Microbial-derived transglutaminase enzyme preparation (Ajinomoto Activa GS, İstanbul, Türkiye) was used in the study. The Longissimus thoracis et lumborum obtained from a beef carcass (24 h post mortem) were divided into two after the trimming process and thus, four pieces of meat were obtained for sırt pastırma production. One of these pieces was used as a control. The other three pieces were used for the enzyme treatment. The experiment was conducted twice, and a total of two beef carcasses were used. In production, 150 mg/kg sodium nitrite was used as the curing agent. Red pepper, Trigonella foenum graecum flour and garlic were also used in the preparation of pasta (çemen).
2.2. The Production of Pastırma
Pastırma was produced in a climate chamber (Reich, Thermoprozestechnik GmbH, Schechingen, Germany) to provide controlled conditions. Meat pieces were subjected to the incision process at an angle of 45°, and the curing process was applied. In the process of curing, sucrose (0.3%), salt (5%) and sodium nitrite (150 mg/kg) were used by weight of the meat. The cured meat pieces were kept for 48 h at 4 °C. The meat pieces were then subjected to the 1st drying process at a relative humidity of 88 ± 2% and at 15 °C. This stage continued for 8 days. During the 1st drying, the relative humidity was gradually reduced to 80 ± 2%. After this stage, one of the four pieces of pastırma meat was used as a control. The other three parts were treated with transglutaminase enzyme preparation at different rates (0.25%, 0.50% and 1% by weight of meat). The calculated amount of transglutaminase for each muscle was diluted prior to use by taking a ratio of 1:4 (enzyme/water) as a basis. The preparation prepared in this way was applied to the surface of the muscle. After this process, both the control and enzyme-treated meat strips were pressed at a relative humidity of 82 ± 2%, for 20 h at 7 °C and with a weight of 15 kg per 1 kg of meat (the 1st pressing). After this process, the partially dried and pressed pieces of meat were applied to the 2nd drying process at a relative humidity of 72 ± 2% for 9 days at 20 °C. This meat was then subjected to the 2nd pressing process at a relative humidity of 70 ± 2% at 25 °C. The 2nd pressing process lasted for 7 h. Afterward, the 3rd drying stage was started. This stage lasted at a relative humidity of 70 ± 2% for 5 days at 25 °C. After the 3rd drying, the dry-cured pieces were kept in çemen (500 g Trigolella foenum graecum flour, 1200 mL water, 350 g garlic and 150 g red pepper) at 7 °C for one day, then they were covered with this çemen with a thickness of 2–3 mm and dried at 15 °C.
2.3. Determination of pH, aw and Thiobarbituric Acid Reactive Substance Content (TBARS) Values
To detect the pH value of pastırma, distilled water (100 mL) was added to 10 g of the sample and after homogenization, the pH value was determined using a pH meter (Mettler Toledo Ion S220, Greifensee, Switzerland).
In order to detect the water activity (aw) of pastırma, an aw meter (Novasina AG CH-8853, Lachen, Switzerland) was calibrated using calibration salts. After approximately 3 g of sample was taken and placed in a special container, it was put in the measuring cell. The measurement was carried out at 25 °C.
For determining the TBARS value in the pastırma samples, the method suggested by Kilic and Richards [] was performed, and the TBARS value was given as µmol MDA/kg sample. For TBARS analysis, 12 mL of 7.5% trichloroacetic acid solution was added onto 2 g pastırma samples and homogenized with the Ultra Turrax. Filter paper (Whatman no 1) was used for filtration of the homogenate. After that, the same amount of filtrate and TBA (thiobarbituric acid solution–0.02 M) were mixed. For 40 min, the mixture was placed in a boiling water bath. Then, they were centrifuged at 2000 G (MR23G, Thermo Fisher Scientific, Waltham, MA, USA) for 5 min. Finally, the absorbances were measured against the blank at 530 nm.
2.4. Determination of Color Values
In order to determine the color values (L*, a* and b*) in the 2 mm-sliced pastırma samples, a Chroma Meter (CR-400 Konica Minolta, Osaka, Japan) was used with a* C D65 illuminant, an aperture size of 8 mm and standard observed of 2°. All measurements were performed in triplicate.
2.5. Stress–Relaxation and Cutting Tests
The textural properties of the pastırma samples were determined by stress–relaxation and cutting tests with a TA.XT plus Texture Analyzer (Stable, Micro Systems Ltd., Godalming, Surrey, UK). The test conditions and samples sizes are given in Table 1. Stress–relaxation and cutting tests were performed twice and in triplicate, respectively.

Table 1.
Application parameters of cutting test.
2.6. Determination of Volatile Compounds
Five g samples from each group were taken and subjected to an analysis of volatile compounds in 40 mL vials (Supelco, Bellefonte, PA, USA). These vials were put into a 30 °C thermal block (Supelco, Bellefonte, PA, USA) in order to gather the volatile compounds in the headspace. After 1 hour, CAR/PDMS fiber (Supelco 75 μm, USA) was put in the vials and kept for 2 h in order to adsorb the volatile compounds onto the fiber. The absorbed compounds on the fiber were then identified using GC/MS (Gas Chromatography (GC), Agilent Technologies 6890 N /Mass Spectrometry (MS), Agilent Technologies 5973, USA) with a column of DB-624 (60 m × 0.25 mm × 1.4 μm film, J&W Scientific, Folsom, CA, USA). As the carrier gas, helium was used with a flow rate of 1 mL/min. Having the initial value of 40 °C for 6 min, the oven temperature was increased gradually up to 110 °C, 150 °C and 210 °C (kept for 12 min) with the rate values of 3 °C/min, 4 °C/min and 10 °C/min, respectively. The compounds were identified by comparing their retention times and mass spectra of volatiles to those of authentic compounds, as well as to mass spectra obtained from the NIST, WILEY and FLAVOR libraries. Kovats’ retention indexes were also determined using a standard mix (Supelco 44585-U, Bellefonte, PA, USA) [].
2.7. Determination of Free Amino Acid Composition
Each pastırma sample of 10 g was mixed with 75% MeOH (75% methanol + 25% ultra-pure water). After homogenization, it was put into a volumetric flask (100 mL) and the volume of the flask was filled with 75% MeOH up to 100 mL. After 1 h incubation at 4 °C and centrifugation at 15,000 rpm for 40 min, the samples were filtered using 0.2 µm diameter membrane filters []. For derivatization, 250 µL of borate buffer and 50 µL of the filtrate were mixed and 50 µL of OPA reagent (phthaldialdehyde reagent, Sigma, St. Louis, MO, USA), 50 µL FMOC reagent (Agilent Technologies, Santa Clara, CA, USA) and 3200 mL distilled water were added into the mixture. By use of HPLC with a fluorescence detector (Agilent Technologies 1100 Series, USA), the content of free amino acids was determined. Sodium phosphate solution (pH 7.8) was used for mobile phase A and methanol/acetonitrile/water (45:45:10) for mobile phase B. Mobile phase A was initially set to 100%, and mobile phase B was gradually increased. The flow rates of the mobile phases were adjusted as 2 mL/min with a column (Zorbax 4.6 × 150 mm (Agilent, USA)) temperature of 40 °C. An amino acid standard was used as a mixture and diluted to be 10, 25, 50, 100 and 250 pmol in order to generate the standard curve. The result was given as mg/100 g dry matter.
2.8. Statistical Analysis
Analysis of variance (ANOVA) was applied to the entire analyzed dataset using a general linear model. Enzyme treatment was considered as the main factor and replicates were considered as a random factor for randomized complete block design. The experiments were repeated twice. The individual standard errors of the means are presented in the tables. Differences between the means were determined using Duncan’s multiple range tests at the p < 0.05 level. SPSS version 20 was used for all the statistical analyses (SPSS Inc., Chicago, IL, USA). In statistical evaluation, the following mathematical model was used:
where Yij is the value of parameter; µ is the mean of population; ai is the effect of enzyme application on parameters; bj is the effect of block on parameters; and eij is the error based on randomness.
Yij = µ + ai + bj + eij
To determine the relationship between the MTGase groups and volatile compounds, and between the MTGase groups and free amino acid composition, the correlation heat map was plotted using ChiPlot (https://www.chiplot.online/ (accessed on 25 May 2025)) []. In the algorithm, complete was used as the method and correlation was used as the distance.
3. Results and Discussion
The results of the aw, pH, TBARS and color analyses of pastırma (sırt type) with and without the transglutaminase enzyme at different rates are presented in Table 2. The enzyme treatment showed no crucial effect on the aw value (p > 0.05). By being in the food class of intermediate moisture, the aw value for pastırma is a notable hurdle for microbiological stability and should be below 0.90 []. In this study, the aw value was found to be below 0.90 in all groups. Similarly, Hazar et al. [] reported mean aw values of 0.872 in a control and 0.858 in a group with 0.5% MTGase. The MTGase treatment showed an important effect on pH value (p < 0.05). The pH values of pastırma groups ranged from 5.87 to 5.93. The lowest pH value was determined in the 0.50% MTGase group (Table 2). In another study conducted on different pastırma types, in a group using 0.5% MTGase, lower pH values were determined compared with the control in traditionally produced sırt, kuşgömü and şekerpare pastırma types []. However, as in this study, the differences between the groups were very low. Similarly, in a study conducted on restructured ham, the average pH value was determined as 5.99 in samples using 3% TGase and 6.07 in the control group, and it was emphasized that the difference between the average values had no statistically significant effect []. The pH value usually varies between 5.5 and 6.0 for pastırma []. The Turkish Food Codex Communique on Meat, Prepared Meat Mixtures, and Meat Products states that the pH value should be at most 6.0 for pastırma [].

Table 2.
Effect of transglutaminase application on aw, pH, TBARS and color (L*, a* and b*) values.
In dry-cured meat products such as pastırma, lipid oxidation is one of the biochemical changes that occurs during processing and contributes to the flavor of the product [,]. However, advanced lipid oxidation may adversely affect sensory quality []. MTGase had a crucial impact on TBARS value (p < 0.01). Using MTGase in the pastırma production resulted in limited lipid oxidation. While the highest TBARS value was detected in the control group, this value decreased with an increasing amount of enzyme use. The TBARS value with the lowest mean was specified in the presence of MTGase at the level of 1% (Table 2). Similarly, Hazar et al. [] determined that using MTGase at the level of 0.50% decreased the TBARS value in different pastırma types. They emphasized this result due to the partial protection of the MTGase enzyme from oxygen in the pastırma []. In this study, the TBARS values in pastırma produced using 0.50% and 1% transglutaminase enzyme were also determined to be at lower levels than the TBARS values found in a study related to pastırma []. On the other hand, it has been reported that the modification of food proteins such as myosin and actomyosin by transglutaminase leads to textured products, and protects lysine from various chemical reactions. Furthermore, it retards oxidation by encapsulating lipids and/or lipid-soluble substances, and exhibits various functional properties []. It has also been reported that the use of MTGase in cooked beef meatballs delays lipid oxidation [].
Color is a significant quality criterion for pastırma []. MTGase enzyme treatment did not show a notable effect on the L* and b* values, while the a* value, indicating the intensity of red color, was found to be higher in the 0.25% enzyme treatment (Table 2). However, this a* value was similar to the results obtained by Çakıcı et al. []. Hazar et al. [] reported that the addition of 0.50% MTGase caused a decrease in the a* value of traditionally produced pastırma. Additionally, it was found that the a* value of pastırma under modified atmosphere conditions (30% CO2 + 70% N2) decreased as storage time progressed. These differences in a* values are thought to be due to differences in curing conditions and process conditions. On the other hand, Lee and Chin [] determined that the use of 3% TGase had no significant effect on the color values of restructured ham.
Curing, pressing and drying, which are the production stages of pastırma, cause water loss of the product and some changes in proteins and thus affect the texture of the product []. The results of the cutting and stress–relaxation tests of the pastırma samples with and without MTGase are presented in Table 3. The maximum cutting force and maximum stress–relaxation force values of pastırma samples were statistically affected by enzyme treatment. There was an increase in the maximum cutting force value with the enzyme treatment, and while the lowest mean value was determined in the control group, the other groups did not vary statistically from each other. According to these results, including the ratio of 0.25%, the use of transglutaminase in pastırma increases the value of the maximum cutting force. This has a positive effect on the texture and consistency of the product. Another study related to traditional pastırma types suggested that the maximum cutting force increased in the presence of 0.50% MTGase []. This result may be due to the formation of cross-links in proteins caused by transglutaminase []. MTGase catalyzes the acyl transfer reaction, deamidation, and cross-linking of lysine and glutamine residues []. It, by catalyzing the formation of isopeptide bonds between proteins, has significant potential to improve the textural properties of foods []. Indeed, it was stated that MTGase’s effect on functional properties such as the binding capacity of proteins may be responsible for enhancing the springiness and cohesiveness of dry-cured ham [,]. Another parameter giving information about the hardness of the product is the maximum stress–relaxation force. The lowest mean value for maximum cutting force was found in the control group (Table 3). This value increased with the increase in the enzyme usage rate. However, the difference between 0.50 and 1% was found to be insignificant statistically (p > 0.05) (Table 3).

Table 3.
Effect of transglutaminase application on cutting and stress–relaxation properties.
A total of 48 volatile compounds were identified in the pastırma samples such as acid, alcohols, aldehydes, aliphatic hydrocarbons, aromatic hydrocarbons, esters, furans, ketones, nitrogenous compounds and sulfur compounds—depicted in Table 4. Among the compounds, the highest abundance was for aldehydes followed by aliphatic hydrocarbons, sulfur compounds and ketones. The other two important groups in pastırma were alcohols and esters. Among the alcohols, ethanol exhibited a higher abundance (Table 4). Similar results have been found by Kaban []. Microorganisms and endogenous enzymes are important factors that play a role in the quality and flavor of meat products made from whole pieces such as dry-cured ham and pastırma [,]. The changes that occur in the proteins and lipids during ripening contribute to the flavor of dry-cured meat products including pastırma []. As can be seen from Table 4, the enzyme treatment showed no crucial impact on the volatile compounds of pastırma (p > 0.05). Another study conducted on traditional pastırma types found that the use of MTGase was effective on a few volatile compounds [].

Table 4.
Effect of transglutaminase treatment on the volatile compound profile of pastırma (AU × 106).
A correlation heat map was used to study the relationship between using MTGase and volatile compounds is shown in Figure 1. Although the use of different rates of MTGase did not lead to a statistically significant difference in the volatile compound profile of pastırma, the correlation analysis showed that there was a difference between the groups. The use of different amounts of MTGase resulted in the formation of two clusters. The groups containing 0% (control) and 0.25% MTGase and the groups containing 0.5% and 1% MTGase were in the same clusters and showed closer correlations. It was also observed that the use of MTGase caused differences in the volatile compounds (Figure 1). Hexanal, one of the secondary products produced during the oxidation of linoleic acid and linolenic acid that has a characteristic odor of green grass [], had a higher abundance in the 0.5% and 1% MTGase groups compared with the other groups.
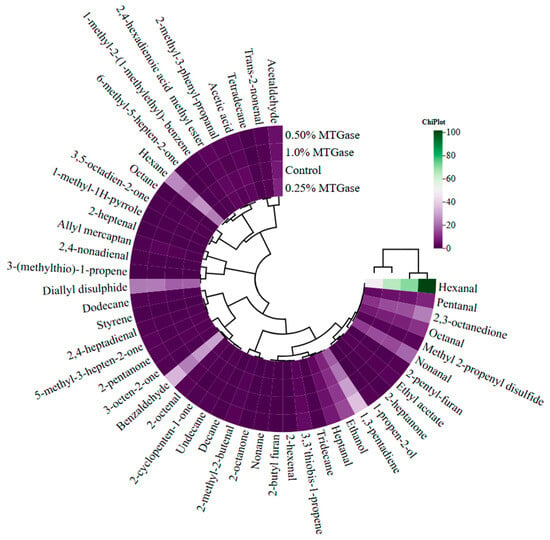
Figure 1.
Cluster analysis of correlation heat map showing the relationship between MTGase and volatile compounds.
The free amino acid composition of pastırma containing transglutaminase enzyme at different levels is presented in Table 5. Alanine, arginine, cystine, glutamic acid and valine were determined to be the dominant free amino acids. The effect of the enzyme treatment on cystine and phenylalanine was determined at the p < 0.01 level, and on arginine and proline at the p < 0.05 level. The lowest amounts of proline and arginine in the pastırma samples were determined in pastırma containing 0.25% enzyme. In terms of cystine, the lowest mean value was given by the control group, but the mean value of the pastırma containing 0.25% enzyme did not vary from the mean value of the control group. It was observed that the mean phenylalanine content of the control group and pastırma produced using 0.25% enzyme were higher than those of other groups (samples containing 0.50% and 1% enzyme) (Table 5). On the other hand, Hazar et al. [] reported that MTGase application (0.50%) in different pastırma type productions increased the level of histidine, alanine and proline. Additionally in the same study, the presence of MTGase decreased the levels of arginine, isoleucine, threonine and valine. These differences can be clarified by fiber type, the production conditions (traditional or industrial) of pastırma and the anatomical location of the muscle(s). The taste of the product is directly affected by the free amino acids and peptides formed as a result of proteolysis. Free amino acids are the precursor of some volatile compounds and therefore affect the aroma of dry-cured meat products. The degree of proteolysis in this type of meat product varies according to the raw material, process conditions (temperature, time, pH, aw and redox potential) and curing components (salt, nitrate nitrite and ascorbic acid) []. In addition, endogenous muscle enzymes cause the degradation of proteins [].

Table 5.
Effect of transglutaminase application on free amino acid composition (mg/100 g dry matter).
Figure 2 shows the two main clusters between MTGAse groups. The first cluster contains the control and 0.25% MTGase groups, while the second cluster includes the 0.5% and 1% MTGase groups. Two main clusters were formed in terms of free amino acids. Within these clusters, two more subclusters formed. Among the high number of amino acids found in the same cluster, glutamic acid showed a close correlation with valine; lysine with arginine; and cystine with serine (Figure 2). MTGase can cause changes in amino acids by catalyzing acyl transfer reactions, deamidation, and cross-linking (polymerization) between protein intra- or inter-chain glutamine and lysine peptide residues [].
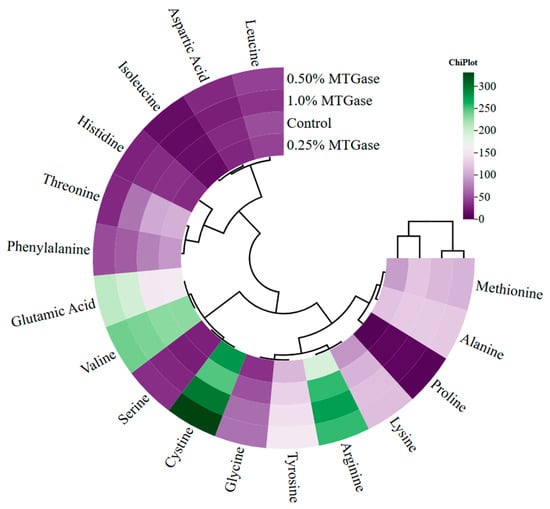
Figure 2.
Custer analysis of correlation heat map showing the relationship between MTGase groups and free amino acid composition.
4. Conclusions
In pastırma production, MTGase application increased the maximum cutting force even at the 0.25% level. For maximum force, the 0.5% MTGase level was sufficient. While MTGase use did not affect the L* value, 0.25% MTGase use increased the a* value. In addition, the use of MTGase limited lipid oxidation. Regarding volatile compounds, the control and 0.25% level exhibited a similar profile. In addition, MTGase caused a change in the content of some amino acids, and the control and 0.25% level showed a closer correlation. In addition, it was determined that MTGase application did not cause a significant difference in terms of total free amino acid level. In the production of pastırma, incisions cause textural defects and this situation may negatively affect consumer preference. In this study, the fact that the use of transglutaminase does not cause significant changes in the characteristic properties of the product shows that this enzyme has a potential for use in production.
Author Contributions
Conceptualization, M.K.; methodology, F.Y.H.S. and M.K.; validation, G.K. and F.Y.H.S.; formal analysis, F.Y.H.S. and G.K.; investigation, F.Y.H.S.; resources, F.Y.H.S. and M.K.; data curation, M.K.; writing—original draft preparation, F.Y.H.S.; writing—review and editing, G.K. and M.K.; visualization, G.K.; supervision, M.K.; project administration, M.K.; funding acquisition, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Republic of Türkiye Ministry of Agriculture and Forestry General Directorate of Agricultural Research and Policies (TAGEM 16/AR-GE/29).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vasić, K.; Knez, Ž.; Leitgeb, M. Transglutaminase in foods and biotechnology. Int. J. Mol. Sci. 2023, 24, 12402. [Google Scholar] [CrossRef] [PubMed]
- Motoki, M.; Seguro, K. Transglutaminase and its use for food processing. Trends Food Sci. Technol. 1998, 9, 204–210. [Google Scholar] [CrossRef]
- Aktaş, N.; Kilic, B. Effect of microbial transglutaminase on thermal and electrophoretic properties of ground beef. LWT-Food Sci. Technol. 2005, 38, 815–819. [Google Scholar] [CrossRef]
- Gaspar, A.L.C.; Góes-Favoni, S.P. Action of microbial transglutaminase (MTGase) in the modification of food proteins: A review. Food Chem. 2015, 171, 315–322. [Google Scholar] [CrossRef]
- Fulladosa, E.; Serra, X.; Gou, P.; Arnau, J. Effects of potassium lactate and high pressure on transglutaminase restructured dry-cured hams with reduced salt content. Meat Sci. 2009, 82, 213–218. [Google Scholar] [CrossRef]
- Romero de Ávila, M.D.; Ordóñez, J.A.; de la Hoz, L.; Herrero, A.M.; Cambero, M.I. Microbial transglutaminase for cold-set binding of unsalted/salted pork models and restructured dry ham. Meat Sci. 2010, 84, 747–754. [Google Scholar] [CrossRef]
- Bergamin Filho, W.; Costa, M.D.R.; Felício, P.E.D.; Silveira, E.T.F. Accelerated method of dry-cured ham processing. Food Sci. Technol. 2010, 30, 494–500. [Google Scholar] [CrossRef]
- Jira, W.; Sadeghi-Mehr, A.; Brühhrmann, D.A.; Schwägele, F. Production of dry-cured formed ham with different concentrations of microbial transglutaminase: Mass spectrometric and sensory evaluation. Meat Sci. 2017, 129, 81–87. [Google Scholar] [CrossRef]
- Lee, H.C.; Chin, K.B. Evaluation of various salt levels and different dairy proteins in combination with microbial transglutaminase on the quality characteristics of restructured pork ham. Int. J. Food Sci. Technol. 2011, 46, 1522–1528. [Google Scholar] [CrossRef]
- Lee, C.H.; Chin, K.B. Effect of pork skin gelatin on the physical properties of pork myofibrillar protein gel and restructured ham with microbial transglutaminase. Gels 2022, 8, 822. [Google Scholar] [CrossRef]
- Müller, W.D. Using phosphate and transglutaminase—Influence to quality parameter of cooked ham. Fleischwirtsch 2003, 83, 114–117. [Google Scholar]
- Dimitrakopoulou, M.A.; Ambrosiadis, J.A.; Zetou, F.K.; Bloukas, J.G. Effect of salt and transglutaminase (TG) level and processing conditions on quality characteristics of phosphate-free, cooked, restructured pork shoulder. Meat Sci. 2005, 70, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Kilic, B. Effect of microbial transglutaminase and sodium caseinate on quality of chicken döner kebab. Meat Sci. 2003, 63, 417–421. [Google Scholar] [CrossRef]
- Choi, Y.S.; Jeong, T.J.; Kim, H.W.; Hwang, K.E.; Sung, J.M.; Seo, D.H.; Kim, Y.B.; Kim, C.J. Combined effects of sea mustard and transglutaminase on the quality characteristics of reduced-salt frankfurters. J. Food Process. Preserv. 2017, 41, e12945. [Google Scholar] [CrossRef]
- Palhares, P.C.; do Carmo, L.R.; Andrade, B.F.; Ramos, A.D.L.S.; Piccoli, R.H.; Ramos, E.M. Use of glucono-delta-lactone in the accelerated processing of boneless dry-cured lamb hams. Int. J. Food Sci. Technol. 2023, 58, 2270–2279. [Google Scholar] [CrossRef]
- Hazar, F.Y.; Kaban, G.; Kaya, M. The effects of transglutaminase on the qualitative properties of different pastırma types. LWT 2021, 145, 111289. [Google Scholar] [CrossRef]
- Kaban, G. Changes in the composition of volatile compounds and in microbiological and physicochemical parameters during pastırma processing. Meat Sci. 2009, 82, 17–23. [Google Scholar] [CrossRef]
- Kaya, M.; Oral, Z.F.Y.; Kaban, G. Pastırma. Production of Traditional Mediterranean Meat Products; Lorenzo, J.M., Domínguez, R., Pateiro, M., Munekata, P.E.S., Eds.; Springer, Humana Press: New York, NY, USA, 2022; pp. 143–152. [Google Scholar]
- Kilic, B.; Richards, M.P. Lipid oxidation in poultry doner kebap: Pro-oxidative and anti-oxidative factors. J. Food Sci. 2003, 68, 686–689. [Google Scholar] [CrossRef]
- Antoine, F.R.; Wei, C.I.; Littell, R.C.; Marshall, M.R. HPLC method for analysis of free amino acids in fish using ο-phthaldialdehyde precolumn derivatization. J. Agric. Food Chem. 1999, 47, 5100–5107. [Google Scholar] [CrossRef]
- ChiPlot. Available online: https://www.chiplot.online/ (accessed on 25 May 2025).
- Turkish Food Codex Regulation. The Communique on Meat, Prepared Meat Mixtures, and Meat Products; No: 30670, 2018/52. 29.01; Ministry of Agricultural and Forestry: Ankara, Türkiye, 2019. [Google Scholar]
- Lorenzo, J.M.; Carballo, J. Changes in physico-chemical properties and volatile compounds throughout the manufacturing process of dry-cured foal loin. Meat Sci. 2015, 99, 44–51. [Google Scholar] [CrossRef]
- Hazar, F.Y.; Kaban, G.; Kaya, M. Volatile compounds of pastırma under different curing processes. J. Food Process. Preserv. 2019, 43, e14040. [Google Scholar] [CrossRef]
- Zhu, Y.; Rinzema, A.; Tramper, J.; Bol, J. Microbial transglutaminase—A review of its production and application in food processing. Appl. Microbiol. Biotechnol. 1995, 44, 277–282. [Google Scholar] [CrossRef]
- Ersöz, F.; Aykın Dinçer, E.; Türkanoğlu Özçelik, A.; İnan, M. Effect of recombinant transglutaminase on the quality characteristics of cooked beef meatballs. Kafkas Univ. Vet. Fak. Derg. 2021, 27, 209–215. [Google Scholar] [CrossRef]
- Çakıcı, N.; Aksu, M.I.; Erdemir, E. A survey of the physico-chemical and microbiological quality of different pastırma types: A dry-cured meat product. CyTA-J. Food 2015, 13, 196–203. [Google Scholar] [CrossRef]
- Akköse, A.; Kaban, G.; Karaoğlu, M.M.; Kaya, M. Characteristics of pastırma types produced from water buffalo meat. Kafkas Univ. Vet. Fak. Derg. 2018, 24, 179–185. [Google Scholar] [CrossRef]
- Andres-Bello, A.; García-Segovia, P.; Ramíres, J.A.; Martínez-Monzo, J. Production of cold-setting restructured fish products from gilthead sea bream (Sparus aurata) using microbial transglutaminase and regular and low-salt level. CyTA-J. Food 2011, 9, 121–125. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, X. Modification of myofibrillar protein functional properties prepared by various strategies: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 458–500. [Google Scholar] [CrossRef]
- Kieliszek, M.; Misiewicz, A. Microbial transglutaminase and its application in the food industry. A review. Folia Microbiol. 2014, 59, 241–250. [Google Scholar] [CrossRef]
- Costa, M.R.; Bergamin, W.; Silveira, E.T.F.; Felício, P.E. Colour and texture profiles of boneless restructured dry-cured hams compared to traditional hams. Sci. Agric. 2008, 65, 169–173. [Google Scholar] [CrossRef]
- Li, G.; Li, S.; Wen, Y.; Yang, J.; Wang, P.; Wang, H.; Cui, Y.; Wu, W.; Li, L.; Liu, Z. Correlation between the characteristic flavour and microbial community of Xuanwei ham after ripening. Fermentation 2024, 10, 392. [Google Scholar] [CrossRef]
- Beigbabaei, A.; Dara, A.; Borji, N.; Beigbabaei, K. Surveying the hexanal levels in chips as an indicator of lipid oxidation through “microextraction from the upper space in a microdrop” via gas chromatography. J. Food Meas. Charact. 2025, 19, 4506–4515. [Google Scholar] [CrossRef]
- Toldrá, F.; Flores, M. The role of muscle proteases and lipases in flavor development during the processing of dry-cured ham. Critic. Rev. Food Sci. 1998, 38, 331–352. [Google Scholar] [CrossRef] [PubMed]
- Deniz, E.; Mora, L.; Aristoy, M.C.; Candoğan, K.; Toldrá, F. Free amino acids and bioactive peptides profile of pastırma during its processing. Food Res. Int. 2016, 89, 194–201. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).