Abstract
Critical-sized bone defects (CSBDs) are injuries that exceed the body’s natural capacity for repair and require external intervention. These defects are particularly challenging in the mandible, often resulting from trauma, tumor resection, or implant-related complications. Effective treatment involves scaffold designs that support vascularization, bone formation, and sufficient mechanical strength. This systematic review aims to assess whether ceramic-based scaffold properties, including porosity, pore size, and macroscopic characteristics, improve vascularization, bone formation, and the mechanical properties in the treatment of CSBDs in large animal models. A search of databases (PubMed, Embase, and Web of Science) identified 11 in vivo studies involving CSBDs (>2 cm), ceramic scaffolds, and histological analysis. Findings indicate that scaffolds with porosity exceeding 50% yield optimal outcomes by striking a balance between cell infiltration and mechanical stability. Pore sizes ranging from 300 μm to 700 μm are ideal for vascularization and bone ingrowth. Three-dimensional (3D) printing shows promise in creating scaffolds with precise and reproducible features. However, the studies varied significantly in their methodologies and outcomes, with no consensus on the optimal scaffold properties for mandibular CSBDs. Scaffold porosity and pore size play key roles in promoting vascularization and bone regeneration. Various animal models reinforce this finding, suggesting that scaffold architecture is crucial for biological integration and functional outcomes. This review highlights the importance of standardized research protocols and clear design criteria in enhancing the success of bone regeneration. Future research should investigate emerging biomaterials and new scaffold technologies to overcome current limitations in clinical applications.
1. Introduction
The reconstruction of large bone defects in the jaws is one of the most complex challenges in Oral and Maxillofacial Surgery (OMS) []. These defects are caused by metabolic diseases, chronic inflammatory conditions, high-energy traumas, congenital anomalies, and oncologic surgeries, among other causes, and require sophisticated approaches to restore both the form and function of the maxillary bones [,]. Bone is a complex tissue, highly vascularized, with an organized and multifunctional structure that must support loads and mechanical stress. It comprises a mineralized matrix and specialized cells in blood cell production and mineral storage. Therefore, reproducing this structure on both macro- and microscales and ensuring that the newly regenerated bone tissue can develop an adequate vascular network is challenging [,,,].
Critical-sized bone defects (CSBDs) are defined as bone injuries that exceed the intrinsic capacity for spontaneous regeneration and therefore require surgical intervention for healing []. In OMS, the reconstruction of critical mandibular defects presents unique challenges due to the mandible’s complex anatomy, high mechanical demands, and essential role in aesthetics and function [,]. However, defining what constitutes a CSBD in preclinical research is highly variable, influenced by species-specific, anatomical, and defect-related factors []. In large animal models commonly used for translational research—including pigs, sheep, dogs, and non-human primates—mandibular CSBDs are typically defined as defects exceeding 2–3 cm in length or corresponding to volumes greater than 800–3000 mm3, depending on the species []. Segmental defects that involve a loss of periosteum are particularly critical, as the absence of this vascularized membrane impairs osteogenic potential and complicates spontaneous healing [,]. This variability highlights the need for standardized criteria that integrate defect size, geometry, continuity, and the biological environment. Despite continuous advancements in bone tissue engineering, the lack of a universally accepted definition of CSBDs complicates the comparability of preclinical studies and hinders their translation into clinical practice. Current definitions often rely on simple dimensional thresholds, insufficiently accounting for the complex biological and biomechanical factors that determine regenerative outcomes [,,]. Therefore, rigorous preclinical models that accurately simulate the regenerative challenges of the human mandible are essential to evaluate scaffold design, optimize pore architectures, and develop clinically relevant solutions. This systematic review adopts the definition of CSBDs as segmental mandibular defects exceeding 2 cm in large animal models and investigates how ceramic-based scaffold microarchitectures—specifically porosity, pore size, and macroscopic characteristics—influence vascularization, bone formation, and mechanical strength in the treatment of these defects [,,].
Bone regeneration in CSBDs is often limited by factors such as defect size, inadequate vascularization, and the absence of a repair-friendly environment. In this context, ceramic-based scaffolds, such as hydroxyapatite (HA), tricalcium phosphate (TCP), and bioactive glass, emerge as promising solutions. These materials serve as three-dimensional matrices that mimic the natural properties of bone, promoting osteoconduction and supporting bone regeneration. However, significant challenges remain, such as improving the integration between the scaffold and native bone, optimizing porosity to support vascularization, and adapting mechanical properties to meet biomechanical demands [,,].
Ceramic-based synthetic bone scaffolds (e.g., HA, TCP [], bioactive glasses, beta-tricalcium phosphate [β-TCP], bioactive silicates, and their derivatives [,]) are designed with porous architectures featuring interconnected macro- and microstructures that mimic the mechanical and structural properties of natural bone [,]. This design ensures robust mechanical support during healing, particularly in load-bearing or stress-prone areas. Additionally, these scaffolds can support cell proliferation, migration, and differentiation. Some even act as vehicles for the controlled release of growth factors, further promoting bone regeneration [,,,,,,,,].
These scaffolds are highly suitable for maxillofacial bone regeneration due to their exceptional biocompatibility, bioactivity, and structural similarity to natural bone. They mimic the mineral phase of bone, promoting osteoconduction and integration with surrounding tissues. Their porous architecture enables vascularization and cellular infiltration, which are essential for new bone formation and nutrient exchange. Furthermore, ceramic scaffolds can be engineered to degrade at a controlled rate, to align with the body’s natural bone healing process [,].
Despite lacking osteoinductive and osteogenic properties, the wide variety of ceramic materials with osteoconductive properties offers great potential to advance bone regeneration [,,]. The choice of specific ceramic material depends on the desired properties, such as degradation rate, cell adhesion, and mechanical requirements [,,,].
The advent of 3D printing and other additive manufacturing (AM) techniques has revolutionized the creation of customized scaffolds tailored to the specific needs of each application. This is particularly relevant in OMS, where anatomical complexity demands personalized solutions that also address important aesthetic requirements, such as reconstruction after oncologic resection. Three-dimensional printing enables the fabrication of scaffolds with complex, controlled geometries, allowing for adjustments to parameters such as porosity and spacing between printing lines [].
Despite advances, no ideal scaffold material has been identified, and research continues to focus on optimizing the integration between the scaffold and native bone [,]. Animal models, especially large animals, are essential for preclinical evaluation, as they serve as a significant analog to the human body, thereby facilitating translational research. These models enable the assessment of critical scaffold properties, including pore size and interconnectivity, as well as mechanical strength, and facilitate the analysis of osseointegration processes [].
Although many studies have investigated ceramic-based scaffolds for bone regeneration, there is no consensus on the optimal combination of porosity, pore size, and macroscopic design to achieve effective vascularization, bone formation, and mechanical performance in CSBDs. The variability in study designs, animal models, and outcome measures further complicates comparisons and limits clinical translation. Therefore, this systematic review aims to assess whether ceramic-based scaffold properties (e.g., porosity, pore size, and macroscopic characteristics) enhance vascularization, bone formation, and mechanical strength in the treatment of CSBDs in large animal models.
2. Materials and Methods
2.1. Protocol
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) []. The review was not registered in PROSPERO.
2.2. Focused Question
This systematic review was performed to answer the question: How does ceramic-based scaffold microarchitecture impact maxillofacial bone regeneration?
2.3. Search Strategy (Supplementary Materials)
A search was conducted for scientific articles published in the databases of the US National Library of Medicine (PubMed), Web of Science, and Scopus, from which articles (limited to English) were collected without restriction on the publication year. The following terms were used to conduct a comprehensive search across databases: ((mandible OR maxilla OR jaw OR “craniofacial bone”)) AND (“large bone defect” OR “critical-sized bone defect” OR “critical size defect” OR “segmental bone defect” OR CSBD OR “segmental defect”))) AND (bioengineering OR “bone tissue engineering” OR “bone regeneration” OR bone reconstruction OR “guided bone regeneration” OR GBR OR biomaterials OR “bone graft” OR grafts)) AND ((scaffold* OR ceramic* OR “calcium phosphate” OR hydroxyapatite OR bioceramic* OR “3D printing” OR “3D-printed” OR “additive manufacturing” OR “layer-by-layer fabrication”)) AND ((“large animal model” OR “preclinical animal model” OR sheep OR goat OR porcine OR pig OR swine OR minipig OR dog OR canine OR primate* OR chimpanzee OR macaque* OR baboon OR cow OR bovine OR horse OR equine)). The last search in all databases was conducted in September 2024.
2.4. Eligibility Criteria
The PICO strategy used for this study is described below:
(P) Population: large animals (all ages and all sexes) with CSBDs;
(I) Intervention: jaw reconstruction using ceramic bone scaffolds;
(C) Comparison: not applicable;
(O) Outcome: primary—histological evaluation of vascularization and bone healing;
Secondary—clinical evaluation and biomechanical strength.
Included in this systematic review were the following: (a) papers published and indexed in the mentioned research platforms, including in vivo large animal models (swine, sheep, canine, primate) with CSBDs (>2 cm), and (b) treatment with ceramic scaffolds (at least one group of the study), (c) with at least a histological analysis of the changes occurring after the intervention.
Studies conducted in small animal models (rodents and rabbits), or with a bone defect (<2 cm) or treated with another type of scaffolds (non-ceramic), or descriptive studies, in vitro studies, review articles, clinical trials (case reports, case series, controlled trials, human studies, randomized controlled clinical trials), or unavailable full texts were excluded.
2.5. Study Selection
The Rayyan platform (available at www.rayyan.ai, accessed on 15 August 2024) was used to screen and select studies. Initially, crosschecking eliminated all duplicates, and two reviewers (A.M.P.B. and F.R.S.M.) selected the included studies through a two-step reviewing protocol. Both reviewers independently screened records for inclusion and exclusion, and they were blinded to each other’s decisions. Disagreements were resolved by a third reviewer (Y.M.S.).
2.6. Data Extraction
The following information was recorded: author(s), year of publication, animal characteristics (animal model, strain/breed, age, gender, weight), type and size of bone defect, experimental groups, time points of the study (weeks), general characteristics of the scaffolds (methods of fabrication and composition), geometrical parameters of the scaffolds (size, porosity, pore size, macroscopy), imaging analysis, histology, histomorphometry, and biomechanical testing. Prior to full data extraction, the reviewers independently piloted the extraction form on a random subset of studies to ensure consistency in interpretation and data capture. Discrepancies were discussed and resolved by consensus, and minor adjustments were made to the form accordingly. This calibration step aimed to enhance the reliability of the data and reduce the risk of bias.
The results of individual studies are presented in tables summarizing key parameters for each study.
2.7. Quality and Risk-of-Bias Assessments
All studies were reviewed for adherence to the Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines for quality assessment []. A grading system was employed to evaluate the 20 items included in the ARRIVE checklist, based on the criteria established by Schwarz et al. (2012) [] and Monteiro et al. (2021) []. Additionally, the risk of bias in the included studies was assessed using the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) tool, which provides a risk-of-bias assessment for animal studies [].
2.8. Effect Measures
For each outcome, appropriate effect measures were considered. Histological and imaging outcomes, such as bone regeneration and vascularization, were presented as qualitative assessments or, when available, as quantitative values (e.g., bone volume and area of new bone), using mean and standard deviation. Biomechanical outcomes were reported in terms of compressive or tensile strength, with mean differences used when data allowed comparison. Clinical observations were narratively described due to their qualitative nature.
3. Results
3.1. Article Selection
A flow diagram, according to the PRISMA 2020 guidelines, is illustrated in Figure 1. A total of 1996 articles were initially identified using the search strategy, comprising 1069 in PubMed, 858 in Web of Science, and 69 in Embase. After 485 duplicates were removed and 415 records were identified as ineligible by automatic tools, 1096 records were screened by title and abstract, resulting in the selection of 19 articles. The full text of these articles was assessed, and eight of these articles were excluded for at least one of the following reasons: bone defect <2 cm (n = 4), not a large animal model (n = 2), no treatment of a bone defect (n = 1), and histological analysis was not performed (n = 1). In total, 11 studies were included in the final qualitative synthesis.
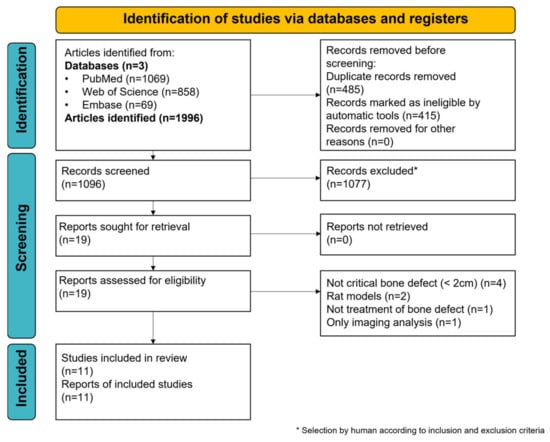
Figure 1.
PRISMA flow diagram. Search strategy and selection process of the included studies. * selection by a human according to inclusion and exclusion criteria.
3.2. Animal Model’s Characteristics
The animal models used in the studies included in this review varied among canine (37%), porcine (27%), sheep (18%), and primate (18%) (Figure 2).
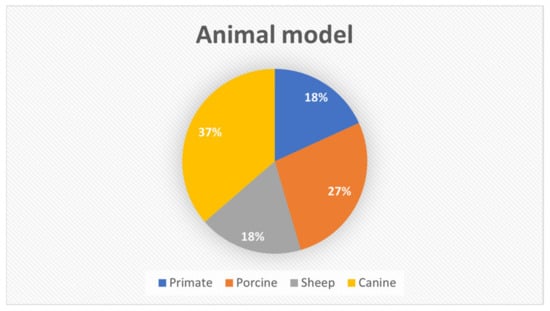
Figure 2.
Distribution of animal models used in the studies included in this review.
The age of the animals was reported in eight studies. Cao et al. (2021) [] used primates aged between 6 and 9 years. Johnson et al. (2021) [], Henkel et al. (2006) [], Wu et al. (2006) [], and Zhang et al. (2019) [] used animals that were 1 year of age or younger. Zeng et al. (2017) [] used 2- and 3-year-old sheep, while Paré et al. (2022) [] used 4- to 7-year-old sheep. Yao et al. (2009) [] and Bachtiar et al. (2016) [] did not report the age of the animals but specified skeletal-mature and young adult animals, respectively. Yuan et al. (2007) [] reported canines at 1 year and 6 months of age. Only six studies mentioned the gender of the animals (n = 4 males [,,,] and n = 2 females [,]).
The creation of CSBDs and the implantation of ceramic scaffolds were performed in the mandibular region in nine [,,,,,,,,] out of the eleven studies (82%), located in either the anterior or posterior mandibular region.
Yao et al. (2009) [] and Zhang et al. (2019) [] created bone defects in the bilateral posterior mandibular region, while Johnson et al. (2021) [] and Zeng et al. (2017) [] utilized the calvaria region (18%). The mean follow-up period was 20 weeks in the studies using the mandible region to create the critical bone defect and 24 weeks in the calvaria region. The animal model and defect characteristics are presented in Table 1.

Table 1.
Animal model’s characteristics.
3.3. Outcomes Analysis
The outcomes were evaluated using histology, imaging, and biomechanical testing (Table 2). Only Bachtiar et al. (2016) [] and Henkel et al. (2006) [] did not perform imaging analysis. Cao et al. (2021) [], Johnson et al. (2021) [], Paré et al. (2022) [], and Zeng et al. (2017) [] utilized Micro-Computed Tomography (Micro-CT). Additionally, Cao et al. (2021) [] and Paré et al. (2022) [] performed PET-CT (Positron Emission Tomography) imaging post-implantation. After removing the fixation plate, a computed tomography (CT) scan was also performed. Three studies [,,] utilized only CT scans as the analysis tool. Yao et al. (2009) [] performed imaging analysis using Technetium 99m-methyl diphosphonate (Jiangsu Institute of Nuclear Medicine, Wuxi, China) single-photon emission computed tomography (99mTc-MDP SPECT/CT) (Siemens Healthineers, Erlangen, Germany) after 8 h and 8 weeks of implantation in muscle and mandible. Only Zhang et al. (2019) [] used radiographs for imaging.

Table 2.
Outcome parameters and main findings.
Qualitative histological analysis was performed in all included studies, utilizing various staining techniques to examine newly formed bone and osteoblastic cells. Hematoxylin and eosin (H&E) staining was employed in five studies [,,,,]. Henkel et al. (2006) [] performed histological analysis using Giemsa–toluidine, H&E, or the Goldner technique for staining; Zeng et al. (2017) [] used 1% methylene blue and 0.3% basic fuchsine; Paré et al. (2022) [] used hematoxylin and eosin saffron (HES) and Movat pentachrome to analyze newly formed bone and osteoblastic cells. Zhang et al. (2019) [] performed the staining using toluidine blue dye, while Wu et al. (2006) [] and Yao et al. (2009) [] also used Masson’s trichrome in addition to the H&E staining.
Regarding biomechanical testing, only five studies performed biomechanical tests [,,,,] using the scaffolds. Cao et al. (2021) [] performed uniaxial compressive strength testing, Johnson et al. (2021) [] performed compressive and mechanical strength testing, Yuan et al. (2007) [] performed biomechanical testing through a bending system, and Yao et al. (2009) [] also performed compressive and bending testing using an electric universal testing machine (AG-10 TA Type; Shimadzu, Japan). Zhang et al. (2019) [] exposed the implants to a pullout test measuring the maximum force in Newtons (N) (Table 3).

Table 3.
Geometrical parameters.
3.4. Scaffold Fabrication
All included studies described the techniques used for fabricating ceramic scaffolds. The methods for fabricating ceramic scaffolds can vary depending on the desired characteristics of the final product and the specific properties of the ceramic material in question [,]. Seven of the eleven included studies employed a 3D printing method to fabricate ceramic scaffolds [,,,,,,], ensuring controlled porosity and the standardization of the printing process. Cao et al. (2021) [] employed a Robotic Deposition process (where a paste or gel is extruded through a robotically controlled nozzle to build a structure layer by layer) to fabricate the TCP scaffolds. For the poly(lactic-co-glycolic acid (PLGA)/TCP scaffolds, the LTRP (Low-Temperature Rapid Prototyping) was used. This technique prints the mixture at low temperatures, followed by freeze-drying to remove the solvent []. Johnson et al. (2021) [] opted for computer-aided design (CAD) 3D printing, which combines computer-aided modeling and design techniques with AM to create customized scaffolds (HA/TCP combined with DPNCC/BMA) that meet the specific needs of the bone defect and biological requirements []. Paré et al. (2022) [] employed the drop-on-demand 3D printing method to fabricate βCP scaffolds, where molds were designed and constructed layer by layer using a 3D printer with drop-on-demand deposition. Once printed, the molds were impregnated with biphasic calcium phosphate (BCP) ceramic slurry and sintered to consolidate the ceramic structure []. The Polycaprolactone (PCL)/β-TCP scaffolds utilized by Nokhbatolfoghahaei et al. (2022) [] were fabricated using the Fused Deposition Modeling (FDM) 3D printing method. On the other hand, the TCP scaffolds were manufactured using the foam-casting method, a traditional technique that uses foam as a support to create a porous structure [].
Zhang et al. (2019) [] and Yao et al. (2009) [] utilized scaffolds manufactured via the foaming method using hydrogen peroxide (H2O2) and high-temperature sintering. Henkel et al. (2006) [] tested materials manufactured by a sol–gel process to fabricate biomaterials with nanoporous structures and controlled porosity. Chemical [] and mechanical [,] methods were also employed to produce the ceramic scaffolds.
Calcium phosphates were the most widely used ceramics, with β-TCP (31%) and BCP (23%) being the most prevalent. Figure 3 illustrates the distribution of the ceramic materials used for scaffold fabrication.
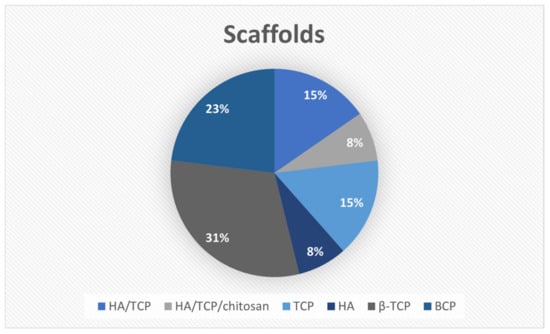
Figure 3.
Distribution of the ceramic materials used for scaffold fabrication.
3.5. Scaffold’s Geometrical Parameters and Main Findings
3.5.1. Scaffold Size
Except for three papers [,,], all other reviewed papers reported the size of the ceramic scaffolds. Table 3 shows the geometrical parameters of the ceramic scaffolds.
Comparing the scaffold size and the primary outcome, some studies observed a relationship, while others did not [,]. Cao et al. (2021) [] used ceramic scaffolds measuring 5 mm × 5 mm × 5 mm, yielding specific results, including the formation of soft calluses and low bone density at the periphery of the poly(lactic-co-glycolic acid)/β-tricalcium phosphate–bone morphogenetic protein (PLGA/TCP-BMP) structures. On the other hand, scaffolds with a diameter of 3 cm yielded different results, forming bone tissue similar to that of native bone.
Therefore, Cao et al. (2021) [] also concluded that smaller scaffolds (e.g., 5 mm long × 5 mm high × 5 mm thickness) tend to show specific results in small areas, such as forming irregular bone layers. In comparison, larger scaffolds (e.g., 54.80 mm × 34.4 mm × 11.58 mm) demonstrated long-term results, including complete bone maturation and extensive integration, in a study conducted by Paré et al. (2022) [].
3.5.2. Porosity
In this review, it was possible to observe that, except for the study of Paré et al. (2022) [], which used BCP scaffolds with a porosity of 40%, all other studies opted to use scaffolds with more than 50%, with Henkel et al. (2006) [] utilizing scaffolds with the highest porosity (60–80%) (CaP).
According to Henkel et al. (2006) [], a CaP matrix with high porosity can be beneficial for osteoconduction and bone integration, as it facilitates cell migration and bone growth. However, balancing porosity with the material’s mechanical strength and structural integrity is crucial. The sintering of CaP ceramics at high temperatures can negatively affect the porous structure by destroying pores of different sizes and reducing connectivity between them []. This reduces the material’s osteoconductive effectiveness, as partially connected pores hinder cell migration. Henkel et al. (2006) [] observed that when exposed to a maximum temperature of 700 °C, CaP ceramics largely maintain their natural crystal structure and degree of porosity, resulting in better biological properties. However, high sintering temperatures can make the production of CaP ceramics more challenging, compromising the structural integrity necessary for adequate performance []. Three of the eleven studies [,,] did not mention the scaffolds’ porosity.
3.5.3. Pore Size
Of all the included studies, only one [] did not report scaffold pore sizes. Some studies [,] show varied results with different pore sizes, while others [] indicate that smaller pores may lead to better bone formation. Cao et al. (2021) [] observed that scaffolds with pores of 345 ± 10 μm and 365 ± 30 μm had different results under SEM (Scanning Electron Microscopy). The scaffolds with larger pores (365 ± 30 μm) (PLGA/TCP) showed irregular bone formation on the surface. In comparison, the scaffolds with smaller pores (345 ± 10 μm) (TCP) primarily presented fibrous tissue and infiltrated blood vessels. Although the PLGA/TCP scaffolds (365 ± 30 μm) also exhibited a structurally similar porous structure, the surface of the TCP scaffolds was more regular and smoother compared to the TCP scaffolds. In the Micro-CT analysis, the pore sizes were measured as 358 ± 39 μm (PLGA/TCP) and 335 ± 11 μm (TCP) [].
Zeng et al. (2017) [] recognized that scaffolds with pore sizes in the range of 200–435 μm yielded similar results, with minimal new bone formation, and vascularization was not directly mentioned in this specific study. Nokhbatolfoghahaei et al. (2022) [] used scaffolds with pores of 500 μm (PCL/β-TCP) and 191.9 ± 74.6 μm (βTCP). They concluded that they had significantly higher bone formation rates than the parallel groups of supports with larger pores (500 μm).
In a study by Zhang et al. (2019) [], implants with intermediate pore sizes (300–400 μm) exhibited greater bone formation compared to those with smaller pores. Additionally, angiogenesis-related genes were positively regulated in implants with intermediate pores, suggesting a relationship between pore size and vascularization. It was also observed that pore sizes between 200 and 435 μm [] demonstrate efficient scaffold degradation and the formation of new bone. According to Yuan et al. (2007) [], scaffolds with high porosity and large pore sizes (450 ± 50 μm) facilitate better nutrient exchange and enhanced cell penetration. However, this structure also results in a decrease in the mechanical properties of the scaffold, which can lead to fragility. In this study, the authors observed a β-TCP scaffold with large pores (450 ± 50 μm) and no seeded cells fractured during surgery due to fixation with a titanium plate. In contrast, all constructs seeded with BMSCs maintained their shape without fractures []. None of the studies included in this review mentioned the pore shape.
3.5.4. Scaffold’s Macroscopy
Based on the different scaffold formats and their respective primary outcomes reported by the authors, scaffold morphology plays a crucial role in bone regeneration. For instance, in the study by Cao et al. (2021) [], 3D-printed rectangular scaffolds showed callus formation and low bone density at the periphery. In contrast, Johnson et al. (2021) [] demonstrated that circular and conical scaffolds support regeneration with trabecular structures similar to those of native bone. Henkel et al. (2006) [] showed that granular scaffolds facilitated complete ossification with a regular spongy structure. Zeng et al. (2017) [] compared TCP and poly(trimethylene carbonate) (PTMC)-CaP disc-shaped scaffolds, with TCP scaffolds demonstrating an increased osteoconductive effect. Additionally, cubic-shaped scaffolds, such as those used by Yuan et al. (2007) [] and Wu et al. (2006) [], provided a uniform distribution of mechanical stress and structural support, favoring the formation of irregular osteons and the efficient repair of bone continuity in the jaw. These observations highlight the significant impact of scaffold morphology on bone regeneration, as it accommodates the specific needs of the bone defect, thereby facilitating cellular integration and vascularization for optimal long-term outcomes.
Only Bachtiar et al. (2016) [] did not mention the macroscopic characteristics of the scaffolds.
3.5.5. Pore Shape
Although pore size was extensively reported in the included studies, none of them directly evaluated the pore shape or its influence on biological outcomes. Most studies described only the macroscopic shape of the scaffold (Table 3), without detailing the three-dimensional morphology of the internal pores. This lack of information represents a significant limitation, as the pore shape can greatly influence fluid flow patterns, cell migration, and vascular formation within the scaffold []. The literature suggests that regular and interconnected pore geometries support bone regeneration by promoting better perfusion and cell distribution [], but this relationship was not investigated in the reviewed studies. Therefore, future research should include the morphological characterization of pore structures and explore their correlation with biological parameters to optimize scaffold design for bone reconstruction.
3.5.6. Interconnectivity
Scaffold interconnectivity was addressed variably across the studies included in this review. This parameter is critical for successful bone regeneration, as it directly influences nutrient diffusion, vascularization, and cell migration [,,]. Quantitative data on interconnectivity were reported only by Nokhbatolfoghahaei et al. 2022 [], who described 100% interconnectivity in PCL/βTCP scaffolds (50% porosity) and βTCP scaffolds (70.6% porosity). These findings suggest an open and interconnected pore network, facilitating the efficient transport of fluids and cells.
Although not all studies provided quantitative measurements, some employed 3D printing techniques that favor the construction of interconnected porous architectures, as seen in Cao et al. (2021) [] and Johnson et al. (2021) []. Additionally, the presence of macropores ranging from 0.1 to 1 mm, reported by Henkel et al. 2006 [], suggests a high interconnectivity potential, despite the lack of precise numerical data. The variability in methodology and the lack of standardization in reporting interconnectivity highlight the need for consistent evaluation criteria in future studies.
3.5.7. Biomechanical Properties
Five studies [,,,,] conducted biomechanical tests on the scaffolds, all evaluating compressive strength (Table 3 and Table 4). Other parameters, such as toughness and energy absorption, were assessed only by Yao et al. (2009) [] and Zhang et al. (2019) [], respectively. None of the studies measured stiffness (Table 4). The methodology used by each author, along with the main findings, is presented in Table 5.

Table 4.
Biomechanical testing parameters.

Table 5.
Methods, systems, and main findings of the biomechanical tests.
Cao et al. (2021) [] observed a significant increase in compressive strength when BMP was added to PLGA/TCP scaffolds, with the TCP-BMP group showing the highest strength (57.6 MPa). Johnson et al. (2021) [] tested the compressive strength of regenerated bone with HA/TCP scaffolds, observing strength similar to native bone.
Yao et al. (2009) [] found a substantial increase in the compressive strength of Ca-P ceramics implanted in living tissues, suggesting significant improvement after biological integration. The Ca-P ceramic showed remarkable improvements due to the integration of organic and inorganic elements, resulting in higher toughness. Yuan et al. (2007) [] found that the BMSCs/b-TCP scaffold also exhibited bending properties similar to native bone, demonstrating its good biomechanical performance for bone regeneration in mandibles. Zhang et al. (2019) [] found that the BCP implant had significantly better mechanical fixation than HA, suggesting better bone integration and long-term stability (Table 5).
Table 6 shows a comparative summary of bone regeneration findings by animal model and defect site.

Table 6.
Comparative summary of bone regeneration findings by animal model and defect site.
3.6. Compliance with the ARRIVE Guidelines
Figure 4 illustrates the compliance with the ARRIVE guidelines for all studies included in the qualitative synthesis. All studies provided clear details about their design, including the groups being compared. None of the studies provided clear information regarding the randomization method used to allocate experimental units, although Paré et al. (2022) [] and Wu et al. (2006) [] offered partial details. Nokhbatolfoghahaei et al. (2022) [] partially described whether and where study data are available, while the remaining studies did not provide any information on this matter. All studies included an adequate scientific background to understand the rationale and context of the research and detailed their experimental approaches.
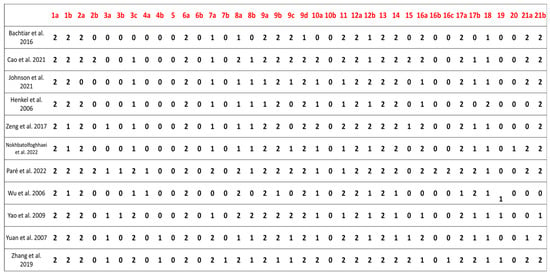
Figure 4.
This figure shows the level of compliance with the ARRIVE guidelines [] among the studies included in the qualitative synthesis. The evaluated elements were: study design, referring to the description of the experimental setup and comparison groups; randomization, regarding whether the method of random allocation was reported; data availability, indicating if the study stated whether and where its data could be accessed; scientific background, assessing the clarity of the rationale and context provided for the research; and experimental approach, referring to the detailed description of the procedures used. Each item was scored on a scale from 0 to 2, where 0 indicates the item was not reported, 1 indicates it was partially reported, and 2 indicates full reporting in accordance with the guidelines [,,].
3.7. SYRCLE Risk-of-Bias Tool
The risk-of-bias assessment [] using the SYRCLE tool is summarized in Figure 5. Overall, the methodological quality of the included studies was moderate to low, with a predominance of unclear or high-risk judgments across several domains. Specifically,
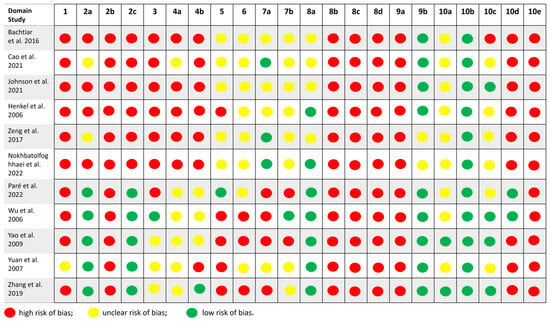
Figure 5.
Assessment of the risk of bias []. The tool evaluates ten domains related to methodological rigor and internal validity, including sequence generation, baseline characteristics, allocation concealment, random housing, blinding of caregivers and investigators, random outcome assessment, blinding of outcome assessors, incomplete outcome data, selective outcome reporting, and other potential biases. Each domain was judged as ““low risk,”” ““high risk,”” or ““unclear risk”” based on the information reported in the articles. The figure summarizes the distribution of risk levels across all studies.
- Two studies (Paré et al. 2022 []; Yao et al. 2009 []) were judged as having a low risk of bias in most domains, indicating relatively robust study designs.
- Three studies (Johnson et al. 2021 [], Zhang et al. 2019 [], Zeng et al. 2017 []) showed a moderate risk of bias, mainly due to unclear reporting on randomization procedures and blinding.
- Six studies (Cao et al. 2021 [], Henkel et al. 2006 [], Wu et al. 2006 [], Zhang et al. 2019 [], Zeng et al. 2017 [], Bachtiar et al. 2016 []) were rated as having a high risk of bias, with multiple domains marked as high or unclear, particularly regarding allocation concealment, random housing, and the blinding of outcome assessors.
Most studies did not clearly report the methods used for random sequence generation, housing conditions, or the handling of incomplete outcome data, which limited the ability to fully assess internal validity.
4. Discussion
Each bone defect has a unique shape and size, making it difficult to achieve a perfect fit with grafts or implants. In large animals, the determination of CSBDs is multifactorial, involving considerations such as species, age, anatomical location, and defect geometry. While no universally accepted definition exists, segmental bone defects exceeding 1–2 cm are frequently regarded as critical in clinical and preclinical research [,,]. Specifically for the mandible, the current literature predominantly describes defects larger than 2–3 cm as critical, as they do not heal independently [,].
CSBDs represent a fundamental challenge in mandibular bone tissue engineering, as they surpass the bone’s natural regenerative capacity and require scaffold-based intervention [,]. However, variability in how CSBDs are defined across preclinical studies complicates direct comparisons and slows clinical translation []. This systematic review defined CSBDs as segmental mandibular defects greater than 2 cm in length. This pragmatic threshold was chosen to strike a balance between scientific rigor and the need to include a representative number of relevant large animal studies. While more differentiated criteria based on species, defect volume, or periosteal status would have offered finer resolution, such stratification would have excessively narrowed the available literature and impeded meaningful synthesis. A 2 cm cutoff ensures that all included studies examined defects unlikely to heal spontaneously under standard conditions, providing a robust foundation for evaluating scaffold performance across different designs and biomaterials [,,].
A significant inconsistency was observed in the reporting of the characteristics of the experimental animals in the included studies. Information such as age, sex, and weight of the animals was described in a heterogeneous manner or, in some cases, omitted entirely. This lack of standardization limits the ability to directly compare results across studies and may substantially impact the interpretation of findings, particularly in the context of bone regeneration, which is highly dependent on the animal’s age and physiological condition.
Furthermore, notable differences were observed in how scaffold porosity and pore size were measured and reported. While some studies utilized Micro-CT, others provided only theoretical values based on design specifications. In several cases, measurement methods were unspecified or unclear. Pore size reporting also varied considerably, with some studies presenting average values, others reporting ranges, and some omitting this data entirely (Table 3). This lack of consistency hinders the direct comparison of scaffold performance and complicates the identification of optimal architectural parameters for bone regeneration.
Across the included studies, defects exceeding 2 cm consistently exhibited impaired spontaneous healing, validating the critical nature of the defects and the necessity for engineered scaffolds. Moreover, scaffold properties—such as a porosity greater than 50% and pore sizes between 300 and 700 μm—proved essential for promoting vascularization, bone formation, and mechanical strength within these large, non-healing defects. These observations emphasize that scaffold design must be tailored to the unique biological and mechanical demands of critical defects, particularly when translating regenerative strategies from preclinical models to human applications [,,].
Current bone reconstruction strategies rely on filling defects with autologous, allogenic, or xenogenic bone grafts, which promote healing through osteoinduction, osteoconduction, and osseointegration. However, autologous grafts are associated with higher donor site morbidity, while allogenic and xenogenic grafts face issues of availability, compatibility, and the risk of rejection or disease transmission. Furthermore, none of these grafts are tailored to the shape and size of the defect, which also hinders regeneration in extensive areas, increases surgical time and, consequently, operational costs [,,,,,,].
Tissue engineering has focused on developing alternative biological substitutes with the structural properties of bone and the necessary mechanical properties to aid in regenerating large bone defects that do not spontaneously heal [,,,]. In this context, AM techniques, such as 3D printing, are considered innovative and promising for producing advanced materials, as they enable the printing of various biomaterials. Additionally, they offer several advantages over conventional techniques, including rapid prototyping, high repeatability, freeform fabrication, and precise fidelity to the shape and size of the defect [,]. These printing technologies have enabled the manufacturing of scaffolds with standardized geometric properties, including size, shape, pore distribution, and porosity, which directly influence vascularization and, consequently, the regeneration process [,].
The clinical success of scaffolds in tissue engineering depends on a complex combination of factors, including the biocompatibility of these materials, vascularization capacity, bone neoformation capacity, porosity and interconnectivity, mechanical stability, controlled degradability, and interaction with the host tissue [,,,]. Therefore, the careful design and manufacturing processes of these scaffolds are crucial for optimizing these factors and promoting effective bone regeneration, as both macro- and microarchitecture influence these properties and the performance of the scaffold [,].
Scaffolds serve as temporary support structures that mimic the extracellular matrix of bones, providing a framework for cells to attach, proliferate, and differentiate. In addition to structural support, scaffolds are designed to be biocompatible, meaning they do not cause an adverse immune response and can integrate well with the surrounding tissue [,].
Ideally, scaffolds degrade as new bone tissue forms, ensuring a gradual replacement by natural bone without requiring removal, while also promoting bone growth (osteoconductivity). Scaffolds can be functionalized with cells, growth factors, drugs, or other bioactive molecules to enhance their regenerative properties and guide tissue growth [,,]. They are available in various forms, including porous structures, fibers, and meshes, each tailored to specific applications in bone regeneration [,].
The selection and development of suitable biomaterials that mimic the properties of native bone and promote regeneration are complex. From a mechanical perspective, matching the characteristics of the scaffold with the tissue to be repaired is crucial to ensure the effectiveness of regeneration, structural stability, and long-term functional integration. Resembling the mechanical properties of the native tissue, such as elasticity modulus and strength, ensures that the scaffold can support physiological loads without excessive deformation or failure. This uniform load distribution minimizes stress points and promotes better integration and tissue regeneration. Additionally, the appropriate mechanical properties can positively influence cellular behavior, promoting the differentiation of stem cells into osteoblasts—the cells responsible for forming new bone tissue [,,,,].
The use of ceramic materials such as CaP, particularly TCP, BCP, and HA, in regenerative processes for critical defects in large animals is extensively documented in the scientific literature [,]. This aligns with the findings of this review, as most studies opted for the use of scaffolds made from these bioceramics. Due to their composition being similar to bone, they exhibit excellent biocompatibility and good osteoconductive and regenerative properties, promoting effective integration [], which justifies their widespread use. In particular, TCP has a controlled degradation rate that can be adjusted to match the rate of bone formation [].
Comparing the different compositions of scaffolds and their relationship with the inflammatory response (secondary outcome), scaffolds composed of CaP in various ratios [] exhibit a low inflammatory response characterized by osteoclasts aiding in material degradation. On the other hand, scaffolds containing β-TCP [] demonstrate a high potential for bone formation, particularly when combined with additional treatments, such as recombinant bone morphogenetic protein (rhBMP2) or mesenchymal stem cells (MSCs), despite potentially triggering a moderate inflammatory response. Some cases [] mention an association between chronic inflammatory reactions and the use of certain materials (e.g., β-TCP []). Henkel et al. (2006) [] observed biodegradation without significant inflammation associated with calcium phosphate (CaP) microparticles. Many reviewed studies [,,,] do not provide explicit details on the inflammatory reaction, instead focusing more on bone formation and the overall biocompatibility of the scaffolds.
The results found in this review show that β-TCP scaffolds were frequently associated with better bone formation outcomes. The addition of cells or growth factors also improved the results. Wu et al. (2006) [] and Yuan et al. (2007) [] utilized β-TCP scaffolds with different sizes and porosities. Both showed good bone regeneration; however, the combination of β-TCP with other materials, such as cells [,], resulted in better bone formation than pure β-TCP, suggesting that the type of cell or the combination with the scaffold may influence bone regeneration. Cao et al. (2021) [] reported that PLGA/TCP scaffolds exhibited callus formation and low peripheral bone density. TCP scaffolds maintained their structure but were more infiltrated with fibrous tissue and blood vessels. This suggests that material combinations can affect the structure and quality of regenerated bone. Johnson et al. (2021) [] reported effective bone regeneration with HA/TCP in combination with different treatments (dental pulp neural crest mesenchymal stem cells (DPNCCs) and bone marrow aspirate (BMA)). Paré et al. (2022) [] and Zhang et al. (2019) [] also utilized BCP, demonstrating its effectiveness in promoting bone formation. Notably, Zhang et al. (2019) [] observed greater mineralization compared to HA.
In addition to the material used in fabrication, the macro- and micro-characteristics of scaffolds significantly influence their behavior. The geometry of the scaffold can influence its integration with the host tissue and the colonization of cells within its structure. Porosity is crucial for vascularization and cellular growth within the scaffold []. Larger pores can facilitate the entry of nutrients and cells, while smaller pores may be more effective in retaining cells []. Structurally, the scaffold needs to be porous, featuring an interconnected network of pores with an appropriate pore size. This is vital for facilitating the transport of essential nutrients to cells, the exchange of nutrients and waste products, and the cellular migration necessary for the bone regeneration process [,,].
The scaffold’s porosity is a crucial factor for promoting cell infiltration, nutrient exchange, and vascularization, which are fundamental to the success of bone tissue engineering. However, excessive porosity can have negative consequences. For example, high porosity compromises the material’s mechanical strength, making the scaffold more fragile and prone to failure, as shown by Yuan et al. (2007) []. This is especially problematic in load-bearing areas, such as the mandible [,,]. There is a consensus that a scaffold porosity of over 50% can promote vascularization []. However, too large or numerous pores can hinder cell adhesion, reducing cell density and limiting tissue regeneration. Vascularization can also be impaired, as excessive porosity makes it more difficult to form stable vascular networks [,,].
Another important point is that scaffolds with high porosity can be challenging to handle during implantation, as they may lose their shape or collapse under their own weight. Additionally, osteoconductivity can be compromised if porosity is not adequately controlled, preventing the adhesion and proliferation of bone cells. Therefore, it is crucial to balance porosity to ensure the scaffold’s effectiveness in bone regeneration [].
The data show that scaffolds with high porosity (e.g., 70%) [,,] promote greater bone formation, accompanied by significant vascular invasion and bone regeneration within the pores. Nokhbatolfoghahaei et al. (2022) [] evaluated the masseter muscle as a natural bioreactor for β-TCP scaffolds (porosity = 70.6% ± 1.8%) or PCL/β-TCP scaffolds (porosity = 50%) in terms of bone regeneration. The study also investigated the effects of pedicle preservation and the isolated or combined application of mesenchymal stem cells (MSCs) or rhBMP2 on the scaffolds. After 12 weeks, a histological analysis revealed that all β-TCP scaffold groups (higher porosity) showed significantly higher rates of new bone formation, regardless of being subjected to a pedicled or non-pedicled surgical approach, compared to parallel groups with β-TCP/PCL scaffolds (lower porosity) (p ≤ 0.05).
According to Paré et al. (2022) [], lower porosity (e.g., 40%) allowed for fragmented physiological bone regeneration. Despite the continuous formation of new physiological tissues being observed throughout the volume of the critical bone defect, the authors [] correlated the fragmentation of this tissue with the slow biodegradation of the bioceramic, as approximately 40% of the bone defect volume remained occupied by the ceramic after 12 months. Therefore, this suggests that porosity is not the sole factor influencing regenerative capacity, and that other factors, such as material composition, also impact the results [,].
Zeng et al. (2017) [] also reported that scaffolds with different levels of porosity (PTMC-CaP vs. β-TCP) affected the amount of new bone formed. Due to the 70% porosity in the PTMC-CaP scaffolds, the concentration of CaP particles in the matrices is reduced to only 9%, which is insufficient to generate significant bioactivity. This low concentration explains the reduced new bone formation in these scaffolds compared to the β-TCP scaffolds, which have 60% porosity []. Cao et al. (2021) [] demonstrated that scaffolds with higher porosity (TCP) (74,2% ± 2,2%) and larger pores (365 ± 30 μm) maintained better structure, while PLGA/TCP (63,7% ± 4,0%) (345 ± 10 μm) scaffolds exhibited lower peripheral bone density. Furthermore, the compressive strengths of the PLGA/TCP scaffolds were substantially lower (0.7 ± 0.06 MPa) than those of the TCP scaffolds (20.56 ± 1.81 MPa). Henkel et al. (2006) [] demonstrated that the porosity (60% ± 80%) and pore size (0.1–1 mm, granulated) of CaP matrices facilitated good bone integration and appropriate biodegradation.
Zeng et al. (2017) [] observed the most significant bone formation associated with β-TCP scaffolds with pore sizes ranging from 400 to 700 μm, compared to PTMC scaffolds with 200–435 μm pore sizes. This suggests that scaffolds with larger pores may be more effective in promoting bone regeneration. However, determining the optimal pore size for a bone regeneration scaffold requires a more comprehensive analysis, as it depends on various factors, including the type of application, the specific biological environment, and the characteristics of the biomaterial. Based on the reviewed studies and considering that intermediate-sized pores seem to promote favorable biological responses, we infer that pore sizes in the 300–400 μm range are particularly promising [,,,]. Consequently, scaffolds with pore sizes ranging from medium to large (400–700 μm) appear to support more efficient bone formation. Furthermore, Zhang et al. (2019) [] suggest that pores within the 300–400 μm range are associated with the upregulation of genes related to osteogenesis and angiogenesis. However, it is essential to note that the ideal pore size may vary depending on patient-specific factors, the location of the bone defect, and the properties of the biomaterial.
Based on the methodologies and results presented by each author, several correlations and trends can be identified in the biomechanical characteristics of scaffolds used for bone regeneration. Studies show that adding BMP [] and ceramic materials such as HA/TCP [] enhances the mechanical strength of the scaffolds, bringing their properties closer to those of native bone. The interaction of these materials with the biological environment is also crucial for improving mechanical properties, as observed in the studies by Yao et al. (2009) [] and Zhang et al. (2019) []. Their analysis highlights that scaffold strength can be significantly enhanced when implanted in the body. For example, Ca-P ceramics demonstrate a remarkable improvement in strength due to biological integration, whereas BCP exhibits superior mechanical fixation compared to HA, which is crucial for implant stability.
There is a relationship between porosity and mechanical strength, where higher porosities may reduce strength but promote bone regeneration by providing a larger interface area for vascularization and bone growth []. Pore size also appears to be an important factor, with sizes around 300–400 μm being effective for promoting bone regeneration without significantly compromising strength [].
The compressive and bending strengths of Ca-P ceramics increased significantly after implantation in muscle in the study by Yao et al. (2009) [], which also utilized scaffolds with 300–400 μm pores, indicating that the in vivo process enhanced the mechanical properties. A porosity of 60% can be considered intermediate, thus providing a good balance between mechanical strength and bone regeneration. The Ca-P ceramic benefited from the integration of both organic and inorganic components, which helped increase its strength [].
Cao et al. (2021) [] used scaffolds with porosities of 63.7% ± 4.0% (PLGA/TCP) and 74.2% ± 2.2% (TCP). The higher porosity observed for TCP (74.2%) may have reduced the mechanical strength, as evidenced by the comparison between TCP and PLGA/TCP scaffolds (63.7%). Adding BMPs may have improved cell adhesion and mineralization, potentially increasing the material’s strength. According to Zhu et al. (2022) [], BMPs have significant chondrogenic, osteogenic, and tendinous activity. The porous structure of the scaffolds not only promotes cell adhesion, proliferation, and differentiation but also allows cells to interact with the extracellular matrix, providing essential mechanical support for tissue growth. This porous structure facilitates rapid cell diffusion and offers a larger area for vascularization and bone growth, ultimately improving implant fixation and promoting bone integration [].
In the study by Johnson et al. (2021) [], the compressive strength of the regenerated bone with the HA/TCP scaffold was equivalent to that of native bone. The large size of the scaffold (30 mm in diameter) may have influenced the compressive strength, providing a robust structure to support compressive forces. This may have contributed to the equivalence in compressive strength with native bone. The pore size of 4 mm is relatively large, which may indicate that the scaffold favors vascularization and bone regeneration but could reduce mechanical strength. However, the scaffold successfully supported bone regeneration, suggesting that other factors, such as the quality of the regenerated bone, were also important [].
Yuan et al. (2007) [] showed that the BMSCs/b-TCP group had bending biomechanical properties similar to normal mandibles, suggesting that the scaffold provided adequate biomechanical support for bone regeneration. The scaffold’s high porosity (70%) may have facilitated bone regeneration, but this could have compromised its mechanical strength. However, the combination with BMSCs may have compensated for this loss of strength, resulting in suitable biomechanical properties. The pore size of 450 ± 750 μm is relatively large, which may have favored cell regeneration but likely reduced the scaffold’s strength compared to other materials with lower porosity. This indicates a trade-off between bone regeneration and mechanical strength. The size and cuboid shape (30 mm × 15 mm × 10 mm) of the BMSCs/b-TCP scaffold suggest that it was designed to support a significant load, which is important for testing bending mechanical properties. A larger and more robust scaffold may better withstand bending forces and stresses than smaller scaffolds, which could explain the results similar to native and autogenous bone in bending tests [,].
Based on the results found by Zhang et al. (2019) [], it can be concluded that a pore size of 300–400 μm is suitable for mechanical fixation and bone regeneration, corroborating the findings of Cao et al. (2021) [] and Yao et al. (2009) []. These correlations indicate that the geometry of the scaffold, including size, porosity, and pore size, has a significant impact on the material’s mechanical properties, especially on bone regeneration and the scaffold’s mechanical strength. However, other factors, such as the presence of BMPs or stem cells, may also modify mechanical outcomes independently of the geometric characteristics.
Macroscopic features that provide a larger surface area of contact (such as granular and disc-shaped) [,] or are adaptable to specific defects tend to favor better integration and bone formation. Additionally, combining macroscopic features with other characteristics, such as material composition and porosity, is crucial in determining the overall effectiveness of scaffolds in bone regeneration. Therefore, when designing scaffolds for specific applications, the consideration of macroscopic features is essential for optimizing bone regeneration outcomes [,,,].
The preclinical studies analyzed in this review demonstrate significant advances in applying ceramic biomaterials for maxillofacial bone regeneration, particularly in critical-sized defects. A key factor influencing the translational relevance of these findings is the choice of animal model. Rodents, especially rats, have proven to be efficient for early-stage mechanistic studies in small defects, such as calvarial models. However, their limited anatomical and biomechanical similarity to humans restricts direct clinical extrapolation. Furthermore, rodents exhibit a markedly accelerated bone healing and remodeling process compared to large animals and humans, which can overestimate the regenerative potential of tested scaffolds. In contrast, larger animals such as canines provide closer anatomical analogs for mandibular reconstruction, allowing for more realistic functional and mechanical evaluations. These models also better replicate the slower bone healing kinetics and immune response seen in humans, offering more clinically relevant insights. Notably, as demonstrated by Paré et al. (2022) [], minipigs emerge as an ideal translational platform due to their bone density, vascularization, and load-bearing characteristics, which closely resemble human physiology. Recognizing these species-specific biological differences is crucial for interpreting preclinical outcomes and optimizing scaffold design for successful clinical translation.
In terms of biomaterials and scaffold design, the incorporation of β-TCP, HA, and BCP with osteoinductive agents, such as rhBMP-2, MSCs, or BMSCs, has significantly enhanced osteogenesis and mechanical properties across various studies [,,,]. Additionally, customized 3D-printed scaffolds [,] enabled better anatomical adaptation and more efficient bone regeneration, thereby reinforcing the potential for personalized reconstruction strategies. These advances are especially relevant for clinical applications in orthodontic and orthognathic contexts, where the integration of tissue engineering techniques can enhance treatment outcomes by supporting skeletal corrections and improving bone healing in complex craniofacial procedures [].
Potential clinical applications are further strengthened by findings indicating that bone substitutes can achieve biomechanical performance comparable to native bone, as observed by Yuan et al. (2007) [] and Johnson et al. (2021) []. This suggests that such materials could represent viable alternatives to autologous grafts, thereby minimizing donor site morbidity. Furthermore, innovative strategies, such as pre-vascularization or pre-implantation in muscle tissue, demonstrated by Yao et al. (2009) [], highlight promising approaches for addressing complex clinical scenarios that require functional bone regeneration.
Nevertheless, despite these encouraging results, several challenges remain. Variability in scaffold compositions, animal models, defect types, and evaluation methodologies across studies underscores the need for greater standardization. Moreover, longer-term studies assessing scaffold degradation and bone remodeling are crucial for fully validating the translational potential of these biomaterials.
Research focused on identifying the optimal biomaterial and its ideal characteristics has revealed a significant gap in the standardization of geometric parameters. There needs to be a consensus on the ideal dimensions and shapes of the biomaterials used, which complicates the comparison between studies and the reproducibility of results. Studies must rigorously address these characteristics to ensure consistency and achieve an ideal standard. Although conventional techniques often do not precisely control these characteristics, advancements in manufacturing methods offer a unique opportunity to standardize scaffold designs, allowing for the more precise control of essential properties and bringing us closer to more effective and reproducible solutions.
Limitations
This study has some limitations. Although ceramic scaffolds have shown promising results in preclinical models, it is essential to consider certain limitations when interpreting these findings. Some studies [,] have reported improvements in bone formation and mechanical strength. However, the wide variation in material compositions (e.g., β-TCP, BCP, HA composites), pore architectures, and biofunctionalization strategies hampers direct comparisons between studies. Additionally, factors such as defect size, anatomical location, and the choice of animal model, ranging from rodents to larger species like canines and minipigs, introduce biological variability that may not accurately reflect human bone regeneration processes.
Another critical issue is the generally short duration of follow-up in most experiments. Studies by Wu et al. (2006) [] and Yuan et al. (2007) [] focused on relatively short-term observation periods, which limited their ability to assess slower processes, such as scaffold degradation and long-term bone remodeling. Furthermore, the lack of standardized mechanical testing protocols hampers the establishment of clear comparative benchmarks for scaffold performance. Given the small number of included studies and the substantial heterogeneity in study design and outcomes, a formal assessment of publication bias was not feasible; however, the potential for such bias cannot be excluded and should be considered when interpreting the overall findings.
Therefore, despite the clear clinical potential of ceramic scaffolds, there is a need for more standardized studies with uniform evaluation protocols and longer follow-up periods to validate the experimental findings and support their translation into clinical practice.
5. Conclusions
In conclusion, the evidence consistently indicates that scaffold porosity and pore size play key roles in promoting vascularization and bone regeneration. Various animal models reinforce this finding, suggesting that scaffold architecture is crucial for biological integration and functional outcomes. Studies highlight that a porosity above 50% is beneficial for promoting cell infiltration and vascularization. Pore sizes in the range of 300–400 μm appear particularly effective for enhancing osteogenesis while maintaining sufficient mechanical strength. Additionally, larger pore sizes—between 400 and 700 μm—are associated with greater vascular invasion and more mature bone formation, although excessive porosity may compromise scaffold stability. Therefore, scaffolds with 50–70% porosity and pore sizes of 300–700 μm offer a promising balance between degradation, mechanical support, and biological performance.
Despite these advances, the diversity in surgical techniques and evaluation criteria, as well as the scarcity of studies extending beyond 12 months, leaves important questions unanswered regarding long-term material resorption and mechanical stability. Despite the clear clinical potential of ceramic scaffolds, more standardized studies with uniform evaluation protocols and longer follow-up periods are needed to validate experimental findings and support their translation into clinical practice.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15126899/s1. Detailed description of the search strategy. PRISMA 2020 Checklist: The updated PRISMA 2020 checklist used for reporting this systematic review.
Author Contributions
Conceptualization, A.M.P.B., Y.M.S. and F.P.S.G.; methodology, A.M.P.B.; software, Y.M.S. and P.E.; validation, A.M.P.B. and F.P.S.G.; formal analysis, A.M.P.B., Y.M.S., P.E., F.R.S.M., J.L.G.C.M. and A.P.F.B.; investigation, A.M.P.B., Y.M.S., P.E., F.R.S.M. and J.L.G.C.M.; resources, F.P.S.G.; data curation, A.M.P.B., Y.M.S. and P.E.; writing—original draft preparation, A.M.P.B. and Y.M.S.; writing—review and editing, A.M.P.B., Y.M.S., P.E., F.R.S.M., J.L.G.C.M., A.P.F.B. and F.P.S.G.; visualization, F.P.S.G.; supervision, F.P.S.G.; project administration, F.P.S.G.; funding acquisition, F.P.S.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partly supported by the Educational and Research Fund, Department of Oral and Maxillofacial Surgery, Massachusetts General Hospital (Boston, MA, USA).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CSBDs | Critical-sized bone defects |
| 3D | Three-dimensional |
| μm | Micrometer |
| OMS | Oral and Maxillofacial Surgery |
| HA | Hydroxyapatite |
| TCP | Tricalcium Phosphate |
| β-TCP | Beta-Tricalcium Phosphate |
| AM | Additive Manufacturing |
| mm3 | Cubic millimeters |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| ARRIVE | Animal Research: Reporting of In Vivo Experiments |
| SYRCLE | Systematic Review Centre for Laboratory Animal Experimentation |
| Micro-CT | Micro-Computed Tomography |
| PET-CT | Positron Emission Tomography–Computed Tomography |
| CT | Computed Tomography |
| 99mTc-MDP SPECT/CT | Technetium-99m Methyl Diphosphonate Single-Photon Emission Computed Tomography/Computed Tomography |
| H&E | Hematoxylin–Eosin Saffron |
| SPECT | Single-Photon Emission Computed Tomography |
References
- Huang, X.; Lou, Y.; Duan, Y.; Liu, H.; Tian, J.; Shen, Y.; Wei, X. Biomaterial scaffolds in maxillofacial bone tissue engineering: A review of recent advances. Bioact. Mater. 2024, 33, 129–156. [Google Scholar] [CrossRef] [PubMed]
- Pillia, M.; Guda, T.; Appleford, M. Development of Composite Scaffolds for Load-Bearing Segmental Bone Defects. Biomed. Res. Int. 2013, 2013, 458253. [Google Scholar] [CrossRef]
- Dussault, A.; Pitaru, A.A.; Weber, M.H.; Haglund, L.; Rosenzweig, D.H.; Villemure, I. Optimizing Design Parameters of PLA 3D-Printed Scaffolds for Bone Defect Repair. Surgeries 2022, 3, 162–174. [Google Scholar] [CrossRef]
- Liu, W.; Liu, S.; Li, Y.; Zhou, P.; Ma, Q. Biomimetic Design of 3D Printed Tissue-Engineered Bone Constructs. Curr. Nanosci. 2021, 17, 223–240. [Google Scholar] [CrossRef]
- Basyuni, S.; Ferro, A.; Santhanam, V.; Birch, M.; McCaskie, A. Systematic scoping review of mandibular bone tissue engineering. Br. J. Oral. Maxillofac. Surg. 2020, 58, 632–642. [Google Scholar] [CrossRef]
- Bahraminasab, M. Challenges on optimization of 3D-printed bone scaffolds. BioMed. Eng. OnLine 2020, 19, 69. [Google Scholar] [CrossRef]
- Schemitsch, E.H. Size Matters: Defining Critical in Bone Defect Size! J. Orthop. Trauma 2017, 31 (Suppl. 5), S20–S22. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Chen, X.; Xu, Y.; Shi, L.; Zhang, M.; Nie, M.; Liu, X. Significance and considerations of establishing standardized critical values for critical size defects in animal models of bone tissue regeneration. Heliyon 2024, 10, e33768. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dalfino, S.; Savadori, P.; Piazzoni, M.; Connelly, S.T.; Giannì, A.B.; Del Fabbro, M.; Tartaglia, G.M.; Moroni, L. Regeneration of Critical-Sized Mandibular Defects Using 3D-Printed Composite Scaffolds: A Quantitative Evaluation of New Bone Formation in In Vivo Studies. Adv. Healthc. Mater. 2023, 12, e2300128. [Google Scholar] [CrossRef]
- Döbelin, N.; Luginbühl, R.; Bohner, M. Synthetic Calcium Phosphate Ceramics for Treatment of Bone Fractures. Chimia 2010, 64, 723. [Google Scholar] [CrossRef]
- Valtanen, R.S.; Yang, Y.P.; Gurtner, G.C.; Maloney, W.J.; Lowenberg, D.W. Synthetic and Bone Tissue Engineering Graft Substitutes: What Is the Future? Injury 2021, 52 (Suppl. 2), S72–S77. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Ghayor, C.; Siegenthaler, B.; Schuler, F.; Rüegg, J.; De Wild, M.; Weber, F.E. Lattice Microarchitecture for Bone Tissue Engineering from Calcium Phosphate Compared to Titanium. Tissue Eng. Part A 2018, 24, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Busch, A.; Jäger, M.; Mayer, C.; Sowislok, A. Functionalization of Synthetic Bone Substitutes. Int. J. Mol. Sci. 2021, 22, 4412. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.; Yin, S. Compressive Strength of β-TCP Scaffolds Fabricated via Lithography-Based Manufacturing for Bone Tissue Engineering. Ceram. Int. 2022, 48, 15516–15524. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Li, J.; Liu, C.; Lao, C.; Fu, Y.; Liu, C.; Li, Y.; Wang, P.; He, Y. 3D Printing of Ceramics: A Review. J. Eur. Ceram. Soc. 2019, 39, 661–687. [Google Scholar] [CrossRef]
- Zocca, A.; Colombo, P.; Gomes, C.M.; Günster, J. Additive Manufacturing of Ceramics: Issues, Potentialities, and Opportunities. J. Am. Ceram. Soc. 2015, 98, 1983–2001. [Google Scholar] [CrossRef]
- Shuai, C.; Feng, P.; Zhang, L.; Gao, C.; Hu, H.; Peng, S.; Min, A. Correlation between Properties and Microstructure of Laser Sintered Porous β-Tricalcium Phosphate Bone Scaffolds. Sci. Technol. Adv. Mater. 2013, 14, 055002. [Google Scholar] [CrossRef]
- Baino, F.; Novajra, G.; Vitale-Brovarone, C. Bioceramics and Scaffolds: A Winning Combination for Tissue Engineering. Front. Bioeng. Biotechnol. 2015, 3, 202. [Google Scholar] [CrossRef]
- De Carvalho, A.B.G.; Rahimnejad, M.; Oliveira, R.L.M.S.; Sikder, P.; Saavedra, G.S.F.A.; Bhaduri, S.B.; Gawlitta, D.; Malda, J.; Kaigler, D.; Trichês, E.S.; et al. Personalized bioceramic grafts for craniomaxillofacial bone regeneration. Int. J. Oral. Sci. 2024, 16, 62. [Google Scholar] [CrossRef]
- Cancedda, R.; Giannoni, P.; Mastrogiacomo, M.A. Tissue Engineering Approach to Bone Repair in Large Animal Models and in Clinical Practice. Biomaterials 2007, 28, 4240–4250. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Iglhaut, G.; Becker, J. Quality assessment of reporting of animal studies on pathogenesis and treatment of peri-implant mucositis and peri-implantitis. A systematic review using the ARRIVE guidelines. J. Clin. Periodontol. 2012, 39 (Suppl. 12), 63–72. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.L.G.C.; Guastaldi, F.P.; Troulis, M.J.; McCain, J.P.; do Egito Vasconcelos, B.C. Induction, Treatment, and Prevention of Temporomandibular Joint Ankylosis-A Systematic Review of Comparative Animal Studies. J. Oral. Maxillofac. Surg. 2021, 79, 109–132.e6. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Cao, S.; Li, S.; Geng, Y.; Kapat, K.; Liu, S.; Perera, F.H.; Li, Q.; Terheyden, H.; Wu, G.; Che, Y.; et al. Prefabricated 3D-Printed Tissue-Engineered Bone for Mandibular Reconstruction: A Preclinical Translational Study in Primate. ACS Biomater. Sci. Eng. 2021, 7, 5727–5738. [Google Scholar] [CrossRef]
- Johnson, Z.M.; Yuan, Y.; Li, X.; Jashashvili, T.; Jamieson, M.; Urata, M.; Chen, Y.; Chai, Y. Mesenchymal Stem Cells and Three-Dimensional-Osteoconductive Scaffold Regenerate Calvarial Bone in Critical Size Defects in Swine. Stem Cells Transl. Med. 2021, 10, 1170–1183. [Google Scholar] [CrossRef]
- Henkel, K.O.; Gerber, T.; Lenz, S.; Gundlach, K.K.H.; Bienengräber, V. Macroscopical, Histological, and Morphometric Studies of Porous Bone-Replacement Materials in Minipigs 8 Months after Implantation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2006, 102, 606–613. [Google Scholar] [CrossRef]
- Wu, W.; Chen, X.; Mao, T.; Chen, F.; Feng, X. Bone Marrow-Derived Osteoblasts Seeded into Porous Beta-Tricalcium Phosphate to Repair Segmental Defect in Canine’s Mandibula. Ulus. Travma Acil Cerrahi Derg. 2006, 12, 268–276. [Google Scholar]
- Zhang, Z.; Wang, P.; Li, X.; Wang, Y.; Qin, Z.; Zhang, C.; Li, J. Reconstruction of Mandibular Bone Defects Using Biphasic Calcium Phosphate Bone Substitutes with Simultaneous Implant Placement in Mini-Swine: A Pilot In Vivo Study. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 2071–2079. [Google Scholar] [CrossRef]
- Zeng, N.; van Leeuwen, A.C.; Grijpma, D.W.; Bos, R.R.M.; Kuijer, R. Poly(Trimethylene Carbonate)-Based Composite Materials for Reconstruction of Critical-Sized Cranial Bone Defects in Sheep. J. Cranio-Maxillofac. Surg. 2017, 45, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Paré, A.; Charbonnier, B.; Veziers, J.; Vignes, C.; Dutilleul, M.; De Pinieux, G.; Laure, B.; Bossard, A.; Saucet-Zerbib, A.; Touzot-Jourde, G.; et al. Standardized and Axially Vascularized Calcium Phosphate-Based Implants for Segmental Mandibular Defects: A Promising Proof of Concept. Acta Biomater. 2022, 154, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Li, X.; Bao, C.; Fan, H.; Zhang, X.; Chen, Z. A Novel Technique to Reconstruct a Boxlike Bone Defect in the Mandible and Support Dental Implants with In Vivo Tissue-Engineered Bone. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91B, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Bachtiar, E.W.; Amir, L.R.; Suhardi, P.; Abas, B. Scaffold Degradation During Bone Tissue Reconstruction in Macaca Nemestrina Mandible. Interv. Med. Appl. Sci. 2016, 8, 77–81. [Google Scholar] [CrossRef]
- Yuan, J.; Cui, L.; Zhang, W.J.; Liu, W.; Cao, Y. Repair of canine mandibular bone defects with bone marrow stromal cells and porous β-tricalcium phosphate. Biomaterials 2007, 28, 1005–1013. [Google Scholar] [CrossRef]
- Nokhbatolfoghahaei, H.; Bastami, F.; Farzad-Mohajeri, S.; Rezai Rad, M.; Dehghan, M.M.; Bohlouli, M.; Farajpour, H.; Nadjmi, N.; Khojasteh, A. Prefabrication technique by preserving a muscular pedicle from masseter muscle as an in vivo bioreactor for reconstruction of mandibular critical-sized bone defects in canine models. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 1675–1686. [Google Scholar] [CrossRef]
- Sillmann, Y.M.; Eber, P.; Orbeta, E.; Wilde, F.; Gross, A.J.; Guastaldi, F.P.S. Milestones in Mandibular Bone Tissue Engineering: A Systematic Review of Large Animal Models and Critical-Sized Defects. J. Clin. Med. 2025, 14, 2717. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.-K.; Li, L.; Qin, L.; Wang, X.-L.; Lai, Y.-X. Bone defect animal models for testing efficacy of bone substitute biomaterials. J. Orthop. Transl. 2015, 3, 95–104. [Google Scholar] [CrossRef]
- Roohani-Esfahani, S.I.; Dunstan, C.R.; Davies, B.; Pearce, S.; Williams, R.; Zreiqat, H. Repairing a critical-sized bone defect with highly porous modified and unmodified baghdadite scaffolds. Acta Biomater. 2012, 8, 4162–4172. [Google Scholar] [CrossRef]
- Pérez-Sánchez, L.; Ortiz de la O, M.A.; Álvarez-Pérez, M.A.; Llaguno-Munive, M.; Chanes-Cuevas, O.A.; Serrano-Bello, J. Standardization of 3D printing parameters to control the size and shape of pores in polylactic acid scaffolds. MedComm 2024, 3, e74. [Google Scholar] [CrossRef]
- Velasco, M.A.; Lancheros, Y.; Garzón-Alvarado, D.A. Geometric and mechanical properties evaluation of scaffolds for bone tissue applications designing by a reaction-diffusion models and manufactured with a material jetting system. J. Comput. Des. Eng. 2016, 3, 385–397. [Google Scholar] [CrossRef]
- Feng, Y.; Zhu, S.; Mei, D.; Li, J.; Zhang, J.; Yang, S.; Guan, S. Application of 3D printing technology in bone tissue engineering: A review. Curr. Drug Deliv. 2021, 18, 847–861. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhang, L.; Zhou, Z.; Luo, X.; Wang, T.; Zhao, X.; Lu, B.; Chen, F.; Zheng, L. Calcium phosphate-based biomaterials for bone repair. J. Funct. Biomater. 2022, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Pires, L.C.A.; da Silva, R.C.; Poli, P.P.; Ruas Esgalha, F.; Hadad, H.; Palin, L.P.; Piquera Santos, A.F.; Teixiera Colombo, L.; Kawamata de Jesus, L.; Bassi, A.P.F.; et al. Evaluation of osteoconduction of a synthetic hydroxyapatite/β-tricalcium phosphate block fixed in rabbit mandibles. Materials 2020, 13, 4902. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, Y.; Wang, A.; Zhu, Z.; Li, Y.; Zhu, C.; Che, Z.; Mon, T.; Liu, H.; Huang, L. Application of BMP in bone tissue engineering. Front. Bioeng. Biotechnol. 2022, 10, 810880. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Farjaminejad, R.; Farjaminejad, S.; Hasani, M.; Garcia-Godoy, F.; Sayahpour, B.; Marya, A.; Jamilian, A. The Role of Tissue Engineering in Orthodontic and Orthognathic Treatment: A Narrative Review. Oral 2025, 5, 21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).