Abstract
Ground beetles (Coleoptera, Carabidae) are common predators found in agricultural ecosystems. They feed on crop pests and help reduce pest population. Additionally, they are used as bioindicators to determine the impact of human activities on entomofauna and habitat conditions. The aim of this study was to investigate the ground beetles that inhabit chemically protected (CP) and non-chemically protected (NCP) potato crops and to assess the impact of pesticide use on these beneficial insects. This study was conducted in Poland, on potato fields where ground beetles were caught during four-year crop rotation cycles in 2004, 2008, 2012, and 2016. Two fields with potato crops were chosen: one without chemical protection and the other with chemical protection. Soil traps were used to catch insects, resulting in 7095 individuals of Carabidae, belonging to 41 species, caught throughout the study. The abundance and species richness of ground beetles fluctuated depending on the year of the study and the type of crop protection. Results showed that pesticide use in potato crops decreased ground beetle abundance while species richness remained unaffected. Furthermore, the use of chemical plant protection (CP) induced changes in some life traits of the carabids, leading to a decrease in the abundance of hemizoophages and autumn-breeding carabids. The abundance of the other ecological groups of Carabidae was also year-dependent.
1. Introduction
Agriculture is constantly changing and progressing, and a fundamental factor shaping the direction and effectiveness of these changes is biodiversity [1,2]. However, the unquestionable productive success of industrial agriculture (which began in the 18th century), directed solely at economic purposes, has pushed the limits of reproduction and the sustainability of the natural environment [3]. Consequently, the prospect of the growing demand for food due to demographic growth and increasing environmental and climate problems is one of the most critical challenges of the 21st century [4,5,6]. Global anthropopressure is an inevitable consequence of the development of civilization. However, the strength and extent of this challenge can be corrected by making the right decisions, taking into account both economic and environmental welfare aspects [7]. The need to transform conventional agriculture towards sustainable agriculture has become a priority objective of the European Union’s agricultural policy. Restrictive reduction in pesticide use, a strategic element in determining crop yields, necessitates the search for alternative methods in agricultural production [4,5,8]. Among the pro-ecological farming practices that increase crop production potential is crop rotation [9,10]. A planned crop rotation system that varies year to year creates a diverse soil with balanced nutrient cycling and good structure. In addition, a properly implemented crop rotation can eliminate or reduce the appearance of diseases, pests, and weeds without the use of mineral fertilisers and pesticides. This ensures high yields and the resilience of agricultural ecosystems [9].
It is important to carefully select the crops that will follow each other to ensure successful crop rotation. This decision significantly affects the outcome of the rotation [8,11]. The potato is an essential component of global food security [12,13,14,15]. Due to its versatile use, the potato is one of the most important food crops in the world, cultivated in 150 countries (over 20 million hectares) [12,16]. In recent years, the cultivation of potatoes in Europe has become less profitable due to climate change and high storage costs. As a result, the acreage dedicated to potato cultivation has decreased. However, the potato can nevertheless be an important alternative to agricultural landscapes impoverished by cereal monocultures, as its cultivation leaves weed-free soil, rich in nutrients. This makes potato cultivation a good forecrop for following crops and is the basis for a rational crop rotation [15,16,17].
Changes in the natural environment are assessed by analyzing the responses of living organisms [18,19]. A model group used to understand the functioning and direction of ongoing changes in agricultural ecosystems is ground beetles (Coleoptera, Carabidae). These insects are both predators of crop pests and effective bioindicators [20,21,22,23,24].
This study aimed to evaluate the impact of pesticide use on the ground beetle assemblages in potato crops grown under a four-year crop rotation system.
The following hypotheses were tested: (i) ground beetle abundance and species richness are lower in chemically protected fields due to increased habitat disturbance and reduced food availability, and (ii) the use of chemical plant protection reduces the abundance of larger carnivores, which require more prey and are more sensitive to disturbance; macropterous species, which tend to colonize disturbed habitats; and autumn-breeding species, whose larvae are exposed longer to chemically treated soils.
2. Materials and Methods
2.1. Study Area
This study was carried out at the Agricultural Experimental Station in Winna Góra, near Środa Wielkopolska, (52°12′32.0″ N 17°26′16.0″ E), western Poland. The experimental fields consisting of four-year crop rotations (potato, spring barley, yellow lupin, and winter wheat) have been in use since the 1960s. This study comprised a block of control fields where no chemical plant protection preparations were applied and a second block where a plant protection programme was implemented according to conventional or integrated agricultural production guidelines. Crops were grown under a ploughing system. In fields without chemical protection (NCP), mechanical weeding was used instead of herbicides. The same fertilization method was used in both blocks. The surface area of each field was 0.5 ha. The soils in the experiment were similar and belonged to the good wheat complex (class IIIa and IIIb) in the Polish soil taxonomy system [25].
2.2. Data Collection
This study was carried out on potato fields grown in a four-year rotation in 2004, 2008, 2012, and 2016. Two fields with potato crops were selected: without chemical protection (NCP—no chemical protection) and with chemical protection (CP—chemical protection). The studied fields were separated from others by isolation strips sown with phacelia or clover. During the four years chosen for our study, the field under chemical protection was treated with insecticides, herbicides, and fungicides, as specified in Table S1. Ground beetles were collected between May and September using soil traps made from plastic cups, 10 cm in diameter and 15 cm deep, filled with ethylene glycol. These traps were emptied every two weeks. Two transects with ten traps were established in each field. The details of the study are shown in Figure S1.
2.3. Data Analysis
The species composition, abundance, and richness of ground beetles were assessed. Because of the different requirements, the ground beetles were divided into groups based on the following traits: feeding strategy and body size, and the type of breeding and dispersion capability. These life traits are considered the best for describing ground beetle assemblages in field crops. Due to their essential role as the predators of plant pests, the ground beetles were divided in terms of food preference and body size. The following groups were distinguished: phytophages (feeding on plant food), hemizoophages (generalists, feeding on both plants and animals), large carnivores (body length over 12 mm), medium carnivores (5- 12 mm), and small carnivores (body length less than 5 mm). The categorization into large, medium, and small carnivores was performed according to Aleksandrowicz [26], based on the average body length of each species given by Hůrka [27]. Additionally, the ground beetles were classified as autumn breeders, which reproduce in autumn and hibernate as larvae, or spring breeders, which hibernate as adults and reproduce in spring [28]. The presence of ground beetles of different breeding types is also a reflection of field conditions [22]. The ability to disperse, especially in distorted habitats, is another crucial aspect in the study of ground beetles [29]. Using Hůrka’s [27] description, the following groups were distinguished among the ground beetles: macropterous, with fully developed wings; brachypterous, with reduced second pair wings; and dimorphic, whose second pair wings can be developed or reduced.
The Shapiro–Wilk test was used to analyze the distribution of the data. Considering the data distribution, we used a generalized linear model (GLM) with a Poisson distribution to analyze differences in the mean species richness and abundance of assemblages, factoring in plant protection and the study year. A non-metric multidimensional scaling (NMDS) analysis was used to visualize and evaluate the patterns of dissimilarity within the ground beetle assemblages in the years of study in the different types of plant protection based on their species composition. NMDS was calculated in PAST 4.17 software [30] on a Bray–Curtis similarity matrix. The significance of the differences between the analyzed assemblages in the NMDS method was carried out using the ANOSIM non-parametric statistical test [31]. An investigation of correlations between the ground beetles and the following environmental variables: the type of protection (with or without chemical plant protection), chemical treatments applied (herbicides, insecticides, and fungicides), and the years of study, was completed using redundancy analysis (RDA) [32]. The Monte Carlo permutation test was conducted with 499 permutations, where p < 0.05 was considered statistically significant.
The temperature and distribution of rain precipitation in the years of the study were also analyzed. ANOVA analysis of variance did not demonstrate statistically significant differences in the temperature or rainfall between the years examined.
All analyses were carried out using untransformed data. Statistical calculations and their graphic presentation were performed using the Statistica 13.3, PAST 4.17, and Canoco 4.5 software programs.
3. Results
During the four years of study, 7095 ground beetles representing 41 species were caught (Table 1). More specifically, 3127 specimens representing 38 species were captured in the fields with chemical plant protection (CP), while 3968 individuals belonging to 39 species were captured in fields without chemical plant protection (NCP). The species composition of the ground beetles in both field types was similar (Table 1). In the total material collected, Harpalus rufipes (46.4%) and Calathus ambiguus (11.4%) had the highest contribution.

Table 1.
List of the Carabid species and their feeding preferences (Hz—hemizoophages, Sc—small carnivores, Mc—medium carnivores, Lc—large carnivores, Ph—phytophages), breeding types (Ab—autumn breeding, Sb—spring breeding), dispersion capability (Dpt—dimorphics, Mpt—macropterous, Bpt—brachypterous), and total abundance and species richness in the analyzed study fields (CP—chemically protected, NCP—non-chemically protected) in the years of study (2004, 2008, 2012, 2016).
Significant differences between the analyzed study variants in the research years were observed with respect to the abundance of ground beetles (Table 2). A significantly higher number of ground beetles was determined in the NCP fields (Figure 1). Regarding the number of species, no significant differences were observed between fields with different protection variants. The factor indicating the differences in the number of collected species was the year of the study (Table 2, Figure 1).

Table 2.
Generalized linear model (GLM, Wald statistics) for changes in total abundance, species richness, and life trait distribution in relation to the year of study and type of plant protection.
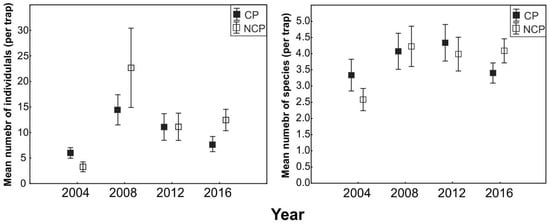
Figure 1.
Mean abundance and species richness of ground beetles (Coleoptera, Carabidae) depending on plant protection type (CP—chemically protected, NCP—non-chemically protected) in years of study.
ANOSIM analysis revealed no significant differences in the ground beetle assemblages in the protected (CP) and unprotected (NCP) fields in only two of the years studied (2012, 2016) (Table 3). The non-metric multidimensional scaling (NMDS) analysis for individual objects also showed differences in the analyzed ground beetle assemblages (ANOSIM R = 0.64; p < 0.001) connected not only with the application of pesticides in the experimental fields but also with the research year (Figure 2). The assemblages of the ground beetles caught in 2004 in chemically protected (CP) and unprotected (NCP) fields differed significantly from those in other years.

Table 3.
R statistics of ANOSIM analysis comparing ground beetle variation between plant protection type (CP—chemically protected, NCP—non-chemically protected) in years of study (2004, 2008, 2012, and 2016), * p < 0.05, ** p < 0.01, *** p < 0.001, ns—not significant; significance after Bonferroni correction.
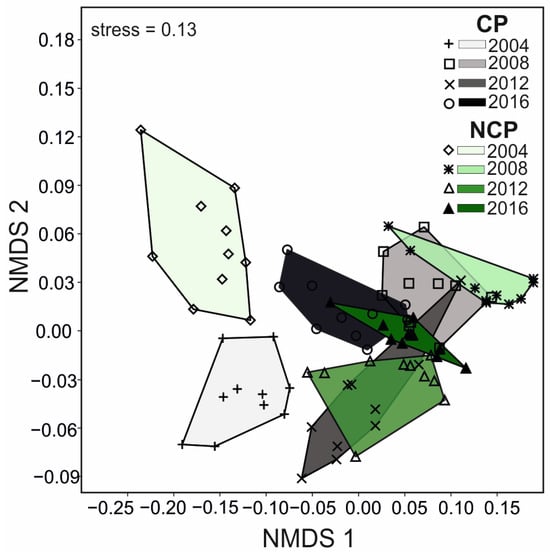
Figure 2.
DA diagram of non-metric multidimensional scaling (NMDS) performed on the Bray–Curtis similarity matrix of ground beetles in the years of study with different types of plant protection (CP—chemically protected in the shades of grey, NCP—non-chemically protected in the shades of green).
The redundancy analysis (RDA) demonstrated relationships between the ground beetle species and environmental variables such as the form of plant protection; the application of insecticides, fungicides, herbicides; and the study year (Figure 3). The first and the second ordination axes described 96.6% of the variation. The first axis (92.1% of the variation) was correlated with the fungicide application. The Monte Carlo permutation test showed that fungicides (F-ratio = 12.14, p = 0.002) and herbicides (F-ratio = 3.67, p = 0.05) had the highest effects on the carabids community. Also, some carabid species demonstrated the strongest correlation with the tested axis: Microlestes minutulus, Carabus cancellatus, Harpalus smaragdinus, Amara bifrons, Clivina fossor, Calathus melanocephalus, Harpalus tardus, and Harpalus rufipes (Figure 3). Fields without chemical protection (NCP) were associated with a large number of ground beetle species, mainly with large and medium carnivores. The application of herbicides and insecticides in chemically protected (CP) fields was correlated with the occurrence of Poecilus cupreus and some species classified as small carnivores.
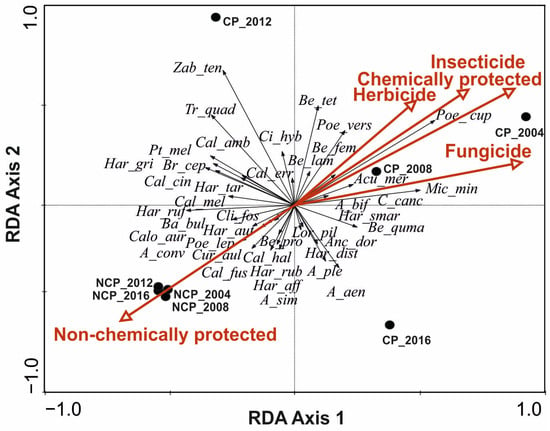
Figure 3.
RDA analysis demonstrating the relationships between the analyzed environmental variables: the type of plant protection (CP—chemically protected, NCP—non-chemically protected); the use of insecticides, herbicides, fungicides; the year of study; and the species of Carabidae (abbreviations are listed in Table 1).
Analysis of the effect of chemical protection on the carabid feeding strategy indicated that the application of pesticides significantly affected the abundance of hemizoophages and medium and small carnivores (Table 2). A decrease in the number of hemizoophages was found in chemically protected fields (CP). In the case of carnivores, increases and decreases in their abundance differed between years. Small carnivores in 2004 were more numerous in NCP fields, while in subsequent years, they were more numerous in CP fields. The mean abundance of medium carnivores was higher in CP fields in most of the years studied (Figure 4). Due to their small number, phytophages were excluded from the above analysis. Our results indicated that chemical protection had a significant effect on the breeding strategy of ground beetles. The number of autumn breeders was significantly higher in the field not treated with pesticides (NCP). In contrast, in chemically protected fields (CP), spring breeders were more abundant (Figure 4). In terms of the dispersal ability, no unambiguous effect of the use of chemical crop protection on this group of beetles was observed. Brachypterous species were very scarce and, therefore, excluded from the analysis. The plant protection technology did not have a significant effect on the abundance of dipterous carabids but did influence the number of macropterous carabids (Table 2). The year of study was an important determinant of the differences in the abundance of both dimorphic and macropterous carabids. Macropterous carabids were more abundant in chemically protected (CP) fields during the first two years of the study, whereas in the subsequent years, their numbers were higher in fields without chemical protection. In contrast, the pattern for dimorphic species was the opposite (Figure 4).
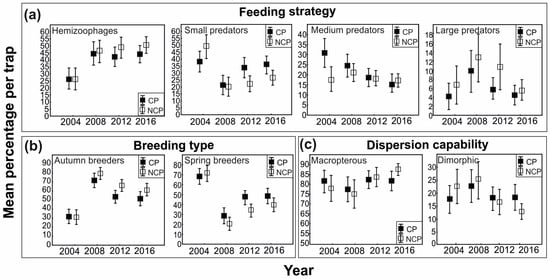
Figure 4.
Percentages of statistically significant ecological groups of carabids: (a) feeding strategy (hemizoophages, large carnivores, medium carnivores, small carnivores), (b) breeding type (autumn and spring breeders), and (c) dispersion capability (macropterous and dimorphic species), depending on plant protection type (CP—chemically protected, NCP—non-chemically protected) in years of study (mean ± SE).
4. Discussion
Organic potato plantations are prone to the massive incidences of pests, causing economic losses and yield deterioration [33,34]. Rempelos et al. [35], analyzing the metadata, indicated a decrease in the yields in organic crops of up to 20–25%. To prevent crop loss, various agricultural practices, including crop rotation and vegetation management to enhance natural pest enemies, are used, with chemical control as a last resort [36]. The use of chemical plant protection products still remains a controversial topic [37] due to their impact on beneficial entomofauna also present in fields, e.g., [38,39,40,41,42]. Nonetheless, the implementation of integrated pest management (IPM) and advancements in plant protection products, characterized by increased selectivity and faster degradation rates, seem to be mitigating the extent of this harmful progress.
Our studies carried out on the effects of pesticides on ground beetles provide important information on the abundance, species richness, and structure of the assemblages of these insects. Analysis of the species composition of ground beetles in both field types showed considerable similarity. Despite this, the RDA diagram indicated that the majority of ground beetle species avoided fields in which chemical plant protection was used. The results revealed that ground beetles were more abundant in potato fields without chemical plant protection (NCP). However, in terms of the species richness, the method of plant protection was not significant. These results were in line with studies indicating that agricultural practices, such as pesticide use, can affect the abundance and structure of ground beetle assemblages [43]. However, the results obtained were inconclusive. Although the experimental treatments did not have a statistically significant effect on species richness, other parameters of the ground beetle assemblages, such as abundance or ecological traits, showed clear differences between chemically protected and unprotected fields. Our results partially supported the hypothesis that chemical protection affects ground beetle assemblages. However, they also suggested that commonly used parameters, such as species richness and abundance, may not fully reflect the scale of ecological change. Most similar to these obtained results were from the studies of Dritschilo and Wanner [44], who recorded a much higher abundance of Carabidae in organic fields with a comparable level of diversity. Clark et al. [45] also reported higher Carabidae abundance in organic fields. In addition to higher abundance, higher species richness was observed in organic fields [46]. On the other hand, Rondon et al. [47] did not confirm the above results entirely, pointing to a higher abundance of ground beetles in conventional potato crops. A small effect of insecticide on Carabidae was also reported by Kalushkov et al. [48]. In view of such different data on the abundance and species richness of ground beetles in fields where pesticides were applied and in organic fields, we also followed the data in the various years of potato cultivation in a four-year rotation. The data obtained, which varied greatly over the years of the study, also did not provide a clear answer on how the use of pesticides in potato cultivation affected the ground beetle assemblages. The ANOSIM analysis showed no significant differences in the ground beetle assemblages in potato fields with chemical plant protection (CP) and without chemical plant protection (NCP) in 2012 and 2016. However, NMDS and RDA analysis revealed significant differences in the ground beetle assemblages. Fields where chemical protection (NCP) was not applied correlated positively with the occurrence of mainly large and medium-sized carnivores. These species are generally more sensitive to pesticide use due to their predatory lifestyle and greater exposure to contaminated prey [42], which most likely explained their limited occurrence in fields under chemical protection. The use of herbicides and insecticides in chemically protected (CP) fields correlated mainly with the presence of Poecilus cupreus, a field-specific species with a high tolerance to disturbance including chemical ones. Sowa et al. [49] suggested that exposure to pesticides leads to the selection of more resistant individuals, resulting in their dominance in fields where pesticides are applied. Differences in the studied ground beetle assemblages were related not only to pesticide application but also to the year of the study. According to Holland and Luff [24], this variability may have been due to differences in climatic and agronomic conditions from one year to the next. In our study, no significant differences in the average temperatures and precipitation were noted across the years. However, the use of different pesticides in each year of the study may have affected the carabid fauna. As reported by Pearsons and Tooker [50] some active compounds may have different effects on carabids.
Lundgren and Mc Cravy [51] pointed out that agricultural practices can directly or indirectly impact ground beetle assemblages. Crucial information can be gathered by examining certain aspects of their life traits. The use of chemical protection has had a significant impact on the feeding strategy of ground beetles. The vast majority of ground beetles are active predators feeding on molluscs and other invertebrates, and they may also be omnivorous or partially herbivorous, e.g., [39,52,53,54]. In the studies conducted, the abundance of hemizoophages decreased in potato fields with chemical plant protection (CP), which may be due to the direct effect of pesticides on their populations. In contrast, the abundance of medium carnivores was higher in CP fields in most years of the study. According to Sądej and Nietupski [55], ground beetles can feed on insects, such as aphids, that fall from leaves during rainstorms or as a result of chemical treatments. The increased availability of dead prey, in particular, may attract predatory insects that do not exclusively hunt live animals but also feed on freshly dead prey. These results were consistent with the second hypothesis we established and suggested that different groups of ground beetles respond differently to pesticide use, which may affect the structure of the agrocenosis ecosystem [39]. Theis study also showed that the abundance of autumn-breeding species was higher in NCP fields, while spring breeders dominated CP fields. This pattern may indicate that NCP fields provided more stable habitats, allowing autumn breeders to develop their larvae successfully. In contrast, CP fields, subjected to pesticide applications may be less suitable for these species, favouring spring breeders with shorter development cycles [24]. Additionally, the abundance of macropterous ground beetles was higher in CP fields during the first two years of the study, and later increased in NCP fields. Meijer [29] stated that macropterous carabids are the first to colonize new habitats. Once a habitat is colonized, individuals with reduced wings begin to appear in the population, which partially aligned with our observations. These results suggested that pesticides may influence the dispersal abilities and life-history strategies of ground beetles, potentially by selecting for species with greater dispersion capability in more disturbed environments [23].
5. Conclusions
These results showed that the use of pesticides in potato crops did not significantly affect the species richness of carabids, but led to changes in the structure of their assemblages and a decrease in the abundance of some ecological groups, such as hemizoophages and autumn breeders. This could disrupt natural pest control mechanisms and the ecological balance of agroecosystems.
For the sustainable management of agroecosystems, it is important not only to monitor the species diversity of ground beetles but also to analyze their functional groups, as these provide insights into trophic relationships and overall ecosystem health. Conducting long-term ecological monitoring will allow us to better understand interannual variability and the effects of pesticide use on epigeic fauna.
Based on these results, it is recommended to integrate ecological knowledge into agricultural practice by, among others, reducing chemical plant protection, using crop rotation, and increasing habitat diversity to protect beneficial entomofauna and maintain ecological balance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15126572/s1, Figure S1. Diagram of the distribution of traps for trapping carabids in a field experiment conducted on chemically protected (CP) and non-chemically protected (NCP) potato plantations. Table S1. Characterization of the potato crops in the consecutive years alongside the specification of pesticides used in chemically protected (CP) fields.
Author Contributions
Conceptualization, A.K., R.K. and M.N.; methodology, A.K. and D.D.; software, A.K., R.K. and M.N.; validation, A.K., R.K. and M.N.; formal analysis, A.K.; investigation, A.K., D.D. and M.N.; resources, A.K.; data curation, A.K.; writing—original draft preparation, A.K., R.K., M.N., E.L. and B.B.; writing—review and editing, A.K., R.K., D.D., M.N., E.L. and B.B.; visualization, A.K. and R.K.; supervision, A.K. and R.K.; project administration, A.K. and D.D.; funding acquisition, A.K. All authors have read and agreed to the published version of the manuscript.
Funding
The results presented in this paper were obtained as a part of a comprehensive study financed by the University of Warmia and Mazury in Olsztyn, Faculty of Agriculture and Forestry, Department of Entomology Phytopathology and Molecular Diagnostics, no 30.610.010-110. This study was financially supported by the Minister of Science under the “Regional Initiative of Excellence” Program.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data relating to the species composition and abundance of ground beetles presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank Katarzyna Nijak, from the Plant Protection Institute in Poznań, for the collection of entomological material. The authors thank the unknown reviewers for valuable comments on a former draft of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CP | Chemically protected field |
| NCP | Non-chemically protected field |
References
- Diyaolu, C.O.; Folarin, I.O. The Role of Biodiversity in Agricultural Resilience: Protecting Ecosystem Services for Sustainable Food Production. Int. J. Res. Publ. Rev. 2024, 5, 1560–1573. [Google Scholar] [CrossRef]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2023. In Urbanization, Agrifood Systems Transformation and Healthy Diets Across the Rural–Urban Continuum; FAO: Rome, Italy, 2023. [Google Scholar] [CrossRef]
- Crippa, M.; Solazzo, E.; Guizzardi, D.; Monforti-Ferrario, F.; Tubiello, F.N.; Leip, A. Food Systems Are Responsible for a Third of Global Anthropogenic GHG Emissions. Nat. Food 2021, 2, 198–209. [Google Scholar] [CrossRef]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2022. In Repurposing Food and Agricultural Policies to Make Healthy Diets More Affordable; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- WHO—World Health Organization. Towards Stronger Food Safety Systems and Global Cooperation; WHO: Geneva, Switzerland, 2022; ISBN 978-92-4-005768-5.
- Jerzak, M.A.; Śmiglak-Krajewska, M. Globalization of the Market for Vegetable Protein Feed and Its Impact on Sustainable Agricultural Development and Food Security in EU Countries Illustrated by the Example of Poland. Sustainability 2020, 12, 888. [Google Scholar] [CrossRef]
- Lankauskienė, R.; Simonaitytė, V.; Gedminaitė-Raudonė, Ž.; Johnson, J. Addressing the European Green Deal with Smart Specialization Strategies in the Baltic Sea Region. Sustainability 2022, 14, 11912. [Google Scholar] [CrossRef]
- Hakeem, K.R.; Akhtar, M.S.; Abdullah, S.N.A. Plant, Soil and Microbes: Volume 1: Implications in Crop Science; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–366. [Google Scholar] [CrossRef]
- He, D.C.; Ma, Y.L.; Li, Z.Z.; Zhong, C.S.; Cheng, Z.B.; Zhan, J. Crop Rotation Enhances Agricultural Sustainability: From an Empirical Evaluation of Eco-Economic Benefits in Rice Production. Agriculture 2021, 11, 91. [Google Scholar] [CrossRef]
- Darguza, M.; Gaile, Z. The Productivity of Crop Rotation Depending on the Included Plants and Soil Tillage. Agriculture 2023, 13, 1751. [Google Scholar] [CrossRef]
- Benini, M.; Blasi, E.; Detti, P.; Fosci, L. Solving Crop Planning and Rotation Problems in a Sustainable Agriculture Perspective. Comput. Oper. Res. 2023, 159, 106316. [Google Scholar] [CrossRef]
- Mickiewicz, B.; Volkova, E.; Jurczak, R. The Global Market for Potato and Potato Products in the Current and Forecast Period. Eur. Res. Stud. J. 2022, XXV, 740–751. [Google Scholar] [CrossRef]
- Barbaro, L.; Pontcharraud, L.; Vetilllard, F.; Guyon, D.; Jactel, H. Comparative Responses of Bird, Carabid, and Spider Assemblages to Stand and Landscape Diversity in Maritime Pine Plantation Forests. Ecoscience 2005, 12, 110–121. [Google Scholar] [CrossRef]
- Barbaś, P.; Noaema, A.H.; Sawicka, B. Potato (Solanum tuberosum L.) As A Rich Source Of Nutrients And Bioactive Compounds. J. Cell Tissue Res. 2023, 23, 7337–7355. [Google Scholar]
- Campos, H.; Ortiz, O. The Potato Crop: Its Agricultural, Nutritional and Social Contribution to Humankind; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; ISBN 9783030286835. [Google Scholar]
- Eurostat The EU Potato Sector Statistics on Production. Prices and Trade—Statistics Explained; European Union: Brussels, Belgium, 2021; Volume 16. [Google Scholar]
- Maciejczak, M.; Filipiak, T.; Gołębiewska, B.; Urbanowicz, J.; Osowski, J.; Treder, K. Differentiation of Profitability of Traditional and Innovative Potatoes Cultivation in Poland. Zagadnienia Ekon. Rolnej 2023, 377, 70–85. [Google Scholar] [CrossRef]
- Shende, V.A.; Janbandhu, K.S.; Patil, K.G. Impact of Human Beings on Environment. Int. J. Res. Biosci. Agric. Technol. 2015, 1, 23–28. [Google Scholar]
- Manickavasagam, S.; Sudhan, C.; Subramaniam, B.; Aanand, S. Bioindicators In Aquatic Environment And Their Significance. J. Aquac. Trop. 2019, 34, 73–79. [Google Scholar] [CrossRef]
- Rainio, J.; Niemela, J. Ground Beetles (Coleoptera: Carabidae) as Bioindicators. Biodivers. Conserv. 2003, 12, 487–506. [Google Scholar] [CrossRef]
- Avgın, S.S.; Luff, M.L. Ground Beetles (Coleoptera: Carabidae) as Bioindicators of Human Impact. Munis Entomol. Zool. J. 2010, 5, 209–215. [Google Scholar]
- Johan Kotze, D.; Brandmayr, P.; Casale, A.; Dauffy-Richard, E.; Dekoninck, W.; Koivula, M.J.; Lövei, G.L.; Mossakowski, D.; Noordijk, J.; Paarmann, W.; et al. Forty Years of Carabid Beetle Research in Europe—From Taxonomy, Biology, Ecology and Population Studies to Bioindication, Habitat Assessment and Conservation. Zookeys 2011, 100, 55–148. [Google Scholar] [CrossRef]
- Thiele, H.U. Carabid Beetles in Their Environments; Springer: Berlin/Heidelberg, Germany, 1977. [Google Scholar]
- Holland, J.M.; Luff, M.L. The Effects of Agricultural Practices on Carabidae in Temperate Agroecosystems. Integr. Pest Manag. Rev. 2000, 5, 109–129. [Google Scholar] [CrossRef]
- Kabała, C.; Charzyński, P.; Chodorowski, J.; Drewnik, M.; Glina, B.; Greinert, A.; Hulisz, P.; Jankowski, M.; Jonczak, J.; Łabaz, B.; et al. Polish Soil Classification, 6th Edition—Principles, Classification Scheme and Correlations. Soil Sci. Annu. 2019, 70, 71–97. [Google Scholar] [CrossRef]
- Aleksandrowicz, O.R. Biegaczowate (Carabidae). In Fauna Polski—Charakterystyka I Wykaz Gatunków; Bogdanowicz, W., Chudzicka, E., Filipiuk, I., Skibińska, E., Eds.; Muzeum i Instytut Zoologii PAN: Warszawa, Poland, 2004; Volume I, pp. 28–42. [Google Scholar]
- Hurka, K. Carabidae of the Czech and Slovak Republics; Kabournek: Zlin, Czech Republic, 1996; pp. 1–566. [Google Scholar]
- Larsson, S.G. Entwicklungstypen Und Entwicklungszeiten Der Dän Carabiden. Entomol. Meddelels 1939, 15, 270–560. [Google Scholar]
- Meijer, J. A Comparative Study of the Immigration of Carabids (Coleoptera, Carabidae) into a New Polder. Oecologia 1974, 16, 185–208. [Google Scholar] [CrossRef]
- Hammer, D.A.T.; Ryan, P.D.; Hammer, Ø.; Harper, D.A.T. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Anderson, A. New Method for Non-parametric Multivariate Analysis of Variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and User’s Guide to Canoco for Windows; Centre for Biometry: Wageningen, The Netherlands, 1998; p. 352. [Google Scholar]
- Kowalska, J.; Kühne, S. Effect of biological plant protection products on beneficial insects in organic potato crops. J. Res. Appl. Agric. Eng. 2010, 55, 191–194. [Google Scholar]
- Palmer, M.W.; Cooper, J.; Tétard-Jones, C.; Średnicka-Tober, D.; Barański, M.; Eyre, M.; Shotton, P.N.; Volakakis, N.; Cakmak, I.; Ozturk, L.; et al. The Influence of Organic and Conventional Fertilisation and Crop Protection Practices, Preceding Crop, Harvest Year and Weather Conditions on Yield and Quality of Potato (Solanum Tuberosum) in a Long-Term Management Trial. Eur. J. Agron. 2013, 49, 83–92. [Google Scholar] [CrossRef]
- Rempelos, L.; Barański, M.; Sufar, E.K.; Gilroy, J.; Shotton, P.; Leifert, H.; Średnicka-Tober, D.; Hasanaliyeva, G.; Rosa, E.A.S.; Hajslova, J.; et al. Effect of Climatic Conditions, and Agronomic Practices Used in Organic and Conventional Crop Production on Yield and Nutritional Composition Parameters in Potato, Cabbage, Lettuce and Onion; Results from the Long-Term NFSC-Trials. Agronomy 2023, 13, 1225. [Google Scholar] [CrossRef]
- Gabryś, B.; Kordan, B. Cultural Control and Other Non-Chemical Methods. In Insect Pests of Potato: Global Perspectives on Biology and Management; Elsevier: Amsterdam, The Netherlands, 2022; pp. 297–314. ISBN 9780128212370. [Google Scholar]
- Tang, F.H.M.; Lenzen, M.; Mcbratney, A.; Maggi, F. Risk of pesticide pollution at the global scale. Nat. Geosci. 2021, 14, 206–210. [Google Scholar] [CrossRef]
- Kromp, B. Carabid beetles (Coleoptera, Carabidae) as bioindicators in Biological and conventional farming in Austrian potato fields. Biol. Fertil. Soils 1990, 9, 182–187. [Google Scholar] [CrossRef]
- Kromp, B. Carabid Beetles in Sustainable Agriculture: A Review on Pest Control Efficacy, Cultivation Impacts and Enhancement. Agric. Ecosyst. Environ. 1999, 74, 187–228. [Google Scholar] [CrossRef]
- Birkhofer, K.; Fließbach, A.; Wise, D.H.; Scheu, S. Generalist Predators in Organically and Conventionally Managed Grass-Clover Fields: Implications for Conservation Biological Control. Ann. Appl. Biol. 2008, 153, 271–280. [Google Scholar] [CrossRef]
- Kosewska, A.; Nijak, K.; Nietupski, M.; Kędzior, R.; Ludwiczak, E. Effect of Plant Protection on Assemblages of Ground Beetles (Coleoptera, Carabidae) in Sugar Beet Crops in Four-Year Rotation. Acta Zool. Acad. Sci. Hung. 2020, 66, 49–68. [Google Scholar] [CrossRef]
- Šerić Jelaska, L.; Jelić, M.; Anđelić Dmitrović, B.; Kos, T. Bioaccumulation of Pesticides in Carabid Beetles in a Vineyard and Olive Grove under Integrated Pest Management. Eur. J. Entomol. 2024, 121, 269–279. [Google Scholar] [CrossRef]
- Geiger, F.; Bengtsson, J.; Berendse, F.; Weisser, W.W.; Emmerson, M.; Morales, M.B.; Ceryngier, P.; Liira, J.; Tscharntke, T.; Winqvist, C.; et al. Persistent Negative Effects of Pesticides on Biodiversity and Biological Control Potential on European Farmland. Basic Appl. Ecol. 2010, 11, 97–105. [Google Scholar] [CrossRef]
- Dritschilo, W.; Wanner, D. Ground Beetle Abundance in Organic and Conventional Corn Fields. Environ. Entomol. 1980, 9, 629–631. [Google Scholar] [CrossRef]
- Clark, S.; Szlavecz, K.; Cavigelli, M.A.; Purrington, F. Ground Beetle (Coleoptera: Carabidae) Assemblages in Organic, No-Till, and Chisel-Till Cropping Systems in Maryland. Environ. Entomol. 2006, 35, 1304–1312. [Google Scholar] [CrossRef]
- Clark, M.S.; Luna, J.M.; Stone, N.D.; Youngman, R.R. Habitat Preferences of Generalist Predators in Reduced-Tillage Corn. J. Entomol. Sci. 1993, 28, 404–416. [Google Scholar] [CrossRef]
- Rondon, S.I.; Pantoja, A.; Hagerty, A.; Horneck, D.A. Ground Beetle (Coleoptera: Carabidae) Populations in Commercial Organic and Conventional Potato Production. Fla. Entomol. 2013, 96, 1492–1499. [Google Scholar] [CrossRef]
- Kalushkov, P.; Gueorguiev, B.; Spitzer, L.; Nedved, O. Biodiversity of Ground Beetles (Coleoptera: Carabidae) in Genetically Modified (Bt) and Conventional (Non-Bt) Potato Fields in Bulgaria. Biotechnol. Biotechnol. Equip. 2009, 23, 1346–1350. [Google Scholar] [CrossRef]
- Sowa, G.; Bednarska, A.J.; Ziółkowska, E.; Laskowski, R. Homogeneity of Agriculture Landscape Promotes Insecticide Resistance in the Ground Beetle Poecilus cupreus. PLoS ONE 2022, 17, e0266453. [Google Scholar] [CrossRef]
- Pearsons, K.A.; Tooker, J.F. Acute Toxicity of Neonicotinoid Insecticides to Ground Beetles (Coleoptera: Carabidae) from Pennsylvania. Environ. Entomol. 2025, 54, nvaf048. [Google Scholar] [CrossRef]
- Lundgren, J.; McCravy, K. Carabid Beetles (Coleoptera: Carabidae) of the Midwestern United States: A Review and Synthesis of Recent Research. Terr. Arthropod Rev. 2011, 4, 63–94. [Google Scholar] [CrossRef]
- Lovei, G.L.; Sunderland, K.D. Ecology and behavior of ground beetles (Coleoptera: Carabidae). Annu. Rev. Entomol. 1996, 41, 231–256. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, K.D. Invertebrate Pest Control by Carabids. In The agroecology of Carabid Beetles; Holland, J.M., Ed.; Intercept: Andover, UK, 2002; pp. 165–214. [Google Scholar]
- Saska, P.; Martinkova, Z.; Honek, A. Temperature and Rate of Seed Consumption by Ground Beetles (Carabidae). Biol. Control 2010, 52, 91–95. [Google Scholar] [CrossRef]
- Sądej, W.; Nietupski, M. Occurrence of Pea Aphid (Acyrthosiphon pisum Harris) on Faba Bean and Some Biotic Factors Reducing Its Numbers. Nat. Sci. 2000, 5, 73–82. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).