Abstract
This study compared the efficacy of different solutions in achieving patency in teeth filled with AH Plus Bioceramic sealer. Eighty-five premolars with a straight canal were prepared. After sealer placement, a master gutta-percha cone was introduced 2 mm short of the working length. The teeth were stored at 37 °C and 100% humidity for five weeks before retreatment. Filling materials were removed up to the gutta-percha cone’s length. The canals were then randomly assigned to groups: G1 (control, no solution), G2 (5.25% NaOCl), G3 (17% EDTA), G4 (10% citric acid), and G5 (10% formic acid). The apical patency was attempted with a 10 K file within a period of 10 min, by a blinded operator. Additionally, sealer samples were immersed in the solutions, followed by scanning electron microscopy analysis. The Kruskal–Wallis test was used for statistical analysis. Patency was achieved in all canals except one in the control and one in the NaOCl groups. No significant differences were found in the time required to achieve patency. Acid solutions had a greater impact on the sealer’s structural integrity, and a decalcifying effect of EDTA and citric acid was registered. Apical patency in straight canals obturated with AH Plus Bioceramic sealer was consistently achieved regardless of the solution used.
1. Introduction
Non-surgical endodontic retreatment (NSER) is a therapeutic option to consider in the presence of persistent apical periodontitis after endodontic treatment, following an appropriate follow-up period. Its main goal is to clean and eliminate biofilms concealed by remnants of the old fillings and debris, as well as to reseal all entry points with a new, highly hermetic obturation, preventing recurrence [1]. Removing the old filling material is crucial because it may harbor bacteria in biofilms, preventing disinfecting procedures from reaching them [2].
The apical third of the root canal is the most critical area for effective disinfection due to its anatomical complexities and the persistence of accumulated debris, filling material remnants, and bacterial biofilms [3]. Proper removal of the filling material, disinfection of this zone, and re-establishment of apical patency seem to contribute to a successful outcome after NSER [4]. The difficulty in removing root canal materials and achieving patency may be influenced by factors such as the instrument design, the age of the root canal filling, the obturation technique, the type of sealer used, and the canal anatomy [1]. Despite advancements in technology, achieving complete removal of the previously placed obturation remains challenging [5,6]. Techniques for removing filling materials include rotary files, ultrasonic instruments, heat, lasers, hand files, and solvent solutions [5,7]. Although a combination of these methods is often required, the introduction of retreatment rotary systems has greatly enhanced the process, leading some clinicians to overlook the use of solvent solutions [5,7]. Nevertheless, the effectiveness of combining solvents for filling removal remains controversial [5,8,9]. Some authors suggest that this approach can help reduce complications, particularly when the filling material has hardened over time or in cases involving thin and curved canals [1]. However, other studies argue that solvents should be reserved for situations where reaching the working length is challenging (e.g., in curved canals), as they may slow down the retreatment process and contribute to the accumulation of filling remnants on canal walls and within dentinal tubules [10,11,12].
Chloroform has historically been regarded as the most effective agent for softening gutta-percha due to its strong solvency properties [13]. However, concerns regarding its cytotoxicity and potential carcinogenicity have led to limitations on its clinical use. Consequently, alternatives such as orange oil have gained interest for their lower toxicity, although they typically exhibit slower and less effective softening capabilities compared to chloroform [14]. Traditionally, these chemical agents have been used indiscriminately for both gutta-percha and sealer removal. More recently, specialized formulations have been developed to selectively target specific types of sealers. For example, EndoSolv E (a tetrachloroethylene-based solution) is tailored for zinc oxide-eugenol sealers, while EndoSolv R (containing formamide and phenyl ethylic alcohol) is intended for resin-based sealers. Both products, previously manufactured by Septodont, have now been replaced by a newer version—EndoSolv—which is indicated for use with both types of sealers. Additionally, combined solutions such as “methyl ethyl ketone + tetrachloroethylene” and “methyl ethyl ketone + orange oil” have been proposed as dual-action options capable of softening both gutta-percha and resin sealers simultaneously [7,15].
Solvents are typically applied in the early stages of NSER by placing a few drops into the space created after the removal of the coronal filling [1]. Alternately, some approaches suggest flooding the canal with solvent after removing most of the remaining gutta-percha and further enlarging the canal [7]. In this context, Ferreira, et al. [16] highlighted the enhanced effectiveness of novel specific solvents (like methyl ethyl ketone/tetrachloroethylene) when combined with agitation, particularly in targeting gutta-percha and the epoxy resin-based sealer, AH Plus.
Hydraulic calcium silicate-based sealers (CCSs), collectively referred to as bioceramics, have gained popularity in recent years. These materials exhibit a wide range of chemical compositions, which may influence their physical, chemical, and biological properties [17]. However, concerns regarding their solubility and retrievability remain a topic of discussion among clinicians [18]. In a recent survey, 55.5% of dentists considered that these sealers may influence the ability to re-establish apical patency during NSER [18]. CCSs are most commonly used with the single-cone obturation technique, resulting in a higher sealer-to-gutta-percha ratio compared to other techniques [17]. This approach simplifies clinical procedures while maintaining high success rates [19]. When NSER is necessary, gutta-percha can typically be removed using rotary instruments; however, the removal of CCSs can be more challenging due to their hardness [20].
Several parameters are used to assess CCS retreatability, including the ability to regain the working length (WL) and apical patency, the time required to reach the WL, and the amount of residual filling material left in the canal [20]. Studies on CCSs retreatability have yielded inconsistent results. While some have demonstrated the advantages of using solvents [21], others suggest specific solvent solutions targeting sealer chemistry [22], while some report better outcomes without any solution [23]. Various solutions, including formic acid (FA), ethylenediamide tetraacetic acid (EDTA), citric acid (CA), acetic acid, carbonated water, chloroform, and hydrochloric acid, have been tested [22,23,24], but a definitive solvent for CCSs has yet to be identified. It is expected that the ideal solvent should be able to dissolve and remove the CCSs without causing structural damage to the dentin and perirradicular tissues.
AH Plus Bioceramic (Dentsply Sirona, Charlotte, NC, USA) is a newly developed, premixed calcium silicate-based sealer available in an injectable syringe. Its composition includes zirconium dioxide, tricalcium silicate, dimethyl sulfoxide, lithium carbonate, and a thickening agent [25]. Recent studies have shown that AH Plus Bioceramic possesses key physical properties required for an effective sealer, including adequate flow, setting time, radiopacity, and a high pH, which contributes to its antibacterial activity [25]. Additionally, it exhibits significantly higher cytocompatibility and bioactive potential than AH Plus sealer, while demonstrating a cytocompatibility level comparable to Endosequence BC sealer [26]. One concern is its high solubility, which may negatively impact the quality of the obturation [22,25]. However, research on the retreatability of this sealer remains limited.
Currently, to the best of our knowledge, no study has evaluated the ability to regain patency in teeth obturated with the AH Plus Bioceramic sealer. This study aimed to compare the efficacy of different solutions in achieving patency during NSER in straight canals filled with AH Plus Bioceramic sealer and their effects on the sealer’s microstructure. The null hypotheses were that (i) acid-based solutions do not significantly enhance the rate of patency achievement in teeth filled with AH Plus Bioceramic sealer and (ii) do not affect the microstructure of the sealer.
2. Materials and Methods
This ex vivo study complies with PRILE 2021 guidelines [27]. The research process and the main results are presented in the PRILE flowchart (Figure 1). The study was approved by the Institutional Research Ethics Committee of the Faculty of Dental Medicine of the University of Porto (nº 11/2023).

Figure 1.
The PRILE 2021 flowchart.
The selected sealer was AH Plus Bioceramic (Dentsply Sirona, Charlotte, NC, USA), composed of zirconium dioxide (50–75%), tricalcium silicate (5–15%), dimethyl sulfoxide (10–30%), lithium carbonate (<0.5%), and a thickening agent (<6%).
2.1. Ex Vivo Study
2.1.1. Sample Collection and Standardization
Eighty-five single-rooted premolars with straight canals, extracted for reasons unrelated to this study—mostly due to periodontal conditions—were selected. Teeth with caries, curvatures, or calcifications were excluded. Radiographs were taken in the buccolingual and mesiodistal directions to select only teeth with a single root canal and a circular shape. The space corresponding to the root canal lumen was measured 5 mm from the apex. When the mesiodistal diameter was similar to the buccolingual diameter, the teeth were classified as having round-shaped canals [28].
All included teeth were disinfected in a 0.1% thymol solution for 24 h and subsequently stored in distilled water for one month, which was the necessary time to collect the required number of samples. The time between extraction and immersion in the thymol solution did not exceed two hours. Immediately after extraction, all teeth were placed in a moist environment and transferred to the disinfectant within this time frame to prevent dehydration and preserve tissue integrity.
All procedures in this study were performed under magnification using loupes (4.6×, ExamVision Akura Medical, NovaMed Concepts S.L., Madrid, Spain). Samples were standardized using a diamond disk with a length of 19 ± 0.5 mm. Teeth were stabilized during all procedures.
2.1.2. Endodontic Treatment
After access preparation, the working length (WL) was established 1 mm short of the apex. The patency was confirmed using a K file 10 (Dentsply Sirona, Ballaigues, Switzerland). Each root canal was instrumented to a final size of 30/.04 using ProTaper Ultimate (Dentsply Sirona, Ballaigues, Switzerland) and irrigated with 10 mL of 5.25% of sodium hypochlorite (NaOCl), delivered with a 30 G needle (Max-I-Probe, Dentsply International, Inc., York, PA, USA). The final irrigation protocol consisted of 2 mL of 17% ethylenediamine tetracetic acid (EDTA, Coltene, Whaledent AG, Altstatten, Switzerland) followed by 2 mL of 5.25% NaOCl (Cerkamed, Stalowa Wola, Poland) and finally distilled water. The canals were dried using sterile absorbent paper points. Patency was reconfirmed after chemo-mechanical preparation, prior to obturation. Root canals were filled using the single-cone technique with a ProTaper Ultimate F3 gutta-percha cone (Dentsply Sirona, Ballaigues, Switzerland) which was intentionally adjusted 2 mm short of the working length to ensure that the apical 2 mm were filled exclusively with AH Plus Bioceramic (Dentsply Sirona, Ballaigues, Switzerland). Radiographs were taken to confirm the position of the gutta-percha cone. The bioceramic sealer was applied by positioning the syringe tip inside the root canal and injecting the sealer according to the manufacturer’s recommendation followed by distribution with a lentulo spiral. The teeth were radiographed again, sealed with a temporary restorative material (Coltosol F (Coltene/Whaledent AG, Altstatten, Switzerland). They were then were stored in 15 mL Hank’s Balance Salt Solution (HBSS) for 28 days at 37 °C and 100% humidity [29].
2.1.3. Non-Surgical Endodontic Retreatment Protocol
All procedures were performed by a single operator in a blinded manner regarding the solutions, except for Group 1 (no solution). The syringes containing the different solutions were masked with dark paper. Each tooth was assigned a number and was randomly distributed into the groups (n = 17) using the website https://www.random.org/. The operator wore an N95 respirator to prevent recognition of the solutions by odor.
Filling material removal, particularly gutta-percha, was carried out using Reciproc (R25, VDW, Munich, Germany), with only saline solution used as the irrigant, stopping 2 mm short of the WL. Radiographs and magnification were used in combination to ensure proper filling removal, as outlined. Samples were then randomly assigned to the groups based on the solution used to achieve patency:
- Group 1: no solution exposure (n = 17; control)
- Group 2: 5.25% NaOCl (n = 17; Cerkamed, Stalowa Wola, Poland)
- Group 3: 17% EDTA (n = 17; Coltene, Whaledent AG, Altstatten, Switzerland)
- Group 4: 10% citric acid (n = 17; CA; 40% citric acid, Cerkamed, Stalowa Wola, Poland). The 10% CA was obtained by diluting 40%CA with distilled water.
- Group 5: 10% formic acid (n = 17; FA; Sigma Aldrich, Gillingham, UK)
For each group, 0.3 mL of the corresponding solution was placed in the apical third of the canal, and the time required to regain patency through the AH Plus Bioceramic sealer using a size 10 K file (Dentsply Sirona, Ballaigues, Switzerland) was recorded in seconds. Apical patency was visually confirmed by observing the stopper reaching the coronal reference point (1 mm beyond the working length) and observing the tip of the instrument becoming visible in the apical foramen under magnification. Short-amplitude in and out movements and light pecking strokes were performed A maximum time limit of 10 min was set to re-establish patency. The time was recorded using a digital chronometer (precision of 0.01 s).
All procedures are outlined in Figure 2.
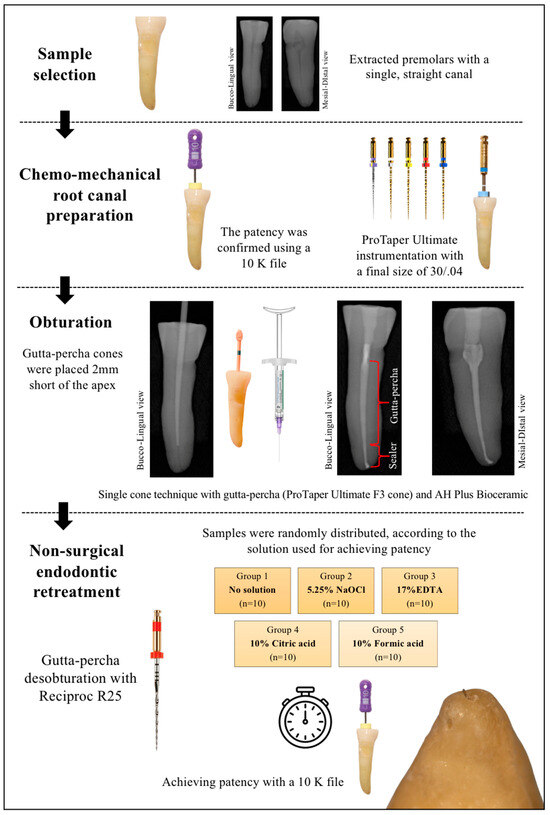
Figure 2.
The flow diagram of the ex vivo part of the study.
2.2. Microstructure of Sealer Samples
Sealer specimens were prepared using plastic molds with a diameter of 10 mm and a thickness of 2 mm. To prevent evaporation and drying, the specimens were placed in a humidity chamber at 37 °C, covered with moist gauze, and sealed in a plastic bag. After setting, excess material was trimmed to the surface level of the washer using silicon carbide paper (600 grit). Sealer samples (n = 3) were then immersed in the different solutions (5.25% NaOCl, 17% EDTA, 10% CA, and 10% FA) for 5 min.
Scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS) were performed to assess the microstructural changes induced by the solutions on AH Plus Bioceramic sealer. Samples were coated with Au/Pd thin film by sputtering, using the SPI Module Sputter Coater. SEM images were taken at the center of the specimen using the backscatter mode and 100x magnification. A blinded co-author performed the analysis.
2.3. Statistical Analysis
The G*Power v3.1.9.6 program was used to determine an a priori sample size. The procedure used was an analysis of variance (ANOVA) with Fixed effects, using an alpha-type error of 0.05 with a power (1-ß) of 0.80, with an effect size of 0.4. Seventeen specimens per group were established as the ideal size.
Data were analyzed using the IBM SPSS Statistic 29.0. software (SPSS Inc., Chicago, IL, USA, EUA). The Shapiro–Wilk test determined that the data were not normally distributed. Therefore, the Kruskal–Wallis test was used to compare the different groups. The level of significance was set at 5%.
3. Results
3.1. Achieving Patency—Ex Vivo Study
Patency was achieved in all root canals, except for one in Group 1 (no solution) and one in Group 2 (5.25% NaOCl). The time to achieve patency is shown in (Figure 3). Briefly, the median times for the no solution, 5.25% NaOCl, 17% EDTA, 10% CA, and 10% FA groups were 28.5, 47.0, 30.0, 27.0, and 37.0 s, respectively. No significant differences were observed among the five groups (Kruskal–Wallis test, p = 0.542).
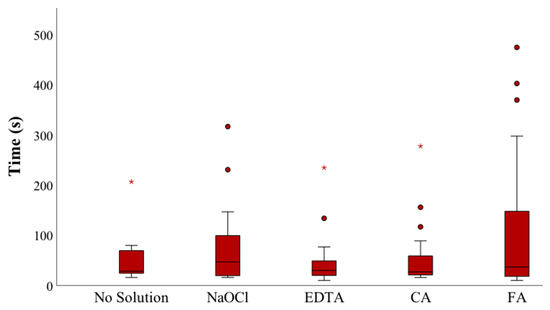
Figure 3.
The time to achieve patency (seconds) in the different groups (NaOCl—sodium hypochlorite; EDTA—ethylenediamide tetraacetic acid; CA—citric acid; FA—formic acid). Symbols (star and circle) indicate outliers of the study sample.
3.2. Microstructure of Sealer Samples
The microstructure of sealer samples treated with different solutions is illustrated in Figure 4.
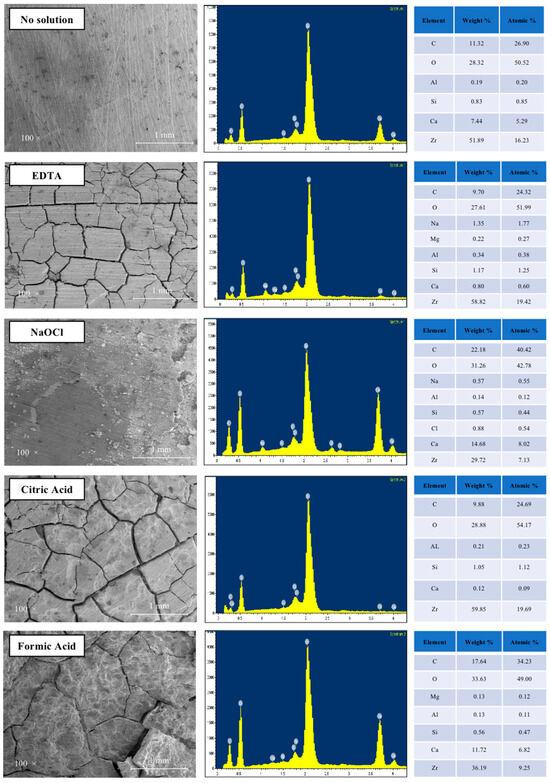
Figure 4.
SEM images of AH Plus Bioceramic sealer immersed in the different solutions: control (no exposure), and after immersion for 5 min in 17% EDTA, 5.25% NaOCl, 10% citric acid, and 10% formic acid (magnification 100×). Energy dispersive spectroscopic (EDS) plots show the elemental composition of each sample.
A 5 min exposure to the tested solutions produced different effects on the sealer surface. EDTA, CA, and FA caused cracks, leaving the sealer without the evident surface carbonation seen in the control. Dissolution of the sealer and the presence of pores were observed in the samples exposed to the acids. The damage to the structural integrity induced by NaOCl was considerably less pronounced compared to the other solutions.
EDS analysis indicated that the observed structural changes were due to the decalcifying effect of EDTA and CA, as significantly lower amounts of calcium were detected in the acid-treated specimens. The main components of etched surfaces were zirconium and oxygen. The calcium/silica ion ratio from the EDS analysis showed a decrease in specimens treated with EDTA and CA, with a similar pattern observed for NaOCl and FA.
4. Discussion
In the present investigation, the efficacy of achieving patency in straight canals obturated with AH Plus Bioceramic sealer was evaluated. The study assessed the use of NaOCl and three acid solutions as an adjunct to the mechanical action of a 10 K file, in comparison to the control group (no solution). The results indicated that, although the acid solutions produced changes in the microstructure of the sealer, this effect was not related to a higher occurrence of canals with patency. Thus, among the two null hypotheses tested, only hypothesis (i) was supported by the results, suggesting no significant effect of acid-based solutions on patency rates. Hypothesis (ii), however, was not confirmed, as the microstructure of the sealer was affected. Although previous studies have investigated CSSs, including comparisons with the conventional AH Plus sealer [21,29], one of the objectives of the present study was to determine whether some of the solvents known to be effective in retrieving CSSs could also efficiently dissolve AH Plus Bioceramic sealer. To the best of our knowledge, this is one of the first studies to specifically assess the ability of various solutions to regain patency in teeth obturated with AH Plus Bioceramic sealer.
Apical patency, as defined by the American Association of Endodontists, refers to the procedure in which the apical portion of the canal is kept free of debris by recapitulating with a small file through the apical foramen [30]. In addition to other anatomical complexities within the root canal system, the apical part of the main canal is considered the “critical zone” due to its strategic position, which enables microorganisms to cause or maintain apical periodontitis [31]. During NSER, re-establishing patency can influence the prognosis [32]. As reported in another study [23], the time to achieve patency was measured after gutta-percha removal, simulating a short filling, with the cone intentionally left 2 mm short of the WL. This approach increased the difficulty of retreatment and allowed for a more objective assessment of sealer removal in the absence of gutta-percha. A ten-minute time limit was set to re-establish apical patency, though this may not fully reflect clinical conditions.
Traditional solvents, such as chloroform, which are effective against gutta-percha and resin-based sealers, exhibit unclear action against CSSs [33]. Hess, et al. [33] compared the efficacy of regaining patency in teeth obturated with either AH Plus or EndoSequence BC Sealer. The samples were divided into four groups: in two groups, the gutta-percha was placed to the full WL, while in the other two, it was positioned 2 mm short. The retreatment protocol included the use of heat, chloroform, rotary files, and hand instruments. When gutta-percha extended to the WL, complete success (100%) in re-establishing WL was achieved in all groups, regardless of the sealer used. In samples where the gutta-percha was short, WL was re-established in 100% of canals filled with AH Plus, but only in 30% of those filled with EndoSequence BC Sealer. Rezaei, et al. [24] compared the efficacy of 20% hydrochloric acid (HCl), 10% FA, and chloroform as solvents for regaining patency in teeth filled with EndoSequence BC Sealer. Their results revealed that all three solvents were generally effective, but 20% HCl achieved patency significantly faster than FA and chloroform. However, scanning electron microscopy demonstrated that HCl may have caused erosion of dentinal tubules, raising concerns about its potential for structural compromise. Oltra, et al. [21], in their micro-CT-based study, demonstrated that AH Plus resulted in substantially less residual filling material and allowed for complete re-establishment of canal patency in all samples, particularly when used with chloroform. In contrast, BC Sealer exhibited higher volumes of residual material, especially when chloroform was not used, and patency could only be regained in 14% of such cases. These studies underscore the importance of solvent selection during retreatment and suggest that the physical and chemical properties of CSSs, including their potential for dentin bonding and intratubular precipitation, may hinder their complete removal.
Nevertheless, the use of chloroform has been questioned due to its cytotoxicity and carcinogenic potential, and other alternatives have been explored [7]. This study evaluated the efficacy of four different solutions (NaOCl, CA, FA, and EDTA) for achieving patency. Given that NaOCl is routinely used in endodontic procedures, it was evaluated for its ability to dissolve CSSs, to determine whether its application could affect the action of the other solutions tested. CSSs appear to be more soluble at low pH, suggesting that acids may act as solvents for these materials [34]. The selection of 10% CA was based on the study by Drukteinis, et al. [35] which demonstrated that CA at concentrations of 10% can effectively dissolve hydraulic calcium silicate materials without causing adverse effects on dentin [36]. Additionally, Garrib and Camilleri [22] proposed using 17% EDTA or 10% FA as a chemical adjunct to mechanical instrumentation for retreating teeth filled with TotalFill BC Sealer, while minimizing damage to the dentine structure.
As in previous studies [37], our choice to use the Reciproc R25 instrument rather than the R40 was based on both clinical and methodological considerations. The R25 was primarily selected to standardize the retreatment procedure and simulate the initial stage of canal re-entry commonly encountered in clinical retreatments, where a smaller instrument is typically used to safely negotiate blockages or resistance. Furthermore, the objective of this study was specifically to evaluate the ability to regain patency in canals filled with AH Plus Bioceramic sealer, rather than to assess the overall cleanliness or extent of material removal within the root canal system. In this context, the use of an instrument such as the R25 was considered more appropriate and representative of clinical practice.
The present study showed that apical patency was re-established in all five groups using a 10 K hand file. Our results corroborate previous findings [22], confirming the retreatment efficacy of CSSs used in the single-cone obturation technique in straight monorradicular teeth. Garrib and Camilleri [22] concluded that patency was achieved in the majority of cases obturated with Totalfill BC sealer, regardless of whether an adjuvant solution was used (17% EDTA or 10% FA). These findings are also consistent with those of Carrillo, et al. [23], who reported that the apical patency achievement rate, in teeth obturated with different EndoSequence BC, EdgeBioceramic, or NeoSEALERFlo sealers, was significantly higher when no solution was used, compared to 6% NaOCl, 5% acetic acid, and carbonated water. It was suggested that a possible effect of pore-liquid on the strength of CSSs could have a negative impact on their retrievability. However, contrary to our results, Carrillo’s group concluded that retrievability decreased with the use of solutions (6% NaOCl, 5% acetic acid, and carbonated water). The differences in the solutions and sealers studied could explain the contrasting outcomes.
Pre-mixed calcium silicate-based sealers have been clinically reported as successful in root canal treatment [38]. Their advantageous properties regarding antimicrobial ability and bioactivity have led to a wide consensus that they are an alternative to the standard epoxy resin sealers, namely through the single-cone technique. In the present investigation, a recently introduced pre-mixed calcium silicate-based sealer, AH Plus Bioceramic, was evaluated. As confirmed by EDS analysis, it is mainly composed of zirconium. Although it contains reactive tricalcium silicate, its overall calcium silicate content is comparatively lower (5–15%) than that of conventional CSSs due to the absence of di-calcium silicate in its composition [39]. The lower calcium silicate content in AH Plus Bioceramic may contribute to its high solubility and lower compression modulus and strength, making it much easier to remove [39,40].
SEM/EDS analysis provided detailed information on the elemental composition and the microstructural characteristics of the sealer surface after exposure to the different solutions. The objective was to visualize surface changes, such as erosion patterns and structural breakdown, potentially induced by the tested agents. Although the microstructural alterations observed did not directly correlate with the lack of statistically significant differences in regaining patency, this analysis was conducted in an effort to identify possible explanations for the outcomes. Garrib and Camilleri [22] observed the presence of pores and dissolution of sealer material in response to acid exposure, which aligns with our findings. Specifically, exposure to 17% EDTA caused cracks in Total Fill samples, a feature also observed in this study with AH Plus Bioceramic sealer. Cracks were evident in SEM images after exposure to 17% EDTA, as well as to 10% CA and 10% FA (Figure 4). In the study by Raman and Camilleri [41], cracks observed in aged samples were attributed to an artifact resulting from the dehydration process required for coating and imaging. The authors [41] also emphasized the full disintegration of AH Plus Bioceramic when in contact with the storage solution over a period of 7 and 28 days. Chen, et al. [42] reported that AH Plus Bioceramic exhibited early-stage porosity, indicating displacement of particles from the set structure overtime. A reduction in material strength could result from this pore formation. Additionally, as mentioned by a previous study [33], voids within the sealer or unset sealer may be the reason that hand files can navigate through the sealer to gain patency. Nevertheless, further studies are needed to confirm and expand upon the effects of these solutions on the microstructure of AH Plus Bioceramic sealer.
The lack of differences between the various solutions tested in this study highlights the importance of other factors, such as the chemical composition of the sealer and its bond strength to dentin. The higher solubility and weak bond strength to dentin presented by AH Plus Bioceramic [25,43] may facilitate its removal, thereby aiding in the re-establishment of patency. Additionally, NSER was performed four weeks after canal filling, which may have been insufficient for the formation of a mineralized layer between the root canal dentin and the sealer. It is also important to note that this study only assessed straight, single-root canals. Therefore, future studies should focus on investigating the re-establishment of apical patency in teeth obturated with different CSSs in more challenging canal anatomies.
5. Conclusions
Within the limitations of the present study, patency in straight canals obturated with AH Plus Bioceramic sealer was consistently achieved regardless of the solution used. These findings suggest that the type of irrigant or solvent may not significantly influence the ability to regain patency in these conditions.
Author Contributions
Conceptualization, I.F. and I.P.-V.; methodology, I.F.; data curation, I.F., A.C.B. and M.A.L.; formal analysis, I.F., A.C.B. and M.A.L.; Visualization, I.F. and B.F; Resources, M.A.L.; writing—original draft preparation, I.F. and I.P.-V.; writing— review and editing, I.F., B.F., A.C.B., M.A.L. and I.P.-V.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Faculty of Dental Medicine of the University of Porto (research project nº 11/2023; approved on 22 April 2024).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Duncan, H.F.; Chong, B.S. Removal of root filling materials. Endod. Top. 2008, 19, 33–57. [Google Scholar] [CrossRef]
- Ricucci, D.; Siqueira, J.F., Jr. Biofilms and apical periodontitis: Study of prevalence and association with clinical and histopathologic findings. J. Endod. 2010, 36, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr.; Pérez, A.R.; Marceliano-Alves, M.F.; Provenzano, J.C.; Silva, S.G.; Pires, F.R.; Vieira, G.C.S.; Rôças, I.N.; Alves, F.R.F. What happens to unprepared root canal walls: A correlative analysis using micro-computed tomography and histology/scanning electron microscopy. Int. Endod. J. 2018, 51, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Negishi, J.; Kawanami, M.; Ogami, E. Risk analysis of failure of root canal treatment for teeth with inaccessible apical constriction. J. Dent. 2005, 33, 399–404. [Google Scholar] [CrossRef]
- Rossi-Fedele, G.; Ahmed, H.M. Assessment of Root Canal Filling Removal Effectiveness Using Micro-computed Tomography: A Systematic Review. J. Endod. 2017, 43, 520–526. [Google Scholar] [CrossRef]
- Ferreira, I.; Babo, P.S.; Braga, A.C.; Gomes, M.E.; Pina-Vaz, I. Effect of Sonic Agitation of a Binary Mixture of Solvents on Filling Remnants Removal as an Alternative to Apical Enlargement-A Micro-CT Study. J. Clin. Med. 2020, 9, 2465. [Google Scholar] [CrossRef]
- Ferreira, I.; Pina-Vaz, I. The Novel Role of Solvents in Non-Surgical Endodontic Retreatment. Appl. Sci. 2022, 12, 5492. [Google Scholar] [CrossRef]
- Canakci, B.C.; Er, O.; Dincer, A. Do the Sealer Solvents Used Affect Apically Extruded Debris in Retreatment? J. Endod. 2015, 41, 1507–1509. [Google Scholar] [CrossRef]
- Kfir, A.; Tsesis, I.; Yakirevich, E.; Matalon, S.; Abramovitz, I. The efficacy of five techniques for removing root filling material: Microscopic versus radiographic evaluation. Int. Endod. J. 2012, 45, 35–41. [Google Scholar] [CrossRef]
- Takahashi, C.M.; Cunha, R.S.; de Martin, A.S.; Fontana, C.E.; Silveira, C.F.; da Silveira Bueno, C.E. In vitro evaluation of the effectiveness of ProTaper universal rotary retreatment system for gutta-percha removal with or without a solvent. J. Endod. 2009, 35, 1580–1583. [Google Scholar] [CrossRef]
- Horvath, S.D.; Altenburger, M.J.; Naumann, M.; Wolkewitz, M.; Schirrmeister, J.F. Cleanliness of dentinal tubules following gutta-percha removal with and without solvents: A scanning electron microscopic study. Int. Endod. J. 2009, 42, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.J.; Rhodes, J.S.; Ford, T.R. The efficacy of gutta-percha removal using ProFiles. Int. Endod. J. 2001, 34, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Tamse, A.; Unger, U.; Metzger, Z.; Rosenberg, M. Gutta-percha solvents—A comparative study. J. Endod. 1986, 12, 337–339. [Google Scholar] [CrossRef]
- Martos, J.; Bassotto, A.P.; Gonzalez-Rodriguez, M.P.; Ferrer-Luque, C.M. Dissolving efficacy of eucalyptus and orange oil, xylol and chloroform solvents on different root canal sealers. Int. Endod. J. 2011, 44, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.; Grenho, L.; Gomes, P.; Braga, A.C.; Fernandes, M.H.; Lopes, M.A.; Pina-Vaz, I. Efficacy and Cytotoxicity of Binary Mixtures as Root Canal Filling Solvents. Materials 2020, 13, 3237. [Google Scholar] [CrossRef]
- Ferreira, I.; Babo, P.S.; Braga, A.C.; Lopes, M.A.; Gomes, M.E.; Pina-Vaz, I. Supplementary solvent irrigation efficacy on filling remnants removal comparing XP-endo Finisher R vs IrriSafe. Sci. Rep. 2021, 11, 12659. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, F.; Camilleri, J. A critical review of the material properties guiding the clinician’s choice of root canal sealers. Clin. Oral Investig. 2023, 27, 4147–4155. [Google Scholar] [CrossRef]
- Guivarc’h, M.; Jeanneau, C.; Giraud, T.; Pommel, L.; About, I.; Azim, A.A.; Bukiet, F. An international survey on the use of calcium silicate-based sealers in non-surgical endodontic treatment. Clin. Oral Investig. 2020, 24, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Drukteinis, S.; Bilvinaite, G.; Tusas, P.; Shemesh, H.; Peciuliene, V. Porosity Distribution in Single Cone Root Canal Fillings Performed by Operators with Different Clinical Experience: A microCT Assessment. J. Clin. Med. 2021, 10, 2569. [Google Scholar] [CrossRef]
- Al Akam, H.; Kim, H.C.; Jeong, J.W. Retreatment Strategies for Cases Containing Calcium Silicate-Based Root Canal Sealers: A Comprehensive Review. Dent. J. 2024, 12, 41. [Google Scholar] [CrossRef]
- Oltra, E.; Cox, T.C.; LaCourse, M.R.; Johnson, J.D.; Paranjpe, A. Retreatability of two endodontic sealers, EndoSequence BC Sealer and AH Plus: A micro-computed tomographic comparison. Restor. Dent. Endod. 2017, 42, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Garrib, M.; Camilleri, J. Retreatment efficacy of hydraulic calcium silicate sealers used in single cone obturation. J. Dent. 2020, 98, 103370. [Google Scholar] [CrossRef]
- Carrillo, C.A.; Kirkpatrick, T.; Freeman, K.; Makins, S.R.; Aldabbagh, M.; Jeong, J.W. Retrievability of Calcium Silicate-based Root Canal Sealers During Retreatment: An Ex Vivo Study. J. Endod. 2022, 48, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, G.; Liu, X.; Jalali, P. Efficacy of Different Solvents for Achieving Patency in Teeth Obturated Using Bioceramic Sealer. J. Endod. 2023, 49, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.C.; Neves, G.S.T.; Kirkpatrick, T.; Letra, A.; Silva, R. Physicochemical and Biological Properties of AH Plus Bioceramic. J. Endod. 2023, 49, 69–76. [Google Scholar] [CrossRef]
- Sanz, J.L.; López-García, S.; Rodríguez-Lozano, F.J.; Melo, M.; Lozano, A.; Llena, C.; Forner, L. Cytocompatibility and bioactive potential of AH Plus Bioceramic Sealer: An in vitro study. Int. Endod. J. 2022, 55, 1066–1080. [Google Scholar] [CrossRef] [PubMed]
- Nagendrababu, V.; Murray, P.E.; Ordinola-Zapata, R.; Peters, O.A.; Rôças, I.N.; Siqueira, J.F., Jr.; Priya, E.; Jayaraman, J.; Pulikkotil, S.J.; Suresh, N.; et al. PRILE 2021 guidelines for reporting laboratory studies in Endodontology: Explanation and elaboration. Int. Endod. J. 2021, 54, 1491–1515. [Google Scholar] [CrossRef] [PubMed]
- De-Deus, G.; Barino, B.; Zamolyi, R.Q.; Souza, E.; Fonseca, A., Jr.; Fidel, S.; Fidel, R.A. Suboptimal debridement quality produced by the single-file F2 ProTaper technique in oval-shaped canals. J. Endod. 2010, 36, 1897–1900. [Google Scholar] [CrossRef]
- Pedullà, E.; Iacono, F.; Pitrolo, M.; Barbagallo, G.; La Rosa, G.R.M.; Pirani, C. Assessing the impact of obturation techniques, kinematics and irrigation protocols on apical debris extrusion and time required in endodontic retreatment. Aust. Endod. J. 2023, 49, 623–630. [Google Scholar] [CrossRef]
- AAE. American Association of Endodontists. Glossary of Endodontic Terms. Available online: https://www.aae.org/specialty/clinical-resources/glossary-endodontic-terms/ (accessed on 2 February 2025).
- Ricucci, D.; Loghin, S.; Gonçalves, L.S.; Rôças, I.N.; Siqueira, J.F., Jr. Histobacteriologic Conditions of the Apical Root Canal System and Periapical Tissues in Teeth Associated with Sinus Tracts. J. Endod. 2018, 44, 405–413. [Google Scholar] [CrossRef]
- Ng, Y.L.; Mann, V.; Gulabivala, K. A prospective study of the factors affecting outcomes of nonsurgical root canal treatment: Part 1: Periapical health. Int. Endod. J. 2011, 44, 583–609. [Google Scholar] [CrossRef] [PubMed]
- Hess, D.; Solomon, E.; Spears, R.; He, J. Retreatability of a bioceramic root canal sealing material. J. Endod. 2011, 37, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.; Ferreira, C.M.; Pinto, K.P.; Barbosa, A.F.A.; Colaço, M.V.; Sassone, L.M. Influence of variations in the environmental pH on the solubility and water sorption of a calcium silicate-based root canal sealer. Int. Endod. J. 2021, 54, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Drukteinis, S.; Bilvinaite, G.; Sakirzanovas, S. The Impact of Citric Acid Solution on Hydraulic Calcium Silicate-Based Sealers and Root Dentin: A Preliminary Assessment. Materials 2024, 17, 1351. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Che, Y.; Leng, F. Calcium leaching behavior of cementitious materials in hydrochloric acid solution. Sci. Rep. 2018, 8, 8806. [Google Scholar] [CrossRef] [PubMed]
- De-Deus, G.; Belladonna, F.G.; Zuolo, A.S.; Simões-Carvalho, M.; Santos, C.B.; Oliveira, D.S.; Cavalcante, D.M.; Silva, E.J.N.L. Effectiveness of Reciproc Blue in removing canal filling material and regaining apical patency. Int. Endod. J. 2019, 52, 250–257. [Google Scholar] [CrossRef]
- Chybowski, E.A.; Glickman, G.N.; Patel, Y.; Fleury, A.; Solomon, E.; He, J. Clinical Outcome of Non-Surgical Root Canal Treatment Using a Single-cone Technique with Endosequence Bioceramic Sealer: A Retrospective Analysis. J. Endod. 2018, 44, 941–945. [Google Scholar] [CrossRef]
- Kharouf, N.; Sauro, S.; Eid, A.; Zghal, J.; Jmal, H.; Seck, A.; Macaluso, V.; Addiego, F.; Inchingolo, F.; Affolter-Zbaraszczuk, C.; et al. Physicochemical and Mechanical Properties of Premixed Calcium Silicate and Resin Sealers. J. Funct. Biomater. 2022, 14, 9. [Google Scholar] [CrossRef]
- Alouda, M.; Akil, S.; Eid, A.; Cardinali, F.; Achour, H.; Haikel, Y.; Kharouf, N. Retreatment of Two Bioceramic Sealers Included Two Different Percentages of Calcium Silicate Using Two Endodontic File Systems: An In Vitro Study. Eur. J. Dent. 2025. [Google Scholar] [CrossRef]
- Raman, V.; Camilleri, J. Characterization and Assessment of Physical Properties of 3 Single Syringe Hydraulic Cement-based Sealers. J. Endod. 2024, 50, 381–388. [Google Scholar] [CrossRef]
- Chen, J.H.; Raman, V.; Kuehne, S.A.; Camilleri, J.; Hirschfeld, J. Chemical, Antibacterial, and Cytotoxic Properties of Four Different Endodontic Sealer Leachates Over Time. J. Endod. 2024, 50, 1612–1621. [Google Scholar] [CrossRef] [PubMed]
- Creazzo, G.; de Barros Ciribelli Alves, B.M.; de Assis, H.C.; Villamayor, K.G.G.; de Sousa-Neto, M.D.; Mazzi-Chaves, J.F.; Lopes-Olhê, F.C. Bond Strength and Adhesive Interface Quality of New Pre-Mixed Bioceramic Root Canal Sealer. Microsc. Res. Tech. 2025. early view. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).