Abstract
Stapling devices have emerged as a widespread and effective option for soft tissue surgery, offering promising outcomes for patients by reducing complication rates and surgery time. This review aims to provide an exhaustive analysis of commercially available alternatives in the market, incorporating insights from market analysis, patent landscape, and the existing literature. The main focus lies in identifying and evaluating the most widely adopted and innovative stapling devices, including linear, linear cutting, circular, and powered staplers. In addition, this review delves into the realm of bioabsorbable staples, exploring the materials utilized and the surgical fields where these advanced staples find applications. To facilitate easy comprehension, the gathered information is presented in tables, highlighting the essential parameters for each stapling device. This comprehensive research about stapling devices is intended to aid healthcare practitioners and researchers in making informed decisions when choosing the most appropriate instrument for specific surgical procedures.
1. Introduction
In this work, surgical staplers, which are internal use devices employed to deliver staplers to tissues inside the body during surgery for removing part of an organ, cutting through and sealing organs, and creating connections between structures, are covered.
Modern surgical staplers are designed to be disposable, with a maximum number of firing actions, as well as cartridges and other products used for surgical stapling. Today’s staples have a rectangular cross-section and become "B" shaped when compressed against the anvil of the stapler to reduce the risk of ischemia, allowing blood to flow through them to the tissue edges. The specific geometry has been developed to ensure hemostasis and to allow sufficient vascularization of the tissue, supporting wound healing [1].
Three major types of stapling devices used both in open and laparoscopic surgery can be identified: linear, linear cutting, and circular. Linear instruments form a straight staple line and may or may not include a blade, while circular instruments have staples set in a crown shape and always include a blade. The ever-increasing diffusion of laparoscopy and robotic surgery led to the diffusion of stapling devices dedicated to endoscopic techniques; linear and circular stapling devices are employed mostly in gastrointestinal procedures such as complex gasterectomies, bariatric treatments, and colorectal oncological resections. Devices have been compared in terms of procedure duration, hospital stay, wound infection, anastomotic leakage, and anastomotic structures.
The outcome of the repair made by staples or suture techniques has attracted much interest in the scientific community. Recently, the mechanical behavior of repairs made with staples, sutures, and hybrid methods (staple and suture together) was evaluated carefully, showing that staples can resist high loads but are less deformable and rigid than handsewn sutures. This suggests safer employment in the case of small defects or diastasis, where the presumed tissue displacement is minimal. A hybrid repair improves its efficiency, becoming crucial in case of larger defects where the expected tissue displacement is maximal [2].
Although being more complex to operate and resulting in longer procedure times, in several studies covering gastric, bariatric, and colorectal surgery, linear staplers appeared to be preferred in clinical use due to their lower rate of anastomosis leakage and anastomosis strictures, with a sensible reduction in patients’ postoperative morbidity [3,4,5,6].
Regardless of its specific configuration, a surgical stapler is always characterized by an actuation handle: the surgeon can operate the device manually through a sequence of squeezing a handle or sliding a knob. The effective function of stapling, cutting, and ejecting the staples is performed by the loading unit: two jaws are coupled in different ways to act, one as a cartridge loader for the staples and the other as an anvil to allow the closing of the staples. In the case of circular staplers, the anvil jaw is detachable, granting its insertion into the anastomosis site. The actuation handle and the loading unit are usually connected to each other: the input from the surgeon is translated into the mechanical or electro-mechanical firing system through the use of various keyed shafts [7]. Additional pins can be inserted into the jaws or be comprised into the loading unit to serve as alignment or safety devices, ensuring the correct compression of the tissue before firing or avoiding retention after staple delivery.
Each device is completely in accordance with the aims of surgical stapling, which consist, from a medical point of view, of creating an adequate lumen, preserving adequate vascularization, preventing tension in tissues, avoiding leakage, providing hemostasis, and ensuring mechanical reliability [8].
Non-absorbable staples are the ones most widely used in clinics thanks to their low artifact production in CT and their non-magnetic behavior, which causes minimal distortions in MRI. Titanium is the leading material, both pure or in alloys, thanks to its high resistance and lighter weight compared to stainless steel. Furthermore, titanium is corrosion-resistant but, most importantly, anti-allergic, and it can also be employed in patients suffering from chromate-nickel allergies [1,9].
The general design of stapling devices includes an anvil, a cartridge assembly to support an array of staples, a mechanism to push the cartridge close to the anvil, an alignment pin for capturing tissue between the cartridge and the anvil, and a firing mechanism for ejecting the surgical staples. The firing process is guided by a sequence of pressing the handle: the first press grants tissue compression and allows the surgeon to verify the correct alignment of the jaws, while the second press releases the staples. A button placed on the stapler permits the opening of the jaws at the end of the operation, setting the tissue free and allowing the reloading process.
The main particularity of cutting staplers is the addition of a blading element that allows for making a resection in the middle of the rows of staples. Instead, for endo cutting staplers, the instrument is composed of a disposable articulating end effector comprising the cartridge, the anvil, and a disposable handling unit containing the firing mechanism and the safety control system. The circular staplers have the same components with the particularity of a circular anvil and arrangement of the staples with a blading element. The powered stapling devices have the distinction of a powered actuation, meaning that the firing mechanism is controlled by an electro-mechanical system that gains energy from a battery. Figure 1 shows a description and representation of each type of device.
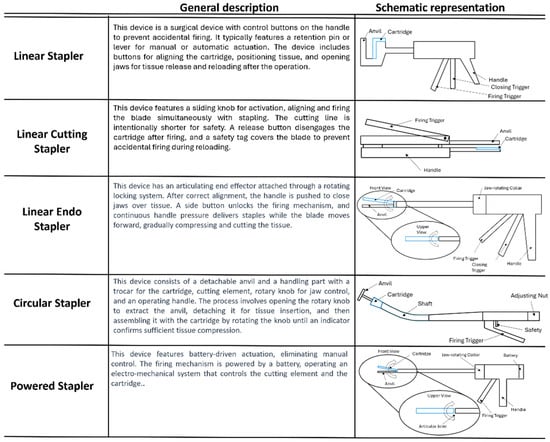
Figure 1.
Description of key components for each type of device. The cartridge is highlighted in blue.
Absorbable Staples
The use of absorbable staples is mainly spreading in dermal suturing and in performing ligations [10,11,12,13,14,15]. Currently, considering the bariatric, gastrointestinal, vascular, and thoracic surgery fields, which are the main fields of application of staplers, the only commercially available absorbable staple is Medtronic Polysorb [16,17,18], while other bioresorbable magnesium and zinc alloys are still a matter of research. Although studies claim to match the clinical requirements for a secure anastomosis, their technology is yet to be implemented by manufacturers on a production scale [19,20].
Another field of application for staplers is deep dermal suturing. In particular, in plastic surgery, the use of staplers has been adopted and provides wound healing outcomes that are better than those of conventional suturing [15].
Staplers are also used in orthopedics to secure soft tissues to bone [21,22,23]. Few applications have been found comprising staples in the process of suturing soft tissues in meniscal or rotator cuff repair, the latter involving scaffold placement [24,25].
When comparing devices of each type, a variety of features must be considered since each manufacturer proposes its own combination of specifications. In this work, all available characteristics of surgical staplers will be presented to compare the available alternatives for the three different classes of staplers.
2. Materials and Methods
The first step was the analysis of the current surgical stapling market through ECRI Institute archives, which allowed us to identify the main competitors on the global scene (Ethicon (Johnson & Johnson, New Brunswick, NJ, USA) endo-Surgery and Covidien (Medtronic, Minneapolis, MN, USA)). The Italian market was then assessed using information accessed from Ministry of Health reports. In light of the Italian public health system’s data, Panther Medical (Beiqijia, Beijing, China) and Sinolinks (Zhonglou Zone, Changzhou, China) were taken into consideration for the analysis.
After choosing the manufacturers, their websites were consulted, and all the available technical documentation was extracted. The main parameters to make a comparison were identified as staple line length for linear devices, staple line diameter for circular devices, open and closed staple height, the maximum number of firings in case of reloadable devices, the maximum number of rows of staples placed on each side of the suturing line, and length of the shaft in case of Endo-related devices.
Each device was identified both by its commercial name and by its product code, allowing for a better univocal identification. To further explore the design and the functioning mechanism of each product, some of the patents have been assessed through OrbitExpress online (www.questel.com) with the search query shown in Table 1 (the search was done in January 2021). Among the 416 retrieved results, 216 have been included based on their International Patent Classification, these being A61B-017/068, A61B-017/072, or A61B-017/115, classes specifically dedicated to surgical staplers. Patents have been scanned on the basis of title and abstract and on the basis of their latest available and granted version, resulting in 38 included documents. The decisional process is described in Figure 2. For all other devices, it was not possible to identify the patent documentation because of a lack of correspondence between commercial and technical denominations or because of intellectual property restrictions and trade secrets. Finally, specifications coming from the FDA medical devices database, which were assessed using manufacturers’ names and product codes as keywords for the query, were recorded.

Table 1.
Patent search queries using OrbitExpress Questel software.
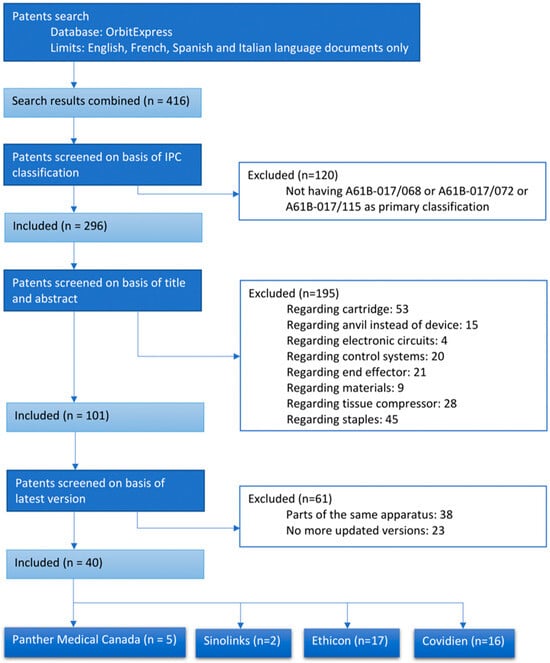
Figure 2.
Patent inclusion flowchart.
3. Results
In this section, the information collected about the different classes of staplers was divided into subsections to organize the findings better. The crucial characteristics of the staplers were organized in tables to summarize. When some information was not available on the website an n.a (not available) was reported.
3.1. Linear Staplers
Linear devices are employed mostly in gastrointestinal procedures [26]. Other uses of linear staplers are abdominal surgery, gynecology, vascular surgery, and pediatric surgery.
Table 2 summarizes the principal characteristics of linear staplers in commerce by manufacturer. In particular, the commercial name and product code are reported according to the manufacturer. Other features that are reported include the staple line length, with values ranging between 30 mm and 60 mm for all manufacturers, while 45 mm and 90 mm sizes are proposed by everyone except Ethicon. Staple height depends on the cartridge mounted on the stapler: values vary from 2.5 mm proposed by Ethicon and Covidien for vascular applications to 4.8 mm for all the manufacturers for thicker tissues. For applications with a medium thickness, 3.5 mm is the chosen value, with only Sinolinks and Ethicon offering 3.8 mm and 3.85 mm alternatives. The height of the closed staple is quite standardized: 1.5 mm is ensured by all manufacturers, and 2 mm is available in every catalog except from Sinolinks, which offers a 1.8 mm alternative. Several rows placed by the instruments are also standardized—only Covidien and Ethicon propose a version of the stapler specific for vascular applications, which grants a height of 1mm but requires three rows of staples instead of two. The exceeding row is determined to be useful in providing a secure anastomosis and avoiding blood leakage issues.
Several studies in the literature analyzed the effects of linear stapler suturing and handsewn methods [26,27,28]. In a review, Choy et al. [27] compare linear stapled versus handsewn methods for ileocolic anastomosis. The authors analyzed different outcome measures like clinical and radiological anastomotic leakage, anastomotic stricture, hemorrhage, and time. Their results showed that stapled functional end-to-end ileocolic anastomosis is associated with fewer anastomotic leaks than handsewn anastomosis and should be considered the gold standard.
A meta-analysis of Gong et al. [26] analyzes stapled vs. hand suture closure of loop ileostomy. The authors affirm that stapled loop ileostomy reversal is superior to hand sutures in reducing postoperative small bowel obstruction, operation time, and hospital stay, and therefore, stapled side-to-side anastomosis should be considered the preferred technique.
According to the study of Giaccaglia et al. [9], in which three linear staplers of different companies were compared, small differences emerged between the devices regarding anastomotic pressure resistance and tensile strength. Many studies have confirmed the advantages of using linear staplers over circular staplers. Regarding a study on 213 patients with gastric cancer who underwent laparoscopic total gastrectomy, although mean operation time in the linear stapler group was longer than the circular stapler group, using linear staplers, anastomosis leakages and costs related to anastomosis were lower, and anastomosis stenosis did not occur. These advantages led scientists to prefer linear staplers in laparoscopic total gastrectomy [6].
Linear staplers are more effective than circular staplers in laparoscopic gastrojejunal (GJ) anastomosis in morbid obesity surgery [3].

Table 2.
Linear stapler characteristics.
Table 2.
Linear stapler characteristics.
| Brand | Commercial Name | Code | Staple Line Length [mm] | Staple Height [mm] | No. of Rows | Closed Staple Height [mm] | Firing Actions | Surgery Application and Relevant Info | Reference |
|---|---|---|---|---|---|---|---|---|---|
| PANTHER HEALTHCARE | Linear Stapler | FLSLF30 FLSLF45 FLSLF60 FLSLF90 | 30.0 45.0 60.0 90.0 | 3.50 4.80 | 2 | 1.5 2.0 | It can be reloaded up to 7 times for a total of 8 firings in a single procedure. | n.a | (Panther Healthcare, Open Linear Staplers) [29] |
| SINOLINKS | Disposable Linear Stapler | DLS B DLS C DALS A DLS A | 30.0 | 3.80 4.80 | n.a | 1.5 1.8 | n.a | In gastrointestinal surgery, they are used for closure of the stump or incision in digestive tract reconstruction and viscera resection. Features: - DLS C: Three rows of staples provide better hemostasis. - DALS A: Two-stage closure for easy tissue adjustment. - DLS A: Manual integrated tissue positioning needle and flexible operation. | (Sinolinks, Linear Staplers) |
| SINOLINKS | Disposable Linear Stapler | DLS B DLS C DALS A DLS A | 45.0 | 3.80 4.80 | n.a | 1.5 1.8 | n.a | (Sinolinks, Linear Staplers) | |
| SINOLINKS | Disposable Linear Stapler | DLS B DLS C DALS A DLS A | 60.0 | 3.80 4.80 | n.a | 1.5 1.8 | n.a | (Sinolinks, Linear Staplers) | |
| SINOLINKS | Disposable Linear Stapler | DLS B DLS C DALS A DLS A | 90.0 | 3.80 4.80 | n.a | 1.5 1.8 | n.a | (Sinolinks, Linear Staplers) | |
| COVIDIEN | DST Series™ TA™ Stapler | TA30V3S TA3035S TA3048S | 30.0 | 2.50 (vascular version) 3.50 4.80 | 3 2 2 | 1.0 1.5 2.0 | It can be reloaded up to 7 times for a total of 8 firings in a single procedure. | The version with a staple height of 2.5 mm is for vascular applications. | (Medtronic, TA™ Stapler with DST Series™ Technology,) [30] |
| COVIDIEN | DST Series™ TA™ Stapler | TA4535S TA4548S | 45.0 | 3.50 4.80 | 2 | 1.5 2.0 | It can be reloaded up to 7 times for a total of 8 firings in a single procedure. | n.a | (Medtronic, TA™ Stapler with DST Series™ Technology) [30] |
| COVIDIEN | DST Series™ TA™ Stapler | TA6035S TA6048S | 60.0 | 3.50 4.80 | 2 | 1.5 2.0 | It can be reloaded up to 7 times for a total of 8 firings in a single procedure. | n.a | (Medtronic, TA™ Stapler with DST Series™ Technology) [30] |
| COVIDIEN | DST Series™ TA™ Stapler | TA9035S TA9048S | 90.0 | 3.50 4.80 | 2 | 1.5 2.0 | It can be reloaded up to 7 times for a total of 8 firings in a single procedure. | n.a | (Medtronic, TA™ Stapler with DST Series™ Technology) [30] |
| ETHICON | PROXIMATE® Reloadable Staplers | TX30V TX30B TX30G | 30.0 | 2.50 (vascular version) 3.50 4.80 | 3 2 2 | 1.0 1.5 2.0 | Max firings: 8 | It has applications throughout the alimentary tract and in thoracic surgery. The vascular version has applications in internal tissue, which can easily be compressed to 1mm in thickness and to ligate pulmonary vessels. | (Ethicon, PROXIMATE Reloadable Linear Stapler) [31] |
| ETHICON | PROXIMATE® Reloadable Staplers | TX60B TX60G | 60.0 | 3.85 4.80 | 2 | 1.5 2.0 | Max firings: 8 | It has applications throughout the alimentary tract and in thoracic surgery. | (Ethicon, PROXIMATE Reloadable Linear Stapler) [31] |
Linear Staplers with Bioresorbable Staples
Among all the manufacturers, only Covidien and CooperSurgical (Trumbull, Connecticut, Stati Uniti) include linear staplers mounting absorbable staples. The device of Covidien, TA Premium Polysorb (see Table 3), has the same design and functioning as traditional linear staplers. The available staple length is declared to be 55 mm. Each cartridge is intended to deliver a double staggered row of staples with a closed staple height that can be either 1.5 mm or 5 mm, depending on the staple filament width. The proposed device by CooperSurgical, INSORB®, contains 20 individual, horseshoe-shaped staples with the following features: the staple is nominally 5 mm long × 3.5 mm wide × 0.7 mm thick. The staple is composed of an absorbable copolymer of predominantly polylactide and a lesser component of polyglycolide [32]. A study that compares the use of INSORB® and Subcuticular Skin Closure showed that the two treatment arms did not appear to differ [15,33].

Table 3.
Linear staplers with absorbable characteristics.
During the search for bioresorbable staplers, a promising device for staple line reinforcement was found. In particular, FOREseal is made of polysaccharidic polyglycuronate biopolymers (highly purified fractions from calcium alginates) originating from seaweeds. It creates an interface between the staples and the organ wall and then forms a hemostatic, healing bioadhesive gel through the release of its calcium ions. The preliminary study on pancreatic parenchyma highlighted that the use of this new device to reinforce the staple line during pancreatectomy was well tolerated [33] and showed promising results for lung staple line reinforcement [34].
In a study conducted in France on six hundred sixty-four patients regarding the dermal suture, it was seen that the suture with semiautomatic stapler is a potential alternative to the one with conventional thread, and regarding patient scar assessment scale score at 3 months or after 18 months, the medium operating time and complication rates were equivalent. Instead, the average suture time and occupational exposure to blood were significantly lower in the stapler [9]. A study conducted on 176 patients confirmed the effectiveness of bioresorbable staples, showing a decreased incidence of composite wound complications with subcuticular staple closure versus traditional staple closure in patients undergoing cesarean section [35].
Another study conducted on 150 patients who underwent distal pancreatectomy affirms that the use of bioresorbable staples, in particular absorbable Lactomer staples for distal pancreatic resection, can be a safe alternative to the standard closure technique [18].
3.2. Linear Cutting Staplers
Linear cutting staplers are employed in open surgery to seal and resect tissues at the same time. The device allocates two or four rows of staggered staples while making a resection in the middle of the rows through the blading element.
Table 4 reports the main characteristics of linear cutting staplers. Panther Medical and Sinolinks offer the same staple line lengths of 60 mm, 80 mm, and 100 mm, while Covidien (part of Medtronic) offers 64 mm, 84 mm, and 104 mm staple line lengths, and Ethicon (part of Johnson & Johnson) proposes different lines of devices varying from the 45 mm staple line length of ENDOPATH® ETS Articulating Linear Cutters to the 102 mm of PROXIMATE® Linear Cutters series. Ethicon ENDOPATH was used in a novel technique that avoids entry to the chest cavity and minimizes the use of electrocautery on the diaphragm [36].
Panther Medical and Sinolinks provide cartridges having the same staple height of 3.8 mm or 4.8 mm, Covidien provides a 2.5 mm option for vascular purposes, while Ethicon offers a variety of sizes covering an interval from 2.6 mm to 4.4 mm depending on the considered series of devices and on the application. As can be seen in Table 3, all producers ensure the stapler reloading a maximum of seven times, resulting in a maximum of eight firings during the same operation. Only the Ethicon brand proposes two devices reaching 12 maximum firings. The Ethicon endo-Surgery Linear Cutter was adopted for gracillis muscle flap splitting in facial reanimation, allowing for a perfect resection of the gracillis muscle [37].
The closed staple height is 1.5 and 2 mm for3.8 and 4.8 mm staple height, respectively, but it reaches a minimum of 1 mm for Ethicon and Covidien cartridges with vascular purposes. Ethicon also offers a 1.8 mm option for medium-thickness tissue application. Covidien linear cutting staplers DST series GIA have been used to prevent blood loss during cesarean delivery [38].
Recently, Meditulip developed a Novel Asymmetrical Linear Stapler (NALS). In comparison with the other liner cutting staplers, on the resected organ side, there is a single row of titanium fasteners located farthest from the endocutter in the stapler device. Thanks to the new design, the stapler can provide tissue for a frozen section biopsy at the true resection margin without the titanium fasteners injuring the tissue [39].
Standard Bariatrics (Cincinnati, OH, USA) has developed a novel linear stapler called Standard Titan to perform a sleeve gastrectomy with a single stapler firing. This device aims to decrease technical and mechanical failure by eliminating junctions along the staple line, decreasing tissue manipulation and operative time while optimizing gastric tissue compression [40].
The performance of the linear cutter stapler (Ethicon Endo-Surgery Linear Cutters) for muscle transferring was tested by Nasir [37]. The results showed a very clear and precise division of the muscle edges; the titanium clips prevent muscle fibril separation, resulting in an easy and safe procedure even after splitting the muscle [37]. The advantages of Ethicon Endo-Surgery Linear Cutters were also reported in a new approach for cutting and closing the pancreas during pancreatojejunostomy with positive results [41] and to perform cervical esophagogastric anastomosis using the triangulating stapling technique [42].
Only one study in the literature reported on the performance of the NALS device developed by Meditulip (Cheongju-si, Chungcheongbuk-do, Republic of Korea). In particular, the NALS stapler was evaluated in the examination of animal lung tissue after the procedure of resection. In comparison with the same typology of staplers, the NALS device showed no squeezing artifact at the resection margin on microscopic examination, and all of the alveolar structures for evaluation were preserved [39]. However, no other information was found on this new linear cutter stapler, and a modification was reported to improve the pathologic evaluation of the true surgical margin after the use of the Ethicon ENDOPATH stapler [43].
Linear cutting staplers are widely used in gastrointestinal surgery. The study carried out by Cheng et al. [44] on 10 patients who underwent laparoscopic total gastrectomy highlighted a safe and effective procedure with the linear cutting stapler [44].
In addition, the efficacy of this type of stapler in a new procedure for anastomosis between the ileum and colon was evaluated and continues to show promising results [39].
Another surgery in which linear cutting staplers are well established consists of laparoscopic sleeve gastrectomy (established standard of care), the most common bariatric procedure. In particular, the stapler is used to resect the lateral portion of the stomach [40]. In comparison with the other two staplers adopted in this surgery, Standard Titan highlighted the mechanical benefits of withstanding higher burst pressure with the notable advantage of single-load functionality. Clearly, the new single staple load eliminates staple line junctions without sacrificing the integrity of staple formation along the staple line [40].

Table 4.
Linear cutting stapler characteristics.
Table 4.
Linear cutting stapler characteristics.
| Brand | Commercial Name | Code | Staple Line Length [mm] | Staple Height [mm] | No. of Rows | Close Staple Height [mm] | Firing Actions | Surgery Application and Relevant Info | Reference |
|---|---|---|---|---|---|---|---|---|---|
| PANTHER HEALTHCARE | Linear Cutter Stapler | SSAB-60 | 60.0 | 3.8 4.8 | 2 | 1.5 2.0 | It can be reloaded up to 7 times for a total of 8 firings in a single procedure. | n.a | (Panther Healthcare) [45] |
| PANTHER HEALTHCARE | Linear Cutter Stapler | SSAB-80 | 80.0 | 3.8 4.8 | 2 | 1.5 2.0 | (Panther Healthcare) [45] | ||
| PANTHER HEALTHCARE | Linear Cutter Stapler | SSAB-100 | 100.0 | 3.8 4.8 | 2 | 1.5 2.0 | (Panther Healthcare) [45] | ||
| SINOLINKS | Disposable Linear Cutter Stapler | DLC B-60 | 63.0 | 3.8 4.8 | n.a | n.a | n.a | Used for removing and suturing organs in gastrointestinal surgery and general surgery. | (Sinolinks, Disposable Linear Cutter Stapler) |
| SINOLINKS | Disposable Linear Cutter Stapler | DLC B-80 | 83.0 | 3.8 4.8 | n.a | n.a | (Sinolinks, Disposable Linear Cutter Stapler) | ||
| SINOLINKS | Disposable Linear Cutter Stapler | DLC B-100 | 103.0 | 3.8 4.8 | n.a | n.a | (Sinolinks, Disposable Linear Cutter Stapler) | ||
| COVIDIEN | GIA™ Stapler with DST Series™ Technology | GIA6025S GIA6038S GIA6048S | 66.0 | 2.5 3.8 4.8 | 4 | 1.0 1.5 2.0 | It can be reloaded for a total of 8 firings. | n.a | (Medtronic, GIA™ Stapler with DST Series™ Technology) [46] |
| COVIDIEN | GIA™ Stapler with DST Series™ Technology | GIA8038S GIA8048S | 86.0 | 3.8 4.8 | 4 | 1.5 2.0 | It can be reloaded for a total of 8 firings. | n.a | (Medtronic, GIA™ Stapler with DST Series™ Technology) [46] |
| COVIDIEN | GIA™ Stapler with DST Series™ Technology | GIA10038S GIA10048S | 106.0 | 3.8 4.8 | 4 | 1.5 2.0 | It can be reloaded for a total of 8 firings. | n.a | (Medtronic, GIA™ Stapler with DST Series™ Technology) [46] |
| COVIDIEN | GIA™ Stapler with Tri-Staple™ Technology | (Thin/medium) GIA60TMS GIA60TMC (Medium/thick) GIA60MTS GIA60MTC (Extra thick) GIA60XTS GIA60XTC (Thin/medium) GIA80TMS GIA80TMC (Medium/thick) GIA80MTS GIA80MTC (Extra thick) GIA80XTS GIA80XTC | 60 80 | 2.4 2.7 3.0 3.5 4.0 4.5 5.0 | 3 | n.a | It can be reloaded for a total of 8 firings. | Features: - Stronger staple line leak pressure compared to two-row staplers. - Less stress on tissue during compression and clamping. - A stepped cartridge face and varied-height staples that may provide greater perfusion to the staple line. - Consistent performance over a broad range of tissue thickness. | (Medtronic, GIA™ Stapler with DST Series™ Technology) |
| ETHICON | Ethicon Surgery Linear Cutters | NTLC55 SR55 | 55.0 | 4.4 | 6 | 1.5 1.8 2.0 | It can be reloaded for a total of 12 firings. | It has applications in gastrointestinal, gynecologic, thoracic, and pediatric surgery for transection, resection, and the creation of anastomoses and can be used with staple line or tissue buttressing materials. | (Ethicon, Linear Cutters) [47] |
| ETHICON | Ethicon Surgery Linear Cutters | NTLC75 SR75 | 75.0 | 4.4 | 6 | 1.5 1.8 2.0 | (Ethicon, Linear Cutters) [48] | ||
| ETHICON | PROXIMATE® Linear Cutters | TVC55 TLC55 TCT55 | 57.0 | 2.6 3.8 4.5 | 4 | 1.0 1.5 2.0 | It can be reloaded for a total of 8 firings. | It has applications in gastrointestinal, gynecologic, thoracic, and pediatric surgery for transection, resection, and the creation of anastomoses and can be used with staple line or tissue buttressing materials. | (Ethicon, PROXIMATE® Linear Cutters) [49] |
| ETHICON | PROXIMATE® Linear Cutters | TLC75 TCD75 TCT75 | 77.0 | 3.8 4.2 4.5 | 4 | 1.5 1.8 2.0 | (Ethicon, PROXIMATE® Linear Cutters) [49] | ||
| ETHICON | PROXIMATE® Linear Cutters | TLC10 TCT10 | 102.0 | 3.8 4.5 | 4 | 1.5 2.0 | (Ethicon, PROXIMATE® Linear Cutters) [49] | ||
| ETHICON | ENDOPATH® ETS Articulating Linear Cutters (laparoscopic stapler) | ATS45-TR45W ATS45-6R45G ATS45-TR45G | 45.0 | 2.5 3.5 4.1 | 4 (TR45G) 6 (TR45W or 6R45B) | 1.0 1.5 2.0 | It can be reloaded for a total of 8 firings. | It has applications in multiple open or minimally invasive general, gynecologic, urologic, thoracic, and pediatric surgical procedures for transection, resection, and/or creation of anastomoses. | (Ethicon, ENDOPATH™ ETS Articulating Linear Cutter) [50] |
| STANDARD BARIATRICS | Standard Titan | n.a | 230 | n.a | n.a | 1.2 2.2 | n.a | The device is designed to help achieve a more consistent and symmetrical performing sleeve gastrectomy. Furthermore, the company may provide a staple line reinforcement with GORE® SEAMGUARD®, a bioabsorbable staple line reinforcement. | (Standard Bariatrics, Standard Titan) [51] |
Linear Cutting Staplers with Bioresorbable Staples
Covidien is the only manufacturer to provide a linear cutting stapler having the same design as those described in the previous paragraph but mounting absorbable staples (see Table 5). Poly GIA 75 places two double staggered rows of staples while a knife divides the tissue in between; the staple line length is declared to be 75 mm, and with a staple section width of 0.060 mm, it is possible to reach a closed staple height of 1.5 mm. Covidien also produces Premium Poly CS 57, specifically intended to be used in C-section suturing processes, mounting Polysorb absorbable staples. The design resembles that of linear staplers: the squeezing of a handle activates the firing and cutting mechanism, placing two rows of staples while a knife blade operates a resection between them.

Table 5.
Linear cutting staplers with bioresorbable staple characteristics.
For over 30 years, the standard of care for hysterotomy in the gravid uterus has been the Premium Poly CS stapler. Premium Poly CS stapler was developed for fetal surgery in the 1980s; the benefits of this stapler included simple use, hemostasis, membrane sealing, and fewer adverse effects upon future reproduction than metallic staplers [52].
Church et al. [53] compared the performance of a bipolar electrocautery device, the LigaSure Impact (Medtronic), and an ultrasonic dissection device, the Harmonic ACE + 7 Shears (Ethicon part of Johnson & Johnson), with the Premium Poly CS Stapler. As a result, the Harmonic ACE could create a hysterotomy that healed comparably to the CS Stapler after repair [53].
3.3. Endo Linear Cutting Staplers
The Endo linear cutting staplers are specialized medical instruments with a crucial role in various surgeries, particularly in gastrointestinal, colorectal, and thoracic procedures. In addition, the use of these devices has been reported for pregnant patients who have undergone surgery, with a low rate of complications reported [54].
The device features an adjustable articulating element controlled by a lever located on the upper part of the shaft. Most manufacturers allow for a 22° range of movement in one direction and 23° in the other, providing a total articulation movement of 45°.
In this work, Table 6 outlines the main characteristics of the Endo linear staplers. In detail, Panther Medical, Sinolinks, and Covidien offer a consistent triplet of shaft length options, ranging from 6 cm to 26 cm. However, Ethicon stands out with shaft length options of 34 cm and 44 cm and an additional 32 cm alternative specifically designed for vascular applications. On the other hand, the staple line length varies depending on the cartridge in use, with the standard options being 30, 45, and 60 mm, except for Sinolinks, which ensures staple line lengths of 31, 46, and 61 mm. Ethicon’s PVE35A model offers a specific staple line length of 35 mm, tailored for vascular applications, as evidenced above, which explains the difference in comparison to other models. Except for Ethicon, which presents a range of values from 2.6 mm for the vascular cartridge to 4.2 mm for the thick tissue cartridge, the open staple height is relatively standardized among manufacturers. The Endo GIA linear cutting stapler is reported to be used in vascular applications [55].
Each manufacturer employs a varying number of staple rows for their devices. Sinolinks and Ethicon use three rows on each side of the cutting line, whereas Panther Medical and Covidien opt for two rows on each side of the blade. This results in a total of four lines. To prioritize safety and prevent blood leakage while ensuring successful anastomosis, the staple line length exceeds the cutting line length by at least 5 mm. All the devices under consideration guarantee a 45° degree of reached articulation.
Endoscopic linear cutting staplers have garnered considerable attention and discussion within the scientific community due to their remarkable applications across various types of endoscopic surgeries. In particular, The Endo GIA™ linear stapler has proven to be highly effective and superior to its competitors, specifically in gastric laparoscopy surgery, surpassing products like ETS flex™. This superiority is attributed to its outstanding materials, durability, and reusability after multiple surgeries, resulting in improved clinical outcomes. Notably, it exhibits exceptional efficacy in reducing excessive blood loss and minimizing human errors during procedures [4].
Endoscopic staplers are adopted for the treatment of patients with advanced lung emphysema. In particular, Akil et al. [56] evaluated the surgical performance of a traditional Endo linear stapler compared to a powered stapling system, reporting no difference in the amount of postoperative air leakage, the consecutive length of chest tube drainage, and the time of surgery [56].
In addition to this, some authors have further highlighted the innovative applications of Endo GIA™ linear staplers in pancreaticoduodenectomy and total pancreatectomy, addressing the challenges faced by many surgeons in these complex surgeries. Its use in these procedures offers significant benefits, including reduced bleeding, enhanced surgical precision, shorter operative times, and ultimately, decreased postoperative morbidity and mortality rates [57].
With regard to other manufacturers, Ethicon offers a range of device instruments specifically designed for minimally invasive surgical procedures, including gastrointestinal, vascular, and thoracic procedures, in comparison to Covidien products. In particular, recent studies have emphasized the remarkable efficacy of powered vascular staplers (PVS) in intricate surgical procedures, such as thoracoscopic anatomical surgery, where endoscopic staplers play a critical role in transecting vessels, bronchi, and lung tissue. The ECHELON FLEX™ (Ethicon PSE45A) powered articulating linear instrument stands out as the preferred choice for closing targeted bronchi, consistently leading to highly satisfactory clinical outcomes. Its advantage becomes evident in the context of limited surgical space, making it significantly advantageous over other commercially available powered vascular staplers. The ECHELON FLEX™ has proven its superiority and ease of use, offering equivalent levels of effectiveness and security. By integrating the ECHELON FLEX™ into surgical procedures, the overall complexity of the operation is reduced, and the potential for intraoperative complications is minimized [58,59].
Notably, the use of Ethicon powered staplers has been associated with lower rates of transfusion, as evident in comparison with Medtronic manual staplers. Moreover, recent studies have compellingly supported the use of the ECHELON FLEX™ for pulmonary artery stapling, showcasing its promise in preventing tissue damage and intraoperative bleeding during pulmonary artery transection procedures [60].
On the other hand, some authors’ reports have compellingly showcased the efficacy of the Panther and Sinolink families of linear staplers in laparoscopic total gastrectomy for cancer treatments. Among these breakthroughs, the intracorporeal construction of the anastomosis stands out, where endoscopic linear staplers have emerged as the preferred reconstruction method among esteemed surgeons worldwide, surpassing circular staplers in popularity and reliability. The overwhelming preference for linear stapled anastomosis over circular stapled anastomosis stems from its numerous advantages and simplified execution. Notably, the linear approach is widely regarded as being easier to perform, making it an attractive choice for surgeons seeking optimal outcomes and reduced surgical complexity [61,62].

Table 6.
Endo linear cutting stapler characteristics.
Table 6.
Endo linear cutting stapler characteristics.
| Brand | Commercial Name | Code | Shaft Length [cm] | Staple Line Length [mm] | Staple Height [mm] | No. of Rows | Close Staple Height [mm] | Firing Actions | Surgery Application and Relevant Info | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| PANTHER HEALTHCARE | Universal Endo Linear Cutter | CEAC 30 45 60 | 6 16 26 | 30 45 60 | 2.5 3.5 4.2 4.8 5.0 | 4 | n.a | It can be reloaded for a total of 25 firings. | (Panther Healthcare) [63] | |
| PANTHER HEALTHCARE | Endo Linear Stapler and P2G Cartridges | CADF-30T CADF-30N CADF-45T CADF-45N CADF-45S CADF-45D CADF-45R CADF-60T CADF-60N CADF-60N CADF-60S CADF-60D CADF-60R | 6 16 26 | 30 45 60 | 2.5 3.5 4.2 4.8 5.0 | n.a | n.a | n.a | Features: Dog bone-shaped staple buckets enhance tissue stability and provide improved staple formation in thick tissue. | (Panther Healthcare) [63] |
| SINOLINKS | Disposable Endoscopic Linear Cutter, GEN I and GEN II | DEC A/B 60 160 260 | 26 | 31 46 61 | 2 2.5 3.5 4.8 | 6 | 1.5 1.9 | It can be reloaded for a total of 25 firings. | It has applications in thoracic surgery, abdominal surgery, pediatric surgery, and gynecologic endoscopy procedures for transection, resection, and/or creation of anastomoses. | (Sinolinks, Endoscopic linear stapler) |
| COVIDIEN | Multifire Endo TA™ 30 Staplers and Reloads | n.a | n.a | 30 | 2.5 (vascular) 3.5 | 3 | n.a | It can be reloaded 7 times for a total of 8 firings. | n.a | (Medtronic, Multifire Endo TA™) [64] |
| COVIDIEN | Endo GIA™ Universal Staplers with Tri-Staple | SIG30AVM SIG30AVT SIG45AXT SIG60AXT | n.a | 30 45 60 | 2.0 2.5 3.0 3.5 4.0 | 3 | n.a | n.a | n.a | (Medtronic, Endo GIA™ Ultra Universal Staplers) [64] |
| COVIDIEN | Endo Gia™ Ultra Universal Stapler | EGIAUSHT EGIAUSTD EGIAUXL | 6 16 26 | 30 45 60 | 2.0 2.5 3.5 4.8 | 6 | 0.75 0.88 1.5 2.25 3.0 | n.a | n.a | (Medtronic, Endo GIA™ Ultra Universal Staplers) [65] |
| ETHICON | ECHELON FLEX™ ENDOPATH™ Staplers | EC45A EC45AL EC60A LONG60A | 34 44 | 45 60 | 2.6 3.6 3.8 4.1 4.2 | 6 | 1 1.5 1.8 2 2.3 | n.a | It has applications in multiple open or minimally invasive general, gynecologic, urologic, thoracic, and pediatric surgical procedures. It can be used with tissue buttressing materials. The instrument may also be used for transection and resection of liver parenchyma (hepatic vasculature and biliary structures), pancreas, kidney, and spleen. | (Ethicon, Echelon Flex Endopath) [66] |
3.4. Circular Staplers
Specialists use them for end-to-side, end-to-end, and side-to-side anastomoses. The applications of circular staplers involve the anastomosis of the proximal colon with the distal colon during colostomy, laparoscopic distal gastrectomy, and gastrojejunal anastomosis in Roux-en-Y gastric bypass [67,68,69,70,71,72,73]. Circular staplers also play an increasingly important role in vascular anastomoses [74]. The instrument is composed of a detachable anvil and a handling part, the last comprising a shaft that allocates the stapling cartridge and cutting element, a wing nut for opening and closing, and a handle to perform the firing and cutting operation [75]. This class of devices’ cartridges contain a circular knife and a crown of staples of different heights that are available in a range of diameters to adapt to the dimension of the lumen to suture.
Table 7 shows the principal characteristics of circular staplers, sorting devices according to their producers and identifying them both through their commercial name and through their univocal product code. Panther Medical offers a device with a 26 cm shaft and cartridges with 21 mm, 24 mm, 26 mm, 29 mm, 32 mm, and 34 mm diameters. No information on the shaft length has been found for Sinolinks. Still, it is known that their staplers are available in 17 mm, 19 mm, 21 mm, 23 mm, 25 mm, and 27 mm diameters. Covidien offers 22 cm and 35 cm shafts with diameters of 21 mm, 25 mm, 28 mm, 31 mm, and 33 mm. Finally, Ethicon circular staplers have 26 and 37 cm shafts with 21 mm, 25 mm, 29 mm, and 33 mm diameters. As can be seen in Table 7, all manufacturers, except for Sinolinks, for which data were not available, provide cartridges allocating two rows of staples whose height depends on the chosen diameter; only Covidien uses its technology Tri-Staple, with three rows of different height staples. Circular staplers’ cartridges contain a circular knife and a crown of staples of different heights, and they are available in a range of diameters to adapt to the dimension of the lumen. Staple height varies in a wide range depending on the considered manufacturer, going from 3 mm of EEA™ circular stapler with Tri-Staple™ technology to 5.5 mm of Ethicon circular staplers. Close staple heights also vary in the data, including a range that goes from 1.4 mm for Panther Medical to 3mm for Ethicon circular staplers. Ethicon circular staplers with product codes beginning with CDHA and ECS-A provide the maximum closed staple height, indicating they are specifically intended for use in deeper, thicker bowels.
Despite the widespread use of staplers, surgeons are still in search of the most suitable technique to perform surgery. Circular staplers are typically used in colorectal anastomosis, gastrojejunal anastomosis, and other procedures. In the meta-analysis of Milone et al. [73], the literature regarding the use of circular and linear stapler techniques for gastrojejunostomy was analyzed. The results showed that by analyzing the overall anastomotic complications, there was a significantly lower rate of complications in the linear stapler technique compared to the circular stapler one. Other studies in the literature showed similar results. Edholm et al. [76] found that linear staplers, unlike circular staplers, are associated with short operative times, reduced wound infection risks, and shorter lengths of stay.
Mazaki et al. [77] compared the pressure resistance of triple-row circular stapler (EEA™ circular stapler with Tri-Staple™ technology, 28 mm Medium/Thick, Covidien) and double-row circular stapler (EEA™ circular stapler with DST series™ technology, 28 mm, 4.8 mm, Covidien) in a porcine model, reporting that the pressure resistance of the triple-row circular stapler is higher compared to DCS, suggesting that the triple-row circular stapler may reduce the rate of anastomotic leakage [77].
Strassner et al. (2023) [78] evaluated the performance of the Tri-Staple technology (Medtronic) with two-row staplers (Ethicon), comparing them in terms of perfusion, withstanding tension and intraluminal pressure, and reduced damage to the staple line during removal. The results of the tests show that Tri-Staple technology shows a median leak pressure that was 73% higher, better perfusion preservation, a 20% higher failure force, and a median removal force 78% lower than the double-row stapler [78].

Table 7.
Circular stapler characteristics.
Table 7.
Circular stapler characteristics.
| Brand | Commercial Name | Code | Shaft Length [cm] | Diameter [mm] | Staple Height [mm] | No. of Rows | Close Staple Height [mm] | Staple Quantity | Surgery Application and Relevant Info | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| PANTHER HEALTHCARE | Disposable Circular Stapler | FCSME21 FCSME24 FCSME26 FCSME29 FCSME32 FCSLWAF21 FCSLWAF25 FCSLWAF29 FCSLWAF32 FCSLWAF33 (Extra Long) | 26.0 | 21.0 24.0 26.0 29.0 32.0 21.0 25.0 29.0 32.0 33.0 | 4.5 4.8 5.0 | 2 | 1.4-2.2 (range) | n.a | n.a | (Panther Healthcare, Circular Stapler) [79] |
| SINOLINKS | Disposable Circular Stapler | DCS H 17 DCS H 19 | n.a | 17.0 19.0 | 4.5 | n.a | 2.0 2.2 | n.a | It has applications in gastrointestinal surgery. Used for the whole digestive tract for end-to-end, end-to-side, and side-to-side anastomoses. | (Sinolinks, Disposable Circular Stapler) |
| SINOLINKS | Disposable Circular Stapler | DCS A/E/F21 DCS A/E/F23 DCS A/E/F25 DCS A/E/F27 | n.a | 21.0 23.0 25.0 27.0 | 4.8 5.2 | n.a | 2.0 2.2 | 18 18 20 22 | (Sinolinks, Disposable Circular Stapler) | |
| SINOLINKS | Disposable Circular Stapler | DCS A/E/F29 DCS A/E/F31 DCS A/E/F33 | n.a | 29.0 31.0 33.0 | 4.8 5.2 | n.a | 2.0 2.2 | 24 28 30 | (Sinolinks, Disposable Circular Stapler) | |
| COVIDIEN | EEA™ Stapler with DST Series™ Technology | EEA21 EEA25 EEA28 | 22.0 35.0 | 21.0 25.0 28.0 | 3.5 4.8 | 2 | n.a | 18 22 26 30 32 | It has applications in bariatric, colorectal, and general surgery. | (Medtronic, EEA™ Circular Stapler with DST™ Series Technology) [80] |
| COVIDIEN | EEA™ Stapler with DST Series™ Technology | EEA31 EEA33 | 22.0 35.0 | 31.0 33.0 | 4.8 | 2 | n.a | (Medtronic, EEA™ Circular Stapler with DST™ Series Technology) [80] | ||
| COVIDIEN | EEA™ Stapler with Tri-Staple Technology | TRIEEA28MT TRIEEA28XT TRIEEAXL28MT TRIEEAXL28XT TRIEEA31MT TRIEEA31XT TRIEEAXL31MT TRIEEAXL31XT | n.a | 28.0 31.0 | MT 3.0 3.5 4.0 XT 4.0 4.5 5.0 XL 3.0 3.5 4.0 | 3 | n.a | n.a | n.a | (Medtronic, Suturatrice Circolare EEA™ con Tri-Staple™ Technology) [81] |
| ETHICON | Ethicon Circular Stapler | CDH21A CDH25A CDH29A CDH33A | 26.0 | 21.0 25.0 29.0 33.0 | 5.5 | 2 | 1.5 3.0 | 16 | n.a | (Ethicon, Circular Stapler) [82] |
| ETHICON | Ethicon Circular Stapler | ECS21A ECS25A ECS29A ECS33A | 37.0 | 21.0 25.0 29.0 33.0 | 5.5 | 2 | 1.5 3.0 | 16 20 24 28 | n.a | (Ethicon, Circular Stapler) [83] |
| ETHICON | ETHICON™ Circular Stapler | ECS21B ECS25B ECS29B ECS33B | 37.0 | 21.0 25.0 29.0 33.0 | 5.2 | 2 | 1.5 2.2 | 16 20 24 28 | It has applications in surgery fields such as bariatric, colorectal, and thoracic surgeries to perform esophagectomy, gastric bypass, left colectomy, lower anterior resection, and sigmoidectomy procedures. | (Ethicon, Circular Stapler) [84] |
Over the years, researchers and surgeon specialists performed different studies to assess which type of stapler was the most suitable for different surgery procedures. Gastrojejunostomy is one of the most analyzed techniques for anastomosis. Typically, two different types of staplers’ outcomes are compared, and the authors consider linear and circular staplers’ techniques for the outcome comparison [3,68,69,70,71,72,73,76].
Other studies analyze the safety and efficacy of laparoscopy gastrectomy in patients with gastric cancer using linear stapler and circular stapler techniques [85]. Circular staplers were also used for restorative surgery for rectal cancer [86].
A variant of this class of device is hemorrhoidal staplers, which work exactly as circular staplers but have different diameters and employ different cartridges, ensuring a lower closed staple height. Table 8 below displays the different manufacturers that offer staplers for this specific purpose. The disposable hemorrhoidal stapler by Sinolinks has diameters of 32 mm and 34 mm; Covidien has the EEA™ hemorrhoid and prolapse stapler with DST Series™ technology that has a diameter of 33 mm, the same as Ethicon PROXIMATE® PPH3 Hemorrhoidal Circular Stapler Set; and Golden Stapler has the PPHD Single-Use Hemorrhoidal Circular Stapler line, with diameters of 32 mm, 34 mm, and 36 mm. The staple height is different in each of the four staplers: 3.8 mm for Sinolinks staples, 3.5 mm and 4.8 mm for Covidien staples, 4 mm for Ethicon, and finally, 4.3 and 4.5 for Golden Stapler. The number of rows of staggered staples, as the closed staple height, is also listed in Table 8.

Table 8.
Hemorrhoidal stapler characteristics.
3.5. Powered Stapling System
Both linear and circular powered stapling systems are commercially available (see Table 9). For linear systems, today, Ethicon and Covidien are the only manufacturers on the market offering powered surgical staplers. Ethicon has two powered stapling systems, one being the ECHELON CIRCULAR™ Powered Stapler (ECPS) already mentioned in the previous section. The other one is the ECHELON FLEX™ Powered Vascular Stapler (PVS) (Ethicon, Echelon Flex Powered Vascular Stapler) [90].
The PVS has a powered actuation, meaning that the firing mechanism is no more controlled by the swing of a knob or the compression of a spring but through a battery providing power to an electro-mechanical system maneuvering the cutting element if present and the cartridge. The stapler has a curved anvil tip, an introducer-like cartridge body tip, and active articulation with high articulation angles to facilitate access in surgical procedures with small surgical spaces or difficult-to-reach vessels.
Medtronic Covidien Signia™ Stapling System (SIG) is relatively more complex. Not only does it have powered actuation, but the articulation, opening, and closing of the end effector are also powered through electrical motors. The compression of the tissue is sensed thanks to a strain-gage transducer located on the shaft, providing indications to the surgeon through a display.
Regarding circular powered stapling solutions, Ethicon produces the ECHELON CIRCULAR™ Powered Stapler (ECPS) (Ethicon, Echelon Circular Powered Vascular Stapler) [91].
This represents the powered variant of the already-discussed ETHICON™ Circular Stapler product and shares most of its technical specifications, such as lumen diameter (available in four sizes), staple rows, open staple leg length, and closed staple height. The iDrive™ staplers provided by Covidien showed superior performance in terms of access, visibility, and ease of placement in the deep pelvis [92].
Both the ECHELON FLEX™ Powered Vascular Stapler (PVS) and the Covidien Signia™ Stapling System (SIG) have been extensively validated by the scientific community. In particular, SIG was tested for video-assisted thoracic surgery by Shimada et al. [93], which also introduced a new procedure, named “sliding technique”, to optimize safe surgical margins with minimal stapler movement.
The advantages of PVS in comparison to manual endoscopy are highlighted in Roy et al. [94] for laparoscopic bariatric surgery, in Park et al. [95] for thoracoscopic lobectomy lung cancer, and video-assisted thoracic surgery lobectomy in Miller et al. [59].
In comparison to the non-powered alternatives, these studies showed, respectively, a decrease of 47% (3.05% to 1.61%) in terms of hemostasis-related complications, a decrease of 56% in terms of intraoperative blood loss, and a decrease of 47% in terms of bleeding complications. The PVS device has also been applied for scientific investigations on a novel physiologic lung model by Eckert et al. [96].
Some direct comparisons of PVS and SIG have been performed by Rawlins et al. [97] in laparoscopic sleeve gastrectomy. The authors report a lower observed incidence of hemostasis-related complications during the surgical admission in the ECHELON FLEX™ group in comparison with the SIG group (3 events vs. 11 events).
Regarding the ECHELON CIRCULAR™ Powered Stapler (ECPS), this has been proven to reduce the risk of anastomotic leakage rates in left-sided colorectal anastomosis from 11.8% to 1.7% in a single institution clinical study [98]. Postoperative anastomotic leaks were experienced in 2.4% of subjects in a cohort study of left-sided colorectal surgery performed by Herzig et al. [99]. Similarly, it has been successfully benchmarked against manual operation for left-sided colorectal resections by Pollack et al. [100] and Sylla et al. [101].

Table 9.
Powered stapler characteristics.
Table 9.
Powered stapler characteristics.
| Brand | Commercial Name | Code | Dimension [mm] | Staple Height [mm] | No. of Rows | Closed Staple Height [mm] | Firing Actions | Surgery Application and Relevant Info | Reference |
|---|---|---|---|---|---|---|---|---|---|
| COVIDIEN | Signia™ Stapling Platform | n.a | 30 30 45 | 4.5 | n.a. | n.a. | n.a | It adapts to different surgical conditions, properly adjusting the drive speed according to force measurements. | (Medtronic, Signia™ Stapling Platform) [102] |
| COVIDIEN | Signia™ Stapling Platform Small Diameter (8 mm shaft) | SIGSDS30CTV SIGSDS30CTVT SIGSDS45CTVT | 30 30 45 | n.a | n.a | n.a | n.a | Compatibility with all Medtronic handles. | (Medtronic, Signia™ Stapling Platform) [102] |
| ETHICON | FLEX™ Powered Vascular Stapler | PVE35A VASECR35 (Only for vascular/thin tissue) | 32 | 5.2 | 4 | 1.5 mm–2.2 mm | It can be reloaded for a total of 12 firings. | It has applications in surgery fields such as general, gynecologic, pediatric, thoracic, and urologic to perform lung resection, nephrectomy, appendectomy, splenectomy, and oophorectomy procedures, as well as for vessel transection and stapling. | (Ethicon, Echelon Flex Powered Vascular Stapler) [90] |
| ETHICON | CIRCULAR™ Powered Vascular Stapler | CDH23P, CDH25P, CDH29P, CDH31P | 23 mm, 25 mm, 29 mm, 31 mm | 5.2 | 2 | 1.5 mm–2.2 mm | n.a | It has applications in surgery fields such as colorectal, gastric, and thoracic to perform colorectal resections, gastrectomies, gastric bypass, and esophagectomies procedures. | (Ethicon, Echelon Circular Powered Vascular Stapler) [91] |
| COVIDIEN | iDrive™ | n.a | n.a | n.a | n.a | n.a | n.a | The iDrive Ultra powered stapling device, with Endo GIA stapling reloads, provides precision in surgical stapling, enabling surgeons to position and keep the stapler exactly where they intend. | (Covidien, iDrive™) |
4. Conclusions
Stapling devices have gained widespread adoption in various surgical fields, demonstrating excellent results in terms of surgical outcomes and reduced operation time. Manufacturers and the existing literature provide valuable information on the specific applications of each stapling device, with a range of staple cartridges tailored to different surgical targets. The variety of staple sizes, designed to accommodate diverse tissue thicknesses and lumen diameters, surgical spaces, ensures effective hemostasis while minimizing ischemia and tissue damage. However, it is important to acknowledge that stapling can disrupt the structural continuity of tissues, introducing stress and strain that may not be entirely physiological but still lead to favorable outcomes. Despite this, only a limited number of articles have explored the strength of anastomotic techniques involving stapling devices. It is important to highlight that staple configurations are under constant improvement, and the experimental tests to evaluate their performance are performed by different research groups, as reported in [78].
Therefore, further studies should focus on understanding the impact of adopting specific stapling devices, also in comparison with manual or mixed operations as documented in [2] regarding suture resistances, for different tissues and explore the potential influence of staple height and the number of staple rows on the overall surgical outcome. By undertaking such investigations, we can better optimize the use of stapling devices, tailor their applications to specific tissues, and potentially enhance the overall success and safety of surgical procedures. This research will contribute to the advancement of surgical techniques and the improvement of patient outcomes in the future.
This review aims to gather information from the literature published in previous years regarding surgical stapling devices. Furthermore, specific sections are dedicated to the use of bioresorbable staples to highlight where bioresorbable materials are used in this specific field of surgery. The use of bioresorbable devices in medicine has been increasing in the last few years, both in clinical and research settings. However, few devices adopt the usage of bioresorbable staples and only for specific fields of surgery. The collected information indicates a growing interest in new stapling devices using bioresorbable staples.
While this suggests the potential future of bioresorbable staples, their applicability across all surgical fields might be limited only to skin closure. Indeed, non-bioresorbable staples will remain the gold standard for applications that require permanent closure of internal organs, such as gastrointestinal procedures, abdominal surgery, gynecology, and vascular surgery.
Author Contributions
V.B.: Investigation, Writing—Original Draft Preparation, Writing—Review and Editing; J.B.: Investigation, Writing—Original Draft Preparation; M.R.R.: Investigation, Writing—Review and Editing, Writing—Original Draft Preparation, Formal analysis; M.C.: Investigation, Writing—Original Draft Preparation, Writing—Review and Editing, Data Curation, Formal analysis; O.G.R.: Investigation, Writing—Original Draft Preparation, Writing—Review and Editing, Formal analysis; C.S.: Conceptualization, Writing—Review and Editing, Methodology, Supervision, Funding acquisition, Resources; N.M.P.: Conceptualization, Methodology, Writing—Review and Editing, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank Giorgio Tropiano for their precious help in the first draft of the paper and Jacobacci & Partners (Turin) for the help in the patent landscape.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yang, J.; Soltz, M.; Russell, H.; Beres, J.; Zhao, J.; Liao, D.; Gregersen, H. Surface Deformation Analysis of End-to-End Stapled Intestinal Anastomosis. Surg. Innov. 2012, 19, 281–287. [Google Scholar] [CrossRef]
- Lauro, E.; Corridori, I.; Luciani, L.; Di Leo, A.; Sartori, A.; Andreuccetti, J.; Trojan, D.; Scudo, G.; Motta, A.; Pugno, N.M. Stapled Fascial Suture: Ex Vivo Modeling and Clinical Implications. Surg. Endosc. 2022, 36, 8797–8806. [Google Scholar] [CrossRef] [PubMed]
- Penna, M.; Markar, S.R.; Venkat-Raman, V.; Karthikesalingam, A.; Hashemi, M. Linear-Stapled Versus Circular-Stapled Laparoscopic Gastrojejunal Anastomosis in Morbid Obesity: Meta-Analysis. Surg. Laparosc. Endosc. Percutaneous Tech. 2012, 22, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Champion, J.K.; Williams, M.D. Prospective Randomized Comparison of Linear Staplers during Laparoscopic Roux-En-Y Gastric Bypass. Obes. Surg. 2003, 13, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, R.; Fujimoto, Y.; Sugiyama, M.; Hisamatsu, Y.; Nakanoko, T.; Ando, K.; Ota, M.; Kimura, Y.; Oki, E.; Yoshizumi, T. Clinical Impact of the Triple-Layered Circular Stapler for Reducing the Anastomotic Leakage in Rectal Cancer Surgery: Porcine Model and Multicenter Retrospective Cohort Analysis. Ann. Gastroenterol. Surg. 2022, 6, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, H.; Song, J.H.; Choi, S.; Cho, M.; Son, T.; Kim, H.-I.; Hyung, W.J. Intracorporeal Esophagojejunostomy Using a Linear Stapler in Laparoscopic Total Gastrectomy: Comparison with Circular Stapling Technique. BMC Surg. 2020, 20, 100. [Google Scholar] [CrossRef]
- Lim, J.J.B.; Erdman, A.G. A Review of Mechanism Used in Laparoscopic Surgical Instruments. Mech. Mach. Theory 2003, 38, 1133–1147. [Google Scholar] [CrossRef]
- Von Maszewski, M.; Sucher, J.F.; MacFadyen, B.V. Laparoscopic Instrumentation: Linear Cutters, Clip Appliers, and Staplers. Semin. Laparosc. Surg. 2001, 8, 69–76. [Google Scholar] [CrossRef]
- Giaccaglia, V.; Antonelli, M.S.; Addario Chieco, P.; Cocorullo, G.; Cavallini, M.; Gulotta, G. Technical Characteristics Can Make the Difference in a Surgical Linear Stapler. or Not? J. Surg. Res. 2015, 197, 101–106. [Google Scholar] [CrossRef]
- Yao, D.B.; Wu, S.D. Application of Stapling Devices in Liver Surgery: Current Status and Future Prospects. World J. Gastroenterol. 2016, 22, 7091–7098. [Google Scholar] [CrossRef]
- Tajirian, A.L.; Goldberg, D.J. A Review of Sutures and Other Skin Closure Materials. J. Cosmet. Laser Ther. 2010, 12, 296–302. [Google Scholar] [CrossRef]
- Duteille, F.; Rouif, M.; Alfandari, B.; Andreoletti, J.B.; Sinna, R.; Laurent, B.; Perrot, P. Reduction of Skin Closure Time without Loss of Healing Quality: A Multicenter Prospective Study in 100 Patients Comparing the Use of Insorb Absorbable Staples with Absorbable Thread for Dermal Suture. Surg. Innov. 2013, 20, 70–73. [Google Scholar] [CrossRef]
- Partecke, L.I.; Kessler, W.; Von Bernstorff, W.; Diedrich, S.; Heidecke, C.D.; Patrzyk, M. Laparoscopic Appendectomy Using a Single Polymeric Clip to Close the Appendicular Stump. Langenbecks Arch. Surg. 2010, 395, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Kuppersmith, R.B.; Atkins, J.H.; Tami, T.A. The Use of Bioresorbable Staples for Mucoperichondrial Flap Coaptation in Septoplasty. Otolaryngol. Head. Neck Surg. 2009, 140, 599–600. [Google Scholar] [CrossRef] [PubMed]
- Malard, O.; Duteille, F.; Darnis, E.; Espitalier, F.; Perrot, P.; Ferron, C.; Planche, L.; Hardouin, J.B.; Tessier, P.; Bellanger, M.; et al. A Novel Absorbable Stapler Provides Patient-Reported Outcomes and Cost-Effectiveness Noninferior to Subcuticular Skin Closure: A Prospective, Single-Blind, Randomized Clinical Trial. Plast. Reconstr. Surg. 2020, 777E–789E. [Google Scholar] [CrossRef] [PubMed]
- Farkas, G.; Leindler, L.; Farkas, G. Safe Closure Technique for Distal Pancreatic Resection. Langenbecks Arch. Surg. 2005, 390, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Hirashima, T.; Eto, T.; DenBesten, L. Lactomer Copolymer Absorbable Staples in Gastrointestinal Surgery. Am. J. Surg. 1985, 150, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Farkas, G. PolysorbR (an Absorbable Lactomer) Staples, a Safe Closure Technique for Distal Pancreatic Resection. World J. Gastroenterol. 2014, 20, 17185. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Amano, H.; Miyake, K.; Hinoki, A.; Yokota, K.; Kinoshita, F.; Nakazawa, A.; Tanaka, Y.; Seto, Y.; Uchida, H. Novel Zinc Alloys for Biodegradable Surgical Staples. World J. Clin. Cases 2020, 8, 504–516. [Google Scholar] [CrossRef]
- Amano, H.; Hanada, K.; Hinoki, A.; Tainaka, T.; Shirota, C.; Sumida, W.; Yokota, K.; Murase, N.; Oshima, K.; Chiba, K.; et al. Biodegradable Surgical Staple Composed of Magnesium Alloy. Sci. Rep. 2019, 9, 14671. [Google Scholar] [CrossRef]
- Cole, B.J.; Sayegh, E.T.; Yanke, A.B.; Chalmers, P.N.; Frank, R.M. Fixation of Soft Tissue to Bone. J. Am. Acad. Orthop. Surg. 2016, 24, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Barca, F.; Busa, R. Resorbable Poly-l-Lactic Acid Mini-Staples for the Fixation of Akin Osteotomies. J. Foot Ankle Surg. 1997, 36, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Rethnam, U.; Kuiper, J.; Makwana, N. Mechanical Characteristics of Three Staples Commonly Used in Foot Surgery. J. Foot Ankle Res. 2009, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Oberlander, M.A.; Chisar, M.A. Meniscal Repair Using the Polysorb Meniscal Stapler XLS. Arthrosc. J. Arthrosc. Relat. Surg. 2005, 21, 1148.e1–1148.e5. [Google Scholar] [CrossRef] [PubMed]
- McMillan, S.; Ford, E. Management of Rotator Cuff Defects After Calcific Tendinopathy Debridement Using a Bioinductive Collagen Implant. Arthrosc. Tech. 2016, 5, e1373–e1379. [Google Scholar] [CrossRef]
- Gong, J.; Guo, Z.; Li, Y.; Gu, L.; Zhu, W.; Li, J.; Li, N. Stapled vs Hand Suture Closure of Loop Ileostomy: A Meta-Analysis. Color. Dis. 2013, 15, e561–e568. [Google Scholar] [CrossRef] [PubMed]
- Choy, P.Y.G.; Bissett, I.P.; Docherty, J.G.; Parry, B.R.; Merrie, A. Stapled versus Handsewn Methods for Ileocolic Anastomoses. In Cochrane Database of Systematic Reviews; Bissett, I.P., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2007. [Google Scholar]
- Kano, M.; Hanari, N.; Gunji, H.; Hayano, K.; Hayashi, H.; Matsubara, H. Is “Functional End-to-End Anastomosis” Really Functional? A Review of the Literature on Stapled Anastomosis Using Linear Staplers. Surg. Today 2017, 47, 1–7. [Google Scholar] [CrossRef]
- Panther Healthcare Canada, Linear Stapler and Cartridges. Available online: https://pantherhealthcarecanada.com/products/open-linear-stapler/ (accessed on 19 July 2023).
- Medtronic, TATM Sta-Pler with DST Se-RiesTM Technology. Available online: https://www.medtronic.com/covidien/en-us/support/products/surgical-stapling/dst-series-ta-single-use-staplers-and-reloads.html (accessed on 19 July 2023).
- Ethicon, PROXIMATE Reloadable Linear Stapler. Available online: https://www.jnjmedtech.com/en-us/product/proximate-tx-reloadable-linear-stapler (accessed on 19 July 2023).
- Pineros-Fernandez, A.; Salopek, L.S.; Rodeheaver, P.F.; Drake, D.B.; Edlich, R.; Rodeheaver, G.T. A Revolutionary Advance in Skin Closure Compared to Current Methods. J. Long. Term. Eff. Med. Implant. 2006, 16, 19–27. [Google Scholar] [CrossRef]
- Hornez, E.; Garnier, E.; Sastre, B.; Garcia, S.; Mayet, A.; Berdah, S.V. Bioabsorbable Staple-Line Reinforcement for Pancreatectomy in a Porcine Model: A Preliminary Study. Eur. Surg. Res. 2012, 48, 48–53. [Google Scholar] [CrossRef]
- Thomas, P.; Massard, G.; Porte, H.; Doddoli, C.; Ducrocq, X.; Conti, M. A New Bioabsorbable Sleeve for Lung Staple-Line Reinforcement (FOREsealTM): Report of a Three-Center Phase II Clinical Trial. Eur. J. Cardio-Thorac. Surg. 2006, 29, 880–885. [Google Scholar] [CrossRef][Green Version]
- Schrufer-Poland, T.L.; Ruiz, M.P.; Kassar, S.; Tomassian, C.; Algren, S.D.; Yeast, J.D. Incidence of Wound Complications in Cesarean Deliveries Following Closure with Absorbable Subcuticular Staples versus Conventional Skin Closure Techniques. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 206, 53–56. [Google Scholar] [CrossRef][Green Version]
- Hanna, D.N.; Schlegel, C.; Ghani, M.O.; Hermina, A.; S Mina, A.; McKay, K.; Bailey, C.E.; Magge, D.; Idrees, K. Stapled Full-Thickness Diaphragm Resection: A Novel Approach to Diaphragmatic Resection in Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy. J. Am. Coll. Surg. 2022, 234, e1–e6. [Google Scholar] [CrossRef]
- Nasir, S. Using Linear Cutting Stapler for Gracillis Muscle Flap Splitting in Facial Reanimation. Microsurgery 2018, 38, 122–123. [Google Scholar] [CrossRef]
- Belfort, M.A.; Shamshiraz, A.A.; Fox, K. Minimizing Blood Loss at Cesarean-Hysterectomy for Placenta Previa Percreta. Am. J. Obstet. Gynecol. 2017, 216, 78.e1–78.e2. [Google Scholar] [CrossRef]
- Kang, S.-K.; Bok, J.S.; Cho, H.J.; Kang, M.-W. Novel Asymmetrical Linear Stapler (NALS) for Pathologic Evaluation of True Resection Margin Tissue. J. Thorac. Dis. 2018, 10, S1631–S1636. [Google Scholar] [CrossRef]
- Salyer, C.; Spuzzillo, A.; Wakefield, D.; Gomaa, D.; Thompson, J.; Goodman, M. Assessment of a Novel Stapler Performance for Laparoscopic Sleeve Gastrectomy. Surg. Endosc. 2021, 35, 4016–4021. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Matsumoto, S.; Matsushita, A.; Yoshioka, M.; Shimizu, T.; Yamahatsu, K.; Uchida, E. Pancreaticojejunostomy with Closure of the Pancreatic Stump by Endoscopic Linear Stapler in Laparoscopic Pancreaticoduodenectomy: A Reliable Technique and Benefits for Pancreatic Resection. Asian J. Endosc. Surg. 2012, 5, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Noshiro, H.; Urata, M.; Ikeda, O.; Iwasaki, H.; Nabae, T.; Uchiyama, A.; Nagai, E.; Tanaka, M. Triangulating Stapling Technique for Esophagogastrostomy after Minimally Invasive Esophagectomy. Surgery 2013, 154, 604–610. [Google Scholar] [CrossRef]
- Tsujimoto, H.; Tsuda, H.; Hiraki, S.; Nomura, S.; Ito, N.; Kanematsu, K.; Horiguchi, H.; Aosasa, S.; Yamamoto, J.; Hase, K. In Vivo Evaluation of a Modified Linear Stapling Device Designed to Facilitate Accurate Pathologic Examination of the Surgical Margin. Gastric Cancer 2016, 19, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, D.; Zhao, Q.; Yang, P.; Ding, P.; Fan, H.; Dong, T.; Liu, Z.; Yang, X.; Ren, L.; et al. A Case Series of 10 Patients Undergone Linear Cutter/Stapler Guiding Device-Led Overlapped Esophagojejunostomy: A Preliminary Study. J. Gastrointest. Oncol. 2023, 14, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Panther Healthcare Canada, Open Linear Cutter and Cartridges. Available online: https://pantherhealthcarecanada.com/products/open-linear-cutter/ (accessed on 19 July 2023).
- Medtronic, GIATM Stapler with DST SeriesTM Technology. Available online: https://www.medtronic.com/covidien/en-us/support/products/surgical-stapling/dst-series-gia-single-use-reloadable-staplers.html (accessed on 19 July 2023).
- Ethicon, Linear Cutters. Available online: https://www.ethicon.com/na/epc/code/ntlc55?lang=en-default (accessed on 19 July 2023).
- Ethicon, Linear Cutters. Available online: https://www.ethicon.com/na/epc/code/ntlc75?lang=en-default (accessed on 19 July 2023).
- Ethicon, PROXI-MATE® Linear Cutters. Available online: https://www.jnjmedtech.com/en-us/product/ethicon-proximate-linear-cutters (accessed on 19 July 2023).
- Ethicon, ENDOPATHTM ETS Articulating Linear Cutter. Available online: https://www.jnjmedtech.com/en-us/product/endopath-ets-articulating-linear-cutter (accessed on 19 July 2023).
- Standard Bariatrics, Standard Titan. Available online: https://standardbariatrics.com/ (accessed on 19 July 2023).
- Perrone, E.E.; Galganski, L.A.; Tarantal, A.F.; Olstad, K.J.; Treadwell, M.C.; Berman, D.R.; Jarboe, M.D.; Mychaliska, G.B.; Farmer, D.L. Fetal Surgery in the Primate 4.0: A New Technique 30 Years Later. Fetal Diagn. Ther. 2021, 48, 43–49. [Google Scholar] [CrossRef]
- Church, J.T.; McLeod, J.S.; Coughlin, M.A.; Bergin, I.L.; Perkins, E.M.; Hoffman, H.R.; Bilger, M.; Rojas-Peña, A.; Treadwell, M.C.; Berman, D.R.; et al. An Early Investigation into Possible Alternatives to Stapled Hysterotomy in Open Fetal Surgery. Am. J. Perinatol. 2019, 36, 742–750. [Google Scholar] [CrossRef]
- Anthimidis, G. Laparoscopic Excision of a Pedunculated Uterine Leiomyoma in Torsion as a Cause of Acute Abdomen at 10 Weeks of Pregnancy. Am. J. Case Rep. 2015, 16, 505–508. [Google Scholar] [CrossRef]
- Tatekawa, Y.; Kanehiro, H.; Nakajima, Y. Laparoscopic Extirpation of Splenic Hamartoma. Pediatr. Surg. Int. 2007, 23, 911–914. [Google Scholar] [CrossRef]
- Akil, A.; Semik, M.; Freermann, S.; Reichelt, J.; Redwan, B.; Görlich, D.; Fischer, S. Use of a Powered Stapling System for Minimally Invasive Lung Volume Reduction Surgery: Results of a Prospective Double-Blind Single-Center Randomized Trial. Thorac. Cardiovasc. Surg. 2019, 67, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Povoski, S.P. Novel Applications of Endo GIA Linear Staplers during Pancreaticoduodenectomy and Total Pancreatectomy. Am. J. Surg. 2001, 182, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Qiu, B.; Gao, S. The Powered Vascular Staple (PVS) versus Conventional Powered Linier Cutter (PLC) for the Application of Bronchial Transection in Thoracoscopic Anatomic Segmentectomy. J. Thorac. Dis. 2019, 11, 4647–4653. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.L.; Roy, S.; Kassis, E.S.; Yadalam, S.; Ramisetti, S.; Johnston, S.S. Impact of Powered and Tissue-Specific Endoscopic Stapling Technology on Clinical and Economic Outcomes of Video-Assisted Thoracic Surgery Lobectomy Procedures: A Retrospective, Observational Study. Adv. Ther. 2018, 35, 707–723. [Google Scholar] [CrossRef]
- Tsunezuka, Y.; Tanaka, N.; Fujimori, H. The Impact of Endoscopic Stapler Selection on Bleeding at the Vascular Stump in Pulmonary Artery Transection. Med. Devices Evid. Res. 2020, 13, 41–47. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Du, J.; Wei, J.; Wang, K.; Zhang, S.; Zhang, N. Pre-Expansion of Posterior Gastric Fascia during Laparoscopic Radical Gastrectomy for Gastric Cancer Prevents Injuries to Upper Short Gastric Vessels. Transl. Cancer Res. 2020, 9, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Charalabopoulos, A.; Davakis, S.; Paraskeva, P.; Machairas, N.; Kapelouzou, A.; Kordzadeh, A.; Sakarellos, P.; Vailas, M.; Baili, E.; Bakoyiannis, C.; et al. Feasibility and Short-Term Outcomes of Three-Dimensional Hand-Sewn Esophago-Jejunal Anastomosis in Completely Laparoscopic Total Gastrectomy for Cancer. Cancers 2021, 13, 4709. [Google Scholar] [CrossRef]
- Panther Healthcare Canada, Endo Linear Cutter and P2G Cartridges. Available online: https://pantherhealthcarecanada.com/products/endo-linear-cutter/ (accessed on 19 July 2023).
- Medtronic. Available online: https://www.medtronic.com/covidien/en-us/products/surgical-stapling/laparoscopic-staplers.html#endo-gia-ultra-universal-staplers-and-reloads.html (accessed on 19 July 2023).
- Medtronic, Endo GIATM Ultra Universal Staplers. Available online: https://www.medtronic.com/covidien/en-us/support/products/surgical-stapling/endo-gia-ultra-universal-staplers-and-reloads.html (accessed on 19 July 2023).
- Ethicon, ECHELON FLEXTM ENDOPATH®. Available online: https://www.jnjmedtech.com/en-us/product/echelon-flex-endopath-staplers (accessed on 19 July 2023).
- Guweidhi, A.; Steffen, R.; Metzger, A.; Teuscher, J.; Flückiger, P.; Z’graggen, K. Circular Stapler Introducer. Dis. Colon. Rectum 2009, 52, 746–748. [Google Scholar] [CrossRef] [PubMed]
- Burla, L.; Weibel, P.; Baum, C.; Huber, M.; Gürtler, T.; Weber, M. Linear versus Circular Stapler for Gastrojejunal Anastomosis in Laparoscopic Roux-En-Y Gastric Bypass: An Analysis of 211 Cases. Surg. Res. Pract. 2020, 2020, 4090797. [Google Scholar] [CrossRef]
- Bohdjalian, A.; Langer, F.B.; Kranner, A.; Shakeri-Leidenmühler, S.; Zacherl, J.; Prager, G. Circular- vs. Linear-Stapled Gastrojejunostomy in Laparoscopic Roux-En-Y Gastric Bypass. Obes. Surg. 2010, 20, 440–446. [Google Scholar] [CrossRef]
- Barr, A.C.; Lak, K.L.; Helm, M.C.; Kindel, T.L.; Higgins, R.M.; Gould, J.C. Linear vs. Circular-Stapled Gastrojejunostomy in Roux-En-Y Gastric Bypass. Surg. Endosc. 2019, 33, 4098–4101. [Google Scholar] [CrossRef]
- Giordano, S.; Tolonen, P.; Victorzon, M. Comparison of Linear versus Circular Stapling Techniques in Laparoscopic Gastric Bypass Surgery—A Pilot Study. Scand. J. Surg. 2010, 99, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Tan, Y.; Xue, W.; Wang, Y.; Xu, X.-Z. Comparison of the Short-Term Outcomes between Delta-Shaped Anastomosis and Conventional Billroth I Anastomosis after Laparoscopic Distal Gastrectomy. Medicine 2018, 97, e0063. [Google Scholar] [CrossRef] [PubMed]
- Milone, M.; Elmore, U.; Manigrasso, M.; Vertaldi, S.; Aprea, G.; Servillo, G.; Parise, P.; De Palma, G.D.; Rosati, R. Circular versus Linear Stapling Oesophagojejunostomy after Laparoscopic Total Gastrectomy. A Systematic Review and Meta-Analysis. Am. J. Surg. 2022, 223, 884–892. [Google Scholar] [CrossRef]
- Raza, S.T. A Circular Surgical Stapler Designed to Anastomose Aorta and Dacron Tube Graft. AORTA 2013, 1, 71–77. [Google Scholar] [CrossRef]
- Giaccaglia, V.; Antonelli, M.S.; Franceschilli, L.; Salvi, P.F.; Gaspari, A.L.; Sileri, P. Different Characteristics of Circular Staplers Make the Difference in Anastomotic Tensile Strength. J. Mech. Behav. Biomed. Mater. 2016, 53, 295–300. [Google Scholar] [CrossRef]
- Edholm, D. Systematic Review and Meta-Analysis of Circular- and Linear-Stapled Gastro-Jejunostomy in Laparoscopic Roux-En-Y Gastric Bypass. Obes. Surg. 2019, 29, 1946–1953. [Google Scholar] [CrossRef]
- Mazaki, J.; Katsumata, K.; Udo, R.; Tago, T.; Kasahara, K.; Kuwabara, H.; Enomoto, M.; Ishizaki, T.; Nagakawa, Y.; Tsuchida, A. Comparison of Pressure Resistance of Double-Rows and Triple-Rows Circular Stapler in Rectal Double Stapling Technique: In Vitro Study. Medicine 2022, 101, e29600. [Google Scholar] [CrossRef]
- Strassner, H.; Caulk, A.; Reher, N.; Petrescu, S.; Vasanji, A. Evaluating Performance of Circular Staplers Us-ing Comparative Test Methods for Evidence-Based Surgery. Surg. Innov. 2023, 30, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Golden Stapler, Single Use Hemorrhoidal Circular Stapler. Available online: http://en.goldenstapler.com/open-surgical-instruments/5.html (accessed on 19 July 2023).
- Medtronic, EEATM Circular Stapler with DSTTM Series Technology. Available online: https://www.medtronic.com/covidien/en-us/support/products/surgical-stapling/eea-circular-stapler-dst-series-technology.html (accessed on 19 July 2023).
- Medtronic, Suturatrice Circolare EEATM Con Tri-StapleTM Technology. Available online: https://www.medtronic.com/covidien/it-it/products/surgical-stapling/eea-circular-stapler.html# (accessed on 19 July 2023).
- Ethicon, Circular Stapler. Available online: https://www.ethicon.com/emea/epc/code/cdh21a?lang=en-default (accessed on 19 July 2023).
- Ethicon, Circular Stapler. Available online: https://www.ethicon.com/emea/epc/code/ecs21a?lang=en-default (accessed on 19 July 2023).
- Ethicon, Circular Stapler. Available online: https://www.ethicon.com/na/epc/code/ecs21b?lang=en-default (accessed on 19 July 2023).
- Sun, D.; Zhang, R.; Wei, M.; Liu, P.; Zhong, X.; Liang, Y.; Chen, Y.; Huang, Y.; Yu, W. Comparison Between Linear Stapler and Circular Stapler After Laparoscopic-Assisted Distal Gastrectomy in Patients With Gastric Cancer. Front. Surg. 2022, 9, 858236. [Google Scholar] [CrossRef]
- Brisinda, G.; Chiarello, M.M.; Pepe, G.; Cariati, M.; Fico, V.; Mirco, P.; Bianchi, V. Anastomotic Leakage in Rectal Cancer Surgery: Retrospective Analysis of Risk Factors. World J. Clin. Cases 2022, 10, 13321–13336. [Google Scholar] [CrossRef]
- Sinolinks, Disposable Circular Stapler. Available online: https://sinolinks.com.cn/stapler-en/102.html (accessed on 19 July 2023).
- Covidien, EEATM Hemorrhoid and Prolapse Stapler Set. Available online: https://www.medtronic.com/covidien/en-us/support/products/surgical-stapling/eea-hemorrhoid-and-prolapse-stapler-set.html (accessed on 19 July 2023).
- Ethicon, PROXIMATETM PPH Hemorrhoidal Circular Stapler Set. Available online: https://www.jnjmedtech.com/en-us/product/proximate-pph-hemorrhoidal-circular-stapler-set (accessed on 19 July 2023).
- Echelon Flex Powered Vascular Stapler. Ethicon. Cincinnati. Available online: https://www.jnjmedtech.com/en-us/product/echelon-flex-powered-vascular-stapler (accessed on 19 July 2023).
- Echelon Circular Powered Vascular Stapler. Ethicon. Cincinnati. Available online: https://www.jnjmedtech.com/en-us/product/echelon-circular-powered-stapler (accessed on 19 July 2023).
- Sonoda, T.; Verdeja, J.C.; Rivadeneira, D.E. Stapler Access and Visibility in the Deep Pelvis: A Comparative Human Cadaver Study between a Computerized Right Angle Linear Cutter versus a Curved Cutting Stapler. Ann. Surg. Innov. Res. 2011, 5, 7. [Google Scholar] [CrossRef]
- Shimada, Y.; Maehara, S.; Osawa, J.; Hagiwara, M.; Ohira, T.; Ikeda, N. Powered articulation by the SigniaTM stapling system for stapling position adjustments: Optimizing safe surgical margins in thoracoscopic sublobar resection. Surg. Today 2021, 51, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Yoo, A.; Yadalam, S.; Fegelman, E.J.; Kalsekar, I.; Johnston, S.S. Comparison of Economic and Clinical Outcomes between Patients Undergoing Laparoscopic Bariatric Surgery with Powered versus Manual Endoscopic Surgical Staplers. J. Med. Econ. 2017, 20, 423–433. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, D.J.; Mo Nam, C.; Park, G.; Byun, G.; Park, H.; Choi, J.H. Clinical and Economic Benefits Associated with the Use of Powered and Tissue-Specific Endoscopic Staplers among the Patients Undergoing Thoracoscopic Lobectomy for Lung Cancer. J. Med. Econ. 2019, 22, 1274–1280. [Google Scholar] [CrossRef]
- Eckert, C.E.; Harris, J.L.; Wong, J.; Thompson, S.; Kassis, E.S.; Tsuboi, M.; Ott, H.C.; Force, S. Preclinical Quantification of Air Leaks in a Physiologic Lung Model: Effects of Ventilation Modality and Staple Design. Med. Devices Evid. Res. 2018, 11, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Rawlins, L.; Johnson, B.H.; Johnston, S.S.; Elangovanraaj, N.; Bhandari, M.; Cohen, R.V.; Rheinwalt, K.P.; Fryrear, R.; Roy, S. Comparative Effectiveness Assessment of Two Powered Surgical Stapling Platforms in Laparoscopic Sleeve Gastrectomy: A Retrospective Matched Study. Med. Devices Evid. Res. 2020, 13, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Pla-Martí, V.; Martín-Arévalo, J.; Moro-Valdezate, D.; García-Botello, S.; Mora-Oliver, I.; Gadea-Mateo, R.; Cozar-Lozano, C.; Espí-Macías, A. Impact of the Novel Powered Circular Stapler on Risk of Anastomotic Leakage in Colorectal Anastomosis: A Propensity Score-Matched Study. Tech. Coloproctol. 2021, 25, 279–284. [Google Scholar] [CrossRef]
- Herzig, D.O.; Ogilvie, J.W.; Chudzinski, A.; Ferrara, A.; Ashraf, S.Q.; Jimenez-Rodriguez, R.M.; Van der Speeten, K.; Kinross, J.; Schimmelpenning, H.; Sagar, P.M.; et al. Assessment of a Circular Powered Stapler for Creation of Anastomosis in Left-Sided Colorectal Surgery: A Prospective Cohort Study. Int. J. Surg. 2020, 84, 140–146. [Google Scholar] [CrossRef]
- Pollack, E.; Johnston, S.; Petraiuolo, W.J.; Roy, S.; Galvain, T. Economic Analysis of Leak Complications in Anastomoses Performed with Powered versus Manual Circular Staplers in Left-Sided Colorectal Resections: A US-Based Cost Analysis. Clin. Outcomes Res. 2021, 13, 531–540. [Google Scholar] [CrossRef]
- Sylla, P.; Sagar, P.; Johnston, S.S.; Dwarakanathan, H.R.; Waggoner, J.R.; Schwiers, M.; Roy, S. Outcomes Associated with the Use of a New Powered Circular Stapler for Left-Sided Colorectal Reconstructions: A Propensity Score Matching-Adjusted Indirect Comparison with Manual Circular Staplers. Surg. Endosc. 2022, 36, 2541–2553. [Google Scholar] [CrossRef]
- Medtronic Covidien Signia Stapling Platform. Covidien Japan, Tokyo. Available online: https://www.medtronic.com/covidien/it-it/products/surgical-stapling/signia-stapling-system.html# (accessed on 19 July 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).