Effectiveness of Ultrasonic and Manual Instrumentation in Nonsurgical Periodontal Therapy: Are Additional Therapies More Effective? A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Focused Question
2.2. Eligibility Criteria
2.3. Search Strategy
2.4. Research
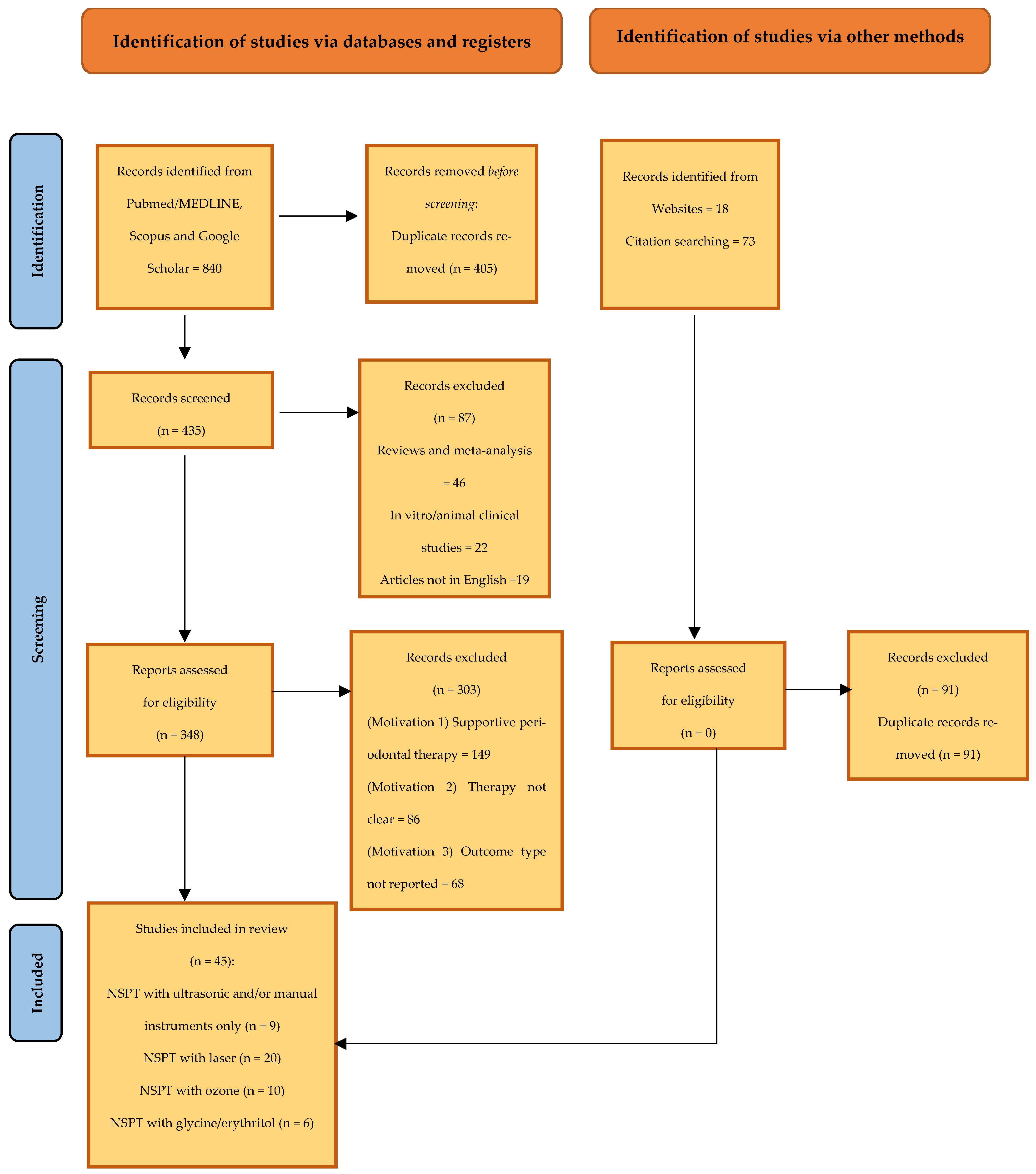
2.5. Screening and Selection of Articles
2.6. Risk of Bias and Results
3. Results
3.1. RCTs (Ultrasonic and Manual Instruments)
3.2. RCTs (Laser)
3.3. RCTs (Ozone)
3.4. RCTs (Glycine(Erythritol))
3.5. Risk of Bias of RCTs Included
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Relvas, M.; López-Jarana, P.; Monteiro, L.; Pacheco, J.J.; Braga, A.C.; Salazar, F. Study of Prevalence, Severity and Risk Factors of Periodontal Disease in a Portuguese Population. J. Clin. Med. 2022, 11, 3728. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. (Qassim) 2017, 11, 72–80. [Google Scholar] [PubMed]
- Aimetti, M. Nonsurgical periodontal treatment. Int. J. Esthet. Dent. 2014, 9, 251–267. [Google Scholar] [PubMed]
- Drisko, C.H. Nonsurgical periodontal therapy. Periodontol. 2000 2001, 25, 77–88. [Google Scholar] [CrossRef]
- Kučič, A.C.; Gašperšič, R. Minimally invasive non-surgical therapy (MINST) in stage III periodontitis patients: 6-month results of a split-mouth, randomised controlled clinical trial. Clin. Oral Investig. 2023, 27, 2075–2087. [Google Scholar] [CrossRef]
- Drisko, C.L.; Cochran, D.L.; Blieden, T.; Bouwsma, O.J.; Cohen, R.E.; Damoulis, P.; Fine, J.B.; Greenstein, G.; Hinrichs, J.; Somerman, M.J.; et al. Research, Science and Therapy Committee of the American Accademy of Periodontology. Position paper: Sonic and ultrasonic scalers in periodontics. Research, Science and Therapy Committee of the American Academy of Periodontology. J. Periodontol. 2000, 71, 1792–1801. [Google Scholar]
- Migliario, M.; Franchignoni, M.; Soldati, L.; Melle, A.; Carcieri, P.; Ferriero, G. Analisi ergonomica del manico di strumenti manuali per igiene dentale [Ergonomic analysis of the handle of manual instruments for dental hygiene]. G. Ital. Med. Lav. Ergon. 2012, 34, 202–206. [Google Scholar]
- Mittal, A.; Nichani, A.S.; Venugopal, R.; Rajani, V. The effect of various ultrasonic and hand instruments on the root surfaces of human single rooted teeth: A Planimetric and Profilometric study. J. Indian Soc. Periodontol. 2014, 18, 710–717. [Google Scholar] [CrossRef]
- Chung, W.C.; Huang, C.F.; Feng, S.W. Clinical Benefits of Minimally Invasive Non-Surgical Periodontal Therapy as an Alternative of Conventional Non-Surgical Periodontal Therapy-A Pilot Study. Int. J. Environ. Res. Public Health 2022, 19, 7456. [Google Scholar] [CrossRef]
- Zhou, X.; Lin, M.; Zhang, D.; Song, Y.; Wang, Z. Efficacy of Er:YAG laser on periodontitis as an adjunctive non- surgical treatment: A split-mouth randomized controlled study. J. Clin. Periodontol. 2019, 46, 539–547. [Google Scholar] [CrossRef]
- Sağlam, M.; Köseoğlu, S.; Taşdemir, I.; Erbak Yılmaz, H.; Savran, L.; Sütçü, R. Combined application of Er:YAG and Nd:YAG lasers in treatment of chronic periodontitis. A split-mouth, single-blind, randomized controlled trial. J. Periodontal Res. 2017, 52, 853–862. [Google Scholar] [CrossRef] [PubMed]
- El Meligy, O.A.; Elemam, N.M.; Talaat, I.M. Ozone Therapy in Medicine and Dentistry: A Review of the Literature. Dent. J. 2023, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Kshitish, D.; Laxman, V.K. The use of ozonated water and 0.2% chlorhexidine in the treatment of periodontitis patients: A clinical and microbiologic study. Indian J. Dent. Res. 2010, 21, 341–348. [Google Scholar] [PubMed]
- Moraschini, V.; Kischinhevsky, I.C.C.; Calasans-Maia, M.D.; Shibli, J.A.; Sartoretto, S.C.; Figueredo, C.M.; Granjeiro, J.M. Ineffectiveness of ozone therapy in nonsurgical periodontal treatment: A systematic review and metaanalysis of randomized clinical trials. Clin. Oral Investig. 2020, 24, 1877–1888. [Google Scholar] [CrossRef] [PubMed]
- Wenzler, J.S.; Krause, F.; Böcher, S.; Falk, W.; Birkenmaier, A.; Conrads, G.; Braun, A. Antimicrobial Impact of Different Air-Polishing Powders in a Subgingival Biofilm Model. Antibiotics 2021, 10, 1464. [Google Scholar] [CrossRef] [PubMed]
- Adriaens, P.A.; Adriaens, L.M. Effects of nonsurgical periodontal therapy on hard and soft tissues. Periodontol. 2000 2004, 36, 121–145. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ricciardi, W.; La Torre, G. Health Technology Assessment. Principi, Dimensioni e Strumenti; Seed Editor: Turin, Italy, 2010. [Google Scholar]
- Arpağ, O.F.; Dağ, A.; İzol, B.S.; Cimitay, G.; Uysal, E. Effects of vector ultrasonic system debridement and conventional instrumentation on the levels of TNF-α in gingival crevicular fluid of patients with chronic periodontitis. Adv. Clin. Exp. Med. 2017, 26, 1419–1424. [Google Scholar] [CrossRef]
- Puglisi, R.; Santos, A.; Pujol, A.; Ferrari, M.; Nart, J.; Pascual, A. Clinical comparison of instrumentation systems for periodontal debridement: A randomized clinical trial. Int. J. Dent. Hyg. 2022, 20, 328–338. [Google Scholar] [CrossRef]
- Petelin, M.; Perkič, K.; Seme, K.; Gašpirc, B. Effect of repeated adjunctive antimicrobial photodynamic therapy on subgingival periodontal pathogens in the treatment of chronic periodontitis. Lasers Med. Sci. 2015, 30, 1647–1656. [Google Scholar] [CrossRef]
- Malali, E.; Kadir, T.; Noyan, U. Er:YAG lasers versus ultrasonic and hand instruments in periodontal therapy: Clinical parameters, intracrevicular micro-organism and leukocyte counts. Photomed. Laser Surg. 2012, 30, 543–550. [Google Scholar] [CrossRef]
- Kargas, K.; Tsalikis, L.; Sakellari, D.; Menexes, G.; Konstantinidis, A. Pilot study on the clinical and microbiological effect of subgingival glycine powder air polishing using a cannula-like jet. Int. J. Dent. Hyg. 2015, 13, 161–169. [Google Scholar] [CrossRef]
- Tomasi, C.; Liss, A.; Welander, M.; Alian, A.Y.; Abrahamsson, K.H.; Wennström, J.L. A randomized multi-centre study on the effectiveness of non-surgical periodontal therapy in general practice. J. Clin. Periodontol. 2022, 49, 1092–1105. [Google Scholar] [CrossRef]
- Johnston, W.; Paterson, M.; Piela, K.; Davison, E.; Simpson, A.; Goulding, M.; Ramage, G.; Sherriff, A.; Culshaw, S. The systemic inflammatory response following hand instrumentation versus ultrasonic instrumentation-A randomized controlled trial. J. Clin. Periodontol. 2020, 47, 1087–1097. [Google Scholar] [CrossRef]
- Dilsiz, A.; Sevinc, S. Trauma from instrumentation after non-surgical periodontal treatment with ultrasonic scalers and Nd:YAG laser. Acta Odontol. Scand. 2015, 73, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Meulman, T.; Giorgetti, A.P.; Gimenes, J.; Casarin, R.C.; Peruzzo, D.C.; Nociti, F.H., Jr. One stage, full-mouth, ultrasonic debridement in the treatment of severe chronic periodontitis in smokers: A preliminary, blind and randomized clinical trial. J. Int. Acad. Periodontol. 2013, 15, 83–90. [Google Scholar]
- Dukić, W.; Bago, I.; Aurer, A.; Roguljić, M. Clinical effectiveness of diode laser therapy as an adjunct to non-surgical periodontal treatment: A randomized clinical study. J. Periodontol. 2013, 84, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- de Melo Soares, M.S.; D’Almeida Borges, C.; de Mendonça Invernici, M.; Frantz, F.G.; de Figueiredo, L.C.; de Souza, S.L.S.; Taba, M., Jr.; Messora, M.R.; Novaes, A.B., Jr. Antimicrobial photodynamic therapy as adjunct to non-surgical periodontal treatment in smokers: A randomized clinical trial. Clin. Oral Investig. 2019, 23, 3173–3182. [Google Scholar] [CrossRef] [PubMed]
- Gurpegui Abud, D.; Shariff, J.A.; Linden, E.; Kang, P.Y. Erbium-doped: Yttrium-aluminum-garnet (Er:YAG) versus scaling and root planing for the treatment of periodontal disease: A single-blinded split-mouth randomized clinical trial. J. Periodontol. 2022, 93, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Pai, B.S.J.; Krishnan, N.R.; Walveker, A.; Keeneri, S.; Emmanuel, A.; Krishnan, N.R.; Lira, M.A. Comparative Evaluation of Sclerostin Levels in Gingival Crevicular Fluid in the Treatment of Chronic Periodontitis Patients Using Diode Laser as an Adjunct to Scaling and Root Planing: A Clinico-biochemical Study. Contemp. Clin. Dent. 2021, 12, 276–281. [Google Scholar] [PubMed]
- Dilsiz, A.; Canakci, V.; Aydin, T. Clinical effects of potassium-titanyl-phosphate laser and photodynamic therapy on outcomes of treatment of chronic periodontitis: A randomized controlled clinical trial. J. Periodontol. 2013, 84, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Dereci, O.; Hatipoglu, M.; Sindel, A.; Tozoglu, S.; Üstün, K. The efficacy of Er,Cr:YSGG laser supported periodontal therapy on the reduction of peridodontal disease related oral malodor: A randomized clinical study. Head Face Med. 2016, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Hatipoglu, M.; Aytekin, Z.; Daltaban, Ö.; Felek, R.; Firat, M.Z.; Üstün, K. The effect of diode laser as an adjunct to periodontal treatment on clinical periodontal parameters and halitosis: A randomized controlled clinical trial. Cumhur. Dent. J. 2017, 20, 152–160. [Google Scholar] [CrossRef]
- Matarese, G.; Ramaglia, L.; Cicciù, M.; Cordasco, G.; Isola, G. The Effects of Diode Laser Therapy as an Adjunct to Scaling and Root Planing in the Treatment of Aggressive Periodontitis: A 1-Year Randomized Controlled Clinical Trial. Photomed. Laser Surg. 2017, 35, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Ustun, K.; Hatipoglu, M.; Daltaban, O.; Felek, R.; Firat, M.Z. Clinical and biochemical effects of erbium, chromium: Yttrium, scandium, gallium, garnet laser treatment as a complement to periodontal treatment. Niger. J. Clin. Pract. 2018, 21, 1150–1157. [Google Scholar] [PubMed]
- Alwaeli, H.A.; Al-Khateeb, S.N.; Al-Sadi, A. Long-term clinical effect of adjunctive antimicrobial photodynamic therapy in periodontal treatment: A randomized clinical trial. Lasers Med. Sci. 2013, 30, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Pourabbas, R.; Kashefimehr, A.; Rahmanpour, N.; Babaloo, Z.; Kishen, A.; Tenenbaum, H.C.; Azarpazhooh, A. Effects of Photodynamic Therapy on Clinical and Gingival Crevicular Fluid Inflammatory Biomarkers in Chronic Periodontitis: A Split-Mouth Randomized Clinical Trial. J. Periodontol. 2014, 85, 1222–1229. [Google Scholar] [CrossRef]
- Birang, R.; Shahaboui, M.; Kiani, S.; Shadmehr, E.; Naghsh, N. Effect of Nonsurgical Periodontal Treatment Combined With Diode Laser or Photodynamic Therapy on Chronic Periodontitis: A Randomized Controlled Split-Mouth Clinical Trial. Lasers Med. Sci. 2015, 6, 112–119. [Google Scholar] [CrossRef]
- Queiroz, A.C.; Suaid, F.A.; Andrade, P.F.; Oliveira, F.S.; Novaes, A.B., Jr.; Taba, M., Jr.; Palioto, D.B.; Grisi, M.F.; Souza, S.L. Adjunctive effect of antimicrobial photodynamic therapy to nonsurgical periodontal treatment in smokers: A randomized clinical trial. Lasers Med. Sci. 2015, 30, 617–625. [Google Scholar] [CrossRef]
- Balata, M.L.; Andrade, L.P.D.; Santos, D.B.N.; Cavalcanti, A.N.; Tunes, U.D.R.; Ribeiro, E.D.P.; Bittencourt, S. Photodynamic therapy associated with full-mouth ultrasonic debridement in the treatment of severe chronic periodontitis: A randomized-controlled clinical trial. J. Appl. Oral Sci. 2013, 21, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Malgikar, S.; Reddy, S.H.; Babu, P.R.; Sagar, S.V.; Kumar, P.S.; Reddy, G.J. A randomized controlled clinical trial on efficacy of photodynamic therapy as an adjunct to nonsurgical treatment of chronic periodontitis. J. Dent. Lasers 2015, 9, 75–79. [Google Scholar] [CrossRef]
- Gündoğar, H.; Şenyurt, S.Z.; Erciyas, K.; Yalım, M.; Üstün, K. The effect of low-level laser therapy on non-surgical periodontal treatment: A randomized controlled, single-blind, split-mouth clinical trial. Lasers Med. Sci. 2016, 31, 1767–1773. [Google Scholar] [CrossRef]
- Manjunath, S.; Singla, D.; Singh, R. Clinical and microbiological evaluation of the synergistic effects of diode laser with nonsurgical periodontal therapy: A randomized clinical trial. J. Indian Soc. Periodontol. 2020, 24, 145–149. [Google Scholar] [PubMed]
- Euzebio Alves, V.T.E.; de Andrade, A.K.P.; Toaliar, J.M.; Conde, M.C.; Zezell, D.M.; Cai, S.; Pannuti, C.M.; De Micheli, G. Clinical and microbiological evaluation of high intensity diode laser adjutant to non-surgical periodontal treatment: A 6-month clinical trial. Clin. Oral Investig. 2013, 17, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Bundidpun, P.; Srisuwantha, R.; Laosrisin, N. Clinical effects of photodynamic therapy as an adjunct to full-mouth ultrasonic scaling and root planing in treatment of chronic periodontitis. Laser Ther. 2018, 27, 33–39. [Google Scholar] [CrossRef]
- Mishra, A.; Schergill, N. The effect of low-level laser therapy on nonsurgical periodontal therapy: A clinic-biochemical study. J. Dent. Lasers 2018, 12, 14–17. [Google Scholar] [CrossRef]
- Dengizek, E.S.; Serkan, D.; Abubekir, E.; Bay, K.A.; Onder, O.; Arife, C. Evaluating clinical and laboratory effects of ozone in non-surgical periodontal treatment: A randomized controlled trial. J. Appl. Oral Sci. 2019, 27, e20180108. [Google Scholar] [CrossRef]
- Tasdemir, Z.; Oskaybas, M.N.; Alkan, A.B.; Cakmak, O. The effects of ozone therapy on periodontal therapy: A randomized placebo-controlled clinical trial. Oral Dis. 2019, 25, 1195–1202. [Google Scholar] [CrossRef]
- Rapone, B.; Ferrara, E.; Santacroce, L.; Topi, S.; Gnoni, A.; Dipalma, G.; Mancini, A.; Di Domenico, M.; Tartaglia, G.M.; Scarano, A.; et al. The Gaseous Ozone Therapy as a Promising Antiseptic Adjuvant of Periodontal Treatment: A Randomized Controlled Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 985. [Google Scholar] [CrossRef]
- Piva, A.; Avantaggiato, P.; Candotto, V.; Pellati, A.; Moreo, G. The use of ozone therapy for treatment of periodontal disease: A split-mouth, randomized, controlled clinical trial. J. Biol. Regul. Homeost. Agents 2020, 34, 91–98. [Google Scholar] [PubMed]
- Uraz, A.; Karaduman, B.; Isler, S.Ç.; Gönen, S.; Çetiner, D. Ozone application as adjunctive therapy in chronic periodontitis: Clinical, microbiological and biochemical aspects. J. Dent. Sci. 2019, 14, 27–37. [Google Scholar] [CrossRef]
- Al Habashneh, R.; Alsalman, W.; Khader, Y. Ozone as an adjunct to conventional nonsurgical therapy in chronic periodontitis: A randomized controlled clinical trial. J. Periodontal Res. 2015, 50, 37–43. [Google Scholar] [CrossRef]
- Issac, A.V.; Mathew, J.J.; Ambooken, M.; Kachappilly, A.J.; Pk, A.; Johny, T.; Vk, L.; Samuel, A. Management of chronic periodontitis using subgingival irrigation of ozonized water: A clinical and microbiological study. J. Clin. Diagn. Res. 2015, 9, Zc29–Zc33. [Google Scholar] [CrossRef] [PubMed]
- Pandya, D.J.; Manohar, B.; Mathur, L.K.; Shankarapillai, R. Comparative evaluation of two subgingival irrigating solutions in the management of periodontal disease: A clinicomicrobial study. J. Indian Soc. Periodontol. 2016, 20, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Bhavikatti, S.K.; Das, S.S.; Khanna, S.; Jain, M.; Kaur, A. Efficacy of Ozonised Water and 0.2% Chlorhexidine Gluconate in the Management of Chronic Periodontitis when Used as an Irrigant in Conjugation with Phase I Therapy. J. Contemp. Dent. Prac. 2019, 20, 318–323. [Google Scholar]
- Hayakumo, S.; Arakawa, S.; Mano, Y.; Izumi, Y. Clinical and microbiological effects of ozone nano-bubble water irrigation as an adjunct to mechanical subgingival debridement in periodontitis patients in a randomized controlled trial. Clin. Oral Investig. 2013, 17, 379–388. [Google Scholar] [CrossRef]
- Divnic-Resnik, T.; Pradhan, H.; Spahr, A. The efficacy of the adjunct use of subgingival air-polishing therapy with erythritol powder compared to conventional debridement alone during initial non-surgical periodontal therapy. J. Clin. Periodontol. 2022, 49, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-J.; Kwon, E.-Y.; Kim, H.-J.; Lee, J.-Y.; Choi, J.; Joo, J.-Y. Clinical and microbiological effects of the supplementary use of an erythritol powder air-polishing device in non-surgical periodontal therapy: A randomized clinical trial. J. Periodontal Implant Sci. 2018, 48, 295–304. [Google Scholar] [CrossRef]
- Jentsch, H.F.R.; Flechsig, C.; Kette, B.; Eick, S. Adjunctive air-polishing with erythritol in nonsurgical periodontal therapy: A randomized clinical trial. BMC Oral Health 2020, 20, 364. [Google Scholar] [CrossRef]
- Caygur, A.; Albaba, M.R.; Berberoglu, A.; Yilmaz, H.G. Efficacy of glycine powder air-polishing combined with scaling and root planing in the treatment of periodontitis and halitosis: A randomised clinical study. J. Int. Med. Res. 2017, 45, 1168–1174. [Google Scholar] [CrossRef]
- Tsang, Y.C.; Corbet, E.F.; Jin, L.J. Subgingival glycine powder air-polishing as an additional approach to nonsurgical periodontal therapy in subjects with untreated chronic periodontitis. J. Periodontal Res. 2018, 53, 440–445. [Google Scholar] [CrossRef]
- Stein, J.M.; Yekta-Michael, S.S.; Schittenhelm, F.; Reichert, S.; Kupietz, D.; Dommisch, H.; Kasaj, A.; Wied, S.; Vela, O.C.; Stratul, S.I. Comparison of three full-mouth concepts for the non-surgical treatment of stage III and IV periodontitis: A ran-domized controlled trial. J. Clin. Periodontol. 2021, 48, 1516–1527. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Z.; Li, W.; Chen, J. Treating periodontitis-a systematic review and meta-analysis comparing ultrasonic and manual subgingival scaling at different probing pocket depths. BMC Oral Health 2020, 20, 176. [Google Scholar] [CrossRef]
- Muniz, F.W.M.G.; Langa, G.P.J.; Pimentel, R.P.; Martins, J.R.; Pereira, D.H.; Rösing, C.K. Comparison Between Hand and Sonic/Ultrasonic Instruments for Periodontal Treatment: Systematic Review with Meta-Analysis. J. Int. Acad. Periodontol. 2020, 22, 187–204. [Google Scholar] [PubMed]
- Suvan, J.; Leira, Y.; Moreno Sancho, F.M.; Graziani, F.; Derks, J.; Tomasi, C. Subgingival instrumentation for treatment of periodontitis. A systematic review. J. Clin. Periodontol. 2020, 47, 155–175. [Google Scholar] [CrossRef]
- Oza, R.R.; Sharma, V.; Multani, P.; Balsara, K.; Bajaj, P.; Dhadse, P. Comparing the Effectiveness of Ultrasonic Instruments Over Manual Instruments for Scaling and Root Planing in Patients With Chronic Periodontitis: A Systematic Review and Meta-Analysis. Cureus 2022, 14, e31463. [Google Scholar] [CrossRef] [PubMed]
- Darby, I. Non-surgical management of periodontal disease. Aust. Dent. J. 2009, 54, S86–S95. [Google Scholar] [CrossRef]
- Kwon, T.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef]
- Tomasi, C.; Abrahamsson, K.H.; Apatzidou, D. Subgingival instrumentation. Periodontol. 2000 2023. ahead of print. [Google Scholar] [CrossRef]
- Graeber, J.J. Scaling and root planing. J. Am. Dent. Assoc. 2015, 146, 865. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A. Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontol. 2000 2018, 76, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Christgau, M.; Männer, T.; Beuer, S.; Hiller, K.A.; Schmalz, G. Periodontal healing after non-surgical therapy with a new ultrasonic device: A randomized controlled clinical trial. J. Clin. Periodontol. 2007, 34, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Kozlovsky, A.; Rapaport, A.; Artzi, Z. Influence of operator skill level on the clinical outcome of non-surgical periodontal treatment: A retrospective study. Clin. Oral Investig. 2018, 22, 2927–2932. [Google Scholar] [CrossRef]
- Obeid, P.; Bercy, P. Loss of tooth substance during root planing with various periodontal instruments: An in vitro study. Clin. Oral Investig. 2005, 9, 118–123. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liu, Z.; Zhu, X.; Xia, H.; Gao, H.; Lu, J. Ultrasonic Microbubble Cavitation Enhanced Tissue Permeability and Drug Diffusion in Solid Tumor Therapy. Pharmaceutics 2022, 14, 1642. [Google Scholar] [CrossRef] [PubMed]
- Christgau, M.; Männer, T.; Beuer, S.; Hiller, K.A.; Schmalz, G. Periodontal healing after non-surgical therapy with a modified sonic scaler: A controlled clinical trial. J. Clin. Periodontol. 2006, 33, 749–758. [Google Scholar] [CrossRef]
- Drisko, C.H. Root instrumentation. Power-driven versus manual scalers, which one? Dent. Clin. N. Am. 1998, 42, 229–244. [Google Scholar] [CrossRef]
- Liss, A.; Wennström, J.L.; Welander, M.; Tomasi, C.; Petzold, M.; Abrahamsson, K.H. Patient-reported experiences and outcomes following two different approaches for non-surgical periodontal treatment: A randomized field study. BMC Oral Health 2021, 21, 645. [Google Scholar] [CrossRef]
- Daly, S.; Newcombe, R.G.; Claydon, N.C.A.; Seong, J.; Davies, M.; West, N.X. A randomised controlled trial to determine patient experience of a magnetostrictive stack scaler as compared to a piezoelectric scaler, in supportive periodontal therapy. J. Dent. 2020, 93, 103279. [Google Scholar] [CrossRef]
- Walmsley, A.D.; Lea, S.C.; Landini, G.; Moses, A.J. Advances in power driven pocket/root instrumentation. J. Clin. Periodontol. 2008, 35, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H.; Sato, S.; Kishida, M.; Ito, K. A comparison of root surface instrumentation using two piezoelectric ultrasonic scalers and a hand scaler in vivo. J. Periodontal Res. 2007, 42, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.A.; Pochapski, M.T.; Leal, P.C.; Gimenes-Sakima, P.P.; Marcantonio, E., Jr. Comparative study on the effect of ultrasonic instruments on the root surface in vivo. Clin. Oral Investig. 2008, 12, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Casarin, R.C.V.; Pinto, F.R.; Júnior, F.H.N.; Sallum, E.A.; Sallum, A.W.; Casati, M.Z. Assessment of ultrasonic root surface scaling with different power settings. roughness evaluation. Braz. J. Oral Sci. 2006, 5, 996–1000. [Google Scholar]
- Mahiroglu, M.B.; Kahramanoglu, E.; Ay, M.; Kuru, L.; Agrali, O.B. Comparison of Root Surface Wear and Roughness Resulted from Different Ultrasonic Scalers and Polishing Devices Applied on Human Teeth: An In-Vitro Study. Healthcare 2020, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Uppoor, A.; Nayak, D. A comparative evaluation of the efficacy of manual, magnetostrictive and piezoelectric ultrasonic instruments—An in vitro profilometric and SEM study. J. Appl. Oral Sci. 2012, 20, 21–26. [Google Scholar] [CrossRef]
- Vaziri, F.; Maybodi, F.R.; Farashahi, M.A. Evaluation of root surface roughness produced by hand instruments and ultrasonic scalers: An in vivo study. J. Adv. Periodontol. Implant Dent. 2022, 14, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Das, S.J.; Sonowal, S.T.; Chawla, J. Comparison of Root Surface Roughness Produced By Hand Instruments and Ultrasonic Scalers: An Invitro Study. J. Clin. Diagn. Res. 2015, 9, ZC56–ZC60. [Google Scholar] [CrossRef]
- Salvi, G.E.; Stähli, A.; Schmidt, J.C.; Ramseier, C.A.; Sculean, A.; Walter, C. Adjunctive laser or antimicrobial photodynamic therapy to non-surgical mechanical instrumentation in patients with untreated periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 22, 176–198. [Google Scholar] [CrossRef]
- Ren, C.; McGrath, C.; Jin, L.; Zhang, C.; Yang, Y. The effectiveness of low-level laser therapy as an adjunct to non-surgical periodontal treatment: A meta-analysis. J. Periodontal Res. 2017, 52, 8–20. [Google Scholar] [CrossRef]
- Schwarz, F.; Aoki, A.; Becker, J.; Sculean, A. Laser application in non-surgical periodontal therapy: A systematic review. J. Clin. Periodontol. 2008, 35, 29–44. [Google Scholar] [CrossRef]
- Onisor, F.; Mester, A.; Mancini, L.; Voina-Tonea, A. Effectiveness and Clinical Performance of Erythritol Air-Polishing in Non-Surgical Periodontal Therapy: A Systematic Review of Randomized Clinical Trials. Medicina 2022, 58, 866. [Google Scholar] [CrossRef] [PubMed]
| Articles | Type of Study | Problem | Intervention/Comparison | Clinical Indeces/Follow-Up | Outcome |
|---|---|---|---|---|---|
| Arpağ et al., 2017 [19] | RCT | Determine the effect of vector ultrasonic system (VUS) on the levels of TNF-α in gingival crevicular fluid (GCF) and the clinical parameters in patients with chronic periodontitis | 30 patients were included for a split-mouth treatment: (A) scaling and root planing with hand instrument; (B) vector ultrasonic systems | PPD, CAL, PI, GI 6 months | Significant clinical improvements were seen with all treatments, yet there was no discernible variation across the groups. |
| Puglisi et al., 2022 [20] | RCT | Examine the clinical effectiveness, chairside duration and hypersensitivity following treatment of four instruments utilized in subgingival periodontal debridement | This split-mouth design was used to enroll 17 patients with stage II and III periodontitis in a randomized clinical trial. Four treatment groups were randomly selected from the quadrants: Group D, piezosurgery ultrasonic with PP1 insert; Group C, diamond burs, 40 µm; Group D, piezoelectric ultrasonic with No.1S insert; Group A, gracey curettes. | PPD, CAL, PI, REC 2 months | All treatments resulted in a significant clinical improvement, but there were no significant differences among groups. |
| Petelin et al., 2015 [21] | RCT | Compare the effect of subgingival ultrasonic scaling followed by repeated (three times) antimicrobial photodynamic therapy (PDT), ultrasonic scaling alone (US), and scaling and root planing with hand instruments (SRP) for initial periodontal treatment | 27 non-smoking, systemically healthy chronic periodontitis patients were included. Residual pockets ≥ 4 mm deep and bleeding on probing were debrided either with SRP, US alone or US followed by a single episode of PDT during supportive periodontal treatment. | PPD, CAL BoP 12 months | All treatments resulted in a significant clinical improvement, but there were no significant differences among groups. |
| Malali et al., 2012 [22] | RCT | Compare the clinical parameters and crevicular cell population, particularly leukocyte counts, and changes after initial periodontal therapy with different instruments in severe periodontitis patients | 30 systemically healthy subjects with severe chronic periodontitis were randomly assigned to three groups (n = 10) and were treated either with hand curettes, ultrasonic scalers or an Er:YAG laser alone. | PPD, CAL, BoP, PI, GI 3 months | All treatments resulted in a significant clinical improvement, but there were no significant differences among groups. |
| Kargas et al., 2015 [23] | RCT | Evaluate the efficacy of glycine powder air-polishing in treatment periodontal pockets equal or up to 4 mm | Each quadrant of 25 subjects was randomly assigned to the following treatments: subgingival scaling with hand instruments (SRP), GPAP, subgingival ultrasonic debridement (UD) and no subgingival treatment (NT). | PPD, CAL, PI, GI, REC | All treatments resulted in a significant clinical improvement, but there were no significant differences among groups. |
| Tomasi et al., 2022 [24] | RCT | Evaluate the effectiveness of two nonsurgical treatment protocols for periodontitis patients in general dental practice | 615 patients were divided into two groups: (A) single session of ultrasonic instrumentation (guided periodontal infection control [GPIC]) or (B) conventional nonsurgical therapy (CNST), including patient education and scaling and root planing integrated in multiple sessions. | PPD, BoP 6 months | All treatments resulted in a significant clinical improvement, but there were no significant differences among groups. |
| Johnston et al., 2020 [25] | RCT | Investigate whether the immediate systemic inflammatory response following full-mouth debridement differs following use of hand compared with ultrasonic instruments | 37 periodontitis patients were randomized to treatment with full-mouth debridement using either hand or ultrasonic instrumentation completed within 24 h. | PPD, CAL, FMBS, FMPS 3 months | All treatments resulted in a significant clinical improvement, but there were no significant differences among groups. |
| Dilsiz et al., 2015 [26] | RCT | Examine and contrast the immediate impact of tool trauma on the clinical attachment level following nonsurgical periodontal therapy using Nd:YAG lasers and ultrasonic scalers | 24 patients with untreated chronic periodontitis were entered into the study. Each quadrant was randomly allocated in a split-mouth design either to treatment with Nd:YAG laser using an energy of 1 W, 100 mj and 1064 nm (test group) or to periodontal treatment using ultrasonic scalers (control group). | PPD, CAL, BoP, PI No clear follow-up | All treatments resulted in a significant clinical improvement, but there were no significant differences among groups. |
| Meulman et al., 2013 [27] | RCT | Evaluate the effectiveness of a full-mouth ultrasonic debridement technique in treating smokers’ severe chronic periodontitis in contrast to a quadrant-wise therapy involving scaling and root planing | 30 patients with periodontitis were split into three groups: Group SRP (scaling and root planing performed in a quadrant-wise manner for smokers; n = ten); Group FMUD (full-mouth ultrasonic debridement); and Group Control (scaling and root planing for nonsmokers; n = ten), treated using the same protocol as the SRP group. | PPD, CAL, BoP, PI, REC 6 months | Significant clinical improvements were seen with all treatments, yet there was no discernible variation across the groups. |
| Articles | Type of Study | Problem | Intervention/Comparison | Clinical Indices/Follow-Up | Outcome |
|---|---|---|---|---|---|
| Dukic et al., 2013 [28] | RCT | 35 patients with chronic periodontitis | Test: SRP + diode laser Control: SRP | CAL, PPD, BOP, API; 18 weeks | The current study shows that only significant periodontal pockets demonstrated PD improvements with several adjunctive administrations of a 980 nm diode laser combined with SRP, as opposed to SRP alone. |
| De Melo Soares et al., 2019 [29] | RCT | 30 patients with chronic periodontitis | Test: SRP + PDT (diode laser) Control: SRP | CAL, PPD, BOP, PI, MB; 3 months | When compared to SRP alone, aPDT as an adjuvant to SRP did not show statistically significant advantages on clinical parameters. |
| Gurpegui et al., 2022 [30] | RCT | 30 patients with generalized moderate-to-severe chronic periodontitis of generalized stages II or III and Grade B periodontitis | Test: SRP + Er:YAG laser Control: SRP | CAL, PPD, BOP, PI, GR; 3 months | Both treatments resulted in overall improvement, but no significant differences were found between modalities for clinical attachment gain or probing depth reduction. |
| Pai et al., 2021 [31] | RCT | 15 patients with chronic periodontitis | Test: SRP + diode laser Control: SRP | CAL, PPD, BI, GI; 3 months | After three months, this study revealed a statistically significant decrease in clinical indices in both the test and control groups. After three months, the sclerostin levels in GCF were statistically significant in both groups, with the test group exhibiting a highly significant drop. |
| Dilsiz et al., 2013 [32] | RCT | 24 patients with chronic periodontitis | Test 1: SRP + KTP laser Test 2: SRP + PDT Control: SRP | CAL, PPD, BOP, PI, GI; 6 months | KTP laser group showed a greater reduction in PD compared to the other groups (p < 0.05). In addition, KTP laser group showed a greater CAL gain compared to the other groups (p < 0.05). |
| Dereci et al., 2016 [33] | RCT | 60 patients with chronic periodontitis | Test: SRP + Er,Cr:YSGG laser Control: SRP + placebo | CAL, PPD, BOP, PI; 6 months | Er,Cr:YSGG laser assisted conventional periodontal therapy is more effective in improving periodontal healing compared to conventional periodontal therapy alone. |
| Hatipoglu et al., 2017 [34] | RCT | 40 patients with chronic periodontitis | Test: SRP + indium–gallium–aluminium–phosphate diode laser Control: SRP | CAL, PPD, BOP, PI, GI; 6 months | The present study indicates that compared to SRP solely, adjunctive applications of a 940 nm diode laser with SRP showed lower bleeding on probing. |
| Matarese et al., 2017 [35] | RCT | 31 patients with a diagnosis of GAgP | Test: SRP + diode laser Control: SRP + placebo | CAL, PPD, BOP, FMPS; 1 month | At 1 year, SRP + diode laser yielded a significant reduction in some clinical parameters. |
| Ustun et al., 2018 [36] | RCT | 40 patients with chronic periodontitis | Test: SRP + Er,Cr:YSGG laser Control: SRP | CAL, PPD, BOP, PI, GI; 6 months | After periodontal treatment, CAL, PD, BOP, GI and PI, which are clinical parameters analyzed, decreased significantly (p < 0.05) in both test and control groups. |
| Alwaeli et al., 2013 [37] | RCT | 16 patients with chronic periodontitis | Test: SRP + PDT (diode laser) Control: SRP | CAL, PPD, BOP; 12 months | For all three time points in both groups, there was a significant decrease in PPD and BOP and a significant increase in CAL from the baseline. Furthermore, at all three time points, there were noticeably more reductions and gains for SRP + aPDT than for SRP. |
| Pourabbas et al., 2014 [38] | RCT | 22 patients with moderate-to-severe chronic periodontitis | Test: SRP + PDT (diode laser) Control: SRP | CAL, PPD, BOP, GR; 3 months | In patients with CP, a single application of PDT did not provide any additional benefit to SRP in terms of clinical parameters or inflammatory markers 3 months following the intervention. |
| Birang et al., 2015 [39] | RCT | 20 patients with chronic periodontitis | Test 1: diode laser Test 2: SRP + PDT Control: SRP | CAL, PPD, PI, PBI; 3 months | The 6-week evaluation revealed a statistically significant increase in CAL in the laser groups compared to the control group (p < 0.05). Additionally, Test 2 with the laser group showed a higher PPD reduction than the other treatment methods (p < 0.05). |
| Queiroz et al., 2015 [40] | RCT | 20 patients with chronic periodontitis | Test: SRP + PDT (diode laser) Control: SRP | CAL, PPD, BOP, PI, GR; 3 months | The adjunctive effect of aPDT did not warrant improvements on clinical parameters. |
| Balata et al., 2013 [41] | RCT | 22 patients with severe chronic periodontitis | Test: SRP + PDT (diode laser) Control: SRP | CAL, PPD, GR, BOP, GBI, VPI; 6 months | While there were notable clinical benefits with both methods for treating severe chronic periodontits, the PDT did not yield any further advantages over full-mouth ultrasonic debridement alone. |
| Malgikar et al., 2015 [42] | RCT | 24 patients with chronic periodontitis | Test: SRP + PDT (diode laser) Control: SRP | CAL, PPD, SBI, GBI, PI; 6 months | In terms of clinical factors, a single PDT session may be applied in conjunction with scaling and root planing for the nonsurgical treatment of chronic periodontitis. |
| Gundogar et al., 2016 [43] | RCT | 25 with generalized chronic periodontitis | Test: SRP + LLT Control: SRP | CAL, PPD, PI, GI; 1 month | LLLT as an adjunct to nonsurgical periodontal treatment has a positive impact on clinical parameters. |
| Manjunath et al., 2020 [44] | RCT | 40 patients with chronic periodontitis | Test: SRP + diode laser Control: SRP | CAL, PPD, BOP; 3 months | There was a significant improvement in clinical parameters in the test group (SRP + laser) as compared to the control group (SRP alone). |
| Euzebio et al., 2013 [45] | RCT | 36 patients with severe chronic periodontitis | Test: SRP + diode laser Control: SRP + placebo | CAL, PPD, BOP, PI; 6 months | After 6 months of evaluation, the high-intensity diode laser has not shown any additional benefits compared to the conventional periodontal treatment. |
| Bundidpun et al., 2020 [46] | RCT | 20 patients with generalized moderate-to-severe chronic periodontitis | Test: SRP + PDT (diode laser) Control: SRP | CAL, PPD, PI, GBI, GI; 6 months | One visit full-mouth ultrasonic SRP seems to have a good enough effort for the periodontal status till 6 months. The adjunct treatment of PDT provided a positive effect in terms of GBI and GI. |
| Mishra et al., 2018 [47] | RCT | 20 patients with chronic generalized periodontitis | Test: SRP + LLT (diode laser) Control: SRP | CAL, PPD, MGI, SBI; 3 months | Only in the test group did MGI, SBI and PD exhibit a significant difference; however, from baseline to three months, every parameter showed a statistically significant difference. MGI, SBI and CRP levels varied statistically significantly throughout the groups in intergroup comparison. |
| Articles | Type of Study | Problem | Intervention/Comparison | Clinical Indices/Follow-Up | Outcome |
|---|---|---|---|---|---|
| Seydanur Dengizek et al., 2019 [48] | RCT | 40 patients with moderate generalized chronic periodontitis | Test: SRP + ozone gas Control: SRP | CAL, PPD, PI, GI; 1 month | SRP plus gaseous ozone versus SRP alone does not correlate to a significant improvement in periodontal recovery. |
| Tasdemir et al., 2019 [49] | RCT | 36 patients with moderate and/or severe generalized periodontitis | Test: SRP + ozone gas Control: SRP | CAL, PPD, BOP, PI, GI; 3 months | Ozone therapy did not have any additional effect on periodontal parameters. |
| Rapone et al., 2022 [50] | RCT | 90 patients with moderate or severe generalized chronic periodontitis | Test: SRP + ozone gas Control: SRP | CAL, PPD, BOP; 6 months | When SRP and ozone therapy were used together to treat periodontitis, the results were better than when SRP was used alone. From the baseline, there was a statistically significant difference between the groups’ scores for CAL, PPD and BOP. |
| Piva et al., 2020 [51] | RCT | 10 patients with chronic periodontitis | Test: SRP + ozonated water Control: SRP | Microbiological samples; 7 days | T. forsythia and T. denticola were eradicated in the ozone group. |
| Uraz et al., 2019 [52] | RCT | 18 patients with generalized chronic periodontitis | Test: SRP + ozone gas Control: SRP | PPD, BOP, PI, GI; 3 months | Ozone therapy did not provide additional benefits to clinical, microbiological and biochemical parameters over SRP in chronic periodontitis patients. |
| Al Habashneh et al., 2015 [53] | RCT | 41 patients with chronic periodontitis | Test: SRP + ozonated water Control: SRP | CAL, PPD, BOP, PI, GI; 3 months | As an adjuvant therapy to SRP, ozonated water irrigation yields no statistically significant benefit. |
| Issac et al., 2015 [54] | RCT | 30 patients with chronic periodontitis | Test: SRP + ozonated water Control: SRP | CAL, PPD, GI; microbiological samples; 4 weeks | When combined with cleaning and root planing, ozonized water subgingival irrigation can help patients with chronic periodontitis by improving their clinical and microbiological parameters. |
| Pandya et al., 2016 [55] | RCT | 10 patients with chronic periodontitis | Test: SRP + chlorhexidine Test: SRP + ozonated water Test: SRP + saline solution Control: SRP | PPD, GI; microbiological samples; 1 month | When compared to the other two subgingival irrigants, ozonated water and 0.2% chlorhexidine gluconate were found to be the most efficacious, while saline proved to be useless. |
| Kaur et al., 2019 [56] | RCT | 20 patients with chronic periodontitis | Test: SRP + chlorhexidine Test: SRP + ozonated water | CAL, PPD, PI; 3 months | Utilizing ozonized water for subgingival irrigation is more advantageous than current traditional treatment approaches. By limiting the production of tooth plaque and lowering the quantity of subgingival bacteria, ozonated water helps treat periodontal diseases. |
| Hayakumo et al., 2013 [57] | RCT | 22 patients that exhibited mild to moderate chronic periodontitis | Test: SRP + ozonated water Control: SRP | CAL, PPD, BOP; 8 weeks | Subgingival irrigation with ozonated water may be a valuable adjunct to periodontal treatment. |
| Articles | Type of Study | Problem | Intervention/Comparison | Clinical Indices/Follow-Up | Outcome |
|---|---|---|---|---|---|
| Divnic-Resnik et al., 2022 [58] | RCT | 21 patients with generalized stage 2 and 3 grade B periodontitis | Test: SRP + erythritol Control: SRP | CAL, PPD, BOP, API, SBI; 6 months | After 6 months, there was a greater attachment gain and a substantial reduction in the number of initially deep pockets (PPD > 5.5 mm) to shallow pockets (PPD ≤ 3.4 mm) in the test group. |
| Park et al., 2018 [59] | RCT | 21 patients with moderate chronic periodontitis | Test: SRP + erythritol Control: SRP | CAL, PPD, BOP, PI, REC; 3 months | Clinically and microbiologically, SRP and SRP + EPAP proved to be efficacious nonsurgical periodontal treatments. With P. gingivalis in particular, the SRP + EPAP group demonstrated an antibacterial effect. |
| Jentsch et al., 2020 [60] | RCT | 42 patients with chronic periodontitis | Test: SRP + erythritol Control: SRP | CAL, PPD, BOP, API; 6 months | BOP is not decreased by subgingival instrumentation with adjunctive erythritol air-polishing powder. However, as compared to subgingival instrumentation alone, it might have additional positive effects, such as lowering the probing depth as indicated by the frequency of residual periodontal pockets with PD ≥ 5 mm. |
| Caygur et al., 2017 [61] | RCT | 60 patients with moderate chronic periodontitis | Test: SRP + glycine Control: SRP | CAL, PPD, BOP, PI, GI; 1 month | There are no advantages to using GPAP in addition to mechanical instruments for halitosis or periodontal parameters. |
| Tsang et al., 2018 [62] | RCT | 27 systematically health non-smoking patients with chronic periodontitis | Test: SRP + glycine Control: SRP | CAL, PPD, BOP, GR; 6 months | When determined by gingival crevicular fluid volume, GPAP may be helpful as an additional nonsurgical periodontal therapy strategy for the temporary alleviation of subclinical inflammation. |
| Stein et al., 2021 [63] | RCT | 172 patients with stage 3 and 4 periodontitis | Test 1: full mouth Test 2: full-mouth disinfection Test 3: full-mouth disinfection + erythritol Control: SRP | CAL, PPD, BOP; 6 months | Every treatment modality was successful, and there were no appreciable variations between full-mouth techniques. After six months, FMDAP (erythritol) outperformed Q-SRP in terms of clinical outcomes for both moderate and deep pockets. |
| Adequate Sequence Generated | Allocation Concealment | Blinding | Incomplete Outcome Data | Registration Outcome Data | |
|---|---|---|---|---|---|
| Arpağ et al., 2017 [19] |  |  |  |  |  |
| Puglisi et al., 2022 [20] |  |  |  |  |  |
| Petelin et al., 2015 [21] |  |  |  |  |  |
| Malali et al., 2012 [22] |  |  |  |  |  |
| Kargas et al., 2015 [23] |  |  |  |  |  |
| Tomasi et al., 2022 [24] |  |  |  |  |  |
| Johnston et al., 2020 [25] |  |  |  |  |  |
| Dilsiz et al., 2015 [26] |  |  |  |  |  |
| Meulman et al., 2013 [27] |  |  |  |  |  |
| Adequate Sequence Generated | Allocation Concealment | Blinding | Incomplete Outcome Data | Registration Outcome Data | |
|---|---|---|---|---|---|
| Dukic et al., 2013 [28] |  |  |  |  |  |
| De Melo Soares et al., 2019 [29] |  |  |  |  |  |
| Gurpegui et al., 2022 [30] |  |  |  |  |  |
| Pai et al., 2021 [31] |  |  |  |  |  |
| Dilsiz et al., 2013 [32] |  |  |  |  |  |
| Dereci et al., 2016 [33] |  |  |  |  |  |
| Hatipoglu et al., 2017 [34] |  |  |  |  |  |
| Matarese et al., 2017 [35] |  |  |  |  |  |
| Ustun et al., 2018 [36] |  |  |  |  |  |
| Alwaeli et al., 2013 [37] |  |  |  |  |  |
| Pourabbas et al., 2014 [38] |  |  |  |  |  |
| Birang et al., 2015 [39] |  |  |  |  |  |
| Queiroz et al., 2015 [40] |  |  |  |  |  |
| Balata et al., 2013 [41] |  |  |  |  |  |
| Malgikar et al., 2015 [42] |  |  |  |  |  |
| Gundogar et al., 2016 [43] |  |  |  |  |  |
| Manjunath et al., 2020 [44] |  |  |  |  |  |
| Euzebio et al., 2013 [45] |  |  |  |  |  |
| Bundidpun et al., 2020 [46] |  |  |  |  |  |
| Mishra et al., 2018 [47] |  |  |  |  |  |
| Adequate Sequence Generated | Allocation Concealment | Blinding | Incomplete Outcome Data | Registration Outcome Data | |
|---|---|---|---|---|---|
| Seydanur Dengizek et al., 2019 [48] |  |  |  |  |  |
| Tasdemir et al., 2019 [49] |  |  |  |  |  |
| Rapone et al., 2022 [50] |  |  |  |  |  |
| Piva et al., 2020 [51] |  |  |  |  |  |
| Uraz et al., 2019 [52] |  |  |  |  |  |
| Al Habashneh et al., 2015 [53] |  |  |  |  |  |
| Issac et al., 2015 [54] |  |  |  |  |  |
| Pandya et al., 2016 [55] |  |  |  |  |  |
| Kaur et al., 2019 [56] |  |  |  |  |  |
| Hayakumo et al., 2013 [57] |  |  |  |  |  |
| Adequate Sequence Generated | Allocation Concealment | Blinding | Incomplete Outcome Data | Registration Outcome Data | |
|---|---|---|---|---|---|
| Divnic-Resnik et al., 2022 [58] |  |  |  |  |  |
| Park et al., 2018 [59] |  |  |  |  |  |
| Jentsch et al., 2020 [60] |  |  |  |  |  |
| Caygur et al., 2017 [61] |  |  |  |  |  |
| Tsang et al., 2018 [62] |  |  |  |  |  |
| Stein et al., 2021 [63] |  |  |  |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabatini, S.; Maiorani, C.; Bassignani, J.; Cotellessa, S.; Di Trani, G.; Fulgenzi, E.; Iacono, R.; Mercogliano, I.; Butera, A. Effectiveness of Ultrasonic and Manual Instrumentation in Nonsurgical Periodontal Therapy: Are Additional Therapies More Effective? A Systematic Review. Appl. Sci. 2024, 14, 1950. https://doi.org/10.3390/app14051950
Sabatini S, Maiorani C, Bassignani J, Cotellessa S, Di Trani G, Fulgenzi E, Iacono R, Mercogliano I, Butera A. Effectiveness of Ultrasonic and Manual Instrumentation in Nonsurgical Periodontal Therapy: Are Additional Therapies More Effective? A Systematic Review. Applied Sciences. 2024; 14(5):1950. https://doi.org/10.3390/app14051950
Chicago/Turabian StyleSabatini, Silvia, Carolina Maiorani, Jessica Bassignani, Silvia Cotellessa, Giuseppe Di Trani, Elisa Fulgenzi, Roberta Iacono, Ilaria Mercogliano, and Andrea Butera. 2024. "Effectiveness of Ultrasonic and Manual Instrumentation in Nonsurgical Periodontal Therapy: Are Additional Therapies More Effective? A Systematic Review" Applied Sciences 14, no. 5: 1950. https://doi.org/10.3390/app14051950
APA StyleSabatini, S., Maiorani, C., Bassignani, J., Cotellessa, S., Di Trani, G., Fulgenzi, E., Iacono, R., Mercogliano, I., & Butera, A. (2024). Effectiveness of Ultrasonic and Manual Instrumentation in Nonsurgical Periodontal Therapy: Are Additional Therapies More Effective? A Systematic Review. Applied Sciences, 14(5), 1950. https://doi.org/10.3390/app14051950









