Abstract
Nowadays, drug delivery has an important role in medical therapy. The use of biopolymers in developing drug delivery systems (DDSs) is increasingly attracting attention due to their remarkable and numerous advantages, in contrast to conventional polymers. Biopolymers have many advantages (biodegradability, biocompatibility, renewability, affordability, and availability), which are extremely important for developing materials with applications in the biomedical field. Additionally, biopolymers are appropriate when they improve functioning and have a number of positive effects on human life. Therefore, this review presents the most used biopolymers for biomedical applications, especially in drug delivery. In addition, by combining different biopolymers DDSs with tailored functional properties (e.g., physical properties, biodegradability) can be developed. This review summarizes and provides data on the progress of research on biopolymers (chitosan, alginate, starch, cellulose, albumin, silk fibroin, collagen, and gelatin) used in DDSs, their preparation, and mechanism of action.
1. Introduction
Nowadays, technology is continuously developing, and the highest priority of researchers is the standard of medical care. Medication distribution and therapeutics are an important concern for scientists because the effectiveness of many drug delivery systems (DDSs) is a constant issue. The major problems experienced in drug delivery include different and many side effects, toxicity, non-specificity, low bioavailability, brief drug delivery, and rapid degradation [1,2,3]. To date, promising studies have been performed for the development of DDSs based on biopolymers due to their multiple characteristics and advantages.
Natural or plant-based resources, such as various bio-wastes from horticulture and crops, are used to make biopolymers, which are then produced as byproducts [4]. These materials are readily biodegradable because they contain atoms of carbon, oxygen, and nitrogen that compose their structural backbone. They are broken down into carbon dioxide, water, biomass, organic macromolecular material (humic matter), and other natural substances during the biodegradation process. Therefore, materials that are naturally recycled through biological processes are known as biopolymers [5]. Among the advantages of biopolymers are renewability, biocompatibility, affordability, and the release of less carbon (Figure 1). Also, a very important advantage of biopolymers is that they are environmentally friendly [4]. Biopolymers are proven to be non-toxic, non-carcinogenic, non-thrombogenic, and easy to extract [6,7,8].
Figure 1.
The main characteristics of biopolymers.
Numerous factors, like the kind of material employed as a structural matrix (conformation and distribution), the preparation circumstances (pH, concentration, temperature, solvent, etc.), and the type and concentration of additives (antimicrobials, antioxidants, plasticizers, crosslinking agents, etc.), all have a precise impact on these qualities [9].
The presence of hydroxyl, amino, or carboxyl functional groups in the structure of natural biopolymers provides good chemical reactivity and adaptability, which are comparable with synthetic biopolymers. For example, it was demonstrated that these reactive groups allow for changes that lead to an increase in the stability of biopolymers in different biological media. Also, cross-linking procedures with aldehydes and polyethylene glycol were studied for stability enhancement in order to avoid the rapid degradation of various biopolymeric coatings [10,11,12].
Even though biopolymers have many benefits, there are still a number of processing-related restrictions, beginning with the extraction and concluding with the isolation of the finished biomaterial. Because it is a completely natural material, the final properties of the biopolymer significantly depend on the source of the primary starting material [13]. To obtain and reach comparable results with DDSs in which conventional materials are used, research on DDSs based on biopolymers is still developing [14]. In controlled drug delivery, biopolymers are used as hydrogels, microcapsules, micro/nanospheres, and liposomes (Figure 2).
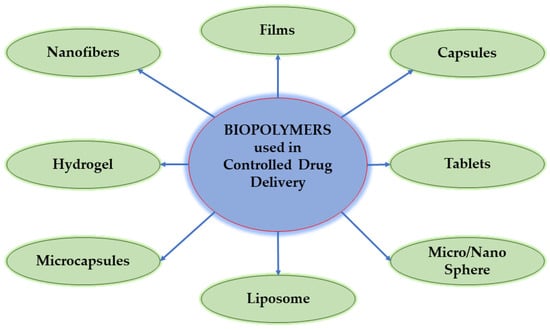
Figure 2.
The application form of biopolymers in the pharmaceutical field.
Researchers are interested in nanotechnology as a potential remedy for the issues with traditional DDSs. The interest is therefore shifted towards nanotechnology drug delivery systems (NDDSs). The main advantages of these systems are their flexibility in composition; the fact that, due to their design at the nano-level, they present free movement; biocompatibility; and non-toxicity, which is also very important [15,16].
This review aims to summarize the most commonly used biopolymers in the biomedical field, especially in DDSs. It also includes the progress so far in this field and the potential applications of biopolymer nanocomposites. Emphasis was placed on the mechanisms of action of DDSs developed so far—a less detailed aspect addressed in previous reviews.
2. Types of Biopolymers Used in Drug Delivery Systems
Based on their origin, biodegradable polymers are classified into four major categories: (i) from biomass (agro-biopolymers), (ii) from microorganisms, (iii) from biotechnology, and (iv) from petrochemical products.
Biopolymers obtained from biomass products, which are by far the most studied, consist of various compounds, such as polysaccharides [17], proteins of animal and vegetal origin [18,19], and lipids [20,21]. It is possible to create a large variety of biopolymers from natural sources such as agro-wastes, plants, animals, and microorganisms, including algae [22]. Biopolymers have been prepared from different agro-sources (e.g., bananas, rice, maize, corn, etc.), vegetable waste (e.g., from tomatoes, apples, pineapples, etc.), and animal sources (e.g., pigs, cattle, etc.) [23,24]. The materials obtained from these products present elasticity, softness, and gel-like properties, combining the characteristics of a solid and fluid [25,26].
The features of biopolymers based on polysaccharides and proteins—such as their biodegradability, biocompatibility, low immunogenicity, and antibacterial activity—make them more interesting than synthetic ones [27].
The selection of the biomaterial to be used for drug delivery is a very important step because of the potential toxicity of the products resulting from their degradation. For example, polysaccharides are known to have high biocompatibility and are non-toxic, although they are biodegraded by enzymes (e.g., guar gum is degraded in the human colon by enzymes of colonized bacteria), leading to different degradation byproducts whose biocompatibility needs to be understood assiduously before using them in DDSs [28].
2.1. Polysaccharides
Polysaccharides are derivates of monosaccharides with repeating units and have a high molecular weight. The most used surface modified polysaccharides (Figure 3) in DDSs are chitosan, alginate, starch, and cellulose [29].
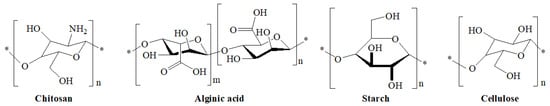
Figure 3.
The chemical structures of the most used polysaccharides for developing DDSs.
2.1.1. Chitosan
One of the most prevalent and non-toxic biopolymers that has been thoroughly studied for a variety of medicinal uses is chitosan [30,31,32]. This cationic polysaccharide, which is composed of N-acetyl and D-glucosamine, is produced from the naturally occurring polymer chitin. Naturally, chitosan is found in the exoskeletons of crustaceans, fungi [33], annelids, mollusks, and insects. Also, chitosan is biocompatible, adhesive, hemostatic, and mucoadhesive [31,32,34].
Chitosan is non-toxic, odorless, biocompatible, biodegradable, and has antibacterial properties. Frequently, this biopolymer is used for microencapsulation for cells that need a cationic medium. Chitosan has been used in DDSs in different forms, such as gels, films, beads, oral tablets, and microspheres for oral, nasal, ocular, and transdermal routes [35,36]. Also, the anti-tumor and fungal properties of chitosan play an important role in the development of bio-dental materials and the treatment of periodontitis, as well as dental pulp regeneration [37]. Chitosan can be administered parenterally, intravenously, nasally, vaginally, or via injection. One major benefit of utilizing chitosan in DDSs is that drug absorption and stability can be achieved while controlling drug diffusion from the material to the target dosages [38].
By mixing chitosan with various natural and synthetic polymers, such as sodium alginate, polylactic acid, polycaprolactone, poly(ethylene oxide), poly(vinyl pyrrolidine), and graphene oxide, one can alter the material’s characteristics [39,40]. Chitosan’s -NH2 and -OH groups allow it to interact with biological molecules and other polymers. As a result, chitosan alone or combined with other materials represent an applicable substrate for obtaining different new nanocarriers (e.g., films, hydrogels, foams, beads, gels, nanoparticles, nanofibers, and sponges [41,42]). These combinations improve the drug properties regarding the release process and improve their mechanical and physical properties [43]. Chen and coworkers developed a hydrogel prepared from chitosan using sodium dialdehyde alginate and dopamine via grafting, crosslinking, and compounding for drug delivery and bladder cancer treatment. The obtained drug-delivery hydrogel demonstrated strong organ-wall adhesion and targeting capacity. Also, the developed hydrogel showed antibacterial and antimicrobial properties (98%) and biocompatibility (99%) [44]. To treat breast cancer, a hybrid nanoparticle of calcium phosphate and folate-functionalized carboxymethyl chitosan loaded with curcumin was created. The resulting materials showed good biocompatibility, stability, and pH-responsive drug release. The outcomes demonstrated that the materials based on carboxymethyl chitosan had an organelle-targeting cancer therapeutic method [45].
A recent study performed by Aranda-Barradas et al. demonstrated that the molecular weight (20.6 and 57.5 kDa) of chitosan has an important role in the physicochemical, morphological, and biological properties of polyplex nanoparticles designed for gene delivery. Their research demonstrated that the low molecular weight of chitosan and the low nitrogen/phosphorus ratio were suitable for designing chitosan-based nonviral vectors for gene therapy because of their physicochemical and biological properties. Also, the stability of the obtained nanoparticles was greater than those formulated with chitosan of a higher molecular weight [46]. An important role of the nanoparticle assembly for gene therapy is the degree of deacetylation to form poly-D-glucosamine [47]. Thus, the amine groups of D-glucosamine make its conjugation possible with some crosslinkers to form covalent bonds with molecules, which permits directed gene therapy to specific cell types. In conclusion, these very important characteristics qualify chitosan as a suitable polymer for designing new nonviral vectors [48].
Due to their excellent functional properties in DDSs and decisive, non-invasive, and focused tissue locations, injectable hydrogels have attracted attention lately. Thus, designing DDSs to be responsive to hydrogel stimuli and release a drug to an external stimulus while having different advantages is an ambitious task. In this regard, chitosan-based hydrogels offer high potential for tissue engineering and drug delivery due to their biocompatibility, mucosal adhesion, and hemostatic activity [49].
2.1.2. Alginate
Because of its remarkable encapsulating qualities and function in the healing of bruises, alginate is also a highly utilized biopolymer in the pharmaceutical and medical sectors. Since its first isolation in the 1980s, alginate has found numerous uses because of its many benefits, including being a mucoadhesive, biodegradable, biocompatible, renewable, and easily accessible substance. It is also non-toxic and immunogenic. This biopolymer is used to treat reflux esophagitis and is regarded as an excipient in the pharmaceutical sector [50,51].
Alginate is extracted from brown algae (Macrocystis pyrifera, Laminaria hyperborea, Saccharina japonica, and Ascophyllum nodosum) and is found as sodium, calcium, and magnesium salts of alginic acid. Its synthesis can be performed by different species of bacteria (Azotobacter vinelandii and various Pseudomonas species) [52]. Alginate can be extracted by grinding the raw material from algae, cleaning it with acid, and then using heated alkali to extract it. The obtained alginic acid is filtered, precipitated with calcium, and then acidified. By processing insoluble alginic acid with oxides, metallic carbonates, and hydroxides, the required alginate salt can be produced [53].
Solutions of alginate quickly form gels in the presence of various divalent cations, calcium being the most extensively used. These gels are stable in the temperature range of 0 to 100 °C. These gels may dissociate in the presence of acids. The experimental conditions of forming gels (e.g., alginate concentration, temperature, cation type, etc.) need to be correctly controlled in order to produce gels with a homogeneous composition. In contact with intestinal fluids, the alginate particles break down due to the presence of Na+ ions and acids. To avoid this disintegration, most of the time, alginate is used in combination with one or more biopolymers for developing DDSs [54].
Hoang et al. developed dual cross-linked chitosan/alginate hydrogels for pH-responsive drug delivery. The hydrogels that were developed demonstrated a hydrophobic drug loading capacity of 44% (wt./wt.) and did not cause any cytotoxicity on human cells when evaluated, showing their biocompatibility [55]. Li and colleagues created a pH- and temperature-responsive pectin/chitosan biopolymer hydrogel for use in medication delivery systems. The hydrogels that were created had a strong ability to mend themselves and were shown to be biocompatible, causing no adverse reactions in mouse fibroblast cells [56].
2.1.3. Starch
The use of natural and modified starches as biodegradable, renewable, biocompatible, and non-toxic polysaccharides has been widely used in several medicinal applications, including tissue engineering and drug delivery [57,58,59,60,61,62]. Natural sources including wheat, rice, corn, and potatoes can be utilized to separate starch, which is then employed primarily as a carrier and in some applications like bone replacement and healing [29]. Besides these advantages, native starch also presents some disadvantages such as low water solubility, the formation of gel or pastes with non-uniform texture and viscosity, deterioration in different conditions of high temperature and pHs, and freeze–thaw variations [63,64]. Therefore, in order to improve native starch’s physicochemical or biological properties and make it suitable for producing DDSs, several modifications (chemical, physical, and enzymatic) are required [65].
Using broken rice, Xiao and colleagues created a novel nanoparticulate system for acetylated starch nanocrystals. Acetylated starch nanocrystals with different degrees of substitution were prepared using acetic anhydride as an acetylating agent through a reaction with starch nanocrystals. These findings suggest that the acetylated starch nanocrystals made from broken rice are a potentially useful tool for the regulated administration of doxorubicin in cancer treatment [66]. Other examples of starch derivatives that demonstrated benefits for drug delivery applications are presented in Table 1.

Table 1.
Starch types used for intravenous drug delivery.
Starch-modified alginate nanoparticles for drug delivery were developed by Thomas and coworkers using an environmentally friendly method. For the determination of their potential in controlled drug delivery applications, theophylline and bovine serum albumin were used as model drugs. The obtained nanoparticles had good encapsulation efficiency, and the in vitro drug release studies showed pH dependency characteristics. Also, it was shown that the created nanoparticles were biocompatible with L929 fibroblast cell lines. Thus, the developed materials were demonstrated to be a promising tool for drug delivery application [70].
2.1.4. Cellulose
As the most prevalent biopolymer, cellulose exhibits biodegradability, renewability, and high strength. It is composed of glucose units, and it has abundant hydroxyl groups in its backbone. Some of the disadvantages of cellulose are its very low solubility in water and common organic solvents, thus having limited applicability [71,72,73]. Because it is environmentally friendly, simple, and presents low cost and toxicity, an aqueous sodium hydroxide (NaOH)/urea solution is frequently used to dissolve cellulose [74,75]. Cellulose from different and diverse sources represents an adaptable and adequate material for DDSs [76,77]. AL-Rajabi and Teow developed a sustainable synthesis method for a thermoresponsive Pluronic F127 composite hydrogel reinforced with cellulose taken from empty fruit clusters of oil palm for silver sulfadiazine drug delivery. Their study demonstrated that the developed material from rich agricultural biomass is sustainable, environmentally friendly, and cost-effective for being used as DDSs [78].
2.1.5. Hyaluronan
Hyaluronan is a linear polysaccharide with disaccharide repeats of d-glucuronic acid and N-acetyl-d-glucosamine. Even though it is a simple linear chain, it has several important biomechanical properties [79]. Hyaluronan was demonstrated to present biocompatibility, biodegradability, high viscoelasticity, and can also be mixed with specific receptors on the cell surface [80].
Hyaluronan has attracted attention as a drug delivery vehicle because it can recognize specific receptors that are overexpressed on the surface of tumor cells, and cancer drugs can be targeted to the tumor cells to better destroy them. Hyaluronan has been used extensively in controlled-release and targeted DDSs. Until now, most studies are only in an in vitro experimental phase; studies utilizing in vivo tests are very rare. However, it is believed that the prospect of hyaluronan as a drug carrier will be larger with the discovery of new materials and the development of new technologies. The literature presents much research on hyaluronan as a carrier for various drugs, but most of them are just in a laboratory study phase. Because of the complex processes involved, hyaluronan is difficult to industrialize [81].
2.1.6. Dextran/Cyclodextrins
Among the mentioned polysaccharides, dextran has earned great interest for nanoscale drug carriers due to its availability, hydrophilicity, biocompatibility, non-toxicity, non-immunogenicity, and biodegradability [82]. Dextran is biosynthesized intra- or extra-cellularly by several microorganisms. Commercial dextran is usually obtained from L. mesenteroides or L. dextranicum fermentation in a media with sucrose and is an important nitrogen source. For obtaining highly biocompatible DDSs, it is recommended that dextran obtained by fermentation to be minimally modified. Contrarily, if the l structure of dextran is affected, a decrease in the biocompatibility or an increase in cytotoxicity is observed. Thus, many DDSs containing acetylated, diethyl aminoethyl-dextran, carboxymethyl, or the sodium salt of dextran sulphate have been eliminated from in vivo or clinical studies. Many DDSs with dextran were developed in the form of microspheres, micro- and nanoparticles, micelles, liposomes, hydrogel, and medical adhesives for medical and pharmaceutical applications [83]. Lately, the formation of micelles using grafted dextran for DDSs in anticancer therapy has received great attention [84]. For obtaining amphiphilic polymers with the capacity to form micelles and trap chemotherapeutic agents, dextran is bound with lipids such as oleic acid, stearic acid, and cholesterol. These kinds of polymers have great stability and rapidly reach the target cell and avoid kidney extraction [85].
Cyclodextrins are biocompatible and biodegradable materials produced by the enzymatic degradation of starch. In recent decades, cyclodextrins and derivates represent an important class of pharmaceutical excipients that contribute to increasing the therapeutic efficiency of many drugs. They are the smallest nanocarrier used in DDSs [86]. Pedotti et al. developed a release system using β-cyclodextrin for the antiviral drug Acyclovir. They evaluated its hydrolysis in simulated physiological media to analyze the potentiality of this prodrug for its use through different ways of administration. The release of Acyclovir was tested in both acidic and neutral conditions and in the presence of porcine liver esterase. In all cases, the 100% release of free Acyclovir and Aciyclovir succinate at differing rates as a function of hydrolysis conditions was observed within 7 days [87].
Di Cagno et al. evaluated the potential of novel β-cyclodextrin -dextran polymers for drug delivery. The results concluded that all the studied polymers had suitable sizes for parenteral administration. The presence of the dextran backbone structure did not influence the stability of the polymer/drug complex, in comparison to the native polymer and other commercially available derivatives. The drug release studies showed that the diffusion of the hydrocortisone drug was influenced by the solubilization induced by the developed polymer derivatives [88].
2.2. Proteins
Proteins are compounds with high molecular weight and are frequently used in DDSs. Silk fibroin, collagen, gelatin, and albumin are the most used animal-originated proteins for DDSs [29].
2.2.1. Albumin
Albumin plays a crucial physiological role in the human body and is the protein found in plasma with the largest amount (it comprises 50% of all plasma proteins) [89,90]. More specifically, albumin is an internal source of amino acids. It also contributes to preserving the constant osmotic pressure of plasma. Albumin has up to 40% water solubility at a pH of 7. Thus, albumin can be combined in vivo with various insoluble chemical compounds and inorganic ions to generate soluble complexes using albumin as a non-specific transport protein. Among the advantageous characteristics of albumin are its biocompatibility, non-immunogenicity, and biodegradability [91].
Lately, research on protein-based delivery systems with albumin for use in ocular applications has shown an increase. However, proteins require certain designing strategies to deliver at specific places because they are always susceptible to enzymatic destruction [92,93]. Interestingly, albumin is a highly charged protein that is suitable for the electrostatic adsorption of charged bioactive compounds. Thus, drug delivery with albumin is intensely researched and explored. Among various albumin, and compared with ovalbumin and human serum albumin, bovine serum albumin was demonstrated to have therapeutic and medicinal applications [94].
The use of mixtures of albumin and hyaluronic acid was demonstrated to be suitable nanocarriers with significant advantages such as efficient targeting, pH- and/or hyaluronidase-sensitive drug release, reducible particle size, mixing capacity for different drugs, and great stability. Additionally, skin tissue, joints, cancers, and the vitreum have all been treated with drugs delivered via albumin and hyaluronic acid-based nanoparticles. Additionally, this combination has prospects for both theranostics and combined therapy [95].
2.2.2. Silk Fibroin
Silk fibroin is composed of a collection of proteins which originate from silkworms and spiders. Silk consists of a crystalline structure (fibroin) and an amorphous protein (sericin) [96]. Silk is a naturally occurring protein that is thought to be a beneficial biomaterial for creating DDSs [97]. Silk has multiple advantages (e.g., biocompatibility, biodegradability, non-toxic degradation products, a versatility of options for sterilization, soft aqueous processing that keeps the medication’s bioactive qualities, mechanical stability, and self-assembly properties), which are applicable for developing sustainable DDSs [98]. Also, multiple preparation methods are used to obtain silk delivery system applications, (microspheres, lyophilized sponges, silk-coated polymeric particles, nanoparticles, hydrogels, films, and nanofibers) [99].
To reduce the medication’s dosage and adverse effects on healthy tissues, studies are developing DDSs in which silk is combined with other polymers or nanoparticles. For example, the drugs cis-dichloro diamino platinum and Paclitaxel, inserted in silk nanoparticles, reduced tumor growth and efficiency. More than that, due to the silk’s pH sensitivity, DDSs based on silk are a suitable alternative because drug release kinetics appear regularly at low pH values [100]. A silk fibroin-polymethacrylate copolymer coating was recently developed for oral dosage forms. In vitro and in vivo research showed that the capsules coated with the newly developed silk fibroin formulation facilitate pancreatin-dependent drug release. This novel formulation and its extensions demonstrated the ability to produce more effective and tailored DDSs for sensitive patients that have affected and variable intestinal physiology [101]. Other research presents a silk fibroin hydrogel used as a carrier for vincristine in Wilms tumors and the ultrasound as a method to accelerate the release of the drug. The study demonstrated that the ultrasound started increasing cell death rates, but the Wilms tumor cells were resistant to higher concentrations of released drugs [102].
2.2.3. Collagen and Gelatin
Collagen is a natural protein, richly present in animals. This biopolymer has been used in different DDS applications [103], mostly in oral drug delivery due to its biodegradability and biocompatibility. Also, collagen is non-antigenic, non-toxic, and presents synergism with bioactive compounds. Collagen has functional groups that can be quickly modified to obtain suitable properties for oral drug delivery. Moreover, it was demonstrated that collagen is the major component of some tumor microenvironments. This property is favorable in cancer therapy because the collagen compound is capable of infiltrating the tumor area to deliver anti-cancer agents [104].
Gelatin is a natural biopolymer derived from collagen by acid/alkaline hydrolysis. Gelatin has suitable properties for the delivery of chemotherapeutics, and it is simple to cross-link with other compounds [105,106]. The bioavailability of gelatin increases when it is modified to enhance drug encapsulation efficiency. Also, gelatin can be tailor modified to be appropriate for loading a desired drug. Thus, gelatin is excellent for the oral drug delivery of hydrophobic chemotherapeutics [107].
The co-solvent method was used to create nanometric vesicles using a poly(styrene-b-ethylene oxide) block copolymer that included adapalene. The vesicles were combined with free adapalene and silver sulfadiazine and incorporated into collagen and gelatin matrices. The created material was demonstrated to function as a skin dressing that combined a longer and slower release of adapalene to promote skin healing with a gradual release of significant amounts of medicines within the initial hours of use (to stop the growth of the infection) [108]. In another study, ionic medication (ionized cromoglicate sodium and ipratropium bromide) release via inhalable dry powders including gelatin was investigated. It was demonstrated that the developed drug-loaded gelatin microspheres have excellent aerodynamic performances that are highly dispersible and biocompatible. Additionally, the materials’ swelling profiles showed that, by preventing macrophage absorption, particle size can lengthen lung residence time. Therefore, because of its adjustable charge and swelling properties, gelatin may be a suitable and authorized excipient for pulmonary DDSs [109].
2.3. Nucleic Acids
Nucleic acids are supramolecular biopolymers that exist universally in living organisms. The best known are DNA and RNA that play fundamental roles in biological processes. DNA stores, encodes, and transfers genetic information to other components of cells, while RNA serves as an intermediate messenger for gene expression [110,111]. In addition to this, they participate in the catalysis of certain biochemical reactions and in the regulation of certain activities in the cells [112].
Nucleic acids have utility in various biomedical applications. Unmodified nucleic acids cannot be used in drug delivery. However, multivalent nucleic acid nanostructures and nucleic acid aptamers have led to a rapid increase in the number of drug delivery systems with a potential for controlled release [113].
3. Other Polymers Used in DDSs
3.1. Polyamide/Poly(amino acid) Polymers
Among the above-mentioned polymers, polyamides or poly(amino acid)s present distinct properties such as great biocompatibility, gradual degradability, and adjustable physicochemical properties. Polyamide has repeating units linked by amide bonds. A group of small polyamides containing multiple amino acids of the same types linked through amide bonds are generally called poly(amino acid)s. Polyamide appears naturally or can be obtained synthetically [114]. Among naturally occurring polyamides are mentioned proteins (e.g., silk and wool), while synthetically obtained ones include different materials such as nylons, sodium poly(aspartate), and aramids [115]. Also, polyamides can be classified into homopolyamides (with one kind of monomer) and copolyamides (with different constituents) [116].
The use of these polymers in DDSs presents several important advantages, such as localized target site action, constant release, and stabilization. There are also some disadvantages, such as the possibility of microbial contamination, extreme hydration, and reduced viscosity in storage [117].
Synthetic poly(amino acid)s are also used in designing DDSs because they have a similar structure to the naturally occurring ones [118]. Poly(amino acid)s are mostly used in the design of chemotherapeutics for obtaining selective delivery for an acceptable duration of time. These polymers used in DDSs increase anti-tumor efficacy and lessen drug-related side effects [119].
Polyaspartamide biopolymer has recently gained attention as being non-toxic, extremely biocompatible, and biodegradable. Supplementarily, for different biological applications, its physicochemical properties present flexibility in functionalizing with different components, such as DNA and pharmaceuticals (doxorubicin or biotin) [120,121]. For cancer diagnosis and therapy, Nguyen et al. recently synthesized superparamagnetic iron oxide nanoparticles by a thermal decomposition method and encapsulated them in a polyaspartamide biopolymer to form a hydrophilic and biocompatible construct. In addition, polyaspartamide was conjugated with biotin and doxorubicin functional groups to increase the targeting of cancer cells. In vivo tests demonstrated that the developed bio-construct decreased the magnitude of cancer tumor volume growth by three times, compared to the control cells [122].
Di Meo et al. developed a new polymeric product based on α,β-poly(N-2-hydroxyethyl)(2-aminoethylcarbamate)-d,l-aspartamide copolymer covalently linked with doxorubicin for its application in anticancer treatment. In vitro tests demonstrated that the newly developed polymers had a retarded cytotoxic effect on tumor cells. Also, there was a noticeable improvement in the in vivo antitumor activity of the newly developed polymer and a survival advantage of the treated NOD-SCID mice [123].
3.2. Dendritic Polymers
Dendritic polymer architectures show promising therapeutic properties, with potential applications in drug-delivery systems. Also, the dendritic polymer architecture is more advantageous for delivery applications than linear polymers, having mono-dispersity, high symmetricity, and surface polyvalency [124].
Depending on the mechanism, a dendrimer can capture a drug through empty spaces (molecular capture), through branching points (hydrogen bonds), or external surface groups (charge–charge interactions). The most used dendrimers for drug delivery systems are PAMAM dendrimers (Poly(amidoamine) dendrimers), commercially available with amine, hydroxyl, carboxylate, and pyrrolidinone surfaces, along with corresponding generations [125].
Also, core–shell architectures of easily accessible hyperbranched polymers have been reported. Thus, based on commercially available dendritic structures (polyglycerol and poly-(ethylene imine)), pH-sensitive shells were attached through acetal or imine bonds, obtaining pH-sensitive nanocarriers [126]. Such dendritic structures are dendritic polyglycerol-co-polycaprolactone (PG-co-PCL)-derived block copolymers used for gemcitabine delivery for pancreatic ductal adenocarcinoma therapy [127].
Functional dendritic polymer architectures as stimuli-responsive nanocarriers for the delivery of bioactive agents were obtained using several chemical linkers that respond to external stimuli [128].
Narayanan et al. developed a fourth-generation poly-lysine dendritic nanocarrier to target epidermal growth factor receptor-overexpressed breast cancer for methotrexate delivery. The methotrexate was incorporated into the nanocarrier using a cathepsin B cleavable spacer (glycine-phenylalanine-leucine-glycine). The in vitro analysis showed that developed DDSs were highly effective. The efficacy analysis using NOD-SCID mice also demonstrated that the DDS reduced tumor volume. The mice treated with the DDSs developed gained weight faster than those treated with the free drug, which allowed for a conclusion that dendrimer is more tolerated by mice than the free drug [129].
4. Preparation Methods for Obtaining Biomaterials Used in DDSs
Multiple methods are used for the preparation of the biomaterials that are further used in DDSs. Among them, important to be mentioned, are supercritical fluid extraction [130], electrospraying [131], desolvation [132], spray freeze-drying [133,134], layer-by-layer self-assembly [135], and microemulsion [136].
These multiple types of preparation methods allow for the obtaining of important carriers for different drugs (e.g., different molecule sizes, protein, and gene drugs). Properties such as swelling and crosslinking can be tailored by changing the ratio between biomaterials and the modifying materials [29].
Generally, every preparation method has advantages and disadvantages. Thus, desolvation using solvents (e.g., ethanol, acetone) is the easiest method that can be used for the preparation of protein-based nanoparticles. The amount of additional desolvating chemicals supplied and the flow rate can regulate the size of the nanoparticles [137,138].
Electrospray is an electrohydrodynamic single step technique that is adaptable and has very good reproducibility. Through this technique, small-sized particles are formed from a macroscopic mass, a process controlled by electrostatic forces that break up a dielectric liquid [139]. This technique is an emerging technology in fabricating drug carriers that can produce highly homogeneous materials at room temperature [140]. Electrospraying demonstrated benefits for preparing particles. Most of the time, a regulated and tight particle size distribution is seen together with a high drug-loading efficiency [141]. Another advantage of the electrospray technique is that biopharmaceuticals are not degraded during the encapsulation process [142].
Table 2 outlines various DDSs, containing different biopolymers loaded with various active drugs.

Table 2.
Procedures for preparation and a list of medications included in the created biopolymer-based nanoparticles.
For compounds that are very sensitive to temperature and pressure, the spray freeze-drying technique is used [160]. This technique involves a two-step process, involving the spray freezing step and freeze-drying step. This technique is highly used in the food and pharmaceutical industries [161,162]. In the spray freezing step, the pharmaceutical dissolved or suspended in the liquid is further atomized to obtain fine droplets that are directly frozen, generally in liquid nitrogen. The resulting frozen particles are then put through a process called freeze-drying, which produces dried and porous particles by sublimating the solvents at low pressure and temperature [160]. This technique enhances drying efficiency and increases the production yield and capacity. The solutions used for spray freeze-drying are non-toxic aqueous-based suspensions with content of dispersed nano- or micro-sized hydrophobic active pharmaceutical ingredients. Liquid nitrogen has been the most popular refrigerant for spray freeze-drying up until now [163,164].
Multilayer sequential film formation is performed with a novel layer-by-layer self-assembly method. This is achieved through electrostatic, hydrophobic interactions, and hydrogen bonding between the layers. Through these kinds of interactions, a deposition of alternate layers of oppositely charged biomaterials can be performed, which offers precise control of nanoscopic features (e.g., thickness, composition, and surface characteristics) of the film by using additional buffers, such as acid, base, and salt buffers [165,166]. This technology is frequently used for fabricating hollow microcapsules with certain sizes, compositions, and properties. Among the advantages of this technique are simple preparation conditions, various loading methods, and favorable surface functionalization [5]. Obtainable capsules with particular structures and small wall-to-diameter ratios have an exceptional capacity for encapsulation, good stability, fluidity, and deformability [6]. Additionally, their semipermeable nature is also a decisive trait that allows them to communicate with external enclosing structures [167].
Microemulsions are obtained via dispersion of the biopolymer in two immiscible liquids, with the use of emulsifiers or surfactants. The nanomaterials produced by this process typically have a diameter of 10–100 nm, are optically transparent, isotropic, thermodynamically stable, and have strong drug-solubilizing qualities [168,169]. With a polar head and a non-polar tail, surfactants are amphiphilic substances that adjust at the interface between the water and oil phases. Thus, surfactants reduce the overall tension and promote miscibility. Microemulsions are predominantly used in lipophilic drug delivery (79.4%) through oil-in-water microemulsions and non-ionic surfactants (90%) used for oral or topical administration [169]. Microemulsions are versatile DDSs due to their different advantages (small particle size, capacity to incorporate hydrophilic and hydrophobic drugs, and easy emulsification preparation process) [170].
5. Mechanisms Involved in Drug Delivery Systems
Innovative drug delivery systems are widely studied nowadays due to the increasing demands on their quality [171]. DDSs are preferred due to reasons such as the fact that drugs can be administered in their active form or, on the other end, can be stabilized by the functionalization of the carrier. Moreover, the presence of a DDS ensures administration through body cavities that in other ways could be impractical. Therefore, these qualities lead these systems to be characterized by low costs, higher efficiency, and better therapies [172]. Drugs can be administrated using different systems depending on the necessities of the patient. The most common are the ones that ensure an immediate release or a controlled and prolonged release. An immediate release is accomplished through pills, capsules, and injections, and even though they have very important clinical applications, they do not have a good pharmacokinetic profile, meaning they cannot be used for different therapies [173]. A prolonged release is obtained using implantable devices that can allow the drug to be released over weeks, months, or even years. The most common applications are in contraception and cancer therapies. However, the installed devices are mostly made of non-biodegradable polymers, meaning that, after the end of the therapy, they need to be surgically removed [174]. The controlled release systems made of biopolymers or other biomolecules are preferred to the others due to their advantages compared to conventional ones, such as improved efficacy, improved patient compliance, and reduced toxicity [175,176]. The first parameters to study in order to develop a highly efficient drug delivery system are the parameters that characterize the drug, such as its physicochemical properties, pharmacokinetics, and pharmacodynamics [177].
The study of the physicochemical properties of the drug is crucial for the optimization of the delivery system; some of the key properties are, for example, drug solubility, molecular weight, chemical stability, and surface tension. For instance, the diffusivity of a drug is strictly dependent on its molecular weight; thus, the carrier chosen for a small molecule and a polypeptide should differ to enhance the delivery capacity [178]. Once these parameters are studied, the right carrier must be found, and the drug must be loaded. One of the most crucial aspects of the system is drug loading, which is the process of integrating the medication into the carrier. The ideal loading strategies for the introduction have to be determined through a study of the compatibility between the elements that characterize the entire system (drug, carrier, and excipients), but release environment should be also taken into account for a better experiment yield.
Therapeutics can be introduced by both covalent and non-covalent interactions, such as ionic interactions, dipole interactions, hydrogen bonding, etc., but also via physical encapsulation and surface absorption [179]. Covalent bond-based systems, which are used, for example, in small drug–drug complexes, linear polymer prodrugs, and dendritic drugs, are mostly used when the drug’s solubility and biocompatibility need to be improved. These properties are achieved by linking hydrophobic or hydrophilic segments, forming an amphiphilic structure. Not all covalent bonds can be used for this strategy because, to ensure the release, these bonds must be susceptible to enzymes, pH, T, or other possible external stimulations [180].
Non-covalent systems are valid alternatives to covalent ones due to qualities such as their reversibility, biocompatibility, bioactivity, and their natural responsive behavior to stimuli, allowing the system to be adaptable to any changes in physiological conditions, offering targeted and stimuli-responsive release. Examples of these systems are micelles and dendrimers. Non-covalent interactions are the driving force for the formation of stable micelles that can ensure, due to the presence of both a hydrophilic and hydrophobic part, kinetic stability, increased drug loading efficiency, and release capacity [181]. A large number of micelles made with biopolymers have already been studied with protein, peptides, and polysaccharides [71].
Dendrimers are equally important and have similar characteristics to micelles, which makes them very useful for the controlled and targeted release of a drug. However, dendrimers are synthetic polymers, and their combination with biopolymers to create hybrid materials is still being explored [182,183].
It is important to wisely choose which type of loading is needed by keeping in mind the type of release—immediate, non-immediate, sustained, and in situ-specific—and the dosage wanted [184]. Once the drug is loaded on the most compatible and efficient system, the last step is the release of it. Many release mechanisms can occur in a solution, and usually, there are more happening at the same time, but the final classification and kinetic study of controlled drug delivery systems depends on the primary mechanism that occurs. The simplest is dissolution, a process in which the molecules of a solute are dissolved in a solvent. In the case of drug delivery systems, this means that the drug molecules pass from solute to solvent until the solution reaches the solubility limit at the established pressure and temperature conditions. The rate of dissolution is directly proportional to the solubility of the drug when no chemical reaction occurs, and the solubility coefficient of the therapeutic molecule decreases with its increasing melting point and increases with increasing chemical compatibility with the solution. Consequently, the dissolving process may be expressed numerically as [185]
where the surface area of solid A, the diffusivity coefficient of solute D, and the difference between solute concentration Cs and solid solubility C determine the dissolving rate dC/dt.
In the work of Salamanca et al., it is evidenced that the dissolution rate depends on the characteristics of the biopolymer, such as its molecular weight and chemical structure. The authors have done a comparative study on the delivery mechanism by dissolution, using DDSs with xanthan gum and tragacanth gum, which are both biopolymers but with different properties. They observed different drug release profiles that are dependent on the biopolymer used. In the case of tragacanth gum, due to its molecular weight of 840 kDa and polarity, the polymer is poorly soluble in aqueous media, resulting in a release profile of the drug that was similar to the behavior of classical tablets, which ensured an immediate release of the therapeutics and was not compatible with a prolonged and sustained release. However, the opposite results were obtained with xanthan gum, which was characterized by the presence of anions in its chains and has a molecular weight of 2000–16,000 kDa. Due to these characteristics, in the case of xanthan gum, the release of the drug was sustained, prolonged, and pH-dependent because, at neutral pH values, the repulsion of the carboxyl groups in the chain causes the polymer’s backbone to extend, which decreases interaction with the drug, favoring the release. However, at that pH, the interaction with the medium is more beneficial, leading to an apparent gelation process that further modulates the release; at acidic pH values, on the other hand, this process is unfavorable, decreasing swelling and increasing matrix erosion [186].
Another mechanism usually involved in the release of therapeutic molecules is diffusion. This process is defined as the mass transfer of molecules from one part of the system to another carried by random molecular motions such as a concentration gradient.
The equation that describes this process is [185]
where J—the rate of diffusion per unit area on the section studied, C—the concentration of the molecule diffused, x—the distance between the two points of the section considered, the minus means that diffusion occurs in the direction of concentration decreasing, and D—the coefficient of diffusion that, in very diluted solutions, can be considered constant, but in the case of polymers, is highly dependent on concentration, and it is a measure of the molecule’s mobility in the medium. Since large molecules diffuse more slowly than small ones, viscous liquids should slow this diffusion. Furthermore, Brownian motion is driven by the heat agitation intensity.
Although diffusion is not effective over longer distances and is strictly related to the geometry of the carrier, this mechanism is a simple, efficient, and secure strategy to reach a sustained and controlled release of a drug and can usually happen when paired with dissolution and swelling. Moreover, it can produce a zero-order model of kinetic release, making it very advantageous [185]. Diffusion can occur in different phases of the release, as shown in the work by Harting et al. where the authors analyzed the release from polyester blends. It was shown that there is a four-phase drug release mechanism where dissolution was observed in the first initial burst of release and again in the second burst release, although the release was mostly modulated by the degradation process of the carrier [187].
What can be considered a crucial mechanism for drug delivery in polymers is swelling. Swelling is a process where a material put in a liquid tends to increase in size and volume due to absorption phenomena. It is observed in various materials, including biopolymers, and can result in changes in the physical properties of the material (permeability, strength, flexibility, etc.), leading to the release of the drug. Hydrogels are especially affected by this mechanism and can absorb many times their weight in water. In glassy hydrogels, the slow structural relaxations of the swelling polymer affect the kinetics of water uptake, which influences the release of the drug. The forces of osmotic, electrostatic, and entropy-favored dissolving of the polymer in water are typically balanced to drive the process of water intake and swelling [188]. These systems strictly refer to cases where water not only activates the release of the drug but is also the rate-controlling mechanism of drug release. Especially for hydrophilic polymers, swelling is coupled with diffusion, leading to the stabilization of an otherwise dissolution-controlled drug release rate as already seen in the work by Salamanca et al. [186]. Swelling behavior has been largely studied for both biopolymers and hybrid-enhanced biopolymers, leading to many publications over the years.
Osmosis is another important mechanism that is also part of the swelling mechanism. It is defined as the process in which a solvent is transferred through a membrane that is permeable to smaller solvent molecules and not permeable by larger ones in an attempt to equalize the concentrations of the not-permeable solute on both sides of the semipermeable membrane. Osmotic pressure is the energy that powers the process and directs the release, and it is regulated by the equation [185]
where h is the thickness of the membrane, A is the cross-sectional area for transport, k is the effective permeability of the membrane, dV/dt is the volume flow of the solvent through the membrane, and ∆π is the osmotic pressure. Osmotic systems are typically more complicated to build and less volume efficient than other drug delivery systems since they typically consist of a drug core surrounded by a membrane that allows water to permeate and create osmotic pressure, which causes the release of the medication. However, osmotic methods also often offer higher percentages of medication loading at zero-order delivery rates and superior zero-order delivery [189]. It is possible to notice in the literature that drug delivery systems characterized by an osmotic driving force are usually made of cellulose and its derivatives, due to their structural and chemical characteristics that allow for the controlled permeability of the membrane [190].
Erosion and degradation were mentioned before as “assistants” to sustained drug delivery, but these processes can also be the leading driving force. When diffusion is the main driving force of the process, it can be said that the polymer has a relatively passive role, but when the carriers go through a process of degradation or erosion, these processes can delay the velocity of the distribution, leading to a more active role. Erosion/degradation can be caused by many reasons—the more common being chemical reactions or due to the application of external stimulation (pH, T, mechanical stress, etc.). These systems are very useful for implantable or injectable therapies where the removal of the carrier can be avoided, leading to less discomfort for the patient [185].
Because they may offer consistent and adjustable release kinetics, the impacts of degradation and erosion on drug delivery are the subject of research. Additionally, the use of combinatory materials in the design of drug release systems may increase drug bioavailability [176].
Another way to stimulate the release of a drug is the application of external stimulation over the system to induce a process that allows for the release. The application of internal stimuli has already been studied, especially for the therapy of tumors via utilization of smart nanocarriers [191]. The problem with internal stimuli such as pH, temperature, and enzymatic and redox activities is the difficulty in controlling them [192]. Suffice to say, problems in the use of external stimuli such as ultrasounds [193], magnetic fields [194], lights [191], and electrochemical devices connected to apps are being explored [195]. The choice of the external stimulus applied is strictly related to the carrier employed because it triggers a reaction that is related to the chemical and/or physical structure of the polymer.
6. Conclusions
Even if the outstanding research regarding DDSs using biopolymers has revolutionized the medical and pharmaceutical fields, studies are still needed to explore the constant and economical environmental changes. Biopolymers contained in developed DDSs were demonstrated to be effective and appropriate in many different medical conditions. Even so, there are ongoing studies to develop reliable guidelines for (bio)polymer applications for improving adaptability, safety, and biopotency and avoiding contamination. Also, the same regime of necessity involves studies on the optimization of technical parameters, different formulations regarding encapsulations, the capacity of product loading, and metabolic activity.
Author Contributions
Conceptualization, M.-L.S. and O.O.; writing—original draft preparation, O.O., C.M., I.L., A.S. (Adina Stegarescu), A.S. (Albert Soran) and M.-L.S.; writing—review and editing, O.O. and A.S. (Albert Soran); supervision, M.-L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
This project was funded by the Ministry of Research, Innovation, and Digitalization through Programme 1—Development of the National Research and Development System, Subprogramme 1.2—Institutional Performance—Funding Projects for Excellence in RDI, contract No. 37PFE/30.12.2021, and the Core Program within the National Research Development and Innovation Plan 2022–2027, carried out with the support of MCID, project No. 27N/03.01.2023, component project code PN 23 24 01 03.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cui, X.; Li, X.; Xu, Z.; Guan, X.; Ma, J.; Ding, D.; Zhang, W. Fabrication and characterization of chitosan/poly (lactic-co-glycolic acid) core-shell nanoparticles by coaxial electrospray technology for dual delivery of natamycin and clotrimazole. Front. Bioeng. Biotechnol. 2021, 9, 635485. [Google Scholar] [CrossRef]
- Du, X.; Wei, D.; Huang, L.; Zhu, M.; Zhang, Y.; Zhu, Y. 3D printing of mesoporous bioactive glass/silk fibroin composite scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2019, 103, 109731. [Google Scholar] [CrossRef]
- Khwaza, V.; Buyana, B.; Nqoro, X.; Ngonidzashe, R.; Oyedeji, O.O.; Aderibigbe, B.A. 2-Polymeric beads for targeted drug delivery and healthcare applications. In Woodhead Publishing Series in Biomaterials, Polymeric Biomaterials for Healthcare Applications; Varaprasad, K., Ed.; Woodhead Publishing: Cambridge, UK, 2022; pp. 41–70. [Google Scholar]
- Taib, N.-A.A.B.; Rahman, M.R.; Huda, D.; Kuok, K.K.; Hamdan, S.; Bakri, M.K.B.; Julaihi, M.R.M.B.; Khan, A. A review on poly lactic acid (PLA) as a biodegradable polymer. Polym. Bull. 2023, 80, 1179–1213. [Google Scholar] [CrossRef]
- Yadav, P.; Yadav, H.; Shah, V.G.; Shah, G.; Dhaka, G. Biomedical biopolymers, their origin and evolution in biomedical sciences: A systematic review. J. Clin. Diagn. Res. 2015, 9, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Jummaat, F.; Bashir Yahya, E.; Khalil H.P.S., A.; Adnan, A.S.; Mohammed Alqadhi, A.; Abdullah, C.K.; Sofea, A.A.; Olaiya, N.G.; Abdat, M.; Hps, K. The role of biopolymer-based materials in obstetrics and gynecology applications: A review. Polymers 2021, 13, 633. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A comparative review of natural and synthetic biopolymer composite scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Olivia, M.; Jingga, H.; Toni, N.; Wibisono, G. Biopolymers to improve physical properties and leaching characteristics of mortar and concrete: A review. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Bangkok, Thailand, 24–26 February 2018; Volume 345, p. 012028. [Google Scholar]
- Vieira, M.G.A.; da Silva, M.A.; dos Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Birlik Demirel, G.; Aygul, E.; Dag, A.; Atasoy, S.; Cimen, Z.; Cetin, B. Folic acid-conjugated pH and redox-sensitive ellipsoidal hybrid magnetic nanoparticles for dual-triggered drug release. ACS Appl. Bio Mater. 2020, 3, 4949–4961. [Google Scholar] [CrossRef]
- Che, E.; Gao, Y.; Wan, L.; Zhang, Y.; Han, N.; Bai, J.; Li, J.; Sha, Z.; Wang, S. Paclitaxel/gelatin coated magnetic mesoporous silica nanoparticles: Preparation and antitumor efficacy in vivo. Microporous Mesoporous Mater. 2015, 204, 226–234. [Google Scholar] [CrossRef]
- Dumontel, B.; Conejo-Rodríguez, V.; Vallet-Regí, M.; Manzano, M. Natural biopolymers as smart coating materials of mesoporous silica nanoparticles for drug delivery. Pharmaceutics 2023, 15, 447. [Google Scholar] [CrossRef]
- Mtibe, A.; Motloung, M.P.; Bandyopadhyay, J.; Ray, S.S. Synthetic biopolymers and their composites: Advantages and limitations—An overview. Macromol. Rapid Commun. 2021, 42, 2100130. [Google Scholar] [CrossRef]
- Adeyeye, O.A.; Sadiku, E.R.; Reddy, A.B.; Ndamase, A.S.; Makgatho, G.; Sellamuthu, P.S.; Perumal, A.B.; Nambiar, R.B.; Fasiku, V.O.; Inrahim, I.D.; et al. The use of biopolymers in food packaging. In Green Polymers and Their Nanocomposites; Gnanasekaran, D., Ed.; Springer: Singapore, 2019; pp. 137–158. [Google Scholar]
- Gurnani, K.; Singh, Y.; Satpute, G. Nanotech drug delivery system: The perfect physio-chemical deal for biological command. J. Pharm. Biol. Sci. 2021, 9, 73–80. [Google Scholar]
- Ahmed, S.; Ahmad, M.; Ikram, S. Chitosan: A natural antimicrobial agent—A review. J. Appl. Chem. 2014, 3, 493–503. [Google Scholar]
- Qasim, U.; Osman, A.I.; Al-Muhtaseb, A.H.; Farrell, C.; Al-Abri, M.; Ali, M.; Vo, D.V.N.; Jamil, F.; Rooney, D.W. Renewable cellulosic nanocomposites for food packaging to avoid fossil fuel plastic pollution: A review. Environ. Chem. Lett. 2021, 19, 613–641. [Google Scholar] [CrossRef]
- Hamouda, T. Sustainable packaging from coir fibers. In Biopolymers and Biocomposites from Agro-Waste for Packaging Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 113–126. [Google Scholar]
- Shivam, P. Recent developments on biodegradable polymers and their future trends. Int. Res. J. Sci. Eng. 2016, 4, 17–26. [Google Scholar]
- Chen, H.; Wang, J.; Cheng, Y.; Wang, C.; Liu, H.; Bian, H.; Pan, Y.; Sun, J.; Han, W. Application of protein-based films and coatings for food packaging: A review. Polymers 2019, 11, 2039. [Google Scholar] [CrossRef]
- Swain, S.K.; Pattanayak, A.J.; Sahoo, A.P. Functional biopolymer composites. In Functional Biopolymers; Thakur, V.K., Thakur, M.K., Eds.; Springer: Berlin, Germany, 2018; pp. 159–182. [Google Scholar]
- Varma, K.; Gopi, S. Biopolymers and their role in medicinal and pharmaceutical applications. In Biopolymers and Their Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 175–191. [Google Scholar]
- Temesgen, S.; Rennert, M.; Tesfaye, T.; Nase, M. Review on spinning of biopolymer fibers from starch. Polymers 2021, 13, 1121. [Google Scholar] [CrossRef]
- Gustafsson, J.; Landberg, M.; Bátori, V.; Akesson, D.; Taherzadeh, M.J.; Zamani, A. Development of bio-based films and 3D objects from apple pomace. Polymers 2019, 11, 289. [Google Scholar] [CrossRef]
- Pattanashetti, N.A.; Heggannavar, G.B.; Kariduraganavar, M.Y. Smart biopolymers and their biomedical applications. Procedia Manuf. 2017, 12, 263–279. [Google Scholar] [CrossRef]
- Torres, F.G.; Troncoso, O.P.; Pisani, A.; Gatto, F.; Bardi, G. Natural polysaccharide nanomaterials: An overview of their immunological properties. Int. J. Mol. Sci. 2019, 20, 5092. [Google Scholar] [CrossRef] [PubMed]
- Gopi, S.; Amalraj, A.; Thomas, S. Effective drug delivery system of biopolymers based on nanomaterials and hydrogels—A review. Drug Des. 2016, 5, 1–7. [Google Scholar] [CrossRef]
- Vishakha, K.S.; Kishor, B.D.; Sudha, R.S. Natural polymers—A comprehensive review. Int. J. Pharm. Biomed. Res. 2019, 3, 1597–1613. [Google Scholar]
- Jacob, J.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Biopolymer based nanomaterials in drug delivery systems: A review. Mat. Today Chem. 2018, 9, 43–55. [Google Scholar] [CrossRef]
- Yen, M.-T.; Yang, J.-H.; Mau, J.-L. Physicochemical characterization of chitin and chitosan from crab shells. Carbohydr. Polym. 2009, 75, 15–21. [Google Scholar] [CrossRef]
- Heragh, B.K.; Javanshir, S.; Mahdavinia, G.R.; Jamal, M.R.N. Hydroxyapatite grafted chitosan/laponite RD hydrogel: Evaluation of the encapsulation capacity, pH-responsivity, and controlled release behavior. Int. J. Biol. Macromol. 2021, 190, 351–359. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Kang, P.-L.; Xin, W.; Yen, C.-S.; Hwang, L.-C.; Chen, C.-J.; Liu, J.-T.; Chang, S.J. Preparation and evaluation of chitosan biocompatible electronic skin. Comput. Ind. 2018, 100, 1–6. [Google Scholar] [CrossRef]
- Machodi, M.J.; Daramola, M.O. Synthesis and performance evaluation of PES/ chitosan membranes coated with polyamide for acid mine drainage treatment. Sci. Rep. 2019, 9, 17657. [Google Scholar] [CrossRef]
- Szymańska, E.; Winnicka, K. Stability of chitosan—A challenge for pharmaceutical and biomedical applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef]
- Varshosaz, J. The promise of chitosan microspheres in drug delivery systems. Expert Opin. Drug Deliv. 2007, 4, 263–273. [Google Scholar] [CrossRef]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef]
- Fakhri, E.; Eslami, H.; Maroufi, P.; Pakdel, F.; Taghizadeh, S.; Ganbarov, K.; Yousefi, M.; Tanomand, A.; Yousefi, B.; Mahmoudi, S. Chitosan biomaterials application in dentistry. Int. J. Biol. Macromol. 2020, 162, 956–974. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.C.; Jony, B.; Nandy, P.K.; Chowdhury, R.A.; Halder, S.; Kumar, D.; Ramakrishna, S.; Hassan, M.; Ahsan, M.A.; Hoque, M.E.; et al. Recent advancement of biopolymers and their potential biomedical applications. J. Polym. Environ. 2022, 30, 51–74. [Google Scholar] [CrossRef]
- Chaves, L.L.; Silveri, A.; Vieira, A.C.C.; Ferreira, D.; Cristiano, M.C.; Paolino, D.; Di Marzio, L.; Lima, S.C.; Reis, S.; Sarmento, B.; et al. pH-responsive chitosan based hydrogels affect the release of dapsone: Design, set-up, and physicochemical characterization. Int. J. Biol. Macromol. 2019, 133, 1268–1279. [Google Scholar] [CrossRef] [PubMed]
- Hogan, K.J.; Mikos, A.G. Biodegradable thermoresponsive polymers: Applications in drug delivery and tissue engineering. Polymer 2020, 211, 123063. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Fundamentals and applications of chitosan. In Sustainable Agriculture Reviews; Springer: Berlin/Heidelberg, Germany, 2019; pp. 49–123. [Google Scholar]
- HPS, A.K.; Saurabh, C.K.; Adnan, A.S.; Fazita, M.R.N.; Syakir, M.I.; Davoudpour, Y.; Rafatullah, M.; Abdullah, C.K.; MHaafiz, M.K.; Dungani, R. A review on chitosan-cellulose blends and nanocellulose reinforced chitosan biocomposites: Properties and their applications. Carbohydr. Polym. 2016, 150, 216–226. [Google Scholar]
- Kaur, M.; Sharma, A.; Puri, V.; Aggarwal, G.; Maman, P.; Huanbutta, K.; Nagpal, M.; Sangnim, T. Chitosan-based polymer blends for drug delivery systems. Polymers 2023, 15, 2028. [Google Scholar] [CrossRef]
- Chen, L.; Deng, X.; Tian, L.; Xie, J.; Xiang, Y.; Liang, X.; Jiang, L.; Jiang, L. Preparation and properties of chitosan/dialdehyde sodium alginate/dopamine magnetic drug-delivery hydrogels. Coll. Surf. A Physicochem. Eng. Asp. 2024, 680, 132739. [Google Scholar] [CrossRef]
- Lv, Y.; Chen, X.; Shen, Y. Folate-modified carboxymethyl chitosan-based drug delivery system for breast cancer specific combination therapy via regulating mitochondrial calcium concentration. Carbohydr. Polym. 2024, 323, 121434. [Google Scholar] [CrossRef]
- Aranda-Barradas, M.E.; Trejo-LÓpez, S.E.; Del Real, A.; Álvarez-Almazán, S.; Méndez-Albores, A.; García-Tovar, C.G.; González-Díaz, F.R.; Miranda-Castro, S.P. Effect of molecular weight of chitosan on the physicochemical, morphological, and biological properties of polyplex nanoparticles intended for gene delivery. Carbohydr. Polym. Technol. Appl. 2022, 4, 100228. [Google Scholar] [CrossRef]
- Alameh, M.; De Jesus, D.; Jean, M.; Darras, V.; Thibault, M.; Lavertu, M.; Merzouki, A. Low molecular weight chitosan nanoparticulate system at low N:P ratio for nontoxic polynucleotide delivery. Int. J. Nanomed. 2012, 7, 1399–1414. [Google Scholar]
- Mao, H.Q.; Roy, K.; Troung-Le, V.L.; Janes, K.A.; Lin, K.Y.; Wang, Y.; Leong, K. Chitosan-DNA nanoparticles as gene carriers: Synthesis, characterization and transfection efficiency. J. Control. Release 2001, 70, 399–421. [Google Scholar] [CrossRef]
- Garshasbi, H.; Salehi, S.; Naghib, S.M.; Ghorbanzadeh, S.; Zhang, W. Stimuli-responsive injectable chitosan-based hydrogels for controlled drug delivery systems. Front. Bioeng. Biotechnol. 2023, 10, 1126774. [Google Scholar] [CrossRef]
- Paques, J.P.; Van Der Linden, E.; Van Rijn, C.J.M.; Sagis, L.M.C. Preparation methods of alginate nanoparticles. Adv. Colloid Interface Sci. 2014, 209, 163–171. [Google Scholar] [CrossRef]
- Severino, P.; da Silva, C.F.; Andrade, L.N.; de Lima Oliveira, D.; Campos, J.; Souto, E.B. Alginate nanoparticles for drug delivery and targeting. Curr. Pharm. Des. 2019, 25, 1312–1334. [Google Scholar] [CrossRef] [PubMed]
- Martau, G.A.; Mihai, M.; Vodnar, D.C. The use of chitosan, alginate, and pectin in the biomedical and food sector-biocompatibility, bioadhesiveness, and biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Miri, T. Alginates in foods. In Practical Food Rheology: An Interpretive Approach; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 113–132. [Google Scholar]
- Joye, I.J.; McClements, D.J. Biopolymer-based nanoparticles and microparticles: Fabrication, characterization, and application. Curr. Opin. Colloid Interface Sci. 2014, 19, 417–427. [Google Scholar] [CrossRef]
- Hoang, H.T.; Vu, T.T.; Karthika, V.; Jo, S.-H.; Jo, Y.-J.; Seo, J.-W.; Oh, C.-W.; Park, S.-H.; Lim, K.T. Dual cross-linked chitosan/alginate hydrogels prepared by Nb-Tz ‘click’ reaction for pH responsive drug delivery. Carbohydr. Polym. 2022, 288, 119389. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-Q.; Wang, S.-Y.; Meng, Y.-J.; Guo, Z.-W.; Cheng, M.-M.; Li, J. Fabrication of self-healing pectin/chitosan hybrid hydrogel via Diels-Alder reactions for drug delivery with high swelling property, pH-responsiveness, and cytocompatibility. Carbohydr. Polym. 2021, 268, 118244. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, D.H.; Kim, J.Y. Effect of heat-moisture treatment under mildly acidic condition on fragmentation of waxy maize starch granules into nanoparticles. Food Hydrocoll. 2017, 63, 59–66. [Google Scholar] [CrossRef]
- Patel, C.M.; Chakraborty, M.; Murthy, Z.V.P. Fast and scalable preparation of starch nanoparticles by stirred media milling. Adv. Powder Technol. 2016, 27, 1287–1294. [Google Scholar] [CrossRef]
- Acevedo-Guevara, L.; Nieto-Suaza, L.; Sanchez, L.T.; Pinzon, M.I.; Villa, C.C. Development of native and modified banana starch nanoparticles as vehicles for curcumin. Int. J. Biol. Macromol. 2018, 111, 498–504. [Google Scholar] [CrossRef]
- Chan, P.S.K.; Chen, J.S.; Ettelaie, R.; Law, Z.; Alevisopoulos, S.; Day, E.; Smith, S. Study of the shear and extensional rheology of casein, waxy maize starch and their mixtures. Food Hydrocoll. 2007, 21, 716–725. [Google Scholar] [CrossRef]
- Mundargi, R.C.; Shelke, N.B.; Rokhade, A.P.; Patil, S.A.; Aminabhavi, T.M. Formulation and in-vitro evaluation of novel starch-based tableted microspheres for controlled release of ampicillin. Carbohydr. Res. 2008, 71, 42–53. [Google Scholar] [CrossRef]
- Mao, S.R.; Chen, Z.M.; Wei, Z.P.; Liu, H.; Bi, D.Z. Intranasal administration of melatonin starch microspheres. Int. J. Phytorem. 2004, 272, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Lemos, P.V.F.; Marcelino, H.R.; Cardoso, L.G.; de Souza, C.O.; Druzian, J.I. Starch chemical modifications applied to drug delivery systems: From fundamentals to FDA-approved raw materials. Int. J. Biol. Macromol. 2021, 184, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, L.; Zhang, C.; Deng, Y.; Xie, P.; Liu, L.; Cheng, J. Research advances in chemical modifications of starch for hydrophobicity and its applications: A review. Carbohydr. Polym. 2020, 240, 116292. [Google Scholar] [CrossRef] [PubMed]
- Ab’lah, N.; Yusuf, C.Y.L.; Rojsitthisak, P.; Wong, T.W. Reinvention of starch for oral drug delivery system design. Int. J. Biol. Macromol. 2023, 241, 124506. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Yang, T.; Lin, Q.; Liu, G.Q.; Zhang, L.; Yu, F.; Chen, Y. Acetylated starch nanocrystals: Preparation and antitumor drug delivery study. Int. J. Biol. Macromol. 2016, 89, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Yasar, H.; Ho, D.K.; De Rossi, C.; Herrmann, J.; Gordon, S.; Loretz, B.; Lehr, C.M. Starch-chitosan polyplexes: A versatile carrier system for anti-infectives and gene delivery. Polymers 2018, 10, 252. [Google Scholar] [CrossRef]
- Okunlola, A.; Ghomorai, T. Development of ibuprofen microspheres using acetylated plantain starches as polymer for sustained release. J. Pharm. Investig. 2018, 48, 551–564. [Google Scholar] [CrossRef]
- Zhao, K.; Li, D.; Xu, W.; Ding, J.; Jiang, W.; Li, M.; Wang, C.; Chen, X. Targeted hydroxyethyl starch prodrug for inhibiting the growth and metastasis of prostate cancer. Biomaterials 2017, 116, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Mathew, N.; Nath, M.S. Starch modified alginate nanoparticles for drug delivery application. Int. J. Biol. Macromol. 2021, 173, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Atanase, L.I. Micellar drug delivery systems based on natural biopolymers. Polymers 2021, 13, 477. [Google Scholar] [CrossRef] [PubMed]
- Bhaladhare, S.; Das, D. Cellulose: A fascinating biopolymer for hydrogel synthesis. J. Mater. Chem. B 2022, 10, 1923–1945. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Tukhani, M.; Hussain, C.M. Recent advancements in 3D bioprinting technology of carboxymethyl cellulose-based hydrogels: Utilization in tissue engineering. Adv. Colloid Interface Sci. 2021, 292, 102415. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Medronho, B.; Lindman, B.; Norgren, M. Simple one pot preparation of chemical hydrogels from cellulose dissolved in cold LiOH/Urea. Polymers 2020, 12, 373. [Google Scholar] [CrossRef] [PubMed]
- Zainal, S.H.; Mohd, N.H.; Suhaili, N.; Anuar, F.H.; Lazim, A.M.; Othaman, R. Preparation of cellulose-based hydrogel: A review. J. Mater. Res. Technol. 2020, 10, 935–952. [Google Scholar] [CrossRef]
- Zafar, A.; Afzal, M.; Quazi, A.M.; Yasir, M.; Kazmi, I.; Al-Abaasi, F.A.; Alruwaili, N.K.; Alharbi, K.S.; Alzarea, S.I.; Sharma, S.; et al. Chitosan-ethyl cellulose microspheres of domperidone for nasal delivery: Preparation, in-vitro characterization, in-vivo study for pharmacokinetic evaluation and bioavailability enhancement. J. Drug Deliv. Sci. Technol. 2021, 63, 102471. [Google Scholar] [CrossRef]
- Kahya, N.; Gölcü, A.; Erim, F.B. Barium ion cross-linked alginate-carboxymethyl cellulose composites for controlled release of anticancer drug methotrexate. J. Drug Deliv. Sci. Technol. 2019, 54, 101324. [Google Scholar] [CrossRef]
- AL-Rajabi, M.M.; Teow, Y.H. Synthesis of thermoresponsive composite hydrogel from Pluronic F127 reinforced by oil palm empty fruit bunches-extracted cellulose for silver sulfadiazine drug delivery. Sustain. Chem. Pharm. 2023, 31, 100939. [Google Scholar] [CrossRef]
- Passi, A.; Vigetti, D. Hyaluronan as tunable drug delivery system. Adv. Drug Deliv. Rev. 2019, 146, 83–96. [Google Scholar] [CrossRef]
- Widjaja, L.K.; Bora, M.; Chan, P.N.; Lipik, V.; Wong, T.T.L.; Venkatraman, S.S. Hyaluronic acid-based nano-composite hydrogels for ocular drug delivery applications. J. Biomed. Mater. Res. A 2014, 102, 3056–3065. [Google Scholar] [CrossRef]
- Huang, G.; Huang, H. Hyaluronic acid-based biopharmaceutical delivery and tumor-targeted drug delivery system. J. Control. Release 2018, 278, 122–126. [Google Scholar] [CrossRef]
- Maity, S.; Saha, S.; Sarkar, S.; Kumar, P.; Gautam, A.K.; Sonkar, A.B. Chapter 12—Dextran-based nanomaterials in drug delivery applications. In Biopolymer-Based Nanomaterials in Drug Delivery and Biomedical Applications; Bera, H., Hossain, C.M., Saha, S., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 293–312. [Google Scholar]
- Petrovici, A.R.; Pinteala, M.; Simionescu, N. Dextran formulations as effective delivery systems of therapeutic agents. Molecules 2023, 28, 1086. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, G.; Nichifor, M.; Sacarescu, L. Dextran based polymeric micelles as carriers for delivery of hydrophobic drugs. Curr. Drug Deliv. 2017, 2017. 14, 406–415. [Google Scholar] [CrossRef]
- Ghosh, B.; Biswas, S. Polymeric micelles in cancer therapy: State of the art. J. Control. Release 2021, 332, 127–147. [Google Scholar] [CrossRef] [PubMed]
- Kali, G.; Haddadzadegan, S.; Bernkop-Schnürch, A. Cyclodextrins and derivatives in drug delivery: New developments, relevant clinical trials, and advanced products. Carbohydr. Polym. 2024, 324, 121500. [Google Scholar] [CrossRef] [PubMed]
- Pedotti, S.; Pistarà, V.; Cannavà, C.; Carbone, C.; Cilurzo, F.; Corsaro, A.; Puglisi, G.; Ventura, C.A. Synthesis and physico-chemical characterization of a β-cyclodextrin conjugate for sustained release of Acyclovir. Carbohydr. Polym. 2015, 131, 159–167. [Google Scholar] [CrossRef]
- di Cagno, M.; Nielsen, T.T.; Larsen, K.L.; Kuntsche, J.; Bauer-Brand, A. β-Cyclodextrin-dextran polymers for the solubilization of poorly soluble drugs. Int. J. Pharm. 2014, 468, 258–263. [Google Scholar] [CrossRef]
- Kratz, F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control. Release 2008, 132, 171–183. [Google Scholar] [CrossRef]
- Larsen, M.T.; Kuhlmann, M.; Hvam, M.L.; Howard, K.A. Albumin-based drug delivery: Harnessing nature to cure disease. Mol. Cell. Therap. 2016, 4, 1–12. [Google Scholar] [CrossRef]
- Preeti, N.; Shobha, W.; Michelle, M.; Shishanka, W.; Pooja, C.; Dhiman, S. Graphene quantum dots conjugated albumin nanoparticles for targeted drug delivery and imaging of pancreatic cancer. J. Mater. Chem. B 2014, 2, 3190–3195. [Google Scholar]
- Qiu, C.; Wang, C.; Gong, C.; McClements, D.J.; Jin, Z.; Wang, J. Advances in research on preparation, characterization, interaction with proteins, digestion and delivery systems of starch-based nanoparticles. Int. J. Biol. Macromol. 2020, 152, 117–125. [Google Scholar] [CrossRef]
- Kanugo, A.; Misra, A. New and novel approaches for enhancing the oral absorption and bioavailability of protein and peptides therapeutics. Ther. Deliv. 2020, 11, 713–732. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Wehling, R.L.; Ciftci, O.; Zhang, Y. Formation of complexes between tannic acid with bovine serum albumin, egg ovalbumin and bovine beta-lactoglobulin. Food Res. Int. 2017, 102, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Liu, X.-R.; Chen, Q.-B.; Li, Y.; Zhou, J.-L.; Zhou, L.-Y.; Zou, T. Hyaluronic acid and albumin based nanoparticles for drug delivery. J. Control. Release 2021, 331, 416–433. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Yu, Y.; Liu, Y.; Chen, Z.; Kong, T.; Zhao, Y. Spinning and applications of bioinspired fiber systems. ACS Nano 2019, 13, 2749–2772. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.; Nguyen, Q.V.; Nguyen, V.; Le, T.; Van Le, Q. Silk fibroin-based biomaterials for biomedical. Polymers 2019, 11, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Yucel, T.; Lovett, M.L.; Kaplan, D.L. Silk-based biomaterials for sustained drug delivery. J. Control. Release 2014, 190, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. Silk fibroin as a functional biomaterial for drug and gene delivery. Pharmaceutics 2019, 11, 494. [Google Scholar] [CrossRef]
- Farahani, A.; Zarei-Hanzaki, A.; Abedi, H.R.; Daryoush, S.; Ragheb, Z.D.; Mianabadi, F.; Shahparvar, S.; Akrami, M.; Mostafavi, E.; Khanbareh, H.; et al. Silk-based biopolymers promise extensive biomedical applications in tissue engineering, drug delivery, and BioMEMS. J. Polym. Environ. 2023, 31, 4559–4582. [Google Scholar] [CrossRef]
- Navamajiti, N.; Gardner, A.; Cao, R.; Sugimoto, Y.; Yang, J.W.; Lopes, A.; Phan, N.V.; Collins, J.; Hua, T.; Damrongsakkul, S.; et al. Silk fibroin-based coatings for pancreatin-dependent drug delivery. J. Pharm. Sci. 2023, in press. [CrossRef]
- Gharehnazifam, Z.; Dolatabadi, R.; Baniassadi, M.; Shahsavari, H.; Kajbafzadeh, A.-M.; Abrinia, K.; Gharehnazifam, K.; Baghani, M. Multiphysics modeling and experiments on ultrasound-triggered drug delivery from silk fibroin hydrogel for Wilms tumor. Int. J. Pharm. 2022, 621, 121787. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Visht, T.; Chak, V. A review on collagen-based drug delivery systems. Int. J. Pharm. Teach. Pract. 2013, 4, 811–820. [Google Scholar]
- Le, V.M.; Lang, M.D.; Shi, W.B.; Liu, J.W. A collagen-based multicellular tumor spheroid model for evaluation of the efficiency of nanoparticle drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 540–544. [Google Scholar] [CrossRef]
- Solanki, P.R.; Kaintura, P.S.R.; Singh, K.R.S. Gelatin nanoparticles as a delivery system for proteins. J. Nanomed. Res. 2015, 2, 1–3. [Google Scholar] [CrossRef]
- Lammers, J.T.; Kiessling, F.; Hennink, W.E.; Storm, G. Drug targeting to tumors: Principles, pitfalls and (pre-) clinical progress. J. Control. Release 2012, 161, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Madan, J.J.; Pandey, R.S.S.; Jain, U.K.; Katare, O.P.; Aneja, R.; Katyal, A. Sterically stabilized gelatin microassemblies of noscapine enhance cytotoxicity, apoptosis and drug delivery in lung cancer cells. Colloids Surf. B Biointerfaces 2013, 107, 235–244. [Google Scholar] [CrossRef]
- Gil, V.S.B.; Gil, C.S.B.; Goulart, G.A.C.; Oréfice, R.L. Multi-drug hybrid delivery systems with distinct release profiles based on gelatin/collagen containing vesicles derived from block copolymers. Int. J. Biol. Macromol. 2019, 139, 967–974. [Google Scholar]
- Behrend-Keim, B.; Castro-Muñoz, A.; Monrreal-Ortega, L.; Àvalos-León, B.; Campos-Estrada, C.; Smyth, H.D.C.; Bahamondez-Canas, T.F.; Moraga-Espinoza, D. The forgotten material: Highly dispersible and swellable gelatin-based microspheres for pulmonary drug delivery of cromolyn sodium and ipratropium bromide. Int. J. Pharm. 2023, 644, 123331. [Google Scholar] [CrossRef]
- Zhao, Y.; Zuo, X.; Li, Q.; Chen, F.; Chen, Y.-R.; Deng, J.; Han, D.; Hao, C.; Huang, F.; Huang, Y. Nucleic acids analysis. Sci. China Chem. 2021, 64, 171–203. [Google Scholar] [CrossRef]
- Wang, F.; Li, P.; Chu, H.C.; Lo, P.K. Nucleic acids and their analogues for biomedical applications. Biosensors 2022, 12, 93. [Google Scholar] [CrossRef]
- Serganov, A.; Patel, D.J. Ribozymes, riboswitches and beyond: Regulation of gene expression without proteins. Nat. Rev. Genet. 2007, 8, 776–790. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Jia, F.; Wang, P.; Zhang, K. Nucleic acid-based drug delivery strategies. J. Control. Release 2020, 323, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Sabard, M.; Gouanvé, F.; Espuche, E.; Fulchiron, R.; Fillot, L.-A.; Trouillet-Fonti, L. Erasure of the processing effects in polyamide 6 based cast films by the introduction of montmorillonite: Effect on water and oxygen transport properties. J. Membr. Sci. 2014, 456, 11–20. [Google Scholar] [CrossRef]
- Winnacker, M. Polyamides and their functionalization: Recent concepts for their applications as biomaterials. Biomater. Sci. 2017, 5, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Bankar, S.B.; Nimbalkar, P.R.; Chavan, P.V.; Singhal, R.S. Chapter 12—Microbial polyamino acids: An overview for commercial attention. In Role of Materials Science in Food Bioengineering; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: London, UK, 2018; pp. 381–412. [Google Scholar]
- Khattab, S.N.; Naim, S.E.A.; El-Sayed, M.; El Bardan, A.A.; Elzoghby, A.; Bekhit, A.; El-Faham, A. Design and synthesis of new s-triazine polymers and their application as nanoparticulate drug delivery systems. New J. Chem. 2016, 40, 9565–9578. [Google Scholar] [CrossRef]
- González-Aramundiz, J.; Lozano, M.V.; Sousa-Hervés, A.; Fernandez-Megia, E.; Csaba, N. Polypeptides and polyaminoacids in drug delivery. Expert Opin. Drug Deliv. 2012, 9, 183–201. [Google Scholar] [CrossRef] [PubMed]
- Boddu, S.H.S.; Bhagav, P.; Karl, P.K.; Jacob, S.; Adatiya, M.D.; Dhameliya, T.M.; Ranch, K.M.; Tiwari, A.K. Polyamide/poly(amino acid) polymers for drug delivery. J. Funct. Biomater. 2021, 12, 58. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Ray, S.S. Preparation, characterization and in vitro release kinetics of polyaspartamide-based conjugates containing antimalarial and anticancer agents for combination therapy. J. Drug Deliv. Sci. Technol. 2016, 36, 34–45. [Google Scholar] [CrossRef]
- Huynh, N.-T.; Jeon, Y.-S.; Kim, D.; Kim, J.-H. Preparation and swelling properties of ‘click’ hydrogel from polyaspartamide derivatives using tri-arm PEG and PEG-co-poly(amino urethane) azides as crosslinking agents. Polymer 2013, 54, 1341–1349. [Google Scholar] [CrossRef]
- Nguyen, M.P.; Thuy, V.T.T.; Kim, D. Integration of iron oxide nanoparticles and polyaspartamide biopolymer for MRI image contrast enhancement and an efficient drug-delivery system in cancer therapy. Nanotechnology 2020, 31, 335712. [Google Scholar] [CrossRef]
- Di Meo, C.; Cilurzo, F.; Licciardi, M.; Scialabba, C.; Sabia, R.; Paolino, D.; Capitani, D.; Fresta, M.; Giammona, G.; Villani, C.; et al. Polyaspartamide-doxorubicin conjugate as potential prodrug for anticancer therapy. Pharm. Res. 2015, 32, 1557–1569. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Qiu, L.; Qiao, X.; Yang, H. Dendrimer-based drug delivery systems: History, challenges, and latest developments. J. Biol. Eng. 2022, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.S. Dendrimers for drug delivery. Molecules 2018, 23, 938. [Google Scholar] [CrossRef]
- Haag, R. Supramolecular drug-delivery systems based on polymeric core–shell architectures. Angew. Chem. Int. Ed. 2004, 43, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.; Ferraro, M.; Haag, R.; Quadir, M. Dendritic polyglycerol-derived nano-architectures as delivery platforms of gemcitabine for pancreatic cancer. Macromol. Biosci. 2019, 19, 1900073. [Google Scholar] [CrossRef]
- Calderón, M.; Quadir, M.A.; Strumia, M.; Haag, R. Functional dendritic polymer architectures as stimuli-responsive nanocarriers. Biochimie 2010, 92, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, P.; Anitha, A.K.; Ajayakumar, N.; Kumar, K.S. Poly-lysine dendritic nanocarrier to target epidermal growth factor receptor overexpressed breast cancer for methotrexate delivery. Materials 2022, 15, 800. [Google Scholar] [CrossRef]
- Salerno, H.; Verdolotti, L.; Raucci, M.G.; Saurina, J.; Domingo, C.; Lamanna, R.; Iozzino, V.; Lavorgna, M. Hybrid gelatin-based porous materials with a tunable multiscale morphology for tissue engineering and drug delivery. Eur. Polym. J. 2018, 99, 230–239. [Google Scholar] [CrossRef]
- Brito-Pereiraa, R.; Correia, D.M.; Ribeiro, C.; Francesko, A.; Etxebarria, I.; Pérez-Álvarez, L.; Vilasc, J.L.; Martins, P.; Lanceros-Mendez, S. Silk fibroin-magnetic hybrid composite electrospun fibers for tissue engineering applications. Compos. Part B 2018, 141, 70–75. [Google Scholar] [CrossRef]
- Fu, C.; Ding, C.; Sun, X.; Fu, A. Curcumin nanocapsules stabilized by bovine serum albumin-capped gold nanoclusters (BSA-AuNCs) for drug delivery and theranosis. Mater. Sci. Eng. C 2018, 87, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, F.; Catanzano, O.; d’Angelo, I.; Diaz-Gomez, L.; Concheiro, A.; Miro, A.; Alvarez-Lorenzo, C.; Quaglia, F. Microparticle-embedded fibroin/alginate beads for prolonged local release of simvastatin hydroxyacid to mesenchymal stem cells. Carbohydr. Polym. 2017, 175, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Iwao, Y.; Tomiguchi, I.; Domura, A.; Mantaira, Y.; Minami, A.; Suzuki, T.; Ikawa, T.; Kimura, S.I.; Itai, S. Inflamed site-specific drug delivery system based on the interaction of human serum albumin nanoparticles with myeloperoxidase in a murine model of experimental colitis. Eur. J. Pharm. Biopharm. 2018, 125, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Shon, Y.; Kim, H.; Hwang, H.S.; Bae, E.S.; Eom, T.; Park, E.J.; Ahn, W.S.; Wie, J.J.; Shim, B.S. A nanostructured cell-free photosynthetic biocomposite via molecularly controlled layer-by-layer assembly. Sens. Actuat. B Chem. 2017, 244, 1–10. [Google Scholar] [CrossRef]
- Bonilla, P.; Arias, E.M.; Solans, C.; García-Celma, M.J. Influence of crosslinked alginate on drug release from highly concentrated emulsions. Colloids Surf. A. Physicochem. Eng. Asp. 2018, 536, 148–155. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Dastmalchi, S.; Davaran, S. A simple improved desolvation method for the rapid preparation of albumin nanoparticles. Int. J. Biol. Macromol. 2016, 91, 703–709. [Google Scholar] [CrossRef]
- von Storp, B.; Engel, A.; Boeker, A.; Ploeger, M.; Langer, K. Albumin nanoparticles with predictable size by desolvation procedure. J. Microencapsul. 2012, 29, 138–146. [Google Scholar] [CrossRef]
- Moreno, J.A.S.; Mendes, A.C.; Stephansen, K.; Engwer, C.; Goycoolea, F.M.; Boisen, A.; Nielsen, L.H.; Chronakis, I.S. Development of electrosprayed mucoadhesive chitosan. Carbohydr. Polym. 2018, 190, 240–247. [Google Scholar] [CrossRef]
- Mendes, A.C.; Chronakis, I.S. Electrohydrodynamic encapsulation of probiotics: A review. Food Hydrocoll. 2021, 117, 106688. [Google Scholar] [CrossRef]
- Zamani, M.; Prabhakaran, M.P.; Ramakrishna, S. Advances in drug delivery via electrospun and electrosprayed nanomaterials. Int. J. Nanomed. 2013, 8, 2997–3017. [Google Scholar]
- Xie, J.; Wang, C.H. Electrospray in the dripping mode for cell microencapsulation. J. Colloid Interface Sci. 2007, 312, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Ataabadi, F.V.; Oveissi, F.; Etebari, M.; Taheri, A. Preparation of chitosan nanoparticles for simultaneous drug delivery of dacarbazine and enoxaparin in melanoma. Carbohydr. Polym. 2023, 316, 121041. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kong, Y.; Liu, F.; Shou, D.; Tao, Y.; Qin, Y. Construction of pH-responsive drug delivery platform with calcium carbonate microspheres induced by chitosan gels. Ceram. Int. 2018, 44, 7902–7907. [Google Scholar] [CrossRef]
- Rao, K.M.; Kumar, A.; Suneetha, M.; Han, S.S. pH and near-infrared active; chitosan-coated halloysite nanotubes loaded with curcumin-Au hybrid nanoparticles for cancer drug delivery. Int. J. Biol. Macromol. 2018, 112, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Jaiturong, P.; Sirithunyalug, B.; Eitsayeam, S.; Asawahame, C.; Tipduangta, P.; Sirithunyalug, J. Preparation of glutinous rice starch/polyvinyl alcohol copolymer electrospun fibers for using as a drug delivery carrier. AJPS 2018, 13, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wang, Y.; Li, H.; Duan, Y. A facile one-pot synthesis of starch functionalized graphene as nano-carrier for pH sensitive and starch-mediated drug delivery. Colloids Surf. B Biointerfaces 2015, 128, 86–93. [Google Scholar] [CrossRef]
- Rao, Z.; Ge, H.; Liu, L.; Zhu, C.; Min, L.; Liu, M.; Fan, L.; Li, D. Carboxymethyl cellulose modified graphene oxide as pH-sensitive drug delivery system. Int. J. Biol. Macromol. 2018, 107, 1184–1192. [Google Scholar] [CrossRef]
- Wijaya, C.J.; Saputra, S.N.; Soetaredjo, F.E.; Putro, J.N.; Lin, C.X.; Kurniawan, A.; Ju, Y.-H.; Ismadji, S. Cellulose nanocrystals from passion fruit peels waste as antibiotic drug carrier. Carbohydr. Polym. 2017, 175, 370–376. [Google Scholar] [CrossRef]
- Solanki, A.; Thakore, S. Cellulose crosslinked pH-responsive polyurethanes for drug delivery: A-hydroxy acids as drug release modifiers. Int. J. Biol. Macromol. 2015, 80, 683–691. [Google Scholar] [CrossRef]
- Taneja, N.; Singh, K.K. Rational design of polysorbate 80 stabilized human serum albumin nanoparticles tailored for high drug loading and entrapment of irinotecan. Int. J. Pharm. 2018, 536, 82–94. [Google Scholar] [CrossRef]
- Nosrati, H.; Sefidi, N.; Sharafi, A.; Danafar, H.; Kheiri Manjili, H. Bovine serum albumin (BSA) coated iron oxide magnetic nanoparticles as biocompatible carriers for curcumin-anticancer drug. Bioorg. Chem. 2018, 76, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Taguchi, K.; Yamasaki, K.; Sakuragi, M.; Kuroda, S.; Otagiri, M. Albumin-encapsulated liposomes: A novel drug delivery carrier with hydrophobic drugs encapsulated in the inner aqueous core. J. Pharm. Sci. 2018, 107, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.; Liu, C.; Zheng, W.; Li, X.; Ge, R.; Shen, H.; Guo, X.; Lian, Q.; Shen, X.; Li, C. Cyclic cRGDfk peptide and Chlorin e6 functionalized silk fibroin nanoparticles for targeted drug delivery and photodynamic therapy. Biomaterials 2018, 161, 306–320. [Google Scholar] [CrossRef]
- Zhou, Y.; Liang, K.; Zhao, S.; Zhang, C.; Li, J.; Yang, H.; Liu, X.; Yin, X.; Chen, D.; Xu, W.; et al. Photopolymerized maleilated chitosan/methacrylated silk fibroin micro/nanocomposite hydrogels as potential scaffolds for cartilage tissue engineering. Int. J. Biol. Macromol. 2018, 108, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.A.; Mallepally, R.R.; McHugh, M.A. Silk fibroin aerogels for drug delivery applications. J. Supercrit. Fluids 2014, 91, 84–89. [Google Scholar] [CrossRef]
- Anandhakumar, S.; Krishnamoorthy, G.; Ramkumar, K.M.; Raichur, A.M. Preparation of collagen peptide functionalized chitosan nanoparticles by ionic gelation method: An effective carrier system for encapsulation and release of doxorubicin for cancer drug delivery. Mater. Sci. Eng. C 2017, 70, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Nejat, H.; Rabiee, M.; Varshochian, R.; Tahriri, M.; Jazayeri, H.E.; Rajadas, J.; Ye, H.; Cui, Z.; Tayebi, L. Preparation and characterization of cardamom extractloaded gelatin nanoparticles as effective targeted drug delivery system to treat glioblastoma. React. Funct. Polym. 2017, 120, 46–56. [Google Scholar] [CrossRef]
- Dolci, L.S.; Liguori, A.; Panzavolta, S.; Miserocchi, A.; Passerini, N.; Gherardi, M.; Colombo, V.; Bigi, A.; Albertini, B. Non-equilibrium atmospheric pressure plasma as innovative method to crosslink and enhance mucoadhesion of econazole-loaded gelatin films for buccal drug delivery. Colloids Surf. B. Biointerfaces 2018, 163, 73–82. [Google Scholar] [CrossRef]
- Liang, W.; Chan, A.Y.L.; Chow, M.Y.T.; Lo, F.F.K.; Qiu, Y.; Kwok, P.C.L.; Lam, J.K.W. Spray freeze drying of small nucleic acids as inhaled powder for pulmonary delivery. AJPS 2018, 13, 163–172. [Google Scholar] [CrossRef]
- Ishwarya, S.P.; Anandharamakrishnan, C.; Stapley, A.G.F. Sprayfreeze-drying: A novel process for the drying of foods and bioproducts. Trends Food Sci. Technol. 2015, 41, 161–181. [Google Scholar] [CrossRef]
- Wanning, S.; Suverkrup, R.; Lamprecht, A. Pharmacetical spray freeze drying. Int. J. Pharmaceut. 2015, 488, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Morishita, M.; Mizutani, K.; Shibayama, A.; Okazaki, M.; Okamoto, H. Development of spray-freeze-dried siRNA/PEI powder for inhalation with high aerosol performance and strong pulmonary gene silencing activity. J. Control. Release 2018, 279, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Fukuhara, M.; Okuda, T.; Okamoto, H. Naked pDNA/hyaluronic acid powder shows excellent long-term storage stability and gene expression in murine lungs. Int. J. Pharm. 2020, 574, 118880. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Jeong, H.; Hong, J. CO2 bubble assisted layer-by-layer self-assembly of graphene oxide multilayer film. Colloids Surf. A 2017, 533, 76–80. [Google Scholar] [CrossRef]
- Liu, G.; Li, L.; Huo, D.; Li, Y.; Wu, Y.; Zeng, L.; Cheng, P.; Xing, M.; Zeng, W.; Zhu, C. A VEGF delivery system targeting MI improves angiogenesis and cardiac function based on the tropism of MSCs and layer-by-layer self-assembly. Biomaterials 2017, 127, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Tan, X.; Sheng, R.; Xiao, J. Layer-by-layer self-assembly of giant polyelectrolyte microcapsules templated by microbubbles as potential hydrophilic or hydrophobic drug delivery system. Colloid Interface Sci. Commun. 2022, 47, 100603. [Google Scholar] [CrossRef]
- Kupper, S.; Kłosowska-Chomiczewska, I.; Szumała, P. Collagen and hyaluronic acid hydrogel in water-in-oil microemulsion delivery systems. Carbohydr. Polym. 2017, 175, 347–354. [Google Scholar] [CrossRef]
- Callender, S.P.; Mathews, J.A.; Kobernyk, K.; Wettig, S.D. Micro emulsion utility in pharmaceuticals: Implications for multi-drug delivery. Int. J. Pharm. 2017, 526, 425–442. [Google Scholar] [CrossRef]
- Yadav, K.S.; Soni, G.; Choudhary, D.; Khanduri, A.; Bhandari, A.; Joshi, G. Microemulsions for enhancing drug delivery of hydrophilic drugs: Exploring various routes of administration. Med. Drug Discov. 2023, 20, 100162. [Google Scholar] [CrossRef]
- Ding, C.; Li, Z. A review of drug release mechanisms from nanocarrier systems. Mater. Sci. Eng. C 2017, 76, 1440–1453. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled drug delivery systems: Current status and future directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Laracuente, M.-L.; Yu, M.H.; McHugh, K.J. Zero-order drug delivery: State of the art and future prospects. J. Control. Release 2020, 327, 834–856. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.A.; Domínguez-Robles, J.; McIlorum, V.J.; Mancuso, E.; Lamprou, D.A.; Donnelly, R.F.; Larrañeta, E. Development of a biodegradable subcutaneous implant for prolonged drug delivery using 3D printing. Pharmaceutics 2020, 12, 105. [Google Scholar] [CrossRef]
- Uhrich, K.E.; Cannizzaro, S.M.; Langer, R.S.; Shakesheff, K.M. Polymeric systems for controlled drug release. Chem. Rev. 1999, 11, 3181–3198. [Google Scholar] [CrossRef]
- Macha, I.J.; Ben-Nissan, B.; Vilchevskaya, E.N.; Morozova, A.S.; Abali, B.E.; Müller, W.H.; Rickert, W. Drug delivery from polymer-based nanopharmaceuticals-an experimental study complemented by simulations of selected diffusion processes. Front. Bioeng. Biotechnol. 2019, 7, 37. [Google Scholar] [CrossRef]
- Daya, R. A note on neural, astrocyte and oligodendrocyte markers for central nervous system. J. Cell. Mol. Pharmacol. 2021, 5, 107. [Google Scholar]
- Wang, S.; Liu, R.; Fu, Y.; Kao, W.J. Release mechanisms and applications of drug delivery systems for extended-release. Expert Opin. Drug Deliv. 2020, 17, 1289–1304. [Google Scholar] [CrossRef] [PubMed]
- Nafo, W. Hydrogel biomaterials for drug delivery: Mechanisms, design, and drugs. In Hydrogels—From Tradition to Innovative Platforms with Multiple Applications; Popa, L., Ghica, M.V., Dinu-Pîrvu, C.-E., Eds.; IntechOpen: London, UK, 2023. [Google Scholar]
- Liu, C.; Liu, C.; Bai, Y.; Wang, J.; Tian, W. Drug self-delivery systems: Molecule design, construction strategy, and biological application. Adv. Healthc. Mater. 2023, 12, e2202769. [Google Scholar] [CrossRef]
- Ke, X.; Ng, V.W.L.; Ono, R.J.; Chan, J.M.W.; Krishnamurthy, S.; Wang, Y.; Hedrick, J.L.; Yang, Y.Y. Role of non-covalent and covalent interactions in cargo loading capacity and stability of polymeric micelles. J. Control. Release 2014, 193, 9–26. [Google Scholar] [CrossRef]
- Cheng, Y.; Xu, T. The effect of dendrimers on the pharmacodynamic and pharmacokinetic behaviors of non-covalently or covalently attached drugs. Eur. J. Med. Chem. 2008, 43, 2291–2297. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yao, Y. Incorporation with dendrimer-like biopolymer leads to improved soluble amount and in vitro anticancer efficacy of paclitaxel. J. Pharm. Sci. 2019, 108, 1984–1990. [Google Scholar] [CrossRef] [PubMed]
- Sandeep, N.; Gupta, M.M. Immediate drug release dosage form: A review introduction. J. Drug Deliv. Ther. 2011, 2013, 155–161. [Google Scholar]
- Bruschi, M.L. Main mechanisms to control the drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Elsevier: Amsterdam, The Netherlands, 2015; pp. 37–62. [Google Scholar]
- Salamanca, C.H.; Yarce, C.J.; Moreno, R.A.; Prieto, V.; Recalde, J. Natural gum-type biopolymers as potential modified nonpolar drug release systems. Carbohydr. Polym. 2018, 189, 31–38. [Google Scholar] [CrossRef]
- Harting, R.; Johnston, K.; Petersen, S. Correlating in vitro degradation and drug release kinetics of biopolymer-based drug delivery systems. Int. J. Biobased Plast. 2019, 1, 8–21. [Google Scholar] [CrossRef]
- Siegel, R.A.; Rathbone, M.J. Overview of controlled release mechanisms. In Fundamentals and Applications of Controlled Release Drug Delivery; Springer: New York, NY, USA, 2012; pp. 19–43. ISBN 9781461408819. [Google Scholar]
- Sanopoulou, M.; Papadokostaki, K.G. Controlled drug release systems: Mechanisms and kinetics (Chapter 1). In Biomedical Membranes and (Bio)artificial Organs; Stamatialis, D., Ed.; World Scientific: Singapore, 2018; pp. 1–33. [Google Scholar]
- Jeevanandam, J.; Pan, S.; Rodrigues, J.; Elkodous, M.A.; Danquah, M.K. Medical applications of biopolymer nanofibers. Biomater. Sci. 2022, 10, 4107–4118. [Google Scholar] [CrossRef]
- Salkho, N.M.; Awad, N.S.; Pitt, W.G.; Husseini, G.A. Photo-induced drug release from polymeric micelles and liposomes: Phototriggering mechanisms in drug-delivery systems. Polymers 2022, 14, 1286. [Google Scholar] [CrossRef]
- Amin, M.; Huang, W.; Seynhaeve, A.L.B.; Ten Hagen, T.L.M. Hyperthermia and temperature-sensitive nanomaterials for spatiotemporal drug delivery to solid tumors. Pharmaceutics 2020, 12, 1–23. [Google Scholar] [CrossRef]
- Elnahtawy, A.I.; Elshafei, N.S.; Elzoghby, A.O. Marine polymer-based nano-carriers for drug delivery applications. In Marine Biomaterials; Jana, S., Ed.; Springer: Singapore, 2022; pp. 15–59. [Google Scholar]
- Wu, J.; Shaidani, S.; Theodossiou, S.K.; Hartzell, E.J.; Kaplan, D.L. Localized, on-demand, sustained drug delivery from biopolymer-based materials. Expert. Opin Drug Deliv. 2022, 19, 1317–1335. [Google Scholar] [CrossRef]
- Olvera, D.; Monaghan, M.G. Electroactive material-based biosensors for detection and drug delivery. Adv. Drug Deliv. Rev. 2021, 170, 396–424. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).