Foam-Mat Freeze Drying of Kiwiberry (Actinidia arguta) Pulp: Drying Kinetics, Main Properties and Microstructure
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Technological Processing
2.2.1. Foam Preparation
2.2.2. Drying Process
2.3. Quality Assessment
2.3.1. Dry Matter Content and Water Activity
2.3.2. Hygroscopicity
2.3.3. Water Solubility Index and Water Absorption Index
2.3.4. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3.5. Microstructure
Scanning Electron Microscopy (SEM)
X-ray Micro-Computed Tomography (XRCT)
2.4. Mathematical Modelling
2.5. Statistical Analysis
3. Results and Discussion
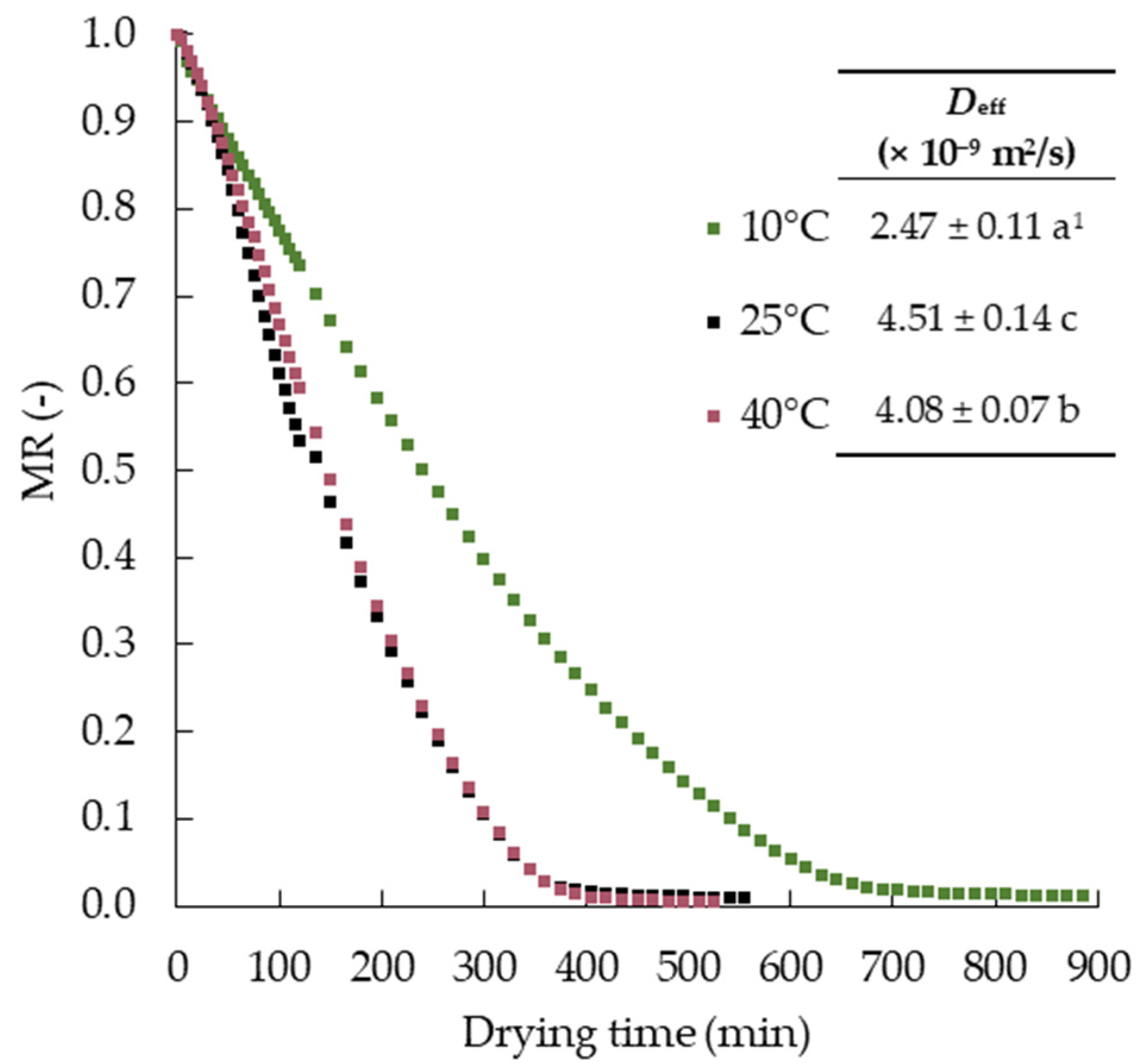
3.1. Drying Kinetics
3.2. Dry Matter Content and Water Activity
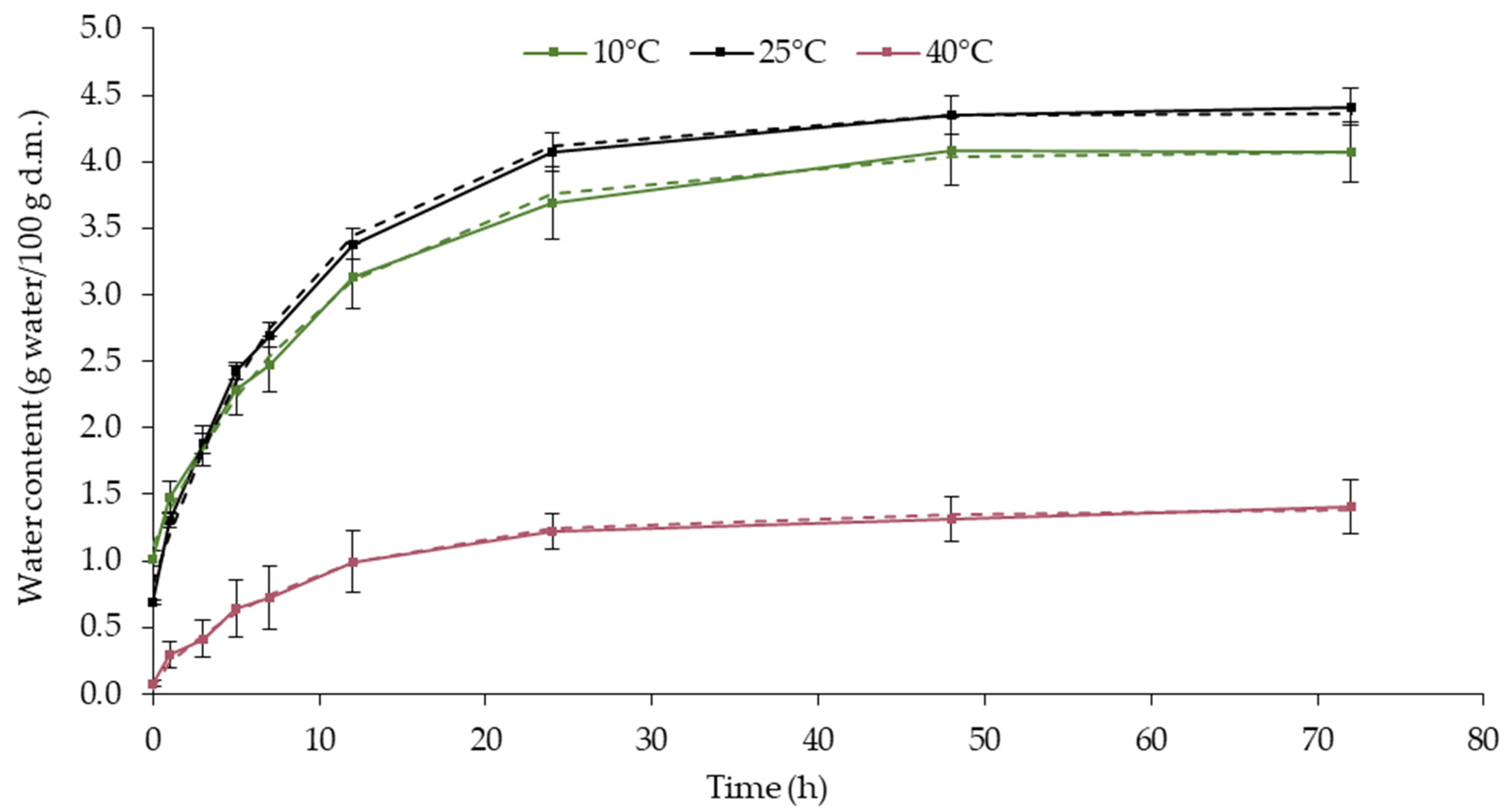
3.3. Hygroscopicity
3.4. Water Solubility Index and Water Absorption Index
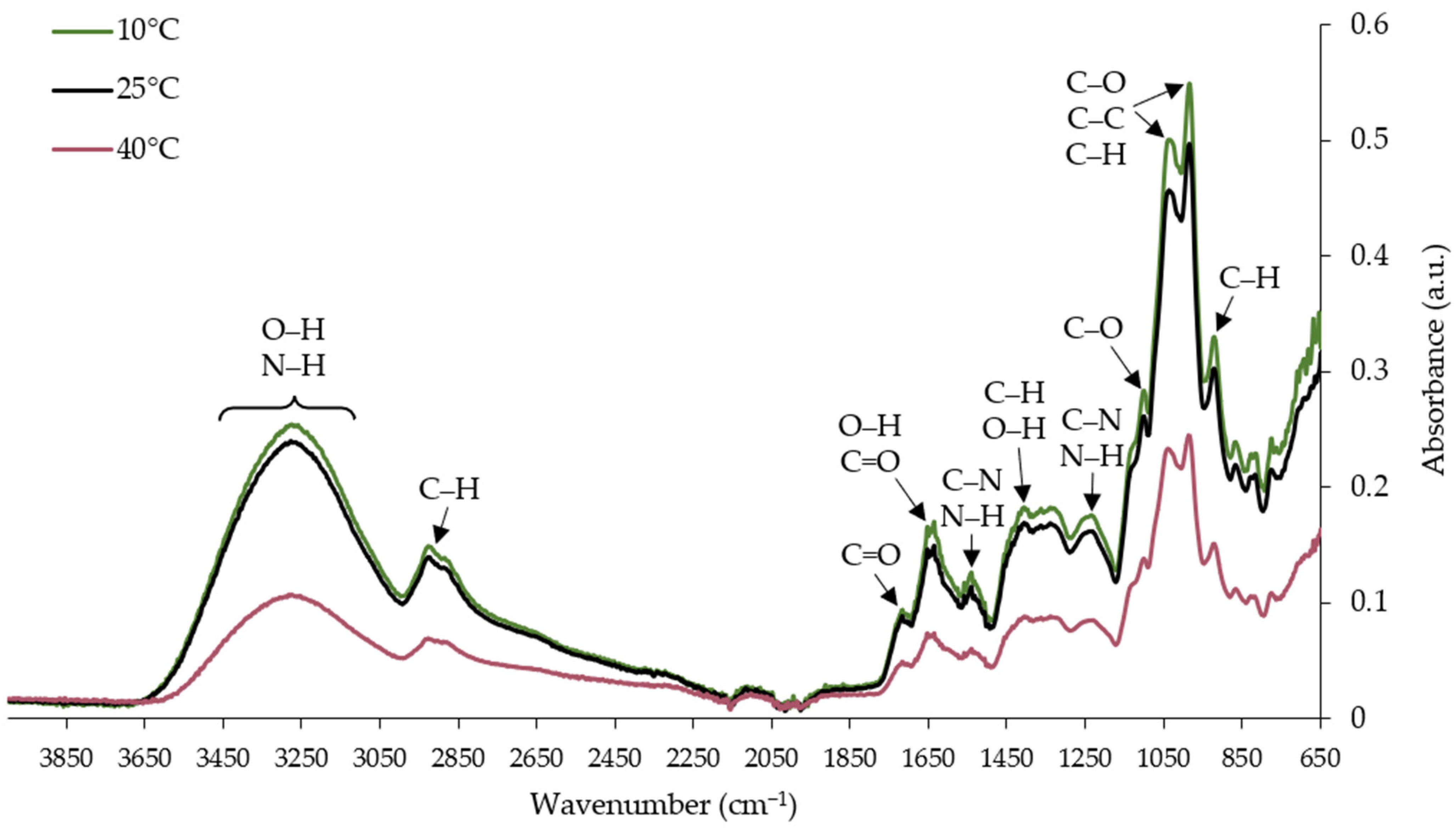
3.5. FTIR Spectra
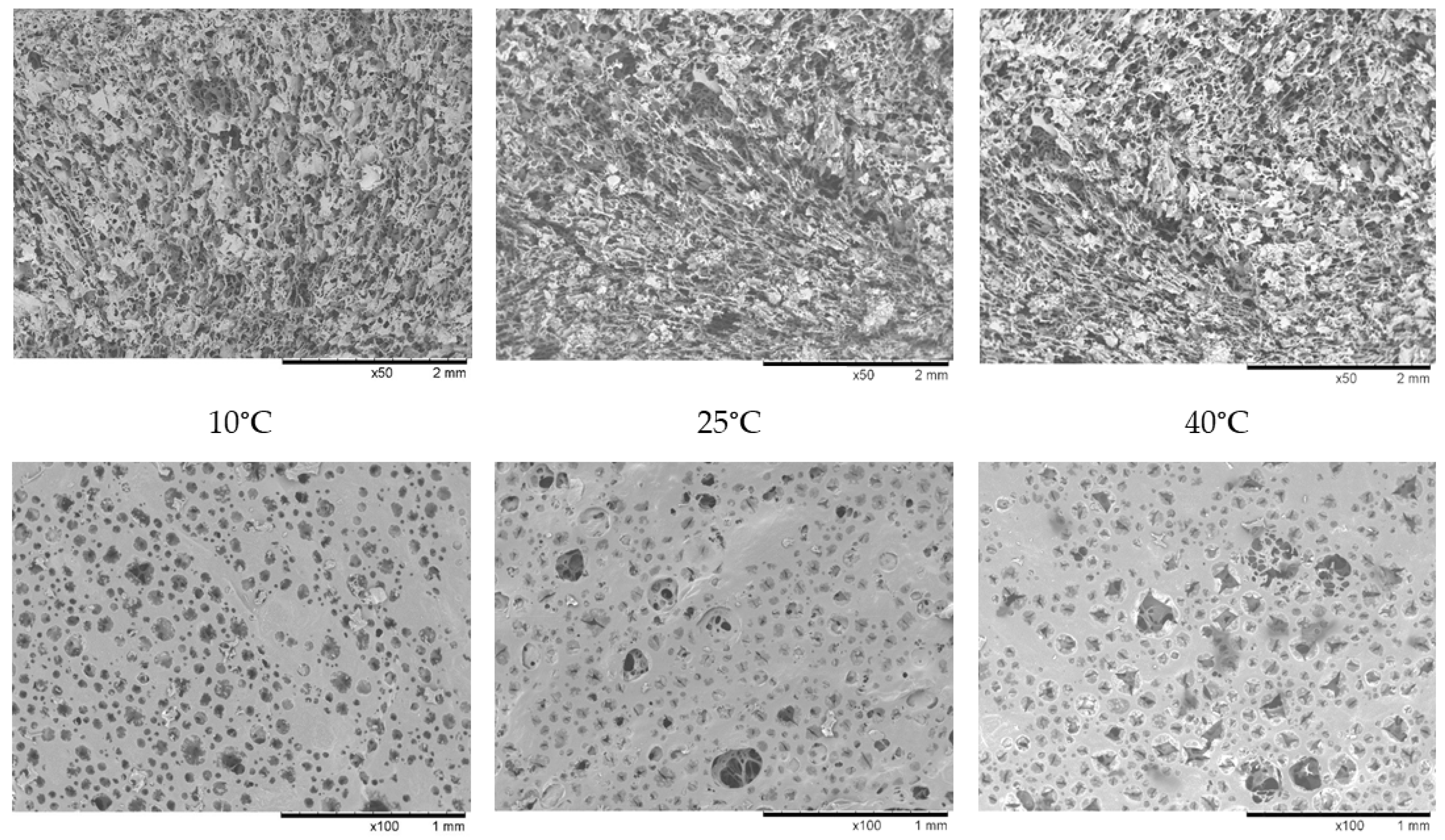
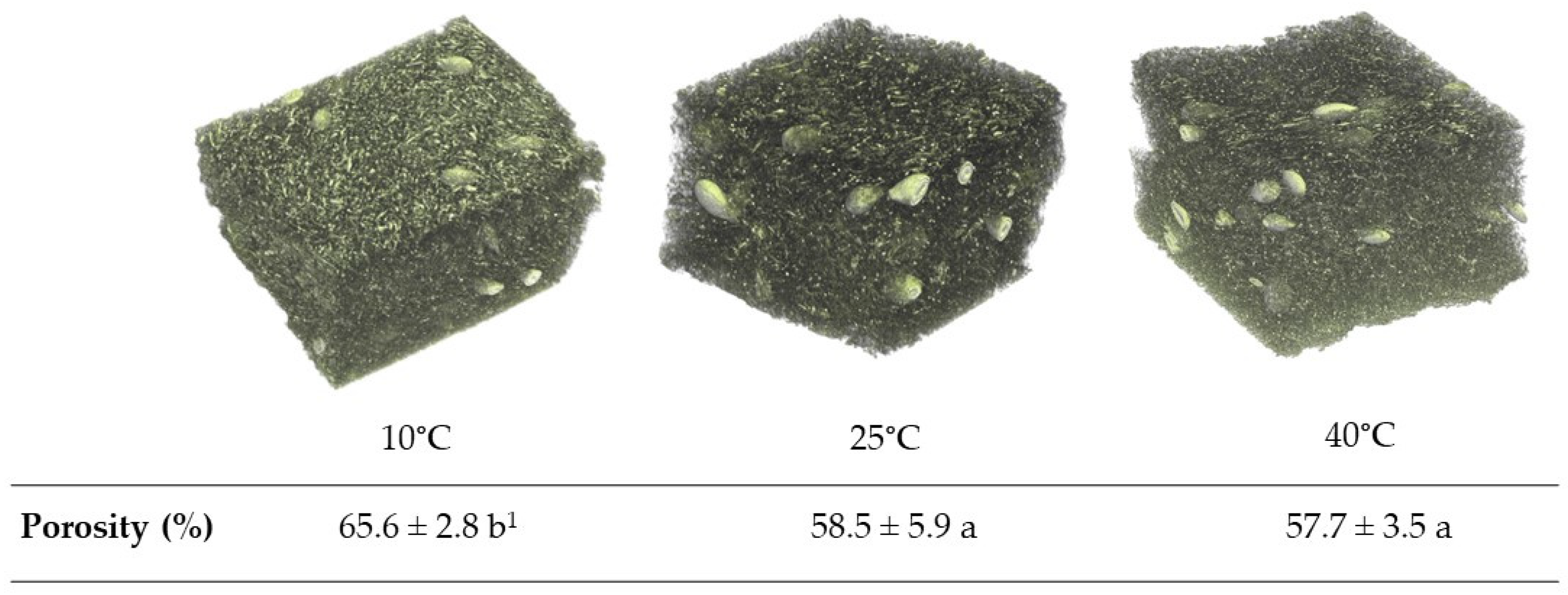
3.6. Microstructure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kostyra, E.; Król, K.; Knysak, D.; Piotrowska, A.; Żakowska-Biemans, S.; Latocha, P. Characteristics of Volatile Compounds and Sensory Properties of Mixed Organic Juices Based on Kiwiberry Fruits. Appl. Sci. 2021, 11, 529. [Google Scholar] [CrossRef]
- Bialik, M.; Wiktor, A.; Latocha, P.; Gondek, E. Mass Transfer in Osmotic Dehydration of Kiwiberry: Experimental and Mathematical Modelling Studies. Molecules 2018, 23, 1236. [Google Scholar] [CrossRef] [PubMed]
- Latocha, P. The Nutritional and Health Benefits of Kiwiberry (Actinidia arguta)—A Review. Plant Foods Hum. Nutr. 2017, 72, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Szpadzik, E.; Zaraś-Januszkiewicz, E.; Krupa, T. Storage Quality Characteristic of Two Minikiwi Fruit (Actinidia arguta (Siebold & Zucc.) Planch. Ex Miq.) Cultivars: ‘Ananasnaya’ and ‘Bingo’—A New One Selected in Poland. Agronomy 2021, 11, 134. [Google Scholar] [CrossRef]
- Latocha, P.; Debersaques, F.; Hale, I. Actinidia Arguta (Kiwiberry): Botany, Production, Genetics, Nutritional Value, and Postharvest Handling. In Horticultural Reviews; Ian Warrington, Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2021; Volume 48, pp. 37–152. ISBN 9781119750802. [Google Scholar]
- Kaveh, M.; Abbaspour-Gilandeh, Y.; Nowacka, M. Optimisation of Microwave-Rotary Drying Process and Quality Parameters of Terebinth. Biosyst. Eng. 2021, 208, 113–130. [Google Scholar] [CrossRef]
- Bialik, M.; Gondek, E.; Wiktor, A.; Latocha, P.; Witrowa-Rajchert, D. Mathematical Modeling of Actinidia Arguta (Kiwiberry) Drying Kinetics. Agric. Eng. 2017, 21, 5–13. [Google Scholar] [CrossRef]
- Dadan, M.; Nowacka, M. The Assessment of the Possibility of Using Ethanol and Ultrasound to Design the Properties of Dried Carrot Tissue. Appl. Sci. 2021, 11, 689. [Google Scholar] [CrossRef]
- Xie, L.; Mujumdar, A.S.; Fang, X.M.; Wang, J.; Dai, J.W.; Du, Z.L.; Xiao, H.W.; Liu, Y.; Gao, Z.J. Far-Infrared Radiation Heating Assisted Pulsed Vacuum Drying (FIR-PVD) of Wolfberry (Lycium barbarum L.): Effects on Drying Kinetics and Quality Attributes. Food Bioprod. Process. 2017, 102, 320–331. [Google Scholar] [CrossRef]
- Piotrowski, D.; Kostyra, E.; Grzegory, P.; Janiszewska-Turak, E. Influence of Drying Methods on the Structure, Mechanical and Sensory Properties of Strawberries. Eur. Food Res. Technol. 2021, 247, 1859–1867. [Google Scholar] [CrossRef]
- Buljat, A.M.; Jurina, T.; Jurinjak Tušek, A.; Valinger, D.; Gajdoš Kljusurić, J.; Benković, M. Applicability of Foam Mat Drying Process for Production of Instant Cocoa Powder Enriched with Lavender Extract. Food Technol. Biotechnol. 2019, 57, 159–170. [Google Scholar] [CrossRef]
- Wiktor, A.; Witrowa-Rajchert, D. Drying Kinetics and Quality of Carrots Subjected to Microwave-Assisted Drying Preceded by Combined Pulsed Electric Field and Ultrasound Treatment. Dry. Technol. 2020, 38, 176–188. [Google Scholar] [CrossRef]
- Darniadi, S.; Ifie, I.; Luna, P.; Ho, P.; Murray, B.S. Foam-Mat Freeze-Drying of Blueberry Juice by Using Trehalose-β-Lactoglobulin and Trehalose-Bovine Serum Albumin as Matrices. Food Bioprocess Technol. 2020, 13, 988–997. [Google Scholar] [CrossRef]
- Kumar, P.S.; Keran, D.A.; Pushpavalli, S.; Shiva, K.N.; Uma, S. Effect of Cellulose and Gum Derivatives on Physicochemical, Microstructural and Prebiotic Properties of Foam-Mat Dried Red Banana Powder. Int. J. Biol. Macromol. 2022, 218, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Bogusz, R.; Wiktor, A.; Nowacka, M.; Ćwintal, J.; Gondek, E. Foam-Mat Convective Drying of Kiwiberry (Actinidia arguta) Pulp. Czech. J. Food Sci. 2022, 40, 187–194. [Google Scholar] [CrossRef]
- Dehghannya, J.; Pourahmad, M.; Ghanbarzadeh, B.; Ghaffari, H. Heat and Mass Transfer Enhancement during Foam-Mat Drying Process of Lime Juice: Impact of Convective Hot Air Temperature. Int. J. Therm. Sci. 2019, 135, 30–43. [Google Scholar] [CrossRef]
- Jakubczyk, E.; Ostrowska-Ligeza, E.; Gondek, E. Moisture Sorption Characteristics and Glass Transition Temperature of Apple Puree Powder. Int. J. Food Sci. Technol. 2010, 45, 2515–2523. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, M.; Mujumdar, A.S.; Liu, W.; Yang, C. Innovative Applications of Freeze-Drying to Produce Compound Formula Instant Foods: A Review. Dry. Technol. 2021, 40, 2583–2597. [Google Scholar] [CrossRef]
- Fongin, S.; Alvino Granados, A.E.; Harnkarnsujarit, N.; Hagura, Y.; Kawai, K. Effects of Maltodextrin and Pulp on the Water Sorption, Glass Transition, and Caking Properties of Freeze-Dried Mango Powder. J. Food Eng. 2019, 247, 95–103. [Google Scholar] [CrossRef]
- Calıskan, G.; Ergun, K.; Dirim, S.N. Freeze Drying of Kiwi (Actinidia deliciosa) Puree and the Powder Properties. Ital. J. Food Sci. 2015, 27, 385–396. [Google Scholar] [CrossRef]
- Watharkar, R.B.; Chakraborty, S.; Srivastav, P.P.; Srivastava, B. Foaming and Foam Mat Drying Characteristics of Ripe Banana [Musa Balbisiana (BB)] Pulp. J. Food Process. Eng. 2021, e13726. [Google Scholar] [CrossRef]
- Silva-Espinoza, M.A.; Ayed, C.; Foster, T.; Del Mar Camacho, M.; Martínez-Navarrete, N. The Impact of Freeze-Drying Conditions on the Physico-Chemical Properties and Bioactive Compounds of a Freeze-Dried Orange Puree. Foods 2020, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Nowak, D.; Jakubczyk, E. The Freeze-Drying of Foods—The Characteristic of the Process Course and the E Ff Ect of Its Parameters On. Foods 2020, 9, 1488. [Google Scholar] [CrossRef] [PubMed]
- Darniadi, S.; Ifie, I.; Ho, P.; Murray, B.S. Evaluation of Total Monomeric Anthocyanin, Total Phenolic Content and Individual Anthocyanins of Foam-Mat Freeze-Dried and Spray-Dried Blueberry Powder. J. Food Meas. Charact. 2019, 13, 1599–1606. [Google Scholar] [CrossRef]
- Nowak, D. The Impact of an Inert Gas Atmosphere on the Kinetics of Changes in the Physical and Chemical Properties of Carrot Lyophilisate. Int. J. Food Eng. 2019, 16, 20180414. [Google Scholar] [CrossRef]
- Ciurzyńska, A.; Popkowicz, P.; Galus, S.; Janowicz, M. Innovative Freeze-Dried Snacks with Sodium Alginate and Fruit Pomace (Only Apple or Only Chokeberry) Obtained within the Framework of Sustainable Production. Molecules 2022, 27, 3095. [Google Scholar] [CrossRef] [PubMed]
- Sramek, M.; Schweiggert, R.M.; van Kampen, A.; Carle, R.; Kohlus, R. Preparation of High-Grade Powders from Tomato Paste Using a Vacuum Foam Drying Method. J. Food Sci. 2015, 80, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Egas-Astudillo, L.A.; Martínez-Navarrete, N.; Camacho, M.M. Impact of Biopolymers Added to a Grapefruit Puree and Freeze-Drying Shelf Temperature on Process Time Reduction and Product Quality. Food Bioprod. Process 2020, 120, 143–150. [Google Scholar] [CrossRef]
- Lang, S.; Ozcelik, M.; Kulozik, U.; Steinhaus, M. Processing of Raspberries to Dried Fruit Foam: Impact on Major Odorants. Eur. Food Res. Technol. 2020, 246, 2537–2548. [Google Scholar] [CrossRef]
- Seerangurayar, T.; Manickavasagan, A.; Al-Ismaili, A.M.; Al-Mulla, Y.A. Effect of Carrier Agents on Physicochemical Properties of Foam-Mat Freeze-Dried Date Powder. Dry. Technol. 2018, 36, 1292–1303. [Google Scholar] [CrossRef]
- Seerangurayar, T.; Manickavasagan, A.; Al-Ismaili, A.M.; Al-Mulla, Y.A. Effect of Carrier Agents on Flowability and Microstructural Properties of Foam-Mat Freeze Dried Date Powder. J. Food Eng. 2017, 215, 33–43. [Google Scholar] [CrossRef]
- Ozcelik, M.; Ambros, S.; Morais, S.I.F.; Kulozik, U. Storage Stability of Dried Raspberry Foam as a Snack Product: Effect of Foam Structure and Microwave-Assisted Freeze Drying on the Stability of Plant Bioactives and Ascorbic Acid. J. Food Eng. 2020, 270, 109779. [Google Scholar] [CrossRef]
- de Carvalho Tavares, I.M.; de Castilhos, M.B.M.; Aparecida Mauro, M.; Mota Ramos, A.; Teodoro de Souza, R.; Gómez-Alonso, S.; Gomes, E.; Da-Silva, R.; Hermosín-Gutiérrez, I.; Silva Lago-Vanzela, E. BRS Violeta (BRS Rúbea × IAC 1398-21) Grape Juice Powder Produced by Foam Mat Drying. Part I: Effect of Drying Temperature on Phenolic Compounds and Antioxidant Activity. Food Chem. 2019, 298, 124971. [Google Scholar] [CrossRef] [PubMed]
- Santacruz-Vázquez, V.; Santacruz-Vázquez, C.; Laguna Cortés, J.O. Physical Characterization of Freeze-Dried Foam Prepared from Aloe Vera Gel and Guar Gum. Rev. Vitae 2015, 22, 75–86. [Google Scholar] [CrossRef]
- Jedlińska, A.; Samborska, K.; Wiktor, A.; Balik, M.; Derewiaka, D.; Matwijczuk, A.; Gondek, E. Spray Drying of Pure Kiwiberry Pulp in Dehumidified Air. Dry. Technol. 2021, 40, 1421–1435. [Google Scholar] [CrossRef]
- Barańska, A.; Jedlińska, A.; Samborska, K. Is It Possible to Produce Carrier-Free Fruit and Vegetable Powders by Spray Drying? Pol. J. Food Nutr. Sci. 2023, 73, 214–223. [Google Scholar] [CrossRef]
- Bogusz, R.; Pobiega, K.; Rybak, K.; Wiktor, A.; Parniakov, O.; Smetana, S.; Nowacka, M. The Pulsed Electric Field Treatment Effect on Drying Kinetics and Chosen Quality Aspects of Freeze-Dried Black Soldier Fly (Hermetia illucens) and Yellow Mealworm (Tenebrio Molitor) Larvae. Appl. Sci. 2023, 13, 10251. [Google Scholar] [CrossRef]
- Kandasamy, P.; Varadharaju, N.; Kalemullah, S.; Maladhi, D. Optimization of Process Parameters for Foam-Mat Drying of Papaya Pulp. J. Food Sci. Technol. 2014, 51, 2526–2534. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, P.; Varadharaju, N.; Dhakre, D.S.; Smritikana, S. Assessment of Physicochemical and Sensory Characteristics of Foam-Mat Dried Papaya Fruit Powder. Int. Food Res. J. 2019, 26, 819–829. [Google Scholar]
- Wilson, R.A.; Kadam, D.M.; Chadha, S.; Sharma, M. Foam Mat Drying Characteristics of Mango Pulp. Int. J. Food Sci. Nutr. Eng. 2012, 2, 63–69. [Google Scholar] [CrossRef]
- Deng, L.Z.; Yang, X.H.; Mujumdar, A.S.; Zhao, J.H.; Wang, D.; Zhang, Q.; Wang, J.; Gao, Z.J.; Xiao, H.W. Red Pepper (Capsicum annuum L.) Drying: Effects of Different Drying Methods on Drying Kinetics, Physicochemical Properties, Antioxidant Capacity, and Microstructure. Dry. Technol. 2018, 36, 893–907. [Google Scholar] [CrossRef]
- Dehghannya, J.; Pourahmad, M.; Ghanbarzadeh, B.; Ghaffari, H. Influence of Foam Thickness on Production of Lime Juice Powder during Foam-Mat Drying: Experimental and Numerical Investigation. Powder Technol. 2018, 328, 470–484. [Google Scholar] [CrossRef]
- Salahi, M.R.; Mohebbi, M.; Taghizadeh, M. Development of Cantaloupe (Cucumis melo) Pulp Powder Using Foam-Mat Drying Method: Effects of Drying Conditions on Microstructural of Mat and Physicochemical Properties of Powder. Dry. Technol. 2017, 35, 1897–1908. [Google Scholar] [CrossRef]
- de Cól, C.D.; Tischer, B.; Hickmann Flôres, S.; Rech, R. Foam-Mat Drying of Bacaba (Oenocarpus bacaba): Process Characterization, Physicochemical Properties, and Antioxidant Activity. Food Bioprod. Process. 2021, 126, 23–31. [Google Scholar] [CrossRef]
- Qadri, T.; Naik, H.R.; Hussain, S.Z.; Naseer, B.; Bhat, T.; Vijaykumar; Wani, F.J. Spray Dried Apple Powder: Qualitative, Rheological, Structural Characterization and Its Sorption Isotherm. LWT 2022, 165, 113694. [Google Scholar] [CrossRef]
- Wanderley, R.d.O.S.; de Figueirêdo, R.M.F.; Queiroz, A.J.d.M.; dos Santos, F.S.; Silva, A.P.d.F.; Paiva, Y.F.; Moura, H.V.; Silva, E.T.d.V.; Carvalho, A.J.d.B.A.; Lima, M.d.S.; et al. Effect of Drying Temperature on Antioxidant Activity, Phenolic Compound Profile and Hygroscopic Behavior of Pomegranate Peel and Seed Flours. LWT 2023, 189, 115514. [Google Scholar] [CrossRef]
- Franco, T.S.; Perussello, C.A.; Ellendersen, L.N.; Masson, M.L. Effects of Foam Mat Drying on Physicochemical and Microstructural Properties of Yacon Juice Powder. LWT—Food Sci. Technol. 2016, 66, 503–513. [Google Scholar] [CrossRef]
- Karwacka, M.; Rybak, K.; Smetana, S.; Galus, S.; Janowicz, M. Analysis of Selected Functional Properties, Resource Demands, and Energy Consumption of Freeze-dried Vegetable Snacks. J. Food Process. Preserv. 2022, 46, e16721. [Google Scholar] [CrossRef]
- Rin-ut, S.; Rattanapitigorn, P. Effect of Foaming Agents on Process Conditions, Characteristics, and Stability of Foam-mat Freeze-dried Pandan (Pandanus amaryllifolius) Powder. J. Food Process. Preserv. 2020, 44, e14690. [Google Scholar] [CrossRef]
- Vimercati, W.C.; Araújo, C.d.S.; Macedo, L.L.; Correa, J.L.G.; Pimenta, C.J. Encapsulation of Coffee Silverskin Extracts by Foam Mat Drying and Comparison with Powders Obtained by Spray Drying and Freeze-drying. J. Food Sci. 2022, 87, 1767–1779. [Google Scholar] [CrossRef]
- Asokapandian, S.; Venkatachalam, S.; Swamy, G.J.; Kuppusamy, K. Optimization of Foaming Properties and Foam Mat Drying of Muskmelon Using Soy Protein. J. Food Process. Eng. 2016, 39, 692–701. [Google Scholar] [CrossRef]
- Wilson, R.A.; Kadam, D.M.; Chadha, S.; Grewal, M.K.; Sharma, M. Evaluation of Physical and Chemical Properties of Foam-Mat Dried Mango (Mangifera indica) Powder during Storage. J. Food Process. Preserv. 2014, 38, 1866–1874. [Google Scholar] [CrossRef]
- Baranowska-Wójcik, E.; Szwajgier, D. Characteristics and Pro-Health Properties of Mini Kiwi (Actinidia arguta). Hortic Env. Biotechnol 2019, 60, 217–225. [Google Scholar] [CrossRef]
- Panato, K.; Muller, C.M.O. Drying Kinetics and Physicochemical and Technological Properties of Pumpkin Purée Flour Dried by Convective and Foam-Mat Drying. Food Process. Preserv. 2022, 46, e16264. [Google Scholar] [CrossRef]
- Rybak, K.; Wiktor, A.; Kaveh, M.; Dadan, M.; Witrowa-Rajchert, D.; Nowacka, M. Effect of Thermal and Non-Thermal Technologies on Kinetics and the Main Quality Parameters of Red Bell Pepper Dried with Convective and Microwave–Convective Methods. Molecules 2022, 27, 2164. [Google Scholar] [CrossRef]
- Samborska, K.; Jedlińska, A.; Wiktor, A.; Derewiaka, D.; Wołosiak, R.; Matwijczuk, A.; Jamróz, W.; Skwarczyńska-Maj, K.; Kiełczewski, D.; Błażowski, Ł.; et al. The Effect of Low-Temperature Spray Drying with Dehumidified Air on Phenolic Compounds, Antioxidant Activity, and Aroma Compounds of Rapeseed Honey Powders. Food Bioproc Tech 2019, 12, 919–932. [Google Scholar] [CrossRef]
- Shanmugavel, D.; KumarYadav, P.; Khadimallah, M.A.; Ramadoss, R. Experimental Analysis on the Performance of Egg Albumen as a Sustainable Bio Admixture in Natural Hydraulic Lime Mortars. J. Clean. Prod. 2021, 320, 128736. [Google Scholar] [CrossRef]
- Durazzo, A.; Kiefer, J.; Lucarini, M.; Camilli, E.; Marconi, S.; Gabrielli, P.; Aguzzi, A.; Gambelli, L.; Lisciani, S.; Marletta, L. Qualitative Analysis of Traditional Italian Dishes: FTIR Approach. Sustainability 2018, 10, 4112. [Google Scholar] [CrossRef]
- Uncu, O.; Ozen, B. A Comparative Study of Mid-Infrared, UV–Visible and Fluorescence Spectroscopy in Combination with Chemometrics for the Detection of Adulteration of Fresh Olive Oils with Old Olive Oils. Food Control. 2019, 105, 209–218. [Google Scholar] [CrossRef]
- Canteri, M.H.G.; Renard, C.M.G.C.; Le Bourvellec, C.; Bureau, S. ATR-FTIR Spectroscopy to Determine Cell Wall Composition: Application on a Large Diversity of Fruits and Vegetables. Carbohydr. Polym. 2019, 212, 186–196. [Google Scholar] [CrossRef]
- Edelmann, A.; Diewok, J.; Schuster, K.C.; Lendl, B. Rapid Method for the Discrimination of Red Wine Cultivars Based on Mid-Infrared Spectroscopy of Phenolic Wine Extracts. J. Agric. Food Chem. 2001, 49, 1139–1145. [Google Scholar] [CrossRef]
- Bureau, S.; Cozzolino, D.; Clark, C.J. Contributions of Fourier-Transform Mid Infrared (FT-MIR) Spectroscopy to the Study of Fruit and Vegetables: A Review. Postharvest Biol. Technol. 2019, 148, 1–14. [Google Scholar] [CrossRef]
- Azizpour, M.; Mohebbi, M.; Khodaparast, M.H.H. Effects of Foam-Mat Drying Temperature on Physico-Chemical and Microstructural Properties of Shrimp Powder. Innov. Food Sci. Emerg. Technol. 2016, 34, 122–126. [Google Scholar] [CrossRef]
- Bialik, M.; Wiktor, A.; Witrowa-Rajchert, D.; Samborska, K.; Gondek, E.; Findura, P. Osmotic Dehydration and Freezing Pretreatment for Vacuum Dried of Kiwiberry: Drying Kinetics and Microstructural Changes. Int. Agrophysics 2020, 34, 265–272. [Google Scholar] [CrossRef]

| Drying Temperature (°C) | Dry Matter Content (%) | Water Activity (-) |
|---|---|---|
| 10 | 97.2 ± 0.1 a 1 | 0.221 ± 0.003 c |
| 25 | 98.2 ± 0.1 b | 0.180 ± 0.003 b |
| 40 | 99.6 ± 0.1 c | 0.159 ± 0.005 a |
| Drying Temperature (°C) | Equilibrium Water Content (g water/100 g d.m.) | Effective Moisture Diffusivity (m2/min) | R2 | χ2 | RMS (%) |
|---|---|---|---|---|---|
| 10 | 4.07 ± 0.24 b 1 | 0.97 × 10−9 ± 0.10 a | 0.997 | 0.005 | 4.20 ± 0.15 |
| 25 | 4.37 ± 0.20 b | 1.17 × 10−8 ± 0.10 b | 0.997 | 0.007 | 7.17 ± 0.60 |
| 40 | 1.59 ± 0.25 a | 1.06 × 10−8 ± 0.01 a | 0.993 | 0.001 | 13.11 ± 5.29 |
| Drying Temperature (°C) | Water Solubility Index (%) | Water Absorption Index (-) |
|---|---|---|
| 10 | 62.68 ± 1.05 a 1 | 1.53 ± 0.03 a |
| 25 | 64.31 ± 0.67 ab | 1.55 ± 0.06 a |
| 40 | 67.55 ± 3.38 b | 1.73 ± 0.10 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogusz, R.; Nowacka, M.; Rybak, K.; Witrowa-Rajchert, D.; Gondek, E. Foam-Mat Freeze Drying of Kiwiberry (Actinidia arguta) Pulp: Drying Kinetics, Main Properties and Microstructure. Appl. Sci. 2024, 14, 5629. https://doi.org/10.3390/app14135629
Bogusz R, Nowacka M, Rybak K, Witrowa-Rajchert D, Gondek E. Foam-Mat Freeze Drying of Kiwiberry (Actinidia arguta) Pulp: Drying Kinetics, Main Properties and Microstructure. Applied Sciences. 2024; 14(13):5629. https://doi.org/10.3390/app14135629
Chicago/Turabian StyleBogusz, Radosław, Małgorzata Nowacka, Katarzyna Rybak, Dorota Witrowa-Rajchert, and Ewa Gondek. 2024. "Foam-Mat Freeze Drying of Kiwiberry (Actinidia arguta) Pulp: Drying Kinetics, Main Properties and Microstructure" Applied Sciences 14, no. 13: 5629. https://doi.org/10.3390/app14135629
APA StyleBogusz, R., Nowacka, M., Rybak, K., Witrowa-Rajchert, D., & Gondek, E. (2024). Foam-Mat Freeze Drying of Kiwiberry (Actinidia arguta) Pulp: Drying Kinetics, Main Properties and Microstructure. Applied Sciences, 14(13), 5629. https://doi.org/10.3390/app14135629







