The authors would like to make the following corrections to this paper [1]:
Error in Figure
In the original publication, there was a mistake in Figure 6a as published. A calculation error caused the bacteriophage titers given to be one decade too high. The corrected Figure 6a appears below.
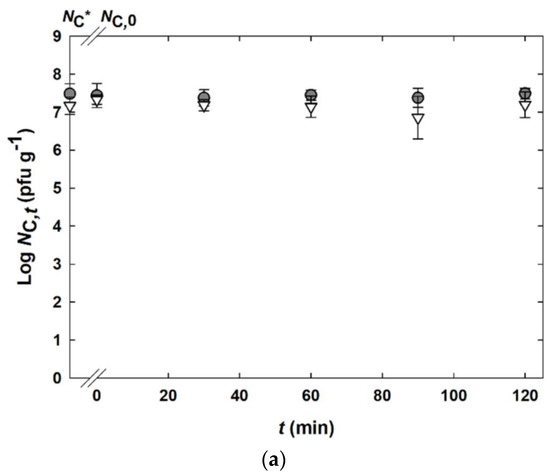
Figure 6.
Static in vitro digestion of bacteriophage K5 capsules composed of alginate and micellar casein ( ) or alginate and whey protein isolate (
) or alginate and whey protein isolate ( ). (a) Incubation in simulated gastric fluid (SGF). Results are given as bacteriophage titer of solid capsules (NC in pfu g–1).
). (a) Incubation in simulated gastric fluid (SGF). Results are given as bacteriophage titer of solid capsules (NC in pfu g–1).
 ) or alginate and whey protein isolate (
) or alginate and whey protein isolate ( ). (a) Incubation in simulated gastric fluid (SGF). Results are given as bacteriophage titer of solid capsules (NC in pfu g–1).
). (a) Incubation in simulated gastric fluid (SGF). Results are given as bacteriophage titer of solid capsules (NC in pfu g–1).
Error in Table
In the original publication, there was a mistake in Table 2 as published. The calculation of the encapsulation efficiency was repeated by using the absolute (non-logarithmized) bacteriophage titers instead of the logarithmized values. The corrected Table 2 is presented below.

Table 2.
Analysis of the bacteriophage K5 capsules regarding physical and biological parameters. Capsules were mainly composed of sodium alginate and milk protein (either micellar casein or whey protein isolate).
Text Correction
With regard to the corrected Figure 6a and Table 2 (see above), the text following passages has been adjusted accordingly:
(1) Abstract:
Powdered capsules with particle sizes of ~10 µm and bacteriophage K5 titers of ~107 plaque-forming units (pfu) g−1 were obtained.
(2) Section 2.4.2., Paragraph 1:
The stability of the encapsulated bacteriophages (Section 2.2) with an initial bacteriophage titer NC* = 1.5 × 107–3.0 × 107 pfu g−1 was analyzed in simulated gastric fluid (SGF).
(3) Section 3.4., Paragraph 8:
The most important quality parameter of the capsules is the encapsulation efficiency, which was roughly 4% in this study (Table 2) (calculated with absolute bacteriophage titers; calculation with logarithmized bacteriophage titers would correspond to an encapsulation efficiency of around 80%).
(4) Section 3.4., Paragraph 10:
The capsules produced in this study had bacteriophage titers of around 107 pfu g−1.
The authors apologize for any inconvenience caused and state that the scientific conclusions are unaffected. This correction was approved by the Academic Editor. The original publication has also been updated.
Reference
- Schubert, C.; Fischer, S.; Dorsch, K.; Teßmer, L.; Hinrichs, J.; Atamer, Z. Microencapsulation of Bacteriophages for the Delivery to and Modulation of the Human Gut Microbiota through Milk and Cereal Products. Appl. Sci. 2022, 12, 6299. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).