Microencapsulation of Bacteriophages for the Delivery to and Modulation of the Human Gut Microbiota through Milk and Cereal Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Host Strain and Bacteriophage
2.1.1. Selection and Morphology
2.1.2. Cultivation, Propagation, and Enumeration
2.1.3. Thermal Inactivation of Bacteriophage K5
2.1.4. Acidic Inactivation of Bacteriophage K5
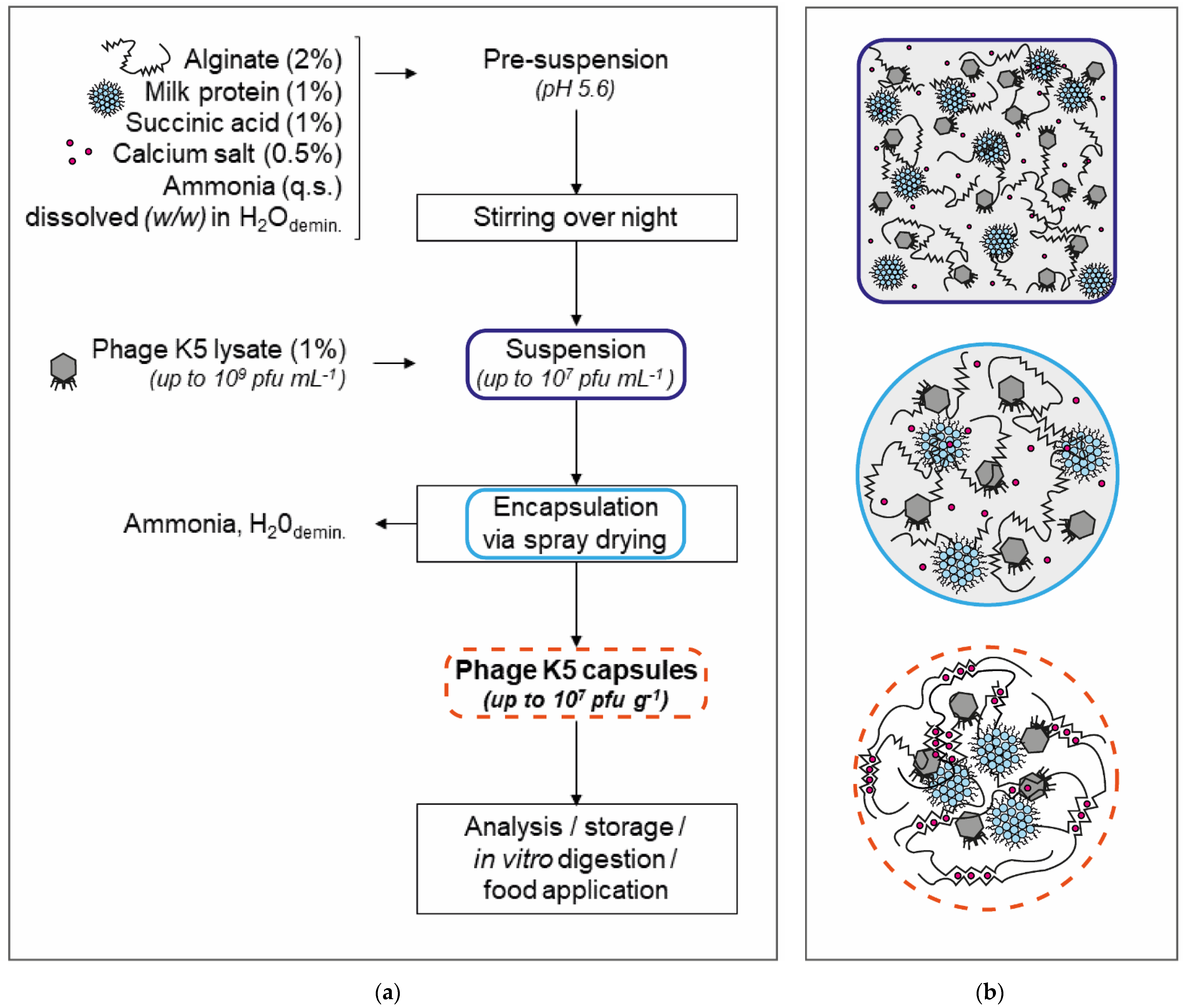
2.2. Encapsulation of Bacteriophage K5 by Spray Drying
2.3. Physical Characterization of Bacteriophage K5 Capsules
2.3.1. Humidity and Water Activity
2.3.2. Particle Size
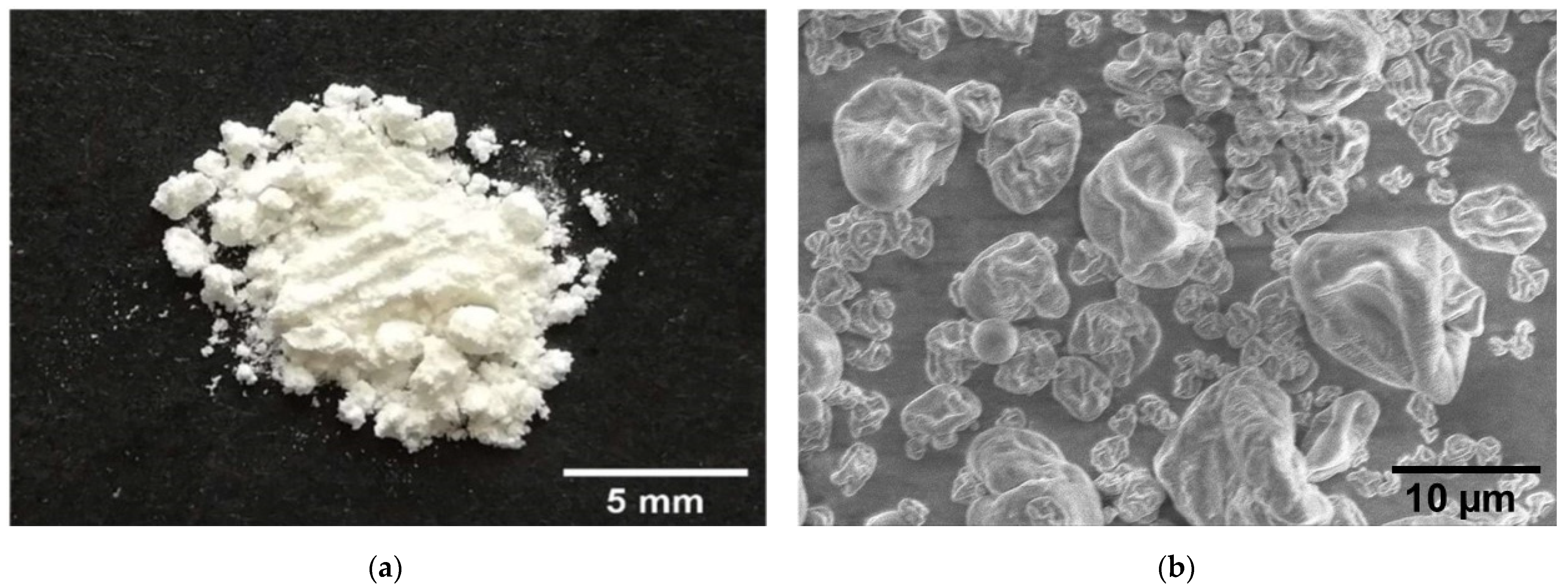
2.3.3. Scanning Electron Microscopy
2.4. Biological Characterization of Bacteriophage K5 Capsules
2.4.1. Capsule Break-Up, Enumeration, and Encapsulation Efficiency
2.4.2. In Vitro Digestion
2.5. Application of Bacteriophage K5 Capsules in Food Matrices
2.5.1. Storage of Capsules
2.5.2. Milk and Cereal Products
2.6. Data Analysis
3. Results and Discussion
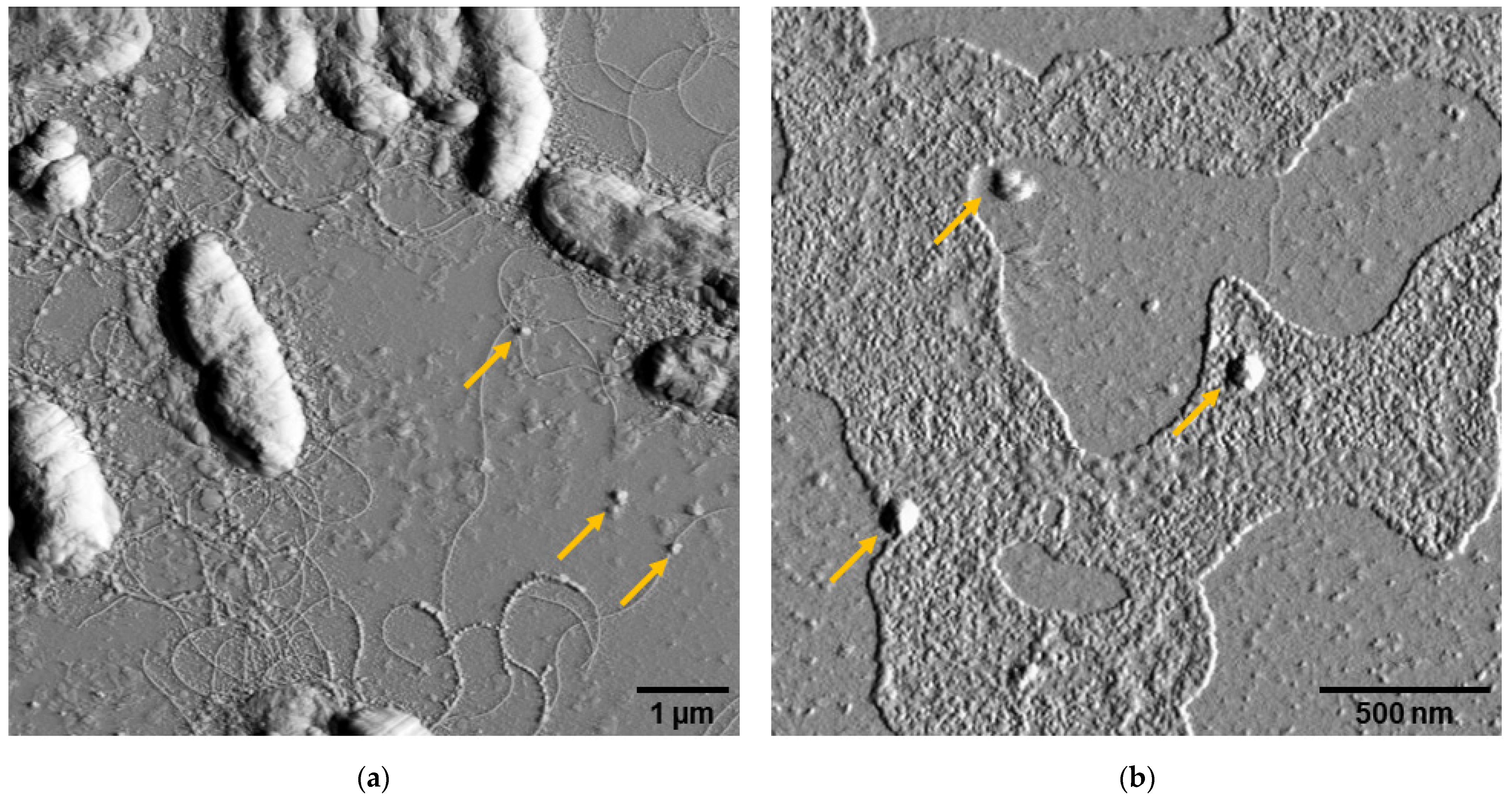
3.1. Morphology of Escherichia coli Nissle 1917 and Bacteriophage K5
3.2. Heat Sensitivity of Bacteriophage K5
3.3. Acid Sensitivity of Bacteriophage K5
3.4. Bacteriophage K5 Capsules Powder and Its Characteristics
3.5. Stability in Simulated Gastric Fluid and Release in Simulated Intestinal Fluid
3.6. Good Survival during Storage at 0 and −20 °C
3.7. Successful Application of Bacteriophage K5 Capsules in Semi-Solid Cereal Matrices and Unsuitable Application in Milk Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schubert, C.; Fischer, S.; Dorsch, K.; Teßmer, L.; Hinrichs, J.; Atamer, Z. Microencapsulation of Bacteriophages for the Delivery to and Modulation of the Human Gut Microbiota through Milk and Cereal Products. Appl. Sci. 2022, 12, 6299. [Google Scholar] [CrossRef]
- O’Hara, A.M.; Shanahan, F. The Gut Flora as a Forgotten Organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Sorboni, S.G.; Moghaddam, H.S.; Jafarzadeh-Esfehani, R.; Soleimanpour, S. A Comprehensive Review on the Role of the Gut Microbiome in Human Neurological Disorders. Clin. Microbiol. Rev. 2022, 35, e00338-20. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the Human Intestinal Microbial Flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Gordon, J.I. The Core Gut Microbiome, Energy Balance and Obesity. J. Physiol. 2009, 587, 4153–4158. [Google Scholar] [CrossRef]
- Lee, J.-H.; Park, J.-H. Host—Microbial Interactions in Metabolic Diseases: From Diet to Immunity. J. Microbiol. 2022, 60, 561–575. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Jernberg, C.; Löfmark, S.; Edlund, C.; Jansson, J.K. Long-Term Impacts of Antibiotic Exposure on the Human Intestinal Microbiota. Microbiology 2010, 156, 3216–3223. [Google Scholar] [CrossRef]
- Dethlefsen, L.; Huse, S.; Sogin, M.L.; Relman, D.A. The Pervasive Effects of an Antibiotic on the Human Gut Microbiota, as Revealed by Deep 16S RRNA Sequencing. PLoS Biol. 2008, 6, e280. [Google Scholar] [CrossRef]
- Schwartz, D.J.; Langdon, A.E.; Dantas, G. Understanding the Impact of Antibiotic Perturbation on the Human Microbiome. Genome Med. 2020, 12, 82. [Google Scholar] [CrossRef]
- Power, S.E.; O’Toole, P.W.; Stanton, C.; Ross, R.P.; Fitzgerald, G.F. Intestinal Microbiota, Diet and Health. Br. J. Nutr. 2014, 111, 387–402. [Google Scholar] [CrossRef] [PubMed]
- McCarville, J.L.; Caminero, A.; Verdu, E.F. Novel Perspectives on Therapeutic Modulation of the Gut Microbiota. Ther. Adv. Gastroenterol. 2016, 9, 580–593. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Mainali, R.; Ahmadi, S.; Wang, S.; Singh, R.; Kavanagh, K.; Kitzman, D.W.; Kushugulova, A.; Marotta, F.; Yadav, H. Gut Microbiome and Aging: Physiological and Mechanistic Insights. Nutr. Heal. Aging 2018, 4, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Zuppi, M.; Hendrickson, H.L.; O’Sullivan, J.M.; Vatanen, T. Phages in the Gut Ecosystem. Front. Cell. Infect. Microbiol. 2022, 11, 822562. [Google Scholar] [CrossRef]
- Kim, M.-S.; Park, E.-J.; Roh, S.W.; Bae, J.-W. Diversity and Abundance of Single-Stranded DNA Viruses in Human Feces. Appl. Environ. Microbiol. 2011, 77, 8062–8070. [Google Scholar] [CrossRef]
- Hoyles, L.; McCartney, A.L.; Neve, H.; Gibson, G.R.; Sanderson, J.D.; Heller, K.J.; van Sinderen, D. Characterization of Virus-like Particles Associated with the Human Faecal and Caecal Microbiota. Res. Microbiol. 2014, 165, 803–812. [Google Scholar] [CrossRef]
- Bikel, S.; López-Leal, G.; Cornejo-Granados, F.; Gallardo-Becerra, L.; García-López, R.; Sánchez, F.; Equihua-Medina, E.; Ochoa-Romo, J.P.; López-Contreras, B.E.; Canizales-Quinteros, S.; et al. Gut DsDNA Virome Shows Diversity and Richness Alterations Associated with Childhood Obesity and Metabolic Syndrome. iScience 2021, 24, 102900. [Google Scholar] [CrossRef]
- Myelnikov, D. An Alternative Cure: The Adoption and Survival of Bacteriophage Therapy in the USSR, 1922–1955. J. Hist. Med. Allied Sci. 2018, 73, 385–411. [Google Scholar] [CrossRef]
- Międzybrodzki, R.; Borysowski, J.; Weber-Dabrowska, B.; Fortuna, W.; Letkiewicz, S.; Szufnarowski, K.; Pawełczyk, Z.; Rogóz, P.; Kłak, M.; Wojtasik, E.; et al. Clinical Aspects of Phage Therapy. Adv. Virus Res. 2012, 83, 73–121. [Google Scholar] [CrossRef]
- Malik, D.J.; Sokolov, I.J.; Vinner, G.K.; Mancuso, F.; Cinquerrui, S.; Vladisavljevic, G.T.; Clokie, M.R.J.; Garton, N.J.; Stapley, A.G.F.; Kirpichnikova, A. Formulation, Stabilisation and Encapsulation of Bacteriophage for Phage Therapy. Adv. Colloid Interface Sci. 2017, 249, 100–133. [Google Scholar] [CrossRef] [PubMed]
- Melo, L.D.R.; Oliveira, H.; Pires, D.P.; Dabrowska, K.; Azeredo, J. Phage Therapy Efficacy: A Review of the Last 10 Years of Preclinical Studies. Crit. Rev. Microbiol. 2020, 46, 78–99. [Google Scholar] [CrossRef] [PubMed]
- García, P.; Martínez, B.; Obeso, J.M.; Rodríguez, A. Bacteriophages and Their Application in Food Safety. Lett. Appl. Microbiol. 2008, 47, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.J. Practical and Theoretical Considerations for the Use of Bacteriophages in Food Systems. In Bacteriophages in the Control of Food- and Waterborne Pathogens; Sabour, P.M., Griffiths, M.W., Eds.; ASM Press: Washington, DC, USA, 2010; ISBN 9781119737896. [Google Scholar]
- O’Sullivan, L.; Bolton, D.; McAuliffe, O.; Coffey, A. Bacteriophages in Food Applications: From Foe to Friend. Annu. Rev. Food Sci. Technol. 2019, 10, 151–172. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.; Moghanjougi, Z.M.; Bari, M.R.; Khaneghah, A.M. Natural Protective Agents and Their Applications as Bio-Preservatives in the Food Industry: An Overview of Current and Future Applications. Ital. J. Food Sci. 2021, 33, 55–68. [Google Scholar] [CrossRef]
- Lewis, R.; Hill, C. Overcoming Barriers to Phage Application in Food and Feed. Curr. Opin. Biotechnol. 2020, 61, 38–44. [Google Scholar] [CrossRef]
- Moye, Z.D.; Woolston, J.; Sulakvelidze, A. Bacteriophage Applications for Food Production and Processing. Viruses 2018, 10, 205. [Google Scholar] [CrossRef]
- Połaska, M.; Sokołowska, B. Bacteriophages—A New Hope or a Huge Problem in the Food Industry. AIMS Microbiol. 2019, 5, 324–346. [Google Scholar] [CrossRef]
- Fernández, L.; Gutiérrez, D.; García, P.; Rodríguez, A. The Perfect Bacteriophage for Therapeutic Applications—A Quick Guide. Antibiotics 2019, 8, 126. [Google Scholar] [CrossRef]
- Jończyk-Matysiak, E.; Łodej, N.; Kula, D.; Owczarek, B.; Orwat, F.; Międzybrodzki, R.; Neuberg, J.; Bagińska, N.; Weber-Dąbrowska, B.; Górski, A. Factors Determining Phage Stability/Activity: Challenges in Practical Phage Application. Expert Rev. Anti-Infect. Ther. 2019, 17, 583–606. [Google Scholar] [CrossRef]
- Ergin, F. Effect of Freeze Drying, Spray Drying and Electrospraying on the Morphological, Thermal, and Structural Properties of Powders Containing Phage Felix O1 and Activity of Phage Felix O1 during Storage. Powder Technol. 2022, 404, 117516. [Google Scholar] [CrossRef]
- Samtlebe, M.; Ergin, F.; Wagner, N.; Neve, H.; Küçükçetin, A.; Franz, C.M.A.P.; Heller, K.J.; Hinrichs, J.; Atamer, Z. Carrier Systems for Bacteriophages to Supplement Food Systems: Encapsulation and Controlled Release to Modulate the Human Gut Microbiota. LWT Food Sci. Technol. 2016, 68, 334–340. [Google Scholar] [CrossRef]
- Liu, H.; Cui, S.W.; Chen, M.; Li, Y.; Liang, R.; Xu, F.; Zhong, F. Protective Approaches and Mechanisms of Microencapsulation to the Survival of Probiotic Bacteria during Processing, Storage and Gastrointestinal Digestion: A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2863–2878. [Google Scholar] [CrossRef] [PubMed]
- Niamah, A.K.; Al-Sahlany, S.T.G.; Ibrahim, S.A.; Verma, D.K.; Thakur, M.; Singh, S.; Patel, A.R.; Aguilar, C.N.; Utama, G.L. Electro-Hydrodynamic Processing for Encapsulation of Probiotics: A Review on Recent Trends, Technological Development, Challenges and Future Prospect. Food Biosci. 2021, 44, 101458. [Google Scholar] [CrossRef]
- Ergin, F.; Atamer, Z.; Göcer, E.M.C.; Demir, M.; Hinrichs, J.; Kucukcetin, A. Optimization of Salmonella Bacteriophage Microencapsulation in Alginate-Caseinate Formulation Using Vibrational Nozzle Technique. Food Hydrocoll. 2021, 113, 106456. [Google Scholar] [CrossRef]
- Liu, H.; Gong, J.; Chabot, D.; Miller, S.S.; Cui, S.W.; Zhong, F.; Wang, Q. Improved Survival of Lactobacillus Zeae LB1 in a Spray Dried Alginate-Protein Matrix. Food Hydrocoll. 2018, 78, 100–108. [Google Scholar] [CrossRef]
- Batalha, L.S.; Gontijo, M.T.G.; de Carvalho Teixeira, A.V.N.; Boggione, D.M.G.; Lopez, M.E.S.; Eller, M.R.; Mendonça, R.C.S. Encapsulation in Alginate-Polymers Improves Stability and Allows Controlled Release of the UFV-AREG1 Bacteriophage. Food Res. Int. 2021, 139, 109947. [Google Scholar] [CrossRef]
- Zuidam, N.J.; Nedović, V.A. Encapsulation Technologies for Active Food Ingredients and Food Processing; Springer: New York, NY, USA, 2009; ISBN 9781441910073. [Google Scholar]
- Jeyakumari, A.; Zynudheen, A.A.; Parvathy, U. Microencapsulation of Bioactive Food Ingredients and Controlled Release—A Review. MOJ Food Process. Technol. 2016, 2, 214–224. [Google Scholar] [CrossRef]
- Parracho, H.M.R.T.; Burrowes, B.H.; Enright, M.C.; McConville, M.; Harper, D.R. The Role of Regulated Clinical Trials in the Development of Bacteriophage Therapeutics. J. Mol. Genet. Med. 2012, 6, 279–286. [Google Scholar] [CrossRef]
- Górski, A.; Międzybrodzki, R.; Łobocka, M.; Głowacka-Rutkowska, A.; Bednarek, A.; Borysowski, J.; Jończyk-Matysiak, E.; Łusiak-Szelachowska, M.; Weber-Dabrowska, B.; Bagińska, N.; et al. Phage Therapy: What Have We Learned? Viruses 2018, 10, 288. [Google Scholar] [CrossRef]
- Michel, C.; Samtlebe, M.; Wagner, N.; Neve, H.; Franz, C.M.A.P.; Hinrichs, J.; Atamer, Z. Orthogonal Processing Strategies to Create “Phage-Free” Whey–Membrane Filtration Followed by Thermal or Ultraviolet C Treatment for the Reduction of Lactococcus Lactis Bacteriophages. Int. Dairy J. 2021, 122, 105149. [Google Scholar] [CrossRef]
- Adams, M. Bacteriophages; Interscience Publishers: New York, NY, USA, 1959. [Google Scholar]
- Atamer, Z.; Dietrich, J.; Müller-Merbach, M.; Neve, H.; Heller, K.J.; Hinrichs, J. Screening for and Characterization of Lactococcus Lactis Bacteriophages with High Thermal Resistance. Int. Dairy J. 2009, 19, 228–235. [Google Scholar] [CrossRef]
- Müller-Merbach, M.; Rauscher, T.; Hinrichs, J. Inactivation of Bacteriophages by Thermal and High-Pressure Treatment. Int. Dairy J. 2005, 15, 777–784. [Google Scholar] [CrossRef]
- Kessler, H.G. Food and Bio Process Engineering—Dairy Techology, 5th ed.; Verlag A. Kessler: Munich, Germany, 2002; ISBN 3980237850. [Google Scholar]
- Jeoh Zicari, T.; Scher, H.B.; Santa-Maria, M.C.; Strobel, S. Spray Dry Method for Encapsulation of Biological Moieties and Chemicals in Polymers Cross-Linked by Multivalent Ions for Controlled Release Applications. U.S. Patent 9,700,519 B2, 11 July 2017. [Google Scholar]
- Strobel, S.A.; Allen, K.; Roberts, C.; Jimenez, D.; Scher, H.B.; Jeoh, T. Industrially-Scalable Microencapsulation of Plant Beneficial Bacteria in Dry Cross-Linked Alginate Matrix. Ind. Biotechnol. 2018, 14, 138–147. [Google Scholar] [CrossRef]
- Krischer, O.; Kast, W. Trocknungstechnik: Die Wissenschaftlichen Grundlagen der Trocknungstechnik, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 1978; ISBN 9783642618796. [Google Scholar]
- Scott, T.A. Refractive Index of Ethanol-Water Mixtures and Densitiy and Refractive Index of Ethanol-Water-Ethyl Ether Mixtures. J. Phys. Chem. 1946, 50, 406–412. [Google Scholar] [CrossRef]
- Ma, Y.; Pacan, J.C.; Wang, Q.; Xu, Y.; Huang, X.; Korenevsky, A.; Sabour, P.M. Microencapsulation of Bacteriophage Felix O1 into Chitosan-Alginate Microspheres for Oral Delivery. Appl. Environ. Microbiol. 2008, 74, 4799–4805. [Google Scholar] [CrossRef]
- Soykut, E.A.; Tayyarcan, E.K.; Evran, Ş.; Boyacı, İ.H.; Çakır, İ.; Khaaladi, M.; Fattouch, S. Microencapsulation of Phages to Analyze Their Demeanor in Physiological Conditions. Folia Microbiol. 2019, 64, 751–763. [Google Scholar] [CrossRef]
- Troge, A.; Scheppach, W.; Schroeder, B.O.; Rund, S.A.; Heuner, K.; Wehkamp, J.; Stange, E.F.; Oelschlaeger, T.A. More than a Marine Propeller—The Flagellum of the Probiotic Escherichia coli Strain Nissle 1917 Is the Major Adhesin Mediating Binding to Human Mucus. Int. J. Med. Microbiol. 2012, 302, 304–314. [Google Scholar] [CrossRef]
- Gupta, D.S.; Jann, B.; Schmidt, G.; Golecki, J.R.; Ørskov, I.; Ørskov, F.; Jann, K. Coliphage K5, Specific for E. Coli Exhibiting the Capsular K5 Antigen. FEMS Microbiol. Lett. 1982, 14, 75–78. [Google Scholar] [CrossRef]
- Soundararajan, M.; von Bünau, R.; Oelschlaeger, T.A. K5 Capsule and Lipopolysaccharide Are Important in Resistance to T4 Phage Attack in Probiotic E. Coli Strain Nissle 1917. Front. Microbiol. 2019, 10, 2783. [Google Scholar] [CrossRef]
- Scholl, D.; Kieleczawa, J.; Kemp, P.; Rush, J.; Richardson, C.C.; Merril, C.; Adhya, S.; Molineux, I.J. Genomic Analysis of Bacteriophages SP6 and K1-5, an Estranged Subgroup of the T7 Supergroup. J. Mol. Biol. 2004, 335, 1151–1171. [Google Scholar] [CrossRef]
- Molineux, I.J. T7 like Phages (Podoviridae). In Encyclopedia of Virology; Granoff, A., Webster, R.G., Eds.; Academic Press: San Diego, CA, USA; London, UK, 1999; ISBN 9780122270307. [Google Scholar]
- Sambrook, J.; Russel, D.W. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001; ISBN 9780879695774. [Google Scholar]
- Vörös, Z.; Csík, G.; Herényi, L.; Kellermayer, M. Temperature-Dependent Nanomechanics and Topography of Bacteriophage T7. J. Virol. 2018, 92, e01236-18. [Google Scholar] [CrossRef]
- Pollard, E.C.; Solosko, W. The Thermal Inactivation of T4 and λ Bacteriophage. Biophys. J. 1971, 11, 66–74. [Google Scholar] [CrossRef]
- Pitaksutheepong, C.; Abhisingha, M.; Dumnin, J.; Visessanguan, W. Isolation, Detection and Inactivation of a Myoviridae Bacteriophage Infecting Bacillus amyloliquefaciens FB11. Ann. Microbiol. 2015, 65, 1841–1846. [Google Scholar] [CrossRef]
- Garneau, J.E.; Moineau, S. Bacteriophages of Lactic Acid Bacteria and Their Impact on Milk Fermentations. Microb. Cell Fact. 2011, 10, S20. [Google Scholar] [CrossRef]
- Atamer, Z. An Extraordinary Dairy Phage and Its Properties: Occurrence, Growth, Inactivation, Survival. Int. Dairy J. 2022, 129, 105336. [Google Scholar] [CrossRef]
- Evans, D.F.; Pye, G.; Bramley, R.; Clark, A.G.; Dyson, T.J.; Hardcastle, J.D. Measurement of Gastrointestinal PH Profiles in Normal Ambulant Human Subjects. Gut 1988, 29, 1035–1041. [Google Scholar] [CrossRef]
- Cook, M.T.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V.V. Microencapsulation of Probiotics for Gastrointestinal Delivery. J. Control. Release 2012, 162, 56–67. [Google Scholar] [CrossRef]
- Dong, Q.Y.; Chen, M.Y.; Xin, Y.; Qin, X.Y.; Cheng, Z.; Shi, L.E.; Tang, Z.X. Alginate-Based and Protein-Based Materials for Probiotics Encapsulation: A Review. Int. J. Food Sci. Technol. 2013, 48, 1339–1351. [Google Scholar] [CrossRef]
- Dussault, P.; Bourgault, J.M.; Verly, W.G. T7 Coliphage Inactivation by Nitrous Acid. Biochim. Biophys. Acta 1970, 213, 312–319. [Google Scholar] [CrossRef]
- Feng, Y.Y.; Ong, S.L.; Hu, J.Y.; Tan, X.L.; Ng, W.J. Effects of PH and Temperature on the Survival of Coliphages MS2 and Qβ. J. Ind. Microbiol. Biotechnol. 2003, 30, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Capra, M.L.; Quiberoni, A.; Reinheimer, J. Phages of Lactobacillus Casei/Paracasei: Response to Environmental Factors and Interaction with Collection and Commercial Strains. J. Appl. Microbiol. 2006, 100, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Campagna, C.; Villion, M.; Labrie, S.J.; Duchaine, C.; Moineau, S. Inactivation of Dairy Bacteriophages by Commercial Sanitizers and Disinfectants. Int. J. Food Microbiol. 2014, 171, 41–47. [Google Scholar] [CrossRef]
- Salo, R.J.; Cliver, D.O. Effect of Acid PH, Salts, and Temperature on the Infectivity and Physical Integrity of Enteroviruses. Arch. Virol. 1976, 52, 269–282. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of Spray-Drying in Microencapsulation of Food Ingredients: An Overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Malik, D.J. Bacteriophage Encapsulation Using Spray Drying for Phage Therapy. Curr. Issues Mol. Biol. 2021, 40, 303–316. [Google Scholar] [CrossRef]
- Cal, K.; Sollohub, K. Spray Drying Technique. I: Hardware and Process Parameters. J. Pharm. Sci. 2010, 99, 575–586. [Google Scholar] [CrossRef]
- Vinner, G.K.; Rezaie-Yazdi, Z.; Leppanen, M.; Stapley, A.G.F.; Leaper, M.C.; Malik, D.J. Microencapsulation of Salmonella-Specific Bacteriophage Felix O1 Using Spray-Drying in a PH-Responsive Formulation and Direct Compression Tableting of Powders into a Solid Oral Dosage Form. Pharmaceuticals 2019, 12, 43. [Google Scholar] [CrossRef]
- Hussein, N.; Omer, H.; Ismael, A.; Alhnan, M.A.; Elhissi, A.; Ahmed, W. Spray-Dried Alginate Microparticles for Potential Intranasal Delivery of Ropinirole Hydrochloride: Development, Characterization and Histopathological Evaluation. Pharm. Dev. Technol. 2020, 25, 290–299. [Google Scholar] [CrossRef]
- Hansen, L.T.; Allan-Wojtas, P.M.; Jin, Y.L.; Paulson, A.T. Survival of Ca-Alginate Microencapsulated Bifidobacterium Spp. in Milk and Simulated Gastrointestinal Conditions. Food Microbiol. 2002, 19, 35–45. [Google Scholar] [CrossRef]
- Matinkhoo, S.; Lynch, K.H.; Dennis, J.J.; Finlay, W.H.; Vehring, R. Spray-Dried Respirable Powders Containing Bacteriophages for the Treatment of Pulmonary Infections. J. Pharm. Sci. 2011, 100, 5197–5205. [Google Scholar] [CrossRef] [PubMed]
- Leung, S.S.Y.; Parumasivam, T.; Gao, F.G.; Carrigy, N.B.; Vehring, R.; Finlay, W.H.; Morales, S.; Britton, W.J.; Kutter, E.; Chan, H.-K. Production of Inhalation Phage Powders Using Spray Freeze Drying and Spray Drying Techniques for Treatment of Respiratory Infections. Pharm. Res. 2016, 33, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- Young, S.L.; Sarda, X.; Rosenberg, M. Microencapsulating Properties of Whey Proteins. 1. Microencapsulation of Anhydrous Milk Fat. J. Dairy Sci. 1993, 76, 2868–2877. [Google Scholar] [CrossRef]
- Rosenberg, M.; Sheu, T.-Y. Microencapsulation of Volatiles by Spray-Drying in Whey Protein-Based Wall Systems. Int. Dairy J. 1996, 6, 273–284. [Google Scholar] [CrossRef]
- Teijeiro, M.; Pérez, P.F.; De Antoni, G.L.; Golowczyc, M.A. Suitability of Kefir Powder Production Using Spray Drying. Food Res. Int. 2018, 112, 169–174. [Google Scholar] [CrossRef]
- Gbassi, G.K.; Vandamme, T.; Ennahar, S.; Marchioni, E. Microencapsulation of Lactobacillus plantarum Spp. in an Alginate Matrix Coated with Whey Proteins. Int. J. Food Microbiol. 2009, 129, 103–105. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Ghosh, D. The Stabilizing Excipients in Dry State Therapeutic Phage Formulations. AAPS PharmSciTech 2020, 21, 133. [Google Scholar] [CrossRef] [PubMed]
- Millqvist-Fureby, A.; Elofsson, U.; Bergenståhl, B. Surface Composition of Spray-Dried Milk Protein-Stabilised Emulsions in Relation to Pre-Heat Treatment of Proteins. Colloids Surfaces B Biointerfaces 2001, 21, 47–58. [Google Scholar] [CrossRef]
- Xavier, A.A.O.; Mariutti, L.R.B. Static and Semi-Dynamic in Vitro Digestion Methods: State of the Art and Recent Achievements towards Standardization. Curr. Opin. Food Sci. 2021, 41, 260–273. [Google Scholar] [CrossRef]
- Tang, Z.; Huang, X.; Baxi, S.; Chambers, J.R.; Sabour, P.M.; Wang, Q. Whey Protein Improves Survival and Release Characteristics of Bacteriophage Felix O1 Encapsulated in Alginate Microspheres. Food Res. Int. 2013, 52, 460–466. [Google Scholar] [CrossRef]
- Shi, L.-E.; Li, Z.-H.; Li, D.-T.; Xu, M.; Chen, H.-Y.; Zhang, Z.L.; Tang, Z.-X. Encapsulation of Probiotic Lactobacillus Bulgaricus in Alginate-Milk Microspheres and Evaluation of the Survival in Simulated Gastrointestinal Conditions. J. Food Eng. 2013, 117, 99–104. [Google Scholar] [CrossRef]
- Tang, Z.; Huang, X.; Sabour, P.M.; Chambers, J.R.; Wang, Q. Preparation and Characterization of Dry Powder Bacteriophage K for Intestinal Delivery through Oral Administration. LWT Food Sci. Technol. 2015, 60, 263–270. [Google Scholar] [CrossRef]
- Salaün, F.; Mietton, B.; Gaucheron, F. Buffering Capacity of Dairy Products. Int. Dairy J. 2005, 15, 95–109. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Ko, S.; Xiao, L. Use of Whey Proteins for Encapsulation and Controlled Delivery Applications. J. Food Eng. 2007, 83, 31–40. [Google Scholar] [CrossRef]
- Colom, J.; Cano-Sarabia, M.; Otero, J.; Aríñez-Soriano, J.; Cortés, P.; Maspoch, D.; Llagostera, M. Microencapsulation with Alginate/CaCO3: A Strategy for Improved Phage Therapy. Sci. Rep. 2017, 7, 41441. [Google Scholar] [CrossRef] [PubMed]
- Agüero, L.; Zaldivar-Silva, D.; Peña, L.; Dias, M. Alginate Microparticles as Oral Colon Drug Delivery Device: A Review. Carbohydr. Polym. 2017, 168, 32–43. [Google Scholar] [CrossRef]
- LeClair, D.A.; Cranston, E.D.; Lichty, B.D.; Xing, Z.; Thompson, M.R. Consecutive Spray Drying to Produce Coated Dry Powder Vaccines Suitable for Oral Administration. ACS Biomater. Sci. Eng. 2018, 4, 1669–1678. [Google Scholar] [CrossRef]
- Leung, S.S.Y.; Parumasivam, T.; Nguyen, A.; Gengenbach, T.; Carter, E.A.; Carrigy, N.B.; Wang, H.; Vehring, R.; Finlay, W.H.; Morales, S.; et al. Effect of Storage Temperature on the Stability of Spray Dried Bacteriophage Powders. Eur. J. Pharm. Biopharm. 2018, 127, 213–222. [Google Scholar] [CrossRef]
- Keogh, B.P.; Pettingill, G. Long-Term Storage of Bacteriophages of Lactic Streptococci. Appl. Microbiol. 1966, 14, 421–424. [Google Scholar] [CrossRef]
- Vandenheuvel, D.; Singh, A.; Vandersteegen, K.; Klumpp, J.; Lavigne, R.; Van Den Mooter, G. Feasibility of Spray Drying Bacteriophages into Respirable Powders to Combat Pulmonary Bacterial Infections. Eur. J. Pharm. Biopharm. 2013, 84, 578–582. [Google Scholar] [CrossRef]
- Mahmood, A.; Usman, S. A Comparative Study on the Physicochemical Parameters of Milk Samples Collected from Buffalo, Cow, Goat and Sheep of Gujrat, Pakistan. Pakistan J. Nutr. 2010, 9, 1192–1197. [Google Scholar] [CrossRef]
- Whitman, P.A.; Marshall, R.T. Characterization of Two Psychrophilic Pseudomonas Bacteriophages Isolated from Ground Beef. Appl. Microbiol. 1971, 22, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Duyvejonck, H.; Merabishvili, M.; Vaneechoutte, M.; de Soir, S.; Wright, R.; Friman, V.P.; Verbeken, G.; De Vos, D.; Pirnay, J.P.; Van Mechelen, E.; et al. Evaluation of the Stability of Bacteriophages in Different Solutions Suitable for the Production of Magistral Preparations in Belgium. Viruses 2021, 13, 865. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Mills, S.; Stanton, C.; Fitzgerald, G.F.; Roos, Y.; Ross, R.P. Effect of Disaccharides on Survival during Storage of Freeze Dried Probiotics. Dairy Sci. Technol. 2008, 88, 19–30. [Google Scholar] [CrossRef]
- Bampi, G.B.; Backes, G.T.; Cansian, R.L.; de Matos, F.E.; Ansolin, I.M.A.; Poleto, B.C.; Corezzolla, L.R.; Favaro-Trindade, C.S. Spray Chilling Microencapsulation of Lactobacillus acidophilus and Bifidobacterium animalis Subsp. Lactis and Its Use in the Preparation of Savory Probiotic Cereal Bars. Food Bioprocess Technol. 2016, 9, 1422–1428. [Google Scholar] [CrossRef]
- Bastos, G.A.; Paulo, E.M.; Chiaradia, A.C.N. Aceitabilidade de Barra de Cereais Potencialmente Probiótica (Acceptability of Potentially Probiotic Cereal Bars). Brazilian J. Food Technol. 2014, 17, 113–120. [Google Scholar] [CrossRef]
- Da Silva, D.C.M.N.; Paulino, V.P.D.A.; de Nascimento, C.P.; Pereira, M.D.G. Desenvolvimento de Barra de Cereais Salgada Enriquecida Com Chia e Fos (Development of a Salty Cereal Bar Enriched Wich Chia and FOS). Rev. Científica Ciências Apl. FAIP 2016, 3, 80–97. [Google Scholar]
- Connolly, M.L.; Lovegrove, J.A.; Tuohy, K.M. In Vitro Evaluation of the Microbiota Modulation Abilities of Different Sized Whole Oat Grain Flakes. Anaerobe 2010, 16, 483–488. [Google Scholar] [CrossRef]
- Wilczak, J.; Błaszczyk, K.; Kamola, D.; Gajewska, M.; Harasym, J.P.; Jałosińska, M.; Gudej, S.; Suchecka, D.; Oczkowski, M.; Gromadzka-Ostrowska, J. The Effect of Low or High Molecular Weight Oat Beta-Glucans on the Inflammatory and Oxidative Stress Status in the Colon of Rats with LPS-Induced Enteritis. Food Funct. 2015, 6, 590–603. [Google Scholar] [CrossRef]
- Nie, Y.; Lin, Q.; Luo, F. Effects of Non-Starch Polysaccharides on Inflammatory Bowel Disease. Int. J. Mol. Sci. 2017, 18, 1372. [Google Scholar] [CrossRef]
- Mills, S.; Shanahan, F.; Stanton, C.; Hill, C.; Coffey, A.; Paul Ross, R. Movers and Shakers: Influence of Bacteriophages in Shaping the Mammalian Gut Microbiota. Gut Microbes 2013, 4, 4–16. [Google Scholar] [CrossRef] [PubMed]
 ), 70 °C (
), 70 °C ( ), 72 °C (
), 72 °C ( ), and 75 °C (
), and 75 °C ( ). Experimental data are displayed by symbols. N* indicates the initial bacteriophage titer before any treatment. Modeled inactivation curves are represented by dashed lines and were calculated using Equation (2) and the following values for the variable parameters (± standard error s): n = 1.42 ± 0.06, kref = (2.8 ± 1.0) × 10−3 s−1 (pfu mL−1)(1−n), Ea = (9.57 ± 1.38) × 102 kJ mol−1, Tref = 345.15 K. The correlation coefficient r2 was 0.98. The limit of quantification was 101 pfu mL−1.
). Experimental data are displayed by symbols. N* indicates the initial bacteriophage titer before any treatment. Modeled inactivation curves are represented by dashed lines and were calculated using Equation (2) and the following values for the variable parameters (± standard error s): n = 1.42 ± 0.06, kref = (2.8 ± 1.0) × 10−3 s−1 (pfu mL−1)(1−n), Ea = (9.57 ± 1.38) × 102 kJ mol−1, Tref = 345.15 K. The correlation coefficient r2 was 0.98. The limit of quantification was 101 pfu mL−1.
 ), 70 °C (
), 70 °C ( ), 72 °C (
), 72 °C ( ), and 75 °C (
), and 75 °C ( ). Experimental data are displayed by symbols. N* indicates the initial bacteriophage titer before any treatment. Modeled inactivation curves are represented by dashed lines and were calculated using Equation (2) and the following values for the variable parameters (± standard error s): n = 1.42 ± 0.06, kref = (2.8 ± 1.0) × 10−3 s−1 (pfu mL−1)(1−n), Ea = (9.57 ± 1.38) × 102 kJ mol−1, Tref = 345.15 K. The correlation coefficient r2 was 0.98. The limit of quantification was 101 pfu mL−1.
). Experimental data are displayed by symbols. N* indicates the initial bacteriophage titer before any treatment. Modeled inactivation curves are represented by dashed lines and were calculated using Equation (2) and the following values for the variable parameters (± standard error s): n = 1.42 ± 0.06, kref = (2.8 ± 1.0) × 10−3 s−1 (pfu mL−1)(1−n), Ea = (9.57 ± 1.38) × 102 kJ mol−1, Tref = 345.15 K. The correlation coefficient r2 was 0.98. The limit of quantification was 101 pfu mL−1.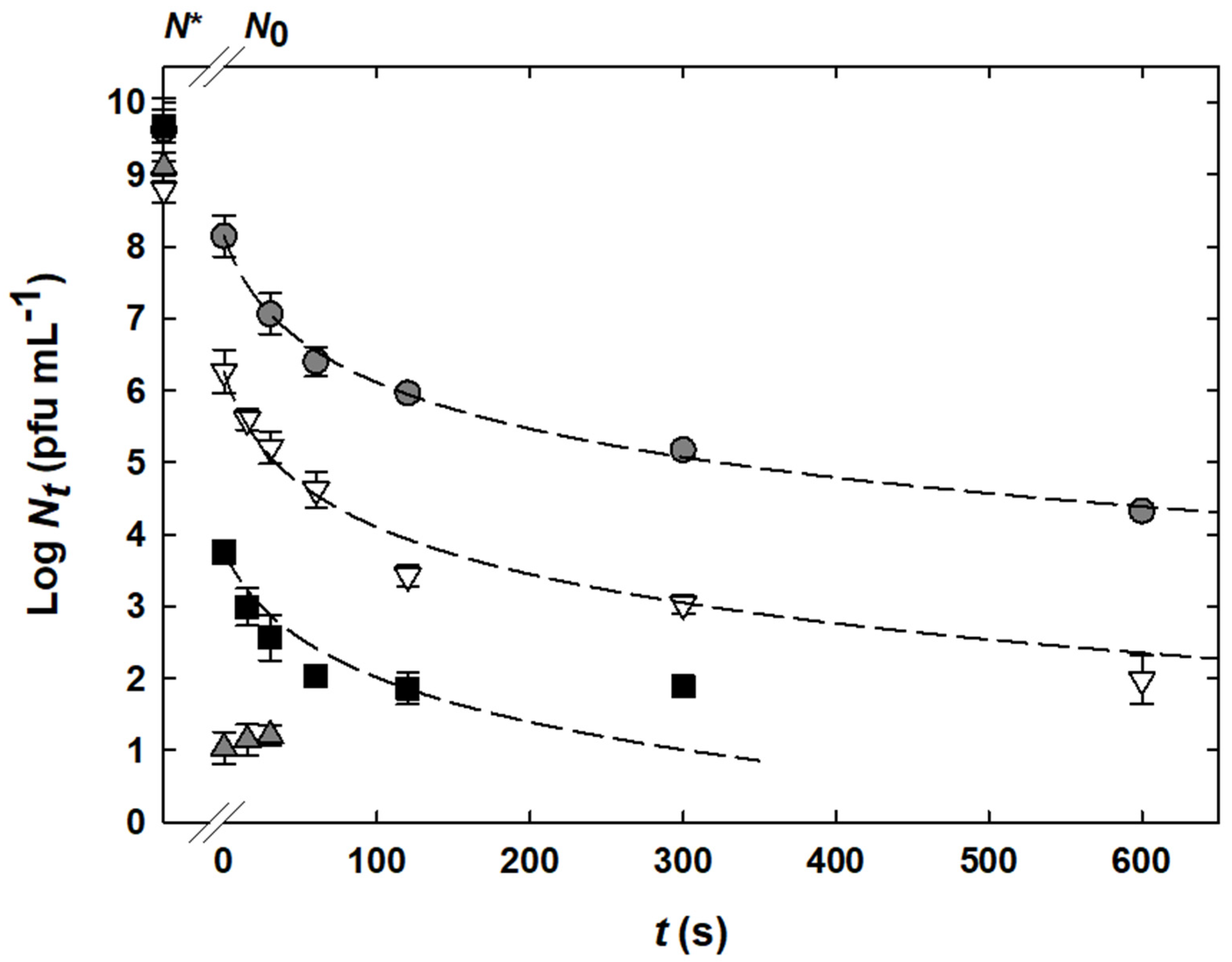
 ), 3.5 (
), 3.5 ( ), 4.0 (
), 4.0 ( ), 5.0 (
), 5.0 ( ), and 6.6 (
), and 6.6 ( ) (reference). Experimental data are displayed by symbols. N* indicates the initial bacteriophage titer before any treatment. Linear regressions are shown by dotted lines. The limit of quantification was 2 × 102 pfu mL−1.
) (reference). Experimental data are displayed by symbols. N* indicates the initial bacteriophage titer before any treatment. Linear regressions are shown by dotted lines. The limit of quantification was 2 × 102 pfu mL−1.
 ), 3.5 (
), 3.5 ( ), 4.0 (
), 4.0 ( ), 5.0 (
), 5.0 ( ), and 6.6 (
), and 6.6 ( ) (reference). Experimental data are displayed by symbols. N* indicates the initial bacteriophage titer before any treatment. Linear regressions are shown by dotted lines. The limit of quantification was 2 × 102 pfu mL−1.
) (reference). Experimental data are displayed by symbols. N* indicates the initial bacteriophage titer before any treatment. Linear regressions are shown by dotted lines. The limit of quantification was 2 × 102 pfu mL−1.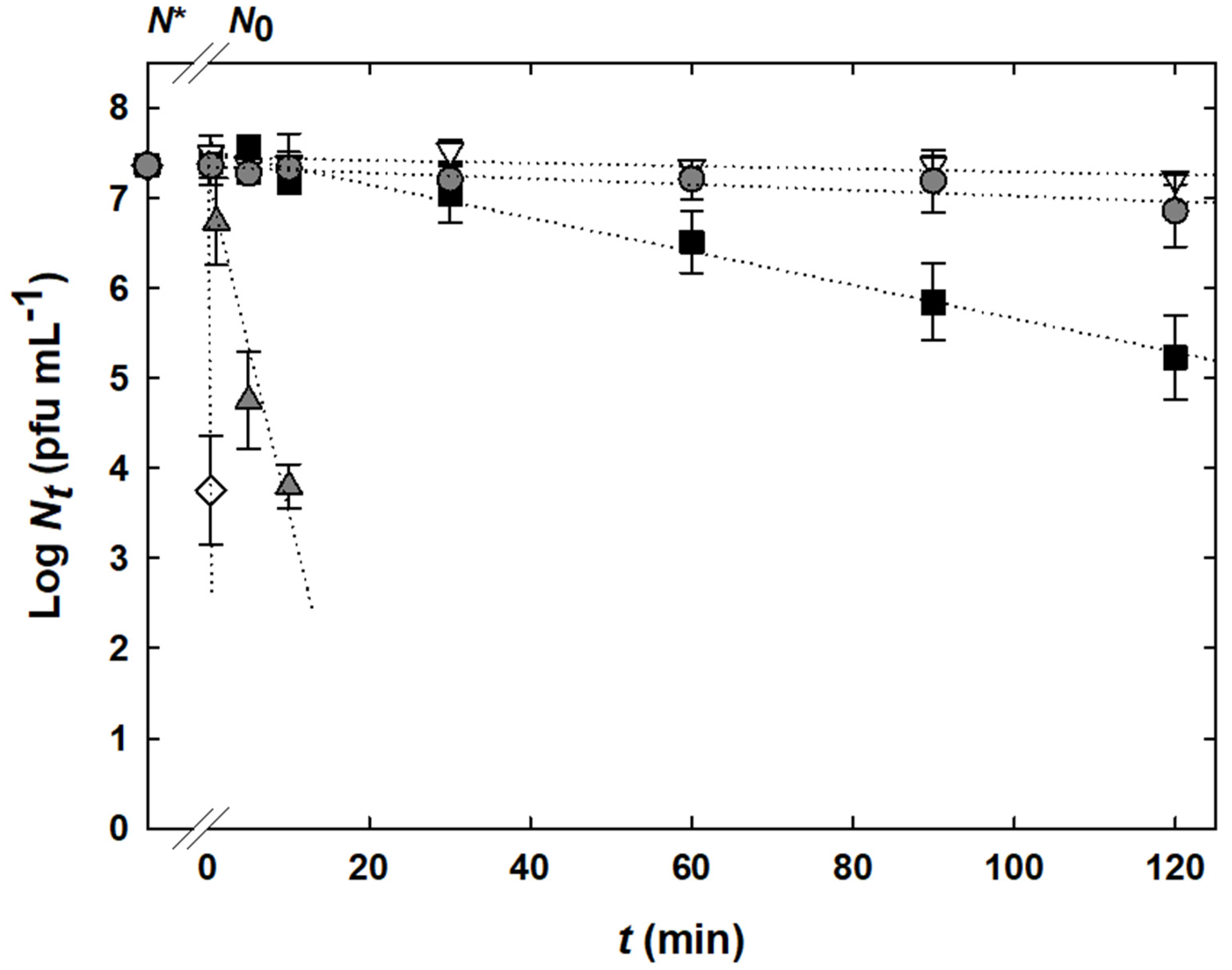
 ) or alginate and whey protein isolate (
) or alginate and whey protein isolate ( ). (a) Incubation in simulated gastric fluid (SGF). Results are given as bacteriophage titer of solid capsules (NC in pfu g−1). (b) Incubation in simulated intestinal fluid (SIF). Results are given as bacteriophage titer of dissolved capsules (1% in SIF) (NC# in pfu mL−1). NC* and NC*,# indicate the initial bacteriophage titers before any treatment, respectively. The limits of quantification were 103 pfu g−1 and 101 pfu mL−1 for bacteriophage titers in SGF and SIF, respectively.
). (a) Incubation in simulated gastric fluid (SGF). Results are given as bacteriophage titer of solid capsules (NC in pfu g−1). (b) Incubation in simulated intestinal fluid (SIF). Results are given as bacteriophage titer of dissolved capsules (1% in SIF) (NC# in pfu mL−1). NC* and NC*,# indicate the initial bacteriophage titers before any treatment, respectively. The limits of quantification were 103 pfu g−1 and 101 pfu mL−1 for bacteriophage titers in SGF and SIF, respectively.
 ) or alginate and whey protein isolate (
) or alginate and whey protein isolate ( ). (a) Incubation in simulated gastric fluid (SGF). Results are given as bacteriophage titer of solid capsules (NC in pfu g−1). (b) Incubation in simulated intestinal fluid (SIF). Results are given as bacteriophage titer of dissolved capsules (1% in SIF) (NC# in pfu mL−1). NC* and NC*,# indicate the initial bacteriophage titers before any treatment, respectively. The limits of quantification were 103 pfu g−1 and 101 pfu mL−1 for bacteriophage titers in SGF and SIF, respectively.
). (a) Incubation in simulated gastric fluid (SGF). Results are given as bacteriophage titer of solid capsules (NC in pfu g−1). (b) Incubation in simulated intestinal fluid (SIF). Results are given as bacteriophage titer of dissolved capsules (1% in SIF) (NC# in pfu mL−1). NC* and NC*,# indicate the initial bacteriophage titers before any treatment, respectively. The limits of quantification were 103 pfu g−1 and 101 pfu mL−1 for bacteriophage titers in SGF and SIF, respectively.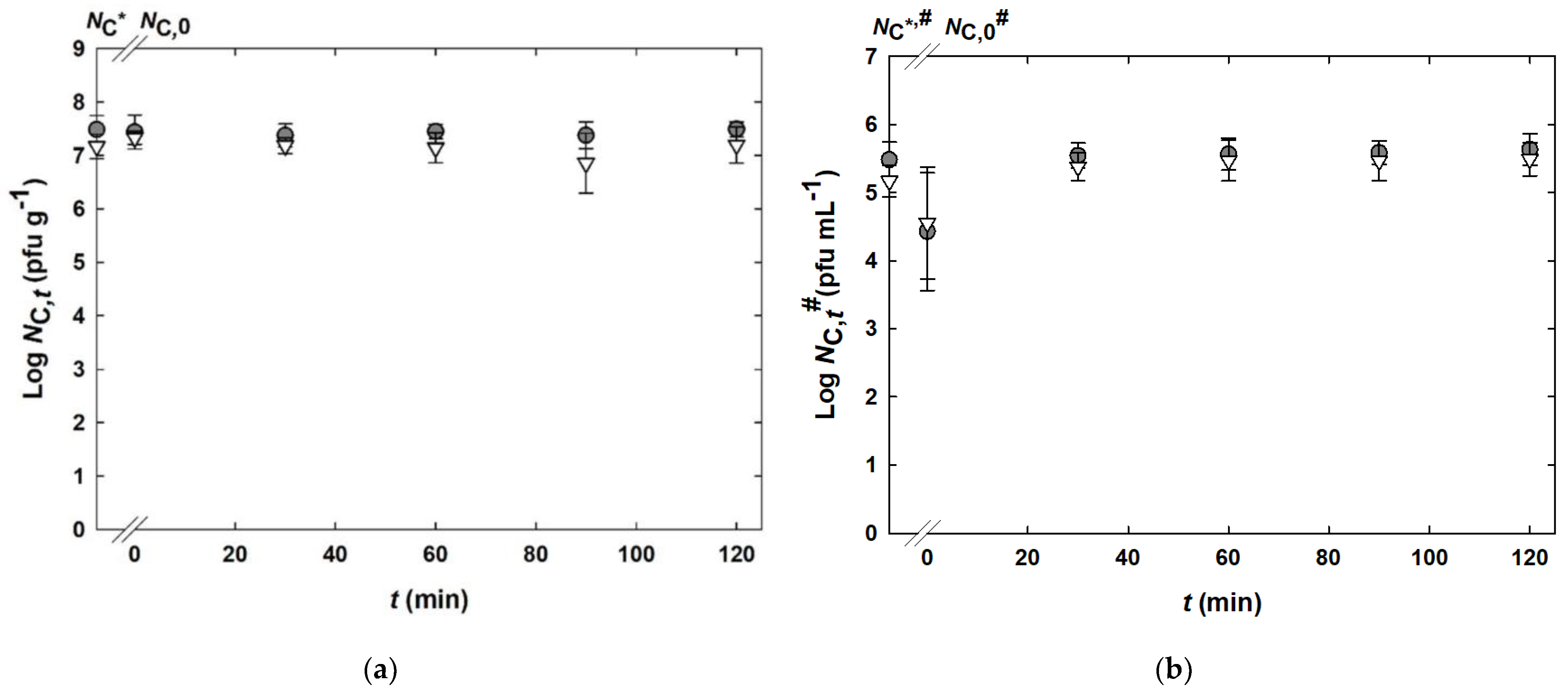
 ). The limit of quantification was 2.3 × 102 pfu (related to one cookie with a mass of 2.505 g). A photograph of the cereal cookie is presented in the lower right corner of the picture.
). The limit of quantification was 2.3 × 102 pfu (related to one cookie with a mass of 2.505 g). A photograph of the cereal cookie is presented in the lower right corner of the picture.
 ). The limit of quantification was 2.3 × 102 pfu (related to one cookie with a mass of 2.505 g). A photograph of the cereal cookie is presented in the lower right corner of the picture.
). The limit of quantification was 2.3 × 102 pfu (related to one cookie with a mass of 2.505 g). A photograph of the cereal cookie is presented in the lower right corner of the picture.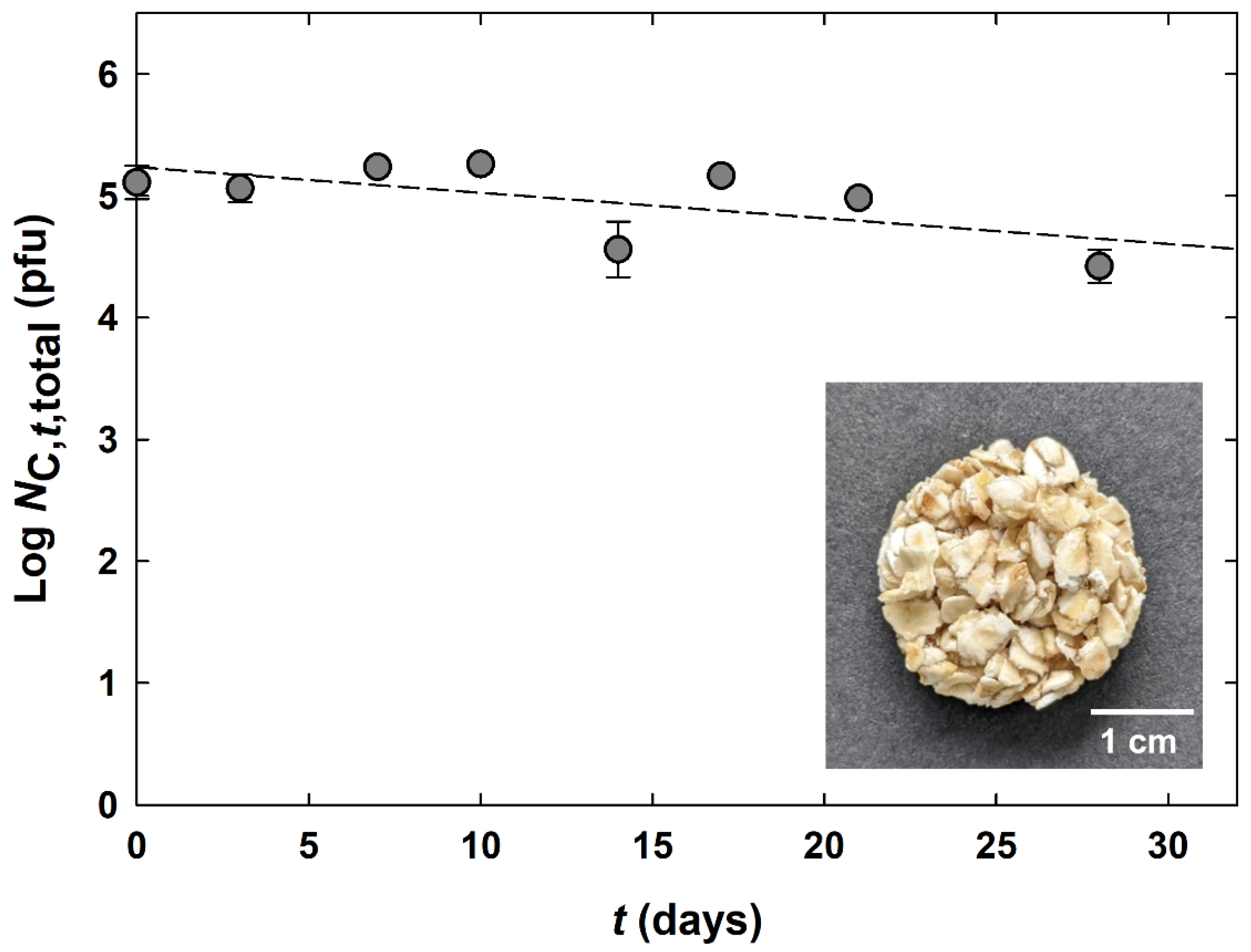
| Parameter | Setting |
|---|---|
| Feed flow volume | 3.6 mL min−1 |
| Gas volume flow | 473–536 L h−1 |
| Aspirator | 90% (35 m3 h−1) |
| Inlet temperature | 110 °C |
| Outlet temperature | 53–59 °C |
| Wet-bulb temperature 1 | 35 °C |
| Capsules Characteristics | Alginate + Micellar Casein Capsules | Alginate + Whey Protein Isolate Capsules |
|---|---|---|
| Humidity (%) | 8.70 ± 0.86 | 9.43 ± 0.32 |
| Water activity (–) | 0.15 ± 0.03 | n.d. * |
| Particle diameter d10,3 (µm) | 3.6 ± 1.2 | 5.1 ± 0.4 |
| Particle diameter d50,3 (µm) | 8.9 ± 0.7 | 12.0 ± 1.9 |
| Particle diameter d90,3 (µm) | 17.5 ± 4.4 | 46.2 ± 2.9 |
| Encapsulation efficiency (%) | 4.2 ± 2.1 ** | 3.6 ± 2.1 *** |
| Bacteriophage titer (pfu g−1) | (3.4 ± 1.7) × 107 | (1.6 ± 0.9) × 107 |
| Log NC,t (pfu g−1) | |||
|---|---|---|---|
| t (Days) | ϑ = 23 °C * | ϑ = 0 °C | ϑ = −20 °C |
| 0 | 7.19 ± n.d. ** | 7.13 ± 0.53 | 7.13 ± 0.53 |
| 15 | 6.37 ± n.d. | 7.04 ± 0.49 | 7.21 ± 0.40 |
| 30 | 4.91 ± n.d. | 7.35 ± 0.14 | 7.35 ± 0.19 |
| 60 | 3.43 ± n.d. | 7.25 ± 0.05 | 7.31 ± 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schubert, C.; Fischer, S.; Dorsch, K.; Teßmer, L.; Hinrichs, J.; Atamer, Z. Microencapsulation of Bacteriophages for the Delivery to and Modulation of the Human Gut Microbiota through Milk and Cereal Products. Appl. Sci. 2022, 12, 6299. https://doi.org/10.3390/app12136299
Schubert C, Fischer S, Dorsch K, Teßmer L, Hinrichs J, Atamer Z. Microencapsulation of Bacteriophages for the Delivery to and Modulation of the Human Gut Microbiota through Milk and Cereal Products. Applied Sciences. 2022; 12(13):6299. https://doi.org/10.3390/app12136299
Chicago/Turabian StyleSchubert, Christina, Sabina Fischer, Kathrin Dorsch, Lutz Teßmer, Jörg Hinrichs, and Zeynep Atamer. 2022. "Microencapsulation of Bacteriophages for the Delivery to and Modulation of the Human Gut Microbiota through Milk and Cereal Products" Applied Sciences 12, no. 13: 6299. https://doi.org/10.3390/app12136299
APA StyleSchubert, C., Fischer, S., Dorsch, K., Teßmer, L., Hinrichs, J., & Atamer, Z. (2022). Microencapsulation of Bacteriophages for the Delivery to and Modulation of the Human Gut Microbiota through Milk and Cereal Products. Applied Sciences, 12(13), 6299. https://doi.org/10.3390/app12136299






