Abstract
This work demonstrates the application of an electronic nose (e-nose) for discrimination between authentic and adulterated honey. The developed e-nose is based on electrodes covered with ionogel (ionic liquid + gelatin + Fe3O4 nanoparticle) films. Authentic and adulterated honey samples were submitted to e-nose analysis, and the capacity of the sensors for discrimination between authentic and adulterated honey was evaluated using principal component analysis (PCA) based on average relative response data. From the PCA biplot, it was possible to note two well-defined clusters and no intersection was observed. To evaluate the relative response data as input for autonomous classification, different machine learning algorithms were evaluated, namely instance based (IBK), Kstar, Trees-J48 (J48), random forest (RF), multilayer perceptron (MLP), naive Bayes (NB), and sequential minimal optimization (SMO). Considering the average data, the highest accuracy was obtained for Kstar: 100% (k-fold = 3). Additionally, this algorithm was also compared regarding its sensitivity and specificity, both being 100% for both features. Thus, due to the rapidity, simplicity, and accuracy of the developed methodology, the technology based on e-noses has the potential to be applied to honey quality control.
1. Introduction
Honey is a natural sweet substance produced by honeybees (Apis mellifera) from plants’ nectar or secretions of their living parts. This animal product is used in various applications, such as in natura; in sweeteners, phytotherapeutics [1], medicines [2], and cosmetics (for improving moisture, cleansing, miscibility, and spreadability) [3]; and as a food industry additive (antioxidant, enzymatic inhibitor, clarifying, and anti-browning agent) [4,5], among others [6]. In general, honey’s composition and physicochemical and organoleptic properties vary according to its botanical origin (the types of flowers used by the bees), geography and climate, processing, and storage [7]. Regarding chemical composition, honey is a mixture of sugars (mainly fructose, followed by glucose and sucrose), amino acids, proteins, enzymes, organic acids, carotenoids, vitamins, antioxidants, phenolic, minerals, trace elements, and volatile organic compounds (VOCs) [7,8]. Focusing on the latter group, hundreds of different compounds have been identified in the volatile fraction of honey, among them being aldehydes, ketones, carboxylic acids, alcohols, hydrocarbons, terpenes, benzene, pyran, and derivatives [9,10].
Due to its natural scarcity, increased demand, high production cost, and commercial value, honey has been one of the most adulterated products worldwide. Nowadays, the decrease in the global honeybee population due to environmental pollution and the spread of insect diseases have further reduced honey availability, encouraging some producers to adulterate their products to obtain a more significant income [11]. Several types of adulteration are possible, such as direct incorporation of foreign substances into honey, blending with lower-cost honey types, or indirect adulteration of honey through bee feeding with industrial sugars [12]. In this sense, to reduce deliberate adulteration and incentivize good practice standards, the quality control of honey is a fundamental step.
However, due to the heterogeneity of samples, with parameters varying considerably among honey types, and the addition of adulterants with physical-chemical properties similar to authentic honey, the quality control of this product involves several tests and a series of steps, which can take from hours to days and add substantial costs to the production chain [13].
Analytical methods used in food adulteration studies are often based on identifying and quantifying specific markers, such as some classes of compounds (carbohydrates, polyphenols, fatty acids). Usually, the techniques to unravel adulteration, such as chromatographic analysis, electrophoretic methods, spectroscopic methods, immunoassays, and isotope ratio mass spectrometry, require complex sample pretreatments, laborious, and time-consuming procedures; expensive analytical instrumentation; and specialized professionals [11,12,14,15,16]. According to the Codex Standard for Honey, for adequate honey quality control, the main parameters to be evaluated are sugars (fructose, glucose, and the ratio between them), moisture and water activity, free acidity and pH, ash and electrical conductivity, color, and 5-hydroxymethylfurfural (5-HMF) content [13,17].
E-noses are a device that mimics the human nose, recognizing gas patterns (smell) and relating to a known sample. In this equipment, gases or vapors interact with sensors, leading to variations in the system’s physical properties according to the atmosphere’s composition to which the sensor is exposed. By studying the variations in these properties through mathematical/statistical tools, it is possible to evaluate the sensor’s performance in identifying or distinguishing VOCs and even in quantifying certain species present in the given sample. Among the advantages of using an e-nose, the low cost of manufacture and operation, easy handling, and low response time have been highlighted [18].
Due to the presence of volatile species and its characteristic olfactory pattern, studies on the characterization of honey with e-noses have been described. There are several works in which e-noses have been used to differentiate between honey according to botanic and geographical origins [19,20,21], which may also be useful for fraud prevention, since mislabeling is also common.
Focusing on adulteration by the addition of components different than honey, Subari et al. studied a method for discrimination between pure Tualang honey and adulteration induced with sugar solutions (from beetroot and sugarcane), reducing the concentrations of pure honey gradually (80%, 60%, 40%, 20%, and 0%). Two techniques were applied, an e-nose and infrared spectroscopy. The data generated by the two techniques were pre-processed using the relative responses or the normalized data and evaluated using different multivariate analyses. Using the merged data from the e-nose and infrared spectroscopy, followed by linear discriminant analysis (LDA), the authors achieved an accuracy of 92.2% for the proposed methodology [22].
Zakaria et al. demonstrated the ability to discriminate and classify honey of different botanical origins, sugar syrup, and adulterated samples using a combination of an e-nose and an electronic tongue (e-tongue). The authors highlighted the importance of data curation using different classifiers to assign samples correctly. Since the nature of the data from the e-nose and e-tongue was non-linear, the probabilistic neural network (PNN) classifier performed better [23]. Other studies have corroborated these findings, drawing attention to the potential of the e-nose [24] and the e-tongue to detect frauds and other features in honey and emphasized the importance of choosing the correct data treatment method to enhance the sensitivity and specificity of the method [22,23,24].
This work demonstrates the application of the e-nose for discrimination between authentic and adulterated honey. Honey samples of different botanic origins (eucalyptus, orange, and wild plants) were obtained from Brazilian producers. The samples were evaluated using conventional techniques (humidity, acidity, diastase enzyme, Lund reaction, Fiehe and Winckler tests), and adulterated samples were related to frauds commonly practiced in the market (addition of different vegetal sugars). The developed e-nose is based on interdigitated electrodes covered with ionogel (ionic liquid + gelatin + Fe3O4) films and on the variation of the sensor’s conductance according to the sample to which it is exposed. Based on conductance data, two strategies were evaluated for the discrimination between authentic and adulterated honey, graphic discrimination (PCA) and machine learning (automated classifiers), the latter being more efficient due to the possibility of automating the treatment of data, reducing subjectivity, and improving the predicting capacity. In this sense, due to the fast response (19 min) and simplicity (non-destructive analysis and without any sample treatment) of the developed methodology, the technology based on the e-nose can be applied to honey quality control.
2. Materials and Methods
2.1. Reagents and Honey Samples
2.1.1. Reagents
The ionic liquid 1-ethyl-3-methylimidazolium dicyanamide (EMIMDCA, purity > 98%) and bovine skin gelatin type B were acquired from Sigma Aldrich (St. Louis, MO, USA). Tetrahydrate iron (II) chloride (FeCl2·4H2O, purity > 99%) was obtained from Vetec (Duque de Caxias, RJ, Brazil). Iron (III) chloride hexahydrate (FeCl3·6H2O, purity ≥ 99%) was obtained from Acros Organic (Geel, Antwerp, Belgium). Ammonium hydroxide and organic solvents were purchased from Labsynth (Diadema, SP, Brazil). The interdigitated electrodes were manufactured by Micropress S.A. (Sao Paulo, SP, Brazil) with a 0.6 cm2 interdigitated area, 200 µm of spacing between the copper digits, and 100 µm of spacing between the nickel digits covered with 0.05 µm of gold.
2.1.2. Honey Samples
All honey samples were from Brazil and obtained after routine analysis from the Laboratory of Inspection of Animal Products and Food Quality Control of the Faculdade de Medicina Veterinária e Zootecnia. Six samples were authentic honey (TH): eucalyptus (TH 1 and TH 6), orange (TH 2 and TH 3), and wild plants (TH 4 and TH 5). Eleven samples were adulterated honey (AH): eucalyptus (AH 1), orange (AH 2, AH 3, AH 4, AH 5, AH 9, AH 10, and AH 11), and wild plants (AH 6, AH 7, and AH 8).
The determination of which honey was adulterated was performed using official methods according to the Brazilian normative. Honey samples whose values disagreed with at least one of the following parameters were considered adulterated: humidity (≥20%), acidity (≤50 mEq·kg−1), the presence of diastase enzyme, the presence of albuminoid substances (Lund reaction, ranging from 0.6 to 3.0 mL), the presence of 5-HMF (Fiehe test), and the quantification of 5-HMF (Winckler’s method, ≤50 mg·kg−1) [17].
2.2. Synthesis of Ionogel and Sensors Assemble
The synthesis and characterization of the material used in this work are described elsewhere [25].
2.2.1. Fe3O4 Nanoparticles
For the synthesis of Fe3O4, 6.0 g of FeCl3·6H2O was dissolved in 100 mL of distilled water (solution A) and 2.7 g of FeCl2·4H2O was dissolved in 11 mL of distilled water (solution B). Solutions A and B were mixed and mechanically stirred under a nitrogen atmosphere until homogenization. Next, 19 mL of ammonium hydroxide aqueous solution (25%) was quickly added to the previous mixture, which was allowed to react for 5 min. Stirring was discontinued, and the Fe3O4 particles were separated with magnetic decantation. The bare particles were washed with water several times and dispersed in water at a final concentration of 78 mg/mL.
2.2.2. Ionogel Preparation
The preparation of ionogel followed the procedure described elsewhere with modifications [25,26]. Briefly, for undoped ionogel (without particles), 30 mg of bovine skin gelatin was added to 75 µL of EMIMDCA, followed by 40 µL of distilled water. The mixture was kept in an ultrasonic bath for 15 min and stored for subsequent use. For doped ionogel, rather than 40 µL of distilled water, the same volume of Fe3O4 suspension was used at different concentrations. The composition of ionogel used in each sensor is presented in Table 1.

Table 1.
Sensor identification and synthesis description according to components and their amounts.
2.3. Sensor Assembly and the e-Nose
The sensor was prepared by depositing 40 µL of ionogel on the active part of an interdigitated electrode, followed by spin coating at 10,000 rpm for 5 s. Next, the freshly prepared thin film was dried in a desiccator under vacuum (550 mmHg) for 15 h.
Concerning the e-nose and software for data acquisition, the system used in this work was the same as previously described [27]. Briefly, the software controls the solenoid valves (SVs) and acquires data. For evaluating the changes in the conductance of the electrode according to the sample, SV1 and SV3 are opened and SV2 is closed; thus, gas passes through the sample chamber and goes to the sensor chamber (exposure). In contrast, for removing the sample VOCs (recovery), valves are set in the inverse way: SV1 and SV3 are closed, and SV2 is opened (Figure 1).
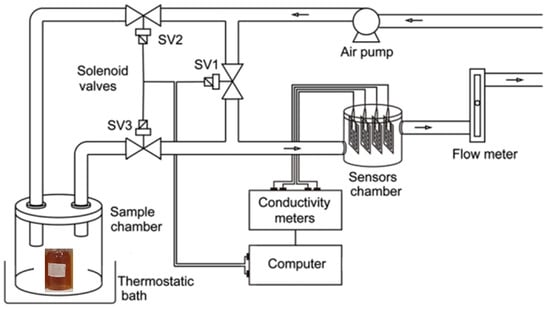
Figure 1.
Schematic representation of the e-nose.
For measurements, a vial containing 5 mL of honey was put inside the sample chamber of the e-nose. The samples were kept at 30.0 ± 0.1 °C using a water bath, and the airflow was 0.5 L·min−1. No further sample treatment was needed. Conductance data were acquired during 7 cycles of exposure (10 s) and recovery (150 s). The whole analysis took 18.7 min.
2.4. Data Processing
Graphical analysis and PCA calculations were performed using Origin software (Origin Lab, Northampton, MA, USA). Weka software (University of Waikato, New Zealand) was used to evaluate the classifiers.
3. Results and Discussion
3.1. Data Acquisition
The samples were analyzed using the sensor array, ionogel doped with different concentrations of bare Fe3O4 nanoparticles. As an example, the conductance data obtained for a given sample (authentic honey 5) by the sensors is shown in Figure 2.
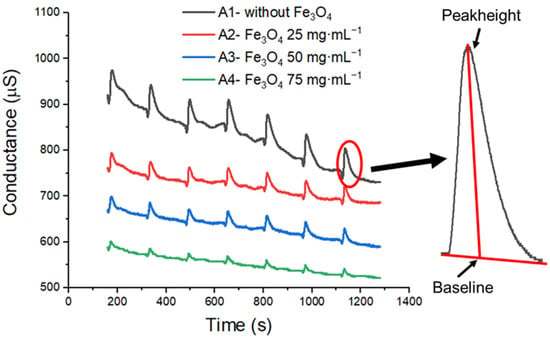
Figure 2.
Data of conductance × time for the sensor array. The inset indicates the determination of peak height and baseline.
Comparing the curves in Figure 2, it is possible to note that in general, increasing the concentration of Fe3O4 led to a decrease in the signal amplitude, which is consistent with our previous work [25]. The peak height and baseline signal (inset Figure 2) were used to calculate the relative response (RR; Equation (1)) of the sensors in each of the cycles. The data of RR obtained for the sensors during each cycle are presented in Table S1.
RR = (peak height − baseline)/baseline
3.2. Data Processing
3.2.1. Graphic Discrimination
Principal component analysis (PCA) is one of the simplest and most widely used multivariate analysis method, an orthogonal vector transformation based on variance criteria, usually applied in dimension reduction and discrimination analysis [28,29]. From the data contained in Table S1, it was possible to calculate the correspondent PCA (Figure S1), which presents huge information and an overlapping region between authentic and adulterated honey. To facilitate the visualization and discussion, the average values of RRs were calculated (Table 2) and used as input variables for the PCA calculation.

Table 2.
RRs average data samples for each sensor.
The PCA biplot obtained for the sensor array is shown in Figure 3. In this biplot, it is possible to note 4 vectors, corresponding to sensors, and 17 square dots, related to samples.
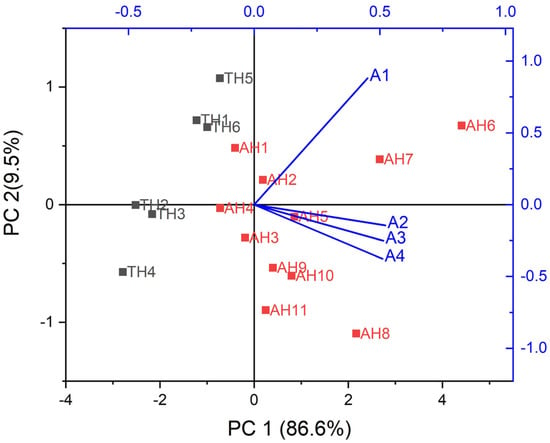
Figure 3.
PCA biplot obtained for authentic honey (TH, ◼) and adulterated honey (AH, ◼) based on average RR data.
In this condition, the variance achieved in PCA was 96.1%, distributed among the two components, PC1 (86.6%) and PC2 (9.5%). The clusters corresponding to authentic and adulterated honey were well defined, and no intersection was observed. In a simplified way, the biplot can be interpreted as follows: the closer the dot is to the vector endpoint, the more significant (higher value) it is for that sensor. For example, from Table 2, sample AH 6 presented the highest values for all sensors and its correspondent dot was closest to the vectors’ extremities. However, the points referring to TH 2 and TH 4 were the farthest and opposite from the vectors’ ends; thus, these samples presented the lowest values for these variables, which agrees with the individual analysis of the data in Table 2.
In this sense, from e-nose data and their correspondent PCA, it is possible to note that in general, for adulterated honey, there was an increase in RR values and, thus, to infer that the developed sensors are sensitive to the adulterating compounds used to defraud honey or their by-products, such as 5-HMF. However, additional studies need to be conducted to confirm this hypothesis.
3.2.2. Machine Learning Classification
Despite the graphical analysis being a valuable alternative for discrimination of the clusters, this task becomes difficult in systems with a large amount of data and containing overlapping regions. Thus, to optimize and automate the treatment of data, reduce subjectivity, and increase the efficiency in the prediction of the results and the robustness of the method, the use of machine learning algorithms for the autonomous classification of the samples is more suitable [30].
In this analysis, the following classifiers were used: instance based (IBK), Kstar, Trees-J48 (J48), random forest (RF), multilayer perceptron (MLP), naive Bayes (NB), and sequential minimal optimization (SMO) [31,32].
These algorithms were selected since they are based on different approaches. IBK and Kstar are based on the K-nearest-neighbor algorithm. These kinds of algorithms use the proximity between the data to classify or group them. The main advantage is that they are non-parametric; thus, they do not need a complex data preparation procedure. J48 and RFs are based on the decision tree algorithm, which uses a set of simple decision rules inferred from the data. This type of algorithm, in addition to being non-parametric, can consider numerical and categorical data. However, they perform well on relatively unbalanced data sets. The MLP classifier is a type of neural network used to solve problems in which the data are not linearly separable or have complex relationships [33,34]. NB is an algorithm based on Bayes’ theorem that consists of conditional probability and takes the input attributes as independent of each other, thus simplifying the calculations and consequently the algorithm implementation [35]. Finally, SMO is an algorithm for solving the quadratic programming problem that arises in support vector machines (SVMs); the latter is a function-based algorithm that uses a hyperplane to separate data from different classes. This hyperplane is chosen considering the closest points between the classes and seeks to maximize the distance between them [36].
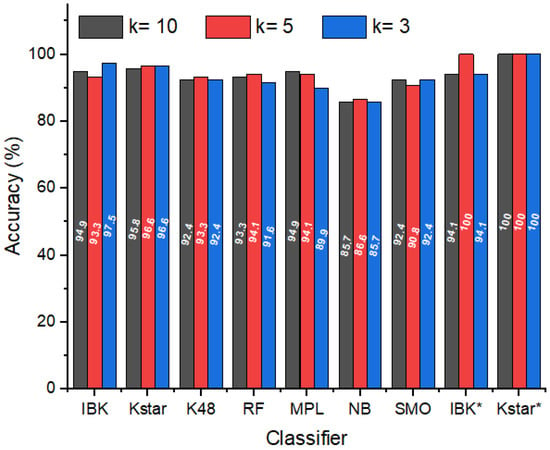
In this sense, based on the RR data for each cycle (Table S1) and considering different values of k-folds for the cross-validation (3, 5, and 10), the accuracy values of the different classifiers were determined and varied from 85.7% to 97.5% (Figure 4). The highest values were obtained for IBK (94.9%, 93.3%, and 97.5% for k = 10, 5, and 3, respectively) and Kstar (95.8%, 96.6%, and 96.6% for k = 10, 5, and 3, respectively). Thus, IBK and Kstar were selected as classifiers for evaluating the average of RRs (Table 2) in the same way as done previously (IBK* and Kstar*). Considering the average data of the measures taken for each sample, for both classifiers, the accuracies reached 100% for at least one k value, IBK* (94.1% for k = 10 and 3 and 100% for k = 5) and Kstar* (100% regardless of the k); in other words, in this case, these classifiers were able to attribute correctly as authentic or adulterated honey all samples considered.
Another way of evaluating the ability of the e-nose-based methodology to discriminate between authentic and adulterated honey is through sensitivity and specificity parameters. Sensitivity is the number of true-positive samples that the applied method identifies, while specificity is the rate of true negatives that are correctly identified. Sensitivity and specificity were calculated according to Equations (2) and (3), respectively [32,37].
Sensitivity = true positive (TP)/((true positive (TP) + false negative (FN))
Specificity = true negative (TN)/(true negative (TN) + false positive (FP))
In this study, the quantity of true positives corresponded to the number of data the sensor assigned as authentic honey, while the number of true negatives corresponded to adulteration. Furthermore, it is necessary to remember that for each sample, seven measurements (seven exposure cycles) were taken. Therefore, in the case of analysis of data from Table S1, to determine sensitivity, the denominator of Equation (2) was 42 (6 samples of authentic honey × 7 measurements), while for the specificity calculation, the denominator of Equation (3) was 77 (11 samples of adulterated honey × 7 measurements). In contrast, for evaluation of the sensitivity and specificity of the average data (Table 1), the denominator of the corresponding equations was 6 and 11, respectively.
Considering the classifiers IBK (k = 3, the accuracy of 97.5%), Kstar (k = 3, accuracy of 96.6%), IBK* (k = 3, the accuracy of 94.1%), and Kstar* (k = 3, accuracy of 100.0%), confusion matrices, sensitivity, and specificity were calculated and are summarized in Figure 5. For IBK (k = 3), the sensitivity was 90.5%, indicating the system could distinguish 38 of the 42 data labeled as authentic honey, attributing 4 measures as adulterated. In the same way, the specificity was 94.8%, which indicates that the sensor discriminated 73 of the 77 measurements as adulterated honey and 4 erroneously as authentic honey. For Kstar (k = 3), the sensitivity was 95.2% and the specificity was 97.4%; in other words, from the total known data, the classifier could discriminate 40 and 75 measures correctly as authentic and adulterated honey, respectively. When the average data were considered, IBK* (k = 3) and Kstar* (k = 3) both correctly attributed all samples concerning authentic honey, with a sensitivity of 100%. Regarding specificity, IBK* (k = 3) erroneously classified 1 of 10 measures (90.9%), while Kstar* did not miss any sample labeled as adulterated honey (100%).
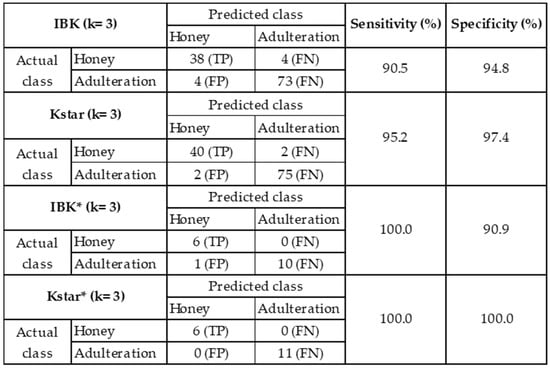
Figure 5.
Confusion matrices, sensitivity, and specificity of classifiers IBK (k = 3), Kstar (k = 3), IBK* (k = 3), and Kstar* (k = 3).
4. Conclusions
This work evaluated the capacity of an e-nose based on thin films of an ionogel composite doped with different concentrations of Fe3O4 nanoparticles for discrimination between authentic and adulterated honey. The sensors were obtained by keeping fixed amounts of ionic liquid and gelatin, while suspensions with different concentrations of Fe3O4 were used (0, 25, 50, and 75 mg·mL−1).
To evaluate the capacity of the e-nose for discrimination between authentic and adulterated honey, two strategies were considered, graphical analysis (PCA) and automated classifiers. In a preliminary analysis using data from individual cycles, PCA presented an overlapping region, while taking the average data, two clusters with no intersection were obtained.
Additionally, based on data from individual cycles, the discrimination accuracy of different machine learning classifiers was calculated; IBK (k = 3) and Kstar (k = 3) presented the highest values of 97.5% and 96.6%, respectively. From the confusion matrix, sensitivity and specificity were calculated for each classifier. In the case of IBK (k = 3), sensitivity was 90.5% and specificity 94.8%, while for Kstar (k = 3), sensitivity and specificity were 95.2% and 97.4%, respectively. Taking the average data of the measures taken for each sample, the accuracy of the classifiers IBK* (k = 3) and Kstar* (k = 3) was 94.1% and 100%, respectively. In the same way, for IBK* (k = 3), the sensitivity was determined as 100% and the specificity as 90.9%. For Kstar* (k = 3), both parameters were 100%.
Finally, the proposed methodology based on e-nose technology is a simple and low-cost alternative for analyzing honey frauds and can be used as a complementary tool for honey quality control, reducing the analysis time and manipulating steps. Nevertheless, further studies must be performed to optimize experimental parameters, data acquisition, and treatment steps. Additionally, more samples must be evaluated for validation of the proposed methodology for discrimination between authentic and adulterated honey.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app13084881/s1, Figure S1: PCA biplot obtained for authentic (TH, ◼) and adulterated honey (AH, ◼) based on individual cycles RRs data. Table S1: RRs data obtained by sensors during individual cycles.
Author Contributions
Conceptualization: W.B.G., W.S.R.T., J.G. and J.G.P.; data curation: W.B.G., W.S.R.T., E.P.C., A.N.d.C.E.S. and O.A.M.; formal analysis: W.B.G., W.S.R.T. and M.d.S.R.M.; funding acquisition: J.G. and J.G.P.; investigation: W.B.G., W.S.R.T., A.N.d.C.E.S. and O.A.M.; methodology: W.B.G., W.S.R.T., M.d.S.R.M., J.G. and J.G.P.; project administration: J.G. and J.G.P.; resources: J.G. and J.G.P.; software: J.G.; supervision: E.P.C., J.G. and J.G.P.; validation: W.B.G., W.S.R.T. and E.P.C.; visualization: W.B.G. and W.S.R.T.; eriting—original draft: W.B.G., W.S.R.T., E.P.C., M.d.S.R.M. and J.G.; writing—review and editing: W.B.G., W.S.R.T., M.d.S.R.M., J.G. and J.G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Brazilian National Council for Scientific and Technological Development (CNPq) through processes 165186/2015-1, 307501/2019-1, and 424027/2018-6.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ahmad, R.S.; Hussain, M.B.; Saeed, F.; Waheed, M.; Tufail, T. Phytochemistry, Metabolism, and Ethnomedical Scenario of Honey: A Concurrent Review. Int. J. Food Prop. 2017, 20 (Suppl. S1), S254–S269. [Google Scholar] [CrossRef]
- Kuropatnicki, A.K.; Kłósek, M.; Kucharzewski, M. Honey as Medicine: Historical Perspectives. J. Apic. Res. 2018, 57, 113–118. [Google Scholar] [CrossRef]
- Burlando, B.; Cornara, L. Honey in Dermatology and Skin Care: A Review. J. Cosmet. Dermatol. 2013, 12, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, C.N.; Wang, X.-H.; Leung, S.; Andrae-Nightingale, L.M.; Schmidt, S.J.; Engeseth, N.J. Selection and Use of Honey as an Antioxidant in a French Salad Dressing System. J. Agric. Food Chem. 2008, 56, 8650–8657. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.; Zhao, Y. Honey in Combination with Vacuum Impregnation to Prevent Enzymatic Browning of Fresh-Cut Apples. Int. J. Food Sci. Nutr. 2005, 56, 165–176. [Google Scholar] [CrossRef]
- Sachdev, S.; Kumar, A.; Ansari, M.I. Health Benefit, Traditional, and Modern Uses of Natural Honey. In Non-Timber Forest Products; Springer International Publishing: Cham, Switzerland, 2021; pp. 281–299. [Google Scholar] [CrossRef]
- Siddiqui, A.J.; Musharraf, S.G.; Choudhary, M.I.; Rahman, A.-U. Application of Analytical Methods in Authentication and Adulteration of Honey. Food Chem. 2017, 217, 687–698. [Google Scholar] [CrossRef]
- Quinto, M.; Miedico, O.; Spadaccino, G.; Paglia, G.; Mangiacotti, M.; Li, D.; Centonze, D.; Chiaravalle, A.E. Characterization, Chemometric Evaluation, and Human Health-Related Aspects of Essential and Toxic Elements in Italian Honey Samples by Inductively Coupled Plasma Mass Spectrometry. Environ. Sci. Pollut. Res. 2016, 23, 25374–25384. [Google Scholar] [CrossRef]
- Manyi-Loh, C.E.; Ndip, R.N.; Clarke, A.M. Volatile Compounds in Honey: A Review on Their Involvement in Aroma, Botanical Origin Determination and Potential Biomedical Activities. Int. J. Mol. Sci. 2011, 12, 9514–9532. [Google Scholar] [CrossRef]
- Derewiaka, D.; Majewska, E.; Kuzak, K.; Szadkowska, D. Comparison of Volatiles and Chemical Composition of Traditional and Non-Traditional Honey Available on the Polish Market. Appl. Sci. 2021, 11, 6371. [Google Scholar] [CrossRef]
- Hong, E.; Lee, S.Y.; Jeong, J.Y.; Park, J.M.; Kim, B.H.; Kwon, K.; Chun, H.S. Modern Analytical Methods for the Detection of Food Fraud and Adulteration by Food Category. J. Sci. Food Agric. 2017, 97, 3877–3896. [Google Scholar] [CrossRef]
- Antônio, D.C.; de Assis, D.C.S.; Botelho, B.G.; Sena, M.M. Detection of Adulterations in a Valuable Brazilian Honey by Using Spectrofluorimetry and Multiway Classification. Food Chem. 2022, 370, 131064. [Google Scholar] [CrossRef]
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical Composition, Stability and Authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Hategan, A.R.; Magdas, D.A.; Puscas, R.; Dehelean, A.; Cristea, G.; Simionescu, B. Machine Learning Algorithms in Corroboration with Isotope and Elemental Profile—An Efficient Tool for Honey Geographical Origin Assessment. Appl. Sci. 2022, 12, 10894. [Google Scholar] [CrossRef]
- Stefas, D.; Gyftokostas, N.; Kourelias, P.; Nanou, E.; Kokkinos, V.; Bouras, C.; Couris, S. A Laser-Based Method for the Detection of Honey Adulteration. Appl. Sci. 2021, 11, 6435. [Google Scholar] [CrossRef]
- Xagoraris, M.; Revelou, P.-K.; Alissandrakis, E.; Tarantilis, P.A.; Pappas, C.S. The Use of Right Angle Fluorescence Spectroscopy to Distinguish the Botanical Origin of Greek Common Honey Varieties. Appl. Sci. 2021, 11, 4047. [Google Scholar] [CrossRef]
- Codex Alimentarius-Standard for Honey. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B12-1981%252Fcxs_012e.pdf (accessed on 14 June 2022).
- Mendez, M.L.R. Electronic Noses and Tongues in Food Science, 16th ed.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Puścion-Jakubik, A.; Borawska, M.H.; Socha, K. Modern Methods for Assessing the Quality of Bee Honey and Botanical Origin Identification. Foods 2020, 9, 1028. [Google Scholar] [CrossRef]
- Tian, H.; Shen, Y.; Yu, H.; Chen, C. Aroma Features of Honey Measured by Sensory Evaluation, Gas Chromatography-Mass Spectrometry, and Electronic Nose. Int. J. Food Prop. 2018, 21, 1755–1768. [Google Scholar] [CrossRef]
- Correa, A.R.; Cuenca, M.M.; Zuluaga, C.M.; Scampicchio, M.M.; Quicazán, M.C. Validation of Honey-Bee Smelling Profile by Using a Commercial Electronic Nose. Ing. Investig. 2017, 37, 45–51. [Google Scholar] [CrossRef]
- Subari, N.; Mohamad Saleh, J.; Md Shakaff, A.; Zakaria, A. A Hybrid Sensing Approach for Pure and Adulterated Honey Classification. Sensors 2012, 12, 14022–14040. [Google Scholar] [CrossRef]
- Zakaria, A.; Shakaff, A.Y.M.; Masnan, M.J.; Ahmad, M.N.; Adom, A.H.; Jaafar, M.N.; Ghani, S.A.; Abdullah, A.H.; Aziz, A.H.A.; Kamarudin, L.M.; et al. A Biomimetic Sensor for the Classification of Honeys of Different Floral Origin and the Detection of Adulteration. Sensors 2011, 11, 7799–7822. [Google Scholar] [CrossRef]
- Faal, S.; Loghavi, M.; Kamgar, S.; Raoufat, M.H.; Golmakani, M.T. Utilizing Pattern Recognition Methods for Detecting the Adulteration of Glucose and Fructose in Honey. J. Res. Innov. Food Sci. Technol. 2018, 7, 419–430. [Google Scholar] [CrossRef]
- Gonçalves, W.B.; Cervantes, E.P.; Pádua, A.C.C.S.; Santos, G.; Palma, S.I.C.J.; Li, R.W.C.; Roque, A.C.A.; Gruber, J. Ionogels Based on a Single Ionic Liquid for Electronic Nose Application. Chemosensors 2021, 9, 201. [Google Scholar] [CrossRef]
- Carvalho, T.; Vidinha, P.; Vieira, B.R.; Li, R.W.C.; Gruber, J. Ion Jelly: A Novel Sensing Material for Gas Sensors and Electronic Noses. J. Mater. Chem. C 2014, 2, 696–700. [Google Scholar] [CrossRef]
- Gruber, J.; Nascimento, H.M.; Yamauchi, E.Y.; Li, R.W.C.; Esteves, C.H.A.; Rehder, G.P.; Gaylarde, C.C.; Shirakawa, M.A. A Conductive Polymer Based Electronic Nose for Early Detection of Penicillium Digitatum in Post-Harvest Oranges. Mater. Sci. Eng. C 2013, 33, 2766–2769. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, I.T.; Cadima, J. Principal Component Analysis: A Review and Recent Developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Lever, J.; Krzywinski, M.; Altman, N. Principal Component Analysis. Nat. Methods 2017, 14, 641–642. [Google Scholar] [CrossRef]
- Netto, M.M.O.; Gonçalves, W.B.; Li, R.W.C.; Gruber, J. Biopolymer Based Ionogels as Active Layers in Low-Cost Gas Sensors for Electronic Noses. Sens. Actuators B Chem. 2020, 315, 128025. [Google Scholar] [CrossRef]
- Olorunshola, O.E.; Irhebhude, M.E.; Evwiekpaefe, A.E.; Ogwueleka, F.N. Evaluation of Machine Learning Classification Techniques in Predicting Software Defects. Trans. Mach. Learn. Artif. Intel. 2020, 8, 1–15. [Google Scholar] [CrossRef]
- Tamizharasi, K.; Umarani, D.V.; Rajasekaran, K. Performance Analysis of Various Data Mining Algorithms. Int. J. Comput. Commun. Inf. Syst. 2014, 6, 118–127. [Google Scholar]
- Kumar, D.; Arora, S. Performance Analysis of Various Data Mining Algorithms: A Review. Int. J. Comput. Appl. 2011, 32, 975–8887. [Google Scholar]
- Lin, W.-C.; Ke, S.-W.; Tsai, C.-F. Top 10 Data Mining Techniques in Business Applications: A Brief Survey. Kybernetes 2017, 46, 1158–1170. [Google Scholar] [CrossRef]
- Chen, S.; Webb, G.I.; Liu, L.; Ma, X. A Novel Selective Naïve Bayes Algorithm. Knowl. Based Syst. 2020, 192, 105361. [Google Scholar] [CrossRef]
- Cervantes, J.; Garcia-Lamont, F.; Rodríguez-Mazahua, L.; Lopez, A. A Comprehensive Survey on Support Vector Machine Classification: Applications, Challenges and Trends. Neurocomputing 2020, 408, 189–215. [Google Scholar] [CrossRef]
- Hossin, M.; Sulaiman, M.N. A Review on Evaluation Metrics for Data Classification Evaluations. Int. J. Data Min. Knowl. Manag. Process 2015, 5, 1–11. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).