Abstract
Microprocessor-controlled prosthetic knees (MPKs) improve the safety and functional capabilities of transfemoral amputees, but there is a lack of information on plantar pressure distribution and effects among individuals who have undergone transfemoral amputation of the sound limb. (1) Background: The aim of this study was to determine possible compensatory mechanisms in gait by evaluating intact extremity foot plantar pressure distribution in young MPK prosthesis users. Twenty-one patients with unilateral transfemoral amputation (TFA) and twenty-four healthy individuals were selected for the study. (2) Methods: The WalkinSense system was used to assess different foot plantar pressure distribution parameters as the participants walked at their chosen walking speed. Plantar pressure peaks and activation percentages in the eight foot regions were measured during the gait cycle. (3) Results: The pressure peaks and activation percentages in the sound limb with TFA patients and healthy subjects were measured, and statistically significant differences between the two groups were identified. The 1-, 2-, 3-, 4-, and 6-point sensor activation percentages significantly increased, whereas the 7- and 8-point sensor activation percentages decreased in the sound limb TFA participants compared with the healthy subjects. Peak plantar pressure sensor points 1, 3, 4, and 6 increased in the TFA sound limb foot in relation to healthy individuals, while they decreased in point 8. (4) Conclusion: In this study, with the use of a microprocessor knee joint TF prosthesis, in the evaluation of the underfoot pressure of intact legs, the maximum pressure point shifted to the forefoot, and it was observed that the forefoot and midfoot were more active during walking compared with the control group. This may indicate that gait compensation and plantar flexion in the sound limb are used more forcefully in the gait cycle.
1. Introduction
Unilateral transfemoral amputees exhibit lower-limb asymmetry because of amputation and distinctive gait characteristics compared with healthy adults [1,2]. They are affected to changes in suspension systems, prosthetic alignment, and prosthetic components, such as the feet, and knee joints [3,4,5,6].
In transfemoral amputees, vaulting may be encountered during the prosthetic swing phase as a gait-compensatory mechanism meant to assist toe clearance. This mechanism is observed with the plantar flexion of the contralateral ankle during the single-limb support phase. Drevelle et al. found that the contralateral limb of transfemoral amputees with vaulting produces higher ankle power during the single-support phase [7]. Borg et al. stated that a significant difference in the plantar pressure–time integral under second–fourth MTP joints maintained in the intact contralateral limb in patients who were transtibial amputees may be the result of changes in gait [8].
The preservation of the contralateral limb is of critical importance, as it may impact the patient’s mobility and quality of life. Mehryar et al. studied muscle synergies between healthy subjects and transfemoral amputees, and statistically significant differences were observed particularly during the stance phase, which mainly activates plantar flexors in the gait cycle in the stance phase in TFA [9].
Technological advances that have led to the emergence of prosthetic knees with electronically controlled stance and swing phases have greatly increased the functional capabilities and safety of transfemoral amputation patients. The use of this technology can improve and increase mobility [10,11,12,13] and walking speed [12,13,14], while reducing the use of walking aids reduces gait asymmetry [15,16,17].
Plantar pressure analysis is clinically effective in the diagnosis of foot deformities, the detection and treatment of gait disturbances, and the prevention of foot ulcers [18,19].
One of the main parameters used in plantar pressure analysis is peak plantar pressure, which is considered to be the highest pressure that occurs in certain parts of the foot during the gait cycle. The instant of pressure peaks can also be calculated, allowing for the recognition of the order in which different parts of the foot are activated. However, the validity of plantar pressure analysis has not yet been determined, and its clinical applicability in field prosthetic use is not clear. Geil and Lay studied the ability of a plantar pressure analysis system to detect changes in the prosthetic alignment of transtibial amputees and concluded that plantar foot pressure analysis is a sensitive and applicable tool that can help clinicians measure gait parameters affected by prosthetic alignment [20].
With regard to the sound limb, although some studies have proven overloading occurs during gait movement [21,22,23], temporal foot roll-over features, plantar pressure distribution patterns, and the identification of specific overloaded zones have yet to be addressed. This information can assist clinicians in preventing foot plantar injuries such as blisters, callosity, and skin ulcers that occur at high-pressure peaks in the foot, and it is beneficial in gait training. Lightweight plantar pressure plates are an effective way to record these data. Castro et al. indicated that unilateral TF amputees showed an asymmetrical plantar pressure distribution and that both lower extremities of patients who had undergone TF amputation were different from healthy subjects, as per plantar pressure analysis; the participants used mechanical knee joints, and the mean age of the participants was 50 [24].
The aim of this study was to measure the plantar pressure distribution under the intact limb of transfemoral amputees using microprocessor prosthetic knees (MPKs) and to evaluate possible gait compensatory mechanisms. Some studies with the rate of loading being higher on the intact side of TFA amputees can be found in the literature, but no articles can be found regarding plantar pressure change on the intact side. We hypothesized that there is a different plantar pressure distribution between healthy subjects and those who underwent TFA. We predicted that different gait patterns reflecting biomechanical demands and adaptation because of amputation may affect the intact side’s plantar pressure distribution. By evaluating the pressure distribution under the intact extremity, compensatory mechanisms in walking can be detected and provide more effective information for gait training.
2. Materials and Methods
2.1. Study Design and Participants
The study participants included 21 subjects with unilateral transfemoral amputations (29.7 ± 5 years old; 5 females and 16 males) and 24 age-matched healthy subjects (21.2 ± 1.7 years old; 9 females and 15 males). The study received approval from the applicable university ethics committee and complied with the principles outlined in the Declaration of Helsinki. The manuscript was prepared according to the Publication Manual of the American Psychological Association using the seventh edition. Each subject signed a written informed consent form before the commencement of any testing. All participants underwent amputation more than 2 years before the experiment. Eighteen amputations were traumatic, two were from diabetes mellitus complications, and one was from vascular disease; in these non-traumatic cases, the individuals did not show difficulty in gait related to the pathology that caused the amputation. Previous walking training and rehabilitation information for the participants was not obtained as this may differ between centers.
The patients were experienced users of microprocessor-controlled (Ottobock, Pilee, Freedom, Rheoknee, Ossur, C-Leg) knee prostheses (able to walk safely with a prosthetic knee for about 1 year) and a prosthetic foot with an energy-storing system. The prosthetic sockets of all participants were of the total contact type, and a pelvic suspension belt was not used. The Medicare Functional Classification Level (MFCL) of the prostheses was 3 or 4. The exclusion criteria were defined as the use of walking aids, incompatible socket fit, recurring skin problems in the stump, and any medical condition that would adversely affect physical performance during the study. The participants used their typical walking sports shoes. Each participant underwent a basic physical assessment, particularly focusing on range of motion and muscle strength.
Manual muscle strength (force-generating capacity) was estimated in the lower extremity muscle groups, including hip flexors, extensors, abductors, and adductors; knee flexors and extensors; and ankle dorsiflexors and plantar flexors. The standard testing positions that are widely used in clinical practice were used for all muscles. A traditional 6-point scale (0–5) was used; half-point scores were allowed between all grades except 4 and 5 [25,26]. The muscle test values varied between 4 and 5 for the intact and amputated sides of the TFA group. In the healthy individuals, the lower extremity manual muscle test score was 5. No limitation was detected in the evaluation of the joint range of motion.
2.2. Procedure
The system used in this study, called “WalkinSense”, consists of a data acquisition and processing unit and eight separate sensors. Underfoot plantar pressure measurements were carried out using the eight-channel WalkinSense device, and the results were evaluated by exporting them from the relevant software (Microelectronics, S.A., Tomorrow Options, WalkinSense version 0.96) [27]. The program consists of a data collection and processing unit that can measure the plantar pressure distribution during the walking phase and the percentage of activation of each sensor during walking thanks to sensors placed under the feet of the person. The participants wore standard and suitable socks equipped with WalkinSense sensors. The sensor placements under the feet were as follows: sensor number 1 was placed under the big toe, as highlighted by the red color seen in the graphic; sensor number 2 was placed under the first metatarsal, as highlighted by the green color seen in the graphic; sensor number 3 was placed under the second metatarsal, as highlighted by blue color seen in the graphic; sensor number 4 was placed under the third and fourth metatarsals, as highlighted by the yellow color seen in the graphic; sensor number 5 was placed between the fourth and fifth metatarsals, as highlighted by the brown color seen in the graphic; sensor number 6 was placed lateral to the midfoot, as highlighted in dark pink; sensor number 7 was placed under the medial of the heel, as highlighted in light blue; and sensor number 8 was placed under the lateral heel, as highlighted in the light brown graphic (Figure 1). Plantar pressure data were recorded during the dynamic gait cycle in participants with TF-sound limb and healthy subjects with the foot of their dominant leg. Pressure values were recorded during the mid-stance phase of the gait cycle when full weight was applied to the foot and all sensors were active. Activation percentage: This percentage shows how active the sensors placed under the feet are throughout the walking cycle. The activation percentage value was also evaluated. The subjects walked at a self-selected speed along a 10 m long walkway. Statistical evaluation was performed using the recorded pressure values in the stance phase (Figure 2).
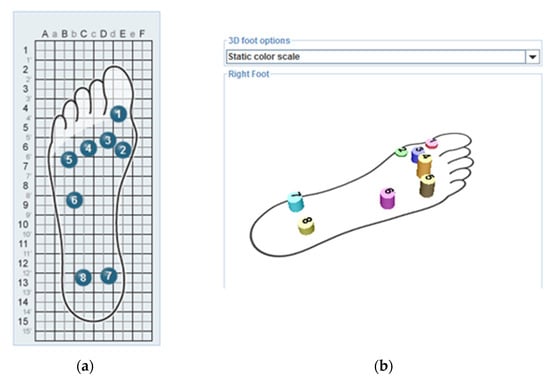
Figure 1.
Underfoot WalkinSense 8-point sensor placements. The placement of the sensors under the feet (a) and the image of the sensors, each in different colors, was reflected on the screen while recording (b).
Figure 2.
Figure showing the activity and peak pressures of the underfoot sensors, each shown separately, during the stance phase.
2.3. Data Analysis
Data were analyzed using a commercial software package made using the SPSS v26 software. Independent samples t-test was used to compare activation percentage and peak plantar pressure values during the gait cycle between TF patient and healthy subjects. Differences were assumed to be statistically significant at p < 0.05. The effect size is based on the context of the study and the conventions for interpreting Cohen’s d (e.g., small = 0.2, medium = 0.5, and large = 0.8).
3. Results
Twenty-one participants with mild TFA (age = 29.7 ± 5 years; weight = 73.8 ± 7.5 kg; height = 169.5 ± 6.6 cm) and twenty-four healthy subjects (age = 21.2 ± 1.7 years; weight = 67.4 ± 8.1 kg; height = 166 ± 6.8 cm) took part in the study (Table 1). A significant difference was detected between the BMI values of the subjects. In the healthy individuals, the BMI value was recorded as a healthy weight (18.5–24.9) at 24, while in the TFA group, a BMI value of 25.4 was considered overweight (25.0–29.9).

Table 1.
Age and anthropometric characteristics of the participants (mean ± standard deviation).
The plantar pressure distribution and sensor activation percentage values obtained from the TF amputees and healthy individuals were examined. The sensor activation percentage shows how active the sensor is during the stance phase, and with an increase in the sensor activation percentage, the activity under the sensor indicated under the foot increases. When we look at the results of the activation percentage of the study, the activation percentages for sensors 1, 2, 3, 4, 6, 7, and 8 were significantly different under the sound limb between the TFA and healthy subjects (Table 2). The 1-, 2-, 3-, 4-, and 6-point sensor activation percentages significantly increased, whereas the 7- and 8-point sensor activation percentages decreased in the sound limb TF participants compared with the healthy subjects. In the TF amputees, the percentage of sensory activation in the sound limb under the foot was higher than that of the control group, especially under sensor numbers 1, 2, 3, 4, and 6. This result shows that the contact activation of these sensor regions during the stance phase in walking is higher; i.e., the percentage of contact in the forefoot and midfoot stance phases is higher than in the control group. The sensor activation percentages for sensors 7 and 8 were found to be less than those for the control group. In these sensor regions, the hindfoot region showed less activation during the gait stance phase. This demonstrates that the contact of the first, second, third, fourth, and sixth metatarsal phalanx points is more intense than the other points in the stance phase and that the forefoot of the foot is used more.

Table 2.
Demonstration of the sensor activation percentage of the eight sensors with the TFA sound limb and the healthy subjects in the stance phase gait cycle (mean ± standard deviation).
When the plantar pressure distribution of the foot was examined, as shown in Table 3, it was determined that the pressure in sensors 1, 3, 4, and 6 in the stance phase of the gait was higher in the TF amputee sound limb than in the control group. In addition, the pressure in sensor number 8 decreased. According to this result, the change in pressure distribution under the sound limb foot in the TF amputees was significant in sensors 1 and 8. The maximum plantar pressure values were higher in the TFA group, especially in the anterior part of the foot. According to the evaluation of the underfoot pressure of the sound leg with the use of a TF prosthesis, the maximum pressure point shifted to the forefoot, and the forefoot and midfoot were more active during walking than in the control group. The rear foot, pressure distribution, and usage percentage were less than those of the control group. Because of the independent-sample t-test, a significant difference was found between the groups, with medium and large effect values.

Table 3.
Demonstration of the plantar pressure distributions of the eight sensors with maximum pressure with the TFA sound limb and the healthy subjects (mean ± standard deviation).
4. Discussion
This study compared and analyzed plantar pressure parameters between the intact limbs of TF amputees and healthy individuals during gait movement. Asymmetrical values in pressure peaks were detected in this study evaluation when the participants who underwent TF amputations walked. This study’s results support the hypothesis that the plantar pressure values of transfemoral amputees with intact limbs and healthy individuals are different. The study found that gait compensations can be seen in TFA individuals using an MPK prosthesis unit, according to plantar pressure data under the foot.
In TF amputees, the function of the knee joint in the prosthesis they use is essential. Devices that can mechanically perform the function of the knee joint have been developed successfully over time up to the present day. In many studies, the effect of prosthetic knee joint components on gait has been examined, and positive effects on gait have been determined in parallel with technological developments [28,29]. In particular, with the use of microprocessor-controlled prosthetic knee (MPK) units, it has been reported that the frequency of falls and tripping decreases, and user satisfaction increases compared with mechanical devices [13,30]. Ranaldi et al. found that the prosthetic side, i.e., the closer proximity of bionic prosthesis users to the SS group, may indicate that this type of prosthetic knee performs better in targeting the physiological kinematic configuration in basic gait events. In addition, the same behavior cannot be identified on the intact side, particularly for the double-support phases. Three different prosthetic knees required similar compensation actions during the weight transfer phases, and activation profiles were recorded and showed that most of the differences in the weight transfer phases were most relevant in the hamstrings and calf muscle activity [31]. According to the results of our study, in TF patients using MPK knee joints, the result of pressure distribution under the foot in the intact limb during the stance phase in the gait cycle was the result of the fact that the pressure is higher in these regions for the duration of weight transfer, whereby the participants use the forefoot and midfoot more actively than healthy individuals; this leads us to surmise that patients can use the compensation mechanism during walking and possibly adopt a vaulting gait. Gait-compensatory mechanisms may occur depending on biomechanical demands, and adaptation mechanisms that may occur in the amputee’s intact leg can also be seen through the difference in plantar pressure distribution under the foot. In transfemoral amputees, intact limb training may be a priority to reduce contraindications that may occur with gait adaptation.
Plantar pressure distribution was used to detect the gait patterns of normal gaits, toe out, toe in, over supination/pronation, and heel-walking gait abnormalities [32]. Plantar pressure has been implemented in foot pathology evaluation [33] and assessments of the feet of diabetic individuals [34]. Plantar pressure data can be used as a guide for detecting gait disorders and gait training in TF prosthesis users. In this study, the forefoot activation percentage and peak plantar pressure value were higher in the sound limb than in the healthy individuals in the stance phase gait cycle. Prosthetic wearers with TF amputations can lift their bodies via the excessive plantar flexion of the intact foot using the compensatory mechanism of vaulting. According to the results, there is higher eccentric muscle work in the knee during the gait stance phase with double support at the beginning of the stance phase [35], and these changes in the knee occur before the plantar flexion of the ankle during the loading response. Higher flexion of the contralateral knee and higher eccentric work help to prepare for the vaulting movement.
As previously mentioned, the purpose of gait compensation is to lift the body up to facilitate toe clearance, and thus, this could emerge as a compensatory mechanism to increase the foot clearance of the prosthetic foot and protect the amputated limb by loading it in a shorter time [8]. This decreased dorsiflexion may also be the result of decreased sound leg deceleration activity and greater hip flexion at the sound leg heel strike [36]. Tatarelli et al. demonstrated that TF amputees increase the muscle co-activation of the sound limb during walking as a compensatory/adaptive mechanism to deal with the prosthetic device. This increased co-activation probably plays a role in prosthetic gait asymmetry and energy consumption [37]. As a result of this study, the increased plantar pressure distribution seen in the intact-side forefoot may have developed for the reasons stated by Carse [1], such as a lack of prosthetic knee flexion in the early stance, a lack of hip extension on the prosthetic side in the late stance, reduced anterior propulsion force on the prosthetic side in the late stance, and reduced prosthetic hip adduction moment in the early stance. Carse et al. showed that unilateral transfemoral amputees experience significant kinetic asymmetries during gait movement as a result of absent loading response knee flexion, and individuals with TFA-intact limbs were shown to be excessive in this regard in comparison with a control group. The authors indicated that the provision of MPKs does not appear to improve any of these marked kinetic asymmetries; therefore, this group continues to walk with reduced energy efficiency [38].
Other studies have shown that 52–89% of unilateral lower limb amputees will frequently encounter lower back pain and that 41–63% will develop osteoarthritis in their sound limbs [39,40]. Rehabilitation programs (during the preoperative and postoperative periods) can be proposed for TFAs in order to improve functional abilities, increase muscle strength, and limit the risk of early degeneration of the musculoskeletal system. The goal of prosthetic rehabilitation is to regain a symmetrical gait. Therapeutic exercises, gait training, and neuromuscular re-education prescribed for this purpose have been reported to be effective in reducing gait deviations and improving functional mobility [41,42,43]. Müßig et al. evaluated trunk and pelvic movement variability in transfemoral amputees using a C-leg system and found that they clearly demonstrated increased kinematic variability in trunk and pelvic movements, indicating that their gait pattern was affected compared with the healthy controls [44]. Villa et al. reported that the vaulting strategy is widely used by people who have undergone transfemoral amputation through their gait analysis results, and the patients in the study showed power generated by the sound ankle at a mid-stance above 0.15 W/kg [45]. This generated power was shown to be a criterion for the vaulting clinical gait strategy of this population during level walking [46]. Esposito et al. stated that individuals who have undergone transfemoral amputation may be at a greater risk of sound knee cartilage or other tissue damage, providing insight into dynamic loading characteristics during walking in young, active individuals exposed to unilateral TFA [47]. According to our results, in the evaluation of the underfoot pressure of the sound leg, the maximum pressure point shifted to the forefoot, and it was observed that the forefoot and midfoot were more active during walking compared with the control group. These results showed that gait compensation and plantar flexion in the sound limb are used more forcefully during the gait cycle, which may affect the biomechanics of the knee joint.
A larger sample size for the TFA group could be considered in future research to confirm and expand upon these results. Future work could also include uneven surfaces and the effects of walking uphill and downhill.
The use of plantar pressure can provide preliminary information about weight transfer between both extremities during the walking phase. Based on our clinical experience in studying transfemoral amputation patients, orthotic insole approaches may be considered according to the results obtained to balance the weight transfer under the foot of the sound limb. Additionally, data may be used for guidance during prosthetic training. On the prosthetic side, considering factors such as prosthetic length, prosthetic alignment, and socket compatibility would be useful in prosthetic rehabilitation and walking training.
5. Conclusions
Prosthetic components and prosthetic alignment used by TF amputees will affect the user’s gait; however, the addition of exercise to rehabilitation programs helps to increase the activity of the foot’s dorsiflexor muscles and may be effective in preventing possible gait disorders. A pressure plate is inexpensive and practical to use, does not require an extensive laboratory environment, and can be used to analyze different aspects of gait. Plantar pressure and activation percentage can provide information on the pressure distribution under the foot throughout the gait cycle and how active the foot parts are during walking. These values can help in better evaluating foot contact and underfoot weight transfer throughout the gait cycle and detecting gait disorders. The use of gait training and prosthetic alignment should be encouraged during prosthetic rehabilitation.
Author Contributions
Conceptualization, S.G. and S.A.; methodology, S.G. and S.A.; software, S.G.; validation and formal analysis, S.G.; investigation and resources, S.G. and S.A.; writing—original draft preparation, S.G.; writing—review and editing, S.G.; supervision, S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially sponsored by the Projects of Scientific Investigation, grant number 11A3630001, from Ankara University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carse, B.; Scott, H.; Brady, L.; Colvin, J. A characterisation of established unilateral transfemoral amputee gait using 3D kinematics, kinetics and oxygen consumption measures. Gait Posture 2020, 75, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Hisano, G.; Namiki, Y.; Hashizume, S.; Hobara, H. Walking characteristics of runners with a transfemoral or knee-disarticulation prosthesis. Clin. Biomech. 2020, 80, 105132. [Google Scholar] [CrossRef] [PubMed]
- Fey, N.P.; Klute, G.K.; Neptune, R.R. The influence of energy storage and return foot stiffness on walking mechanics and muscle activity in below-knee amputees. Clin. Biomech. 2011, 26, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Eshraghi, A.; Abu Osman, N.A.; Karimi, M.; Gholizadeh, H.; Soodmand, E.; Abas WAB, W.; Fasano, A. Gait biomechanics of individuals with transtibial amputation: Effect of suspension system. PLoS ONE 2014, 9, e96988. [Google Scholar] [CrossRef] [PubMed]
- Segal, A.D.; Orendurff, M.S.; Klute, G.K.; McDowell, M.L.; Pecoraro, J.A.; Shofer, J.; Czerniecki, J.M. Kinematic and kinetic comparisons of transfemoral amputee gait using C-Leg and Mauch SNS prosthetic knees. J. Rehabil. Res. Dev. 2006, 43, 857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Bai, X.; Liu, F.; Fan, Y. Effect of prosthetic alignment on gait and biomechanical loading in individuals with transfemoral amputation: A preliminary study. Gait Posture 2019, 71, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Drevelle, X.; Villa, C.; Bonnet, X.; Loiret, I.; Fodé, P.; Pillet, H. Vaulting quantification during level walking of transfemoral amputees. Clin. Biomech. 2014, 29, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Borg, J.; Mizzi, S.; Formosa, C. Peak pressure data and pressure-time integral in the contralateral limb in patients with diabetes and a trans-tibial prosthesis. Gait Posture 2018, 64, 55–58. [Google Scholar] [CrossRef]
- Mehryar, P.; Shourijeh, M.S.; Rezaeian, T.; Khandan, A.R.; Messenger, N.; O’Connor, R.; Farahmand, F.; Dehghani-Sanij, A. Differences in muscle synergies between healthy subjects and transfemoral amputees during normal transient-state walking speed. Gait Posture 2020, 76, 98–103. [Google Scholar] [CrossRef]
- Hahn, A.; Lang, M. Effects of mobility grade, age, and etiology on functional benefit and safety of subjects evaluated in more than 1200 C-leg trial fittings in Germany. J. Prosthet. Orthot. 2015, 27, 86–94. [Google Scholar] [CrossRef]
- Theeven, P.J.; Hemmen, B.; Geers, R.P.; Smeets, R.J.; Brink, P.R.; Seelen, H.A. Influence of advanced prosthetic knee joints on perceived performance and everyday life activity level of low-functional persons with a transfemoral amputation or knee disarticulation. J. Rehabil. Med. 2012, 44, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Hafner, B.J.; Smith, D.G. Differences in function and safety between Medicare Functional Classification Level-2 and -3 trans-femoral amputees and influence of prosthetic knee joint control. J. Rehabil. Res. Dev. 2009, 46, 417–433. [Google Scholar] [PubMed]
- Kahle, J.T.; Highsmith, M.J.; Hubbard, S.L. Comparison of nonmicroprocessor knee mechanism versus C-Leg on prosthesis evaluation questionnaire, stumbles, falls, walking tests, stair descent, and knee preference. J. Rehabil. Res. Dev. 2008, 45, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Eberly, V.J.; Mulroy, S.J.; Gronley, J.K.; Perry, J.; Yule, W.J.; Burnfield, J.M. Impact of a stance phase microprocessor-controlled knee prosthesis on level walking in lower functioning individuals with a transfemoral amputation. Prosthet. Orthot. Int. 2014, 38, 447–455. [Google Scholar] [CrossRef]
- Kaufman, K.R.; Frittoli, S.; Frigo, C.A. Gait asymmetry of transfemoral amputees using mechanical and microprocessor controlled prosthetic knees. Clin. Biomech. 2012, 27, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Schaarschmidt, M.; Lipfert, S.W.; Meier-Gratz, C.; Scholle, H.C.; Seyfarth, A. Functional gait asymmetry of unilateral transfemoral amputees. Hum. Mov. Sci. 2012, 31, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.O.; Comins, J.; Alkjær, T. Assessment of gait symmetry in transfemoral amputees using C-Leg compared with 3R60 prosthetic knees. J. Prosthet. Orthot. 2010, 22, 106–112. [Google Scholar] [CrossRef]
- Rodgers, M. Dynamic foot biomechanics. J. Orthop. Sports Phys. Ther. 1995, 21, 306–316. [Google Scholar] [CrossRef]
- Hessert, M.J.; Vyas, M.; Leach, J.; Hu, K.; Lipsitz, L.A.; Novak, V. Foot pressure distribution during walking in young and old adults. BMC Geriatr. 2005, 5, 8. [Google Scholar] [CrossRef]
- Geil, M.D.; Lay, A. Plantar foot pressure responses to changes during dynamic trans-tibial prosthetic alignment in a clinical setting. Prosthet. Orthot. Int. 2004, 28, 105–114. [Google Scholar] [CrossRef]
- Nolan, L.; Lees, A. The functional demands on the intact limb during walking for active trans-femoral and trans-tibial amputees. Prosthet. Orthot. Int. 2000, 24, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Nolan, L.; Wit, A.; Dudzinski, K.; Lees, A.; Lake, M.; Wychowanski, M. Adjustments in gait symmetry with walking speed in trans-femoral and trans-tibial amputees. Gait Posture 2003, 17, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Royer, T.; Koenig, M. Joint loading and bone mineral density in persons with unilateral, trans-tibial amputation. Clin. Biomech. 2005, 20, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- De Castro, M.P.; Abreu, S.; Pinto, V.; Santos, R.; Machado, L.; Vaz, M.; Vilas-Boas, J.P. Influence of pressure-relief insoles developed for loaded gait (backpackers and obese people) on plantar pressure distribution and ground reaction forces. Appl. Ergon. 2014, 45, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Kendall, F.P.; McCreary, E.K.; Provance, P.G.; Rodgers, M.M.; Romani, W.A. Muscle Testing and Function with Posture and Pain; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2005. [Google Scholar]
- Herbison, G.J.; Isaac, Z.; Cohen, M.E.; Ditunno, J.F. Strength post-spinal cord injury: Myometer vs manual muscle test. Spinal Cord. 1996, 34, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Healy, A.; Burgess-Walker, P.; Naemi, R.; Chockalingam, N. Repeatability of WalkinSense® in-shoe pressure measurement system: A preliminary study. Foot 2012, 22, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Chin, T.; Sawamura, S.; Shiba, R.; Oyabu, H.; Nagakura, Y.; Takase, I.; Machida, K.; Nakagawa, A. Effect of an intelligent prosthesis (IP) on the walking ability of young transfemoral amputees: Comparison of IP users with able-bodied people. Am. J. Phys. Med. Rehabil. 2003, 82, 447–451. [Google Scholar] [CrossRef]
- Johansson, J.L.; Sherrill, D.M.; Riley, P.O.; Bonato, P.; Herr, H. A clinical comparison of variable-damping and mechanically passive prosthetic knee devices. Am. J. Phys. Med. Rehabil. 2005, 84, 563–575. [Google Scholar] [CrossRef]
- Hafner, B.J.; Willingham, L.L.; Buell, N.C.; Allyn, K.J.; Smith, D.G. Evaluation of function, performance, and preference as transfemoral amputees transition from mechanical to microprocessor control of the prosthetic knee. Arch. Phys. Med. Rehabil. 2007, 88, 207–217. [Google Scholar] [CrossRef]
- Ranaldi, S.; De Marchis, C.; Serrao, M.; Ranavolo, A.; Draicchio, F.; Lacquaniti, F.; Conforto, S. Characterization of prosthetic knees through a low-dimensional description of gait kinematics. J. Neuroeng. Rehabil. 2023, 20, 46. [Google Scholar] [CrossRef]
- Chen, M.; Huang, B.; Xu, Y. Intelligent shoes for abnormal gait detection. In Proceedings of the 2008 IEEE International Conference on Robotics and Automation, Pasadena, CA, USA, 19–23 May 2008; pp. 2019–2024. [Google Scholar]
- Tareco, J.M.; Miller, N.H.; Macwilliams, B.A.; Michelson, J.D. Defining flatfoot. Foot Ankle Int. 1999, 20, 456–460. [Google Scholar] [CrossRef]
- Mueller, M.J.; Hastings, M.; Commean, P.K.; Smith, K.E.; Pilgram, T.K.; Robertson, D.; Johnson, J. Forefoot structural predictors of plantar pressures during walking in people with diabetes and peripheral neuropathy. J. Biomech. 2003, 36, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Perry, J. Gait Analysis: Normal and Pathological Function; SLACK Incorporated: San Francisco, CA, USA, 1992. [Google Scholar]
- Ingraham, K.A.; Fey, P.; Simon, A.M.; Hargrove, L.J. Assessing the relative contributions of active ankle and knee assistance to the walking mechanics of transfemoral amputees using a powered prosthesis. PLoS ONE 2016, 11, e0147661. [Google Scholar] [CrossRef] [PubMed]
- Tatarelli, A.; Serrao, M.; Varrecchia, T.; Fiori, L.; Draicchio, F.; Silvetti, A.; Conforto, S.; De Marchis, C.; Ranavolo, A. Global muscle coactivation of the sound limb in gait of people with transfemoral and transtibial amputation. Sensors 2020, 20, 2543. [Google Scholar] [CrossRef] [PubMed]
- Carse, B.; Hebenton, J.; Brady, L.; Davie-Smith, F. Absent loading response knee flexion: The impact on gait kinetics and centre of mass motion in individuals with unilateral transfemoral amputation, and the effect of microprocessor controlled knee provision. Clin. Biomech. 2023, 108, 106061. [Google Scholar] [CrossRef] [PubMed]
- Ephraim, P.L.; Wegener, S.T.; MacKenzie, E.J.; Dillingham, T.R.; Pezzin, L.E. Phantom pain, residual limb pain, and back pain in amputees: Results of a national survey. Arch. Phys. Med. Rehabil. 2005, 86, 1910–1919. [Google Scholar] [CrossRef] [PubMed]
- Morgenroth, D.; Gellhorn, A.C.; Suri, P. Osteoarthritis in the disabled population: A mechanical perspective. PM&R 2012, 4, 20–27. [Google Scholar]
- Highsmith, M.J.; Andrews, C.R.; Millman, C.; Fuller, A.; Kahle, J.T.; Klenow, T.D.; Lewis, K.L.; Bradley, R.C.; Orriola, J.J. Gait training interventions for lower extremity amputees: A systematic literature review. Technol. Innov. 2016, 18, 99–113. [Google Scholar] [CrossRef]
- Gailey, R.; Gaunaurd, I.; Raya, M.; Kirk-Sanchez, N.; Prieto-Sanchez, L.M.; Roach, K. Effectiveness of an evidence-based amputee rehabilitation program: A pilot randomized controlled trial. Phys. Ther. 2020, 100, 773–787. [Google Scholar] [CrossRef]
- Esquenazi, A. Gait analysis in lower-limb amputation and prosthetic rehabilitation. Phys. Med. Rehabil. Clin. N. Am. 2014, 25, 153–167. [Google Scholar] [CrossRef]
- Müßig, J.A.; Brauner, T.; Kröger, I.; Varady, P.A.; Brand, A.; Klöpfer-Krämer, I.; Simmel, S.; Horstmann, T.; Augat, P. Variability in trunk and pelvic movement of transfemoral amputees using a C-leg system compared to healthy controls. Hum. Mov. Sci. 2019, 68, 102539. [Google Scholar] [CrossRef]
- Villa, C.; Drevelle, X.; Bonnet, X.; Lavaste, F.; Loiret, I.; Fodé, P.; Pillet, H. Evolution of vaulting strategy during locomotion of individuals with transfemoral amputation on slopes and cross-slopes compared to level walking. Clin. Biomech. 2015, 30, 623–628. [Google Scholar] [CrossRef]
- Drevelle, X.; Villa, C.; Sauret, C.; Fode, P.; Martinet, N.; Pillet, H.; Lavaste, F. Vaulting quantification for transfemoral amputees in different gait situations. Comput. Methods Biomech. Biomed. Engin. 2013, 16, 126–127. [Google Scholar] [CrossRef]
- Russell Esposito, E.; Aldridge Whitehead, J.M.; Wilken, J.M. Sound limb loading in individuals with unilateral transfemoral amputation across a range of walking velocities. Clin. Biomech. 2015, 30, 1049–1055. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).