Abstract
Successful liver cancer resection requires a comprehensive pre- and intraoperative understanding of the spatial relationships between a patient’s cancer and intrahepatic anatomy. The recent literature has highlighted that patient-specific 3D-printed liver models (3DPLMs) reconstructed from medical imaging data may enhance the comprehension of patients’ liver anatomy and thereby provide a useful preoperative planning and intraoperative guidance tool for liver cancer resection (LCR). The purpose of this systematic review was to critically examine the utility and feasibility of 3DPLMs for LCR surgical planning and intraoperative guidance and explore whether these applications improve patient outcomes. Articles were retrieved from four electronic databases (Scopus, Embase, PubMed, and Curtin University Database) according to predetermined eligibility criteria. In total, 22 eligible articles were identified, including 11 original research articles and 11 case reports. Key concepts were synthesised using an inductive content analysis approach suitable for this heterogeneous body of literature. There is significant descriptive and case-report evidence that 3DPLMs strengthen pre- and intraoperative comprehension of patient liver and liver tumour anatomy and can enhance pre- and intraoperative surgical decision making for LCR. The analysis of these studies presents large variances in the times and costs necessary to produce 3DPLMs, as studies did not provide the full expenses of materials, software, and equipment. Production times were focused on different aspects of the 3D printing process and were not comparable. The review nonetheless demonstrates the potential value of 3DPLMs as preoperative planning and intraoperative guidance tools for LCR. Future studies should detail these economic data points to ensure 3DPLMs’ viability. Further experimental research and randomised controlled trials are also necessary to examine the relationship between 3DPLMs and patient’s intra- and postoperative outcomes.
1. Introduction
Liver cancer is among the most commonly diagnosed malignancies globally and is the third leading cause of cancer deaths worldwide [1,2,3,4]. Its global incidence is predicted to accelerate, implicating significant mortality and burden of disease [1,2]. The most common radical treatment for liver cancer in patients with adequate healthy liver reserves is surgical resection [5,6,7,8,9,10]. Successful resection relies on highly specific preoperative planning due to the liver’s complex anatomy and significant anatomical variability between patients [10,11,12,13]. Hepatic vasculature, for example, may present multiple common variants. The right, middle and left hepatic veins each have three to four independent variants, while the portal veins, hepatic arteries and the biliary tree may also have structural variance [10]. This increases the complexity of the spatial relationships between intrahepatic structures and, therefore, requires adaptive and personalised surgical techniques [10]. During resection, a sufficient future liver remnant must be preserved, while tumours must be resected with an adequate margin to prevent reoccurrence [10,13,14]. This requires a comprehensive pre- and intraoperative understanding of the spatial relationships between a patient’s cancer and hepatic anatomy [10,15,16]. The increasing preference for laparoscopic resection procedures that restrict surgical field overview further emphasises the importance of this understanding [12,17].
Computed tomography (CT) and magnetic resonance imaging (MRI) are fundamental in preoperative planning of liver cancer resection (LCR) [10,11,12,13,18]. CT provides three-dimensional (3D) datasets with high spatial resolution; hence, it is traditionally most commonly used in studies for image processing and segmentation of hepatic structures. In recent years, MRI has also increasingly been performed in liver imaging due to its high contrast-to-noise ratio, and the opportunity of making a clinical diagnosis without using contrast medium. These modalities require the surgeon to mentally reconstruct a 3D visualisation of the liver and its structures from a series of two-dimensional (2D) images [13,15,19]. This can be particularly challenging due to liver’s complex anatomy and the sophisticated spatial relationships between its intrahepatic structures [10,13]. Three-dimensional volume renderings (3DVRs) assist in comprehending these relationships; however, they still require the surgeon to mentally reconstruct depth and spatial location information from a 2D image [16,18,19].
Advances in 3D printing (3DP) may alleviate these difficulties through the introduction of patient-specific 3D-printed liver models (3DPLMs) [20]. 3DP is a form of additive manufacturing where 3D objects are fabricated layer by layer from a digital file [21]. There are several 3DP technologies currently utilised in medicine, and at baseline, these involve a liquid, powder, or filament material being selectively deposited and then fused layer by layer via light, thermal energy, or a bonding agent to produce a solid material [21,22]. Segmentations of patients’ individual liver anatomy and pathology from their medical imaging data can be reconstructed in 3D file formats suitable for 3DP and then used to print representative 3DPLMs [12,17].
Previous literature reviews by Perica and Sun [20], Witowski et al. [23] and Bangeas et al. [24] highlight 3DPLMs’ surgical planning value; however, there is limited systematic research describing whether 3DPLMs enhance LCR surgical planning, or examining whether 3DPLMs improve LCR patients’ intra- and postoperative outcomes. This review, therefore, seeks to build on previous 3DPLM literature by providing an up-to-date critical examination of the utility of 3DPLMs for surgical planning and intraoperative guidance of LCR, exploring whether these applications improve patient outcomes. The rapidly advancing nature of 3DP technology [25,26] justifies an updated review, as does the rising incidence of liver cancer and increasing preference for laparoscopic approaches, which should increase demand for tools that enhance preoperative comprehension of patient liver anatomy.
The following research question will be explored: Do 3DPLMs enhance surgical planning and intraoperative guidance of LCR to improve patient outcomes? To address this question, this review will describe qualitatively the impact of 3DPLMs on pre- and intraoperative LCR surgical decision making, while considering how 3DPLM production methods affect their clinical feasibility, and explore how 3DPLMs affect patients’ LCR outcomes, using several measures, including future liver remnant volume; surgery duration; intraoperative blood loss; postoperative success (i.e., adequate resection margins and lack of reoccurrence) vs. morbidity (i.e., postoperative liver devascularisation or failure); and patients’ LCR literacy/knowledge and ability to provide informed consent.
2. Materials and Methods
2.1. Search Strategy
A thorough literature search was performed following the Preferred Reporting Items for Systematic Review and Meta-Analysis guidelines [27]. Three primary searches were conducted across the Scopus, Embase, and PubMed databases, and the Curtin Library Catalogue (Table 1). The Curtin Library Catalogue contains research articles from an extensive list of scholarly databases beyond Scopus, Embase, and PubMed, and was, therefore, utilised to broaden the search results and achieve a more comprehensive literature search.

Table 1.
Search strategies to identify suitable studies for inclusion in the review.
Search Strategy 1 was designed to identify results discussing 3DPLMs in the context of liver cancer surgery/operations, while Search Strategy 2 used specific search terms to return literature discussing patient outcomes. Search Strategy 3 was a broader search to ensure comprehensive coverage of the literature. The search engine wildcard asterisk (*) symbol was used to further broaden the search results, with the final search for this review being performed in early June 2023.
2.2. Inclusion and Exclusion Criteria
Results were included if they were peer-reviewed, full-text articles published in English in the last five years examining the 3DPLM applications in LCR surgical planning and/or intraoperative guidance. The five-year publishing date threshold was selected to establish currency as 3DP technology has advanced rapidly over recent years. Articles discussing 3DP production methods/materials were included, as these factors directly impact 3DPLMs’ clinical validity and feasibility [28]. Articles describing 3D-printed liver phantoms for surgical simulation were also included, as 3D-printed phantoms in other specialties can provide tangible surgical benefits [28]. The limited original research present in the literature ultimately required the inclusion of case reports, which was a common practice in previous 3DPLM reviews [20,23,24]. To ensure a suitability-focused review, articles were excluded if they concentrated exclusively on liver transplantation or non-cancerous pathology; if they discussed clinical education exclusively without carry over to surgical planning/performance; or if they focused exclusively on 3D bioprinting. Articles were also excluded if they were cadaver studies or if they were not available in English.
2.3. Article Identification, Screening, and Quality Assessment
After performing all searches, duplicate results were removed. The remaining articles were screened by title and abstract to exclude irrelevant results. After full-text screening, 16 eligible articles were identified [9,11,13,15,16,17,19,22,29,30,31,32,33,34,35,36]. These articles’ references were examined to identify five secondary articles [12,14,37,38,39]. One further article was identified serendipitously during a manual search [40]. Altogether, 22 articles were identified for inclusion in the review (Figure 1). The quality of each study was assessed using the “Quality Assessment Tool for Studies with Diverse Designs” (QATSDD), developed by Sirriyeh et al. [41], which evaluates the methodological and reporting quality of the studies by ranking them against 16 quality criteria. The QATSDD is an established tool designed specifically to assess diverse evidence within systematic reviews in healthcare [41] and is, therefore, highly applicable to the literature included in this review. Reference searching and data extraction was conducted by one assessor (T.R.), with results validated by another assessor (A.W.).
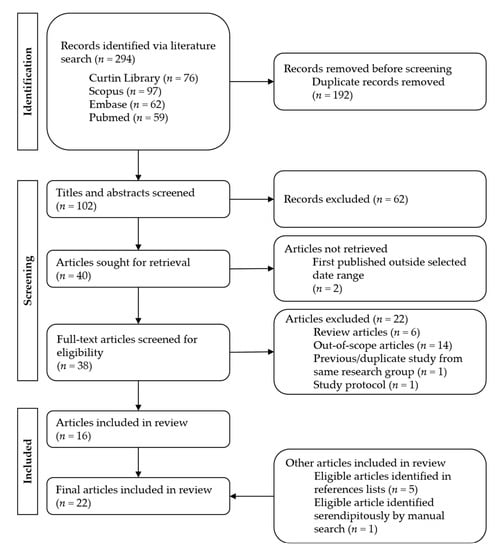
Figure 1.
PRISMA flow chart of literature search process.
2.4. Data Extraction and Synthesis
The articles’ heterogeneity and limited quantitative evidence excluded the use of statistical/quantitative analysis methods in this review [42,43]. Data were instead extracted and synthesised using an inductive content analysis approach [44]. This method is appropriate to distil and synthesise the identified literature in a way that defines key concepts [42,43,44]. Each article was read thoroughly, and open coding was applied. Codes were tabulated and then grouped to define overarching concepts that, when synthesised, describe 3DPLMs’ impacts on LCR surgical planning, intraoperative guidance, and patient outcomes.
3. Results
The articles identified for review constitute a highly heterogeneous body of literature, consisting of 11 case reports, of which 1 is an international conference paper [12] included to increase the review’s comprehensiveness [45]; and 11 original research studies, of which 3 employ a true experimental research methodology, 2 are quasi-experimental studies [15,35], 1 is a descriptive survey study [11], and 5 are observational studies [16,17,19,34,38]. Table 2 further summaries each article’s key characteristics.

Table 2.
Study characteristics and key findings in eligible articles.
The heterogeneous literature prompted quality assessment via the QATSDD from Sirriyeh et al. [41], the results of which are shown in Table 3. The QATSDD highlighted the major limitation of the selected articles—and by extension, the major limitation of this review itself—which is that there is a very limited proportion of methodologically rigorous research describing 3DPLMs’ applications in LCR. The majority of presented evidence is qualitative case-report evidence or is significantly lacking in methodological rigour or statistical significance. It is currently not possible to generalise findings to inform 3DPLM-LCR practice; however, clear themes emerge in this review that may have explanatory power concerning 3DPLMs’ utility in LCR, which may motivate future research efforts to rigorously evaluate the impact of 3DPLMs on LCR patients’ outcomes.

Table 3.
Quality assessment of articles using scoring developed by Sirriyeh et al. [41].
3.1. Surgical Planning and Intraoperative Decision Making
Thirteen articles (59.1%) reported that 3DPLMs enhanced surgical planning for LCR [11,13,14,15,16,17,19,22,29,31,32,33,40]. Seven of these articles were original research studies [11,15,16,17,19,29,31]; however, only two examined actual LCR cases [17,19]. The other five studied how patient-specific 3DPLMs improved resident/intern/medical student surgical planning and anatomical understanding [11,15,16,29,31], and their findings were less transferable to surgeons’ preoperative decision making.
Six articles (27.3%) described the utility of 3DPLMs for intraoperative guidance and navigation of the surgical field during LCR [9,13,19,22,33,40]. Only one original research article supported these findings [19] (the remaining five were case reports), limiting their generalisability.
3.2. 3DPLMs Can Enhance Patient Outcomes
None of the studies demonstrated statistically significant improvements in patients’ surgical outcomes; however, there was still considerable evidence (albeit primarily case-report-based) indicating that 3DPLMs can enhance LCR surgical planning and performance, and by extension, patients’ surgical outcomes [9,13,14,17,19,22,29,30,32,36,37,39,40]. An isolated study by Joo et al. [34] demonstrated that 3DPLMs can improve patients’ postoperative pathological staging and diagnosis by improving the tumour imaging–pathology matching detection rate.
Five articles (22.7%) described how 3DPLMs can enhance patients’ understanding of their pathology and proposed surgical intervention and improve their ability to provide informed consent [22,30,31,32,35]. Only two of these were original research articles [30,35].
3.3. Surgical Simulation and Clinical Education
Four articles (18.2%) described 3DPLMs for surgical simulation of LCR [14,22,38,39], while five articles (22.7%) demonstrated how 3DPLMs may enhance surgical/anatomical teaching [16,29,31,36,37]. These applications are less relevant to this review but suggest that 3DPLMs could improve the preparedness—and, by extension, the surgical performance—of more junior surgical staff.
3.4. Diversity of 3DPLM Production Methods
Table 2 demonstrates the diverse 3DP technologies and materials and varied times and costs involved in 3DPLM production. Fused filament fabrication (FFF) was the most common 3DP technology identified in this review (50%), followed by stereolithography (SLA) (13.6%) and selective laser sintering (SLS) (13.6%). These correlated with the frequently utilised 3DP materials, including acrylonitrile butadiene styrene (ABS) (22.7%), polylactic acid (PLA) (22.7%), and Polymide 12 (PA12, commonly known as nylon) (13.6%). Transparent silicone/silicone-like materials were used in 10 articles (45.5%) to represent liver parenchyma.
The imaging protocols, and segmentation and processing methods used also varied across the included articles. Nevertheless, 3DPLM accuracy was generally adequate for clinical use, with studies reporting CT-validated dimensional error of <0.7–2% [31,37,39] or no greater than a few millimetres [17,22,31]. The majority of studies (68.2%) created 3DPLMs using CT imaging data. Two studies exclusively used MRI datasets as per the preoperative protocol [9,34], and three studies used a combination of CT and MRI [11,14,31] datasets to segment their 3D models without distinction or comment.
3.5. Need for Experimental Research
Most articles described the need for true experimental research to prove the utility and feasibility of 3DPLMs in LCR surgical planning and intraoperative guidance and the benefit of 3DPLMs for patient LCR outcomes [13,15,16,17,19,30,34,35]. Research is also necessary to determine the best-practice approaches to 3DPLM production relative to time and monetary factors [12,16,17,35,36,39,40].
4. Discussion
Analysis of the 22 studies included in this review reveals several key findings, the first being that 3DPLMs can enhance surgical planning and intraoperative guidance of LCR by providing a more cognisable representation of patient liver anatomy and pathology. The improved comprehension of patient liver anatomy granted by 3DPLMs enhances surgical decision making and facilitates more targeted and accurate resections. 3DPLMs may, therefore, provide an overall benefit to LCR patient outcomes; however, further experimental research and clinical trials are necessary to confirm and quantify this relationship definitively.
Traditionally, LCR surgical planning is based on a patient’s CT and/or MRI imaging. The surgeon uses 2D axial/multiplanar images and 3DVRs to determine appropriate resection planes and vascular/biliary reconstruction approaches. This requires using images on a 2D screen to construct a 3D mental visualisation of sophisticated patient liver structures. 3DPLMs can facilitate this task by providing a transparent [13,14,15,16,17,22,31], colour-coded [12,15,16,19,22,32,34,35,36,40], tactile [11,14,15,16,40], manipulable [11,14,16,19,29,31,40] and highly cognisable [14,15,16,17,19,29,31] representation of individualised patient liver anatomy, which improves comprehension of the complex and highly variable 3D spatial relationships between intrahepatic structures [14,16,19,22,29,31,33,40] and enhances cognitive localisation of liver tumours [15,16,17,29], as demonstrated in Figure 2. The improved anatomical perception assists surgeons in selecting the most appropriate surgical strategies [11,14,16,17,19,29,33,40], resection planes [13,16,17,33,40], and vascular reconstruction approaches [13,40].
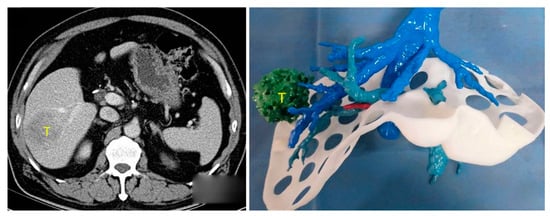
Figure 2.
Three-dimensional spatial location and relationship of liver tumour (T) with intrahepatic anatomy can be better appreciated using 3DPLM (right) compared to CT (left). Reprinted with permission under the open access from Cheng et al. [29].
The above description of 3DPLMs’ preoperative value is drawn primarily from qualitative, case-report evidence, limiting its generalisability. In a prospective observational study, however, Witowski et al. [17] reported that using 3DPLMs in surgical planning of laparoscopic LCR resulted in preoperative changes to surgical strategy for four patients (21% of study sample) due to the models improving cognitive localisation of intrahepatic lesions. Lopez-Lopez et al. [31] performed a descriptive survey of HPB consultants, which indicated unanimously that 3DPLMs could improve preoperative understanding of intrahepatic spatial relationships and assist in defining the most appropriate LCR surgical strategies. These studies were limited by small sample sizes and were not truly experimental; however, they indicate 3DPLMs’ baseline utility in LCR surgical planning.
Further original research supporting 3DPLMs’ surgical planning value is limited to resident/intern populations. In a randomised controlled study, Cheng et al. [29] showed statistically significant improvement in interns’ surgical planning test scores when using 3DPLMs (vs. CT and 3DVRs). Yang et al. [16], in a prospective comparative study involving surgical residents, demonstrated that 3DPLMs (vs. CT and 3DVRs) resulted in statistically significant improvements in tumour localisation and surgical plan design. These studies were more methodologically rigorous than those by Witowski et al. and Lopez-Lopez et al.; however, they only demonstrated 3DPLMs’ surgical planning value for trainee liver surgeons.
This raises the question: Do qualified liver surgeons, who are capable of planning/performing LCR using medical images, actually receive significant benefit from 3DPLMs? In a quasi-experimental study, Huettl et al. [15] found no significant difference in the surgical planning abilities of HPB consultants and fellows when presented with either 3DPLMs or 3D-PDF liver models (equivalent to 3DVRs). This study’s methodology was limited, and it lacked comparison of 3DPLMs and 2D axial/multiplanar imaging, but it supported the idea that 3DPLMs may be superfluous for qualified surgeons [9,19]. 3DPLMs’ value may be limited, therefore, to particularly complex LCR cases, where lesions are located deeply among intrahepatic structures, or where underlying liver disease limits future liver remnant viability [9,13,17,19,31,40].
HPB surgeons must be proficient in correlating mental reconstructions of patient liver anatomy with the actual surgical field during an operation [19]. This is particularly important during laparoscopic procedures where there is restricted anatomical overview [12,17]. Several articles argue, therefore, of the benefits of 3DPLMs for intraoperative guidance during LCR [9,13,19,22,33,40]. 3DPLMs can be oriented and compared directly to the surgical field, improving comprehension and navigation of the viewed anatomy (Figure 3) [9,19,33]. In this application, 3DPLMs can be used to better locate and identify lesions for resection [9,13,19], verify the position of vascular structures to inform potential adjustments to the resection plane [19,33], and better comprehend the locations of deeper anatomical structures hidden within the liver parenchyma [13,19,22]. 3DPLMs may also improve intraoperative detection of smaller metastases to ensure removal [13], and they may decrease the likelihood of intraoperative complications such as damage to intrahepatic vessels [19] and liver devascularisation [13,40].
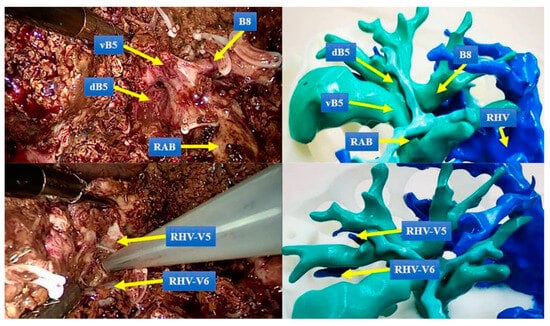
Figure 3.
Comparison of 3DPLM to the surgical field shows that it enhances identification of key structures. vB5: the ventral bile duct of segment 5, dB5: the dorsal bile duct of segment 5, B8: the bile duct of segment 8, RAB: right anterior bile duct, RHV: right hepatic vein, RHV-V5: the venous reflux of hepatic segment 5 to RHV, RHV-V6: the venous reflux of hepatic segment 6 to RHV. Reprinted with permission under the open access from Cheng et al. [19].
Again, these findings are not generalisable as they are drawn primarily from qualitative, case-report evidence. A prospective comparative study by Cheng et al. [19] was the only original research identified that demonstrates direct evidence of 3DPLMs’ intraoperative utility during LCR. In this study, the use of 3DPLMs for intraoperative guidance resulted in real-time changes to the surgical strategy for four patients in the 3DPLM group (16.7% of the study group) due to improved navigation of the surgical field (vs. no changes in the non-3DPLM group). This study was limited by its small sample size and heterogeneity of patient cases. The impact of 3DPLMs on intraoperative decision making in this study could also have been confounded by its surgeons using indocyanine green fluorescent staining as a supplementary navigation tool across the entire study population. Elsewhere, as with surgical planning, 3DPLMs’ utility for intraoperative guidance of LCR was demonstrated qualitatively and through case-report evidence, but currently lacks a methodologically rigorous backing. Similarly, it seems intraoperative use of 3DPLMs is likely mostly relevant for highly complex or laparoscopic cases [9,13,19].
Despite the reported preoperative and intraoperative value of 3DPLMs for LCR, this review identified no statistically significant evidence that 3DPLMs improve patients’ surgical outcomes. Only three studies included in this review recorded patients’ intra- and/or postoperative outcome data [19,30,33]. There were no statistically significant positive correlations identified between the use of 3DPLMs and patients’ future liver remnant volume, duration of surgery, intraoperative blood loss, resection margins, cancer reoccurrence, liver devascularisation, or postoperative liver failure.
The lack of statistical evidence does not disprove the potential benefit of 3DPLMs for patient outcomes. Instead, it is likely a product of there being no exhaustive experimental research with sample sizes large enough to overcome the heterogeneity of individual LCR cases [17,19,30,31]. Ultimately, there is considerable qualitative evidence that 3DPLMs can optimise LCRs and permit more targeted resections, which minimise complications. This should increase the likelihood of postoperative success and reduce the chances of morbidity, implying that 3DPLMs can directly improve LCR patients’ surgical outcomes.
A clear theme across the literature included in this review is the demand for methodologically rigorous, multi-institutional clinical trials to prove the clinical utility of 3DPLMs in LCR surgical planning and intraoperative guidance [13,15,16,17,19,30,34,35]. These trials must incorporate appropriate control groups and enrol sufficient patients to reach statistically significant conclusions [17,19]. One such study has been developed by Huber et al. [46] and is scheduled for completion during 2024. This type of research is crucial to progress 3DPLM technology past its current ‘proof-of-concept’ phase.
3DPLMs may also benefit patient outcomes by improving patients’ understanding of their pathology and surgical intervention [22,30,31,32,35]. In a randomised controlled trial, Giehl-Brown et al. [30] demonstrated statistically significant improvements in LCR patients’ surgical education when using 3DPLMs (vs. education sheets). Yang et al. [35] demonstrated statistically significant improvements in paediatric patients’ parents’ LCR understanding when consulted using 3DPLMs (vs. CT images). The latter study’s pre-test/post-test methodology likely confounds its results, but together these articles’ findings are consistent with other literature examining the patient education value of 3D-printed models [47,48,49].
Improving patients’ LCR knowledge using 3DPLMs should enhance their participation in preoperative decision making and ability to provide informed consent [30]. Greater health literacy following 3DPLM-based education may also improve their postoperative compliance, which then correlates positively with reduced length of hospital stay and decreased postoperative complications [30]. Although limited to gross specimens, 3DPLMs may also benefit patients’ postoperative diagnostic/staging outcomes by improving tumour imaging–pathology matching detection rate [34].
The 3DPLMs demonstrated by articles in this review can be divided into four categories as demonstrated in Figure 4: (1) single/large-piece 3D-printed parenchyma/tumour model; (2) 3D-printed intrahepatic structures model (with/without 3D-printed parenchyma casing); (3) 3D-printed intrahepatic structures model and parenchyma mould with casted/transparent parenchyma; (4) and simulation-specific hollow models.
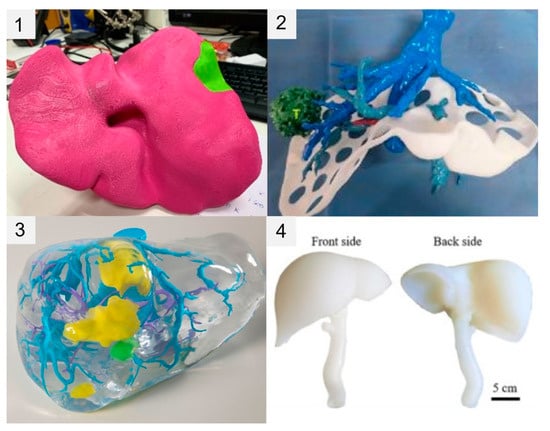
Figure 4.
Comparison of four different 3DPLM construction approaches. (1) Single/large-piece 3D-printed parenchyma/tumour model; (2) 3D-printed intrahepatic structures model (with/without 3D-printed parenchyma casing); (3) 3D-printed intrahepatic structures model and parenchyma mould with casted/transparent parenchyma; (4) simulation-specific hollow models. Image 1 (top left) reprinted with permission under the open access from Tooulias et al. [32]. Image 2 (top right) reprinted with permission under the open access from Cheng et al. [29]. Image 3 (bottom left) reprinted with permission under the open access from Huettl et al. [15]. Image 4 (bottom right) reprinted with permission under the open access from Tan et al. [37].
Construction approach aside, 3DPLM production begins with a patient’s volumetric imaging data (typically CT or MRI) being post-processed (e.g., noise and blur reduction [12] and co-registration of multiphase CT volumes [13]/fusion of CT and MRI volumes [31]) and segmented to produce a 3D virtual liver/intrahepatic structures model [12]. The current literature aligns with the previous findings of Perica and Sun [20], demonstrating the predominant use of CT imaging data compared to MRI for segmentation of the 3D rendered models. Although MRI provides high soft tissue contrast useful in evaluating focal liver lesions, it is utilised less frequently for 3DPLM production due to its inferior spatial resolution and the limited availability of high-resolution 3T scanners [34]. CT is preferred, as its superior spatial resolution and ability to rapidly acquire contrast-enhanced images in multiple phases allows highly accurate and detailed delineation of intrahepatic vasculature while minimising patient motion artifacts [10]. This ultimately produces imaging datasets more conducive to accurate 3DPLM production. Higher spatial resolutions possible via CT also improve the accuracy and effectiveness of automatic and semi-automatic segmentation processes, better supporting 3DPLM production efficiency [10,20].
Segmentation involves digitally extracting the individual voxels within a patient’s imaging volume, which correspond to the liver parenchyma and separate intrahepatic structures and lesions [12,50]. Segmentations are exported in a file format—typically stereolithography (STL)—that uses a tessellated polygon mesh to represent the 3D hepatic structures’ surface geometries [12,14,15,16,22,34,35]. The 3D printer uses these files to produce a 3D-printed model [12]. Figure 5 demonstrates an example of a final STL rendering used for 3DPLM production, compared to a segmented CT image slice and original CT image slice from the corresponding patient imaging data.
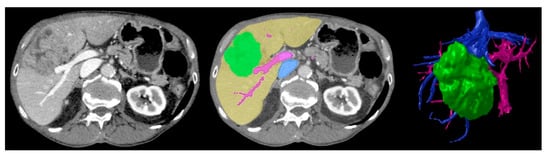
Figure 5.
Baseline CT image (left) vs. segmented image (middle) where green = tumour, yellow = liver parenchyma, pink = arterial vasculature, blue = venous vasculature, vs. final STL 3D rendering (right) demonstrating corresponding anatomical structures. Reproduced with permission under the open access from Witowski et al. [17].
The current ‘gold standard’ for diagnostic-use liver segmentation is a manual approach (i.e., voxel-by-voxel, slice-by-slice); however, this is highly laborious and time consuming [20,51]. Accordingly, the articles in this review use combinations of semi-automatic and automatic segmentation techniques, including adaptive region growing [13,17,31,32,35] and threshold filtering [17,32] (i.e., region-based techniques [50]); and active contour algorithms [13,31] (i.e., boundary-based techniques [50]). These algorithm-based approaches automatically isolate and segment liver/intrahepatic tissues based on homogeneities and/or changes in local voxel/tissue intensity [50]. They increase segmentation efficiency considerably, but still require time-consuming manual validation and correction [11,16,17,35,51,52,53].
A major limitation of 3DPLMs is their time and monetary costs [9,11,13,14,15,16,17,19,31,35,36]. Average 3DPLM production times across the reviewed articles were ~24 h, owing to the laborious nature of segmentation and wait times during the 3DP process [14,15,35]. Ongoing research aims to increase the efficiency and reproducibility of automatic liver segmentation using artificial intelligence to reduce 3DPLM production times [12]. The average cost of 3DPLMs in this review ranged from ~USD 100 to ~USD 2000 (excludes software and 3D printer costs). Costs should decrease as 3DP technology becomes more accessible; however, they currently limit the 3DPLMs’ feasibility for day-to-day clinical use [16,19,31,33,35,40].
3DPLMs’ clinical feasibility must be considered against their 3DP technology [22]. For example, an FFF 3DPLM’s resolution is limited by the FFF printer’s nozzle diameter [21]. Lesions and vessels smaller than this diameter cannot be printed [21]. It is possible to artificially increase the diameter of lesions that are otherwise too small to print, as demonstrated by Joo et al. [34]; however, this approach undermines dimensional accuracy. It could not be used for a 3DPLM used to guide a millimetre-precise LCR—instead, a more advanced, and likely more expensive, 3D printer technology would be required. In contrast, a patient education 3DPLM could sacrifice millimetre accuracy to reduce costs. This illustrates the need for research that standardises and validates which 3DP technologies and 3DPLM types are most feasible and cost-effective for different 3DPLM clinical applications [17,39].
Ultimately, the selection of 3D printing methods and materials depends on the 3DPLM’s clinical application. SLA 3D printing methods use photopolymers to create transparent or opaque resin models with high detail, which may assist in surgical planning, while SLS technology often uses nylon to produce models with high elasticity, strength, and heat resistance for surgical training. Despite the aforementioned limitations of FFF 3D printing, this method is popular as it is suitable to use with a range of materials with different properties and produces minimal waste. ABS and PLA produce tough, rigid models and are some of the most affordable materials used in FFF 3D printing. Brown et al. [30] reported statistically significant improvements in patient education supported by PLA FFF 3DPLMs. The low cost of production in this context is particularly encouraging for further research. Alternatively, FFF can be used to create more flexible 3D models from elastomers. Estermann et al. [38] demonstrated several silicone elastomer materials, and Tejo-Otero et al. [39] demonstrated agarose, or a 1:1 mixture of polyvinyl alcohol and phytagel, as potential 3DPLM materials that mimic biological liver tissue. They point toward an exciting future where patient-specific, tissue-life-like 3DPLMs can be used to preoperatively rehearse and perfect complex patient LCRs [14,22,36,38,39].
5. Conclusions
There is significant qualitative and case-report evidence that 3DPLMs strengthen pre- and intraoperative comprehension of liver and tumour anatomy by providing accurate and highly cognisable 3D representations of patients’ individualised intrahepatic structures. This improved anatomical comprehension can optimise preoperative decision making and enhance intraoperative surgical performance for LCR. Future research should look to determine the clinical value and feasibility of 3DPLMs for LCR surgical planning and intraoperative guidance in terms of patients’ direct surgical outcomes, and the monetary and time costs associated with different 3DPLM production methods.
Author Contributions
Conceptualisation, T.R.; investigation, T.R.; methodology, T.R.; formal analysis, T.R. and A.W.; writing—original draft preparation, T.R.; writing—review and editing, T.R., A.W. and Z.S.; visualisation, T.R.; supervision, Z.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a Perth Radiological Clinic Foundation Grant.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, P.; Henshaw, C.; Youlden, D.R.; Clark, P.J.; Aitken, J.F.; Baade, P.D. Global trends in incidence rates of primary adult liver cancers: A systematic review and meta-analysis. Front. Oncol. 2020, 10, 171. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-G.; He, Z.-Y.; Chen, Y.-Y.; Gao, H.; Du, X.-L. Incidence and survival outcomes of secondary liver cancer: A Surveillance Epidemiology and End Results database analysis. Transl. Cancer Res. 2021, 10, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.; Hydon, K.; Lodge, P. Primary and secondary liver tumours. InnovAiT 2016, 9, 477–482. [Google Scholar] [CrossRef]
- Xiong, J.-J.; Altaf, K.; Javed, M.A.; Huang, W.; Mukherjee, R.; Mai, G.; Sutton, R.; Liu, X.-B.; Hu, W.-M. Meta-analysis of laparoscopic vs open liver resection for hepatocellular carcinoma. World J. Gastroenterol. 2012, 18, 6657–6668. [Google Scholar] [CrossRef] [PubMed]
- Kasai, M.; Cipriani, F.; Gayet, B.; Aldrighetti, L.; Ratti, F.; Sarmiento, J.M.; Scatton, O.; Kim, K.-H.; Dagher, I.; Topal, B.; et al. Laparoscopic versus open major hepatectomy: A systematic review and meta-analysis of individual patient data. Surgery 2018, 163, 985–995. [Google Scholar] [CrossRef]
- Cillo, U.; Fondevila, C.; Donadon, M.; Gringeri, E.; Mocchegiani, F.; Schlitt, H.J.; Ijzermans, J.N.M.; Vivarelli, M.; Zieniewicz, K.; Olde Damink, S.W.M.; et al. Surgery for cholangiocarcinoma. Liver Int. 2019, 39, 143–155. [Google Scholar] [CrossRef]
- Dhir, M.; Sasson, A.R. Surgical management of liver metastases from colorectal cancer. J. Oncol. Pract. 2016, 12, 33–39. [Google Scholar] [CrossRef]
- Rhu, J.; Kim, M.S.; Kim, S.; Choi, G.-S.; Kim, J.M.; Joh, J.-W. Application of three-dimensional printing for intraoperative guidance during liver resection of a hepatocellular carcinoma with sophisticated location. Ann. Hepatobiliary Pancreat. Surg. 2021, 25, 265–269. [Google Scholar] [CrossRef]
- Agostini, A.; Borgheresi, A.; Floridi, C.; Carotti, M.; Grazzini, G.; Pagnini, F.; Guerrini, S.; Pradella, S.; Carrafiello, G.; Vivarelli, M.; et al. The role of imaging in surgical planning for liver resection: What the radiologist needs to know. Acta Biomed. 2020, 91, 18–26. [Google Scholar]
- Streba, C.T.; Popescu, S.; Pirici, D.; Gheonea, I.A.; Vlădaia, M.; Ungureanu, B.S.; Gheonea, D.I.; Ţenea-Cojan, T.S. Three-dimensional printing of liver tumors using CT data: Proof of concept morphological study. Rom. J. Morphol. Embryol. 2018, 59, 885–893. [Google Scholar] [PubMed]
- Guachi, R.; Bici, M.; Bini, F.; Calispa, M.E.; Oscullo, C.; Guachi, L.; Campana, F.; Marinozzi, F. 3D printing of prototypes starting from medical imaging: A liver case study. In Proceedings of the Second International Conference on Design Tools and Methods in Industrial Engineering, Rome, Italy, 9–10 September 2021. [Google Scholar]
- Huber, T.; Huettl, F.; Tripke, V.; Baumgart, J.; Lang, H. Experiences with three-dimensional printing in complex liver surgery. Ann. Surg. 2021, 273, e26–e27. [Google Scholar] [CrossRef] [PubMed]
- Muguruza Blanco, A.; Krauel, L.; Artés, F.F. Development of a patients-specific 3D-printed preoperative planning and training tool, with functionalized internal surfaces, for complex oncologic cases. Rapid Prototyp. J. 2019, 25, 363–377. [Google Scholar] [CrossRef]
- Huettl, F.; Saalfeld, P.; Hansen, C.; Preim, B.; Poplawski, A.; Kneist, W.; Lang, H.; Huber, T. Virtual reality and 3D printing improve preoperative visualization of 3D liver reconstructions—Results from a preclinical comparison of presentation modalities and user’s preference. Ann. Transl. Med. 2021, 9, 1074. [Google Scholar] [CrossRef]
- Yang, T.; Lin, S.; Xie, Q.; Ouyang, W.; Tan, T.; Li, J.; Chen, Z.; Yang, J.; Wu, H.; Pan, J.; et al. Impact of 3D printing technology on the comprehension of surgical liver anatomy. Surg. Endosc. 2019, 33, 411–417. [Google Scholar] [CrossRef]
- Witowski, J.; Budzyński, A.; Grochowska, A.; Ballard, D.H.; Major, P.; Rubinkiewic, M.; Złahoda-Huzior, A.; Popiela, T.J.; Wierdak, M.; Pędziwiatr, M. Decision-making based on 3D printed models in laparoscopic liver resections with intraoperative ultrasound: A prospective observational study. Artif. Intell. Med. 2020, 30, 1306–1312. [Google Scholar] [CrossRef]
- Haberman, D.M.; Andriani, O.C.; Segaran, N.L.; Volpacchio, M.M.; Micheli, M.L.; Russi, R.H.; Pérez Fernández, I.A. Role of CT in two-stage liver surgery. RadioGraphics 2022, 42, 106–124. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, Z.; Liu, J.; Dou, C.; Yao, W.; Zhang, C. Value of 3D printing technology combined with indocyanine green fluorescent navigation in complex laparoscopic hepatectomy. PLoS ONE 2022, 17, e0272815. [Google Scholar] [CrossRef]
- Perica, E.R.; Sun, Z. A systematic review of three-dimensional printing in liver disease. J. Digit. Imaging 2018, 31, 692–701. [Google Scholar] [CrossRef]
- Liacouras, P.; Wake, N. 3D printing principles and technologies. In 3D Printing for the Radiologist; Wake, N., Ed.; Elsevier: St. Louis, MO, USA, 2021; pp. 61–73. [Google Scholar]
- Valls-Esteve, A.; Tejo-Otero, A.; Lustig-Gainza, P.; Buj-Corral, I.; Fenollosa-Artés, F.; Rubio-Palau, J.; Barber, I.; Munuera, J.; Fondevilla, C.; Krauel, L. Patient-specific 3D printed soft models for liver surgical planning and hands-on training. Gels 2023, 9, 339. [Google Scholar] [CrossRef]
- Witowski, J.S.; Coles-Black, J.; Zuzak, T.Z.; Pędziwiatr, M.; Chuen, J.; Major, P.; Budzyński, A. 3D printing in liver surgery: A systematic review. Telemed. e-Health 2017, 23, 941–1022. [Google Scholar] [CrossRef]
- Bangeas, P.; Tsioukas, V.; Papadopoulos, V.N.; Tsoulfas, G. Role of innovative 3D printing models in the management of hepatobiliary malignancies. World J. Hepatol. 2019, 11, 574–585. [Google Scholar] [CrossRef]
- Jakus, A.E.; Huang, Y.-H.; Wake, N. The future of medical 3D printing in radiology. In 3D Printing for the Radiologist; Wake, N., Ed.; Elsevier: St. Louis, MO, USA, 2021; pp. 201–214. [Google Scholar]
- Mitsouras, D.; Liacouras, P.C.; Wake, N.; Rybicki, F.J. RadioGraphics update: Medical 3D printing for the radiologist. RadioGraphics 2020, 40, E21–E23. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Higgins, M.; Leung, S.; Radacsi, N. 3D printing surgical phantoms and their role in the visualization of medical procedures. Ann. 3D Print. Med. 2022, 6, 100057. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, Z.-F.; Yao, W.-F.; Liu, J.-W.; Lu, Y.; Wang, Q.; Cai, X.-J. Comparison of 3D printing model to 3D virtual reconstruction and 2D imaging for the clinical education of interns in hepatocellular carcinoma: A randomized controlled study. J. Gastrointest. Oncol. 2023, 14, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Giehl-Brown, E.; Dennler, S.; Garcia, S.A.; Seppelt, D.; Oehme, F.; Schweipert, J.; Weitz, J.; Riediger, C. 3D liver model-based surgical education improves preoperative decision-making and patient satisfaction—A randomized pilot trial. Surg. Endos. 2023, 37, 4545–4554. [Google Scholar] [CrossRef]
- Lopez-Lopez, V.; Robles-Campos, R.; García-Calderon, D.; Lang, H.; Cugat, E.; Jiménez-Galandes, S.; Férnandez-Cebrian, J.M.; Sánchez-Turrión, V.; Fernández-Fernández, J.M.; Barrera-Gómez, M.A.; et al. Applicability of 3D-printed models in hepatobiliary surgey: Results from “LIV3DPRINT” multicenter study. HPB 2021, 23, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Tooulias, A.; Tsoulfas, G.; Papadopoulos, V.; Alexiou, M.; Karolos, I.-A.; Pikridas, C.; Tsioukas, V. Assisting difficult liver operations using 3D printed models. Livers 2021, 1, 138–146. [Google Scholar] [CrossRef]
- Li, Y.; Yin, X.; Zhu, S.; Liao, C.; Wu, Y.; Liu, Y.; Cai, R.; Yao, L.; Cai, C.; Xie, W. Application value of three-dimensional printing technology assisted laparoscopic anatomic liver resection of segment 8. Chin. J. Dig. Surg. 2021, 20, 548–554. [Google Scholar]
- Joo, I.; Kim, J.H.; Park, S.J.; Lee, K.; Yi, N.-J.; Han, J.K. Personalized 3D-printed transparent liver model using the hepatobiliary phase MRI: Usefulness in the lesion-by-lesion imaging-pathologic matching of focal liver lesions—Preliminary results. Investig. Radiol. 2019, 54, 138–145. [Google Scholar] [CrossRef]
- Yang, T.; Tan, T.; Yang, J.; Pan, J.; Hu, C.; Li, J.; Zou, Y. The impact of using three-dimensional printed liver models for patient education. J. Int. Med. Res. 2018, 46, 1570–1578. [Google Scholar] [CrossRef]
- Smillie, R.W.; Williams, M.A.; Richard, M.; Cosker, T. Producing three-dimensional printed models of the hepatobiliary system from computed tomography imaging data. Ann. R. Coll. Surg. Engl. 2021, 103, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Li, D.; Jeong, M.; Yu, T.; Afat, S.; Grund, K.-E.; Qiu, T. Soft liver phantom with a hollow biliary system. Ann. Biomed. Eng. 2021, 49, 2139–2149. [Google Scholar] [CrossRef] [PubMed]
- Estermann, S.-J.; Pahr, D.H.; Reisinger, A. Quantifying tactile properties of liver tissue, silicone elastomers, and a 3D printed polymer for manufacturing realistic organ models. J. Mech. Behav. Biomed. Mater. 2020, 104, 103630. [Google Scholar] [CrossRef] [PubMed]
- Tejo-Otero, A.; Lustig-Gainza, P.; Fenollosa-Artés, F.; Valls, A.; Krauel, L.; Buj-Corral, I. 3D printed soft surgical planning prototype for a biliary tract rhabdomyosarcoma. Materials 2020, 109, 103844. [Google Scholar] [CrossRef]
- Laureiro, Z.L.; Novelli, S.; Lai, Q.; Mennini, G.; D’andrea, V.; Gaudenzi, P.; Marinozzi, F.; Engelmann, C.; Mookarje, R.; Raptis, D.; et al. There is a great future in plastics: Personalized approach to the management of hilar cholangiocarcinoma using a 3-D-printed liver model. Dig. Dis. Sci. 2020, 65, 2210–2215. [Google Scholar] [CrossRef] [PubMed]
- Sirriyeh, R.; Lawton, R.; Gardner, P.; Armitage, G. Reviewing studies with diverse designs: The development and evaluation of a new tool. J. Eval. Clin. Pract. 2012, 18, 746–752. [Google Scholar] [CrossRef]
- Paré, G.; Kitsiou, S. Methods for literature reviews. In Handbook of eHealth Evaluation: An Evidence-Based Approach; Lau, F., Kuziemsky, C., Eds.; University of Victoria: Victoria, BC, Canada, 2017; Chapter 9. [Google Scholar]
- Mikkonen, K.; Kääriäinen, M. Content analysis in systematic reviews. In The Application of Content Analysis in Nursing Science Research; Kyngäs, H., Mikkonen, K., Kääriäinen, M., Eds.; Springer: Cham, Switzerland, 2019; pp. 105–115. [Google Scholar]
- Elo, S.; Kyngäs, H. The qualitative content analysis process. J. Adv. Nurs. 2008, 62, 107–115. [Google Scholar] [CrossRef]
- Hackenbroich, S.; Kranke, P.; Meybohm, P.; Weibel, S. Include or not to include conference abstracts in systematic reviews? Lessons learned from a large Cochrane network meta-analysis including 585 trials. Syst. Rev. 2022, 11, 178. [Google Scholar] [CrossRef]
- Huber, T.; Hanke, L.I.; Boedecker, C.; Vradelis, L.; Baumgart, J.; Heinrich, S.; Bartsch, F.; Mittler, J.; Schulze, A.; Hansen, C.; et al. Patient-individualized resection planning in liver surgery using 3D print and virtual reality (i-LiVR)—A study protocol for a prospective randomized controlled trial. Trials 2022, 24, 403. [Google Scholar] [CrossRef] [PubMed]
- Povey, M.; Powell, S.; Howes, N.; Vimalachandran, D.; Sutton, P. Evaluating the potential utility of three-dimensional printed models in preoperative planning and patient consent in gastrointestinal cancer surgery. Ann. R. Coll. Surg. Engl. 2021, 103, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.S.; Choi, C.H.; Han, I.H.; Lee, J.H.; Choi, H.J.; Lee, J.I. Obtaining informed consent using patient specific 3D printing cerebral aneurysm model. J. Korean Neurosurg. Soc. 2019, 62, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.-D.; Zhou, M.-C.; Liu, S.-C.; Wu, J.-F.; Wang, R.; Chen, C.-M. Effectiveness of personalized 3D printed models for patient education in degenerative lumbar disease. Patient Educ. Couns. 2019, 102, 1875–1881. [Google Scholar] [CrossRef]
- Chen, J.; Bokacheva, L.; Rusinek, H. Image segmentation and nonuniformity correction methods. In 3D Printing for the Radiologist; Wake, N., Ed.; Elsevier: St. Louis, MO, USA, 2021; pp. 31–43. [Google Scholar]
- Gotra, A.; Sivakumaran, L.; Chartrand, G.; Vu, K.-N.; Vandenbroucke-Menu, F.; Kauffmann, C.; Kadoury, S.; Gallix, B.; de Guise, J.A.; Tang, A. Liver segmentation: Indications, techniques and future directions. Insights Imaging 2017, 8, 377–392. [Google Scholar] [CrossRef]
- Williams, A.; Tillet, C.; Wong, Y.H.; Yeong, C.H.; Sun, Z. How 3D printing software enhances and rewards student learning of brain anatomy. Australas. Med. J. 2023, 16, 552–555. [Google Scholar]
- Alexander, A.E.; Wake, N. 3D printed anatomic models and guides. In 3D Printing for the Radiologist; Wake, N., Ed.; Elsevier: St. Louis, MO, USA, 2021; pp. 75–88. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).