Abstract
The currently available therapeutic options for restoring function and sensitivity in long-term nervous injuries pose challenges. Microsurgery interventions for direct nerve repair often lead to serious complications and limited success. Non-surgical methods, although somewhat effective, have limited benefits. These methods involve drug administration, such as with analgesics or corticosteroids. Photobiomodulation therapy (PBMT) has emerged as a promising approach based on clinical and laboratory studies. PBMT stimulates the migration and proliferation of neuronal fiber cellular aggregates, as reported in the literature. Experimental studies on animal models with peripheral nerve compression injuries have shown that PBMT can enhance the functionality of damaged nerves, preserving their activity and preventing scar tissue formation. The mechanism of action depends on the wavelength, which can positively or negatively affect photo acceptor resonances, influencing their conformation and activities. These findings suggest that photobiomodulation may accelerate and improve nerve regeneration. This review explores various methodologies used in photobiomodulation for regenerating nerve sensitivity after surgical trauma involving nerve structures, in the oral and peri-oral region. Research was conducted to evaluate which laser-assisted therapeutic protocols are used to improve the recovery of nervous sensitivity, using the JBI methodology for scoping reviews and following the PRISMA methodology.
1. Introduction
Nerve lesions of iatrogenic origin can affect both sensory and motor trunks; these lesions can lead to potentially painful symptoms and dysfunctions of the oral and maxillofacial region [1,2,3]. In oral surgery, most of the lesions involve the sensory nerve trunks of the V cranial nerve, the inferior alveolar nerve, the lingual nerve, as well as the greater palatine nerve, albeit less frequently [1,2,3].
These lesions can be caused by a variety of treatments, such as oral or maxillofacial surgery, implant surgery, injection of local anesthetics, or endodontic treatments [1,2,3].
The neurological lesions can be divided into three degrees of increasing severity: neurapraxia, if the interruption is only functional and temporary of the nerve conduction; axonotmesis, if there is an anatomical interruption of the axons but with preservation of the sheaths covering the nerve, with functional recovery which happens if the regeneration takes place completely, but it is necessary for up to a few months. Finally, there is neurotmesis, characterized by the complete interruption of both the axons and the sheaths covering the nerve, where functional recovery of the nerve is rare [1,2,3].
Thus far, the approaches employed to reinstate the functionality of the impaired nerve have demonstrated challenges in achieving enduring outcomes [3,4]. The currently accessible treatments are categorized as either surgical or non-surgical. Surgical methods encompass nerve transfer, nerve conduits, direct nerve repair, and the application of fibrin glue. Conversely, non-surgical therapies encompass pharmacological agents like analgesics, topical remedies such as corticosteroids, and the utilization of phytochemicals. It is necessary to underline that these therapies have disadvantages, as surgical therapies can have non-negligible adverse effects and require a specialized operator for execution, while non-surgical therapies have shown limited efficacy up to now [4]. Neurorrhaphy, the surgical repair of nerves, offers several alternative techniques to restore nerve function. These options include direct suturing (epineurial repair), which involves stitching the nerve ends together, maintaining nerve alignment. Cable grafting involves using a nerve graft or autograft to bridge a gap between nerve ends. Nerve connectors, such as tubes or stents, aid in nerve regeneration by facilitating the growth of nerve fibers across the gap. The use of nerve conduits, synthetic or biological tubes, guides nerve regrowth and prevents scar tissue formation. Nerve transfer involves rerouting a functional nerve to the injured area, allowing the restoration of lost functions. End-to-side neurorrhaphy involves connecting the injured nerve to a donor nerve’s side, promoting axonal growth. Fibrin glue, platelet-rich plasma, and other bioactive substances can enhance nerve repair by promoting cell growth and angiogenesis. Minimally invasive techniques, like robotic-assisted neurorrhaphy, offer precision and reduced scarring. Combining different techniques, tailored to the patient’s condition, can optimize nerve repair outcomes [4].
Among these methodologies, light-based therapy, known as photobiomodulation therapy (PBMT), has exhibited promising outcomes in various clinical and in vitro investigations [4]. Existing literature indicates that PBMT induces the migration and proliferation of clusters of neuronal fibers. In animal models of peripheral nerve compression injuries [5], PBMT has been shown to directly enhance the function of the damaged nerve, sustain nerve activity over time, and reduce or prevent scar tissue formation at the injury site [5,6,7]. Additionally, several researchers have observed that nerve injuries result in escalated oxygen consumption without a corresponding increase in ATP production. This phenomenon may arise from uncoupled respiration and energy production or a reversal of ATP synthetase activity, leading to ATP dissipation [5,6]. The diminished availability of ATP in nerve lesions triggers cell death and neurodegeneration since the low ATP level promotes neuronal depolarization, enhances neurotransmitter release, impairs ATP-dependent reuptake, and heightens the excitability of nociceptors [5,6]. This phenomenon is further linked to the increased production of reactive oxygen species (ROS), which is exacerbated by the inflammation associated with neuronal damage. PBMT enhances the mitochondrial membrane potential (MMP), boosting electron transport. An elevated MMP typically increases ROS production, yet malfunctioning mitochondria also generate ROS via the “ROS-induced ROS release” (RIRR). Excessive oxidative stress triggers channels like the mitochondrial permeability transition pore and inner membrane anion channel, causing MMP to collapse and a heightened ROS via the electron transport chain [7]. This ROS surge may serve as a “second messenger,” activating RIRR in nearby mitochondria, compounding cellular harm. In NF-kB luciferase mice, a 810 nm laser PBM activated NF-kB, inducing ROS generation and a rise in ATP. N-acetylcysteine quenched ROS but not ATP, as PBM’s MMP elevation increased ATP while provoking a ROS burst that likely activated NF-kB via protein kinase D [7].
PBM is thought to enhance mitochondrial function by increasing the mitochondrial membrane potential (MMP), leading to improved electron transport and adenosine triphosphate (ATP) production. Additionally, PBM can influence cellular signaling pathways, such as NF-kB (nuclear factor kappa-light-chain-enhancer of activated B cells), which play a central role in immune responses, inflammation, and cell survival. Transforming Growth Factor Beta (TGF-β) is a multifunctional cytokine that regulates cell growth, differentiation, and immune responses. It is involved in various physiological processes, including tissue repair, wound healing, and immune modulation. TGF-β can stimulate fibroblast activity, leading to tissue remodeling and extracellular matrix production. In the context of PBM, TGF-β may play a role in mediating the effects of light therapy on tissue repair and regeneration, potentially enhancing the healing process and promoting favorable cellular responses [8].
The combination of PBM and TGF-β could have synergistic effects on tissue healing and regeneration. PBM may enhance the cellular responses to TGF-β, leading to improved tissue repair and regeneration outcomes. However, the specific interactions between PBM and TGF-β in various therapeutic contexts would require further research to fully elucidate their combined effects and mechanisms of action [8]. The interaction between red and infrared light and photoacceptors such as cytochromes, water, lipids, S-nitrosylates, nitric oxide, and transient receptor potential channels (TRPC) that regulate that Ca2+ passage, leads to a modification of the bioenergetic properties of mitochondria. This alteration includes adjustments in ATP and ROS generation, as well as the discharge of nitric oxide (NO) and calcium ions (Ca2+) at the mitochondrial level [5,6]. The mechanism of action varies depending on the wavelength, which can either positively or negatively interfere with the photoacceptor resonances, influencing their conformation, properties, and activities. These findings strongly suggest that photobiomodulation plays a pivotal role in accelerating and enhancing the regeneration of damaged nerves [5,6,7,8]. The article of Amaroli et al. [9] explores the effects of photobiomodulation (PBM) on cellular pathways, including calcium regulation, through various interactions with chromophores. While mitochondrial chromophores and ROS signaling are key targets, alternative photoacceptors like water and TRP ion channels also play roles. The interconnectedness of calcium’s roles in cell physiology aligns well with PBM’s consistent effects across different life-forms. PBM-induced effects involve intricate pathways, such as mitochondrial stimulation, leading to ROS generation and calcium uptake. The intricate interactions between ROS, ATP, and calcium signal a complex yet significant impact on cell function and communication. Although PBM’s mechanisms are multifaceted and involve various pathways, the article underscores its potential for beneficial effects in medical applications, while cautioning against unintended cellular damage. Further research is needed to fully comprehend these interactions and their implications [9,10].
Moreover, it was observed that cell proliferation responds differently to various PBM wavelengths: 660 nm and 810 nm enhance proliferation, while 415 nm and 540 nm inhibit it. Based on protein content measurement, the sulforhodamine B assay gauges cell density. The PBM effects were assessed using four wavelengths (3 J/cm2) at five-time points: 810 nm and 660 nm increased hASCs proliferation, while 415 nm and 540 nm inhibited it [11]. Intracellular ATP levels varied: 660 nm and 810 nm raised ATP in a biphasic manner, while 415 nm and 540 nm led to dose-dependent decreases [11]. Flow cytometry and microscopy examined the effects on intracellular calcium, MMP, ROS, and pH. TRPV1 expression influenced proliferation, and CAP promoted proliferation through TRPV1. Inhibitory effects of 415 nm and 540 nm on proliferation were counteracted by TRPV1 inhibitors and ROS blockers [11,12].
Considering these studies, it is possible to state that photobiomodulation is a non-invasive treatment with zero side effects; nowadays, it is a treatment still under study for its therapeutic efficacy in some pathologies, although it is a valid alternative for pathologies of other districts especially in the dental and dermatological. Therefore, this scoping review aims to investigate the effects of various wavelengths and the therapeutic strategies implemented to recover sensitivity in patients who have suffered nerve injuries in the oral and peri-oral nerve branches [7,8,9,10,11,12].
2. Materials and Methods
2.1. Focused Questions
What type of laser and wavelengths are suitable for the photobiomodulation protocol to be applied to patients with nerve injuries in the oral district? What are the results obtained with photobiomodulation in terms of recovery of nervous functions?
2.2. Eligibility Criteria
The following inclusion criteria guided the analysis of the studies:
Type of studies: clinical trials, case-control studies, cross-sectional studies, and cohort studies.
Type of Journal: only articles published in journals indexed on Scopus, Web of Science, or PubMed were considered.
Type of participants: patients with nerve injuries to which a photobiomodulation protocol was applied.
Type of interventions: comparison of the clinical effects of various wavelengths applied and/or different types of lasers used and/or different protocols of application, assessed through clinical trials.
Outcome type: identification of protocols of photobiomodulation that are considered effective in restoring nerve functionality and mid/long term outcomes.
Only research that satisfied all the predefined inclusion criteria was incorporated. Nonetheless, the subsequent exclusion criteria were considered: (I) publications in languages other than English, (II) the existence of concurrent systemic diseases/treatments that could impact the outcomes, (III) animal-based clinical investigations, and (IV) studies lacking approval from an ethics committee.
2.3. Search Strategy
Following the JBI methodology for scoping reviews [13], a three-step search process was conducted: (i) an initial limited search on PubMed (MEDLINE) and Scopus; (ii) identification of key terms from the retrieved articles to devise the search strategy; and (iii) an examination of the reference lists of all included articles for additional research [14].
Moreover, the review applied the Population-Concept-Context (PCC) model, which centers around the following three elements: population (individuals with peripheral nerve lesions), concept (photobiomodulation and its impact on sensory improvement after nerve injury), and context (in this case, the review was not limited to any specific cultural or geographical setting). The abstracts of studies analyzing the effects of photobiomodulation on peripheral nerve injury and sensory recovery, along with their clinical outcomes, were evaluated. Throughout this scoping review of the literature, adherence to the Preferred Reporting Items for Scoping Reviews (PRISMA-ScR) consensus was followed (Table S1 in Supplementary Material) [15].
2.4. Research
The MeSH terms utilized for this study are photobiomodulation, diode laser, laser, oral nerve injury, neurological lesion, and sensibility. An electronic search was performed using PubMed (MEDLINE) and Scopus databases. The targeted publication period was from 2003 to 2023. Data extraction took place between January 2023 and June 2023, with the most recent search conducted on 17 June 2023. During our analysis, 135 articles were selected. Two calibrated reviewers (M.Pe. and F.P.) conducted the search, and any disagreements or discrepancies were resolved through consensus. In case of further issues, three additional reviewers (M.Po., M.B., and F.S.) were consulted. The titles and abstracts of the initially retrieved articles were thoroughly reviewed, and irrelevant studies were excluded. Relevant articles meeting the inclusion criteria were identified and analyzed for any similar studies. Full texts of the included studies were read for the extraction of pertinent results, which were recorded accordingly.
The present protocol has been registered on the Open Science Framework platform (Registration DOI-10.17605/OSF.IO/6X5C7).
The detailed strategies applied for each electronic database can be found in Table S2 (Supplementary Material).
2.5. Quality Assessment of Included Studies
This review was performed by evaluating the risk of bias by conducting a qualitative analysis of the clinical studies via JBI critical appraisal for randomized controlled trials [16].
3. Results
The initial search yielded 135 articles based on MeSH terms, spanning from 2003 to 2023. Subsequently, the focus of the research was narrowed down to clinical trials, meta-analyses, and randomized controlled trials involving human studies, published in the English language, and with full-text availability. As a result, 16 articles were selected and screened for eligibility. Ultimately, these 16 relevant articles were included and thoroughly analyzed in the review. The flowchart depicting the review process is presented in Figure 1.
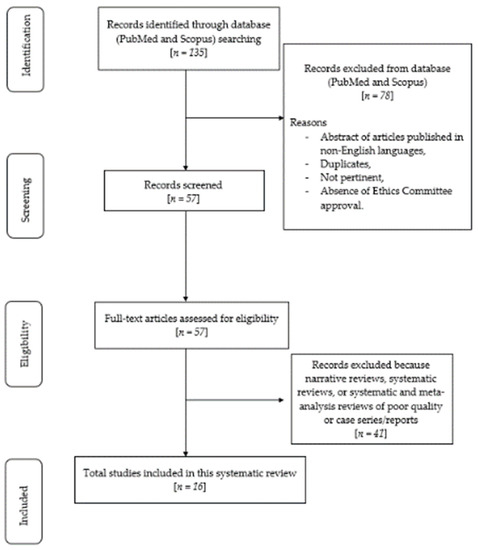
Figure 1.
Flow chart of the review process.
Risk of Bias
The JBI critical appraisal tool was applied to assess the risk of bias in the studies included in this review (Table 1), using the judging criteria for the risk of bias shown in Table S3 (Supplementary Materials). Questions and answers to the criteria for judging the risk of bias in the “JBI Critical Appraisal Checklist for Randomized Controlled Trials” [14] are shown in Table 2.

Table 1.
The risk of bias in case report studies is represented by symbols (green for low risk of bias, yellow for high risk of bias, and blue for uncertain or unavailable data and medium risk of bias).

Table 2.
This review includes criteria for judging the risk of bias in the “JBI Critical Appraisal Checklist for randomized controlled trials”, questions, and answers for each case report. The letter Q indicates the question of the dedicated checklist and the corresponding numbering.
4. Discussion
In the realm of medical innovation, the application of photobiomodulation has emerged as a promising approach for treating nerve injuries in the oral district [17,18,19]. The quest to optimize this therapy hinges upon selecting the appropriate laser type and wavelengths. Researchers delve into the intricate interplay between laser characteristics and their compatibility with the unique anatomical and physiological aspects of the oral nerve network. An exploration of the optimal laser type, such as low-level laser therapy (PBMT), and the most effective wavelengths unfolds as a pivotal pursuit [18,19]. As the medical community strives to unravel the potential of photobiomodulation, questions abound regarding the outcomes that it yields in terms of the recovery of nervous function. Preliminary findings hint at the tantalizing prospect of accelerated nerve regeneration, potentially offering a new ray of hope for patients grappling with nerve injuries in the oral district [20,21,22]. By examining the documented results, insights emerge into the extent of neural recovery, improvements in sensory and motor functions, and the overall quality of life enhancements experienced by individuals subjected to this innovative treatment paradigm. As research continues to illuminate the intricate mechanisms underlying photobiomodulation, a clearer picture emerges of its transformative potential in revitalizing nerve function and ushering in a new era of oral nerve injury rehabilitation [20,21,22].
The potential of laser light to impact the peripheral nervous system has been explored in both the neuromuscular and somatosensory systems. This investigation dates to 1978 when it was discovered that laser radiation directed at exposed nerve tissue had a direct and beneficial effect as a preventive measure and a therapeutic intervention [23]. Currently, the preferred treatment for peripheral nerve injuries is advanced microsurgical repair or autologous nerve grafting. However, functional recovery is often unsatisfactory, and there is a need for new therapeutic approaches. Schwann cells in the peripheral nervous system play a crucial role in nerve repair. Various strategies, like pharmacological and cell-based therapies, have been used to enhance recovery, but none provide a universal cure and may have drawbacks and side effects [24]
Various treatment methods, including exercise, electrical stimulation (ES), magnetic stimulation, low-intensity ultrasound (LIU), and phototherapy, have been explored to enhance peripheral nerve regeneration. Exercise, especially aerobic activities like swimming and walking, promotes axonal growth and synaptic improvement, and when combined with ES, it shows better outcomes. Magnetic stimulation increases axon numbers and diameters, likely through stimulating NGF activity. A low-intensity ultrasound induces positive responses by promoting blood circulation and releasing neurotrophic factors. Phototherapy and photobiomodulation therapy (PBMT) also aid in axonal regeneration and functional recovery. PBMT, utilizing low-level infrared light, stimulates SC proliferation and axonal diameter expansion, yet standardized application parameters remain a challenge [25].
Subsequently, in 1992, Rochkind and Ouaknine [26] demonstrated that photobiomodulation therapy (PBMT) influenced the electrical activity and morphology of injured and intact peripheral nerves in rats. They observed that the action potential of intact nerves increased by up to 33% following a single transcutaneous laser treatment. Similarly, injured nerves subjected to corresponding therapy exhibited a significantly increased action potential amplitude compared to untreated injured nerves [26].
Hakimiha and colleagues [27] conducted an investigation using a rat model to assess the effectiveness of PBMT on nerve regeneration. Their findings revealed that in studies involving highly metabolically active cells like nerve tissue, unfavorable outcomes were more commonly attributed to excessive dosing rather than insufficient dosing. In the current research, a notably expedited nerve recovery was achieved using an energy density of 6 J/cm2. This discovery lends support to the notion of a “biphase dose-response” phenomenon in PBMT, wherein positive biostimulation responses are triggered at doses below 10 J/cm2, while inhibitory responses are prominent at doses surpassing 20 J/cm2 [27]. Nevertheless, this concept of a “window effect” has been extensively examined in existing literature, and the findings from the study under consideration align well with the established data in this field.
The results obtained from the immunoblotting analysis conducted on the second day after the injury indicated elevated concentrations of the nerve growth factor (NGF) in rats that underwent PBMT treatment, in comparison to the control group that did not receive PBMT. This rise in NGF levels corresponded with a swifter restoration of neurosensory functionality [26]. Likewise, a multitude of other investigations, encompassing both controlled and uncontrolled trials, have highlighted favorable subjective enhancements attributed to PBMT across diverse clinical contexts, such as perioral lesions [26], recovery after musculoskeletal surgery [24], and the promotion of osteogenic differentiation [27]. To sum up, PBMT manifests positive influences on distinct nervous, musculoskeletal, and epithelial systems.
In the research carried out by Sharifi et al., the impact of photobiomodulation on the recuperation of the sensory function in the lip and chin following bilateral sagittal split osteotomy (BSSO) was examined [26]. The procedure involved the utilization of a GaAs diode laser emitting continuous waves at a wavelength of 980 nm, generating a power output of 100 mW, and delivering an energy density of 12 J/cm2. Substantial enhancements were noted in the scores recorded on the visual analogue scales (VAS) for general sensitivity, discrimination of pain, recognition of direction, and discrimination of two points, both after a span of 30 days and once more after 60 days [26].
Miloro et al. [27] considered patients with nerve injury due to odontectomy of the third molars or related practices such as anesthetic injection wounds. They used photobiomodulation with a diode laser 830 nm continuous wave, administrated in 20 applications over 3 months for each patient. Instrumental tests such as VAS or 2-point discriminations did not show any significant improvement in sensibility repairment compared to the control group. However, the limitation of this study is considered by the authors themselves to be the fact that they did not categorize the patients according to the degree of severity of the nerve damage, which could have influenced the significance of the statistics [27].
Gasperini et al. [28], Bashiri et al. [29], Salari et al. [30], Pinto et al. [32], Fuhrer-Valdivia et al. [33], and Ozen et al. [34] used the GaAlAs laser in their trials with wavelengths between 789 and 880 nm; according to their studies, their photobiomodulation protocol could significantly improve healing and sensibility could be regained after nerve injury, based on an improvement on pain scales and mechanical test results. Salari et al. [30], however, show that there is no statistical difference between the control group and the treatment group in regards to thermal sensitivity in all the periods examined, while all three authors agree that during the follow-up, the mechanical tests have better results [28,29,30,32,33,34,35,36,37].
The diode laser was used respectively by Miloro et al. [27], Qi et al. [31], Eshghpour et al. [38], De Oliveira et al. [39], Teixera-Santos et al. [36], and Mohajerani et al. [35].
Except for the trial conducted by Miloro et al., the diode laser could be considered as effective as the GaAlAs laser; in fact, in all of the studies, there is a significance in the improvement of pain relief, quality of life, and faster healing. Diode laser wavelengths were used between 632 and 880 nm, in a continuous mode and with the scanning method [27,31,35,36,38,39].
The articles examined in our review are all clinical trials. Peripheral nerve lesions are mainly caused by osteotomies involving the maxillofacial area and, to a lesser extent, nerve lesions following the extraction of the third molars. In the clinical trials examined, the evaluation of nerve sensitivity and the sensation of paraesthesia were evaluated through different tests: mechanosensory tests for routine assessment of surgical trigeminal nerve injuries, such as the brush stroke test, the 2-points discrimination test, and Semmes–Weinsten monofilament test and thermal sensitivity test to evaluate the neurological response to temperature change. Regarding the disturbance the patient perceived, a VAS scale is administered in almost all studies [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40].
As far as photobiomodulation is concerned, the authors mostly used diode lasers or GaAs or GaAlAs lasers, and only in one case, a LED laser is used. The wavelengths used vary from a range from 632 to 910 nm for an application time of at least 10 sessions. Most of the treatments were administrated with a continuous wave. All of the authors report, because of the experimentation, an objective and subjective improvement of the symptoms without side effects, although in some cases, it was a matter of very long therapeutic plans in terms of time. In the cited studies, none of the authors report medium and long-term follow-ups of the effects of photobiomodulation on the patients examined. Table 3 summarizes the studies analyzed, highlighting the type of study, the sample, the type of laser used, and the application protocol. The outcomes and tests conducted to verify the recovery of sensitivity are also reported [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40]. Table 4 indicates the laser parameters used in the clinical trial protocols being reviewed.

Table 3.
Systematic literature review results.

Table 4.
Summary table of the laser parameters used in the clinical trials analyzed.
Photobiomodulation is a non-invasive therapeutic approach, as the literature confirms, that involves the use of light to stimulate cellular activity and promote tissue regeneration. It has shown promising results in the regeneration of peripheral nerves. Studies conducted on animal models, such as rats [41,42,43,44,45,46,47,48,49,50,51,52], have demonstrated that PBM can enhance nerve regeneration by promoting axonal growth, reducing inflammation, and increasing cellular metabolism. These experiments typically involve applying specific wavelengths of light to the injured nerve area, leading to improved nerve function and accelerated healing [53,54,55,56]. While more research is needed to fully understand the underlying mechanisms and optimize treatment protocols, the potential of photobiomodulation as a tool for peripheral nerve regeneration is an exciting avenue for future medical applications [57,58,59]. The studies analyzed in our scoping review confirm encouraging results detectable instrumentally, regarding the recovery of nervous sensitivity of the areas affected by a lesion, confirming the results of the literature regarding the applications of laser-assisted protocols and for the wavelengths tested. Indeed, for a targeted photobiomodulation treatment aimed at recovering sensitivity following peripheral nerve injury, specific wavelengths of light are typically used [57,58,59,60,61,62,63,64]. Wavelengths within the near-infrared range (600–1100 nm) are commonly employed for this purpose. These wavelengths have a greater ability to penetrate tissues and reach the nerve fibers, allowing for deeper stimulation and interaction with cellular components. Wavelengths around 800–850 nm are often favored due to their optimal tissue penetration and interaction with the mitochondrial enzyme cytochrome c oxidase [57,58,59,60,61,62,63,64]. This interaction triggers a cascade of cellular responses, including enhanced ATP production, reduced oxidative stress, and the modulation of signaling pathways involved in nerve repair and regeneration. Furthermore, wavelengths in the red-light range (around 630–670 nm) can also contribute to the stimulation of cellular activity and blood flow, aiding in tissue oxygenation and nutrient supply to support nerve healing. By utilizing these specific wavelengths, photobiomodulation can effectively promote nerve tissue repair, axonal growth, and sensory function recovery after peripheral nerve injury. However, it is important to note that treatment parameters, such as power density, duration, and frequency of exposure, need to be carefully optimized based on the severity of the injury and individual patient characteristics for optimal therapeutic outcomes [64,65,66,67,68,69,70,71].
4.1. Treatment Alternatives
A wide array of therapeutic approaches are employed to mitigate pain, showcasing the versatility of medical interventions in addressing this complex issue. These methods encompass the utilization of painkillers, which encompass both non-opioid analgesics and anti-inflammatory medications, offering rapid relief by targeting pain pathways and reducing inflammation [72,73,74,75,76,77]. Additionally, corticosteroids find application due to their potent anti-inflammatory properties, effectively alleviating pain associated with inflammatory conditions. Painkillers play a pivotal role in managing neuropathic pain, a complex condition characterized by aberrant nerve signaling. Drugs such as tricyclic antidepressants, anticonvulsants, and serotonin-norepinephrine reuptake inhibitors (SNRIs) are commonly prescribed. These medications target specific pathways involved in nerve transmission and modulation, helping to alleviate the distinctive burning, shooting, or tingling sensations associated with neuropathic pain [72,73,74,75,76,77]. While painkillers offer relief, their effectiveness varies among individuals, and a multidisciplinary approach may be needed for comprehensive neuropathic pain management. In cases where nerve-related pain is a concern, nerve denervation, or ablation, emerges as a potential solution. By interrupting the transmission of pain signals along the affected nerves, this technique can provide substantial relief. Similarly, muscle relaxants are employed to ease pain stemming from muscle tension or spasms, allowing for enhanced comfort and improved mobility. Nerve denervation or ablation is considered in cases of severe neuropathic pain that is unresponsive to conservative treatments [72,73,74,75,76,77]. This procedure involves deliberately disrupting the nerve pathways responsible for transmitting pain signals, often providing relief when other methods have failed. The decision to opt for nerve denervation is based on several criteria. Firstly, a thorough evaluation of the patient’s medical history, pain duration, and response to prior treatments is conducted [76]. Candidates for denervation typically exhibit localized neuropathic pain that is well-defined and refractory to conventional interventions. Moreover, imaging studies, such as MRI or nerve blocks, may be utilized to precisely identify the pain source and the nerves involved. A successful nerve denervation outcome hinges on accurate identification and targeting of the specific nerve responsible for the pain. Patient selection is critical, with consideration given to factors such as overall health status, potential risks of the procedure, and the patient’s willingness to undergo a more invasive treatment approach [72,73,74]. The outcomes of nerve denervation can vary, with some patients experiencing significant and sustained pain relief, while others may see only partial improvement. The procedure’s success often depends on the nerve’s regrowth capacity and the underlying cause of neuropathy. Post-denervation rehabilitation, including physical therapy, is crucial to optimize outcomes. The close collaboration between the patient, pain specialist, and interdisciplinary team is vital to making informed decisions regarding nerve denervation as a therapeutic option for refractory neuropathic pain [72,73,74,75,76,77]. Physical therapy is a cornerstone in pain management, focusing on rehabilitation, strengthening, and restoring functional capacity. This approach not only addresses the root causes of pain but also empowers patients with long-term coping mechanisms and an improved overall well-being. An intriguing avenue lies in the administration of parietal hyaluronic acid preparations or biological substances, such as stem cells [72,73,74,75,76,77]. These innovative therapies target tissue regeneration and repair, holding the potential for more sustainable pain relief by addressing underlying structural issues. Stem cells hold considerable promise as a potential therapeutic avenue for neuropathic pain management. These versatile cells have the capacity to differentiate into various cell types and secrete bioactive molecules that can modulate inflammation, tissue repair, and nerve regeneration. In the context of neuropathic pain, stem cell therapy aims to address underlying pathophysiological mechanisms by promoting nerve tissue regeneration and reducing neuroinflammation [72,73,74,75,76,77]. Preclinical studies involving animal models have demonstrated encouraging results, showing improved pain-related behaviors and enhanced nerve function after stem cell transplantation. However, translating these findings into clinical practice requires careful consideration of factors such as the stem cell source, delivery methods, and long-term safety and efficacy. While stem cell-based therapies offer a promising avenue for neuropathic pain treatment, further research and clinical trials are necessary to establish their definitive role and optimize their potential benefits. It is noteworthy, however, that while each of these interventions demonstrates efficacy to varying extents, their impact tends to be temporally constrained, usually offering relief for a limited duration, spanning from several weeks to a few months [72,73,74,75,76,77]. To further the discourse, I propose an expansion of the discussion section, guided by the following essential questions, thereby delving deeper into the long-term effectiveness, potential synergies, and avenues for enhancing the durability of pain management interventions [72,73,74,75,76,77].
4.2. Limits of the Study and Future Perspectives
Nowadays, there is no validated protocol for the use of photobiomodulation in patients who have suffered damage to the nerve fibers and for the recovery of sensitivity, a consensus from the scientific authorities would be needed to establish a single treatment protocol considering the available scientific evidence.
Future research prospects could include further randomized controlled clinical trials that should be conducted to evaluate the effects of different types of lasers and wavelengths on patients. Laboratory studies on cell lines and animal models could be useful to establish what is the cellular and metabolic effect of laser light on tissues, to contribute to the choice of the right wavelength and application according to the pathology and the tissues to be treated.
Another possible research line could be the evaluation of a possible amplification of the photobiomodulation effect through pharmacological treatment.
5. Conclusions
The results of this thorough and extensive review provides substantial evidence that the application of photobiomodulation across various wavelengths has a remarkable and expedited impact on the improvement of Visual Analog Scale (VAS) scores concerning general sensory perception and thermal discrimination. Notably, the utilization of photobiomodulation demonstrates its capacity to accelerate the recovery of these sensory functions. This is particularly significant considering the non-invasive nature of photobiomodulation and its high level of patient tolerance. Therefore, photobiomodulation emerges as a robust and efficacious therapeutic strategy for effectively addressing and managing neurosensory disruptions that may arise postoperatively. The promising outcomes observed in this review highlight its potential as a valuable addition to the array of treatments available for mitigating and treating postoperative neurosensory complications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app13169258/s1, Table S1: PRISMA-ScR Checklist; Table S2: Search strategies for electronic databases; Table S3. JBI critical appraisal checklist for randomized controlled trials, a tool used for risk of bias assessment.
Author Contributions
Conceptualization, F.S.; methodology, F.S.; software, M.P. (Massimo Porrini) and F.P.; validation, M.P. (Massimo Porrini), M.B. and M.P. (Matteo Pellegrini); formal analysis, F.S.; investigation, F.P. and M.P. (Matteo Pellegrini); resources, M.P. (Massimo Porrini), F.S. and F.P.; data curation, F.S.; writing—original draft preparation, F.P. and M.P. (Matteo Pellegrini); writing—review and editing, F.P., F.S., M.P. (Matteo Pellegrini) and A.S.; visualization, M.B., M.P. (Massimo Porrini) and F.S.; supervision, F.S. and A.S.; and project administration, F.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Upon request to the corresponding author, the data are available for use. The protocol of the review was registered with the Open Science Framework (OSF) at https://doi.org/10.17605/OSF.IO/6X5C7 and registered from osf.io/q98zv.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Søren, H. Iatrogenic injury to oral branches of the trigeminal nerve: Records of 449 cases. Clin. Oral. Investig. 2007, 11, 133–142. [Google Scholar]
- Coulthard, P.; Kushnerev, E.; Yates, J.M.; Walsh, T.; Patel, N.; Bailey, E.; Renton, T.F. Interventions for iatrogenic inferior alveolar and lingual nerve injury. Cochrane Database Syst. Rev. 2014, 4, CD005293. [Google Scholar] [CrossRef] [PubMed]
- Weyh, A.; Pucci, R.; Valentini, V.; Fernandes, R.; Salman, S. Injuries of the Peripheral Mandibular Nerve, Evaluation of Interventions and Outcomes: A Systematic Review. Craniomaxillofac Trauma. Reconstr. 2021, 14, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Rivlin, M.; Graham, J.G.; Beredjiklian, P.K. Peripheral nerve injury, scarring, and recovery. Connect. Tissue Res. 2019, 60, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Andreo, L.; Ribeiro, B.G.; Alves, A.N.; Martinelli, A.S.A.; Soldera, C.B.; Horliana, A.C.R.T.; Bussadori, S.K.; Fernandes, K.P.S.; Mesquita-Ferrari, R.A. Effects of Photobiomodulation with Low-level Laser Therapy on Muscle Repair Following a Peripheral Nerve Injury in Wistar Rats. Photochem. Photobiol. 2020, 96, 1124–1132. [Google Scholar] [CrossRef]
- Diker, N.; Aytac, D.; Helvacioglu, F.; Oguz, Y. Comparative effects of photobiomodulation therapy at wavelengths of 660 and 808 nm on regeneration of inferior alveolar nerve in rats following crush injury. Lasers Med. Sci. 2020, 35, 413–420. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and Mitochondrial Redox Signaling in Photobiomodulation. Photochem. Photobiol. 2018, 94, 199–212. [Google Scholar] [CrossRef]
- Arany, P.R. Photobiomodulation-Activated Latent Transforming Growth Factor-β1: A Critical Clinical Therapeutic Pathway and an Endogenous Optogenetic Tool for Discovery. Photobiomodul Photomed. Laser Surg. 2022, 40, 136–147. [Google Scholar] [CrossRef]
- Amaroli, A.; Ferrando, S.; Benedicenti, S. Photobiomodulation Affects Key Cellular Pathways of all Life-Forms: Considerations on Old and New Laser Light Targets and the Calcium Issue. Photochem. Photobiol. 2019, 95, 455–459. [Google Scholar] [CrossRef]
- Colombo, E.; Signore, A.; Aicardi, S.; Zekiy, A.; Utyuzh, A.; Benedicenti, S.; Amaroli, A. Experimental and Clinical Applications of Red and Near-Infrared Photobiomodulation on Endothelial Dysfunction: A Review. Biomedicines 2021, 9, 274. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, Y.Y.; Wang, Y.; Lyu, P.; Hamblin, M.R. Red (660 nm) or near-infrared (810 nm) photobiomodulation stimulates, while blue (415 nm), green (540 nm) light inhibits proliferation in human adipose-derived stem cells. Sci. Rep. 2017, 7, 7781. [Google Scholar] [CrossRef]
- Sommer, A.P. Mitochondrial cytochrome c oxidase is not the primary acceptor for near infrared light-it is mitochondrial bound water: The principles of low-level light therapy. Ann. Transl. Med. 2019, 7 (Suppl. 1), S13. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.D.J.; Marnie, C.; Tricco, A.C.; Pollock, D.; Munn, Z.; Alexander, L.; McInerney, P.; Godfrey, C.M.; Khalil, H. Updated methodological guidance for the conduct of scoping reviews. JBI Evid. Implement. 2021, 19, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Aromataris, E.; Munn, Z. JBI Manual for Evidence Synthesis. 2020. Available online: https://synthesismanual.jbi.global (accessed on 24 April 2023).
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Tufanaru, C.; Munn, Z.; Aromataris, E.; Campbell, J.; Hopp, L. Chapter 3: Systematic reviews of effectiveness. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI: San Diego, CA, USA, 2020. [Google Scholar]
- Hakimiha, N.; Dehghan, M.M.; Manaheji, H.; Zaringhalam, J.; Farzad-Mohajeri, S.; Fekrazad, R.; Moslemi, N. Recovery of inferior alveolar nerve by photobiomodulation therapy using two laser wavelengths: A behavioral and immunological study in rat. J. Photochem. Photobiol. B 2020, 204, 111785. [Google Scholar] [CrossRef]
- El Mobadder, M.; Nammour, S.; Ortega, M.; Grzech-Le´sniak, K. Photobiomodulation Therapy Applied after 6 Months for the Management of a Severe Inferior Alveolar Nerve Injury. Life 2021, 11, 1420. [Google Scholar] [CrossRef]
- Muniz, X.C.; de Assis, A.C.C.; de Oliveira, B.S.A.; Ferreira, L.F.R.; Bilal, M.; Iqbal, H.M.N.; Soriano, R.N. Efficacy of low-level laser therapy in nerve injury repair-a new era in therapeutic agents and regenerative treatments. Neurol. Sci. 2021, 42, 4029–4043. [Google Scholar] [CrossRef]
- Ravera, S.; Colombo, E.; Pasquale, C.; Benedicenti, S.; Solimei, L.; Signore, A.; Amaroli, A. Mitochondrial Bioenergetic, Photobiomodulation and Trigeminal Branches Nerve Damage, What’s the Connection? A Review. Int. J. Mol. Sci. 2021, 22, 4347. [Google Scholar] [CrossRef]
- Kobiela Ketz, A.; Byrnes, K.R.; Grunberg, N.E.; Kasper, C.E.; Osborne, L.; Pryor, B.; Tosini, N.L.; Wu, X.; Anders, J.J. Characterization of Macrophage/Microglial Activation and Effect of Photobiomodulation in the Spared Nerve Injury Model of Neuropathic Pain. Pain. Med. 2017, 18, 932–946. [Google Scholar] [CrossRef][Green Version]
- Abdelkarim Elafifi, H.; Acevedo Carrero, M.; Parada Avendaño, I.; España-Tost, A.; Arnabat-Domínguez, J. Effect of Photobiomodulation (Diode 810 nm) on Long-Standing Neurosensory Alterations of the Inferior Alveolar Nerve: A Case Series Study. Photobiomodul Photomed. Laser Surg. 2021, 39, 4–9. [Google Scholar] [CrossRef]
- Li, B.; Wang, X. Photobiomodulation enhances facial nerve regeneration via activation of PI3K/Akt signaling pathway-mediated antioxidant response. Lasers Med. Sci. 2022, 37, 993–1006. [Google Scholar] [CrossRef] [PubMed]
- Rochkind, S.; Ouaknine, G.E. New trend in neuroscience: Lowpower laser effect on peripheral and central nervous system. Neurol. Res. 1992, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Hakimiha, N.; Bassir, S.H.; Romanos, G.E.; Shamshiri, A.R.; Moslemi, N. Efficacy of photobiomodulation therapy on neurosensory recovery in patients with inferior alveolar nerve injury following oral surgical procedures: A systematic review. Quintessence Int. 2021, 52, 140–153. [Google Scholar] [PubMed]
- Sharifi, R.; Fekrazad, R.; Taheri, M.M.; Kasaeian, A.; Babaei, A. Effect of photobiomodulation on recovery from neurosensory disturbances after sagittal split ramus osteotomy: A triple-blind randomised controlled trial. Br. J. Oral. Maxillofac. Surg. 2020, 58, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Miloro, M.; Criddle, T.R. Does Low-Level Laser Therapy Affect Recovery of Lingual and Inferior Alveolar Nerve Injuries? J. Oral. Maxillofac. Surg. 2018, 76, 2669–2675. [Google Scholar] [CrossRef]
- Gasperini, G.; de Siqueira, I.C.; Costa, L.R. Lower-level laser therapy improves neurosensory disorders resulting from bilateral mandibular sagittal split osteotomy: A randomized crossover clinical trial. J. Craniomaxillofac Surg. 2014, 42, e130–e133. [Google Scholar] [CrossRef]
- Bashiri, S.; Malekzadeh, H.; Fekrazad, R. The effect of delayed photobiomodulation on neurosensory disturbance recovery after zygomatic trauma: A parallel controlled clinical trial. J. Photochem. Photobiol. B 2021, 217, 112153. [Google Scholar] [CrossRef]
- Salari, B.; Nikparto, N.; Babaei, A.; Fekrazad, R. Effect of delayed photobiomodulation therapy on neurosensory recovery in patients with mandibular nerve neurotmesis following traumatic mandibular fracture: A randomized triple-blinded clinical trial. J. Photochem. Photobiol. B 2022, 232, 112460. [Google Scholar] [CrossRef]
- Qi, W.; Wang, Y.; Huang, Y.-Y.; Jiang, Y.; Yuan, L.; Lyu, P.; Arany, P.R.; Hamblin, M.R. Photobiomodulation therapy for management of inferior alveolar nerve injury post-extraction of impacted lower third molars. Lasers Dent. Sci. 2020, 4, 25–32. [Google Scholar] [CrossRef]
- Esteves Pinto Faria, P.; Temprano, A.; Piva, F.; Sant’ana, E.; Pimenta, D. Low-level laser therapy for neurosensory recovery after sagittal ramus osteotomy. Minerva Stomatol. 2020, 69, 141–147. [Google Scholar] [CrossRef]
- Führer-Valdivia, A.; Noguera-Pantoja, A.; Ramírez-Lobos, V.; Solé-Ventura, P. Low-level laser effect in patients with neurosensory impairment of mandibular nerve after sagittal split ramus osteotomy. Randomized clinical trial, controlled by placebo. Med. Oral. Patol. Oral. Cir. Bucal. 2014, 19, e327–e334. [Google Scholar] [CrossRef] [PubMed]
- Ozen, T.; Orhan, K.; Gorur, I.; Ozturk, A. Efficacy of low level laser therapy on neurosensory recovery after injury to the inferior alveolar nerve. Head. Face Med. 2006, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Mohajerani, S.H.; Tabeie, F.; Bemanali, M.; Tabrizi, R. Effect of Low-Level Laser and Light-Emitting Diode on Inferior Alveolar Nerve Recovery After Sagittal Split Osteotomy of the Mandible: A Randomized Clinical Trial Study. J. Craniofac Surg. 2017, 28, e408–e411. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.P.d.A.; Rocha-Junior, W.G.P.; Luz, J.G.C. Effects of light-emitting diode (LED) therapy on sensory changes in the inferior alveolar nerve after surgical treatment of mandibular fractures: A randomized controlled trial. Oral. Maxillofac. Surg, 2022; online ahead of print. [Google Scholar]
- Pol, R.D.; Gallesio, G.D.; Riso, M.D.; Ruggiero, T.D.; Scarano, A.D.; Mortellaro, C.M.; Mozzati, M.M. Effects of Superpulsed, Low-Level Laser Therapy on Neurosensory Recovery of the Inferior Alveolar Nerve. J. Craniofac Surg. 2016, 27, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Eshghpour, M.; Shaban, B.; Ahrari, F.; Erfanian, M.; Shadkam, E. Is Low-Level Laser Therapy Effective for Treatment of Neurosensory Deficits Arising From Sagittal Split Ramus Osteotomy? J. Oral. Maxillofac. Surg. 2017, 75, 2085–2090. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, R.F.; da Silva, C.V.; de Paula, J.T.; de Oliveira, K.D.C.M.; de Siqueira, S.R.D.T.; de Freitas, P.M. Effectiveness of Laser Therapy and Laser Acupuncture on Treating Paraesthesia After Extraction of Lower Third Molars. Photobiomodul Photomed. Laser Surg. 2021, 39, 774–781. [Google Scholar] [CrossRef]
- Santos, F.T.; Sciescia, R.; Santos, P.L.; Weckwerth, V.; Dela Coleta Pizzol, K.E.; Queiroz, T.P. Is Low-Level Laser Therapy Effective on Sensorineural Recovery After Bilateral Sagittal Split Osteotomy? Randomized Trial. J. Oral. Maxillofac. Surg. 2019, 77, 164–173. [Google Scholar] [CrossRef]
- da Silva, T.G.; Ribeiro, R.S.; Mencalha, A.L.; Fonseca, A.d.S. Photobiomodulation at molecular, cellular, and systemic levels. Lasers Med. Sci. 2023, 38, 136. [Google Scholar] [CrossRef]
- Hosseinpour, S.; Tunér, J.; Fekrazad, R. Photobiomodulation in Oral Surgery: A Review. Photobiomodul Photomed. Laser Surg. 2019, 37, 814–825. [Google Scholar] [CrossRef]
- Dompe, C.; Moncrieff, L.; Matys, J.; Grzech-Leśniak, K.; Kocherova, I.; Bryja, A.; Bruska, M.; Dominiak, M.; Mozdziak, P.; Skiba, T.H.I.; et al. Photobiomodulation-Underlying Mechanism and Clinical Applications. J. Clin. Med. 2020, 9, 1724. [Google Scholar] [CrossRef]
- Shoman, A.; Hassan, A.; Kassab, A. A Study on the Effect of 850 nm Low-Level Diode Laser versus Electrical Stimulation in Facial Nerve Regeneration for Patients with Bell’s Palsy. ORL J. Otorhinolaryngol. Relat. Spec. 2022, 84, 370–377. [Google Scholar] [CrossRef]
- De Oliveira, R.F.; de Andrade Salgado, D.M.; Trevelin, L.T.; Lopes, R.M.; da Cunha, S.R.; Aranha, A.C.; de Paula Eduardo, C.; de Freitas, P.M. Benefits of laser phototherapy on nerve repair. Lasers Med. Sci. 2015, 30, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Rochkind, S. Phototherapy in peripheral nerve regeneration: From basic science to clinical study. Neurosurg. Focus. 2009, 26, E8. [Google Scholar] [CrossRef] [PubMed]
- Anders, J.J.; Moges, H.; Wu, X.; Erbele, I.D.; Alberico, S.L.; Saidu, E.K.; Smith, J.T.; Pryor, B.A. In vitro and in vivo optimization of infrared laser treatment for injured peripheral nerves. Lasers Surg. Med. 2014, 46, 34–45. [Google Scholar] [CrossRef]
- Mohammed, I.F.; Al-Mustawfi, N.; Kaka, L.N. Promotion of regenerative processes in injured peripheral nerve induced by low-level laser therapy. Photomed. Laser Surg. 2007, 25, 107–111. [Google Scholar] [CrossRef]
- von Leden, R.E.; Cooney, S.J.; Ferrara, T.M.; Zhao, Y.; Dalgard, C.L.; Anders, J.J.; Byrnes, K.R. 808 nm wavelength light induces a dose-dependent alteration in microglial polarization and resultant microglial induced neurite growth. Lasers Surg. Med. 2013, 45, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Alayat, M.S.M.; Basalamah, M.A.; Elbarrany, W.G.E.A.; El Sawy, N.A.M.; Abdel-Kafy, E.M. Efficacy of multi-wave locked system laser therapy on nerve regeneration after crushing in Wister rats. J. Phys. Ther. Sci. 2021, 33, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Alayat, M.S.M.; Basalamah, M.A.; Elbarrany, W.G.E.A.; El-Sawy, N.A.M.; Abdel-Kafy, E.M.; El-Fiky, A.A. Dose-dependent effect of the pulsed Nd:YAG laser in the treatment of crushed sciatic nerve in Wister rats: An experimental model. Lasers Med. Sci. 2020, 35, 1989–1998. [Google Scholar] [CrossRef]
- Fernandes-Neto, J.A.; Simões, T.M.; Batista, A.L.; Lacerda-Santos, J.T.; Palmeira, P.S.; Catão, M.V. Laser therapy as treatment for oral paresthesia arising from mandibular third molar extraction. J. Clin. Exp. Dent. 2020, 12, e603–e606. [Google Scholar] [CrossRef]
- Buchaim, D.V.; Andreo, J.C.; Ferreira Junior, R.S.; Barraviera, B.; Rodrigues, A.C.; Macedo, M.C.; Rosa Junior, G.M.; Shinohara, A.L.; Santos German, I.J.; Pomini, K.T.; et al. Efficacy of Laser Photobiomodulation on Morphological and Functional Repair of the Facial Nerve. Photomed. Laser Surg. 2017, 35, 442–449. [Google Scholar] [CrossRef]
- Rosso, M.P.O.; Rosa Júnior, G.M.; Buchaim, D.V.; German, I.J.S.; Pomini, K.T.; de Souza, R.G.; Pereira, M.; Favaretto Júnior, I.A.; Bueno, C.R.S.; Gonçalves, J.B.O.; et al. Stimulation of morphofunctional repair of the facial nerve with photobiomodulation, using the end-to-side technique or a new heterologous fibrin sealant. J Photochem Photobiol B. 2017, 175, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Andreo, L.; Soldera, C.B.; Ribeiro, B.G.; de Matos, P.R.V.; Bussadori, S.K.; Fernandes, K.P.S.; Mesquita-Ferrari, R.A. Effects of photobiomodulation on experimental models of peripheral nerve injury. Lasers Med. Sci. 2017, 32, 2155–2165. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, M.A.; Paranhos, L.R.; Martins-Filho, P.R. Low-level laser therapy for treatment of neurosensory disorders after orthognathic surgery: A systematic review of randomized clinical trials. Med. Oral. Patol. Oral. Cir. Bucal. 2017, 22, 780–787. [Google Scholar]
- Brignardello-Petersen, R. Low-level laser therapy may reduce the time of recovery from paresthesia after orthognathic surgery. J. Am. Dent. Assoc. 2018, 149, e44. [Google Scholar] [CrossRef]
- Barbosa, L.M.; de Luna Gomes, J.M.; Laureano Filho, J.R.; do Egito Vasconcelos, B.C.; Dantas Moraes, S.L.; Pellizzer, E.P. Does the use of low-level light therapy postoperatively reduce pain, oedema, and neurosensory disorders following orthognathic surgery? A systematic review. Int. J. Oral. Maxillofac. Surg. 2022, 51, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Prazeres, L.D.; Muniz, Y.V.; Barros, K.M.; Gerbi, M.E.; Laureano Filho, J.R. Effect of infrared laser in the prevention and treatment of paresthesia in orthognathic surgery. J. Craniofac Surg. 2013, 24, 708–711. [Google Scholar]
- da Fonseca, E.V.; Bussadori, S.K.; da Silva Martinho, L.F.C.; de Sousa Melo, M.C.; de Andrade, F.L.; Gonçalves, M.L.L.; Mesquita-Ferrari, R.A.; Horliana, A.C.R.T.; Fernandes, K.P.S. Evaluation of photobiomodulation effects on pain, edema, paresthesia, and bone regeneration after surgically assisted rapid maxillary expansion: Study protocol for a randomized, controlled, and double blind clinical trial. Medicine 2019, 98, e17756. [Google Scholar]
- Leyane, T.S.; Jere, S.W.; Houreld, N.N. Cellular Signalling and Photobiomodulation in Chronic Wound Repair. Int. J. Mol. Sci. 2021, 22, 11223. [Google Scholar] [CrossRef]
- Nascimento, J.J.A.C.; Machado, A.S.D.; Della-Santa, G.M.L.; Fernandes, D.C.; Ferreira, M.C.; Machado, G.A.P.; Chaves, B.C.G.; Costa, K.B.; Rocha-Vieira, E.; Oliveira, M.X.; et al. Effects of photobiomodulation therapy on functional recovery, angiogenesis and redox status in denervated muscle of rats. Einstein 2021, 19, eAO6001. [Google Scholar] [CrossRef]
- Gambarotta, G.; Raimondo, S.; Udina, E.; Phillips, J.B.; Haastert-Talini, K. Editorial: Peripheral nerve regeneration. Front. Cell Neurosci. 2019, 13, 464. [Google Scholar] [CrossRef]
- Araujo, T.; Andreo, L.; Tobelem, D.D.C.; Silva, T.; Malavazzi, T.C.D.S.; Martinelli, A.; Lemes, B.; Fernandes, K.P.S.; Bussadori, S.K.; Mesquita-Ferrari, R.A. Effects of systemic vascular photobiomodulation using LED or laser on sensory-motor recovery following a peripheral nerve injury in Wistar rats. Photochem. Photobiol. Sci. 2023, 22, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Modrak, M.; Talukder, M.A.H.; Gurgenashvili, K.; Noble, M.; Elfar, J.C. Peripheral nerve injury and myelination: Potential therapeutic strategies. J. Neurosci. Res. 2020, 98, 780–795. [Google Scholar] [CrossRef] [PubMed]
- Lopes, B.; Sousa, P.; Alvites, R.; Branquinho, M.; Sousa, A.C.; Mendonça, C.; Atayde, L.M.; Luís, A.L.; Varejão, A.S.P.; Maurício, A.C. Peripheral Nerve Injury Treatments and Advances: One Health Perspective. Int. J. Mol. Sci. 2022, 23, 918. [Google Scholar] [CrossRef]
- Zwick, H.; Beatrice, E.S. Long-term changes in spectral sensitivity after low-level laser (514 nm) exposure. Mod. Probl. Ophthalmol. 1978, 19, 319. [Google Scholar] [PubMed]
- De Rezende, R.A.; Silva, D.N.; Frigo, L. Effect of GaAIAs low-level laser therapy on mouth opening after orthognathic surgery. Lasers Med. Sci. 2018, 12, 1. [Google Scholar]
- Amaroli, A.; Agas, D.; Laus, F.; Cuteri, V.; Hanna, R.; Sabbieti, M.G.; Benedicenti, S. The effects of photobiomodulation of 808 nm diode laser therapy at higher fluence on the in vitro osteogenic differentiation of bone marrow stromal cells. Front. Physiol. 2018, 9, 123. [Google Scholar] [CrossRef]
- Boras, V.V.; Juras, D.V.; Rogul, A.A.; Pandurić, D.G.; Verzak, Z.; Brailo, V.L. Applications of low level laser therapy. In A Textbook of Advanced Oral and Maxillofacial Surgery; Motamedi, M.H.K., Ed.; Intech: London, UK, 2013; pp. 327–339. [Google Scholar]
- Della Santa, G.M.L.; Ferreira, M.C.; Machado, T.P.G.; Oliveira, M.X.; Santos, A.P. Effects of Photobiomodulation Therapy (LED 630 nm) on Muscle and Nerve Histomorphometry after Axonotmesis. Photochem. Photobiol. 2021, 97, 1116–1122. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic Pain: From Mechanisms to Treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef]
- Baron, R.; Binder, A.; Wasner, G. Neuropathic pain: Diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010, 9, 807–819. [Google Scholar] [CrossRef]
- Cavalli, E.; Mammana, S.; Nicoletti, F.; Bramanti, P.; Mazzon, E. The neuropathic pain: An overview of the current treatment and future therapeutic approaches. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419838383. [Google Scholar] [CrossRef]
- Attal, N.; Bouhassira, D. Advances in the treatment of neuropathic pain. Curr. Opin. Neurol. 2021, 34, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Gossrau, G.; Sabatowski, R. Diagnostik und Therapie neuropathischer Schmerzen [Diagnostics and therapy of neuropathic pain]. Anaesthesist 2021, 70, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Alcántara Montero, A.; Ibor Vidal, P.J.; Alonso Verdugo, A.; Trillo Calvo, E. Actualización en el tratamiento farmacológico del dolor neuropático [Update in the pharmacological treatment of neuropathic pain]. Semergen 2019, 45, 535–545. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).

















































































































































































































