Abstract
P-glycoprotein (P-gp) has been implicated in lung cancer development, disease progression, and patient survival. Changes in the ABCB1 expression level may correlate with tumorigenesis and the formation of multidrug resistance (MDR). In addition, epigenetic modifications such as DNA methylation are involved in the regulation of the transcriptional activity of this gene. Therefore, we focused on an analysis of changes in the expression of ABCB1 and its methylation, taking into account their potential associations with the clinicopathological parameters of LUAD and LUSC. The TNMplot, UALCAN, cBioPortal, PrognoScan, and MEXPRESS databases were used to investigate the role of this gene in lung cancer progression. ABCB1 expression in lung tissue was significantly downregulated in cancer cells, but differences also resulted from age, gender, ethnicity, and smoking cessation. Moreover, decreased levels of gene transcript were associated with both a higher stage of cancer and a lower probability of survival. It is worth emphasizing that the presence/direction of ABCB1 expression changes are frequently unique to specific histological tumor subtypes. Finally, it is equally important that the main methylation promoter is one of the causes of decreased gene expression in lung cancer cells. Taken together, these data establish the ABCB1 transporter gene as an important prognostic factor that could alter disease progression and contribute to the survival of cancer patients.
1. Introduction
Lung cancer is the main cause of cancer-related deaths in Poland, Europe, and the world [1,2,3], and it will continue to be the main cause of cancer-related deaths in both Poland and the world; therefore, the greatest emphasis should be placed on the early detection and quick diagnosis of this cancer [4,5].
The non-small-cell lung cancer (NSCLC) group consists of various morphological subtypes of lung cancer, including adenocarcinoma (LUAD) and squamous-cell carcinoma (LUSC) as well as large-cell carcinoma and others. Molecular characterization includes the identification of the genetic features of a tumor (EGFR mutation tests and ALK/ROS1 rearrangements, which may be extended to search for changes in the BRAF, MET, RET, TRK, KRAS, HER2, and NTRK1-3 genes) [5,6] and those useful in targeted drugs such as EGFR, ALK, and ROS1 tyrosine kinase inhibitors or PD-1/PD-L1 inhibitors [5,7]. Although the last two decades have brought great progress in the diagnosis of lung cancer and the identification of molecular changes, which has led to the development of new treatment strategies, the diagnosis of lung cancer is most often made in its advanced clinical stage.
Generally, the risk of lung cancer increases with age. The average age of lung cancer diagnosis is 70 years old [8]. Men are most likely to be diagnosed between the ages of 80 and 84, while women are most likely to be diagnosed between the ages of 75 and 79 [9]. Men are twice as likely to develop lung cancer, which is associated with higher rates of cigarette smoking. Simultaneously, non-smoking women present a higher incidence of this cancer than men who do not smoke, which may be related to the higher rate of EGFR mutations observed in women and the pro-cancer effect of estrogen. African American men also have a higher risk of lung cancer [8].
Lung cancer mortality is strongly related to age; however, the 5-year relative survival rate for lung cancer is higher for women than for men. Moreover, black men have a higher lung cancer death rate than white men. It is worth noting that in recent years, this ethnic group has seen a significant decline in mortality. Currently, the lung cancer death rate in African American women is significantly lower compared to women of Caucasian descent [9]. Total lung cancer mortality rates continue to decline, and this is associated with the detection of lung cancer in the earlier stages of the disease. Current statistics suggest that approximately three quarters of patients with stage I NSCLC can expect to live at least 5 years following their diagnosis. Among patients with stage IV NSCLC that has spread regionally, only a third of all patients survive for 5 years or more after diagnosis [10].
The pathomechanism of cancer development is a long-term and very complex process. In cancer cells, mutations or amplifications are initiated, affecting genes such as BRAF, EGFR, and MET in adenocarcinoma and NOTCH1 and FGFR1 in squamous-cell carcinoma, respectively. TP53 mutations are observed regardless of lung cancer subtype. Subsequent mutations, e.g., in the PIK3CA and NF1 genes, and the acquisition of features of chromosomal instability primarily determine the further survival of cancer cells [11]. The direct cause of the molecular changes in lung tissue cells may be, i.a., a high level of exposure to various toxic compounds [12]. Therefore, human defense mechanisms are of great importance in the pathogenesis of lung cancer, such as the activity of key proteins belonging to the detoxifying metabolic pathway of xenobiotics and other individual characteristics.
One mechanism is the action of transporter proteins from the ABC family (ATP-binding cassette transporters), which capture particles/substances entering a cell by passive transport from the extracellular environment. The first transporter from this family that was identified was P-glycoprotein (P-gp), which has since remained the most studied and most important transporter involved in multidrug resistance (MDR). Due to the wide substrate spectrum, P-gp protects the cellular environment by pumping out numerous xenobiotics of exogenous origin and endogenous ligands, and it also inhibits the transport of compounds through the blood–brain, blood–testis, and blood–placental barriers, while its overexpression leads to the formation of MDR [13]. This phenomenon can be the cause of chemotherapy failure. Inhibitors of this protein are being intensively studied in terms of limiting MDR: from the early generation of P-gp inhibitors with significant toxicity, through newer generations characterized by unsatisfactory clinical effectiveness, to other drugs showing anti-cancer properties and, e.g., mTOR kinase inhibitors that indirectly inhibit P-gp [14,15]. The amount of the transporter in cancer cells may be increased as a result of chemotherapy and the acquisition of resistance to the compounds used as P-gp substrates. However, it has been observed that the process of carcinogenesis itself may be associated with a change in the amount of protein in cells undergoing neoplastic transformations. In the case of cancers of organs in which cells in the physiological state are characterized by high expression of P-gp, i.e., the kidneys or liver, the amount of synthesized protein becomes even greater. On the other hand, in cancers that affect other organs, including the lungs, where the transporter is physiologically at a lower level (https://www.ncbi.nlm.nih.gov/gene/5243, accessed on 15 May 2023), the amount of protein decreases as the disease progresses [16].
P-glycoprotein is encoded by the polymorphic gene ABCB1 (ATP-Binding Cassette Subfamily B Member 1). One of the best-known polymorphisms of this gene is SNP C3435T (rs1045642), which probably affects the transcript level and consequently the amount of protein [17]. Similar correlations were also investigated for other polymorphisms of this gene located in the coding region, introns, and the promoter region [18]. The biological and clinical effects that are associated with these polymorphisms may be the consequences of alternative mRNA splicing, changes in its structure or stability, and modifications in translation kinetics involving rare tRNAs recognizing a modified codon. ABCB1 gene expression can also be modulated by factors such as heat shock, cytokines, chemotherapeutics, UV radiation, oxygen free radicals, and numerous transcription factors that can regulate expression post-transcriptionally by affecting mRNA maturation. Moreover, the p53 protein can positively and negatively affect ABCB1 transcription [19].
The regulatory sequences of the promoter region, which are located upstream of the transcription start site and contain CpG islands that can undergo methylation, are also important elements that modulate the expression of ABCB1 [20]. All these observations suggest that different levels of expression of the P-gp gene should be expected in cancer cells, including lung cancer cells. Therefore, the aim of the study was to assess the clinical significance of the ABCB1 gene in patients with lung cancer using data collected in selected bioinformatics databases. The levels of expression and methylation of the ABCB1 gene in normal and neoplastic lung tissues were analyzed, taking into account their potential correlations with clinicopathological and molecular parameters of the disease.
2. Methods
2.1. TNMplot Database
TNMplot (https://tnmplot.com/analysis/, accessed on 29 July 2022) is an online analysis platform that allows the comparison of gene expression in normal, tumor, and metastatic tissues. The database includes RNA-seq data from The Genotype-Tissue Expression Project (GTEx), The Cancer Genome Atlas (TCGA), and Therapeutically Applicable Research to Generate Effective Treatments (TARGET) and gene chip data from Gene Expression Omnibus (GEO) [21]. In this study, the pan-cancer analysis panel to evaluate the expression of the ABCB1 gene across different tissues was used. The differences between normal and cancer tissues were determined using the Mann–Whitney U test with a 0.01 level of significance.
2.2. UALCAN Database
UALCAN (https://ualcan.path.uab.edu, accessed on 10 August 2022) is an interactive web resource for pan-cancer gene expression analysis; the comparison of relative gene expression levels between tumors and corresponding adjacent normal tissue samples, including the analysis of subgroups according to a tumor’s demographic/clinical/pathologic features; Kaplan–Meier survival analysis; and promoter methylation determination within the TCGA database [22,23]. In this study, we analyzed the expression and methylation of ABCB1 in LUAD/LUSC and its correlations with clinicopathologic parameters estimated using Student’s t-test considering unequal variance. Patients with particular types of cancer were divided into two groups (high- and low-expression groups), and the effect of gene expression on patient survival was measured using a log rank test.
2.3. cBioPortal Database
cBioPortal (http://www.cbioportal.org/, accessed on 5 August 2022) is an open-source platform that enables the processing, analysis, and visualization of large-scale cancer genomic data [24,25]. Using the TCGA data, the association between ABCB1 mRNA expression and the protein levels in the NSCLC histological types was determined. Spearman and Pearson correlation coefficients (r) and their corresponding p values were calculated.
2.4. PrognoScan Database
PrognoScan (http://dna00.bio.kyutech.ac.jp/PrognoScan/index.html, accessed on 17 October 2022) provides an online platform for evaluating the prognostic values of genes [26]. PrognoScan was used to assess the correlations between ABCB1 expression and survival in the main histological types of lung cancer. This tool enabled the analysis of datasets other than those within the TCGA database. For each dataset, samples were divided into two (high and low) expression groups, and then Cox p-values and hazard ratios (with 95% confidence intervals) were calculated.
2.5. MEXPRESS Database
MEXPRESS (https://mexpress.be, accessed on 18 March 2023 ) is an online tool for analyzing and visualizing gene expression, DNA methylation, and clinical TCGA data [27,28]. Our analysis included 574 LUAD samples and 550 LUSC samples for which ABCB1 expression data were available; the samples were sorted by their expression. The p values (t test or ANOVA) and Pearson correlation coefficients (r) for the comparisons between the expression data and the DNA methylation for specific probe/clinical parameters (age at initial pathologic diagnosis, new tumor event after initial treatment, number of pack-years smoked, cigarettes per day, years smoked, sample type, gender, race/ethnicity, histological subtype, simplified tumor stage, particular TNM parameters, and overall survival) were calculated. The presented p values were adjusted based on the Benjamini–Hochberg method. p < 0.05 was considered statistically significant.
3. Results
3.1. Physiologically Low Levels of ABCB1 Expression in Lung Tissue Are Significantly Downregulated in Cancer Cells
First, the level of expression of the ABCB1 gene was compared in different organs (Figure 1), with a particular emphasis on lung tissue (Figure 2). For this purpose, the resources of the TNMplot and UALCAN databases were used. The presented data confirmed the significant variation in the expression of this gene, depending on the type of tissue (Figure 1A,B). Moreover, for almost all analyzed tissues, the neoplastic process was associated with a significant change in the amount of mRNA in the cells, with the exception of colon and gastric cells (Figure 1B).
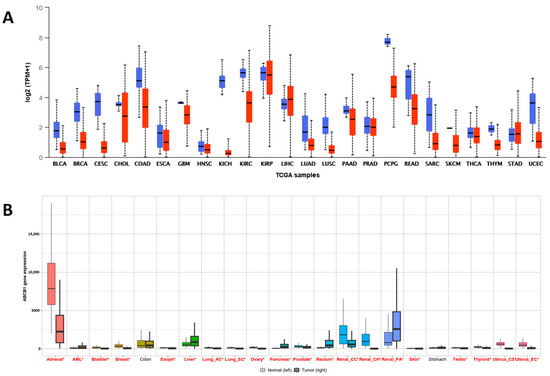
Figure 1.
ABCB1 expression in pan-cancer. (A) Differential ABCB1 expression levels in tumors and normal tissues. Blue box plots—normal samples; red box plots—tumor samples; log2 (TPM + 1)—log2 of transcripts per million transformed to positive values (TPM + 1) (UALCAN). (B) Comparison of ABCB1 expression between cancer and normal tissue. A red * marks those tissues for which the differences in the expression levels were statistically significant (TNMplot).
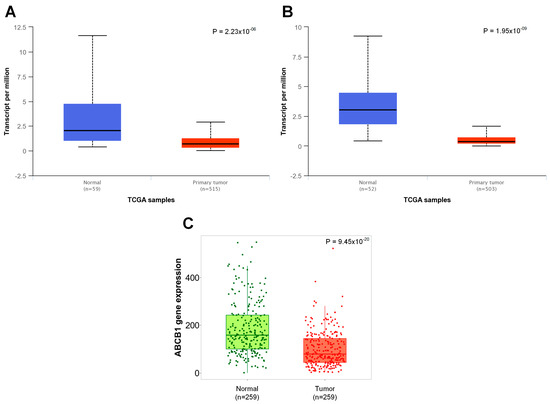
Figure 2.
Comparison of ABCB1 gene expression between lung cancer and normal tissue. (A) The downregulation of ABCB1 expression in LUAD (UALCAN). (B) The downregulation of ABCB1 expression in LUSC (UALCAN). (C) Differentiated ABCB1 expression in cancer tissue and adjacent normal lung tissue (gene chip data and TNMplot).
For most tissues, including lung tissues, the level of expression was higher in normal tissues than in neoplastic ones. This difference was highlighted in the adrenal glands and some kidney tumors. In some cases, an increase in the expression of the ABCB1 gene was observed in the tumor tissue compared to its normal cells. Examples include liver cancer and papillary renal cell carcinoma. Noticeable differences between the data obtained from independent databases may have resulted from the diversity of the histological types and subtypes included in these sources.
As shown in Figure 2A,B, the correlation between the expression of the ABCB1 gene and the malignant transformation of lung cells was present regardless of the histological type of the tumor. This correlation was present in both the glandular type (p < 0.001) and the squamous cell type (p < 0.001). In addition, there was also a difference in the level of expression of this gene between cancer and adjacent normal lung tissue (p < 0.001, Figure 2C).
These observations are clinically significant due to the results of the evaluation of the correlation between the amount of mRNA of the discussed gene and the level of the protein encoded by this gene. As shown in Figure 3, there was a statistically significant correlation between the expression coefficient for mRNA and the amount of P-gp protein. Moreover, this dependence was proportional and occurred in both adenocarcinoma (R (Spearman) = 0.44, p < 0.001; R (Pearson) = 0.62, p < 0.001; Figure 3A) and squamous-cell lung cancer (R (Spearman) = 0.49, p < 0.01; R (Pearson) = 0.50, p < 0.01; Figure 3B). The presented data came from the cBioPortal database collected for this subgroup.
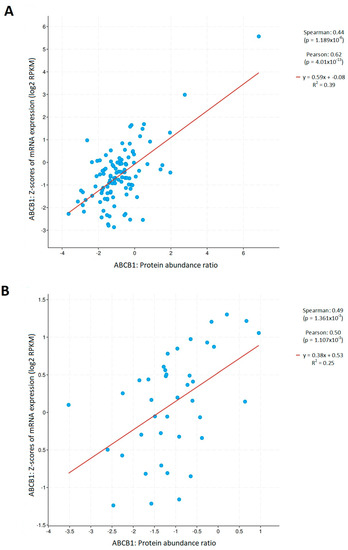
Figure 3.
Correlation between ABCB1 expression and the P-gp amount ratio in (A) LUAD and (B) LUSC (cBioPortal).
3.2. Time Elapsed since Smoking Cessation/Quitting Affects P-gp Gene Expression in Ex-Smokers with Squamous-Cell Lung Cancer
Cigarette smoking is invariably one of the main factors predisposing individuals to the development of lung cancer, and among smokers there is a tendency to develop the squamous histological type of this cancer. Nonsmokers are more likely to be diagnosed with the second type of NSCLC, adenocarcinoma [29]. Therefore, in a further part of the study, it was checked whether there is a relationship between smoking status and the amount of the ABCB1 gene transcript in the cells of both histological types of lung cancer. It was observed that regardless of the status of smoking addiction, the amount of mRNA in adenocarcinoma cells remained at a low level (p < 0.001). In contrast, the time that had elapsed since smoking cessation in ex-smokers seemed to affect ABCB1 expression in squamous-cell carcinoma. A longer time since smoking cessation had a statistically significant effect on the increase in the transcript level of the studied gene (p = 0.0228, Figure 4B).
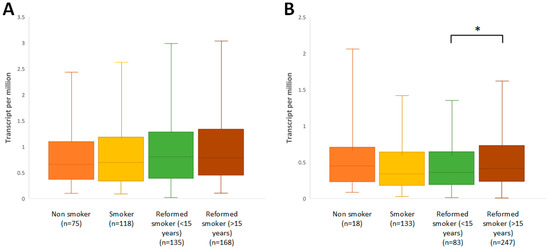
Figure 4.
Comparison of ABCB1 expression between normal tissues and cancer tissues in relation to smoking status in (A) LUAD and (B) LUSC (* p < 0.05; UALCAN).
3.3. Age, Gender, and Ethnicity Influence the Expression of the ABCB1 Gene
The next stage of the study was to analyze the demographic characteristics of patients with non-small-cell lung cancer and how they affected the expression of the ABCB1 gene. The levels of ABCB1 expression in cancer tissues, regardless of the age range, were characterized by similar values that significantly differed from the level of expression in healthy tissue. Moreover, in squamous-cell lung cancer, the lowest level of expression was found in the tumor tissues of patients aged 21–40, and it was significantly lower than the levels in the groups aged 61–80 (p < 0.001) and 81–100 years (p = 0.0106, Figure 5B). This parameter also differed significantly between the 41–60 and 61–80 age groups (p = 0.0136). In the glandular type of cancer, similar dependencies could be observed; however, the differences may have been a consequence of the different sample sizes in the analyzed subgroups (Figure 5A).

Figure 5.
Comparison of ABCB1 expression between normal tissues and cancer tissues (LUAD (A,C,E) and LUSC (B,D,F)), depending on age (A,B), gender (C,D), and race (E,F) (* p < 0.05, ** p < 0.001; UALCAN).
Lower expression of the ABCB1 gene was specific not only to younger patients with lung cancer but also to women and people of African American and Asian descent; however, this only applied to the LUAD subtype. As shown in Figure 5C, the median expression levels of the tested gene in neoplastic tissues differed only slightly between women and men; nevertheless, the expression appeared to be significantly higher in male adenocarcinoma cells (p = 0.0391, Figure 5C). The highest expression in lung adenocarcinoma tissues was observed in representatives of the Caucasian population (p = 0.0294), and the lowest expression was observed in the Asian population, although compared to the others, this ethnic group was represented by only a few individuals in the analysis (Figure 5E). Among patients with squamous-cell lung cancer, the median levels of P-gp gene expression in cancerous tissues were similar regardless of sex in all ethnic groups, without significant differences (Figure 5D,F).
3.4. The Higher Stage of Cancer May Be Associated with a Decrease in the Amount of the ABCB1 Gene Transcript
The next part of the analysis of ABCB1 gene expression in NSCLC was aimed at determining its potential impact on the clinicopathological features of the cancer. By analyzing the relationship between the expression of the ABCB1 gene and the stage of cancer in adenocarcinoma, it was found that between stage I and stage III there was a statistically significant decrease in the expression of the gene for P-gp (p = 0.0387), while in patients with the most advanced stage of cancer, the amount of mRNA in the tumor cells increases again (p = 0.0484, Figure 6A). Similar observations applied to squamous-cell carcinoma (p = 0.0006), although in the highest stage of this type of cancer, a further decrease in ABCB1 expression was seen (p = 0.0062, Figure 6B). However, this may have been due to the small size of this subgroup. This may also indicate a connection between ABCB1 amplifications and the terminal stage of lung cancer as well as patient survival.

Figure 6.
Comparison of ABCB1 expression between normal tissues and cancer tissues (LUAD (A,C,E) and LUSC (B,D,F)), depending on lung cancer staging (A,B), metastatic lymph node status (C,D), and TP53 mutation status (E,F) (* p < 0.05; ** p < 0.01; *** p < 0.001; UALCAN).
Whether the amount of mRNA of the examined gene varied between patients with different degrees of lymph node involvement and different mutation statuses in the TP53 gene was also checked. There were no differences in the amount of mRNA between the individual groups of patients for both of the examined features (p > 0.05, Figure 6C–F). These observations applied to both adenocarcinoma and squamous-cell lung cancer.
3.5. Effect of ABCB1 Expression on Survival in Lung Cancer
Another element of the study was an assessment of the prognostic value of the amount of ABCB1 mRNA in patients with lung cancer. For this purpose, the survival times of patients with high and low expression of the gene were compared for adenocarcinoma and squamous-cell lung cancer. A significant correlation was found between the survival probability of patients with lung adenocarcinoma and the level of expression of the gene encoding P-gp (p = 0.021, Figure 7A). Lower expression of the studied gene was correlated with a lower probability of survival in patients with this type of cancer. Similar associations were not found in squamous-cell carcinoma (p = 0.94, Figure 7B).
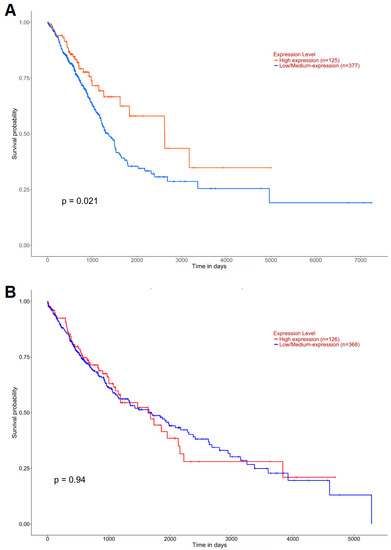
Figure 7.
Kaplan–Meier survival curves for (A) LUAD and (B) LUSC mortality (UALCAN).
In order to analyze the parameters related to the survival of patients with this type of cancer, data from the PrognoScan platform were used (Table 1).

Table 1.
ABCB1 expression and survival data of lung cancer patients (PrognoScan database; OS—overall survival, DSS—disease-specific survival, RFS—recurrence-free survival/relapse-free survival).
On the basis of the analyzed datasets, it was observed that the level of expression of the studied gene was correlated with overall survival and/or recurrence-free survival in 8 out of 35 groups of studied lung cancer patients. For each of these eight studies, the survival curves for patients with high and low expression of the ABCB1 gene are presented (Figure 8). It was shown that patients with lung cancer in the group characterized by a high level of ABCB1 mRNA had a better chance of survival compared to patients with low expression of this gene. An exception was one of the datasets that showed the opposite relationship (Figure 8B). The vast majority of the statistically significant results concerned the LUAD subtype, which confirmed previous observations and indicated the possible use of ABCB1 gene expression as a useful biomarker for predicting the survival of patients with lung adenocarcinoma.
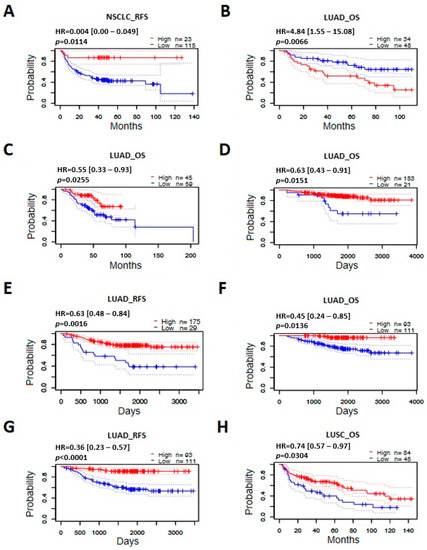
Figure 8.
Prognostic value of ABCB1 mRNA level in lung cancer patients. Kaplan–Meier survival curves for different datasets in relation to the gene expression level (PrognoScan). (A) Recurrence-free survival/relapse-free survival (RFS) in NSCLC; (B–D,F) overall survival (OS) in LUAD; (E,G) recurrence-free survival/relapse-free survival (RFS) in LUAD; (H) overall survival (OS) in LUSC. (A) GSE8894/243951_at; (B) jacob-00182-CANDF/209993_at; (C) jacob-00182-MSK/209994_s_at; (D) GSE31210/209993_at; (E) GSE31210/209993_at; (F) GSE31210/243951_at; (G) GSE31210/243951_at; (H) GSE4573/209994_s_at.
3.6. DNA Methylation Is Correlated with Decreased ABCB1 Gene Expression in Lung Cancer Cells
Gene expression can be regulated by many mechanisms (at both the transcriptional and post-transcriptional levels), including changes in the level of DNA methylation. For the next part of this paper, the methylation status of the ABCB1 gene was compared between healthy and neoplastic lung tissues, and its associations with the clinicopathological and molecular features of LUAD and LUSC were analyzed. Analyses performed using the UALCAN database showed that in tumors, regardless of the histological type, significantly increased methylation of the promoter of this gene was observed in comparison to the normal lung tissue (p < 0.0001, Figure 9A and Figure 10A). In lung adenocarcinoma, increased methylation of the promoter region was also observed in patients over 40 years of age and among members of the Caucasian race.

Figure 9.
The correlations between ABCB1 promoter methylation and (A) the presence of a tumor, (B) smoking status, (C) age, (D) gender, (E) race, (F) cancer staging, (G) metastatic lymph node status, and (H) TP53 mutation status using UALCAN in LUAD (* p < 0.05; ** p < 0.01; *** p < 0.001).

Figure 10.
The correlations between ABCB1 promoter methylation and (A) the presence of a tumor, (B) smoking status, (C) age, (D) gender, (E) race, (F) cancer staging, (G) metastatic lymph node status, and (H) TP53 mutation status using UALCAN in LUSC (** p < 0.01; *** p < 0.001).
Mutations in the TP53 gene also correlated with the level of methylation of the tested gene (Figure 9B–H). In squamous-cell lung cancer, on the other hand, it was observed that the methylation level of the ABCB1 promoter region was significantly lower in stage IV tumors compared to less advanced tumors (Figure 10B–H). Furthermore, to confirm the obtained results, an analysis was performed using the MEXPRESS database. A negative correlation was found between the degree of methylation in one of the regions of the CpG islands and the level of ABCB1 expression in both LUAD and LUSC (areas marked in red in Figure 11 and Figure 12).
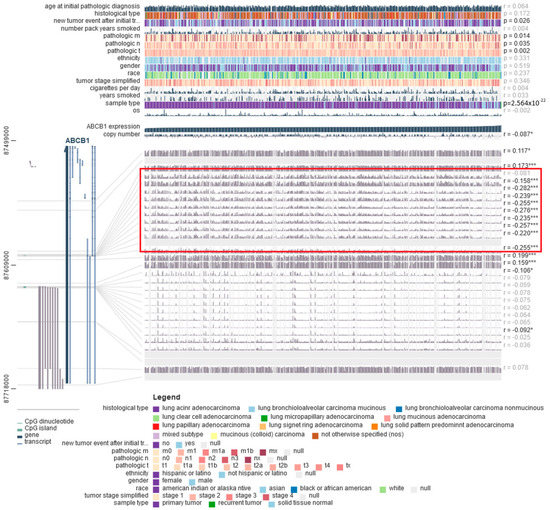
Figure 11.
The correlations between ABCB1 expression, selected clinical parameters, and DNA methylation probes using MEXPRESS in LUAD (* p < 0.05; *** p < 0.001).
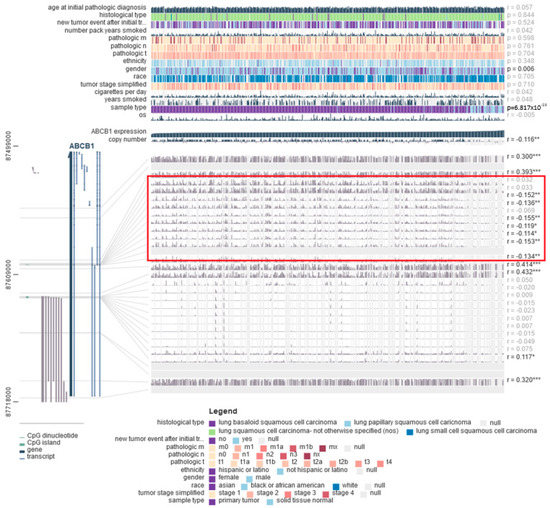
Figure 12.
The correlations between ABCB1 expression, selected clinical parameters, and DNA methylation probes using MEXPRESS in LUSC (* p < 0.05; ** p < 0.01; *** p < 0.001).
4. Discussion
Because of the non-specific symptoms of the disease and diagnosis at a late stage, lung cancer results in a poor prognosis and limited therapeutic options. Members of the ABC transporter superfamily, including P-gp, are involved in the development of resistance to a wide range of drugs that are structurally unrelated and have different mechanisms of action. By increasing the efflux of chemotherapeutic agents, cancer cells can reduce intracellular drug levels and prevent them from reaching therapeutic concentrations at the target site [29]. In studies using cell lines and animals, it has been shown that many substances act as effective P-gp inhibitors. However, the results of clinical trials indicate the low potency and specificity of these substances compared to most clinically used drugs [30].
On the other hand, an increased amount of P-gp was found in many types of cancer cells, including tumors that had not been treated with chemotherapeutic agents. This suggests that the activity of the transporter may be related to the pathomechanism of tumor development. It is probable that the amount of P-gp in a cell is correlated with its exposure to toxins and xenobiotics, which are directly related to DNA damage, leading to the formation of the cancer cell phenotype. Whether the most extensively studied ABCB1 gene polymorphisms affect treatment responses or the side effects of chemotherapeutics remains a matter of speculation. This issue is complex, and it is known that many factors can modulate the process of transcription and protein formation. Therefore, in the presented study, the prognostic significance of the ABCB1 mRNA level in lung cancer was assessed using data collected in publicly available bioinformatics databases.
The obtained results confirmed a large diversity in mRNA levels for ABCB1 depending on the tissue type. High expression of this gene and P-gp in adrenal, kidney, liver, and intestine tissues was confirmed in many previous studies [31,32]. The exceptions were the colon and stomach, where no significant differences in ABCB1 expression were shown between cancer tissues and normal tissues. Additionally, for gastric cells, a negligible level of the transcript was observed. In a study using cell lines, significantly lower ABCB1 mRNA levels in gastric cancers compared to colon cancer were shown [33], reflecting the physiological role of this transporter in the absorption in the small/large intestines. In the presented study, non-cancerous lung tissue indicated higher levels of ABCB1 mRNA compared to NSCLC cells. Our findings were consistent with the results of research related to breast [34] and colorectal cancer [35]. This suggests a close link between the reduction in efflux pump function and the transformation of, i.a., normal lung cells to malignant cells. Furthermore, cell-line-based research indicates that metastatic cells develop a secondary multidrug resistance involving P-gp under the influence of chemotherapy. Several research teams found significant increases in ABCB1 expression after repeated courses of treatment with chemotherapeutic drugs [36,37,38]. These observations are also clinically significant due to the strong positive correlation between the amounts of mRNA and P-gp protein, which was confirmed in the presented study and other clinical lung cancer samples [37].
Tobacco smoking is an important risk factor for lung cancer development. Specifically, this includes SCLC and the squamous histological type of NSCLC cancer. It is thought that chemicals found in tobacco smoke alter the expression or the activity of drug-metabolizing enzymes and xenobiotic transporters and could directly suppress P-gp activity [39]. This may be an explanation for the results obtained in the presented study among LUSC cancer patients. A time longer than 15 years since smoking cessation significantly increased the number of ABCB1 transcripts.
An age-associated upregulation of the investigated gene was another characteristic of NSCLC patients, regardless of the histological cancer subtype. It is possible to hypothesize that the progressive restoration of ABCB1 expression in cancer cells with age may be due to environmental factors, including longer exposure to air pollution or increases in drug consumption. Conversely, older people often have decreased P-gp levels in the blood–brain barrier that are connected, i.a., with concomitant neurodegenerative disease [40]. The results of animal studies indicate that the directions of P-gp expression/function changes are likely to be tissue-specific [41]. It is known that the amounts of P-gp are also different between men and women. For example, hepatic P-gp expression is lower in females than in males [42]. In our analysis, the ABCB1 expression appeared to be upregulated in males but only in adenocarcinoma cells. In summary, differences in the P-gp level may result from tissue diversity, hormonal influences, ethnicity, and interindividual conditions. Due to the limited number of studies addressing the influences of smoking, age, gender, and ethnicity on the ABCB1 transcript levels in lung cancer patients, these observations have not been confirmed elsewhere.
The present analysis demonstrated that ABCB1 gene expression negatively correlates with NSCLC staging. Surprisingly, in the most advanced clinical stage of cancer (IV), the number of transcripts increased, which could suggest an association between ABCB1 amplifications and patient survival. Patients diagnosed with a primary tumor in stage IV could be exposed to the strong effects of harmful environmental compounds or prolonged drug use for reasons other than cancer. Unfortunately, according to our best knowledge, there have been no studies that thoroughly assessed the correlation between the expression of the investigated gene and the lung cancer staging adjusted for data on the degree of environmental influence. Moreover, a high level of ABCB1 mRNA was associated with a longer recurrence-free survival/overall survival for patients with lung adenocarcinoma. Conversely, Zou F et al. showed a trend toward worse survival in tumors with P-gp expression in both LUAD and LUSC; however, the difference for lung squamous-cell carcinoma was not statistically significant [43]. Thus, all these results suggest that ABCB1 expression is useful for predicting prognoses in LUAD patients. However, the level of gene transcripts should be regarded as only one of many factors determining the expression of a protein that requires the interaction of multiple signaling pathways. These, in turn, may undergo interindividual and cancer-specific modifications, e.g., depending on the EGFR mutation status.
On the other hand, many mechanisms have been identified that may alter the ABCB1 expression in tumors, including genomic instability in regions of chromosome 7, the activity of transcription factors/miRNAs, genetic mutations/polymorphisms, and epigenetic regulation. Among the epigenetic modifications, DNA methylation, histone modifications, and the effect of non-coding RNAs are distinguished, but the methylation of the ABCB1 promoter region is analyzed the most often. The organization of the gene encoding P-gp was first described by Chen et al. [44]. According to the aforementioned study, there are two regions in ABCB1 where the transcription of this gene can be initiated. The downstream/proximal promoter acts as the major promoter. A distal promoter is located upstream and usually remains inactive, but under certain conditions it could produce a total transcript in some drug-resistant cell lines. In addition, the upstream promoter is located in a region poor in CpG islands/dinucleotides, and its complementary DNA strand serves as a template for the transcription of the RUNDC3B gene. In studies of breast cancer cell lines, it was observed that increasing concentrations of drugs may trigger many epigenetic mechanisms leading to the development of resistance to chemotherapeutic drugs. Reed K et al. showed that the concentration of docetaxel that induced MDR caused hypermethylation of the main ABCB1 promoter and switched the transcription to the upstream promoter. Further increasing the drug concentration caused a higher mRNA level and preceded a regional chromosome 7 amplification containing, i.a., a gene encoding P-gp [36]. Similarly, ABCB1 expression can be significantly upregulated in lung cancer cells resistant to a cisplatin analogue [37] and paclitaxel [38]. This is accompanied by an increase in the amount of mRNA for surrounding genes (including RUNDC3B or ABCB4), which is proof of the multiplication of the entire ABCB1 amplicon [38]. Similar observations apply to neuroblastoma cancer cell lines as wells as leukemia and myeloma cells [45,46]. The activation of this mechanism probably depends on the types, doses, and concentrations of the chemotherapeutic drugs that are used in experiments with cancer cell lines.
Although it lacks a TATA box, the ABCB1 downstream promoter has an initiator (Inr) motif, an inverted CCAAT box, and two GC boxes. Furthermore, binding motifs for a large number of transcription factors are located in this region. It also contains a CpG island; therefore, the majority of scientific reports concern this ABCB1 promoter, as it is responsible for the epigenetic regulation of the gene’s function. In a study by Spitzwieser M et al. conducted on 19 cancer cell lines (lung, colon, breast, cervix, ovary, prostate, osteosarcoma, multiple myeloma, and leukemia), the differences in the methylation status of the main ABCB1 promoter were observed. In over half of these cell lines, high methylation of the investigated CpG islands was observed, and this was connected with the lack of an encoded mRNA/protein. For lung cancer, three cell lines (HCC827, NCI-H1703, and SW 1573) indicated a high methylation status, while the promoter regions of two other lines (A549 and NCI-H520) were methylated rather heterogeneously [47]. The presence of unique DNA methylation patterns specific to cancer cell line types was also confirmed in other studies, e.g., for lung and prostate cancer cell lines [48,49].
In our study, the analysis of the DNA methylation status of the ABCB1 gene was performed on clinical lung cancer sample data derived from the TCGA database. As was presented in the results, we found that the promoter of ABCB1 was hypo-methylated in both normal tissue and lung cancer tissue (beta-value range: 0.022–0.138). Nevertheless, in both LUAD and LUSC cancer cells, the level of promoter methylation was significantly higher compared to normal lung cells. These observations were consistent with other lung cancer studies [37,48], confirming that the methylation of the promoter region is more frequent in cancers than in non-cancerous tissues. Similarly, Gao et al. detected methylation in the majority of investigated lung cancer samples, but in this study, all normal lung tissue samples also showed promoter methylation [50]. In this case, it cannot be ruled out that the patients with inflammatory pseudotumors, considered as a control group, had an altered DNA methylation pattern due to an inflammatory process.
Our findings are also in line with related studies in other types of cancer. For example, the difference in the frequency of ABCB1 promoter methylation was significant between prostate cancer and benign gland hypertrophy samples. Although the presence of methylated and unmethylated CpG sites was found in both groups, methylated gene regions and weak expression of ABCB1 predominated in the cancer cells [51]. Moreover, hypermethylation in the CpG sites of this gene appears to be quite common in various untreated primary tumors [52]. However, the results of the evaluation of the correlation between promoter methylation and gene/protein expression were not always in accordance. The MEXPRESS data showed a weak negative correlation between gene expression and the methylation of CpG dinucleotides in the region corresponding to the proximal promoter sequence (near 87,600 K; GenBank; area marked in red in Figure 11 and Figure 12). Methylated samples from patients with bronchioloalveolar carcinoma showed no presence of P-gp [50]. On the contrary, a study by Li and colleagues reported that the expression of the ABCB1 gene was enhanced at both the mRNA and protein levels in hypermethylated lung cancer tissue samples [37]. These contradictory results reflect the fact that promoter methylation is only one of several mechanisms affecting gene/protein expression; thus, the level of gene methylation is often not directly related to the amounts of transcripts or protein.
Furthermore, thorough the analysis in the group of lung cancer patients, a slight change in promoter methylation in the course of both LUAD and LUSC was found when taking into account clinicopathological factors. The methylation patterns of the gene for P-gp seem to be reciprocal between LUSC and LUAD in a number of categories. In lung adenocarcinomas, the level of methylation increased with the age of the patient, while a higher stage of the disease was associated with a reduction in the promoter methylation in squamous-cell carcinomas. This may indicate a quite different significance of ABCB1 promoter methylation and/or the strong influence of individual/environmental factors on the course of cancer in these two histological types of NSCLC. To the best of our knowledge, the presented results are not supported by data from other lung cancer studies. So far, findings have suggested that ABCB1 promoter methylation cannot be considered a predictive or prognostic biomarker in lung cancer [48,50]. Data from the TCGA database indicated that promoter methylation of this gene has the potential to become a predicator of patient prognosis, which is consistent with observations for breast carcinomas [53] and other types of solid tumors [52]. In lung squamous-cell carcinoma, the correlation of ABCB1 promoter methylation with the histopathological stage that was observed in this analysis is a novelty of the present report. For LUAD, no similar association could be attributed to the cancer staging.
The present study is not without limitations. The first is the simple model of statistical analysis. This model, which is available in the bioinformatics tools that were used does not include a multi-factor adjustment. Moreover, only bioinformatics techniques were used to analyze DNA methylation in the ABCB1 promoter, which is the second limitation of our research. Nevertheless, the expression of this gene was evaluated through an in vitro study and was previously published [54]. In this preliminary study, there were no statistically significant differences between the level of ABCB1 and the clinicopathological features of the disease, with the exception of histological malignancy. The associations may not be noticeable due to the small number of patients that were included in the experimental analysis.
In summary, the physiologically low levels of ABCB1 expression in lung tissue are significantly downregulated in cancer cells. Thus, a close link between the reduction in efflux pump function and the transformation of normal lung cells to malignant cells was confirmed. In addition, high ABCB1 expression can also be used as an independent prognostic factor for lung cancer patients. Finally, it is equally important that the main methylation promoter is one of the causes of decreased gene expression in lung cancer cells. Taken together, these data establish the ABCB1 transporter gene as an important prognostic factor that could alter disease progression and contribute to the survival of cancer patients.
Author Contributions
Conceptualization, A.M.J. and E.B.; Methodology, A.M.J. and E.B.; Formal Analysis, A.M.J. and E.B.; Investigation, A.M.J. and B.S.; Writing—Original Draft Preparation, A.M.J. and B.S.; Writing—Review and Editing, A.M.J., D.S.-K., E.B., M.P. and B.S.; Visualization, A.M.J., D.S.-K. and B.S.; Supervision, E.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no funding.
Institutional Review Board Statement
Ethical review and additional approval were waived for this study due to presentation of data deposited and compiled by publicly available bioinformatics databases.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are publicly available on bioinformatics platforms.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wojciechowska, U.; Barańska, K.; Michałek, I.; Olasek, P.; Miklewska, M.; Didkowska, J.A. Cancer in Poland in 2020; Polish National Cancer Registry: Warsaw, Poland, 2022; Available online: http://onkologia.org.pl (accessed on 30 January 2023).
- European Respiratory Society. Respiratory Health and Disease in Europe: The European Lung White Book. (Chapter 19: Lung Cancer). Available online: https://www.erswhitebook.org (accessed on 14 August 2022).
- American Cancer Society. Cancer Statistics Center. Available online: http://cancerstatisticscenter.cancer.org (accessed on 30 January 2023).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Adamek, M.; Biernat, W.; Chorostowska-Wynimko, J.; Didkowska, J.A.; Dziadziuszko, K.; Grodzki, T.; Jassem, J.; Kępka, L.; Kowalski, D.; Krawczyk, P.; et al. Lung Cancer in Poland. J. Thorac. Oncol. 2020, 15, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.B.; Alsubait, S. Non-Small Cell Lung Cancer; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK562307/ (accessed on 30 January 2023).
- Jiang, W.; Cai, G.; Hu, P.C.; Wang, Y. Personalized medicine in non-small cell lung cancer: A review from a pharmacogenomics perspective. Acta Pharm. Sin. B. 2018, 8, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Aluru, J.S.; Barsouk, A. Epidemiology of lung cancer. Contemp. Oncol. 2021, 25, 45–52. [Google Scholar]
- Lung Cancer—Non-Small Cell: Statistics. Available online: https://www.cancer.net/cancer-types/lung-cancer-non-small-cell/statistics (accessed on 14 May 2023).
- Flores, R.; Patel, P.; Alpert, N.; Pyenson, B.; Taioli, E. Association of Stage Shift and Population Mortality Among Patients With Non-Small Cell Lung Cancer. JAMA Netw. Open 2021, 4, e2137508. [Google Scholar] [CrossRef]
- Nagl, L.; Pall, G.; Wolf, D.; Pircher, A.; Horvath, L. Molecular profiling in lung cancer. MEMO—Mag. Eur. Med. Oncol. 2022, 15, 201–205. [Google Scholar] [CrossRef]
- Hecht, S.S. Lung carcinogenesis by tobacco smoke. Int. J. Cancer 2012, 131, 2724–2732. [Google Scholar] [CrossRef] [PubMed]
- Pilotto Heming, C.; Muriithi, W.; Wanjiku Macharia, L.; Niemeyer Filho, P.; Moura-Neto, V.; Aran, V. P-glycoprotein and cancer: What do we currently know? Heliyon 2022, 8, e11171. [Google Scholar] [CrossRef]
- Lai, J.I.; Tseng, Y.J.; Chen, M.H.; Huang, C.F.; Chang, P.M. Clinical Perspective of FDA Approved Drugs with P-Glycoprotein Inhibition Activities for Potential Cancer Therapeutics. Front. Oncol. 2020, 10, 561936. [Google Scholar] [CrossRef]
- Wang, J.; Yang, D.H.; Yang, Y.; Wang, J.Q.; Cai, C.Y.; Lei, Z.N.; Teng, Q.X.; Wu, Z.X.; Zhao, L.; Chen, Z.S. Overexpression of ABCB1 Transporter Confers Resistance to mTOR Inhibitor WYE-354 in Cancer Cells. Int. J. Mol. Sci. 2020, 21, 1387. [Google Scholar] [CrossRef]
- Popęda, M.; Płuciennik, M.; Bednarek, A.K. Proteins in cancer resistance. Adv. Hyg. Exp. Med. 2014, 68, 616–632. [Google Scholar]
- Fellay, J.; Marzolini, C.; Meaden, E.R.; Back, D.J.; Buclin, T.; Chave, J.P.; Decosterd, L.A.; Furrer, H.; Opravil, M.; Pantaleo, G.; et al. Swiss HIV Cohort Study. Response to antiretroviral treatment in HIV-1-infected individuals with allelic variants of the multidrug resistance transporter 1: A pharmacogenetics study. Lancet 2002, 359, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Leschziner, G.D.; Andrew, T.; Pirmohamed, M.; Johnson, M.R. ABCB1 genotype and PGP expression, function and therapeutic drug response: A critical review and recommendations for future research. Pharm. J. 2007, 7, 154–179. [Google Scholar] [CrossRef]
- Seelig, A. P-Glycoprotein: One Mechanism, Many Tasks and the Consequences for Pharmacotherapy of Cancers. Front. Oncol. 2020, 10, 576559. [Google Scholar] [CrossRef]
- Mencalha, A.L.; Rodrigues, E.F.; Abdelhay, E.; Fernandez, T.S. Accurate monitoring of promoter gene methylation with high-resolution melting polymerase chain reaction using the ABCB1 gene as a model. Genet. Mol. Res. 2013, 12, 714–722. [Google Scholar] [CrossRef]
- Bartha, A.; Gyorffy, B. TNMplot.com: A Web Tool for the Comparison of Gene Expression in Normal, Tumor and Metastatic Tissues. Int. J. Mol. Sci. 2021, 22, 2622. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Rodriguez, I.P.; Chakravarthi, B.V.S.K.; Varambally, S. UALCAN: A portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Karthikeyan, S.K.; Korla, P.K.; Patel, H.; Shovon, A.R.; Athar, M.; Netto, G.J.; Qin, Z.S.; Kumar, S.; Manne, U.; et al. UALCAN: An update to the integrated cancer data analysis platform. Neoplasia 2022, 25, 18–27. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]
- Mizuno, H.; Kitada, K.; Nakai, K.; Sarai, A. PrognoScan: A new database for meta-analysis of the prognostic value of genes. BMC Med. Genom. 2009, 2, 18. [Google Scholar] [CrossRef]
- Koch, A.; De Meyer, T.; Jeschke, J.; Van Criekinge, W. MEXPRESS: Visualizing expression, DNA methylation and clinical TCGA data. BMC Genom. 2015, 16, 636. [Google Scholar] [CrossRef]
- Koch, A.; Jeschke, J.; Van Criekinge, W.; van Engeland, M.; De Meyer, T. MEXPRESS update 2019. Nucleic Acids Res. 2019, 47, W561–W565. [Google Scholar] [CrossRef]
- Xiao, H.; Zheng, Y.; Ma, L.; Tian, L.; Sun, Q. Clinically-Relevant ABC Transporter for Anti-Cancer Drug Resistance. Front. Pharmacol. 2022, 12, 648407. [Google Scholar] [CrossRef]
- Waghray, D.; Zhang, Q. Inhibit or Evade Multidrug Resistance P-Glycoprotein in Cancer Treatment. J. Med. Chem. 2018, 61, 5108–5121. [Google Scholar] [CrossRef]
- Fojo, A.T.; Ueda, K.; Slamon, D.J.; Poplack, D.G.; Gottesman, M.M.; Pastan, I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc. Natl. Acad. Sci. USA 1987, 84, 265–269. [Google Scholar] [CrossRef]
- Cordon-Cardo, C.; O’Brien, J.P.; Boccia, J.; Casals, D.; Bertino, J.R.; Melamed, M.R. Expression of the multidrug resistance gene product (P-glycoprotein) in human normal and tumor tissues. J. Histochem. Cytochem. 1990, 38, 1277–1287. [Google Scholar] [CrossRef]
- Lee, T.B.; Park, J.H.; Min, Y.D.; Kim, K.J.; Choi, C.H. Epigenetic mechanisms involved in differential MDR1 mRNA expression between gastric and colon cancer cell lines and rationales for clinical chemotherapy. BMC Gastroenterol. 2008, 8, 33. [Google Scholar] [CrossRef]
- Delou, J.M.A.; Vignal, G.M.; Índio-do-Brasil, V.; Accioly, M.T.S.; da Silva, T.S.L.; Piranda, D.N.; Sobral-Leite, M.; de Carvalho, M.A.; Capella, M.A.M.; Vianna-Jorge, R. Loss of constitutive ABCB1 expression in breast cancer associated with worse prognosis. Breast Cancer 2017, 9, 415–428. [Google Scholar] [CrossRef]
- Andersen, V.; Vogel, U.; Godiksen, S.; Frenzel, F.B.; Sæbø, M.; Hamfjord, J.; Kure, E.; Vogel, L.K. Low ABCB1 gene expression is an early event in colorectal carcinogenesis. PLoS ONE 2013, 8, e72119. [Google Scholar] [CrossRef]
- Reed, K.; Hembruff, S.L.; Laberge, M.L.; Villeneuve, D.J.; Côté, G.B.; Parissenti, A.M. Hypermethylation of the ABCB1 downstream gene promoter accompanies ABCB1 gene amplification and increased expression in docetaxel-resistant MCF-7 breast tumor cells. Epigenetics 2008, 3, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Song, J.; Lai, Q.; Liu, B.; Wang, H.; Xu, Y.; Feng, X.; Sun, X.; Du, Z. Hypermethylation of ATP-binding cassette B1 (ABCB1) multidrug resistance 1 (MDR1) is associated with cisplatin resistance in the A549 lung adenocarcinoma cell line. Int. J. Exp. Pathol. 2016, 97, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Yabuki, N.; Sakata, K.; Yamasaki, T.; Terashima, H.; Mio, T.; Miyazaki, Y.; Fujii, T.; Kitada, K. Gene amplification and expression in lung cancer cells with acquired paclitaxel resistance. Cancer Genet. Cytogenet. 2007, 173, 1–9. [Google Scholar] [CrossRef]
- Takano, M.; Naka, R.; Sasaki, Y.; Nishimoto, S.; Yumoto, R. Effect of cigarette smoke extract on P-glycoprotein function in primary cultured and newly developed alveolar epithelial cells. Drug Metab. Pharmacokinet. 2016, 31, 417–424. [Google Scholar] [CrossRef]
- Erdő, F.; Krajcsi, P. Age-Related Functional and Expressional Changes in Efflux Pathways at the Blood-Brain Barrier. Front. Aging Neurosci. 2019, 11, 196. [Google Scholar] [CrossRef]
- Warrington, J.S.; Greenblatt, D.J.; von Moltke, L.L. The effect of age on P-glycoprotein expression and function in the Fischer-344 rat. J. Pharmacol. Exp. Ther. 2004, 309, 730–736. [Google Scholar] [CrossRef]
- van Assema, D.M.E.; Lubberink, M.; Boellaard, R.; Schuit, R.C.; Windhorst, A.D.; Scheltens, P.; Lammertsma, A.A.; van Berckel, B.N. P-glycoprotein function at the blood-brain barrier: Effects of age and gender. Mol. Imaging Biol. 2012, 14, 771–776. [Google Scholar] [CrossRef]
- Zou, F.; Seike, M.; Noro, R.; Kunugi, S.; Kubota, K.; Gemma, A. Prognostic significance of ABCB1 in stage I lung adenocarcinoma. Oncol. Lett. 2017, 14, 313–321. [Google Scholar] [CrossRef]
- Chen, C.; Clark, D.; Ueda, K.; Pastan, I.; Gottesman, M.M.; Roninson, I.B. Genomic organization of the human multidrug resistance (MDR1) gene and origin of P-glycoproteins. J. Biol. Chem. 1990, 265, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Genovese, I.; Ilari, A.; Assaraf, Y.G.; Fazi, F.; Colotti, G. Not only P-glycoprotein: Amplification of the ABCB1-containing chromosome region 7q21 confers multidrug resistance upon cancer cells by coordinated overexpression of an assortment of resistance-related proteins. Drug Resist. Updates 2017, 32, 23–46. [Google Scholar] [CrossRef]
- Trussardi-Regnier, A.; Lavenus, S.; Gorisse, M.C.; Dufer, J. Thalidomide alters nuclear architecture without ABCB1 gene modulation in drug-resistant myeloma cells. Int. J. Oncol. 2009, 35, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Spitzwieser, M.; Pirker, C.; Koblmüller, B.; Pfeiler, G.; Hacker, S.; Berger, W.; Heffeter, P.; Cichna-Markl, M. Promoter methylation patterns of ABCB1, ABCC1 and ABCG2 in human cancer cell lines, multidrug-resistant cell models and tumor, tumor-adjacent and tumor-distant tissues from breast cancer patients. Oncotarget 2016, 7, 73347–73369. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Geng, J.; Ma, K.; Yu, J.; Sun, J.; Shen, Z.; Bao, G.; Chen, Y.; Zhang, H.; He, Y.; et al. RASSF1A, APC, ESR1, ABCB1 and HOXC9, but not p16INK4A, DAPK1, PTEN and MT1G genes were frequently methylated in the stage I non-small cell lung cancer in China. J. Cancer Res. Clin. Oncol. 2009, 135, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Yegnasubramanian, S.; Kowalski, J.; Gonzalgo, M.L.; Zahurak, M.; Piantadosi, S.; Walsh, P.C.; Bova, G.S.; De Marzo, A.M.; Isaacs, W.B.; Nelson, W.G. Hypermethylation of CpG islands in primary and metastatic human prostate cancer. Cancer Res. 2004, 64, 1975–1986. [Google Scholar] [CrossRef]
- Gao, P.; Yang, X.; Xue, Y.W.; Zhang, X.F.; Wang, Y.; Liu, W.J.; Wu, X.J. Promoter methylation of glutathione S-transferase pi1 and multidrug resistance gene 1 in bronchioloalveolar carcinoma and its correlation with DNA methyltransferase 1 expression. Cancer 2009, 115, 3222–3232. [Google Scholar] [CrossRef]
- Enokida, H.; Shiina, H.; Igawa, M.; Ogishima, T.; Kawakami, T.; Bassett, W.W.; Anast, J.W.; Li, L.C.; Urakami, S.; Terashima, M.; et al. CpG hypermethylation of MDR1 gene contributes to the pathogenesis and progression of human prostate cancer. Cancer Res. 2004, 64, 5956–5962. [Google Scholar] [CrossRef]
- Zappe, K.; Cichna-Markl, M. Aberrant DNA Methylation of ABC Transporters in Cancer. Cells 2020, 9, 2281. [Google Scholar] [CrossRef]
- Sharma, G.; Mirza, S.; Parshad, R.; Srivastava, A.; Datta Gupta, S.; Pandya, P.; Ralhan, R. CpG hypomethylation of MDR1 gene in tumor and serum of invasive ductal breast carcinoma patients. Clin. Biochem. 2010, 43, 373–379. [Google Scholar] [CrossRef]
- Zawadzka, I.; Jeleń, A.; Pietrzak, J.; Żebrowska-Nawrocka, M.; Michalska, K.; Szmajda-Krygier, D.; Mirowski, M.; Łochowski, M.; Kozak, J.; Balcerczak, E. The impact of ABCB1 gene polymorphism and its expression on non-small-cell lung cancer development, progression and therapy—Preliminary report. Sci. Rep. 2020, 10, 6188. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).