Anti-Melanogenesis Effect of Rosa rugosa on α-MSH-Induced B16F10 Cells via PKA/CREB Pathway Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Cytotoxicity Assay
2.4. Intracellular Active Tyrosinase and Melanin Levels
2.5. Determination of Secreted Melanin Levels
2.6. RT-qPCR Assay
2.7. Immunoblotting
2.8. Measurement of Total Polyphenol Content
2.9. Statistical Analysis
3. Results
3.1. Effect of R. rugosa Crude Extract on α-MSH-Stimulated Melanogenesis
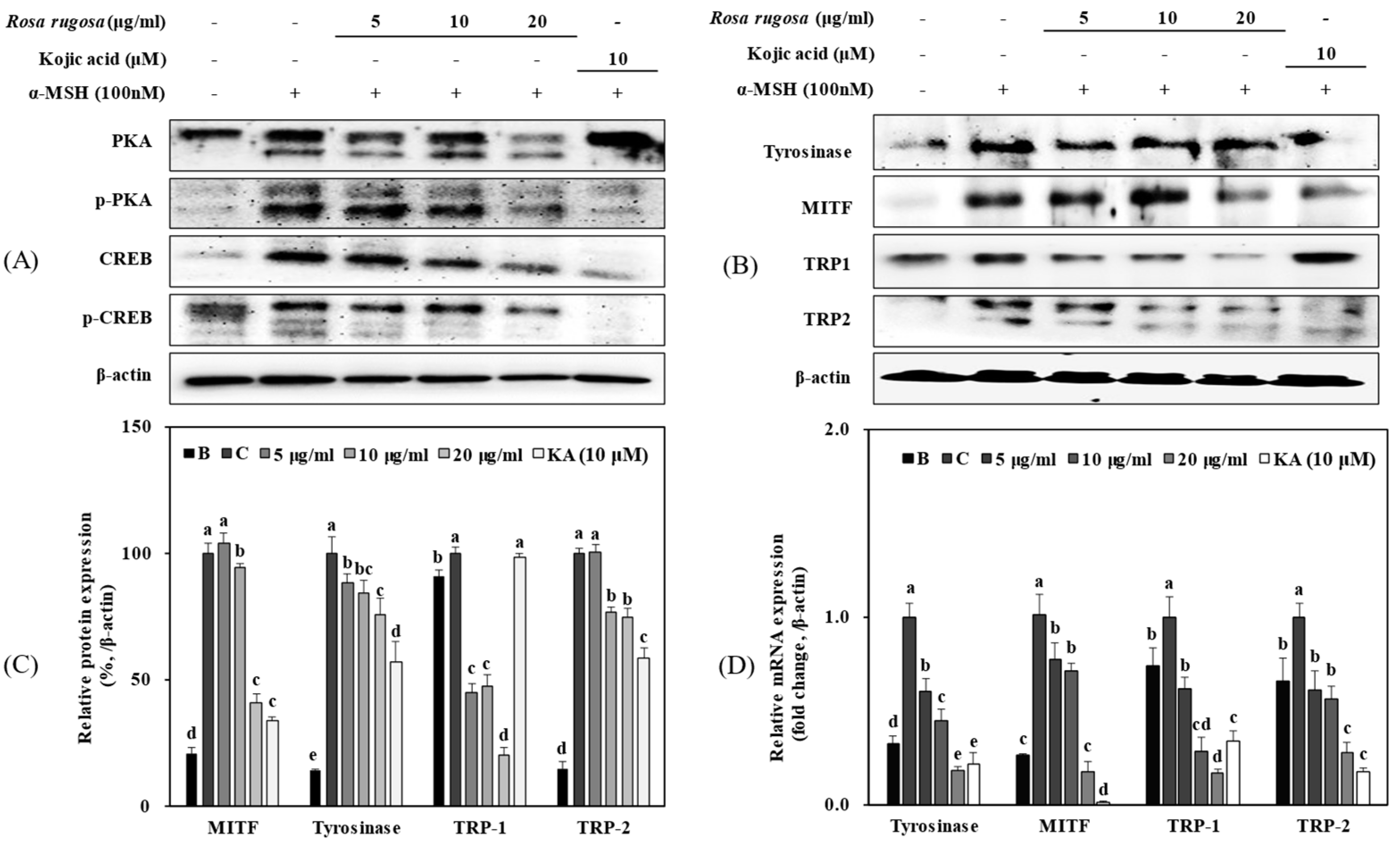
3.2. R. rugosa Crude Extracts Inhibit Melanogenesis by Regulating the PKA/CREB Signaling Pathway
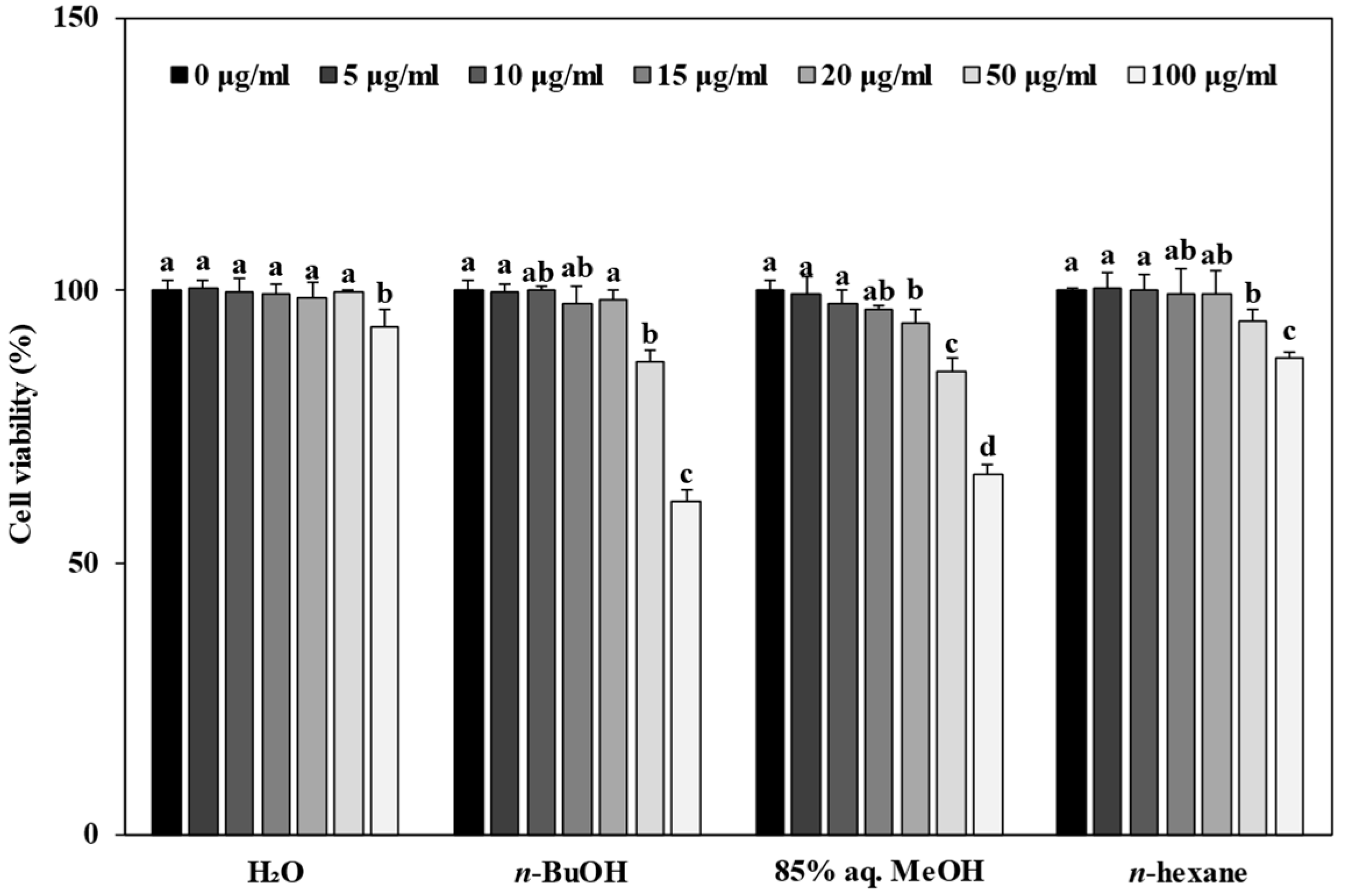
3.3. Effect of Solvent Fractions from R. rugosa Crude Extract on Cell Viability
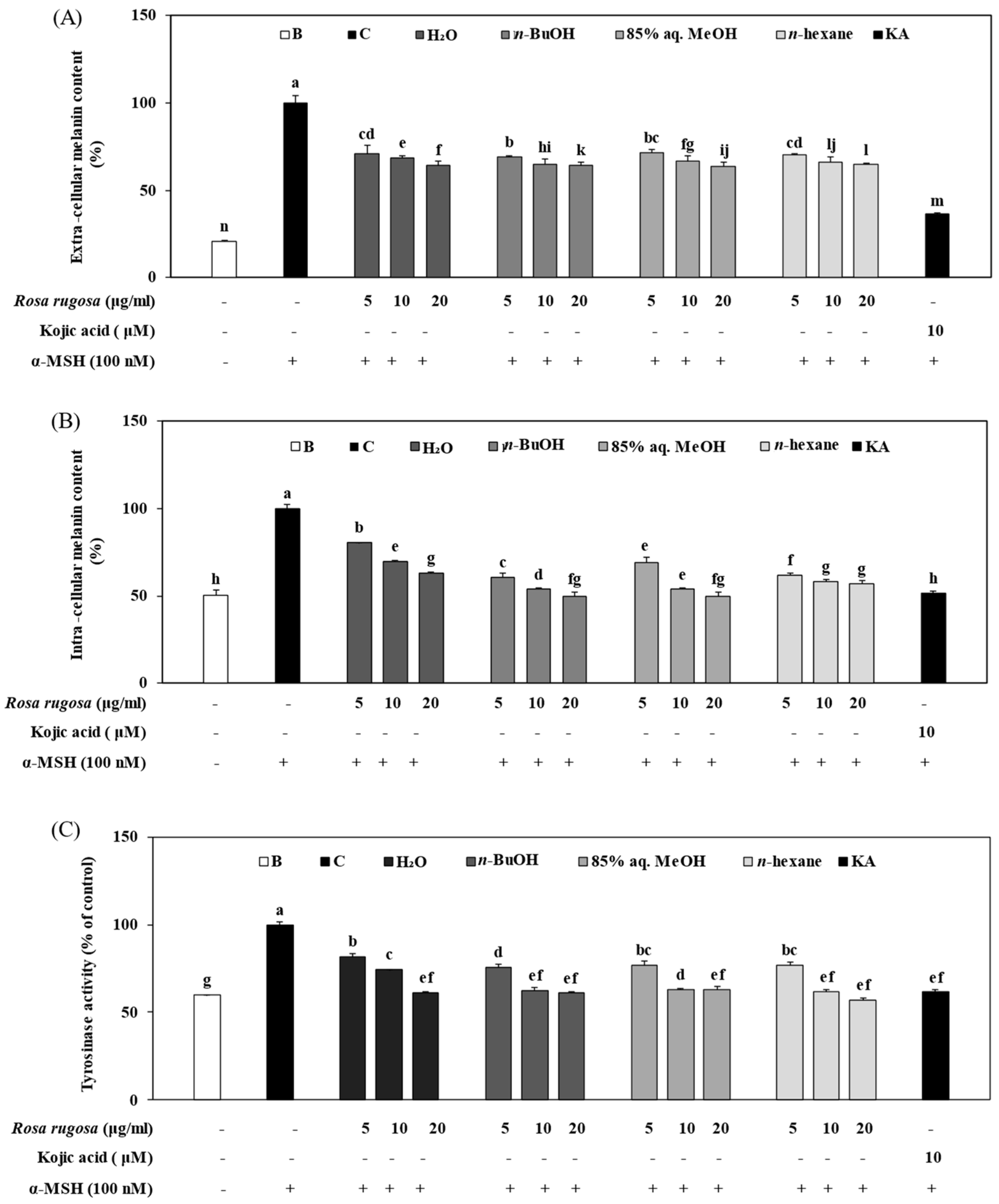
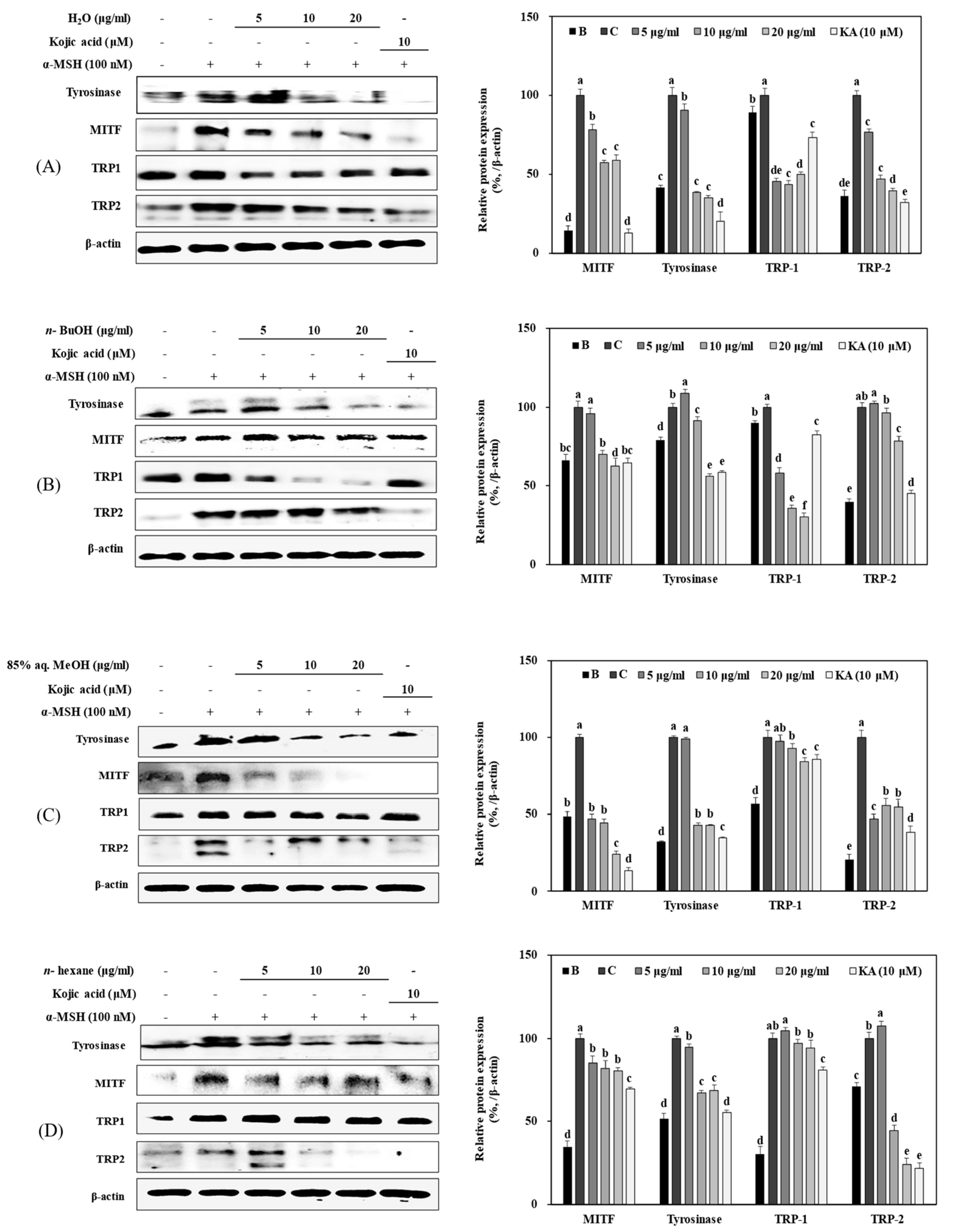
3.4. Effect of Solvent Fractions of R. rugosa Extract on Active Tyrosinase Levels and Extracellular and Intracellular Melanin Content
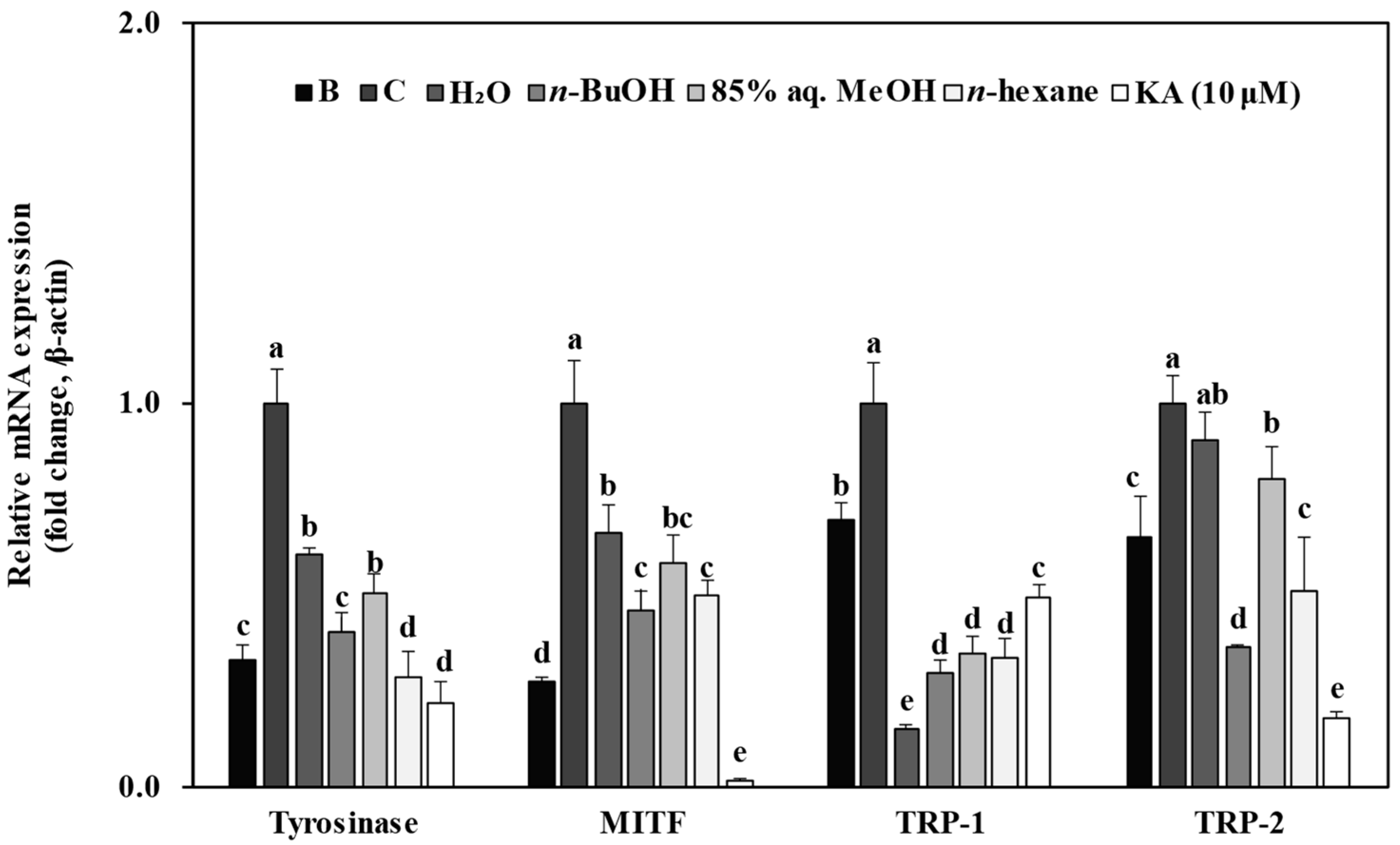
3.5. Effect of Solvent Fractions from R. rugosa Crude Extract on mRNA Expression Levels of Melanogenesis-Related Genes
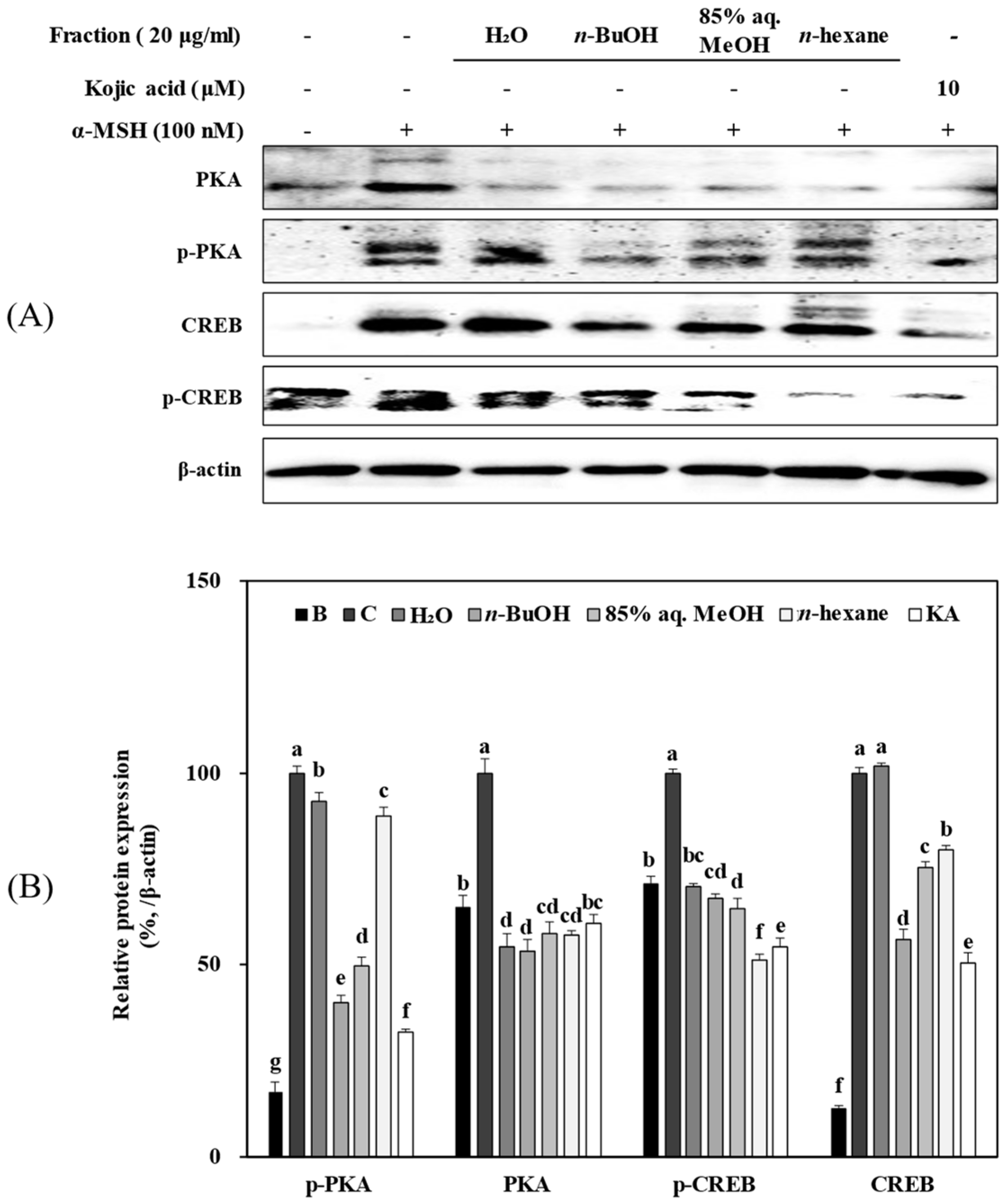
3.6. Solvent Fractions from R. rugosa Inhibit Melanogenesis via Supressed Activation of the PKA/CREB Pathway
3.7. Total Polyphenol Content of Crude Extract of R. rugosa and Its Fractions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, S.; Yotsumoto, H.; Tian, Y.; Sakamoto, K. α-Mangostin suppressed melanogenesis in B16F10 murine melanoma cells through GSK3β and ERK signaling pathway. Biochem. Biophys. Rep. 2021, 26, 100949. [Google Scholar] [CrossRef] [PubMed]
- Merecz-Sadowska, A.; Sitarek, P.; Kowalczyk, T.; Zajdel, K.; Kucharska, E.; Zajdel, R. The Modulation of Melanogenesis in B16 Cells Upon Treatment with Plant Extracts and Isolated Plant Compounds. Molecules 2022, 27, 4360. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Avogadri, F.; Gnjatic, S.; Tassello, J.; Frosina, D.; Hanson, N.; Laudenbach, M.; Ritter, E.; Merghoub, T.; Busam, K.J.; Jungbluth, A.A. Protein Expression Analysis of Melanocyte Differentiation Antigen TRP-2. Am. J. Dermatopathol. 2016, 38, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Riadh, D.; Sakamoto, K. Grape Extract Promoted α-MSH-Induced Melanogenesis in B16F10 Melanoma Cells, Which Was Inverse to Resveratrol. Molecules 2021, 26, 5959. [Google Scholar] [CrossRef]
- Wu, K.C.; Hseu, Y.C.; Shih, Y.C.; Sivakumar, G.; Syu, J.T.; Chen, G.L.; Lu, M.T.; Chu, P.C. Calycosin, a Common Dietary Isoflavonoid, Suppresses Melanogenesis through the Downregulation of PKA/CREB and p38 MAPK Signaling Pathways. Int. J. Mol. Sci. 2022, 3, 1358. [Google Scholar] [CrossRef]
- Ryu, S.; Johnson, A.; Park, Y.; Kim, B.; Norris, D.; Armstrong, C.A.; Song, P.I. The Alpha-Melanocyte-Stimulating Hormone Suppresses TLR2-Mediated Functional Responses through IRAK-M in Normal Human Keratinocytes. PLoS ONE 2015, 10, e0136887. [Google Scholar] [CrossRef]
- Jung, H.J.; Choi, D.C.; Noh, S.G.; Choi, H.; Choi, I.; Ryu, I.Y.; Chung, H.Y.; Moon, H.R. New Benzimidazothiazolone Derivatives as Tyrosinase Inhibitors with Potential Anti-Melanogenesis and Reactive Oxygen Species Scavenging Activities. Antioxidants 2021, 10, 1078. [Google Scholar] [CrossRef]
- Huang, H.C.; Wang, S.S.; Tsai, T.C.; Ko, W.P.; Chang, T.M. Phoenix dactylifera L. Seed Extract Exhibits Antioxidant Effects and Attenuates Melanogenesis in B16F10 Murine Melanoma Cells by Downregulating PKA Signaling. Antioxidants 2020, 9, 1270. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Khongkarat, P.; Ramadhan, R.; Phuwapraisirisan, P.; Chanchao, C. Safflospermidines from the bee pollen of Helianthus annuus L. exhibit a higher in vitro antityrosinase activity than kojic acid. Heliyon 2020, 6, e03638. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.Y.; Kim, D.H.; Son, S.; Ullah, S.; Kim, S.J.; Kim, Y.J.; Yoo, J.W.; Jung, Y.; Chun, P.; Moon, H.R. Design, synthesis, and anti-melanogenic effects of (E)-2-benzoyl-3-(substituted phenyl) acrylonitriles. Drug Des. Dev. Ther. 2015, 9, 4259–4268. [Google Scholar]
- Dashbaldan, S.; Rogowska, A.; Pączkowski, C.; Szakiel, A. Distribution of Triterpenoids and Steroids in Developing Rugosa Rose (Rosarugosa Thunb.) Accessory Fruit. Molecules 2021, 26, 5158. [Google Scholar] [CrossRef] [PubMed]
- Liaudanskas, M.; Noreikienė, I.; Zymonė, K.; Juodytė, R.; Žvikas, V.; Janulis, V. Composition and Antioxidant Activity of Phenolic Compounds in Fruit of the Genus Rosa L. Antioxidants 2021, 10, 545. [Google Scholar] [CrossRef]
- Liu, Y.; Zhi, D.; Wang, X.; Fei, D.; Zhang, Z.; Wu, Z.; Li, Y.; Chen, P.; Li, H. Kushui Rose (R. Setate x R. Rugosa) decoction exerts antitumor effects in C. elegans by downregulating Ras/MAPK pathway and resisting oxidative stress. Int. J. Mol. Med. 2018, 42, 1411–1417. [Google Scholar] [CrossRef]
- Kim, K.H.; Park, Y.J.; Jang, H.J.; Lee, S.J.; Lee, S.; Yun, B.S.; Lee, S.W.; Rho, M.C. Rugosic acid A, derived from Rosa rugosa Thunb.; is novel inhibitory agent for NF-κB and IL-6/STAT3 axis in acute lung injury model. Phytother. Res. PTR 2020, 34, 3200–3210. [Google Scholar] [CrossRef] [PubMed]
- Olech, M.; Nowacka-Jechalke, N.; Masłyk, M.; Martyna, A.; Pietrzak, W.; Kubiński, K.; Załuski, D.; Nowak, R. Polysaccharide-Rich Fractions from Rosa rugosa Thunb.-Composition and Chemopreventive Potential. Molecules 2019, 24, 1354. [Google Scholar] [CrossRef]
- Grajzer, M.; Wiatrak, B.; Gębarowski, T.; Matkowski, A.; Grajeta, H.; Rój, E.; Kulma, A.; Prescha, A. Chemistry, oxidative stability and bioactivity of oil extracted from Rosa rugosa (Thunb.) seeds by supercritical carbon dioxide. Food Chem. 2021, 335, 127649. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, F.; Li, R.; Wu, Y.; Liu, S.; Liang, Q. Purification, characterization, antioxidant and moisture-preserving activities of polysaccharides from Rosa rugosa petals. Int. J. Biol. Macromol. 2019, 124, 938–945. [Google Scholar] [CrossRef]
- Song, M.; Lee, J.; Kim, Y.J.; Hoang, D.H.; Choe, W.; Kang, I.; Kim, S.S.; Ha, J. Jeju Magma-Seawater Inhibits α-MSH-Induced Melanogenesis via CaMKKβ-AMPK Signaling Pathways in B16F10 Melanoma Cells. Mar. Drugs 2020, 18, 473. [Google Scholar] [CrossRef]
- Han, S.M.; Kim, J.M.; Hong, I.P.; Woo, S.O.; Kim, S.G.; Jang, H.R.; Park, K.K.; Pak, S.C. Whitening Effect of Watersoluble Royal Jelly from South Korea. Korean J. Food Sci. Anim. Resour. 2015, 35, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.J.; Zou, J.; Zou, T.; Shang, H.; Sun, L.Y. A Newly Authenticated Compound from Traditional Chinese Medicine Decoction Induces Melanogenesis in B16-F10 Cells by Increasing Tyrosinase Activity. Evid.-Based Complement. Altern. Med. Ecam 2018, 2018, 8485670. [Google Scholar] [CrossRef]
- Ma, S.; Kim, C.; Neilson, A.P.; Griffin, L.E.; Peck, G.M.; O’Keefe, S.F.; Stewart, A.C. Comparison of Common Analytical Methods for the Quantification of Total Polyphenols and Flavanols in Fruit Juices and Ciders. J. Food Sci. 2019, 84, 2147–2158. [Google Scholar] [CrossRef] [PubMed]
- Um, M.; Han, T.H.; Lee, J.W. Ultrasound-assisted extraction and antioxidant activity of phenolic and flavonoid compounds and ascorbic acid from rugosa rose (Rosa rugosa Thunb.) fruit. Food Sci. Biotechnol. 2017, 27, 375–382. [Google Scholar] [CrossRef]
- Garcia-Molina, P.; Garcia-Molina, F.; Teruel-Puche, J.A.; Rodriguez-Lopez, J.N.; Garcia-Canovas, F.; Muñoz-Muñoz, J.L. The Relationship between the IC50 Values and the Apparent Inhibition Constant in the Study of Inhibitors of Tyrosinase Diphenolase Activity Helps Confirm the Mechanism of Inhibition. Molecules 2022, 27, 3141. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.P.; Silva, N.F.; Andrade, E.; Gratieri, T.; Setzer, W.N.; Maia, J.; da Silva, J. Tyrosinase inhibitory activity, molecular docking studies and antioxidant potential of chemotypes of Lippia origanoides (Verbenaceae) essential oils. PLoS ONE 2017, 12, e0175598. [Google Scholar] [CrossRef] [PubMed]
- Azumi, J.; Takeda, T.; Shimada, Y.; Aso, H.; Nakamura, T. The Organogermanium Compound THGP Suppresses Melanin Synthesis via Complex Formation with L-DOPA on Mushroom Tyrosinase and in B16 4A5 Melanoma Cells. Int. J. Mol. Sci. 2019, 20, 4785. [Google Scholar] [CrossRef]
- Baek, S.H.; Nam, I.J.; Kwak, H.S.; Kim, K.C.; Lee, S.H. Cellular Anti-Melanogenic Effects of a Euryale ferox Seed Extract Ethyl Acetate Fraction via the Lysosomal Degradation Machinery. Int. J. Mol. Sci. 2015, 16, 9217–9235. [Google Scholar] [CrossRef]
- Li, X.; Yang, H.W.; Jiang, Y.; Oh, J.Y.; Jeon, Y.J.; Ryu, B. Ishophloroglucin A Isolated from Ishige okamurae Suppresses Melanogenesis Induced by α-MSH: In Vitro and In Vivo. Mar. Drugs 2020, 18, 470. [Google Scholar] [CrossRef]
- Gao, D.; Kim, J.H.; Kim, C.T.; Jeong, W.S.; Kim, H.M.; Sim, J.; Kang, J.S. Evaluation of Anti-Melanogenesis Activity of Enriched Pueraria lobata Stem Extracts and Characterization of Its Phytochemical Components Using HPLC-PDA-ESI-MS/MS. Int. J. Mol. Sci. 2021, 22, 8105. [Google Scholar] [CrossRef]
- Shin, S.; Kim, M.; Song, N.; Sun, S.; Choi, J.; Park, K. Antioxidant and Anti-Melanogenesis Effects of Colloidal Gold Camellia sinensis L. Extracts. Molecules 2022, 27, 5593. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 2007, 445, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Flesher, J.L.; Paterson-Coleman, E.K.; Vasudeva, P.; Ruiz-Vega, R.; Marshall, M.; Pearlman, E.; MacGregor, G.R.; Neumann, J.; Ganesan, A.K. Delineating the role of MITF isoforms in pigmentation and tissue homeostasis. Pigment. Cell Melanoma Res. 2020, 33, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiao, Y.; Guan, Y.; Rui, X.; Zhang, Y.; Dong, M.; Ma, W. An aqueous polyphenol extract from Rosa rugosa tea has antiaging effects on Caenorhabditis elegans. J. Food Biochem. 2019, 43, e12796. [Google Scholar] [CrossRef] [PubMed]
- Chaiprasongsuk, A.; Onkoksoong, T.; Pluemsamran, T.; Limsaengurai, S.; Panich, U. Photoprotection by dietary phenolics against melanogenesis induced by UVA through Nrf2-dependent antioxidant responses. Redox Biol. 2016, 8, 79–90. [Google Scholar] [CrossRef]
- Yang, S.; Qu, Y.; Chen, J.; Chen, S.; Sun, L.; Zhou, Y.; Fan, Y. Bee Pollen Polysaccharide from Rosa rugosa Thunb. (Rosaceae) Promotes Pancreatic β-Cell Proliferation and Insulin Secretion. Front. Pharmacol. 2021, 12, 688073. [Google Scholar] [CrossRef]
- Lee, S.E.; Park, S.H.; Oh, S.W.; Yoo, J.A.; Kwon, K.; Park, S.J.; Kim, J.; Lee, H.S.; Cho, J.Y.; Lee, J. Beauvericin inhibits melanogenesis by regulating cAMP/PKA/CREB and LXR-α/p38 MAPK-mediated pathways. Sci. Rep. 2018, 8, 14958. [Google Scholar] [CrossRef]
- Tsang, T.F.; Chan, B.; Tai, W.C.; Huang, G.; Wang, J.; Li, X.; Jiang, Z.H.; Hsiao, W.L.W. Gynostemma pentaphyllum saponins induce melanogenesis and activate cAMP/PKA and Wnt/β-catenin signaling pathways. Phytomedicine 2019, 60, 153008. [Google Scholar] [CrossRef]
- Yoon, J.H.; Youn, K.; Jun, M. Discovery of Pinostrobin as a Melanogenic Agent in cAMP/PKA and p38 MAPK Signaling Pathway. Nutrients 2022, 14, 3713. [Google Scholar] [CrossRef]
- Wang, H.M.; Qu, L.Q.; Ng, J.P.L.; Zeng, W.; Yu, L.; Song, L.L.; Wong, V.K.W.; Xia, C.L.; Law, B.Y.K. Natural Citrus flavanone 5-demethylnobiletin stimulates melanogenesis through the activation of cAMP/CREB pathway in B16F10 cells. Phytomedicine 2022, 8, 153941. [Google Scholar] [CrossRef]
- Pei, S.; Chen, J.; Lu, J.; Hu, S.; Jiang, L.; Lei, L.; Ouyang, Y.; Fu, C.; Ding, Y.; Li, S.; et al. The Long Noncoding RNA UCA1 Negatively Regulates Melanogenesis in Melanocytes. J. Investig. Dermatol. 2020, 140, 152–163.e5. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jiang, Q. Roles of the Polyphenol-Gut Microbiota Interaction in Alleviating Colitis and Preventing Colitis-Associated Colorectal Cancer. Adv. Nutr. 2021, 12, 546–565. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Badimon, L. Effects of Polyphenol Intake on Metabolic Syndrome: Current Evidences from Human Trials. Oxidative Med. Cell. Longev. 2017, 2017, 5812401. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yang, Y.; Zhou, M.; Luo, L.; Pan, H.; Zhang, Q.; Yu, C. Widely Targeted Metabolic Profiling Reveals Differences in Polyphenolic Metabolites during Rosa xanthina f. spontanea Fruit Development and Ripening. Metabolites 2022, 12, 438. [Google Scholar] [CrossRef]
- Csekes, E.; Račková, L. Skin Aging, Cellular Senescence and Natural Polyphenols. Int. J. Mol. Sci. 2021, 22, 12641. [Google Scholar] [CrossRef]
- Kampatsikas, I.; Bijelic, A.; Pretzler, M.; Rompel, A. Three recombinantly expressed apple tyrosinases suggest the amino acids responsible for mono- versus diphenolase activity in plant polyphenol oxidases. Sci. Rep. 2017, 7, 8860. [Google Scholar] [CrossRef]
- Panzella, L.; Napolitano, A. Natural and bioinspired phenolic compounds as tyrosinase inhibitors for the treatment of skin hyperpigmentation: Recent advances. Cosmetics 2019, 6, 57. [Google Scholar] [CrossRef]

| Sample | Crude Extract | H2O fr. | n-BuOH fr. | 85% aq. MeOH fr. | n-Hexane fr. |
|---|---|---|---|---|---|
| Total polyphenol content 1 | 1383.9 ± 44.5 | 2004.7 ± 43.4 | 7270.3 ± 54.5 | 2064.1 ± 34.8 | 1091.1 ± 26.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Oh, J.H.; Karadeniz, F.; Yang, J.; Lee, H.; Seo, Y.; Kong, C.-S. Anti-Melanogenesis Effect of Rosa rugosa on α-MSH-Induced B16F10 Cells via PKA/CREB Pathway Activation. Appl. Sci. 2023, 13, 184. https://doi.org/10.3390/app13010184
Zhou X, Oh JH, Karadeniz F, Yang J, Lee H, Seo Y, Kong C-S. Anti-Melanogenesis Effect of Rosa rugosa on α-MSH-Induced B16F10 Cells via PKA/CREB Pathway Activation. Applied Sciences. 2023; 13(1):184. https://doi.org/10.3390/app13010184
Chicago/Turabian StyleZhou, Xianrong, Jung Hwan Oh, Fatih Karadeniz, Jiho Yang, Hyunjung Lee, Youngwan Seo, and Chang-Suk Kong. 2023. "Anti-Melanogenesis Effect of Rosa rugosa on α-MSH-Induced B16F10 Cells via PKA/CREB Pathway Activation" Applied Sciences 13, no. 1: 184. https://doi.org/10.3390/app13010184
APA StyleZhou, X., Oh, J. H., Karadeniz, F., Yang, J., Lee, H., Seo, Y., & Kong, C.-S. (2023). Anti-Melanogenesis Effect of Rosa rugosa on α-MSH-Induced B16F10 Cells via PKA/CREB Pathway Activation. Applied Sciences, 13(1), 184. https://doi.org/10.3390/app13010184






