Abstract
Environmentally friendly substrates that are biodegradable may provide an alternative to mineral wool, which is commonly used in hydroponic growing technology. Little is known about the relationship between the physical characteristics of lignite substrate and cucumber yield. The study analyzed the effect of bulk density and water holding capacity of lignite substrate in comparison to mineral wool and EC of nutrient solution on plant morphological parameters, yield and fruit quality of greenhouse cucumber. A positive relation was found between the bulk density of lignite mats and the increase in the number of leaves per week, shoot diameter as well as leaf length and leaf area (calculated as the product of leaf length × width) in cucumbers grown in this medium. Bulk density of lignite growing mats also affected the macro- and micro-nutrient content of cucumber leaves. The physical properties of the substrate and the high EC of the medium had a significant effect on the hardness, color and lutein content of cucumber fruits. The content of biologically active compounds in cucumber fruits depended on the water holding capacity of the medium and the water readily available to plants; these parameters were lower in the lignite medium compared to mineral wool. However, when the lignite substrate was used in hydroponic cucumber cultivation, for a period of 51 days after planting (DAP) there was an increase of more than 23% in the bulk density of the substrate and an increase of nearly 55% in the water readily available compared to the new lignite mats.
1. Introduction
The world population has already reached 7 billion and will reach 9.7 billion by 2050 [1]. As the population grows, so does the demand for arable land and high quality food all year round. To meet this, fertigation is used in conventional farming, which entails problems of excessive soil salinization. Common causes of excessive salinity also include poor agricultural practices, the use of excessive mineral fertilizers and a hot climate [2,3]. The solution to these problems in most countries of the world is soilless cultivation using mineral and organic substrates, inert to plants. Soilless cultivation has many advantages, one of which is isolation of the plant from the soil, which is often heterogeneous. Intensively exploited, the soil may be degraded, become a habitat of pathogenic pathogens, and in addition is often excessively saline [2,3,4]. Controlled growing conditions in hydroponic systems allow a significant increase in production efficiency compared to conventional methods [4]. High efficiency and healthiness of cultivation are ensured by a substrate with appropriate, stable physical properties [1,5]. This is usually mineral wool, which is a versatile substrate, widely used in the commercial cultivation of vegetables and other plants. Mineral wool in year-round cultivation is usually used for one season, which leads to the creation of a large amount of post-production waste, of which only a small part is recycled or reused for cultivation [6,7,8,9]. The process of utilization of mineral wool after cultivation itself is expensive and is not neutral for the environment, and mineral wool stored outside production facilities, without any protection, may adversely affect human health [9]. Annually, from the area of 1 ha of greenhouses, about 150 m3 of used mineral wool remains after cultivation. The production of such an amount of mineral wool emits to the atmosphere approximately 26 tons of CO2 [6,10]. The growing awareness of ecology and problems with mineral wool utilization have contributed to the search for new pro-ecological substrates that will be fully biodegradable [9]. New substrates being investigated for hydroponic cultivation include wood industry waste, composts or biocarbon produced from many types of waste [10,11,12]. Plant residues such as grape pomace and nut and almond shells are also being tested, along with many others [13,14,15]. These substrates are often used in mixtures with other organic or mineral substrates, such as perlite or vermiculite, in order to improve physical properties [8,16]. Another type of organic substrate is lignite, which retains a stable homogeneous structure due to its highly condensed matter. Its suitability has been studied in tomato, cucumber and other vegetable and soft fruit cultivation [17,18,19]. Lignite has good, stable physical properties that allow it to be used for a longer period of time [19]. Additionally, during its production, CO2 emission is reduced by almost 40% in comparison to the production of the substrate from mineral wool [20].
Lignite mining is associated with negative social and environmental impacts, but it also has some benefits. Mining itself can cause rapid development of areas rich in coal resources by creating new jobs and developing infrastructure [21]. However, the exploitation of springs can take years, and during this period, negative effects on agriculture and the environment can occur, as well as have a negative impact on the mental and physical health of neighboring residents [22]. Positive public attitudes about lignite mining are linked to perceptions of employment opportunities, cheaper fuel and energy [23,24]. The increase in CO2 and greenhouse gas emissions from the electricity sector has forced a gradual shift away from the use of lignite as a fossil fuel. Coal extracted from low seams can be used for food production, is not used as a fuel, and, therefore, does not emit CO2 or greenhouse gases. Detman et al. [25] found that lignite is difficult to recycle, and it has been treated as problematic waste. From an agricultural point of view, it is organic matter that contains cellulose, lignin, humins or humic acids and can be used as organic fertilizer in conventional crops. The compounds contained in brown coal (huminous acids, humic acids) play an important role in the absorption of nutrients by plants and act as chelates, preventing leaching and degradation of nutrients. This also contributes to reducing the use of artificial mineral fertilizers. Humic acids have also been found to contain substances such as auxins and gibberellins, which are essential for proper plant growth and development. It has also been proven that soil fertility is improved by the use of humic acids, whose sources include lignite [26,27]. With this in mind, lignite after several seasons of hydroponic cultivation can be intended as an organic fertilizer containing humic acids that improve soil properties. This is a type of cascade proposal for the use of lignite, which was proposed using a substrate of Miscanthus [7], but without combustion, which reduces greenhouse gas emissions.
Despite strictly controlled conditions in hydroponic cultivation, both mineral and organic substrates result in excessive accumulation of salts and ions in the substrate [28]. The accumulation of ions in the substrate and the increase in EC electrolytic conductivity affect plant morphology, may lead to a reduction in leaf area and root and shoot length, and affect the amount of fresh and dry matter of the plant [3,29]. Salinity stress, where there is also a high EC of the substrate, affects physiological activity through changes in the transport of primary and secondary metabolites and disturbances of photosynthesis, including disturbances of the proper functioning of photosynthetic pigments [2,29,30]. The environmental pollution that results from hydroponic cultivation using mineral medium is one of the problems to be solved. Another challenge is to increase the quality of fruits and vegetables by increasing the content of biologically active substances. One way to obtain an increased amount of secondary metabolites and biologically active substances is to apply eustressors in the form of salt stress and high EC of the substrate [20,31,32,33]. Salinity stress can affect the color of cucumber fruit [34], increase the content of vitamin C [35], carotenoids, phenols [36], or acids and antioxidant compounds [37]. On the other hand, salt stress is considered to strongly reduce plant yield [2,33]. By applying salt stress to obtain an increase in biologically active compounds, a strong reduction in yield can be induced [2,36,38] and lead to adverse physical changes, such as the ability to retain water and minerals in the soil [9,39]. Increased nutrient solution ion concentration (high EC) in hydroponic cultivation using lignite substrate increases cucumber fruit quality parameters without significantly reducing yield [20].
There have been no reports to date on the effects of the physical properties of lignite substrate on morphological parameters and plant nutritional status, as well as cucumber fruit yield and quality. The results obtained will help direct research into improving the universal lignite substrate and the use of meters to measure changing physical parameters of the substrate during cultivation. Such solutions will allow future control and management of plant growth and yield in hydroponic crops with lignite substrate.
The aim of this study was to investigate the correlation between bulk density and water holding capacity of the substrate and morphological parameters, macro- and micronutrient content of leaves, and cucumber fruit yield and quality in hydroponic cultivation.
2. Materials and Methods
The trials were conducted at the Greenhouse Experimental Center of the Warsaw University of Life Sciences in the growing rooms of the Department of Vegetable and Medicinal Plants in 2020 and 2021. For testing, we selected greenhouse cucumber (Cucumis sativus L.) cultivar “Mewa” F1 by Rijk Zwaan, which has marketable fruits growing up to 24 cm in length and weighing 200–220 g, with dark, slightly glossy skin. The organic substrate for hydroponic cultivation, carbomat lignite mats from CarboHort, measuring 100 × 20 × 8 cm, further denoted by the symbol L, were tested. The controls were Grotop Master mineral wool mats from Grodan, measuring 100 × 20 × 7.5 cm, further denoted by the symbol MW. The microclimate in the growing rooms was controlled with the Hortimax system. Fertilization was controlled using a DGT Volmatic controller with Dosatron dispensers. Before starting the cultivation, new growing mats were flooded with nutrient solution at pH 5.5 and EC 2.6 dS·m−1, at a rate of approximately 8 dm3 per mat. After 48 h, two 5 cm drainage holes were made in the lignite mats on each side of the longer sides of the mat [19], while in the mineral wool mats, four drainage holes were made (two horizontal in each of the shorter sides of the mat and two vertical in each of the longer sides of the mat). Cucumber seedlings, 28 days after sowing (DAS) plants, in the first year of the study were planted into the growing mats on 10 July 2020, and in the second year, on 12 July 2021, at a rate of 3 per mat. The crop was grown until the 35th week of the year. In 2020, during the growing season, the average daily solar radiation sum was 1474.9 J cm−2, while the average temperature D/N was 25/23 °C, and the experimental camera humidity and CO2 concentration were about 70% and 800 ppm, respectively. In the second year of the experiment, the mean daily solar radiation was 1407.0 J cm−2, D/N temperature 25/22 °C, and humidity at the experimental camera and CO2 concentration were about 70% and 800 ppm, respectively. Plants in the experiment were fed with cucumber standard medium with EC 3.1 dS·m−1 and pH 5.5–5.8, designated as control EC. In each year of the 7 days after planting (DAP) experiment, the EC of the nutrient solution was varied for half of the plants in the experiment by dosing capillary medium with an EC of about 7 dS·m−1 and pH 5.5–5.8, designated as high EC. Four treatments of the experiment were compared: (1) MW/control EC—mineral wool and medium with EC 3.1 dS·m−1, (2) L/control EC—lignite and medium with EC 3.1 dS·m−1, (3) MW/high EC—mineral wool and medium with EC 7 dS·m−1, (4) L/high EC—lignite and medium with EC 7 dS·m−1. The experiments were established using the randomized block method, in 3 replications, with 9 plants in each repetition. The fertigation medium was prepared from one- and two-component mineral fertilizers designed for hydroponic cultivation. The nutrient solution in the control contained (mg·dm−3): N-NO3 230, N-NH4 10, P-PO4 50, K 330, Ca 180, Mg 55, S-SO4 80, Fe 2.5, Mn 0.80, Zn 0.33, Cu 0.15 and B 0.33. Dosatron dosing devices (D25RE2 0.2–2%) were used for diluting the concentrated nutrient solution and for nitric acid to adjust the pH of the working medium. Working medium parameters (pH and EC) were measured daily using a Senmatic portable measuring device. The nutrient solution was dosed into the plants using a capillary system at a rate of 0.5 to 2.5 dm3 per day per plant. The amount and frequency of the nutrient solution applied during the day depended on the developmental stage of the plant and the current solar radiation and humidity of the substrate. Irrigation was started 30 min after sunrise, and the last cycle was started 2 h before sunset. After planting the cucumber seedlings on the growing mats, the first 4 buds were removed from each plant. Plants were string trained, using the one-leader method, in which all side shoots and clinging tendrils were removed.
2.1. Physical and Physico-Chemical Properties of the Substrate
During the experiment, the pH and EC of the substrate were tested in triplicate from each treatment by taking the solution from the cultivation mats, the so-called mat extract, with a syringe at several places on the mat, at a height of 2, 4 and 6 cm, counting from the top surface of the mat—further defined as the top, middle and bottom of the mat, respectively. For the study, medium samples were taken from the substrate (mat extract) with a volume of approximately 100 cm3 (single sample). Measurements were carried out 2 times a week 3 times a day at 9:00 a.m., 12:00 p.m. and 3:00 p.m. The method of preparing the substrate samples for evaluating the physical properties of the growing mats depended on the type of substrate. The physical properties of the lignite substrate were determined in accordance with the current standard PN-EN 10041 [40]. An important element of this method is the method of sample preparation, which is based on the natural settling of a loose substrate (10 cm layer), brought to a water potential of −57 cm H2O. The determination of physical properties of lignite mats was carried out in cylinders of 10 cm diameter and 5 cm height (Figure S1a Supplementary Material) in a sand block (Eijkelkamp) (Figure S1b Supplementary Material). The determination of air–water properties in the vacuum range of 0–100 cm H2O was conducted using a 24 h time to establish water equilibrium at each of the 5 vacuum levels (−4.5, −10, −32, −50 and −100 cm H2O). The samples were dried at 105 °C, and the shrinkage of the media was determined by measuring volume loss. The organic matter content after incineration was determined in accordance with PN-EN 13039 [41] (results are given in % DM), while porosity, bulk density of the substrate, referred to in the paper as BD, and water content were calculated according to the current standard PN-EN 13041 [40]. Physical properties of mineral wool mats, both new and after the experiment, were determined by the method developed at the experimental station Naaldwik in the Netherlands [40,41,42,43,44]. With a sharp knife, 15 cm × 15 cm substrate samples were cut from the mats, placed in a grid box (Figure S1c Supplementary Material) 3 cm from the bottom and filled with distilled water to a level above 1 cm above the samples. The samples were kept in the water for 24 h and then, after removing the water, allowed to stand for 3 h before being flooded again with distilled water. After 30 min, the water was drained, and the samples were transferred to a sand block (Eijkelkamp), establishing a vacuum of −100 cm H2O for 30 min. The samples were then once again flooded with distilled water 3 cm above the surface of the wool for 24 h, and then we proceeded to determine air–water properties in the vacuum range of 0–100 cm H2O, using the 24 h time to establish water equilibrium at each of the 5 vacuum levels (−4.5, −10, −32, −50 and −100 cm H2O). After the sand block determinations were completed, the samples were dried at 103 °C in a laboratory fan dryer, and the shrinkage of the substrate was determined by measuring volume loss. Organic matter and ash content were also determined (PN-EN 13039) [41] in order to calculate the total porosity (PN-EN 13041) [40].
The basic physical parameters of the substrate that have a significant effect on plant growth and yield are bulk density, porosity and air–water properties. These characteristics are closely correlated with each other (density–porosity; water capacity–air capacity), so volumetric density and water capacity were selected for comparison. The volumetric density (BD) results are presented in (kg m−3), while the results for the other substrate parameters including water content pressure at −10 cm H2O, referred to in the paper by the abbreviation WHC, are presented in % vol.
2.2. Morphological Studies
Morphological measurements were made on 9 plants from each treatment, every 7 days to 49 DAP. Weekly cucumber shoot growth in length was studied. For this purpose, the distance from the shoot apex to the location of the shoot apex 7 days earlier (location marked on the string) was measured in cm. The diameter of the shoot was measured with a caliper in mm at two places on the shoot: between the 4th and 5th and 9th and 10th leaves, counting from the top of the plant. The length and width, and the length of the petioles, of the 5th and 10th leaves were measured in cm. The approximate leaf area of the 5th and 10th leaves was calculated as the product of leaf length and width, giving the result in cm2. The weekly increment in the number of leaves per plant (pcs/week) was also determined. The results obtained from the measurements of the 5th and 10th leaves and the shoot diameter measured at two shoot locations were averaged. The results presented in the tables are averages over the 2 years of the study.
2.3. Macro- and Micronutrient Content in Cucumber Leaves
The macronutrient and micronutrient contents of cucumber leaves were examined twice at 20 and 45 DAP; results are given as averages of the two dates. For the study, each time, 3 leaves located at the height of 4.-5. and 3 leaves located at the height of 9.-10. leaf on the shoot, counting from the shoot apex of the plant, were taken randomly from plants in each treatment. Leaf blades were dried at 60 °C in a laboratory air dryer and then ground in a Bosch TSM6A013B grinder. The ground plant material was incinerated in HNO3. Elements (P, K Mg, Na, Ca, Fe, Mn, Cu, Zn, B) were determined using an inductively coupled plasma spectrometer (ICP Model OPTIMA 2000DV, Perkin Elmer, Waltham, MA, USA), giving results in mg·kg−1 DW. For determination of total N, plant material was digested in concentrated sulfuric acid in the presence of copper-potassium catalyst. Nitrogen content was determined using a Kijeldahl apparatus (Vapodest, Gerhardt, Königswinter, Germany). After distillation of nitrogen as NH3, the N content was determined by titration (Official Methods of Analysis of AOAC International. 19th Edition, 2012) [45], giving the results in % DW.
2.4. Fruit Yield and Quality
Cucumber fruits were harvested every 2 days (start of harvest from 15–18 DAP). The number and weight of all harvested fruits, marketable fruits and off-selected fruits were determined. The number of fruits dropped was also estimated. Fruits for the study were collected twice, at 29 and 45 DAP. For the study, marketable fruits were taken randomly, 3 from each treatment. Fruit firmness was measured using an HPE hardness meter with a 5 mm shank diameter, at an angle of 90° to the fruit, averaging the result from 3 measurements: at the peduncle, at the center of the fruit and at the post-flowering part. Results were given on the HPE scale from 0–100 units. Fruit color was measured using a portable reflected light spectrophotometer MiniScan XE PLUS D/8-S calibrated on a standard white plate, on the CIE Lab scale: red share, a*; yellow share, b*; and brightness, L. From the data obtained, the polar coordinates of chroma (saturation) C* = (a*2 + b*2)½, color intensity (hue angle) H* = tan−1 (b*/a*) and color index (ratio a*/b*) were calculated [46,47,48]. Fruit color and firmness were measured in 3 replicates.
2.5. Bioactive Compounds, Nitrate Content, Dry Matter and TSS in Fruit
Fruits for analysis were sampled randomly from the marketable yield at 29 and 45 DAP. In cucumber fruits, β-carotene and lutein contents were determined by high-performance liquid chromatography (HPLC) (Shimadzu Scientific Instruments Company), reporting results in mg of 100 g−1 FW. Three randomly sampled fruits from each treatment were homogenized with 2 g Na2 SO4 per 100 g−1. After weighing 5 g of homogenized material on a laboratory balance to the nearest 2/100 g, the samples were ground in a mortar with cold acetone (−20 °C) and the addition of a small amount of quartz sand. The samples were extracted five times by transferring them into 50 mL volumetric flasks and topping up with cold acetone. The samples in the flasks were then centrifuged (15,000 revolutions), and the resulting supernatant was filtered through a 0.22 μm syringe filter (Supelco IsoDisc™ PTFE 25 mm × 0.22 μm) into 1 mL containers placed in a SIL-20AC HT automatic sample feeder (tray temperature 4 °C). A 5 µL extract was applied to a chromatography column, where compound separation was achieved by isocratic elution with methanol at 40 °C on a Kinetex 2.6 μm C18 100 Å 100 mm × 4.6 mm column from Phenomenex, flow rate 2 mL min−1. In this article, lutein and β-carotene were considered for study. The other compounds analyzed in the fruit are presented in the published article that follows [20]. The retention times for lutein, chlorophyll b, chlorophyll a and β-carotene were 0.75, 1.27, 1.80 and 4.20 min, respectively. The wavelengths at which the signals of the individual mixture components were integrated were 445, 470, 430, 445 and 450 nm. The analysis time was 5 min.
Four marketable fruits with a total weight of about 1 kg were randomly selected for determination of nitrate content in fruits. They were then subjected to homogenization. From the mixed sample prepared in this way, a 10 g sample of plant material was taken three times by adding 0.5 g of activated carbon and 100 mL of 2% acetic acid, and then shaken. After 30 min, the resulting solution was filtered through a fluted filter. Nitrate content in mg N-NO3/100 g−1 FW of fruits was determined spectrophotometrically at 540 nm using a Fiastar 5000 Analyzer. The dry matter content of the fruits was determined by the dry-weighing method at 105 °C, giving the results in %.
The content of soluble components in TSS cell sap was determined using a digital refractometer (Hanna Instruments HI-96800), reporting the results in %.
2.6. Statistical Analysis
Results were analyzed as the average of 2 years of study. The results of the study were statistically processed using Statistica 13.3 software. Data were analyzed for normal distribution and homogeneity of variance, followed by multiple regression analysis for the relationship between plant biometric traits and selected physical characteristics of the substrates. Linear correlation (z) coefficient was calculated separately for each pair of parameters. Numerical data for pH and EC of the mat extracts were checked for homogeneity of variance for the mentioned parameters (Levene’s test), and then analysis of variance (ANOVA) was performed. The means were compared using Tukey’s test (HSD) at a significance level of p < 0.05.
3. Results
The physical properties of mineral wool and lignite mats before and after cucumber cultivation are shown in Table 1. The new mineral wool mat has good physical properties typical of this substrate: density 61.13 kg m−3, porosity 97.70% and high content of readily available water. The air–water properties were favorable for greenhouse cucumber cultivation, and after cultivation (51 DAP), changes were observed in both physical properties and changes in organic matter content compared to the new substrate. There was a 58.7% increase in organic matter in mats after cucumber cultivation fed with standard MW/control EC medium and a 123% increase in MW/high EC mats fed with high EC medium compared to new mats (Table 1). Water content at a potential of −10 cm H2O increased by nearly 19% in MW/control EC mats and by 27.3% in MW/high EC mats. The plant-available water content also increased by an average of about 41%. These changes reduced the air content of the mat by more than 30% in the MW/control EC treatment and by 43% in the MW/high EC treatment, but it was still within the range of optimal levels for cucumber. The results indicate that mat water content increased toward the end of cultivation at the expense of air content. Higher EC did not cause much change compared to the control (Table 1).

Table 1.
Physical properties of mineral wool and lignite mats before and after cucumber cultivation (average of 2 years).
New lignite mat (New L) had high bulk density (378.15 kg m−3) and low porosity (77.07% vol). Its water holding capacity was low (41.32% vol) and it gave up water quickly, which promoted aeration of the mat and was reflected in the high air content regardless of the potential tested (Table 1 and Figure 1a–d). There was a 0.3% decrease in organic matter in mats after cucumber cultivation compared to new lignite mats. The lignite was also characterized by low plant-available water content, which was about 78.7% lower compared to the New MW mats. A beneficial feature of the amendments was the decrease in shrinkage of lignite during cultivation by 20.9% in the L/control EC treatment substrate and by 22.5% in the L/high EC treatment substrate compared to the New substrate (Table 1).
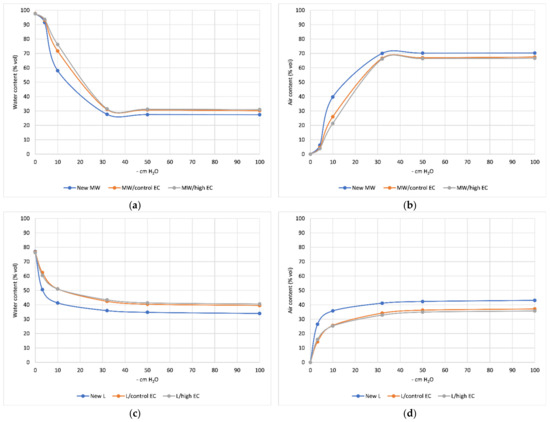
Figure 1.
Air and water content of mineral wool (a,b) and lignite substrate (c,d) before and after cucumber cultivation.
Substrate parameters, measured in the mat extract, depended on substrate type and medium EC. The mineral wool substrate, when cucumbers were fed with standard nutrient solution (MW/control EC), had approximately 14% higher pH compared to the lignite substrate (L/control EC) and 5% higher pH for plants fertilized with high EC nutrient solution, regardless of mat height (Figure 2a,c,e). For ion concentration in the substrate, mineral wool and lignite, when fed capillary nutrient solution with higher EC (7 dS·m−1), had higher EC at the top, middle, and bottom of the growing mat compared to mineral wool and lignite mats fed control nutrient solution (Figure 2b,d,f). The lignite mats in which the growing cucumber was fed with a high EC medium only had higher EC in the upper part compared to the mineral wool, where the cucumber was also fed with a higher EC medium (Figure 2b). In contrast, the middle and lower parts of the lignite mats at high EC of the nutrient solution had lower EC of the substrate compared to the EC of the mineral wool substrate also fed with high EC (Figure 2d,f). The lignite substrate where cucumbers were fed with high EC medium (L/high EC) had about 15% higher EC compared to mineral wool mats, where cucumbers were also fed with high EC medium (Figure 2b). At the middle height and bottom of the mat, the highest substrate EC values were found in the MW/high EC treatment and were 10.5% and 9.3% higher, respectively, compared to L/high EC (Figure 2d,f).
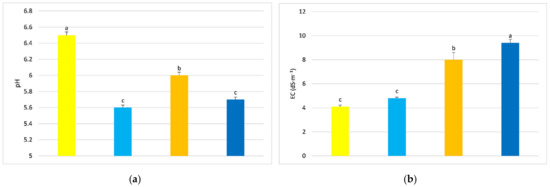
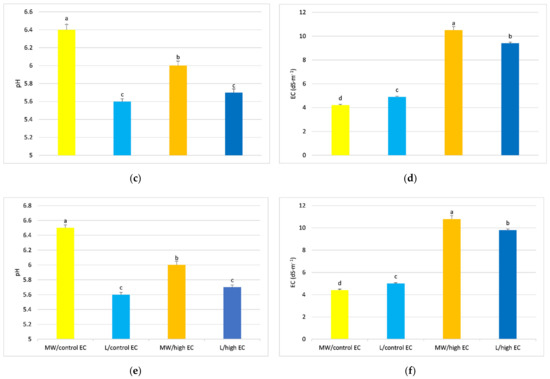
Figure 2.
pH and EC of mineral wool and lignite mat extracts, taken from the top (a,b), middle (c,d) and bottom of the mat (e,f), respectively. Average values marked with the same letters are not significantly different within the analyzed parameter at p < 0.05. Vertical bars indicate ± standard deviation.
3.1. Morphological Studies
Analyzing the results of linear correlation, there was a significant effect of water capacity on the number of leaves emerging per week and petiole length of cucumber in plants grown in mineral wool with both standard and high EC of nutrient solution (Table 2). The high coefficient of determination confirmed these relationships of the studied physical characteristics of mineral wool on the average number of leaves emerging per week (75.19%) and petiole length of cucumber (72.43%) (Table 3).

Table 2.
Linear correlation coefficient(z) between selected morphological parameters of cucumber and physical properties of medium (BD, WHC) depending on medium type and medium EC.

Table 3.
Multiple correlation coefficient (R) and coefficient of determination (D) for selected morphological parameters of cucumber plants and type of medium and EC.
A significant negative correlation was found between the bulk density of lignite growing mats in which cucumber was grown and plants were fed with standard EC (L/control EC) and the number of new leaves per week per shoot, shoot diameter, leaf length and leaf area and petiole length (Table 2). This was confirmed by the high coefficient of determination (D), where physical characteristics had a significant effect on the discussed morphological traits of plants (Table 3). On the other hand, a positive correlation was found between the water holding capacity, both of mineral wool when standard EC and high EC of capillary medium were applied, and the water holding capacity of lignite in the treatment with high EC, and the number of leaves growing weekly on the cucumber shoot. The petiole length of cucumber leaves also depended on the water holding capacity of the mineral wool mats regardless of the EC of the capillary medium (Table 2). When high EC of nutrient solution was applied in lignite substrate, a significant effect of water holding capacity on the number of leaves growing per week was shown as evidenced by the coefficient of determination for this parameter, which was 72.98%. At the same time, other treatments showed a high effect of BD and WHC on the weekly increase in number of leaves in cucumber (Table 3).
3.2. Effect of Bulk Density and Water Capacity of the Substrate on Macro- and Micronutrient Content in Cucumber Leaves
Linear correlation analysis of the relationship between macronutrient content and physical properties of substrates showed a high positive correlation of K content in leaves of cucumber plants grown in mineral wool and fertilized with standard EC nutrient solution, and BD of mineral wool and WHC substrates. No significant relationships of macronutrient concentration in cucumber leaves were found with DB and WHC of mineral wool when high EC of capillary nutrient solution was applied (Table 4 and Table 5). However, in the case of lignite and application of control nutrient solution in cucumber cultivation, a positive relationship was found between K concentration in cucumber leaves and WHC of lignite cultivation mats (Table 4).The significant relationship of these elements was confirmed by the multiple correlation coefficient R and the value of coefficient of determination (D = 96.67%) (Table 5). Significantly higher K concentration in leaves was found in cucumber grown at high EC of mineral wool substrate than at EC standard for cucumber and lignite substrate (Table S1 Supplementary Materials).

Table 4.
Linear correlation coefficient(z) for the relationship between macronutrient content in cucumber leaves and physical parameters of the medium (BD, WHC) depending on the type of medium and EC of the medium.

Table 5.
Multiple correlation coefficient (R) and coefficient of determination (D) for macronutrient content in cucumber leaves vs. medium type and medium EC.
In cucumbers grown in lignite substrate, a significant negative correlation was found between the physical properties of the medium, both BD and WHC, and the leaf concentrations of macronutrients such as calcium and sulfate. Significant correlations were found mainly when lignite was fertilized with high EC medium (Table 4), which was confirmed by multiple correlation analysis and coefficient of determination for these parameters (Table 5). The concentration of calcium, and especially sulfur, in cucumber leaves was higher when plants were grown in lignite substrate than in mineral wool, irrespective of the EC ion concentration of the medium. On the other hand, the concentration of magnesium in cucumber leaves depended more on the concentration of Mg ions in the capillary medium than on the characteristics of the medium because both when cucumber was grown in mineral wool and in lignite mats, more magnesium was in the leaves when plants were fertilized with high EC medium (Table S1 Supplementary Materials).
The results of linear correlation between micronutrient content in cucumber leaves and physical properties of the substrate indicated that there was a significant positive correlation between water holding capacity of the lignite substrate fed with standard EC medium and iron accumulation in cucumber leaves (Table 6). The coefficient of determination for this relationship was 64.06% (Table 7). There was also a significant positive correlation between zinc content in cucumber leaves and water holding capacity of lignite in the treatment with high EC of nutrient solution (Table 6), as confirmed by the significant multiple correlation coefficient R and coefficient of determination (D) of 74.21% (Table 7).

Table 6.
Linear correlation coefficient(z) for the relationship between micronutrient content in cucumber leaves and physical parameters of the medium (BD, WHC) depending on the type of medium and medium EC.

Table 7.
Multiple correlation coefficient (R) and coefficient of determination (D) for micronutrient content in cucumber leaves vs. media type and medium EC.
The accumulation in cucumber leaves of iron, zinc and boron was significantly higher in plants grown in lignite substrate than in mineral wool regardless of the concentration of ions in the drip nutrient solution (Table S1 Supplementary Materials).
3.3. Effect of Bulk Density and Water Holding Capacity on Cucumber Fruit Yield and Quality
The number of harvested cucumber fruits grown in lignite substrate and fed with standard EC medium depended on both BD and WHC of the growing mat. The water holding capacity of this substrate was significantly positively correlated with both the total number of harvested cucumber fruits and the number and weight of marketable fruits and non-choice fruits. There was also a significant relationship of bulk density and water capacity of lignite to total cucumber yield (Table 8). The dependence of cucumber fruit number and fruit weight on the physical properties of lignite was confirmed by significant multiple correlation coefficients and the coefficient of determination for cucumber yield in lignite with standard medium EC. In the case of cucumber grown in mineral wool and fed with high EC of nutrient solution, such relationships were not found (Table 8 and Table 9). Growing cucumber in lignite substrate only with medium of standard EC proved a significant effect of BD and WHC of this substrate on the number of dropped cucumber fruits. This relationship was shown in the positive linear correlation for both these characteristics of the lignite substrate and in the significance of the multiple correlation coefficient and the coefficient of determination for this treatment, which was 96.47% (Table 8 and Table 9).

Table 8.
Linear correlation coefficient(z) for the relationship between cucumber fruit yield and physical parameters of the medium (BD, WHC) depending on the type of medium and medium EC.

Table 9.
Multiple correlation coefficient (R) and coefficient of determination (D) for cucumber yield versus substrate type and medium EC.
A significant relationship was found between cucumber fruit firmness and the physical properties of the medium in hydroponic cultivation (Table 10 and Table 11). The higher the bulk density of lignite substrate fed with standard nutrient solution, the firmer the cucumber fruits were, as confirmed by the high (77.94%) coefficient of determination for this parameter (Table 11). In the case of plants grown in mineral wool and fed with high EC of nutrient solution, the increase in moisture content of this substrate had a decreasing effect on cucumber fruit hardness (Table 10). The multiple correlation coefficient in this case was (R = 0.7569) and the coefficient of determination was 57.29% (Table 11). In both treatments, when lignite growing mats were fed with standard nutrient solution and when in mineral wool mats fed with high EC nutrient solution, a statistically significant negative correlation was found between bulk density of these substrates and cucumber fruit skin color saturation (C*). This was confirmed by the coefficient of determination, which was 75.03% and 75.84%, respectively (Table 10 and Table 11). Cucumber fruit skin color intensity (H*) and coloration index (a*/b*) were positively correlated with the bulk density of the medium, but only in the mineral wool medium treatment, where standard medium was used (Table 10 and Table 11). In the case of lignite substrate and high EC medium, a significant multiple correlation was also found between physical properties of the substrate and color intensity (H*) and color index (a*/b*) of cucumber fruit peel (Table 11).

Table 10.
Linear correlation coefficient(z) for the relationship between firmness of fruit, fruit skin color traits in the CIE Lab system: C* (color saturation), H* (color intensity) and a*/b* color index, and physical parameters of the medium (BD, WHC) depending on the type of medium and EC of the medium.

Table 11.
Multiple correlation coefficient (R) and coefficient of determination (D) for firmness of fruit, fruit skin color traits in CIE Lab system: C* (color saturation), H* (color intensity) and b*/a* color index vs. substrate type and medium EC.
3.4. Effect of Bulk Density and Water Capacity of the Substrate on Dry Matter, Tss and Bioactive Compound Content of Fruit
Using the standard EC of the medium in mineral wool mats, a negative correlation was obtained between bulk density and TSS and β-carotene content in cucumber fruits (Table 12). Coefficients of determination (D) illustrating the influence of selected physical substrate characteristics on dry matter and TSS content in fruit were low and amounted to 15.19% and 12.57%, respectively (Table 13).This indicates a slight influence of the studied substrate characteristics on the discussed parameters (Table 13). Additionally, no significant correlation was obtained between the physical properties of the substrates and the quality of fruits from plants grown in mineral wool and fertilized with high EC medium (Table 12).

Table 12.
Linear correlation coefficient(z) for the relationship between the content of dry matter, TSS, selected bioactive compounds and nitrates (in mg N-NO3/100 g−1 FW) in cucumber fruit and the physical parameters of the medium (BD, WHC) depending on the type of medium and EC of the medium.

Table 13.
Multiple correlation coefficient (R) and coefficient of determination (D) for the content of dry matter, TSS, selected bioactive compounds and nitrates in cucumber fruit depending on the type of substrate and medium EC.
There was a significant positive correlation between WHC and lutein content in fruits from plants grown in lignite substrate, both fertilized with standard and high EC medium (Table 12). It was also confirmed by high coefficient of determination (D) and value of multiple correlation coefficient R, which was 0.8411 for plants fertilized with standard EC and 0.8211 for those fertilized with high EC nutrient solution (Table 13). On the other hand, no relationship was found between cucumber fruit dry matter, TSS, β-carotene and nitrate content and physical properties of both lignite and mineral wool substrates, irrespective of medium EC (Table 12 and Table 13).
4. Discussion
Lignite substrate can be applied in hydroponic cultivation of cucumber. Studies have confirmed that lignite growing mats at the beginning of their use in plant cultivation have low water holding capacity and low content of readily available water [19]. Bulk density (BD) increased in lignite mats after 51 DAP of cucumber cultivation in comparison to new cultivation mats, which was associated with a decrease in air content in mats. The effect of lowering this parameter was an increase in water holding capacity (WHC) of the substrate and water readily available to plants (Table 1). A similar trend was found when lignite substrate was used twice in hydroponic cultivation of greenhouse cucumber [19]. Water holding capacity and water readily available to plants can significantly affect plant growth and yield [19,49,50]. According to a study by Kennard et al. and Dannehl et al. [6,8] the physical properties of the substrate recommended for most plants are in the following ranges: 20–30 vol% readily available water, ≥85 vol% total porosity, and 10–30 vol% total water holding capacity. Despite the low physical parameters of lignite mats, such as water holding capacity and readily available water content, no negative effect of this substrate on plant growth and yield was observed. As shown in previous studies, the yield of cucumber grown in reused lignite mats was higher compared to reused mineral wool mats. This may be due to the better conditions for the root system in the reused growing mats [19]. The results for the nutrient extracts from the growing mats (substrate) indicated that the pH of the lignite substrate was similar to that of the mineral wool substrate. However, the EC level was lower in the lignite substrate compared to the mineral wool substrate. The sorption complex of lignite may to some extent reduce the negative effect of high EC concentration in the substrate as for cucumber (Figure 2a–f). The EC and pH values of the substrate and the physico-chemical characteristics of the plant root system environment are mainly influenced by the material of the growing medium [51,52]. In our study, a negative correlation was found between the water holding capacity of the lignite substrate supplied with the standard EC of the growing medium and the average length and surface area of the leaf. In the study, the average length of a fully developed cucumber leaf was about 22.7 cm, and the calculated leaf area was about 629.8 cm2 (Table S2 Supplementary Materials).Other researchers also reported a negative effect of low water content on leaf area of tomato grown on sheep wool or hemp fiber substrate [6]. A negative linear correlation (R = −0.8082) for water holding capacity of lignite substrate in L/control EC treatment was also reported for shoot diameter of cucumber plants. In the present study, the diameter of the cucumber shoot was on average 7.0 mm (Table S2 Supplementary Materials). Other researchers report that for tomato shoot diameter, varying substrate had no effect on this parameter [53,54]. Different results were obtained by studying the effect of date palm waste substrate on shoot diameter and length of cucumber plants, where this substrate significantly affected the morphological parameters in question compared to, for example, rice husk substrate. According to Sonneveld et al. [55], the date palm substrate had the highest porosity, bulk density and water holding capacity compared to other tested substrates, which translated into good growth conditions for cucumber plants. The availability of nutrients and appropriate physical properties of the substrate affect vegetative growth, and this in turn affects the diameter of the stem. The larger the stem is, the more the vegetative plant growth is. Stems with the thickest stem diameter are less susceptible to damage and other abiotic stresses. Additionally, a thicker stem transports water and nutrients more efficiently [56]. Studies on the effect of lignite substrate on cucumber growth and yield in combination with eustress in the form of high EC showed higher plant stem diameter in the treatment with lignite substrate [20]. It is likely that the lignite substrate, due to its properties, humic acids and nutrient content [57], has a beneficial effect on stem thickness and consequently on plant growth.
In the present study, a significant correlation was found between the WHC of the medium in both MW/control EC and L/control EC combinations to K content in cucumber leaves. The potassium content of cucumber leaves grown in these combinations was 35,252.0 mg·kg−1 DM for MW/control EC and 31,741.7 mg·kg−1 DM for L/control EC. Significantly more K was contained in the leaves of plants grown in mineral wool and fertilized with higher EC of nutrient solution, where this content was on average about 42,275.0 mg·kg−1 DM. On the other hand, higher EC of the nutrient solution in the case of lignite substrate did not result in higher K accumulation in cucumber leaves, as it was only 34,358.3 mg·kg−1 DM (Table S1 Supplementary Materials). However, when using high EC of the medium in the lignite substrate (L/high EC), a significant correlation was found between the physical properties of the medium and the S-SO4 content in cucumber leaves. As reported by Nurzyński et al. [58], the content of mineral components in the substrate changes during cultivation, while changes in the content of components in the leaves are minimal; therefore, proper fertilization and pH in the root environment are important. Similarly to the content of bioactive compounds in fruits, the content of macro- and microelements in leaves may be influenced by many factors, starting from antagonistic relationships of elements [59] through the correct pH of the solution and irrigation strategies, to the right cultivation and plant care practices [55,58]. Micronutrients are elements that are used by plants in small quantities, but a lack or excess of these elements can disrupt the life processes of the plant and consequently reduce the yield [60]. The present study showed a significant correlation of water capacity of lignite mat to Fe (L/control EC) and Zn (L/high EC) content in cucumber leaves (Table 6). Lignite mats, in spite of their stable chemical composition, low chlorine content and high humic acid content, have large amounts of micronutrients in their composition, especially Fe and Zn [17,57]. In the initial stages of cultivation, negative effects on the plant may occur, e.g., excessive accumulation of these elements in the root zone, although this phenomenon was not observed in the conducted studies. In a study by Sonneveld and Voogt [55] it was proved that different Fe concentrations in the nutrient solution had no significant effect on the content of this element in young cucumber leaves. On the other hand, in a study on Zn content in tomato and cucumber leaves, nutrient solution containing different concentrations of Zn was found to have little effect on the Zn content in tomato leaves, while Zn significantly increased in cucumber leaves with increasing concentrations in the nutrient solution. Excessive concentrations of Fe, Zn and other micronutrients may affect the availability of other elements or vice versa [61]. Similarly, salinity stress can affect elemental content in the leaves. Excessive ion accumulation in transpiring tissue may differ between younger and older leaves because the latter transpire the longest, so they accumulate ions the longest. Additionally, ion accumulation in older leaves depends on the properties of these ions and their mode of transport [62]. Despite the introduction of high EC in the nutrient solution, no significant correlation was found between the content of other micronutrients and the physical properties of the media. It is possible that the humic acids present in the lignite substrate reduced the excessive accumulation of ions in the root zone and did not lead to their accumulation in the leaves. Humic substances may mitigate the effects of salinity by altering the absorption of macro- and micronutrients, accelerating root growth and reducing damage to cytoplasmic membranes, the researchers say [59,63].
There was a significant positive correlation between water holding capacity and bulk density and cucumber fruit yield in lignite mats compared to mineral wool, where no such relationships were found. Plants grown in lignite medium fertilized with standard EC (L/control EC) obtained a 10% higher total yield compared to mineral wool (MW/control EC), while in the L/high EC combination, plants obtained a 14.8% higher marketable yield and 10% lower non-marketable yield, respectively, compared to MW/high EC. Allaire et al. [49] found a positive correlation in tomato marketable yield with the content of readily available water in the substrate and a negative correlation with the amount of air in the substrate. Other researchers also confirm that yield correlates with water buffer in the growing medium [64]. Similar results were reported by studying the yield of cucumber grown in perilla, where higher yield was dependent on the availability of water in the substrate [50]. The obtained results confirm the correlation of bulk density (BD) and water holding capacity (WHC) of the lignite substrate with cucumber yield. However, in the studies with mineral wool substrate (MW/control EC), such a correlation of BD and WHC of the substrate with cucumber yield was not proved (Table 8). Physicochemical properties of substrates affect plant yield and growth [65]. However, which parameter affects this and to what extent is difficult to determine. In a study on the yield of cucumber and tomato plants grown on Miscanthus substrate, there were no differences in yield compared to mineral wool [7]. Similar results were reported in tomato plants, where the substrates had no effect on fruit number or weight [54]. According to Luitel et al. [66] fruit number and weight depend on the air and water content of the substrate, because insufficient air in the soil reduces root respiration and negatively affects water and nutrient uptake.
Fruit quality is a very broad concept, and it is not possible to say unequivocally which trait affects the final yield quality more significantly. Fruit firmness, texture, color and organic content are important [32,67]. Table S3 in the Supplementary Materials contains the average values of chosen quality traits of cucumber fruits grown hydroponically in the conducted studies. Despite many studies on new substrates for hydroponic cultivation, there is still little information on the correlation of physical characteristics of the substrate with color and bioactive content. In the study, a significant correlation was found between the bulk density of mineral wool substrate and the color intensity (H*) and color index (a*/b*) of cucumber fruit peel in the CIE Lab system (Table 10). Color saturation (C*) of cucumber fruit was strongly correlated with bulk density of lignite substrate (control EC) and mineral wool (high EC) substrates. The obtained results also confirmed a strong correlation between selected physical substrate characteristics and cucumber fruit firmness. Physical characteristics of the substrate such as bulk density and water holding capacity of lignite mats (BD and WHC) were also strongly correlated with color intensity (H*) and coloration index (a*/b*) of cucumber fruit peel. Many publications indicate that there is no effect from salinity on the color and hardness of cucumber fruit [68,69]. In the case of tomato, no differences were found in the firmness of fruit from plants grown in different organic media [6]. According to Łaźny et al. [19], the substrate and its physical characteristics can affect the dry matter content and TSS in cucumber fruits. In the present study, a high correlation coefficient, but not statistically significant, was obtained between bulk density of lignite growing mats (L/high EC) and dry matter of cucumber fruits. Similar results were obtained in an experiment with twice-used lignite substrate and high EC medium in cucumber cultivation [20]. In studies conducted on tomato plants grown on organic substrates, the highest dry matter content was obtained in fruits grown in sheep wool, but no correlation was found between the physical properties of this substrate and fruit quality [6]. Different results were obtained by examining the sugar extract content (TSS) in tomato fruits, where the lowest level of TSS was in fruits from plants grown in date palm waste substrate [70]. Kraska et al. [7] obtained no differences in TSS content in fruits from tomato and cucumber plants grown in organic medium compared to mineral medium. The content of biologically active compounds may also change under the influence of the growing medium. Analyzing the obtained results, a high correlation was found between the water capacity of the lignite substrate and the lutein content in cucumber fruits (L/control EC and L/high EC) (Table 12 and Table 13). It is likely that the low water content readily available in the lignite mats increased the lutein content in cucumber fruits. These results are consistent with those for carotenoid and phenolic content in tomato, where the low content of readily available water in the medium was correlated with the content of these compounds in fruit [6]. An increase in the content of bioactive compounds in fruit was also observed by studying the effect of reused lignite mats on cucumber fruit quality, where the content of readily available water in the lignite substrate was lower compared to that in mineral wool [19]. As reported by Peet et al. [71], tomato fruit quality and yield depend on the cultivar, maintenance of appropriate substrate moisture level and cultivation method. Despite the use of high EC in the nutrient solution, no significant correlation of selected physical properties with the content of other bioactive compounds in cucumber fruit was observed in both tested substrates. Studies conducted on salinity stress have shown that an appropriate level of salt concentration can lead to an increase in the concentration of carotenoids and phenols in bell pepper fruits [36] or lutein and β-carotene in romaine lettuce [72]. However, the factors affecting the content of compounds in fruit are very complex and also depend on air temperature, sunlight, cultivar or type of fertilizer used.
5. Conclusions
The growing demand for quality food is leading to a search for new alternative substrates that are fully biodegradable and do not place an undue burden on the environment. Unfortunately, many of the materials used as substrates do not have adequate physical properties for plant growth and development. Moreover, knowledge regarding their effects on the plant during the growing season is limited. In the present study, it was found that both the substrate density (BD) and water holding capacity (WHC) affect such morphological features of plants as shoot diameter, leaf and petiole length, as well as the weekly increase in the number of leaves in cucumber. A significant positive correlation was also observed between the density (BD) as well as water capacity (WHC) of the substrate and potassium content in cucumber leaves. It was also found that both the density and water holding capacity (BD and WHC) of the lignite substrate significantly affected the number and weight of fruits in greenhouse cucumber. A positive correlation between water holding capacity (WHC) of lignite substrate and lutein content in cucumber fruits in hydroponic cultivation was also confirmed. The obtained results may contribute to the development of new biodegradable hydroponic growing media, increasing the efficiency of vegetable cultivation. At the same time, they can be used to develop new methods of monitoring and controlling the parameters of the substrate in order to control the quantity and quality of the yield.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app12094480/s1, Figure S1: Apparatus and substrate samples for physical properties determination: (a) lignite substrate sample (b) Eijkelkamp sand block (c) mineral wool substrate sample; Table S1: Contents of macro- and microelements in cucumber leaves; Table S2: Chosen morphological characteristics of cucumber grown hydroponically; Table S3: Chosen yield characteristics of cucumber grown hydroponically, in the period of 8 weeks after planting until the 35th week of the year; Table S4: Chosen quality characteristics of hydroponically grown cucumber fruit.
Author Contributions
Conceptualization, R.Ł., K.K, J.G.-W., J.S.N. and M.M.; methodology, R.Ł. J.S.N., J.L.P., M.N., M.K., W.K. and K.K.; software, J.L.P. and W.K.; validation, J.S.N., J.L.P., M.N. and W.K.; formal analysis, R.Ł., J.S.N., M.N., M.M., M.K. and W.K.; investigation, R.Ł., J.S.N., M.N., M.M., M.K. and W.K.; resources, J.G.-W., K.K. and J.S.N., data curation, R.Ł.; writing—original draft preparation, R.Ł. and J.S.N.; writing—review and editing, R.Ł., K.K., J.S.N. and M.M.; visualization, R.Ł. and J.S.N.; supervision, K.K., J.G.-W., M.M. and J.S.N.; project administration, R.Ł., K.K. and J.G.-W.; funding acquisition, J.S.N. and J.G.-W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Saha, S.; Monroe, A.; Day, M.R. Growth, yield, plant quality and nutrition of basil (Ocimum basilicum L.) under soilless agricultural systems. Ann. Agric. Sci. 2016, 61, 181–186. [Google Scholar] [CrossRef]
- Kamran, M.; Parveen, A.; Ahmar, S.; Malik, Z.; Hussain, S.; Chattha, M.S.; Saleem, M.H.; Adil, M.; Heidari, P.; Chen, J.-T. An overview of hazardous impacts of soil salinity in crops, tolerance mechanisms, and amelioration through selenium supplementation. Int. J. Mol. Sci. 2019, 21, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Farid, I.B.; Marghany, M.R.; Rowezek, M.M.; Sheded, M.G. Effect of salinity stress on growth and metabolomic profiling of Cucumis sativus and Solanum lycopersicum. Plants 2020, 9, 1626. [Google Scholar] [CrossRef] [PubMed]
- Barrett, G.E.; Alexander, P.D.; Robinson, J.S.; Bragg, N.C. Achieving environmentally sustainable growing media for soilless plant cultivation systems—A review. Sci. Hortic. 2016, 212, 220–234. [Google Scholar] [CrossRef] [Green Version]
- Nowak, J.S. Changes of physical properties in rockwool and glasswool slabs during hydroponic cultivation of roses. J. Fruit Ornam. Plant Res. 2010, 18, 349–360. [Google Scholar]
- Dannehl, D.; Suhl, J.; Ulrichs, C.; Schmidt, U. Evaluation of substitutes for rock wool as growing substrate for hydroponic tomato production. J. Appl. Bot. Food Qual. 2015, 88, 68–77. [Google Scholar] [CrossRef]
- Kraska, T.; Kleinschmidt, B.; Weinand, J.; Pude, R. Cascading use of Miscanthus as growing substrate in soilless cultivation of vegetables (tomatoes, cucumbers) and subsequent direct combustion. Sci. Hortic. 2018, 235, 205–213. [Google Scholar] [CrossRef]
- Kennard, N.; Stirling, R.; Prashar, A.; Lopez-Capel, E. Evaluation of recycled materials as hydroponic growing media. Agronomy 2020, 10, 1092. [Google Scholar] [CrossRef]
- Nerlich, A.; Dannehl, D. Soilless Cultivation: Dynamically changing chemical properties and physical conditions of organic substrates influence the plant phenotype of lettuce. Front. Plant Sci. 2021, 11, 601455. [Google Scholar] [CrossRef]
- Gruda, N. Increasing sustainability of growing media constituents and stand-alone substrates in soilless culture systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef] [Green Version]
- Zulfiqar, F.; Allaire, S.E.; Akram, N.A.; Méndez, A.; Younis, A.; Peerzada, A.M.; Shaukat, N.; Wright, S.R. Challenges in organic component selection and biochar as an opportunity in potting substrates: A review. J. Plant Nutr. 2019, 42, 1386–1401. [Google Scholar] [CrossRef]
- Solaiman, Z.M.; Shafi, M.I.; Beamont, E.; Anawar, H.M. Poultry litter biochar increases mycorrhizal colonisation, soil fertility and cucumber yield in a fertigation system on sandy soil. Agriculture 2020, 10, 480. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Moral, R.; Paredes, C.; Pérez-Espinosa, A.; Moreno-Caselles, J.; Pérez-Murcia, M.D. Agrochemical characterisation of the solid by-products and residues from the winery and distillery industry. Waste Manag. 2008, 28, 372–380. [Google Scholar] [CrossRef]
- Dede, O.H.; Ozdemir, S. Development of nutrient-rich growing media with hazelnut husk and municipal sewage sludge. Environ. Technol. 2018, 39, 2223–2230. [Google Scholar] [CrossRef] [PubMed]
- Urrestarazu, M.; Mazuela, P.C.; Martínez, G.A. Effect of substrate reutilization on yield and properties of melon and tomato crops. J. Plant Nutr. 2008, 31, 2031–2043. [Google Scholar] [CrossRef]
- Bilderback, T.E.; Warren, S.L.; Owen, J.S.; Albano, J.P. Healthy substrates need physicals too! Horttechnology 2005, 15, 747–751. [Google Scholar] [CrossRef] [Green Version]
- Dyśko, J.; Kaniszewski, S.; Kowalczyk, W. Lignite as a new medium in soilless cultivation of tomato. J. Elem. 2015, 20, 559–569. [Google Scholar] [CrossRef]
- Amoah-Antwi, C.; Kwiatkowska-Malina, J.; Thornton, S.F.; Fenton, O.; Malina, G.; Szara, E. Restoration of soil quality using biochar and brown coal waste: A review. Sci. Total Environ. 2020, 722, 137852. [Google Scholar] [CrossRef]
- Łaźny, R.; Mirgos, M.; Przybył, J.L.; Nowak, J.S.; Kunka, M.; Gajc-Wolska, J.; Kowalczyk, K. Effect of re-used lignite and mineral wool growing mats on plant growth, yield and fruit quality of cucumber and physical parameters of substrates in hydroponic cultivation. Agronomy 2021, 11, 998. [Google Scholar] [CrossRef]
- Łaźny, R.; Mirgos, M.; Przybył, J.L.; Niedzińska, M.; Gajc-Wolska, J.; Kowalczyk, W.; Nowak, J.S.; Kalisz, S.; Kowalczyk, K. Lignite substrate and EC modulates positive eustress in cucumber at hydroponic cultivation. Agronomy 2022, 12, 608. [Google Scholar] [CrossRef]
- Karasmanaki, E.; Ioannou, K.; Katsaounis, K.; Tsantopoulos, G. The attitude of the local community towards investments in lignite before transitioning to the post-lignite era: The case of Western Macedonia, Greece. Resour. Policy 2020, 68, 101781. [Google Scholar] [CrossRef]
- Hossain, D.; Gorman, D.; Chapelle, B.; Mann, W.; Saal, R.; Penton, G. Impact of the mining industry on the mental health of landholders and rural communities in southwest Queensland. Australas. Psychiatry 2013, 21, 32–37. [Google Scholar] [CrossRef]
- Bec, A.; Moyle, B.D.; McLennan, C.J. Drilling into community perceptions of coal seam gas in Roma, Australia. Extr. Ind. Soc. 2016, 3, 716–726. [Google Scholar] [CrossRef] [Green Version]
- Badera, J.; Kocoń, P. Local community opinions regarding the socio-environmental aspects of lignite surface mining: Experiences from central Poland. Energy Policy 2014, 66, 507–516. [Google Scholar] [CrossRef]
- Detman, A.; Bucha, M.; Simoneit, B.R.T.; Mielecki, D.; Piwowarczyk, C.; Chojnacka, A.; Błaszczyk, M.K.; Jędrysek, M.O.; Marynowski, L.; Sikora, A. Lignite biodegradation under conditions of acidic molasses fermentation. Int. J. Coal Geol. 2018, 196, 274–287. [Google Scholar] [CrossRef] [Green Version]
- Kalaichelvi, K.; Chinnusamy, C.; Swaminathan, A. Exploiting the natural resource—Lignite humic acid in agriculture—A review. Agric. Rev. 2006, 27, 276–283. [Google Scholar]
- Huculak-Mączka, M.; Hoffmann, J.; Hoffmann, K. Evaluation of the possibilities of using humic acids obtained from lignite in the production of commercial fertilizers. J. Soils Sediments 2018, 18, 2868–2880. [Google Scholar] [CrossRef] [Green Version]
- Savvas, D.; Pappa, V.; Kotsiras, A.; Gizas, G. NaCl accumulation in a cucumber crop grown in a completely closed hydroponic system as influenced by NaCl concentration in irrigation water. Eur. J. Hortic. Sci. 2005, 70, 217–223. [Google Scholar]
- Baghel, L.; Kataria, S.; Jain, M. Mitigation of adverse effects of salt stress on germination, growth, photosynthetic efficiency and yield in maize (Zea mays L.) through magnetopriming. Acta Agrobot. 2019, 72, 1757. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.-W.; Gomez Pineda, I.M.; Brand, A.M.; Stützel, H. Determining Ion Toxicity in Cucumber under Salinity Stress. Agronomy 2020, 10, 677. [Google Scholar] [CrossRef]
- Borgognone, D.; Cardarelli, M.; Rea, E.; Lucini, L.; Colla, G. Salinity source-induced changes in yield, mineral composition, phenolic acids and flavonoids in leaves of artichoke and cardoon grown in floating system. J. Sci. Food Agric. 2014, 94, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, M.C.; Rouphael, Y. Towards a new definition of quality for fresh fruits and vegetables. Sci. Hortic. 2018, 234, 463–469. [Google Scholar] [CrossRef]
- Rouphael, Y.; Petropoulos, S.A.; Cardarelli, M.; Colla, G. Salinity as eustressor for enhancing quality of vegetables. Sci. Hortic. 2018, 234, 361–369. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Cardarelli, M.; Massa, D.; Salerno, A.; Rea, E. Yield, fruit quality and mineral composition of grafted melon plants grown under saline conditions. J. Hortic. Sci. Biotechnol. 2006, 81, 146–152. [Google Scholar] [CrossRef]
- Marín, A.; Rubio, J.S.; Martínez, V.; Gil, M.I. Antioxidant compounds in green and red peppers as affected by irrigation frequency, salinity and nutrient solution composition. J. Sci. Food Agric. 2009, 89, 1352–1359. [Google Scholar] [CrossRef]
- Giuffrida, F.; Graziani, G.; Fogliano, V.; Scuderi, D.; Romano, D.; Leonardi, C. Effects of nutrient and NaCl salinity on growth, yield, quality and composition of pepper grown in soilless closed system. J. Plant Nutr. 2014, 37, 1455–1474. [Google Scholar] [CrossRef]
- Keutgen, A.J.; Pawelzik, E. Quality and nutritional value of strawberry fruit under long term salt stress. Food Chem. 2008, 107, 1413–1420. [Google Scholar] [CrossRef]
- Kaya, C.; Higgs, D.; Ince, F.; Amador, B.M.; Cakir, A.; Sakar, E. Ameliorative effects of potassium phosphate on salt-stressed pepper and cucumber. J. Plant Nutr. 2003, 26, 807–820. [Google Scholar] [CrossRef]
- Hajiboland, R.; Aliasgharzadeh, N.; Laiegh, S.F.; Poschenrieder, C. Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum lycopersicum L.) plants. Plant Soil 2010, 331, 313–327. [Google Scholar] [CrossRef]
- Polish Standard. EN 13041; Soil Improvers and Growing Media. Determination of Physical Properties—Dry Bulk Density, Air Volume, Water Volume, Shrinkage Value and Total Pore Space. European Committee for Standardization: Brussels, Belgium, 2002.
- Polish Standards. EN 13039; Soil Improvers and Growing Media. Determination of Organic Matter Content and Ash. PKN: Warszawa, Poland, 2002.
- Nowak, J.S.; Strojny, Z. Changes in physical properties of peat-based substrates during cultivation period of gerbera. Acta Hortic. 2004, 644, 319–323. [Google Scholar] [CrossRef]
- Wever, G. Determination of Dry Matter Content (KIWA); Analysereeks PBG: Naaldwijk, The Netherlands, 2000. [Google Scholar]
- Wever, G. Aangepast Beperkt Fisisch Onderzoek Vaste Substraten; Analysereeks PBG: Naaldwijk, The Netherlands, 2000. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 19th ed.; Academic: Cambridge, MA, USA, 2012. [Google Scholar]
- López Camelo, A.F.; Gómez, P.A. Comparison of color indexes for tomato ripening. Hortic. Bras. 2004, 22, 534–537. [Google Scholar] [CrossRef]
- Barbagallo, R.N.; Di Silvestro, I.; Patanè, C. Yield, physicochemical traits, antioxidant pattern, polyphenol oxidase activity and total visual quality of field-grown processing tomato cv. Brigade as affected by water stress in Mediterranean climate. J. Sci. Food Agric. 2012, 93, 1449–1457. [Google Scholar] [CrossRef]
- Kulapichitr, F.; Borompichaichartkul, C.; Fang, M.; Suppavorasatit, I.; Cadwallader, K.R. Effect of post-harvest drying process on chlorogenic acids, antioxidant activities and CIE-Lab color of Thai Arabica green coffee beans. Food Chem. 2022, 366, 130504. [Google Scholar] [CrossRef]
- Allaire, S.E.; Caron, J.; Ménard, C.; Dorais, M. Potential replacements for rockwool as growing substrate for greenhouse tomato. Can. J. Soil Sci. 2005, 85, 67–74. [Google Scholar] [CrossRef]
- Mazahreh, N.; Nejatian, A.; Mousa, M. Effect of different growing medias on cucumber production and water productivity in soilless culture under UAE conditions. Merit Res. J. Agric. Sci. Soil Sci. 2015, 3, 131–138. [Google Scholar]
- Pinter, I.F.; Fernández, A.S.; Martínez, L.E.; Riera, N.; Fernández, M.; Aguado, G.D.; Uliarte, E.M. Exhausted grape marc and organic residues composting with polyethylene cover: Process and quality evaluation as plant substrate. J. Environ. Manag. 2019, 246, 695–705. [Google Scholar] [CrossRef]
- Savvas, D.; Gruda, N. Application of soilless culture technologies in the modern greenhouse industry—A review. Eur. J. Hortic. Sci. 2018, 83, 280–293. [Google Scholar] [CrossRef]
- Mohammadi-Ghehsareh, A. Effect of plant growth on some physical properties of potting culture media. Int. J. Recycl. Org. Waste Agric. 2015, 4, 205–209. [Google Scholar] [CrossRef] [Green Version]
- Borji, H.; Mohammadi Ghahsareh, A.; Jafarpour, M. Effects of the Substrate on Tomato in Soilless Culture. Res. J. Agric. Biol. Sci. 2010, 6, 923–927. [Google Scholar]
- Sonneveld, C.; De Kreij, C. Response of cucumber (Cucumis sativus L.) to an unequal distribution of salts in the root environment. Plant Soil 1999, 209, 47–56. [Google Scholar] [CrossRef]
- Alam, M. Effect of growing media on rooting response of tomato (Lycopersicum esculentum L.) stem cuttings. Pure Appl. Biol. 2020, 9, 884–896. [Google Scholar] [CrossRef]
- Kwiatkowska, J. Ocena możliwości wykorzystania węgla brunatnego jako efektywnego źródła materii organicznej w gruntach przekształconych antropogenicznie. Inżynieria Ochr. Śr. 2007, 10, 71–85. [Google Scholar]
- Nurzyński, J. Yield and quality of greenhouse tomato fruit grown in rape straw substrates. Acta Sci. Pol. Cultus 2013, 12, 3–11. [Google Scholar]
- Machado, R.; Serralheiro, R. Soil Salinity: Effect on Vegetable crop growth. management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Tavakoli, M.T.; Chenari, A.I.; Rezaie, M.; Tavakoli, A.; Shahsavari, M.; Mousavi, S.R. The Importance of Micronutrients in Agricultural Production. Available online: https://go.gale.com/ps/i.do?p=AONE&sw=w&issn=19950756&v=2.1&it=r&id=GALE%7CA385404507&sid=googleScholar&linkaccess=fulltext (accessed on 22 December 2021).
- Dunlop, S.J.; Arbestain, M.C.; Bishop, P.A.; Wargent, J.J. Closing the Loop: Use of Biochar Produced from Tomato Crop Green waste as a Substrate for Soilless, Hydroponic Tomato Production. HortScience 2015, 50, 1572–1581. [Google Scholar] [CrossRef]
- Munns, R.; Husain, S.; Rivelli, A.R.; James, R.A.; Condon, A.G.T.; Lindsay, M.P.; Lagudah, E.S.; Schachtman, D.P.; Hare, R.A. Avenues for increasing salt tolerance of crops, and the role of physiologically based selection traits. In Progress in Plant Nutrition: Plenary Lectures of the XIV International Plant Nutrition Colloquium; Springer: Dordrecht, The Netherlands, 2002; Volume 247, pp. 93–105. [Google Scholar]
- Ouni, Y.; Ghnaya, T.; Montemurro, F.; Lakhdar, A. The role of humic substances in mitigating the harmful effects of soil salinity and improve plant productivity. Int. J. Plant Prod. 2014, 8, 353–374. [Google Scholar]
- Kirda, C.; Cetin, M.; Dasgan, Y.; Topcu, S.; Kaman, H.; Ekici, B.; Derici, M.R.; Ozguven, A.I. Yield response of greenhouse grown tomato to partial root drying and conventional deficit irrigation. Agric. Water Manag. 2004, 69, 191–201. [Google Scholar] [CrossRef]
- Kumar, K.; Goh, K.M. Crop residues and management practices: Effects on soil quality, soil nitrogen dynamics, crop yield, and nitrogen recovery. Adv. Agron. 1999, 68, 197–319. [Google Scholar]
- Luitel, B.P.; Adhikari, P.B.; Yoon, C.S.; Kang, W.H. Yield and fruit quality of tomato (Lycopersicon esculentum Mill.) cultivars established at different planting bed size and growing substrates. Hortic. Environ. Biotechnol. 2012, 53, 102–107. [Google Scholar] [CrossRef]
- Suthar, R.; Wang, C.; Nunes, M.; Chen, J.; Sargent, S.; Bucklin, R.; Gao, B. Bamboo biochar pyrolyzed at low temperature improves tomato plant growth and fruit quality. Agriculture 2018, 8, 153. [Google Scholar] [CrossRef] [Green Version]
- Colla, G.; Rouphael, Y.; Rea, E.; Cardarelli, M. Grafting cucumber plants enhance tolerance to sodium chloride and sulfate salinization. Sci. Hortic. 2012, 135, 177–185. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Jawad, R.; Kumar, P.; Rea, E.; Cardarelli, M. The effectiveness of grafting to improve NaCl and CaCl2 tolerance in cucumber. Sci. Hortic. 2013, 164, 380–391. [Google Scholar] [CrossRef]
- Mohammadi Ghehsareh, A. Effect of date palm wastes and rice hull mixed with soil on growth and yield of cucumber in greenhouse culture. Int. J. Recycl. Org. Waste Agric. 2013, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Peet, M.M.; Harlow, C.D.; Larrea, E.S. Fruit quality and yield in five small-fruited greenhouse tomato cultivars under high fertilization regime. In Proceedings of the VII International Symposium on Protected Cultivation in Mild Winter Climates: Production, Pest Management and Global Competition, Kissimmee, FL, USA, 23–27 March 2004; International Society for Horticultural Science: Leuven, Belgium, 2004; Volume 659, pp. 811–818. [Google Scholar]
- Kim, H.-J.; Fonseca, J.M.; Choi, J.-H.; Kubota, C.; Kwon, D.Y. Salt in irrigation water affects the nutritional and visual properties of romaine lettuce (Lactuca sativa L.). J. Agric. Food Chem. 2008, 56, 3772–3776. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).