Featured Application
This work will inform the revision process of the ISO Standard ISO 11239:2012 on dose forms of medicinal products and provide tools for training and supporting experts in the pharmaceutical industry and in regulatory affairs, during the standardization of national drug dictionaries to the global terminology for dose forms of EDQM.
Abstract
The aim was (1) to analyse the features of the EDQM terminology, (2) to formulate proposals for minor changes and (3) to create a small ontology of dose forms, based on characteristics of EDQM, and suitable for alignment with other dose form terminologies. The 428 Pharmaceutical Dose Forms (PDF) (“human and veterinary” only) were extracted from the EDQM Standard Terms database. A quantitative and qualitative analysis of the textual definitions of the terms was conducted. Through an analysis of unique combinations of different sets of descriptors and characteristics, a small ontology was built in three levels. For the 143 transformable PDFs, the administrable dose form was made explicit, with 121 requiring only one transformation and 22 multiple transformations, of which 10 include “no transformation”. Different levels of aggregations of the 428 PDFs were tested in 4 analyses, ranging from 206 to 383 unique combinations. An ontology in Webprotégé was created of 22 higher-level concepts (based on the intended site characteristics) and 69 intermediate-level terms (newly created) to accommodate the 428 PDFs of EDQM. EDQM Dose Form terminology is suitable terminology in terms of granularity, for defining dose forms of medicinal products, to enable fair comparison of similar medicinal products, and global identification of medicinal products (IDMP). Recommendations for minor improvements and a simple ontology for dose forms are proposed.
1. Introduction
In recent years, there has been a firm ambition to establish a worldwide system for global identification of medicinal products (IDMP). Each nationally authorized medicinal product anywhere in the world would receive a global identification number, so that the active substance, the dose form and the strength of the medicinal product can be recognised by health care workers and patients, anywhere in the world. This will allow the flow of prescriptions across borders, but also the flow of information about medicinal products, facilitating the development of decision support systems and patient-centred mobile applications.
It started in 2012, with the publication of a series of ISO/CEN IDMP standards, addressing the crucial aspect of information on medicines in research and development, market authorisation, prescribing and dispensing, drug information, and pharmaco-epidemiology [1]. These standards provide guidance for the standardisation of substance representation, dose form terminology and expression of strength, the three basic elements for the identification of a Medicinal Product [2]. The ISO/CEN 13499:2012 standard and its technical specification focuses on dose forms [3]. This triggered, in 1995, a first reorganisation of the European Terminology for Dose forms in the European Pharmacopoeia, managed by the European Directorate for Quality in Medicines & Healthcare (EDQM) [4]. This Directorate is an administrative entity under the Council of Europe, grouping countries from Europe and beyond.
The Food and Drug Administration (FDA) in the US expressed a clear intention to adopt IDMP and to welcome international collaboration [5].
The European Medicines Agency (EMA) developed a close cooperation with the FDA regarding the cleansing of pharmaceutical substance terminology [6,7].
In 2019, the FDA and the World Health Collaborating Centre for Pharmacovigilance in Uppsala (WHO_UMC), and EMA developed a collaboration to test procedures to generate global identification numbers [8], now evolving to a global working group between these parties [9].
In 2020, EMA engaged in an ongoing process of writing an implementation guide for the implementation of IDMP within National Agencies [10]. Further, the pharmaceutical industry prepares for IDMP implementation in the submission of new applications for marketing authorisation. A number of consultancy companies has emerged, specializing in the issue.
Next to substance identification, the identification and descriptive terminology for dose forms of medicinal products is crucial. The EDQM multilingual terminology on dose forms, revised in 2015 to adapt to the ISO/CEN standard, is used in the regulatory sphere in Europe, recommended by the International Council for Harmonisation, and used in many non-European countries [11]. In the US, the less granular RxNorm terminology of the National Library of Medicine is used [12]. In clinical care, the SNOMED-CT terminology has a separate chapter for dose forms, also inspired by the ISO/CEN standard [13].
All these pilot initiatives in the implementation of IDMP led to deeper insight in the theoretical aspects of the CEN/ISO standards. This experience is now fed into a revision cycle of these standards, currently ongoing [14]. This process runs synchronically with the UNICOM project, a large-scale Action Program of 4 years, launched at the end of 2019, to coordinate the efforts of 41 Partners (National Medicines Agencies and stakeholders), and to stimulate transatlantic cooperation [15].
It is clear that in the coming years, a decision will have to be taken on the choice for a global terminology for dose forms, as this is instrumental for creating global identification numbers for national medicinal products, ensuring semantic interoperability across information systems, languages and borders. EDQM is a trusted international terminology, but in this pivotal moment of IDMP implementation, it is important to describe its unique features, which make it fit for purpose as a global terminology, and to examine some possible improvements.
The aim of this study is to propose recommendations for improvements to the current EDQM in the light of its use as a global terminology in the identification of medicinal products, by describing and analysing the features of the EDQM terminology; by formulating possible minor changes, and by creating a small ontology of dose forms, based on the basic attributes of dose forms in EDQM, and suitable for alignment with other dose form terminologies.
2. Materials and Methods
The EDQM Standard Terms database of dose forms is a publicly available database, that is accessible with an application programming interface (API) procedure, after login, free of charge, at the website of the European Pharmacopoeia (Ph. Eur), where a full description is available [16]. Data were extracted from Version 1.2.0—28 January 2019 [17]. The database of dose forms contains 562 single Pharmaceutical Dose Forms (PDF), pertaining to the human and veterinary domain. We did not consider in this analysis the 54 combined PDF (combinations of pharmaceutical dose forms of two manufactured items to be transformed into one pharmaceutical product in one administrable dose form) and the 70 Combined Terms (combinations of administrable dose forms and container). The PDFs pertaining only to the veterinary domain (n = 79), as well as the PDFS with the status of rejected (n = 23) or deprecated (n = 27) were excluded, leaving a final of 428 single current PDFs, for “human and veterinary use” in the analysis. These 428 PDFs with their definitions, descriptors, characteristics, and tags, were extracted from the EDQM Standard Terms database, using the dedicated API with the version of EDQM Standard Terms database [STD] of 2021-03-17 09:52:21. The data were stored in an MS excel file (see Supplementary File/List1-ST) and in a PostgreSQL relational database.
First, a quantitative and qualitative analysis of the EDQM Terminology with its PDFs for manufactured items and administrable dose forms for pharmaceutical products was conducted, together with an analysis of the textual definition; the two descriptors (state of matter, basic dose form) of the pharmaceutical dose form and of the administrable dose form, and the value sets of the 4 characteristics (Release characteristics, Transformation, Intended Site, Method of Administration). PDFs for which a transformation was needed or possible were identified and counted, as well as PDFs with more than one value for method of administration (multiplicity). Second, the congruence between the textual definition and the combined values of the characteristics was checked by the three authors separately. In case these were considered not in accordance with the textual definition, the PDF was discussed until consensus was reached: the combination of characteristics was compatible with definition; contained less elements than in the definition, or more elements than in the definition. Third, a critical analysis was performed, with identification of potential problems or targets for improvement, with the formulation of potential approaches to remediation. A new characteristic was created, to indicate whether or not the PDF was intended for systemic effect. Consequently, a new MS Excel worksheet and PostgreSQL database was constructed with the proposals for remediation implemented. For every line of the 428 PDFs extra columns for administrable dose forms were added, to make the administrable dose form (and its state of matter and basic dose) explicit, in case of transformation (see Supplementary File/List5-basicFile). A first check of the appropriateness of the proposed changes was made by a clinical pharmacologist (RVS) and an EDQM expert (CJ, see acknowledgements). Fourth, an analysis was performed of different sets of the four descriptors (state of matter and basic dose form for PDF and for ADF) and the four characteristics of the PDFs. PDFs with identical values of descriptors and characteristics were grouped. Four different sets of descriptors and characteristics were analysed. These unique combinations, constructed mechanically by selecting different combinations of the characteristics, were then analysed qualitatively for internal consistency and relevance for granularity. Classes were split in case of PDFs with different rules for strength expression. Classes were split to obtain classes which were homogeneous with regard to systemic effect. Some unique combinations were concatenated in a new class if no clinical incentive for separation was present. This ontology-generating process was performed independently by two researchers (RVS and JR), and in case of divergence, discussed until consensus was reached.
Finally, the data in the new file and the results of the ontology construction were then exported to WebProtégé, an open-source ontology manager from Stanford University, Palo Alto, CA, USA [18], with semantic implementation of all the relationships between PDFs, descriptors, characteristics, tags, and ontological class relation.
3. Results
3.1. Describing EDQM as It Is
The 428 Pharmaceutical dose forms were extracted (see Supplementary File/List1-ST). Each Pharmaceutical dose form has a term and a code, a textual definition and a series of structured semantic relationships:
Each Pharmaceutical dose form has two descriptors: State of matter (4 values in the value set: gas; liquid; semi-solid; solid) and a Basic dose form (51 values in the value set: example: capsule; tablet; powder; solution), which are simplified descriptions, allowing crude aggregation of more specific dose form descriptions.
Each Pharmaceutical Dose Form has four characteristics, each with their value set and coding:
- Release characteristics (four values): conventional, prolonged, delayed, modified;
- Transformation (six values): dilution, dissolution, dispersion, mixing, no transformation, unknown;
- Intended Site (25 values): example: auricular; ocular; oral (see Supplementary File for full list);
- Administration method (19 values): example: application; inhalation; injection.
For the full value sets of the descriptors and characteristics, see Supplementary File/List2-IndexEDQM. The ontological relationships between PDF and descriptors and characteristics are given in Figure 1.
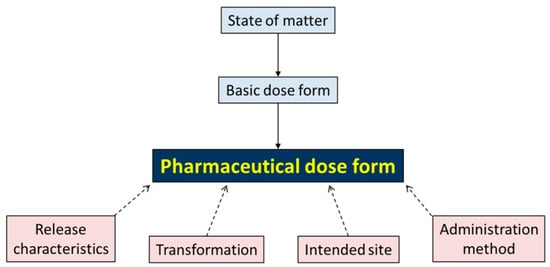
Figure 1.
Hierarchy of the pharmaceutical dose form, arranged according to the state of matter and basic dose form, and further characterised by release characteristics, transformation, intended site, and administration method.
Standard Terms are available in 34 languages: Albanian, Bosnian, Bulgarian, Chinese, Croatian, Czech, Danish, Dutch, English, Estonian, Finnish, French, German, Greek, Hungarian, Icelandic, Italian, Kazakh, Latvian, Lithuanian, Macedonian, Maltese, Norwegian, Polish, Portuguese, Romanian, Russian, Serbian, Slovak, Slovene, Spanish, Swedish, Turkish and Ukrainian.
Pharmaceutical Dose form is an umbrella concept for the manufactured dose form of the manufactured item (the dose form in which the medicinal product is shipped in the distribution from industrial manufacturing company to community and hospital pharmacies) and for the administrable dose form of the Pharmaceutical Product (the dose form in which the medicinal product is administered to the patient, after a process of transformation). All Pharmaceutical Dose Forms that are not subject to transformation and, hence, suitable to be administered as such, are tagged with “AdmDF”, but without specifying explicitly what the resulting administrable dose form(s) will be, or linking to a code of PDFs that are administrable.
In Figure 2, the transformative relationship between manufactured item and pharmaceutical item is given with an example from EDQM.

Figure 2.
Relationship between manufactured item and pharmaceutical product, and the use of pharmaceutical dose forms as the manufactured dose form.
Among the 428 PDFs in EDQM, 295 were labelled with the tag “admDF” (administrable Dose Form), which means that the dose form in which the medicinal product is commercialised requires no transformation to be administered to the patient and, hence, that the manufactured dose form and administrable dose form are one and the same. The other 143 (see Supplementary File/List3-notAdmDF) required at least one transformation. Of those 143, 122 only needed one transformation. In the 23 remaining PDFs, more than one transformation was required or possible; in 12 of them, “no transformation” was one of the possibilities; in 11 of the 23 PDFs with multiple transformation, transformations were consecutive and led to only one possible ADF (e.g., powder for concentrate for dispersion for injection); to vapours and sprays, and resulting in a unclear administrable dose form (see Supplementary File/List4-TransMulti).
3.2. Potential Problems Identified and Approaches to Improvements
For PDFs affected by multiplicity (more than one value possible of a given characteristic), the value set needed to be extended in relational databases with the combination of those specific values and a new code number for this combination needed to be created. The coding procedures of EDQM may not be very robust [18,19] and do not reflect the ISO/CEN standards for coding systems [20,21,22] or value set specifications [23].
Because the requirements for the creation of global identification of medicinal products (as outlined in the ISO/CEN IDMP standard) stipulate that the administrable dose form needs to be used, it was imperative to make explicit the Administrable Dose Form (ADF) for each PDF with transformation. This resulted, often, in a different state of matter and basic dose form for ADF. As mentioned above, this was sometimes problematic, e.g., when multiple transformation processes were possible. This will need remediation with input from experts from EDQM.
While in the description of the PDF, a clear distinction is made between injection, infusion, and injection/infusion, the value for the intended site characteristic is “parenteral”. This might be better synchronised.
One of the values for intended site is “cutaneous/transdermal”, a concatenation of local and systemic effects, present in 48 PDFs. When looking to the textual definition of these PDFs, it was almost always possible to make a clear distinction between either cutaneous or transdermal. Sublingual dose forms (labelled as such in the PDF term and in the definition) were given the value of “oromucosal” for the characteristic “intended site”, amalgamating them with PDFs with only local effect. Further analysis of the textual definitions made it clear that in more than 95% of the PDFs, it is possible to make the distinction between a dose form intended for local effect and a dose form intended for systemic effect. In a few instances, the ambiguity was inherent to the definition and some dose forms in nasal, rectal and pulmonal dose forms can be either local or systemic. A thorough revision of the definitions and characteristics of the affected PDFs might clarify this issue and make it possible to add an extra characteristic, “systemic/local effect” (with three values, “systemic”, “local”, and “systemic/local”).
The textual analysis also revealed that sometimes elements of the definition were not reflected in the characteristics (e.g., soft capsule and hard capsule).
The coding system of the values and terms appears to be weak, needs adaptation for the newly created values, and could contain an indication of the generating system (EDQM).
3.3. Construction of a New Basic File
As an experiment, these proposed improvements were implemented and a basic file (see Supplementary File/List5-basicFilel) was created of 428 PDFs, with 4 additional columns (ADF, ADF_StateofMatter, ADF_basicdoseform, and Systemic/local). All possible combinations of values of characteristics were concatenated to a new combination value (with new code): TRA → TRAC, AME → AMEC. The values of intended site were changed for “cutaneous/transdermal”, whenever possible (ISI → ISIC-Split,), and for “oromucosal” to “sublingual”, when the PDF was a sublingual dose form (see Supplementary File/List6-IndexModified).
3.4. Construction of the Small Ontology
First, the new basic file was analysed by looking at PDFs with identical combinations of the values for each of their descriptors and characteristics. This analysis was repeated with different sets of descriptors and characteristics. In Table 1, the results in terms of number of unique combinations are given for the different sets of descriptors and characteristics, including the new characteristic SYS for systemic effect.

Table 1.
Analysis of unique combinations in different sets of descriptors and characteristics of EDQM dose forms.
When all descriptors and characteristics were taken into account (Analysis 1), 383 unique combinations were observed. This indicates that the descriptors and characteristics are not completely definitional (given the 428 dose forms in the EDQM terminology; see Supplementary File/List7-Analysis1). Analysis 2 (357 unique combinations) may reflect a granularity that is closer to the granularity of Snomed-CT, although Snomed-CT focusses on manufactured dose forms, rather than on administrable dose forms (see Supplementary File/List8/Analysis2). Analysis 3 (based on only the four characteristics) results in a much lower granularity (206 unique combinations), unsuitable for granular identification of dose forms, but used for the further elaboration of the ontology (see Supplementary File/list7-Analysis-1; see Supplementary File/List9-Analysis3). Analysis 4 (195 unique combinations) was based only on the basic dose form of the administrable dose form and three characteristics (release characteristics, intended site, method of administration; see Supplementary File/List10-Analysis4). In analysis 4bis, the same set is used as in analysis 4, without the new variable SYS, hence, only using basic dose form of the administrable dose form, RC, ISIC-split, and AMEC. This mimics the approach used in the FDA/WHO_UMC pilot project to explore the generation of a global identification number [7,9], and resulted in a granularity closer to the granularity of RxNorm (see Supplementary File/List11-Analysis4bis).
The four analyses were repeated, now taking into account the newly created characteristic “systemic/local”, resulting in a slightly higher granularity and a slightly lower level of aggregation, but more clinically relevant collections of PDFs (see Table 1 and Supplementary file/list7-Analysis-1 to List10-Analysis-4).
Next, the basic new file was ordered by identical combinations of values of the four characteristics (the result of Analysis 3), rearranged by intended site, putting the combinations without transformation first, followed by the combinations with transformation (see Supplementary File/List12-Reorg).
For each collection of PDFs with identical combinations, a qualitative evaluation was conducted with regard to the need to further split the collection to create homogeneous classes, with regard to systemic effect or to concatenate collections with another similar collection or collections (to create relevant new classes). These decisions were made based on a lack of clinical relevance to keep them apart, or on the need to separate dose forms with a different expression of strength or effect on the body (systemic/local). Further, the unique combinations with only one occurrence were examined for possible concatenation. Finally, labels were discussed for the retained similar collections of PDFs. This resulted in a simple three-level ontology (merely a taxonomy), with a first level of 22 slightly amended values of “intended site”, and a second level of 69 newly created terms for the collections with similar combinations, and the third level, the 428 granular EDQM PDFs (see Table 2 for the first two levels of the ontology and Supplementary File/List13—Ontology for the three levels). As a final result, this was implemented as an ontology in WebProtégé [24], freely accessible after login procedure (available from the authors) at (https://webprotege.stanford.edu/#projects/a745d3b9-1943-481c-b309-6c920b8385fb/edit/Classes (accessed on 28 January 2022).

Table 2.
Proposal for small ontology of dose form terminology.
3.5. Recommendations
In Table 3, the recommendations for changes in the EDQM dose form Terminology are listed.

Table 3.
Recommendations for EDQM dose form terminology as a global terminology within the implementation of global identification of medicinal products (IDMP), as proposed in the UNICOM project.
4. Discussion
This analysis of the EDQM dose form Terminology was initiated as part of the UNICOM project [15], in preparation for the formulation of procedures to produce global identification numbers for medicinal products, named the pharmaceutical product identifier (PhPID). This procedure must be based on strict rules to define the substance (and its code), the administrable dose form (and its code), and the representation of strength (value and unit of nominator and denominator). As for the PhPID, the focus is on the administrable dose form, this element needed to be made more explicit within the EDQM Terminology.
The strength of this analysis is the focus on what is needed for the implementation of the global identification of medicinal products (IDMP). Its limitation is that the finalising and adoption of the results needs corroboration by internal experts of the European Directorate for the Quality of Medicines and Healthcare (EDQM). The proposal to include the effect on the body in the description of the dose form in terms of local or systemic action is important for allowing automated determination of polypharmacy in drug utilisation dispensing data, which lack posology and route of administration information.
The analysis was made at a time when the ISO/CEN standard for dose form is under revision, and the European Directorate for the Quality of Medicines and Healthcare must decide whether it will accept the challenge of becoming a global terminology, as preconised by the International Council of Harmonisation. The current results are intended to inform these revision processes and contribute to the resolution of remaining controversies in the implementation of IDMP in Europe and globally.
The use of EDQM standard terms is already adopted to some extent in the regulatory sphere, but not always in an interoperable way. This terminology is complex and needs expertise to be correctly implemented. Research has shown that inter-rater variability when assigning Standard Terms for dose forms to national medicinal products can be high, especially when the rating is performed by inexperienced agents [25].
The development of the simple ontology of dose forms may contribute to the production of tools and didactic efforts to enhance the expertise of standardisation in regulatory agencies. It may also contribute to the semantic interoperability with other terminologies, such as SNOMED-CT and RxNorm [26,27].
The evolution of European EDQM terminology towards a global terminology will need a governance process, which assures the coordination of IDMP implementation worldwide [28].
5. Conclusions
EDQM Dose Form terminology is the most suitable terminology, in terms of granularity, for defining dose forms of medicinal products, to enable fair comparison of similar medicinal products. A number of minor improvements and a simple ontology for dose forms are proposed. Recommendations are made to inform the processes of the current revision ISO/CEN standard for dose form and of the transition of EDQM from a European to a global terminology.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app12094337/s1: annex_EDQM_Main_article_v1_Feb3.xlsx.
Author Contributions
Conceptualization, R.H.V.S.; methodology, R.H.V.S., J.R., D.v.N.; software, J.R., D.v.N.; validation, R.H.V.S., J.R.; formal analysis, R.H.V.S., J.R., D.v.N.; writing—review and editing, R.H.V.S., J.R.; supervision, R.H.V.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Union’s Horizon 2020 Action Programme under Grant number 875299, and by StandICT.EU (Supporting European Experts Presence in International Standardization Activities in ICT) for Joseph Roumier.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are in the Supplementary File.
Acknowledgments
We thank Christopher Jarvis from the European Directorate for Quality in Medicines and Healthcare, Strasbourg, France, for his assistance in the revision of our proposed minor changes and choices of administrable dose forms, resulting from transformation processes. We thank Dipak Kalra, Christian Hay, and Robert Stegwee for their continued support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- IDMP in a Capsule. Available online: https://unicom-project.eu/wp-content/uploads/2021/10/UNICOM-handboek_A4_04.pdf (accessed on 28 January 2022).
- ISO 11616:2012; Health Informatics—Identification of Medicinal Products—Data Elements and Structures for the Unique Identification and Exchange of Regulated Pharmaceutical Product Information. Available online: https://www.iso.org/standard/55035.html (accessed on 28 January 2022).
- ISO 11239:2012; Health Informatics—Identification of Medicinal Products—Data Elements and Structures for the Unique Identification and Exchange of Regulated Information on Pharmaceutical Dose Forms, Units of Presentation, Routes of Administration and Packaging. Available online: https://www.iso.org/standard/55032.html (accessed on 28 January 2022).
- EDQM Standard Terms. Introduction and Guidance for Use (Version 2.1.3—16 November 2018). Available online: https://www.edqm.eu/sites/default/files/standard_terms_introduction_and_guidance_for_use.pdf (accessed on 28 January 2022).
- Available online: https://www.fda.gov/industry/fda-resources-data-standards/identification-medicinal-products-idmp (accessed on 28 January 2022).
- Available online: https://unicom-project.eu/wp-content/uploads/2021/11/Substance-and-Strenght_Oct.pdf (accessed on 28 January 2022).
- Available online: https://www.fda.gov/drugs/news-events-human-drugs/identification-medicinal-products-path-global-implementation-06112021-06112021 (accessed on 28 January 2022).
- Available online: https://www.ema.europa.eu/en/documents/presentation/presentation-report-who-workshop-idmp_en.pdf (accessed on 28 January 2022).
- Available online: https://unicom-project.eu/pharmaceutical-product-identifier-phpid-is-taking-ground-under-the-leadership-of-who-umc/ (accessed on 28 January 2022).
- EMA Products Management Services—Implementation of International Organization for Standardization (ISO) Standards for the Identification of Medicinal Products (IDMP) in Europe. Introduction—EU Implementation Guide Version 2.1. Available online: https://ema.europa.eu/en/documents/regulatory-procedural-guideline/products-management-services-implementation-international-organization-standardization-iso-standards_en.pdf (accessed on 28 January 2022).
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH); ICH E2B(R3) Expert Working Group/Implementation Working Group. Information Paper Regarding the Use of ISO IDMP Standard in E2B(R3) Messages (Final Version, Approved on 17 June 2021). Available online: https://admin.ich.org/sites/default/files/inline-files/ICH_E2B%28R3%29_EWGIWG_Information_Paper_IDMP_Use_in_E2B%28R3%29_Messages_Final_2021_0617_0_1.pdf (accessed on 28 January 2022).
- Available online: https://www.nlm.nih.gov/research/umls/rxnorm/docs/appendix2.html (accessed on 28 January 2022).
- Available online: https://confluence.ihtsdotools.org/attachments/SNOMED_CT_Medicinal_Product_Model_Specification (accessed on 28 January 2022).
- Revised IDMP Standards to Improve Description of Medicinal Products Worldwide. Available online: https://www.iso.org/news/ref2234.html (accessed on 28 January 2022).
- Available online: https://unicom-project.eu/ (accessed on 28 January 2022).
- Standard Terms Database | EDQM—European Directorate for the Quality of Medicines. Available online: https://www.edqm.eu/en/standard-terms-database (accessed on 28 January 2022).
- EDQM Standard Terms. Internal Controlled Vocabularies for Pharmaceutical Dose Forms (Version 1.2.0—28 January 2019). Available online: https://www.edqm.eu/sites/default/files/standard_terms_internal_vocabularies_for_pharmaceutical_dose_forms.pdf (accessed on 28 January 2022).
- Cimino, J.J. Desiderata for controlled medical vocabularies in the twenty-first century. Methods Inf. Med. 1998, 37, 394–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Keizer, N.F.; Abu-Hanna, A.; Zwetsloot-Schonk, J.H. Understanding terminological systems. I: Terminology and typology. Methods Inf. Med. 2000, 39, 16–21. [Google Scholar] [PubMed] [Green Version]
- ISO 17117-1:2018; Health Informatics—Terminological Resources—Part 1: Characteristics. Available online: https://www.iso.org/standard/61979.html (accessed on 28 January 2022).
- ISO/TS 17117-2; Health Informatics—Terminological Resources—Part 2: Implementation Capability (TIC). Available online: https://www.iso.org/standard/76617.htm (accessed on 28 January 2022).
- ISO/TS 21526:2019; Health Informatics—Metadata Repository Requirements (MetaRep). Available online: https://www.iso.org/standard/71041.html (accessed on 28 January 2022).
- HL7 Specification: Characteristics of a Value Set Definition, Release 1. Available online: http://www.hl7.org/implement/standards/product_brief.cfm?product_id=437 (accessed on 28 January 2022).
- Horridge, M.; Tudorache, T.; Nuylas, C.; Vendetti, J.; Noy, N.F.; Musen, M.A. WebProtégé: A collaborative Web-based platform for editing biomedical ontologies. Bioinformatics 2014, 30, 2384–2385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sass, J.; Becker, K.; Ludmann, D.; Pantazoglou, E.; Dewenter, H.; Thun, S. Intercoder Reliability of Mapping Between Pharmaceutical Dose Forms in the German Medication Plan and EDQM Standard Terms. Stud. Health Technol. Inform. 2018, 247, 845–849. [Google Scholar] [PubMed]
- Mapping Guidance for EDQM to SNOMED CT Pharmaceutical Dose Form Mapping. Available online: https://confluence.ihtsdotools.org/display/USRG/Mapping+Guidance+for+EDQM+to+SNOMED+CT+Pharmaceutical+Dose+Form+Mapping (accessed on 28 January 2022).
- Karapetian, N.; Vander Stichele, R.H.; Quintana, Y. Evaluating the Interoperability of Two Standard Terminologies for Dosage Form: RxNorm from the National Library of Medicine for the United States and EDQM from the European Directorate for the Quality in Medicines and Healthcare for Europe. SSRN. 2022. submitted. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4017191 (accessed on 12 February 2022). [CrossRef]
- Unicom Community of Expertise Webinar on 4 February 2022. Available online: https://unicom-project.eu/all-community-of-expertise-webinars-in-a-nutshell/ (accessed on 12 February 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).