Abstract
The European eel (Anguilla anguilla, Linnaeus 1758) is a catadromous fish with significant cultural, scientific, and commercial value. The protection of this species is particularly difficult because the biology of the eel life cycle remains unknown in many aspects. The European eel (A. anguilla) population has declined alarmingly over the past 30 years; this condition has led to questions about the long-term welfare of this species. This work aims to perform a histological analysis on gonad samples from European eels coming from four different lagoons of the North Adriatic at different stages of silvering, and to evaluate the maturation of the gonads. For this study, a total of 859 wild eels were captured from four different lagoons using the “lavoriero”. Subsequently, the biometric parameters were collected. Seventy-nine female eels were randomly selected, dissected, and the gonads were removed for histological analysis. Sections of 4 µm were cut and stained with hematoxylin and eosin (H&E). Histological observations of germ cells at the light microscopy level allowed for the characterization of six steps of oocyte maturation. Valle di Comacchio had the highest levels of oocyte maturation, while Valle Ca’ Pasta had the lowest. Eels with silver index III had an oocyte maturation nearly equal to that of eels at stages IV and V of silvering. Considering the results, we can affirm that eels from North Adriatic lagoons have high oocyte maturation levels and high GSI value indices at silvering stage III. The following experimental work shows that the levels of oocyte maturation are higher even at lower silver index levels. It can be hypothesized that in these eels, the transition from yellow to silver eel occurs faster in relation to the high trophic availability in North Adriatic lagoons.
1. Introduction
Freshwater eels, genus Anguilla, are distributed widely across much of the globe, from tropical to temperate regions, and they consist of 19 species and subspecies [1,2]. Most studies in recent years have focused on eels of temperate habitats such as the Japanese eel (Anguilla japonica), the European eel (A. anguilla), and the American eel (A. rostrata), which are distributed in regions found between mid and high latitudes [3]. The members of the Anguilla genus are catadromous fish with an oceanic and continental phase. One of the gaps in knowledge concerning the eel life cycle relates to the oceanic phase as it is challenging to observe them during their marine life stages. The life history of this fish depends strongly on oceanic conditions; maturation, migration, spawning, larval transport, and recruitment dynamics are completed in the open ocean [4]. The European eel is distributed from North Cape in Northern Norway, southwards along the coast of Europe, all shores of the Mediterranean, and on the North African Coast [5,6]. The available information indicates that the stock has severely declined in most of its distribution area, and the population has decreased dramatically since the 1980s [7,8]. The European eel is now classified as “critically endangered” according to the International Union for Conservation of Nature (IUCN). Several conservation actions have been applied over the years: in 2007, the European Union adopted a management plan to recover the European eel stock through an EU regulation (EU 1100/2007). In 2010, another measure adopted was the prohibition of the trading of glass eels outside the European Union, and since 2018, 3-month fishing closures have been implemented at the EU level. In the last stage of life in freshwater, eels undergo several morphological and physiological changes to adapt to the long migration [9,10]. These changes are thought to be closely related to gonadal development [4,9,11,12]. Downstream migration is a specific life-history event for eels that marks the end of the passive growth phase (yellow stage) and the beginning of the migratory phase (silver stage). As eels grow and prepare for the long migration, it is of key importance that they accumulate a large amount of lipids, according to Boëtius and Boétius [13]. Optimal trophic and environmental conditions allow for favorable lipid accumulation that is necessary for gonad development and help eels to reach sexual maturation and successfully complete migration [14]. According to this, the process of silvering is also more flexible than generally presumed [14] and can be influenced by several trophic and environmental factors [15,16,17,18]. Information on the gonadal stage and morphological indices are necessary for a better selection and the release of sexually mature individuals that are ready to migrate, as requested by the EU directive. In addition, they can provide guidelines on how to regulate the various factors that influence maturation [19] and thus be of great support for artificial reproduction, which has been showing encouraging results in the last decades [20,21,22]. Over the years, various methods for assessing eel maturity have been developed; in European eels, one of the methods is based only on the change of patterning, and it is used to differentiate the two stages of the yellow and the silver eel, as the “migratory” eels often show a white-silver belly and a black dorsal back. However, most migratory eels often show intermediate characteristics, such as bronze belly and sides. Thus, the color criterion is subjective and unreliable [23].
Pankhurst [9] proposed another method for distinguishing yellow and silver female eels. The author developed an index based on eye surface area, which increases at the migratory stage, and set a threshold of 6.5 for sexually maturing European eels. Another non-invasive method is the silver index, developed by Durif et al. [15,24,25], which divides the maturation process of both female and male adult eels, relying only on external morphological parameters that mainly concern the length and weight of the animals and some characteristics of the eye and the pectoral fin. Five stages were defined for female eels: a growth phase (stages FI and FII), during which eels become sexually differentiated, and a pre-migrating stage (FIII), characterized by the beginning of gonadal development in females. The migratory stage is divided into two groups: stage FIV and stage FV, characterized by a significantly regressed digestive tract, higher gonadotropin levels, and elongated pectoral fins.
Another technique is gonadosomatic index (GSI) measurement, which is an invasive method requiring the killing of eels, often used to compare the reproductive status between individuals or between different groups of individuals [26]; it has been widely used to describe the timing and duration of the breeding season [27]. Several studies have successfully used GSI values to improve accuracy in determining the maturity stage [28,29,30,31]. This technique allows for the identification of mature and immature animals. However, the correlation between morphological changes and gonadal development is still not clear [14]. Thus, integrating morphological and histological analyses might be valuable for a more precise definition of the maturation degree. Histological evaluation is based on the study of cellular structures within the ovaries or testes, proving to be a much more accurate technique for the assessment of gonadal maturation. However, histology is expensive, time-consuming, and requires specialized laboratory equipment.
Italian eel production is characterized by an extensively managed eel population in coastal lagoons, half of them being in the North Adriatic area. In this area, European eels are reared extensively via the traditional Vallicoltura [22,32,33], carried out in the Valli, a sector of a lagoon or enclosed earthen pond [33].
This study aims to perform a histological analysis on samples of European eel gonads from several lagoons of the Po River Delta at different stages of silvering, and to evaluate gonad maturation. This will increase the knowledge on these North Adriatic populations, allowing for the development of a maturation assessment protocol that is as accurate as possible.
2. Materials and Methods
2.1. Sampling of Eels
A total of 854 European eels were caught using the “lavoriero”, a traditional V-shaped weir that captures eels as they begin to move from the lagoon into the open sea. The eels were handled in accordance with the regulations concerning the protection of experimental animals (Dir. 86/609/EEC) and the Ethic Committee of Bologna University regulation (prot. 575/2016). Samplings of A. anguilla were performed in four different lagoons during the autumn/winter period of 2020. More specifically: Valle di Comacchio (44°36′20″ N 12°10′11″ E—10,000 ha, brackish waters), 320 eels; Valle Nuova (44°48′12″ N 12°15′16″ E—200 ha, saltwater), 254 eels; Valle Ca’ Pasta (44°58′07.7″ N 12°19′21.3″ E—240 ha, freshwater), 112 eels; Valle San Carlo (44°59′51.0″ N 12°27′21.3″ E—460 ha, saltwater), 168 eels (Figure 1).
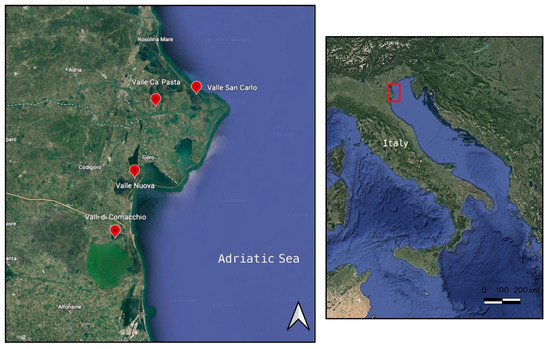
Figure 1.
Map of the sampling areas in lagoons of the Northern Adriatic Sea. The map shows the four lagoons used for sampling: Valle di Comacchio (44°36′20″ N 12°10′11″ E), Valle Nuova (44°48′12″ N 12°15′16″ E), Valle Ca’ Pasta (44°58′07.7″ N 12°19′21.3″ E), Valle San Carlo (44°59′51.0″ N 12°27′21.3″ E).
All the caught eels were anesthetized in a 1:10 solution of carnation oil dissolved in 70% ethanol directly in the field and were subjected to the detection of morphometric parameters: body length (BL—cm), body weight (BW—g), horizontal eye diameter (Edh—mm), vertical eye diameter (Edv—mm), and pectoral fin length (PFL—mm). Then, the Fulton condition factor (K), eye index (EI), and pectoral fin length index (PFLI) were calculated according to the formulae below.
Fulton condition factor (K):
Eye index (EI):
Pectoral fin length index (PFLI):
The initial stage of eels relative to the silvering process (silver index, SI) was determined according to the classification system described by Durif et al. [15], where stages FI and FII correspond to resident eels, FIII to pre-migrant eels, and FIV and FV to migrant eels.
2.2. Gonad Sampling
A total of 10% of eels in each lagoon, proportional to the silver index stage, were transported to the laboratories of the DIMEVET-Cesenatico. The eels were sacrificed with an overdose of 2-phenoxyethanol (800 ppm). The eels were dissected, and the gonads were removed. The gonads were weighed (GW), and the gonadosomatic index (GSI) was calculated as follows:
where GW is the Gonad weight (g), and BW is the Body weight (g).
Fragments of gonads were fixed in 10% formalin. They were then dehydrated in a solution with ethanol and then embedded in paraffin. Sections of 4 µm were cut and stained with hematoxylin and eosin (H&E) [28].
Histological sections were evaluated with a light microscope to estimate the maturation degree of the gonads according to Grier et al. [34], following the division shown in Table 1.

Table 1.
Division of oocyte development into three level: periods, stages, steps [34].
The diameters of 20 oocytes from each specimen were measured randomly, and the corresponding stage was established on the basis of the most advanced oocyte stage observed in the histological sections. All data from each slide were averaged to obtain a single value for each individual. Samples with oocyte maturation starting from the circumnuclear oil droplet (PGod) step, i.e., oocytes in which all lipid droplets are arranged around the germinal vesicle and have a diameter of around 70–80 µm, were considered mature enough. [23,35,36]. The distribution of oocyte maturation values in each lagoon was evaluated with the data obtained. The levels of oocyte maturation within each silver index stage were then represented with a histogram. Lastly, the relationship between oocyte maturation levels/silver index and IGS values was represented with boxplots.
2.3. Statistical Analysis
Normal distribution of data was first evaluated by applying the Shapiro–Wilk test. Given that all data were not normally distributed, non-parametric statistical tests were used. Boxplot figures between maturation level/silver index values and GSI values were made to analyze the trend of observations. A Chi-square test was performed between two categorical variables (i.e., silver index and maturation levels) to test the independence of the two variables. A Pearson’s correlation was conducted to study the correlation between GSI values and the levels of oocyte maturation. All tests were carried out using the software R version 4.1.0 (18 May 2021). A p-value < 0.05 was considered to indicate statistical significance in all tests.
3. Results
3.1. Measurements
Regarding the silver index values, the number of resident eels (FII) in the four lagoons was low, with a higher percentage for Valle Nuova (Table 2). Valle Ca’ Pasta had the highest percentage of pre-migrant eels, followed by Valle Nuova and Valle San Carlo, and the percentage of migrant eels (FIV-FV) in Valle Comacchio was more significant than in other lagoons. The percentage of migrant eels at the FIV stage tended to be low, and in Valle Nuova, there was an absence of migrants at the FIV stage and a lower number of eels at the FV stage as compared to eels at the FIII stage (Table 2). From the analysis of morphometric parameters, the values of BW, BL, and K showed an increasing trend from resident eels and pre-migrants to migrants (FIV). The same general trend was observed in gonadal development (GSI) (Table 2). As regards migrant eels (FIV and FV), those with the largest size and most favorable K-factor for the weight parameter were females from Valle di Comacchio. In contrast, the migrating eels with the highest GSI were FIV females from the San Carlo lagoon.

Table 2.
Morphometric parameters and silvering indices of the four eel populations. Data are reported as mean ± s.d.
Finally, there was a strong reduction in size and K-factor in the transition of the eels from FIV to FV, and partly (Valle Ca’ Pasta and San Carlo) in GSI.
3.2. Histological Analysis
Histological observations of germ cells allowed for the characterization of six steps of oocyte maturation—five steps in primary growth and one in secondary growth (Figure 2):
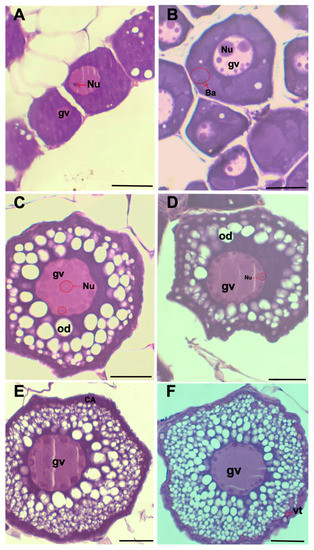
Figure 2.
(A) One-nucleoli stage. (B) Multiple nucleoli stage. (C) Perinucleolar stage. (D) Circumnuclear oil droplets (stage IV of pre-vitellogenesis). (E) Cortical alveolar (stage V of pre-vitellogenesi). (F) Early secondary growth (oocyte in secondary growth with vitellogenin granules). Germinal vesicle (gv). Nucleoli (Nu). Oil droplet (od). Cortical alveoli (CA). Vitellogenin (vt). Scale bar: 20 μm.
- -
- One-nucleoli (PGon): characterized by only one nucleolus inside the germinal vesicle, the absence of lipid droplets, and a reduced oocyte diameter of about 32.5 μm;
- -
- Multiple nucleoli (PGmn): characterized by multiple nucleoli inside the germinal vesicle arranged randomly. There is also the presence of globular masses called Balbiani’s bodies within the cytoplasm;
- -
- Perinucleolar (PGpn): characterized by the presence of the first lipid droplets and the increase of the oocyte diameter to around 60 μm;
- -
- Circumnuclear oil droplet (PGod): characterized both by the position of nucleoli that is arranged peripherally and by the increase of lipid droplets around the germinal vesicle. The oocyte diameter is around 75 μm;
- -
- The cortical stage (PGca) is characterized by an increase in lipid droplets arranged around the germinal vesicle and the presence of cortical alveoli in the periphery. The oocyte diameter is around 80 μm;
- -
- Early secondary growth (SGe): this stage is characterized by the presence of yolk at the periphery of the oocyte. The oocyte diameter is around 95 μm.
In Figure 3, the distribution of maturation levels within the four considered lagoons is shown. Valle di Comacchio has the highest maturation levels, with higher oocyte maturation in 74% of the cases, followed by Valle Nuova with 69%, Valle San Carlo with 62%, and finally, Valle Ca’ Pasta with 45%. In Valle di Comacchio, a greater variability was found for the various levels of oocyte maturation and very low levels of maturation, but the predominant level was PGod. On the other hand, Valle Nuova had high levels of maturation with a prevalence of PGca. In Valle Ca’ Pasta, 55% of the samples had a low level of maturation, and PGpn predominated. Finally, in Valle San Carlo and Valle di Comacchio, the situation was more heterogeneous, with greater variability.
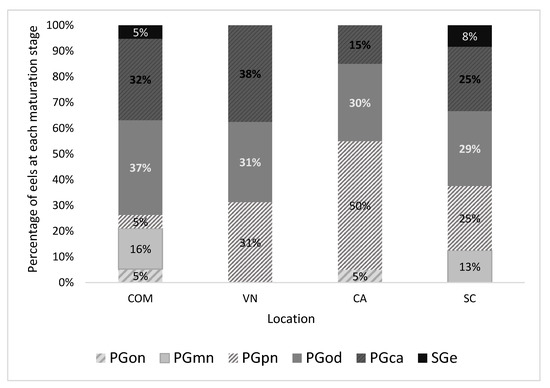
Figure 3.
Distribution of the maturation stages of sampled eels in each lagoon. COM = Valle di Comacchio; VN = Valle Nuova; CA = Valle Ca’ Pasta; SC = Valle San Carlo. PGon = one-nucleoli stage, PGmn = multiple nucleoli stage, PGpn = perinuclear stage, PGod = circumnuclear oil droplet stage, PGca = cortical stage, SGe = early secondary growth stage.
The chi-square test showed a significant relationship between the two categorical variables of silver index and maturation stages (p < 0.023).
As shown in Figure 4, the levels of oocyte maturation vary within the various stages of the silvering index. At silvering stage FII, 50% of oocytes were at PGon and 50% at PGmn. At silvering stage FIII, the situation was heterogeneous, with maturation values ranging from PGmn to SGe and 61% of oocytes showing a degree of maturation equal to or higher than PGod. At silvering stage FIV, the levels of oocyte maturation increased and ranged from PGod (50%) to SGe. Finally, at silvering stage FV, only the maturation stages of PGpn (67%) and PGca (33%) were present.
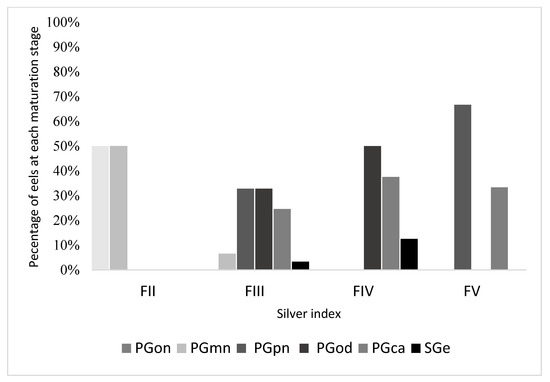
Figure 4.
Percentage of eels at each oocyte maturation stage, at different stages of silvering of the sampled eels. PGon = one-nucleoli stage, PGmn = multiple nucleoli stage, PGpn = perinuclear stage, PGod = circumnuclear oil droplet stage, PGca = cortical stage, SGe = early secondary growth stage.
In Figure 5, the increase in GSI values depending on oocyte maturation is illustrated. Higher variability can be found in the stage of maturation of secondary growth. There are outliers for the stages of PGpn and PGca, but the average values of each maturation level increase with increasing GSI.
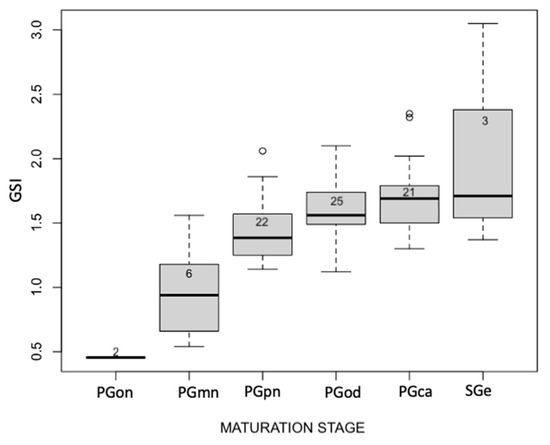
Figure 5.
Boxplot shows the relation between the oocyte maturation stage and the GSI values of sampled eels.
An analysis of the correlation between the maturation stages and the GSI values shows a significant correlation (p < 0.018) and a positive correlation index of 0.65. As shown in Figure 6, the GSI values increase with the silver index. However, the observations at stage V have lower GSI values than stage IV.
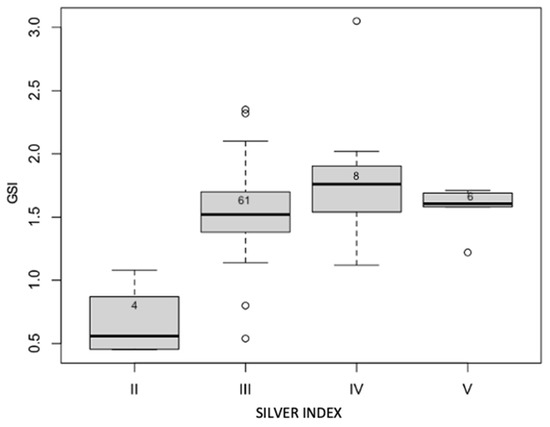
Figure 6.
Relation between silvering stages and GSI values of sampled eels.
4. Discussion
The objective of this study was to evaluate the gonadal maturation stage of European eels at various stages of silvering in four lagoons of the Po River Delta. Generally speaking, the results showed a strong presence of silver eels (SI III–V) in the populations surveyed (>94.5%): the percentage of migrating eels with silver index IV–V was highest in Valle di Comacchio, followed by Valle San Carlo, Valle Nuova, and finally, Valle Ca’ Pasta, with 20% of eels ready to migrate. This difference between the lagoons can be explained by the different environmental and trophic conditions [25,32,37,38] found in each valley despite their proximity and belonging to the same geographical area. De Leo and Gatto [39], for example, have shown that Valle di Comacchio, characterized by strongly brackish waters, has an incredibly large and abundant spectrum of prey (mainly clupeids and engraulids), therefore eels can grow well because there are favorable environmental conditions, not only for growth but probably for gonadal development as well. Likewise, Acou et al. [40] demonstrated that brackish waters such as the Vaccarès lagoon (France) were more favorable growth sites than freshwater environments. This could explain why the eels from Valle Ca’ Pasta, the only freshwater eels, had the lowest GSI values. The results also confirm the hypothesis of Svedäng et al. [14], i.e., environmental properties could lead to large differences in lipid storage metabolism, which is crucial for coping with migration and promoting gonadal maturation [22,41].
Concerning the results obtained on the levels of oocyte maturation, it was seen that the number of mature eels in the four considered lagoons tended to be high. Valle di Comacchio had the highest levels of oocyte maturation, while Valle Ca’ Pasta had the lowest. It should be underlined that eels with silver index FIII had an oocyte maturation nearly equal to that of eels at stages FIV and FV of silvering. Therefore, eels that the Durif et al. [15] classification considered pre-migratory presented a similar oocyte maturation level to that of eels considered to be migratory. Furthermore, a strong, positive correlation was observed between GSI values and maturation levels, thus strengthening histological observation.
Many studies have been conducted on eels from different environments, and all of them show how the status of morphological and histological characteristics at sampling change from area to area [22,25]. Having histological data that confirm high oocyte maturation for eels considered to be pre-migratory is of paramount importance because it allows us to have a deeper understanding of the characteristics of the North Adriatic Italian eel populations. These lagoons are confirmed as favorable environments for the growth of eels; the same study of Durif et al. [15] argued that the process of silvering is conditioned by various environmental factors that play a key role in this process; in fact, favorable growth conditions make the eels’ second metamorphosis quicker [39,42,43] The hypothesis that North Adriatic lagoons are suitable habitats for gonadal growth and maturation is further strengthened by Mordenti et al. (unpublished), who showed that eels at silvering stage FIII are very young eels and superimposed on the age of eels classified as migrants (48% FIII age 7–7+; 50% FIV age 7–7+). Considering the results, we can affirm that eels from North Adriatic lagoons are eels with high levels of oocyte maturation accompanied by high indices of GSI values already at silver stage FIII (pre-migrant). In this regard, considering and comparing the Atlantic eels—on which the silver index protocol has been developed—with those of the North Adriatic, it can be observed that North Adriatic populations appear not to mirror the gonadal maturation pattern of Atlantic eels. Demonstrating this, North Adriatic pre-migrants had GIS values nearly twice that of the Atlantic pre-migrant eels considered in the study by Durif et al. [15] (1.54 ± 0.30 vs. 0.82 ± 0.24, respectively).
Regarding eels at stage FV of silvering, data obtained show that some parameters, including BW, BL, GSI, and oocyte maturation values, were lower than those in eels at lesser stages of silvering. In this context, Svedäng and Wickstrom [14] hypothesized that silvering is a very flexible process, and that eels may stop metamorphosis if the chances of successful migration are compromised or if the so-called environmental window was lost, for example, due to the presence of dams that could stop or delay the run. In A. rostrata Hain [44], eels make several trial runs before finally leaving for the Sargasso Sea, and that migratory characteristic weakens after each false start until the next migratory season.
Additionally, the reduced presence of females at Stage V seems to be a characteristic of all North Adriatic lagoons. This has also been documented in other studies [22,45], which showed that only the presence of eels at Stage V could reach a maximum of 14% among silvering females. This can be viewed favorably as part of a release program, as the highest level of silver eels do not seem to have all the prerequisites to finish their migration. In contrast, eels at stage IV that present a high Fulton’s Condition Factor probably accumulated large amounts of fat that provided the animal with the necessary reserves to reach the Sargasso Sea. Finally, given the characteristics of pre-migrating eels investigated in the Po River Delta, it is possible to assume that female eels at stage FIII are ready to migrate. In support, studies on the artificial reproduction of eels in the Po River Delta [22] have shown that females at stage FIII and kept in captivity can reach full gonadal maturity until the release of eggs.
5. Conclusions
Obtained data show that the levels of oocyte maturation are high even at lower silver index levels. Indeed, eels from the Po River Delta are eels with high levels of oocyte maturation accompanied by high indices of GSI values already at silver stages FIII.
North Adriatic eel populations did not completely mirror the gonadal maturation pattern of Atlantic eels. It can be hypothesized that the transition from yellow to silver eel occurs very quickly in indagating populations due to the high trophic availability in North Adriatic lagoons.
The lagoons of the Po River Delta produce wild female eels with different morphometric parameters but high silvering values. They can successfully contribute to sea release programs and artificial reproduction.
Author Contributions
Conceptualization, O.M.; data curation: P.E., A.C., R.B., and L.G.; methodology, O.M., A.Z., P.E., A.C., R.B., and L.G.; formal analysis, A.Z.; investigation, P.E., A.C., R.B., and L.G.; writing—original draft preparation, L.G., O.M., and A.Z.; writing—review and editing, L.G., O.M., and A.Z.; supervision, O.M. and A.Z.; project administration, O.M.; funding acquisition, O.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the LIFEEL project (LIFE19 NAT/IT/000851).
Institutional Review Board Statement
Eels were handled in accordance with the regulations concerning the protection of experimental animals (Dir. 86/609/EEC) and the regulations of the Ethics Committee of Bologna University (prot. 575/2016).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ege, V. A revision of the genus Anguilla Shaw. Dana Rep. 1939, 16, 8–256. [Google Scholar]
- Watanabe, S.; Aoyama, J.; Tsukamoto, K. A new species of freshwater eel Anguilla luzonensis (Teleostei: Anguillidae) from Luzon Island of the Philippines. Fish. Sci. 2009, 75, 387–392. [Google Scholar] [CrossRef]
- Kuroki, M.; Aoyama, J.; Miller, M.; Yoshinaga, T.; Shinoda, A.; Hagihara, S.; Tsukamoto, K. Sympatric spawning of Anguilla marmorata and Anguilla japonica in the western North Pacific Ocean. J. Fish Biol. 2009, 74, 1853–1865. [Google Scholar] [CrossRef]
- Tesch, F.-W.; Bartsch, P. The Eel; Wiley Online Library: Hoboken, NJ, USA, 2003. [Google Scholar]
- Dekker, W. Status of the European eel stock and fisheries. In Eel Biology; Springer: Berlin, Germany, 2003; pp. 237–254. [Google Scholar]
- Dekker, W. The history of commercial fisheries for European eel commenced only a century ago. Fish. Manag. Ecol. 2019, 26, 6–19. [Google Scholar] [CrossRef]
- Drouineau, H.; Durif, C.; Castonguay, M.; Mateo, M.; Rochard, E.; Verreault, G.; Yokouchi, K.; Lambert, P. Freshwater eels: A symbol of the effects of global change. Fish Fish. 2018, 19, 903–930. [Google Scholar] [CrossRef]
- ICES. European Eel (Anguilla anguilla) Throughout Its Natural Range; ICES Advice: Copenhagen, Denmark, 2020. [Google Scholar]
- Pankhurst, N. Relation of visual changes to the onset of sexual maturation in the European eel Anguilla anguilla (L.). J. Fish Biol. 1982, 21, 127–140. [Google Scholar] [CrossRef]
- Fontaine, Y.; Pisam, M.; Le Moal, C.; Rambourg, A. Silvering and gill “mitochondria-rich” cells in the eel, Anguilla anguilla. Cell Tissue Res. 1995, 281, 465–471. [Google Scholar] [CrossRef]
- Waring, H. Color Change Mechanisms of Cold-Blooded Vertebrates; Academic Press: New York, NY, USA, 1963; Volume 266. [Google Scholar]
- Rohr, D.H.; Lokman, P.M.; Davie, P.S.; Young, G. 11-Ketotestosterone induces silvering-related changes in immature female short-finned eels, Anguilla australis. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 130, 701–714. [Google Scholar] [CrossRef]
- Boëtius, I.; Boétius, J. Lipid and protein content in Anguilla anguilla during growth and starvation. Dana 1985, 4, 1–17. [Google Scholar]
- Svedäng, H.; Wickström, H. Low fat contents in female silver eels: Indications of insufficient energetic stores for migration and gonadal development. J. Fish Biol. 1997, 50, 475–486. [Google Scholar] [CrossRef]
- Durif, C.; Dufour, S.; Elie, P. The silvering process of Anguilla anguilla: A new classification from the yellow resident to the silver migrating stage. J. Fish Biol. 2005, 66, 1025–1043. [Google Scholar] [CrossRef]
- Van Ginneken, V.J.; Maes, G.E. The European eel (Anguilla anguilla, Linnaeus), its lifecycle, evolution and reproduction: A literature review. Rev. Fish Biol. Fish. 2005, 15, 367–398. [Google Scholar] [CrossRef]
- Melià, P.; Bevacqua, D.; Crivelli, A.J.; Panfili, J.; De Leo, G.A.; Gatto, M. Sex differentiation of the European eel in brackish and freshwater environments: A comparative analysis. J. Fish Biol. 2006, 69, 1228–1235. [Google Scholar] [CrossRef]
- Van den Thillart, G.; Dufour, S. How to estimate the reproductive success of European silver eels. In Spawning Migration of the European Eel; Springer: Berlin, Germany, 2009; pp. 3–9. [Google Scholar]
- Okamura, A.; Horie, N.; Mikawa, N.; Yamada, Y.; Tsukamoto, K. Recent advances in artificial production of glass eels for conservation of anguillid eel populations. Ecol. Freshw. Fish 2014, 23, 95–110. [Google Scholar] [CrossRef]
- Gong, X.; Wang, D.; Bao, B.; Zhang, Q.; Jiang, X.; Liu, L. Gonadal development and silvering of the Japanese Eel (Anguilla japonica) in the Yangtze River during downstream migration. Aquac. Fish. 2017, 2, 173–178. [Google Scholar] [CrossRef]
- Mordenti, M.; Di Biase, A.; Sirri, R.; Modugno, S.; Tasselli, A. Induction of Sexual Maturation in Wild Female European Eels (Anguilla anguilla) in Darkness and Light. Isr. J. Aquac. 2012, 64, 1–9. [Google Scholar]
- Mordenti, O.; Di Biase, A.; Bastone, G.; Sirri, R.; Zaccaroni, A.; Parmeggiani, A. Controlled reproduction in the wild European eel (Anguilla anguilla): Two populations compared. Aquac. Int. 2013, 21, 1045–1063. [Google Scholar] [CrossRef]
- Fontaine, Y. L’argenture de l’anguille: Métamorphose, anticipation, adaptation. Bull. Français De La Pêche Et De La Piscic. 1994, 335, 171–185. [Google Scholar] [CrossRef][Green Version]
- Durif, C.M.; Dufour, S.; Elie, P. Impact of silvering stage, age, body size and condition on reproductive potential of the European eel. Mar. Ecol. Prog. Ser. 2006, 327, 171–181. [Google Scholar] [CrossRef]
- Durif, C.M.; Ginneken, V.v.; Dufour, S.; Müller, T.; Elie, P. Seasonal evolution and individual differences in silvering eels from different locations. In Spawning Migration of the European Eel; Springer: Berlin, Germany, 2009; pp. 13–38. [Google Scholar]
- Devlaming, V.; Grossman, G.; Chapman, F. On the use of the gonosomatic index. Comp. Biochem. Physiol. Part A Physiol. 1982, 73, 31–39. [Google Scholar] [CrossRef]
- West, G. Methods of assessing ovarian development in fishes: A review. Mar. Freshw. Res. 1990, 41, 199–222. [Google Scholar] [CrossRef]
- Ganias, K.; Somarakis, S.; Koutsikopoulos, C.; Machias, A. Factors affecting the spawning period of sardine in two highly oligotrophic Seas. Mar. Biol. 2007, 151, 1559–1569. [Google Scholar] [CrossRef]
- Hinton, M.G. Use of gonad indices to estimate the status of reproductive activity of female swordfish, Xiphias gladius: A validated classification method. Fish Bull. 1997, 95, 80–84. [Google Scholar]
- McQuinn, I.H. Identification of spring-and autumn-spawning herring (Clupea harengus harengus) using maturity stages assigned from a gonadosomatic index model. Can. J. Fish. Aquat. Sci. 1989, 46, 969–980. [Google Scholar] [CrossRef]
- Vitale, F.; Svedäng, H.; Cardinale, M. Histological analysis invalidates macroscopically determined maturity ogives of the Kattegat cod (Gadus morhua) and suggests new proxies for estimating maturity status of individual fish. ICES J. Mar. Sci. 2006, 63, 485–492. [Google Scholar] [CrossRef]
- Mordenti, O.; Di Biase, A.; Casalini, A.; Emmanuele, P.; Melotti, P.; Roncarati, A. Growth performances and natural diet of European eel (Anguilla anguilla L.) reared in muddy and sandy ponds. Aquat. Living Resour. 2016, 29, 105. [Google Scholar] [CrossRef]
- Ciccotti, E. Italy. In Management of the European Eel; Moriarty, C., Dekker, W., Eds.; Marine Institute: Dublin, Ireland, 1997; Volume 15, pp. 61–65. [Google Scholar]
- Grier, H.; Uribe-Aranzábal, M.; Patiño, R. The ovary, folliculogenesis, and oogenesis in teleosts. Reprod. Biol. Phylogeny Fishes 2009, 8, 25–84. [Google Scholar]
- Han, Y.-s.; Liao, I.-c.; Huang, Y.-s.; He, J.-t.; Chang, C.-W.; Tzeng, W.-N. Synchronous changes of morphology and gonadal development of silvering Japanese eel Anguilla japonica. Aquaculture 2003, 219, 783–796. [Google Scholar] [CrossRef]
- Pérez, L.; Peñaranda, D.; Dufour, S.; Baloche, S.; Palstra, A.; Van Den Thillart, G.; Asturiano, J. Influence of temperature regime on endocrine parameters and vitellogenesis during experimental maturation of European eel (Anguilla anguilla) females. Gen. Comp. Endocrinol. 2011, 174, 51–59. [Google Scholar] [CrossRef]
- Emmanuele, P.; Casalini, A.; Pisati, D.; Andreini, R.; Guercilena, N.; Parmeggiani, A.; Zaccaroni, A.; Mordenti, O. Artificial reproduction of Anguilla anguilla: Evaluation of biometrics characteristics of a population from Valle Campo Lagoon, Comacchio (Italy). Aquac. Int. 2020, 28, 777–790. [Google Scholar] [CrossRef]
- Di Biase, A.; Lokman, P.M.; Govoni, N.; Casalini, A.; Emmanuele, P.; Parmeggiani, A.; Mordenti, O. Co-treatment with androgens during artificial induction of maturation in female eel, Anguilla anguilla: Effects on egg production and early development. Aquaculture 2017, 479, 508–515. [Google Scholar] [CrossRef]
- De Leo, G.; Gatto, M. Trends in vital rates of the European eel: Evidence for density dependence? Ecol. Appl. 1996, 6, 1281–1294. [Google Scholar] [CrossRef]
- Ackermann, G.E.; Schwaiger, J.; Negele, R.D.; Fent, K. Effects of long-term nonylphenol exposure on gonadal development and biomarkers of estrogenicity in juvenile rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 2002, 60, 203–221. [Google Scholar] [CrossRef]
- Palstra, A.; Cohen, E.; Niemantsverdriet, P.; Van Ginneken, V.; Van den Thillart, G. Artificial maturation and reproduction of European silver eel: Development of oocytes during final maturation. Aquaculture 2005, 249, 533–547. [Google Scholar] [CrossRef]
- Vøllestad, L.A. Tagging experiments with yellow eel, Anguilla anguilla (L.), in brackish water in Norway. Sarsia 1988, 73, 157–161. [Google Scholar] [CrossRef]
- Vøllestad, L.A. Geographic variation in age and length at metamorphosis of maturing European eel: Environmental effects and phenotypic plasticity. J. Anim. Ecol. 1992, 61, 41–48. [Google Scholar] [CrossRef]
- Hain, J. The behaviour of migratory eels, Anguilla rostrata, in response to current, salinity and lunar period. Helgoländer Wiss. Meeresunters. 1975, 27, 211–233. [Google Scholar] [CrossRef]
- Di Biase, A.; Bastone, G.; Casalini, A.; Parmeggiani, A.; Costantini, G.; Mordenti, O. Study of the morpho-physiological char-acteristics of two Anguilla anguilla populations selected for artificial reproduction. In Proceedings of the Global Aquaculture: Securing Our Future—AQUA, Prague, Czech Republic, 1–5 September 2012; pp. 1–5. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).