Abstract
Highly toxic microcystins (MCs) perform complex interactions with many proteins that induce cellular dysregulation, leading to the development of several diseases including cancer. There is significant diversity and chemical complexity among MC congeners, which makes it difficult to identify structure-dependent toxicity outcomes and their long-term effects. The aim of this study was to exploratory identify likely molecular targets of the main MC variants (MC-LA, MC-LR, MC-RR, and MC-LY) by conducting a computational binding affinity analysis using AutoDock Vina to evaluate the interaction of the toxins with 1000 proteins related to different biological functions. All four variants showed strong in silico interactions with proteins that regulate metabolism/immune system, CD38 (top scoring hit, −11.5 kcal/mol); inflammation, TLR4 (−11.4 kcal/mol) and TLR8 (−11.5 kcal/mol); neuronal conduction, BChE; renin–angiotensin signaling, (ACE); thyroid hormone homeostasis (TTR); and cancer-promoting processes, among other biochemical activities. The results show MCs have the potential to bind onto distinct molecular targets which could generate biochemical alterations through a number of signal transduction pathways. In short, this study broadens our knowledge about the mechanisms of action of different variants of microcystins and provides information for future direct experimentation.
1. Introduction
An increase in the frequency and toxicity of cyanobacterial blooms in aquatic environments, mainly in freshwater reservoirs, has been recognized as an emerging health problem [1,2]. Cyanobacterial blooms are often accompanied by a variety of cyanotoxins that have different structures and compositions that define their effects. According to the target organ, they are classified as hepatotoxins, neurotoxins, or dermatoxins, and they are produced by a variety of species from different genera [3,4].
Microcystins (MCs), one of the most common cyanotoxins, are produced by different genera of cyanobacteria and are considered to be a potent hepatotoxin with a wide range of effects [5,6]. They are cyclic heptapeptides, and most of them are hydrophilic due to carboxylic acids at positions 3 and 6, and to arginine, which is frequently found at positions 2 and 4 [5]. However, structural modifications due to amino acid combinations can change the polarity of a peptide. MCs are a diverse group of toxins since variations in functional groups by methylation/demethylation, hydroxylation, and epimerization generate a wide variety of congeners; to date, more than 270 variants have been described [5,6,7].
The marked differences in the toxicity of MC congeners are related to their chemical characteristics and their ability to distribute in different cellular compartments, according to their affinity to specific molecules. Therefore, toxicity among congeners can vary by several orders of magnitude [8]. The diversity of MCs is also related to different effects documented for this group of toxins, which include inducing apoptosis or cell proliferation [9], cancer development [10], inflammatory processes [11], metabolic disorders [12], and reproductive and neuronal alterations [13].
MCs induce DNA damage, mitochondrial dysfunction, cytoskeleton disruption, endoplasmic reticulum disruption, and cell cycle dysregulation, among other processes [4,14,15]. The different toxic effects of MCs appear to be dose- and time-dependent. However, the molecular factors that induce the different biochemical and cellular effects have not been fully elucidated [12,15].
The molecular mechanisms involved in the toxicodynamics of MC congeners need to be determined to understand the processes that regulate signaling pathways, leading to cellular responses. The affinity of MC congeners to specific molecules is one of the principal elements in an evaluation that defines their mechanisms of action and toxicity. Most of these processes are difficult to evaluate through chemical and physiological experimental approaches. In contrast, computational chemistry is an important, rapid, and safe tool to decipher the interaction of toxins with target molecules and to infer their mechanisms of action. This approach evaluates docking interactions between congeners and molecular targets, and affinity scores are generated for different protein sites and configurations. In silico methods can be used to investigate multiple subprocesses and to evaluate accurate predictions of ligand binding energy as well as represent an efficient tool for screening ligand processes [16]. Therefore, potential candidate molecules can be identified for experimental validation.
Currently, one of the most extensively used docking tools is AutoDock Vina (Molecular Graphics Lab, The Scripps Research Institute, La Jolla, CA, USA. https://vina.scripps.edu/). This new generation software has significantly improved the average accuracy of binding mode predictions. Vina software offers a fast search method by using a simple scoring function and provides reproducible results for large systems with more than 20 flexible links [16]. Molecular docking is a direct approach to evaluate each MC congener’s affinity to the active site of enzymes. Although the number of MCs is large, in this work, we focused on MC-LR, MC-RR, MC-LA, and MC-LY (Figure 1) with 1000 human proteins to infer the toxic potential of these MCs. These variants are commonly present in many cyanobacterial blooms worldwide [4], and they bioaccumulate to varying degrees in exposed organisms [5].
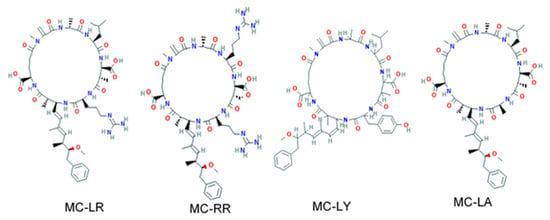
Figure 1.
Chemical structures of microcystin variants MC-LR, MC-RR, MC-LY, and MC-LA. Source: PubChem.
MC-LR and MC-RR are the best characterized congeners due to their ubiquitous presence in many water bodies during blooms of microcystin-producing cyanobacteria. However, MC-LA and MC-LY can become dominant, depending on environmental factors and the level of eutrophication [5,17,18]. Environmental conditions influence the expression of mcyB genes encoding for non-ribosomal peptide synthase involved in variant synthesis [18].
Here, we characterized the interactions of four microcystin congeners with proteins, classified them into different biological functions, and based on theoretical affinities, we evaluated the mechanisms of actions of the biochemical and molecular pathways that could be involved in the induction of pathological processes associated with exposure to these toxins.
2. Materials and Methods
In silico screening for molecular targets of microcystins was based on a search for targets and the evaluation of affinity for protein–ligand coupling protocols using AutoDock Vina. This program improves the accuracy of conformational predictions and calculates grid maps using the value of the scoring function, as well as those derived from the positions and orientations of ligands and twists of flexible residues [16].
The methodological approach implemented in this work was divided into several stages: (1) obtaining the 3D structures of microcystins; (2) optimization of the electronic structures of the compounds; (3) virtual evaluation of the protein–ligand coupling with AutoDock Vina; and (4) characterization of the binding sites, including the identification of the main protein-ligand interactions with Ligand Scout.
Protein–Ligand Coupling Calculations with AutoDock Vina
A high-performance virtual screening was performed docking the microcystin variants MC-LR, MC-LA, MC-LY, and MC-RR with 1000 human proteins, using a blind coupling strategy. Protein structures determined by X-ray crystallography were obtained from Protein Data Bank (PDB) and prepared for docking purposes using AutoDock tools. Selected proteins are related to oxidative stress, cell proliferation, breast cancer, angiogenesis, epigenetics, thyroid function, inflammatory processes, apoptosis, cytochrome P450, insulin, neuroprotection, nuclear receptors, and skin fibrosis, among other biochemically related groups.
The 3D structures of the microcystins were obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov/) for optimization through Gaussian 3.0 (Gaussian Inc., Wallingford, CT, USA). The coupling site was defined by establishing a cube at the geometric center of the native ligand present in each of the evaluated PDB structures, with dimensions of 24 × 24 × 24 Å, covering the coupling site of the ligand, with a grid point spacing of 0.375 Å. Coupling between each protein–ligand pair was evaluated in triplicate, and the average of the best affinity scores (kcal/mol) was used to classify the complexes.
3. Results and Discussion
In molecular modeling, binding energy is the key element to evaluate ligand–receptor interactions. The most negative binding energy represents the best docking position and the most probable binding mode (pose). The affinity predictions of the complexes between MC congeners and the target proteins generate a great diversity of data. A cut-off point of −10.0 kcal/mol was used to identify the most probable MC–protein complexes. Therefore, these complexes presented a binding energy between −10 and −11.9 kcal/mol, which was the minimum coupling energy detected among all interactions. The top 15 poses of MCs with target proteins that are common to all variants and with affinities below −10.0 kcal/mol are presented in Table 1.

Table 1.
Main protein complexes formed with the four microcystin variants (MC-LA, MC-LR, MC-RR, and MC-LY) with binding energies lower than −10 kcal/mol.
3.1. Main Complexes Formed with Microcystins with High Affinity Scores
3.1.1. Metabolic Effects and Insulin Regulation
The computational analysis revealed that all of the analyzed MC variants had high affinities to the CD38 protein, especially MC-LY, which showed the best docking value (−11.5 kcal/mol). CD38 participates in the regulation of cell adhesion, differentiation, and cell proliferation, and has been found to alter the antiapoptotic role of insulin, induce insulin release, and the development of diabetes, and is highly expressed in the immune system [19,20]. CD38 is a transmembrane glycoprotein that, in addition to having functions as an ectoenzyme, serves as a receptor associated with the extracellular domain. It is responsible for mobilizing the Ca2+ at the intracellular level and the production of ADPR, which can covalently bind to many proteins and modify their functions [20,21,22,23,24]. CD38 is made up of 300 amino acids, and their multiple enzymatic functions are executed in a single active site that presents the critical residues Glu 226, Trp 125, Trp 189, and Glu 116 [25].
Most interactions of MC-LY with CD38 are hydrophobic, and binding to the active site of CD38 occurs through Trp 125 and Trp 189 (Figure 2), while connections with highly conserved residues such as Lys 129 and Thr 221 may affect essential ectoenzyme processes such as cADPR hydrolysis, and the necessary folding for cyclization and NAD glycohydrolase (NADasa) enzyme activity [22,26].
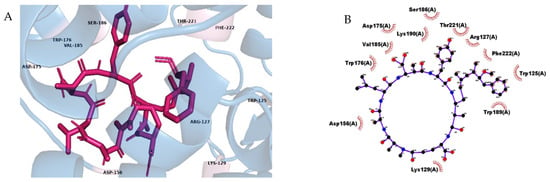
Figure 2.
Three-dimensional view of CD38 in complex with MC-LY (magenta) (A), Atomic level view showing interactions between contact residues on the binding site and the ligand, as predicted by Ligand Scout (B). Atoms are colored with the CPK scheme.
It should be noted that CD38 has not been reported to be a target protein for MCs. However, MC-LR has been shown to impair the insulin receptor signaling pathway, and induce hyperinsulinemia and insulin resistance in mice by altering the expression of genes related to type 2 diabetes mellitus [24,25,26], in addition to the upregulation of several proteins such as Nduf, Ppp3ca, Ide, Marcks, Pgk1, and Suclg1 involved in insulin secretion [19]. It has been found that MC-LR can induce partial islet dysfunction and cause T2 diabetes mellitus; this could be due to the possible binding of MCs to key CD38 residues involved in the loss of important functions such as increasing cADPR production and intracellular Ca2+ release and insulin secretion, and inducing impairment of islet functions as well as activating inflammatory processes [22].
In the case of MC-LA and MC-LR, they showed a strong interrelationship with receptors such as prolactin (PRL-R), MAPK14, and ATP-citrate synthase. These receptors positively regulate cellular proliferation, are activators of mitogenic protein phosphorylation, and participate in lipid synthesis, and their alteration is related to cancer induction [27,28]. The affinities found in MCs indicate that exposure to minority variants such as MC-LA and MC-LY could be high risk as they have a higher affinity for proliferation-inducing factors that alter important processes. It has been shown that these congeners may be dominant during cyanobacteria blooms, and therefore, the risks associated with water consumption by humans and other organisms are increased [17,29].
We found that, among the 189 interactions with insulin receptors, five of the interactions were specific to MC-LA and MC-LY with high affinity, presenting a higher binding energy with two important proteins (i.e., MLL1 and AKT3). MLL1 activates the methylation of histone H3K4 and therefore is involved in the proper development of embryogenesis, differentiation in hematopoiesis, and neurogenesis [30]. In addition, MC-LA and MC-LY bind to the protein kinase serine/threonine AKT3 (a higher affinity of −10.7 kcal/mol to MC-LY than to MC-LA), which regulates metabolism, energy demand, growth, angiogenesis, acts on cell proliferation, and is involved in the up- and downregulation of MMP-13, which is a metastasis activator [30,31]. Clearly, high affinity microcystin variants can alter these processes of important metabolic regulator enzymes, which could explain the proliferation and tumor induction and the indication of apoptosis by these toxins.
One of the most studied effects of MCs has been the inhibition of serine/threonine protein phosphatases, especially PP1 and PP2A in their catalytic units PP-1c/PP-2Ac [32,33].
MCs interact with PPs, resulting in the inhibition effect on adduct formation due to their connection with the catalytic site, the hydrophobic groove, and the C-terminal groove [32,34,35,36]. The inhibition effect is mainly through noncovalent interactions largely by the hydrophobic side chain of the β-amino acid Adda, located at position 5 and the Glu6 residue of the enzyme. This is one of the most important unions of the toxin [5,37].
We found interesting associations with several dual specificity protein phosphatases such as vaccinia H1, dual specificity protein phosphatase 26 (DUSP26), and protein phosphatase 13 (DUSP13). However, all had affinities of approximately −9.00 kcal/mol and insulin-related functions. The effects of MCs on cytoskeleton disruption, cell cycle arrest, metabolic disorders, cell proliferation, and cell death have been linked to PP1/PP2A activity and increased phosphorylation of certain proteins [38].
3.1.2. Interactions Related to Inflammatory Proteins and Neuroprotective Alteration
The clear virtual links among MC-LA, MC-LR, and MC-LY with butyrilcholinesterase (BChE) and β-secrestase 1 (BACE1) could explain another route by which MCs generate neurotoxic effects. The altered behavior of the BChE is related to neurodegenerative diseases and similar to BACE1, and has been related to the generation of beta amyloid-1 protein plaques in the brain [39,40]. The function and regulation of BACE1 at the brain level have not been fully elucidated [41], and there are no reports of the association of MCs with this protein. However, based on the virtual results, we suggest that the interaction between MCs and BACE1 should be investigated experimentally. The β-secretase 1 showed the highest affinities with MC-LY and MC-LA with scores of −10.9 kcal/mol and −10.5 kcal/mol, respectively.
The neurotoxic effects of the MCs are manifested as neuronal loss and morphological changes by hyperphosphorylation of the axon cytoskeleton [42]. Many of these effects have been associated with the obstruction generated by MCs in PP1 and PP2, which leads to oxidative stress, damage to the hippocampus, and hyperphosphorylation of tau protein, the main protein in the neuronal microtubules, thus favoring the development of some neurogenerative diseases [32,33,34]. However, these neurotoxic responses could also be explained as a positive regulation by MCs of the abundant proteins BACE1 and BChE, and not exclusively to the widely referred inhibition of PP1 and PP2.
Alterations in the activity of BChE lead to the release of proinflammatory cytokines [43,44]. In addition, BChE participates in the development of hepatic adiposity that is elevated in type I and II diabetes and hepatocellular carcinoma [45]. The interaction of MCs with this enzyme could also explain the negative effect that MCs generate in the liver as they induce increased hepatic steatosis and binucleation of the hepatocyte, generating strong inflammation and irreversible damage [35,37].
Toll-like receptors (TLRs) were the main representatives of the interaction of MCs with inflammatory proteins (Table 1). TLR receptors are involved in adaptive immune responses and pathogen recognition [46]. The interaction of MCs with these proteins has been reported previously, specifically with TLR4, which generates persistent inflammation through the TLR/MyD88 pathway and the TLR4/NF-kβ pathway [47,48], and therefore can induce hypertension through vascular inflammation, renal and CNS damage, and insulin resistance [49]. We found that in addition to TLR4, the TLR8 receptor generates strong binding with MC-LY and MC-LA (Table 1 and Figure 3). TLR8 contributes to the production of IL-6, IL-12p70, NF-kβ, and TNF-α, which are predominantly expressed on macrophages, dendritic cells, neutrophils, and monocytes [50]. The inflammatory shock produced by MCs may have been caused by the synergistic effect of TLR4 and TLR8, in addition to the disruption of inducible nitric oxide synthase (iNOS), which showed virtual couplings of −11.5 kcal/mol with MC-LA and MC-LY and whose interaction with MC-LR induced genotoxicity [51].
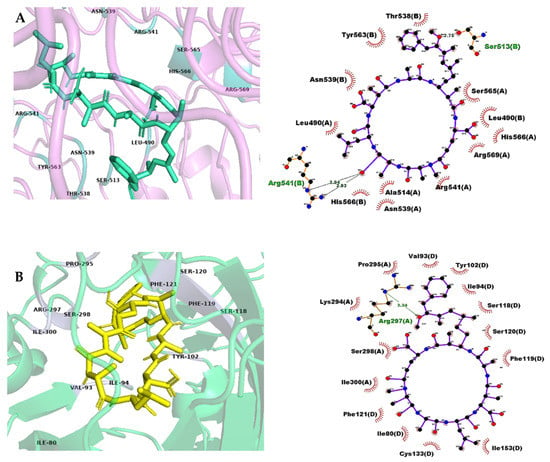
Figure 3.
Interaction of MC-LA with TLR8 (A and B chains) (A), and TLR4 (A and D chains) (B). Figures on the right display atomic level views showing interactions between contact residues on the binding site and the ligand, as predicted by Ligand Scout. Atoms are colored with the CPK scheme.
Angiotensin-I converting enzyme (ACE) is another enzyme that showed strong interactions with the four MC variants analyzed (Figure 4). ACE is a Zn2+ and Cl− dependent metalloprotein responsible for the metabolism of angiotensin I, whose imbalance alters blood pressure and induces congestive heart failure [52]. Microcystins showed superior affinities to this metallopeptide (Table 1), which has also been demonstrated experimentally, generating a variety of mechanisms that affect renal and cardiac function. The mechanisms include oxidative stress, generated by the release of cytochrome c, which triggers apoptosis to other mechanisms not yet elucidated [53,54,55].
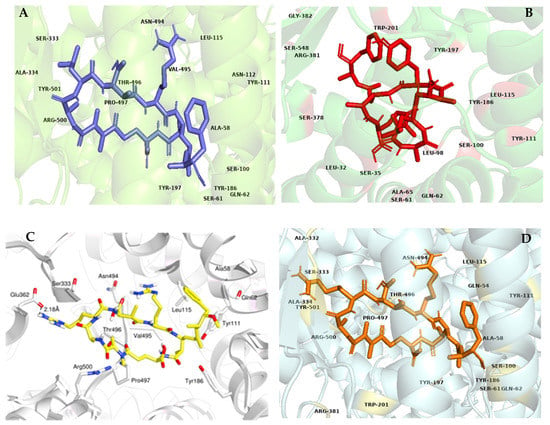
Figure 4.
Three-dimensional representations of angiotensin-I-converting enzyme (ACE)–microcystin complexes. Microcystin-LR (A), MC-LY (B), MC-LA (C) and MC-RR (D) appear in blue, red, yellow, and orange, respectively.
Major residue interactions between the different microcystins and ACE are displayed in Table 2.

Table 2.
Key bonding residues on ACE-microcystin complexes.
3.1.3. Nuclear Receptors and Genotoxicity Potential
The alpha receptor of the thyroid hormone (TRα) was the only nuclear receptor that interacted with the four MC variants, but with affinities of less than −10.0 kcal/mol. This receptor acts as a repressor or activator of transcription, and is involved in heart rate regulation [56]. In contrast, MC-LR and MC-LY presented interactions with 131 receptors, especially with retinoic acid (RA), RXR-alpha receptor, and nuclear receptor coactivator, which stimulated transcriptional activity and recruitment of general hormone-mediated transcription factors [57]. A recent study has demonstrated a linear relationship between the content of RAs and genotoxicity developed by exposure to cyanobacterial extracts [14].
Human transthyretin (TTR), a thyroxine receptor responsible for the major transport of thyroxine in various tissues and associated with the accumulation of abnormal amyloid protein deposits in the brain, has been reported to be altered by MCs [57]. The presence of MC-LR has been associated with a lower expression of TTR, and thus, a reduction in thyroxine levels after acute exposure [58]. The affinity between TTR and MC-LA is similar to that of MC-LR (−10.8 and −10.1 kcal/mol, respectively, Table 1). Therefore, we suggest that the effects are similar between both theoretical complexes.
Regarding the genotoxic potential of MCs, the high affinity found between the four variants with DNA topoisomerases type IIA, (TOP2A) could explain the reduction in the DNA repair system linked to the presence of these toxins [10]. The similar degree of interaction between the different congeners with TOP2A and LIMK1, together with the damage generated by the presence of ROS, suggests that the different variants affect the integrity of the genetic material and cellular homeostasis in the same way. Probably, they induce loss of the control of cellular functions, causing both tumorigenic and carcinogenic activity. The formation of the MC-LY complex with CYP3A4, which presented the lowest binding energy among the complexes found (−11.9 kcal/mol), could also be involved in DNA damage (Figure 5). This can be related to the fact that MCs generate a decrease in the levels of several members of the CYP family, leading to the formation of toxic metabolites, which may be involved in different processes of DNA damage and in the occurrence of ROS [10,59].
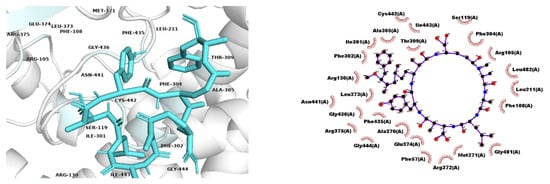
Figure 5.
Three-dimensional representation of the CYP3A4 and MC–LY complex. Contact residues at the binding site and interactions predicted by Ligand Scout are shown on the right. Atoms are colored with the CPK scheme.
3.1.4. Cellular Proliferation and Cancer
The induction of apoptosis or uncontrolled cell proliferation depends on the exposure to different MC concentrations (dose dependent). The expression of the antiapoptotic gene Bcl-2 increases more than 4000-fold at low concentrations of MC-LR. This generates the survival of tumors in response to chemotherapy [60]. In contrast, high concentrations of MC-LR induce a reduction in the expression of the gene Bcl-22, which leads to apoptosis [61].
We found strong interactions between the four variants and MCL-1, a protein that belongs to the Bcl-2 family as well as with kinases MST4, CLK1, and LIMK1. The mechanisms of action of MCs that lead to cell proliferation and carcinogenesis are not well understood. However, MCs seem to share a similar biochemical effect by inducing the activation of antiapoptotic signaling pathways, alteration of the cell cycle, and inhibition of PP1 and PP2A, which causes an altered protein phosphorylation/dephosphorylation balance [33,62].
CLK1 is implicated in the pathophysiology of Alzheimer’s disease through phosphorylation of serine residues in SR proteins [63]. Therefore, these proteins can be considered to be potential targets of the neurological disorders originated by direct interaction with MCs. Downregulation of LIMK1 is associated with fibrosis, psoriatic epidermal proliferation, and different types of carcinogenesis [64,65]. The interaction of MCs with LIMK1 could explain the molecular mechanisms associated with different types of fibrosis, from psoriatic epidermal proliferation [63] to vascular heart disease [53,65].
A strong interaction was found between MC-RR with the selenoprotein thioredoxin reductase TRXR1, which regulates the p53 activity and has a high metastatic potential [66,67]. Alteration of TRXR1 levels or activity is accompanied by pro-oxidative changes in the cell, inducing increased oxidative stress, and thus, damage to different biomolecules [66,68].
The theoretical results obtained in this study are consistent with experimental observations reported by different authors [11,12,26,42,62]. The interactions reported here provide an important perspective of the possible alterations generated by the presence of MC variants or the synergistic effect of them. Furthermore, we were able to quantify binding affinities, describe the types of bonds of MC–enzyme/protein complexes, and thus explore in detail the interactions of the ligand with the active site of the enzyme or protein in general. In short, the affinity of MCs to different receptors that generate similar effects represents a greater potential risk to these toxins.
4. Conclusions
The biochemical and molecular alterations associated with MCs can be elucidated by the virtual interactions found in this study. It is precisely the affinities associated with new molecular targets that could explain the pathways that do not extinguish the anti-apoptotic signals, or that accelerate the irreversible cell damage generated by the presence of this group of toxins, and the redundant events, which lead to the same alterations, provoking complex responses, and accelerating cellular self-deterioration mediated by both intracellular and extracellular factors.
Computational data used in this work suggest that there is an equal or even greater risk of adverse effects after exposure to MC-LA and MC-LY compared with MC-LR. Given the large number of possible molecular targets and the strong theoretical affinity to diverse metabolic important enzymes, the different interactions of these lipophilic variants, especially MC-LA, need to be evaluated experimentally. The limited information on the molecular targets of the 279 MC congeners described so far makes it difficult to identify the impact of chronic exposure and their toxic effects. The in silico approach permits the investigation of possible interactions with proteins and the elucidation of some mechanisms that have not been fully understood, and this information can be used to direct experimental approaches to characterize the toxic effect of MCs.
Author Contributions
Conceptualization, C.T.-L., E.G.M. and J.O.-V.; Methodology, C.T.-L., J.O.-V., E.G.M. and M.C.R.-A.; Software, C.T.-L. and J.O.-V.; Validation, C.T.-L., J.O.-V., M.C.R.-A. and E.G.M.; Formal analysis, C.T.-L. and E.G.M.; Investigation, C.T.-L., J.O.-V., E.G.M. and M.C.R.-A.; Resources, C.T.-L. and J.O.-V.; Writing—original draft preparation, C.T.-L.; Writing—review and editing, C.T.-L., E.G.M., J.O.-V. and M.C.R.-A.; Visualization, C.T.-L., M.C.R.-A., E.G.M. and J.O.-V.; Supervision, J.O.-V.; Funding acquisition, J.O.-V.; Analysis and verification of bibliographic references, C.T.-L. and E.G.M. All authors have read and agreed to the published version of the manuscript.
Funding
Ministry of Science, Technology and Innovation-Minciencias-SGR, Colombia (BPIN 2020000100093, Gobernación de Bolívar); Programme Ecosistema Científico-Colombia Científica, from the Francisco José de Caldas Fund, Grant RC-FP44842-212-2018; University of Cartagena (Plan to Support Research Groups and Graduate Programs).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available after request to the authors.
Acknowledgments
The authors thank the University of Cartagena for providing all the tools required in this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.-L.; Utsumi, M. An overview of the accumulation of microcystins in aquatic ecosystems. J. Environ. Manag. 2018, 213, 520–529. [Google Scholar] [CrossRef]
- Puddick, J.; Prinsep, M.R.; Wood, S.A.; Kaufononga, S.A.F.; Cary, S.C.; Hamilton, D.P. High levels of structural diversity observed in microcystins from Microcystis CAWBG11 and characterization of six new microcystin congeners. Mar. Drugs 2014, 12, 5372–5395. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.; Vasconcelos, V. Molecular mechanisms of microcystin toxicity in animal cells. Int. J. Mol. Sci. 2010, 11, 268–287. [Google Scholar] [CrossRef] [PubMed]
- Bouaïcha, N.; Miles, C.O.; Beach, D.G.; Labidi, Z.; Djabri, A.; Benayache, N.Y.; Nguyen-Quang, T. Structural Diversity, Characterization and Toxicology of Microcystins. Toxins 2019, 11, 714. [Google Scholar] [CrossRef]
- Brózman, O.; Kubickova, B.; Babica, P.; Laboha, P. Microcystin-LR Does Not Alter Cell Survival and Intracellular Signaling in Human Bronchial Epithelial Cells. Toxins 2020, 12, 165. [Google Scholar] [CrossRef] [PubMed]
- Spoof, L.; Catherine, A. Appendix 3: Tables of Microcystins and Nodularins. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 526–537. ISBN 978-1-119-06876-1. [Google Scholar]
- Testai, E.; Buratti, F.M.; Funari, E.; Manganelli, M.; Vichi, S.; Arnich, N.; Biré, R.; Fessard, V.; Sialehaamoa, A. Review and analysis of occurrence, exposure and toxicity of cyanobacteria toxins in food. EFSA Support. Publ. 2016, 13, 998E. [Google Scholar] [CrossRef]
- Zegura, B.; Zajc, I.; Lah, T.T.; Filipic, M. Patterns of microcystin-LR induced alteration of the expression of genes involved in response to DNA damage and apoptosis. Toxicon 2008, 51, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Zegura, B.; Straser, A.; Filipič, M. Genotoxicity and potential carcinogenicity of cyanobacterial toxins—A review. Mutat. Res. 2011, 727, 16–41. [Google Scholar] [CrossRef]
- Zhong, S.; Liu, Y.; Wang, F.; Wu, Z.; Zhao, S. Microcystin-LR induced oxidative stress, inflammation, and apoptosis in alveolar type II epithelial cells of ICR mice in vitro. Toxicon 2020, 174, 19–25. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, K.; Zhou, Y.; Li, X.; Mao, X.; Wei, X.; Xu, Y.; Yang, W.; Chen, G.; Liu, C. Microcystin-LR impairs glucose metabolism in pancreatic β cells in vivo and in vitro. Toxicol. Lett. 2020, 326, 106–113. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Shen, S.; Wang, C.; Xiang, Z.; Han, X.; Li, D. Toxic effects of microcystin-LR on the development of prostate in mice. Toxicology 2017, 380, 50–61. [Google Scholar] [CrossRef]
- Bittner, M.; Štern, A.; Smutná, M.; Hilscherová, K.; Žegura, B. Cytotoxic and Genotoxic Effects of Cyanobacterial and Algal Extracts-Microcystin and Retinoic Acid Content. Toxins 2021, 13, 107. [Google Scholar] [CrossRef]
- Weng, D.; Lu, Y.; Wei, Y.; Liu, Y.; Shen, P. The role of ROS in microcystin-LR-induced hepatocyte apoptosis and liver injury in mice. Toxicology 2007, 232, 15–23. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Díez-Quijada, L.; Prieto, A.I.; Guzmán-Guillén, R.; Jos, A.; Cameán, A.M. Occurrence and toxicity of microcystin congeners other than MC-LR and MC-RR: A review. Food Chem. Toxicol. 2019, 125, 106–132. [Google Scholar] [CrossRef]
- Meyer, S.; Kehr, J.-C.; Mainz, A.; Dehm, D.; Petras, D.; Süssmuth, R.D.; Dittmann, E. Biochemical Dissection of the Natural Diversification of Microcystin Provides Lessons for Synthetic Biology of NRPS. Cell Chem. Biol. 2016, 23, 462–471. [Google Scholar] [CrossRef]
- Ohta, Y.; Kitanaka, A.; Mihara, K.; Imataki, O.; Ohnishi, H.; Tanaka, T.; Taminato, T.; Kubota, Y. Expression of CD38 with intracellular enzymatic activity: A possible explanation for the insulin release induced by intracellular cADPR. Mol. Cell. Biochem. 2011, 352, 293–299. [Google Scholar] [CrossRef]
- Lee, H.C. Structure and enzymatic functions of human CD38. Mol. Med. Camb. Mass 2006, 12, 317–323. [Google Scholar] [CrossRef]
- Liu, Q.; Kriksunov, I.A.; Graeff, R.; Munshi, C.; Lee, H.C.; Hao, Q.Q. Crystal Structure of Human CD38 Extracellular Domain. Structure 2005, 13, 1331–1339. [Google Scholar] [CrossRef]
- Morandi, F.; Airoldi, I.; Marimpietri, D.; Bracci, C.; Faini, A.C.; Gramignoli, R. CD38, a Receptor with Multifunctional Activities: From Modulatory Functions on Regulatory Cell Subsets and Extracellular Vesicles, to a Target for Therapeutic Strategies. Cells 2019, 8, 1527. [Google Scholar] [CrossRef] [PubMed]
- Ting, K.Y.; Leung, C.F.P.; Graeff, R.M.; Lee, H.C.; Hao, Q.; Kotaka, M. Porcine CD38 exhibits prominent secondary NAD(+) cyclase activity. Protein Sci. 2016, 25, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Graeff, R.; Lee, H.C. Roles of cADPR and NAADP in pancreatic cells. Acta Biochim. Biophys. Sin. 2012, 44, 719–729. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moreau, C.; Liu, Q.; Graeff, R.; Wagner, G.K.; Thomas, M.P.; Swarbrick, J.M.; Shuto, S.; Lee, H.C.; Hao, Q.; Potter, B.V.L. CD38 Structure-Based Inhibitor Design Using the N1-Cyclic Inosine 5′-Diphosphate Ribose Template. PLoS ONE 2013, 8, e66247. [Google Scholar] [CrossRef]
- Zhang, Q.; Qin, W.; Yang, L.; An, J.; Zhang, X.; Hong, H.; Xu, L.; Wang, Y. Microcystis bloom containing microcystin-LR induces type 2 diabetes mellitus. Toxicol. Lett. 2018, 294, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, S.R.; Butler, L.M.; Hoy, A.J. The diversity and breadth of cancer cell fatty acid metabolism. Cancer Metab. 2021, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Zwang, Y.; Yarden, Y. p38 MAP kinase mediates stress-induced internalization of EGFR: Implications for cancer chemotherapy. EMBO J. 2006, 25, 4195–4206. [Google Scholar] [CrossRef]
- Zastepa, A.; Pick, F.R.; Blais, J.M. Fate and Persistence of Particulate and Dissolved Microcystin-LA from Microcystis Blooms. Hum. Ecol. Risk Assess. Int. J. 2014, 20, 1670–1686. [Google Scholar] [CrossRef]
- Moriya, C.; Jinnin, M.; Yamane, K.; Maruo, K.; Muchemwa, F.C.; Igata, T.; Makino, T.; Fukushima, S.; Ihn, H. Expression of matrix metalloproteinase-13 is controlled by IL-13 via PI3K/Akt3 and PKC-δ in normal human dermal fibroblasts. J. Investig. Dermatol. 2011, 131, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.L.; Maroulakou, I.G.; Eldridge, J.; Liby, T.L.; Sridharan, V.; Tsichlis, P.N.; Muise-Helmericks, R.C. VEGF stimulation of mitochondrial biogenesis: Requirement of AKT3 kinase. FASEB J. 2008, 22, 3264–3275. [Google Scholar] [CrossRef]
- Pereira, S.R.; Vasconcelos, V.M.; Antunes, A. Computational study of the covalent bonding of microcystins to cysteine residues—A reaction involved in the inhibition of the PPP family of protein phosphatases. FEBS J. 2013, 280, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xie, P. Mechanisms of Microcystin-induced Cytotoxicity and Apoptosis. Mini-Rev. Med. Chem. 2016, 16, 1018–1031. [Google Scholar] [CrossRef] [PubMed]
- Maynes, J.T.; Luu, H.A.; Cherney, M.M.; Andersen, R.J.; Williams, D.; Holmes, C.F.B.; James, M.N.G. Crystal Structures of Protein Phosphatase-1 Bound to Motuporin and Dihydromicrocystin-LA: Elucidation of the Mechanism of Enzyme Inhibition by Cyanobacterial Toxins. J. Mol. Biol. 2006, 356, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Craig, M.; Luu, H.A.; McCready, T.L.; Holmes, C.F.B.; Williams, D.; Andersen, R.J. Molecular mechanisms underlying the interaction of motuporin and microcystins with type-1 and type-2A protein phosphatases. Biochem. Cell Biol. 1996, 74, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Hastie, C.; Borthwick, E.; Morrison, L.; Codd, G.; Cohen, P. Inhibition of several protein phosphatases by a non-covalently interacting microcystin and a novel cyanobacterial peptide, nostocyclin. Biochim. Biophys. Acta BBA-Gen. Subj. 2005, 1726, 187–193. [Google Scholar] [CrossRef]
- Chen, Y.-M.; Lee, T.-H.; Lee, J.; Huang, H.-B.; Huang, R.; Chou, H.-N. Comparison of protein phosphatase inhibition activities and mouse toxicities of microcystins. Toxicon 2006, 47, 742–746. [Google Scholar] [CrossRef]
- Zhou, M.; Tu, W.; Xu, J. Mechanisms of microcystin-LR-induced cytoskeletal disruption in animal cells. Toxicon 2015, 101, 92–100. [Google Scholar] [CrossRef]
- Pope, C.N.; Brimijoin, S. Cholinesterases and the fine line between poison and remedy. Biochem. Pharmacol. 2018, 153, 205–216. [Google Scholar] [CrossRef]
- Li, Q.; Yang, H.; Chen, Y.; Sun, H. Recent progress in the identification of selective butyrylcholinesterase inhibitors for Alzheimer’s disease. Eur. J. Med. Chem. 2017, 132, 294–309. [Google Scholar] [CrossRef]
- Yen, Y.-C.; Kammeyer, A.M.; Jensen, K.C.; Tirlangi, J.; Ghosh, A.K.; Mesecar, A.D. Development of an Efficient Enzyme Production and Structure-Based Discovery Platform for BACE1 Inhibitors. Biochemistry 2019, 58, 4424–4435. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Y.; Zhang, C.; Xiang, Z.; Ding, J.; Han, X. Learning and memory deficits and alzheimer’s disease-like changes in mice after chronic exposure to microcystin-LR. J. Hazard. Mater. 2019, 373, 504–518. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-B.; Zhang, X.; Ju, J.; Li, Y.; Yin, L.; Pu, Y. Alterations in neurobehaviors and inflammation in hippocampus of rats induced by oral administration of microcystin-LR. Environ. Sci. Pollut. Res. Int. 2014, 21, 12419–12425. [Google Scholar] [CrossRef] [PubMed]
- Lampón, N.; Hermida-Cadahia, E.F.; Riveiro, A.; Tutor, J.C. Association between butyrylcholinesterase activity and low-grade systemic inflammation. Ann. Hepatol. 2012, 11, 356–363. [Google Scholar] [CrossRef]
- Lockridge, O. Review of human butyrylcholinesterase structure, function, genetic variants, history of use in the clinic, and potential therapeutic uses. Pharmacol. Ther. 2015, 148, 34–46. [Google Scholar] [CrossRef] [PubMed]
- McGettrick, A.F.; O’Neill, L.A.J. Localisation and trafficking of Toll-like receptors: An important mode of regulation. Curr. Opin. Immunol. 2010, 22, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Adegoke, E.O.; Wang, C.; Machebe, N.S.; Wang, X.; Wang, H.; Adeniran, S.O.; Zhang, H.; Zheng, P.; Zhang, G. Microcystin-leucine arginine (MC-LR) induced inflammatory response in bovine sertoli cell via TLR4/NF-kB signaling pathway. Environ. Toxicol. Pharmacol. 2018, 63, 115–126. [Google Scholar] [CrossRef]
- Lin, W.; Guo, H.; Wang, L.; Zhang, D.; Wu, X.; Li, L.; Qiu, Y.; Yang, L.; Li, D.; Tang, R. Waterborne microcystin-LR exposure induced chronic inflammatory response via MyD88-dependent toll-like receptor signaling pathway in male zebrafish. Sci. Total Environ. 2020, 702, 134969. [Google Scholar] [CrossRef] [PubMed]
- Nunes, K.P.; de Oliveira, A.A.; Mowry, F.E.; Biancardi, V.C. Targeting toll-like receptor 4 signalling pathways: Can therapeutics pay the toll for hypertension? Br. J. Pharmacol. 2019, 176, 1864–1879. [Google Scholar] [CrossRef] [PubMed]
- Tanji, H.; Ohto, U.; Shibata, T.; Miyake, K.; Shimizu, T. Structural reorganization of the Toll-like receptor 8 dimer induced by agonistic ligands. Science 2013, 339, 1426–1429. [Google Scholar] [CrossRef]
- Wang, X.; Huang, P.; Liu, Y.; Du, H.; Wang, X.; Wang, M.; Wang, Y.; Hei, T.K.; Wu, L.; Xu, A. Role of nitric oxide in the genotoxic response to chronic microcystin-LR exposure in human-hamster hybrid cells. J. Environ. Sci. China 2015, 29, 210–218. [Google Scholar] [CrossRef]
- Polakovičová, M.; Jampílek, J. Advances in Structural Biology of ACE and Development of Domain Selective ACE-inhibitors. Med. Chem. 2019, 15, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Massey, I.Y.; Feng, H.; Yang, F. A Review of Cardiovascular Toxicity of Microcystins. Toxins 2019, 11, 507. [Google Scholar] [CrossRef]
- Shi, J.; Zhou, J.; Zhang, M. Microcystins Induces Vascular Inflammation in Human Umbilical Vein Endothelial Cells via Activation of NF-κB. Mediat. Inflamm. 2015, 2015, 942159. [Google Scholar] [CrossRef] [PubMed]
- Alverca, E.; Andrade, M.; Dias, E.; Sam Bento, F.; Batoréu, M.C.C.; Jordan, P.; Silva, M.J.; Pereira, P. Morphological and ultrastructural effects of microcystin-LR from Microcystis aeruginosa extract on a kidney cell line. Toxicon 2009, 54, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Power, D.M.; Elias, N.P.; Richardson, S.J.; Mendes, J.; Soares, C.M.; Santos, C.R. Evolution of the thyroid hormone-binding protein, transthyretin. Gen. Comp. Endocrinol. 2000, 119, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Liu, Z.; Gao, Y.; Jia, D.; Tang, R.; Li, L.; Li, D. Waterborne exposure to microcystin-LR alters thyroid hormone levels, iodothyronine deiodinase activities, and gene transcriptions in juvenile zebrafish (Danio rerio). Chemosphere 2020, 241, 125037. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Z.; Jia, D.; Hu, Q.; Li, L.; Tang, R.; Li, D. Acute microcystin-LR exposure interfere thyroid hormones homeostasis in adult zebrafish (Danio rerio). Chemosphere 2020, 243, 125258. [Google Scholar] [CrossRef]
- Nair, P.C.; McKinnon, R.A.; Miners, J.O. Cytochrome P450 structure-function: Insights from molecular dynamics simulations. Drug Metab. Rev. 2016, 48, 434–452. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, H.; Suganuma, M. Tumor Promoters—Microcystin-LR, Nodularin and TNF-α and Human Cancer Development. Anticancer Agents Med. Chem. 2011, 11, 4–18. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Zuo, X.; Ding, N.; Zeng, H.; Zou, X.; Han, X. Microcystin (-LR) induced testicular cell apoptosis via up-regulating apoptosis-related genes in vivo. Food Chem. Toxicol. 2013, 60, 309–317. [Google Scholar] [CrossRef]
- Dias, E.; Andrade, M.; Alverca, E.; Pereira, P.; Batoréu, M.C.C.; Jordan, P.; Silva, M.J. Comparative study of the cytotoxic effect of microcistin-LR and purified extracts from Microcystis aeruginosa on a kidney cell line. Toxicon 2009, 53, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Karthikeyan, C.; Moorthy, N.S.H.N.; Waiker, D.K.; Jain, A.K.; Trivedi, P. Human CDC2-like kinase 1 (CLK1): A novel target for Alzheimer’s disease. Curr. Drug Targets 2014, 15, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Honma, M.; Shibuya, T.; Fujii, M.; Iinuma, S.; Ishida-Yamamoto, A. Aberrant LIM-kinase 1 expression in hyperproliferative psoriatic epidermis. J. Dermatol. 2017, 44, 91–92. [Google Scholar] [CrossRef]
- Chen, Q.; Gimple, R.C.; Li, G.; Chen, J.; Wu, H.; Li, R.; Xie, J.; Xu, B. LIM kinase 1 acts as a profibrotic mediator in permanent atrial fibrillation patients with valvular heart disease. J. Biosci. 2019, 44, 16. [Google Scholar] [CrossRef] [PubMed]
- Cebula, M.; Schmidt, E.E.; Arnér, E.S.J. TrxR1 as a Potent Regulator of the Nrf2-Keap1 Response System. Antioxid. Redox Signal. 2015, 23, 823–853. [Google Scholar] [CrossRef]
- Woynarowska, B.A.; Woynarowski, J.M. Preferential targeting of apoptosis in tumor versus normal cells. Biochim. Biophys. Acta 2002, 1587, 309–317. [Google Scholar] [CrossRef]
- Schreidah, C.M.; Ratnayake, K.; Senarath, K.; Karunarathne, A. Microcystins: Biogenesis, Toxicity, Analysis, and Control. Chem. Res. Toxicol. 2020, 33, 2225–2246. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).