Abstract
The study of SiO2 nanoparticles (NPs) and their corresponding surface modifications through octadecyltrichlorosilane (OTS) has attracted attention due to their self-cleaning, hydrophobic and superhydrophobic (SHPho) properties, which are desirable for water collection based on the dew condensation effect. Such properties have been addressed by different strategies, of which the development of hybrid superhydrophobic/hydrophilic (SHH) surfaces has shown great promise. In this research, the pairing of OTS-treated and untreated SiO2 NP layers deposited on clay substrates is investigated with the aim of exploring a hybrid SHH surface capable of enhancing dew yield behavior. Infrared analyses were conducted using FTIR to study the interaction between the clay substrate and the OTS-treated and untreated SiO2 NPs. The hybrid SHH surfaces were morphologically characterized, and contact angle (CA) measurements were performed to explore their wettability behavior. The developed hybrid SHH surfaces exhibited hydrophilic (HPhi)/SHPho properties with an improved dew yield performance. The results obtained in this article are of relevance to the development of water-harvesting devices based on hybrid SHH surfaces.
1. Introduction
The replication and imitation of nature have led to our understanding and development of many technologies. This type of research has come to be known as biomimicry, as proposals mimic a certain behavior or property of a natural material, creature or phenomenon. Among these, the engineering of surfaces based on certain animals, such as the Namibia scarab beetle, has received growing interest [1,2,3,4,5,6]. This small creature manifests a unique, tailor-made shell with hydrophobic and hydrophilic properties that allow it to survive in a harsh, dry and water-scarce environment [1,2]. The biomimicry of this creature and other living things with similar attributes has allowed for the development and engineering of surfaces with dewetting, self-cleaning, water-harvesting and superhydrophobic properties [2,6,7,8,9,10,11].
The creation of such surfaces or the treatment of surfaces for the desired properties is a growing field. There are many options of materials and particles capable of being implemented for their corresponding creation [9,10,12,13,14,15,16,17,18,19,20], among which, SiO2 nanoparticles (NPs) have been consistently implemented due to their intrinsic properties [12,21,22,23,24,25,26,27]. Specifically, SiO2 NPs have been extensively investigated because of their capability to manifest superhydrophobic (SHPho) and hydrophilic (HPhi) properties, which are desirable for the engineering of hybrid surfaces [16,21,23,24,25,26,27,28]. Additionally, SiO2 NPs have been demonstrated to possesses excellent adherence to surfaces, such as glass, silane discs, gold (Au), platinum (Pt) and organic substrates (textiles and wood), by being modified or treated with other molecules, such as polyacrylate (PAA), polymethylacrylate (PMA), polydimethylsiloxane (PDMS) and octadecyltrichlorosilane (OTS) [14,16,23,24,25,27,28].
OTS has become increasingly used for its accessibility, consistent results and facile methods for treatment, as well as it being capable of inducing SHPho properties in naturally hydrophilic SiO2 NPs, exhibiting contact angles (CAs) over 150° [24,25,26,29,30,31,32,33]. In addition, the creation of such surfaces can be achieved through different experimental methodologies, such as dip coating and drop casting in different mediums and components for additional benefits [30,32]. For example, Z. G. Bai et al. in ref. [32] managed to coat a surface with OTS-treated SiO2 NPs to achieve a self-healing surface. This versatile pairing allowed for the creation of superhydrophobic/hydrophilic (SHH) surfaces capable of manifesting both properties simultaneously [34,35,36]. In this sense, the creation of surfaces with an SHPho, HPhi or hybrid behavior has attracted scientific attention because of their properties, such as anti-fogging, anti-icing and amphiphobic properties [8,18,37,38,39]. This has created a tendency to view water-harvesting capabilities in the context of experimental studies that quantify this property through the accessible means of fog basking or water recollection and that estimate the yield potential of proposed surface treatments [4,34,37,40,41]. However, the property of dew enhancement is a complex phenomenon, and the required conditions to mimic water harvesting from it can be complicated. Hybrid SHH surfaces have shown promising results as surfaces with water-capturing or dew-enhancing properties [42,43,44]. However, efficient and consistent surface properties are still a significant hurdle that this technology must overcome. A facile method with the ease of reproduction would significantly push passive dew-enhancing technologies forward.
In this study, a surface treatment of SiO2 NPs was investigated to achieve surfaces with SHPho/HPhi behavior via the layered deposition of untreated SiO2 NPs and OTS-treated SiO2 NPs to create a hybrid SHH surface. The SHH surface was created on clay substrates in order to test both functionality and dew-enhancing properties. It was observed that this treated substrate with a hybrid SHH surface manifested the potential for improved water-harvesting capabilities through a proposed surface geometry of the HPhi hexagonal segments. The findings shown in this research demonstrate that the obtained hybrid SHH surface is capable of enhancing dew yield potential for water-harvesting applications.
2. Materials and Methods
SiO2 NPs with an average size distribution between 10 and 20 nm, OTS with an assay ≥90%, toluene (99.8% purity) and isopropyl alcohol (99.7% purity) were purchased from Sigma Aldrich (Toluca, México) and were used without any further purification. Clay material in a roofing tile presentation, used as substrate, was purchased from Ladrillera Mecanizada® (Monterrey, Nuevo León, Mexico).
2.1. Clay Substrate Preparation
Clay material was cut from the roof tile using a Tool-Co Masonry saw purchased from MACTOOL (Monterrey, Nuevo León, Mexico) in order to obtain substrates with dimensions of 2.5 × 3 cm. The obtained clay substrates were rinsed with distilled water to remove residual contaminants or any other undesired particles, and they were dried in a furnace at 100 °C for 8 h prior to use.
2.2. Preparation of the OTS-Treated SiO2 NPs
The OTS-treated SiO2 NPs were obtained by following the methodology described in ref. [25], which consisted of dispersing SiO2 NPs (2 g) in toluene (46 mL) to obtain a 5 wt.% solution, using an ultrasonic homogenizer (Cole-Parmer, Vernon Hills, IL, USA) for 3 min and operating the equipment at 42 KHz with 35% of the maximum amplitude. Then, OTS (1.5 g) was added to the solution and sonicated again to promote the interaction between OTS and SiO2 NPs. Finally, the obtained OTS-treated SiO2 NPs were washed and filtered using a membrane filter with pore size of 0.45 µm and dried in a furnace at 100 °C for 3 h.
2.3. Preparation of the Superhydrophobic (SHPho) Surface
The SHPho surface was created by depositing different number of layers of the OTS-treated SiO2 NPs on clay substrates. For this purpose, OTS-treated SiO2 NPs were immersed in toluene in order to obtain a 5 wt.% solution, and they were dispersed using an ultrasonic bath for 30 min. The layer depositions were carried out through drop casting and by using a micropipette to discharge 500 µL (per layer) of the OTS-treated SiO2 NP solution to create the SHPho surfaces. A schematic representation of the SHPho-layered depositions is shown in Figure 1.
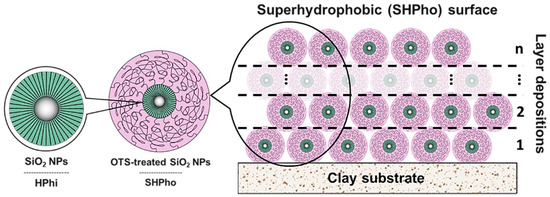
Figure 1.
Schematic representation of the OTS-treated SiO2 NP-layered depositions on clay substrate to create the superhydrophobic (SHPho) surface.
The SHPho behavior was investigated for one-, two- and three-layer depositions as it is described for samples S1, S2 and S3 in Table 1. After each layer deposition, the samples were dried in a furnace at 80 °C for 1 h. The authors limited the investigation of the SHPho surfaces to three layers since, after this condition, the surface became brittle.

Table 1.
Experimental description of the OTS-treated SiO2 NP layer depositions on clay substrate to investigate the SHPho surface behavior.
2.4. Morphology Characterization
The morphology characterization of the NPs was performed using SEM (ZEISS, EVO MA 25, Oberkochen, Germany) and TEM (JEOL, JEM 200 CX, México City, México) microscopes. The SEM micrographs were obtained by considering secondary electron (SE) images taken with an acceleration voltage of 25 KV, and the corresponding TEM micrographs were obtained by operating the system at 200 KV. The preparation of SiO2 NPs for TEM consisted of dispersing them in isopropyl alcohol using an ultrasonic bath for 5 min and then depositing them onto a lacey carbon mesh grid.
2.5. Specific Surface Area Analysis
The nitrogen adsorption/desorption analyses were carried out at −196 °C in a gas-sorption Quantachrome Nova 3200 system (San Luis Potosí, Mexico). Firstly, 50 mg of SiO2 NPs was placed in vacuum at 200 °C for 15 h to remove any adsorbed components. The Brunauer–Emmett–Teller (BET) method was used to calculate the specific surface area value, while for the pore volume and pore diameter values, the Barrett–Joyner–Halenda (BJH) method was used. In both tests, the average of 70 measurements was considered.
2.6. Surface Roughness Analysis
The surface roughness measurements were carried out using an Alicona Infinite Focus G4 microscope (Imaging GmbH, Graz, Austria) and using a 20× lens magnification, which allows a vertical resolution of 50 μm and a lateral resolution of 3.5 μm. The corresponding roughness values (Ra) were retrieved from the average of 10 measurements taken from three different zones (center, left and right) of the samples and using a cut-off length (Lc) filter of 80 µm. Such analyses were performed in accordance with the ISO 4287 standard norm.
2.7. FTIR
The infrared (IR) analyses were carried out using FTIR equipment (PerkinElmer, Frontier 8000, Mexico City, Mexico) and assisted with the ATR accessory. The measurements were performed using a ZnSe–Diamond crystal and a spectral interval ranging from 4000 to 400 cm−1 with a resolution of 2 cm−1. All measurements were carried out by pressing the materials with a tip in order to ensure good contact with the incident IR beam. In the specific case of the clay substrate, a portion was powdered in order to perform the IR measurement.
2.8. TGA
Thermogravimetric characterizations were carried out using TGA equipment (Pyris 8000, Perkin Elmer, Mexico City, Mexico) at a temperature ranging from 30 to 600 °C using nitrogen as purge gas and then by heating it up to 700 °C in oxygen for ultimate thermo-oxidation. The measurements were performed considering a heating rate of 10 °C/min.
2.9. Contact Angle
Contact angle (CA) tests were carried out using optical CA equipment (Data Physics, OCA 15EC, Mexico City, Mexico), and the values were retrieved using SCA20 software. The measurements were performed using a dispenser with a volume capacity of 10 μL and a deposition ratio of 1 μL/s. Once the water droplet was deposited on the sample surface, the CA values were calculated taking into account the average of three measurements and considering the average value taken from the right and left sides of the droplet.
3. Results and Discussion
3.1. Morphological Characterization and Surface Area Analysis of SiO2 NPs
The SEM and TEM morphological characterizations for the OTS-treated and untreated SiO2 NPs are shown in Figure 2. The corresponding SEM micrographs in Figure 2b,c mainly show the presence of SiO2 NP cluster agglomerations. It is possible to note that, in Figure 2c, for the OTS-treated SiO2 NPs, there is an increment in size of the observed NP cluster agglomerations, which may be the result of their entanglement caused by the OTS long-chain alkyl groups. A close up of the SiO2 NPs is given by the TEM micrograph in Figure 2a, revealing dimensions of about 15–20 nm.
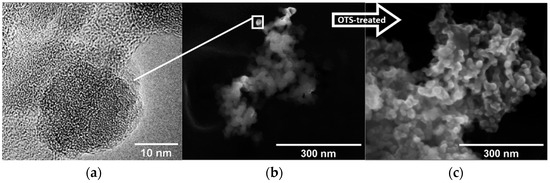
Figure 2.
(a) TEM micrograph of an isolated SiO2 nanoparticle with a size of about 15–20 nm. SEM micrographs for (b) untreated and (c) OTS-treated SiO2 NPs showing the presence of cluster agglomerations.
The BET area values calculated for both the OTS-treated and untreated SiO2 NPs are listed in Table 2. The results for the untreated SiO2 NPs exhibit a surface area average of 483.5 m2/g, while the OTS-treated SiO2 NPs exhibit a value of 81.5 m2/g. However, the pore volume value obtained for the untreated SiO2 NPs is 0.74 cm3/g, with an average pore diameter of 6.7 nm, while for the OTS-treated SiO2 NPs, a pore volume value of 0.27 cm3/g is obtained, suggesting that the OTS long-chain alkyl groups can occupy the surface of the SiO2 NPs and, hence, decrease such a value. The corresponding isotherms can be found in Supplementary Materials (Figure S1).

Table 2.
Specific surface area and pore volume values calculated for untreated and OTS-treated SiO2 NPs.
3.2. Surface Analysis: Contact Angle and Surface Roughness
The CA measurements for samples S1–S3 exhibit values up to 150° as shown in Figure 3. Such values are higher than those measured for the clay substrate, which exhibited a value of 0°, since the water drop was completely absorbed after 5 s. Therefore, it is of relevance to note that, after the first layer deposition of the OTS-treated SiO2 NPs (sample S1), the CA value increased up to 157°, suggesting that such a layer was efficiently deposited on the clay substrate. As a consequence, SHPho behavior was achieved, avoiding the absorption of the water drop. The corresponding CA values for samples S2 and S3 (two- and three-layer depositions) are 156° and 158°, respectively.
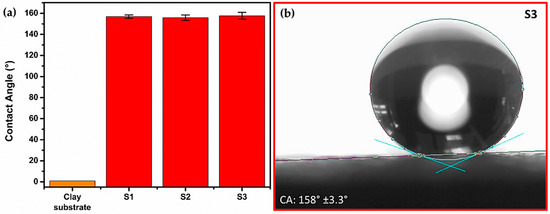
Figure 3.
(a) Contact angle values measured for clay substrate and samples S1–S3, corresponding to one-, two- and three-layer depositions of OTS-treated SiO2 NPs. (b) A water drop stabilized on the surface of sample S3 exhibiting SHPho behavior.
The 3D mapping of the surface roughness for the clay substrate and samples S1–S3 is shown in Figure 4. As expected, the surface morphology is irregular in all samples due to the intrinsic nature of clay material and its manufacturing conditions. Despite the presence of such morphological irregularities, this condition allows one to achieve an effective attachment of the OTS-teated SiO2 NPs in each layer deposition. The latter is corroborated through the CA tests, since the SHPho behavior is achieved after the first layer deposition for sample S1.
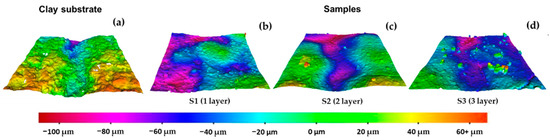
Figure 4.
The 3D mapping of the surface roughness for (a) clay substrate and samples of OTS-treated SiO2 NPs: (b) S1 one-layer deposition, (c) S2 two-layer deposition and (d) S3 three-layer deposition.
Figure 5 shows the roughness values retrieved from the surface morphology mappings. The corresponding Ra value obtained for the clay substrate is about 1.59 µm, which is consistent with the inhomogeneous morphology exhibited in its corresponding 3D mapping. The Ra values for samples S1–S3 are 0.77 µm, 0.56 µm and 0.93 µm, respectively. Such values are similar to one another and slightly different from the value obtained for the clay substrate, suggesting that the surface is slightly modified by the progressive coating of the OTS-treated SiO2 NPs.
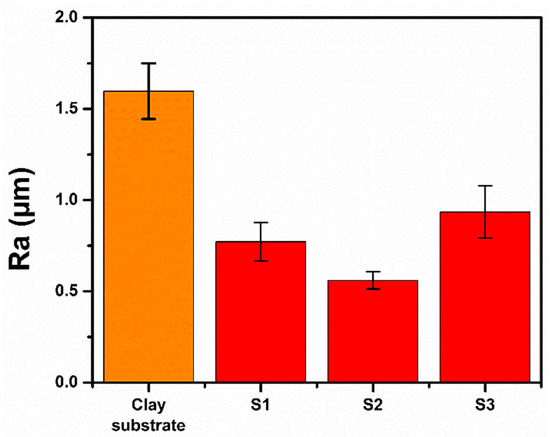
Figure 5.
Average roughness values (Ra) calculated for clay substrate and samples S1–S3.
3.3. FTIR
The infrared spectra for the untreated and OTS-treated SiO2 NPs, as well as for the OTS reference and clay substrate, are shown in Figure 6. The band detected around 1076 cm−1 in the spectrum for the SiO2 NPs is attributed to the siloxane functional group (Si-O-Si), corresponding to the asymmetric stretching and bending vibrations. The band detected at 800 cm−1 is associated with the symmetrical stretching vibration of Si–O, which corresponds to the silicate ion transmittance band. Similarly, the band around 500–450 cm−1 is associated with the siloxane bending modes. However, the double band observed at 564 and 588 cm−1 for the OTS reference corresponds to the Si–Cl stretching mode, which vanishes in the preparation of the OTS-treated SiO2 NPs [45]. The band detected at 1468 cm−1 is associated with asymmetric CH3 bending, and the bands around 2852 and 2924 cm−1 are associated with the OTS CH3 and CH2 long-chain alkyl groups, respectively, which allow us to confirm an interaction between the OTS and SiO2 NPs. The obtained spectrum for the clay substrate shows the contribution from the different materials as it is addressed in refs. [46,47,48]. The broad band 1300–800 cm−1 is attributed to phyllosilicates and tectosilicates, which are characteristic in silica tetrahedron–alumina octahedron–silica tetrahedron (T-O-T) structures. The band around 700–450 cm−1 can be attributed to the presence of quartz, hematite and kaolinite structures. The FTIR spectrum of the OTS-treated SiO2 NPs/clay shows the characteristic bands previously mentioned, such as the long-chain alkyl groups of OTS; it also shows the asymmetric and bending vibrations of the siloxane functional groups (Si-O-Si) and the symmetrical stretching vibration of Si–O, both corresponding to SiO2 NPs, as well as showing the broad band attributed to the T-O-T structures of the clay. With all this information, it is possible to consider that the interaction between the SiO2 NPs and the substrate is due to the rough surface of the clay and to the electrostatic forces between the surface and treated nanoparticles.
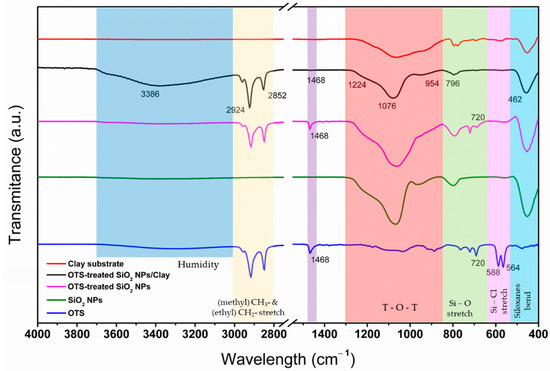
Figure 6.
FTIR spectra for clay substrate, OTS, and treated- and untreated SiO2 NPs, as well as for the OTS-treated SiO2 NPs layered on the clay substrate.
3.4. TGA
The thermogravimetric measurements are shown in Figure 7. The corresponding thermogram for the untreated SiO2 NPs exhibits a small thermal degradation before 100 °C, which can be attributed to water evaporation. The OTS reference shows two steps of degradation around 270 and 480 °C, which are associated with the degradation of octadecyl and alkyl functional groups, respectively. As a result of the first step of degradation, a silane powder is formed, which is almost completely degraded after the second step of degradation. In the case of the OTS-treated SiO2 NPs, it is possible to distinguish the two steps of degradation, with the first one associated with the removal of octadecyl groups around 280–300 °C and the second one associated with the removal of alkyl groups from OTS ≥ 480 °C, forming volatile products [49,50]. The latter corroborates that there is an effective interaction between the SiO2 NPs and OTS.
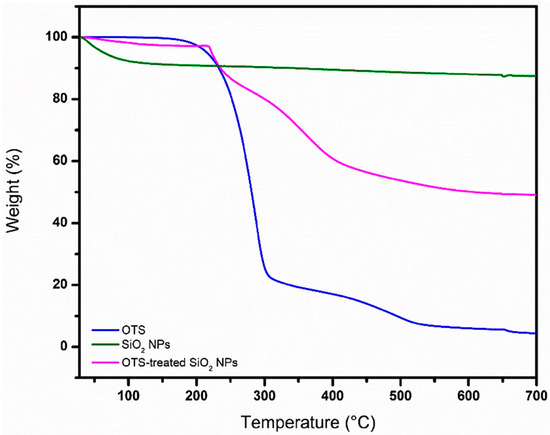
Figure 7.
TGA analysis and comparison of OTS, SiO2 NPs and OTS-treated SiO2 NPs.
3.5. Development of the Hybrid Superhydrophobic/Hydrophilic (SHH) Surface
The SHH surface was developed by using the best experimental conditions achieved in the methodologies previously discussed and by performing alternated layer depositions of OTS-treated (SHPho) and untreated (HPhi) SiO2 NPs. The procedure consisted of depositing two layers of OTS-treated NPs over the clay substrate to create an SHPho surface. The corresponding layer depositions were conducted by following the same procedure previously described in Section 2.3. Subsequently, hexagonal segments containing HPhi NPs were deposited over the SHPho surface using a mask with hexagonal-patterned geometry, as shown in Figure 8a,b. The dimensions of such hexagonal patterns are 5 mm for both the distances across the corners and flats, considering a distance of 7.5 mm between each hexagonal pattern geometry as shown in Figure 8c. Eventually, the developed HPhi-/SHPho-layered depositions exhibited hybrid SHH surfaces as presented in Figure 9d.
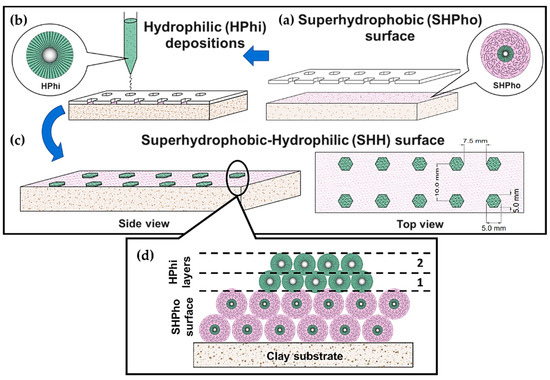
Figure 8.
Schematic of the SHH surface creation process showing (a) the placing of the mask over SHPho surface, (b) deposition layer by layer by drop casting of HPhi layers assisted by a hexagonal-patterned mask, (c) top view of the finished SHH surface with the hexagonal-patterned geometries and (d) a close-up of the HPhi/SHPho layer depositions for the hybrid surface.
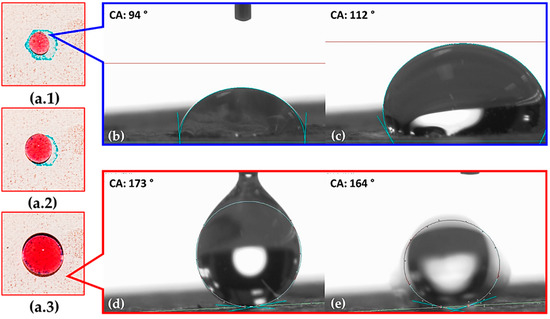
Figure 9.
Hybrid SHH surface development with the HPhi (blue) and SHPho (red) surfaces. Each segment (a.1–a.3) shows the same HPhi surface. Panel (b) shows an initial 10 µL water droplet on hexagonal HPhi segments, (c) shows the water drop CA before collapsing, (d) shows initial contact readings on SHPho surface, and (e) shows SHPho surface CA in an instant as water droplet rolls off the surface.
The surface roughness analysis of the developed hybrid SHH surface can be found in Supplementary Materials (Figure S2), and it obtained Ra values of 3.25 and 2.42 µm for the HPhi and SHPho segments, respectively. As previously discussed in Section 3.2, there is a major dominance from the surface irregularities of the clay substrate, which allow one to achieve a better anchoring of the SHPho layers. However, the deposited untreated SiO2 NPs can interact with the SHPho surface, which, in combination with the irregular surface, create the necessary conditions to form hexagonal HPhi segments.
The wettability behavior of the hybrid SHH surface is shown in Figure 9. The hexagonal HPhi segments were dyed blue in order to distinguish them from the SHPho surface. The corresponding CA value measured for the SHPho surface is around 160° as shown in Figure 9d,e, and it is consistent with the value measured for a similar surface, such as sample S2. The relatively high CA values measured in the hexagonal HPhi segments, shown in Figure 9b,c, are around 94–112°, and this is due to HPhi/SHPho layer interaction. The latter sets the optimal conditions to successfully achieve both water absorption avoidance and the progressive water accumulation to form large drops within the hexagonal HPhi segments, as shown in the sequenced pictures in Figure 9a.1–a.3 for the red-dyed water droplets. Such findings indicate that there is hybrid behavior in the developed SHH surface.
4. Conclusions and Perspectives
In this paper, there was a successful, consistent manifestation and replication of a superhydrophobic/hydrophilic (SHH) surface over clay substrates. This means that OTS-treated SiO2 NPs were paired with untreated SiO2 NPs to create a hybrid SHH surface over a clay substrate, with the aim of application to clay roofing tiles. The annealing process in a furnace at 80 °C after each layer deposition led to improved performance. It is proposed that the rapid evaporation of the deposition medium caused by the annealing allowed for the NPs to be better anchored to the substrate surface. This was observed for both the SHPho (OTS-treated SiO2 NPs) surface and for the hexagonal HPhi (SiO2 NPs) segments over the SHPho-created surface. Hybrid properties were not only observed by CA readings but also by surface roughness profiling. The hexagonal HPhi segments over the SHPho surface showed an increase in roughness (Ra), but it is believed that the roughness manifested by the SHPho coating allowed the SiO2 NPs to anchor themselves and create the hexagonal HPhi segments through an increase in surface area and contact points. This requires further research, but the observed results are promising. There is potential for further insight into and understanding of the relationship between the surface properties.
The perspectives of this work were based on achieving dew-enhancing capabilities from the proposed hexagonal HPhi geometry, which exceeded expectations and motivates further research in this field and on the developed proposal (concept and hypothesis). From the experimental data gathered and recorded, it is believed that the surface morphology or geometry can have a greater influence on water-harvesting capabilities than expected. This can be caused by the occurrence of nucleation or a possible difference in surface energy. Neither of these hypotheses are part of the scope of the current study, but it is strongly believed that the surface geometry proposed (hexagonal) has a considerable impact on the capabilities of the treated substrate. The results of this study were successful in creating an SHH surface capable of manifesting HPhi/SHPho properties simultaneously. Additionally, CA measurements showed that hydrophilic segments could retain water droplets, promoting nucleation and condensation, before naturally coalescing and rolling off the surface. The results of this research on SHH surface characterization encourages further research on the development of surfaces capable of water harvesting through the connection between SHH surfaces, surface properties and dew-enhancing capabilities. Finally, it is proposed that this surface treatment has the potential to reach or even exceed the current water-harvesting technologies in terms of performance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app12031526/s1, Figure S1: Nitrogen adsorption/desorption isotherm analyses, Figure S2: Surface roughness at 5× magnification of the SHH surface with (a) general 3D mapping of the hybrid SHH surface, (b) hydrophilic (HPhi) surface area roughness analysis and (c) superhydrophobic (SHPho) surface area roughness analysis.
Author Contributions
Conceptualization, L.A.B.-J. and A.O.S.; methodology, A.O.S.; software, L.A.B.-J.; validation, L.A.B.-J., A.O.S., N.A.U.-C. and J.I.-E.; formal analysis, L.A.B.-J., A.O.S. and N.A.U.-C.; investigation, L.A.B.-J., A.O.S. and N.A.U.-C.; resources, A.O.S., O.M.-R. and A.E.-Z.; data curation, A.O.S. and N.A.U.-C.; writing—original draft preparation, L.A.B.-J. and A.O.S.; writing—review and editing, A.O.S. and N.A.U.-C.; visualization, A.O.S.; supervision, A.O.S. and N.A.U.-C.; project administration, A.O.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The characterizations were performed in the National Lab in Additive Manufacturing, 3D Digitizing and Computed Tomography (MADiT) LN299129. We also thank V. Rodríguez-González for the support during the use of the laboratory “Nuevos Nanomateriales Catalíticos” at Instituto Potosino de Investigación Científica y Tecnológica (IPICYT).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Parker, A.R.; Lawrence, C.R. Water capture by a desert beetle. Nature 2001, 414, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zeng, J.; Yu, X.; Liang, C.; Zhang, Y. Beetle-like Droplet-Jumping Superamphiphobic Coatings for Enhancing Fog Collection of Sheet Arrays. RSC Adv. 2019, 10, 282–288. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; QI, M.; Qian, Y.F.; Song, R.Y.; DU, B. Hydrophobic and Breathable Nanomembrane for Food Package Material by Mimicking Cocoon’s Structure. Ther. Sci. 2015, 19, 1267–1271. [Google Scholar] [CrossRef]
- Lin, J.; Tan, X.; Shi, T.; Tang, Z.; Liao, G. Leaf Vein-Inspired Hierarchical Wedge-Shaped Tracks on Flexible Substrate for Enhanced Directional Water Collection. ACS Appl. Mater. Interfaces 2018, 10, 44815–44824. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B.; Jung, Y.C. Wetting, Adhesion and Friction of Superhydrophobic and Hydrophilic Leaves and Fabricated Micro/Nanopatterned Surfaces. J. Phys. Condens. Matter 2008, 20, 225010. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Kim, W.; Park, S.; Kim, S.; Gwon, Y.; Kim, J. Leaf-Inspired Micro- and Nanoengineered Surfaces for Controlled Hydrophilic and Hydrophobic Properties. Macromol. Res. 2020, 28, 57–61. [Google Scholar] [CrossRef]
- Malaga, K.; Mueller, U. Relevance of Hydrophobic and Oleophobic Properties of Antigraffiti Systems on Their Cleaning Efficiency on Concrete and Stone Surfaces. J. Mater. Civil Eng. 2013, 25, 755–762. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Shi, L.; Li, J.; Xin, Y.; Yang, T.; Guo, Z. Transparent Superhydrophobic/Superhydrophilic Coatings for Self-Cleaning and Anti-Fogging. Appl. Phys. Lett. 2012, 101, 033701. [Google Scholar] [CrossRef]
- Lai, Y.; Tang, Y.; Gong, J.; Gong, D.; Chi, L.; Lin, C.; Chen, Z. Transparent Superhydrophobic/Superhydrophilic TiO2-Based Coatings for Self-Cleaning and Anti-Fogging. J. Mater. Chem. 2012, 22, 7420–7426. [Google Scholar] [CrossRef]
- Tadanaga, K.; Morinaga, J.; Matsuda, A.; Minami, T. Superhydrophobic−Superhydrophilic Micropatterning on Flowerlike Alumina Coating Film by the Sol−Gel Method. Chem. Mater. 2000, 12, 590–592. [Google Scholar] [CrossRef]
- Fernandes, C.S.; Md Nordin, N.A.H.; Bilad, M.R.; Matsuura, T.; Putra, Z.A.; Wirzal, M.D.H.; Jaafar, J. Explication of Hydrophobic Silica as Effective Pore Former for Membrane Fabrication. Appl. Surf. Sci. Adv. 2021, 3, 100051. [Google Scholar] [CrossRef]
- Rosales, A.; Esquivel, K. SiO2@TiO2 Composite Synthesis and Its Hydrophobic Applications: A Review. Catalysts 2020, 10, 171. [Google Scholar] [CrossRef] [Green Version]
- Subasri, R.; Hima, H. Investigations on the Use of Nanoclay for Generation of Superhydrophobic Coatings. Surf. Coat. Technol. 2015, 264, 121–126. [Google Scholar] [CrossRef]
- Yi, Z.; Zhao, B.; Liao, M.; Qin, Z. Fabrication of Superhydrophobic Wood Surface by Etching Polydopamine Coating with Sodium Hydroxide. Coatings 2020, 10, 847. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Duan, X.; Li, G.; Wang, J. Halloysite Nanotube Supported Ag Nanoparticles Heteroarchitectures as Catalysts for Polymerization of Alkylsilanes to Superhydrophobic Silanol/Siloxane Composite Microspheres. J. Colloid Interface Sci. 2014, 436, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Milionis, A.; Bayer, I.S.; Fragouli, D.; Brandi, F.; Athanassiou, A. Combination of Lithography and Coating Methods for Surface Wetting Control. In Updates in Advanced Lithography; IntechOpen: London, UK, 2013. [Google Scholar]
- Snapp, P.; Kim, J.M.; Cho, C.; Leem, J.; Haque, M.F.; Nam, S.W. Interaction of 2D Materials with Liquids: Wettability, Electrochemical Properties, Friction, and Emerging Directions. NPG Asia Mater. 2020, 12, 22. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, J.; Yu, X.; Zhang, Y. Superamphiphobic Coatings with Polymer-Wrapped Particles: Enhancing Water Harvesting. J. Mater. Chem. A 2019, 7, 5426–5433. [Google Scholar] [CrossRef]
- Pazokifard, S.; Farrokhpay, S.; Mirabedini, M.; Esfandeh, M. Surface Treatment of TiO2 Nanoparticles via Sol-Gel Method: Effect of Silane Type on Hydrophobicity of the Nanoparticles. Prog. Organ. Coat. 2015, 87, 36–44. [Google Scholar] [CrossRef]
- Wang, R.; Wu, F.; Yu, F.; Zhu, J.; Gao, X.; Jiang, L. Anti-Vapor-Penetration and Condensate Microdrop Self-Transport of Superhydrophobic Oblique Nanowire Surface under High Subcooling. Nano Res. 2021, 14, 1429–1434. [Google Scholar] [CrossRef]
- Arkles, B. Hydrophobicity, Hydrophilicity and Silanes. Paint Coat. Ind. 2006, 22, 114. [Google Scholar]
- Ganesh, V.A.; Dinachali, S.S.; Raut, H.K.; Walsh, T.M.; Nair, A.S.; Ramakrishna, S. Electrospun SiO2 Nanofibers as a Template to Fabricate a Robust and Transparent Superamphiphobic Coating. RSC Adv. 2013, 3, 3819–3824. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Kang, Y.; Yang, G.; Yu, L.; Zhang, P. Preparation of Superhydrophobic Poly(Methyl Methacrylate)-Silicon Dioxide Nanocomposite Films. Appl. Surf. Sci. 2010, 257, 1473–1477. [Google Scholar] [CrossRef]
- Mirji, S.A. Octadecyltrichlorosilane Adsorption Kinetics on Si(100)/SiO2 Surface: Contact Angle, AFM, FTIR and XPS Analysis. Surf. Interface Anal. 2006, 38, 158–165. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Ogale, S.B.; Vijayamohanan, K.P. Tuning the Hydrophobic Properties of Silica Particles by Surface Silanization Using Mixed Self-Assembled Monolayers. J. Colloid Interface Sci. 2008, 318, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, H.; Wang, P.; Yang, X.; Mao, H. Thermal Stability of Octadecyltrichlorosilane and Perfluorooctyltriethoxysilane Monolayers on SiO2. Nanomaterials 2020, 10, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurav, A.B.; Xu, Q.; Latthe, S.S.; Vhatkar, R.S.; Liu, S.; Yoon, H.; Yoon, S.S. Superhydrophobic Coatings Prepared from Methyl-Modified Silica Particles Using Simple Dip-Coating Method. Ceram. Int. 2015, 41, 3017–3023. [Google Scholar] [CrossRef]
- Ke, Q.; Fu, W.; Jin, H.; Zhang, L.; Tang, T.; Zhang, J. Fabrication of Mechanically Robust Superhydrophobic Surfaces Based on Silica Micro-Nanoparticles and Polydimethylsiloxane. Surf. Coat. Technol. 2011, 205, 4910–4914. [Google Scholar] [CrossRef]
- Majumdar, S.; Devi, P.S.; Giri, P.K.; Goswami, D.K.; Perumal, A.; Chattopadhyay, A. Synthesis of SnO[Sub 2] Nanoparticles Using Ultrasonication. AIP Conf. Proc. 2010, 1276, 1–7. [Google Scholar]
- Tapasa, K.; Pantulap, U.; Petchareanmongkol, B.; Kaewdang, W. Effect of SiO2 Contents in TEOS-SiO2-OTES Hybrid Coating on Glass. Key Eng. Mater. 2019, 798, 128–133. [Google Scholar] [CrossRef]
- Sinha, S.; Wang, C.-H.; Mukherjee, M. Rubrene on Differently Treated SiO2/Si Substrates: A Comparative Study by Atomic Force Microscopy, X-Ray Absorption and Photoemission Spectroscopies Techniques. Thin Solid Films 2017, 638, 167–172. [Google Scholar] [CrossRef]
- Bai, Z.-G.; Bai, Y.-Y.; Zhang, G.-P.; Wang, S.-Q.; Zhang, B. A Hydrogen Bond Based Self-Healing Superhydrophobic Octadecyltriethoxysilane−lignocellulose/Silica Coating. Prog. Organ. Coat. 2021, 151, 106104. [Google Scholar] [CrossRef]
- Oh, T.; Kim, J. Study on the interfacial properties at the OTS treated SiO2 film. In Proceedings of the 2008 International Conference on Condition Monitoring and Diagnosis, Beijing, China, 21–24 April 2008; pp. 276–279. [Google Scholar]
- Hou, K.; Li, X.; Li, Q.; Chen, X. Tunable Wetting Patterns on Superhydrophilic/Superhydrophobic Hybrid Surfaces for Enhanced Dew-Harvesting Efficacy. Adv. Mater. Interfaces 2020, 7, 1901683. [Google Scholar] [CrossRef]
- Li, F.; Du, M.; Zheng, Z.; Song, Y.; Zheng, Q. A Facile, Multifunctional, Transparent, and Superhydrophobic Coating Based on a Nanoscale Porous Structure Spontaneously Assembled from Branched Silica Nanoparticles. Adv. Mater. Interfaces 2015, 2, 1500201. [Google Scholar] [CrossRef]
- Liu, S.; Latthe, S.S.; Yang, H.; Liu, B.; Xing, R. Raspberry-like Superhydrophobic Silica Coatings with Self-Cleaning Properties. Ceram. Int. 2015, 41, 11719–11725. [Google Scholar] [CrossRef]
- Thickett, S.C.; Neto, C.; Harris, A.T. Biomimetic Surface Coatings for Atmospheric Water Capture Prepared by Dewetting of Polymer Films. Adv. Mater. 2011, 23, 3718–3722. [Google Scholar] [CrossRef]
- Webb, H.K.; Crawford, R.J.; Ivanova, E.P. Wettability of Natural Superhydrophobic Surfaces. Adv. Colloid Interface Sci. 2014, 210, 58–64. [Google Scholar] [CrossRef]
- Qi, C.; Chen, H.; Shen, L.; Li, X.; Fu, Q.; Zhang, Y.; Sun, Y.; Liu, Y. Superhydrophobic Surface Based on Assembly of Nanoparticles for Application in Anti-Icing under Ultralow Temperature. ACS Appl. Nano Mater. 2020, 3, 2047–2057. [Google Scholar] [CrossRef]
- Seo, D.; Lee, J.; Lee, C.; Nam, Y. The Effects of Surface Wettability on the Fog and Dew Moisture Harvesting Performance on Tubular Surfaces. Sci. Rep. 2016, 6, 24276. [Google Scholar] [CrossRef]
- Kotzen, B. Innovation and Evolution of Forms and Materials for Maximising Dew Collection. Ecocycles 2015, 1, 39–50. [Google Scholar] [CrossRef] [Green Version]
- Gürsoy, M. All-Dry Patterning Method to Fabricate Hydrophilic/Hydrophobic Surface for Fog Harvesting. Colloid Polym. Sci. 2020, 298, 969–976. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, J.; Yu, X.; Liang, C.; Zhang, Y. Water Harvesting Method via a Hybrid Superwettable Coating with Superhydrophobic and Superhydrophilic Nanoparticles. Appl. Surf. Sci. 2019, 465, 986–994. [Google Scholar] [CrossRef]
- Lee, J.-W.; Kim, K.; Ryoo, G.; Kim, J.; Vinoth Kumar, J.; Hwang, W. Super-Hydrophobic/Hydrophilic Patterning on Three-Dimensional Objects. Appl. Surf. Sci. 2022, 576, 151849. [Google Scholar] [CrossRef]
- Yegin, C.; Lu, W.; Kheireddin, B.; Zhang, M.; Li, P.; Min, Y.; Sue, H.J.; Murat Sari, M.; Akbulut, M. The Effect of Nanoparticle Functionalization on Lubrication Performance of Nanofluids Dispersing Silica Nanoparticles in an Ionic Liquid. J. Tribol. 2017, 139, 041802. [Google Scholar] [CrossRef]
- Nirmala, G.; Viruthagiri, G. FT-IR Characterization of Articulated Ceramic Bricks with Wastes from Ceramic Industries. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 126, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Adazabra, A.N.; Viruthagiri, G.; Shanmugam, N. Infrared Analysis of Clay Bricks Incorporated with Spent Shea Waste from the Shea Butter Industry. J. Environ. Manag. 2017, 191, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Madejová, J.; Gates, W.P.; Petit, S. IR Spectra of Clay Minerals. In Developments in Clay Science; Elsevier B.V.: Amsterdam, The Netherlands, 2017; Volume 8, pp. 107–149. [Google Scholar]
- Wang, R.; Baran, G.; Wunder, S.L. Packing and Thermal Stability of Polyoctadecylsiloxane Compared with Octadecylsilane Monolayers. Langmuir 2000, 16, 6298–6305. [Google Scholar] [CrossRef]
- McElwee, J.; Helmy, R.; Fadeev, A.Y. Thermal Stability of Organic Monolayers Chemically Grafted to Minerals. J. Colloid Interface Sci. 2005, 285, 551–556. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).