Abstract
Although hearing devices based on cartilage conduction have become more widely used in Japan, methods for evaluating the output volume of such devices have not yet been established. Although the output of air-conduction-based sound-generating devices (e.g., earphones and hearing aids) can be standardized via the head and torso simulator (HATS), this is not applicable to cartilage conduction devices because the simulated pinna is too soft (hardness: A5) compared with human aural cartilage. In this study, we developed polyurethane pinna that had the same shape but different degrees of hardness (A40, A20, and A10). We then compared the HATS results for the new pinna simulators with data from human ears. We found that the spectral shapes of the outputs increasingly approximated those of human ears as the simulated pinna hardness decreased. When a durometer was pressed against the ear tragus of a human ear, the hardness value ranged from A10 to A20. Accordingly, cartilage-conduction-based sound information could be obtained using a HATS that had a simulated pinna with a similar hardness value.
1. Introduction
Aural cartilage gives form to the pinna and the exterior half of the external auditory canal. When a transducer is pressed against the pinna, the cartilage acts as a diaphragm and produces sound in the external auditory canal [1,2]. The generated sounds and propagated vibrations that arise when a transducer is applied to the ear lobe (adipose tissue) and temporal bone are weaker than those at the tragus, scaphoid fossa, and concha because these latter structures contain cartilage [3,4]. As a result, this sound pathway is termed cartilage conduction [2,3,4,5,6,7,8,9]. Hearing devices based on cartilage conduction (e.g., hearing aids [10] and earphones [11]) are currently being marketed in Japan. Although cartilage conduction and bone conduction are both realized by touching a transducer to the user’s body, the vibrations that propagate through the skull bone when cartilage is stimulated are sufficiently small to disregard the contribution of hearing perception [6].
Although cartilage-conduction-based devices are widely used, there are no standardized procedures or indicators for measuring the output volumes of such devices. Because the transmission pathway of cartilage conduction differs from those of air and bone conduction, the calibration methods of the International Organization for Standardization (air conduction [12] and bone conduction [13]) do not apply to cartilage-conduction-based devices. For example, for bone conduction, an artificial mastoid (Type 4130; Brüel & Kjaer, Naerum, Denmark) is used to simulate the mechanical impedance of the human mastoid [14]. The output of an artificial mastoid can be calibrated by pressing a transducer onto the mastoid sensor with a force of 1 N. However, an artificial mastoid is unfit for monitoring sound in the external auditory canal because it does not include a pinna simulator.
To measure air conduction, the sound pressure at the ear drum can be simulated using a pinna simulator [15] embedded in a head-and-torso simulator (HATS) [16]. However, the output from the HATS is different from that obtained in the human external auditory canal by inserting a probe microphone in it, even if the same transducer is used to stimulate the ear at the same position. For instance, when a ring-shaped transducer was placed on the entrance of the external auditory canal, the sound pressure levels (SPLs) in the canal disagreed with those obtained using the HATS, especially in the low-frequency range, i.e., below 1.5 kHz (see Figure 1a) [17]. However, we found that a soft polyurethane pipe attached to a skull bone model could be used to simulate the aural cartilage of the external auditory canal and that this arrangement was able to produce an SPL that was consistent with data from human ears at this low-frequency range (Figure 1b). Polyurethane resin was used to mimic the elasticity of human skin (human skin gel, Exseal Corporation, Mino, Japan). In contrast, the pinna simulator in the HATS (Type 4128; Brüel & Kjaer, Naerum, Denmark) is embedded as a unit in a silicon rubber base (width × height × thickness: 50 × 60 × 10 mm3), and the hardness (Shore 00 35 or Shore A5 from durometer measurements) is lower than that of human aural cartilage.
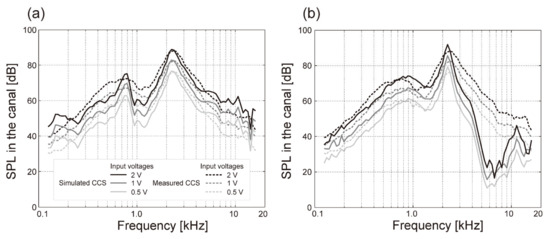
Figure 1.
Simulated cartilage conduction sound (solid line) obtained in the (a) HATS and (b) model aural cartilage. The dashed lines indicate the measured cartilage-conduction sound obtained from the participants. The SPLs obtained in the human canal and by the HATS disagreed, especially in the low-frequency range below 1.5 kHz.
The hardness of the pinna simulator in the HATS is likely to be an important variable in accurately simulating the cartilage conduction sound. Therefore, our research group examined the generated cartilage-conduction sound in five ear simulators, each made of silicon rubber with a different hardness [18]. The ear simulators included the pinna and external auditory canal, and the cartilage-conduction sounds were measured via a probe microphone (type 4182; Brüel & Kjaer, Naerum, Denmark) inserted into the canal. Our results indicated that the spectral characteristics of the cartilage-conduction sounds recorded from the ear simulators approached those from human ears when the hardness of the simulators coincided with that of human aural cartilage (Shore A10 to A20). Accordingly, in the present study, we made HATS-mounted pinna simulators with three different hardness levels and measured the cartilage-conduction sounds. We expect that because the HATS is used in most medical institutes to calibrate normal hearing aids, the ability to upgrade the device by exchanging only the pinna simulator would be convenient for healthcare professionals.
2. Method
2.1. Pinna Simulators
The pinna simulator is made of silicon rubber (Shore 00 35 or Shore A5) and can be easily attached to or removed from the HATS (base: width × height × thickness: 50 × 60 × 10 mm3) via a magnetic backing component. The silicon base has a canal with a diameter of 5 mm and is colinear with respect to the end of the ear canal. The ear canal ends in an occluded ear simulator, which simulates the inner part of the ear canal according to the International Electrotechnical Commission (IEC 60318-4) [16]. The ear simulator contains a half-inch microphone that can be connected to a microphone preamplifier using an adaptor.
We removed the pinna simulator from the HATS and made a mold, which enabled us to make new pinna simulators with three different hardness levels (A40, A20, and A10), as shown in Figure 2a. We used polyurethane resin because the human skin gel, described above, was made of this material [17]. Although the original pinna simulator in the HATS (original HATS pinna after that) had a concavo-convex surface on the reverse side of the base where it joined with the HATS body, the new pinna simulator had a flat surface. Furthermore, the new pinna simulators did not have a magnet to join them to the HATS. Thus, we used rubber cement (Blu Tack, Bostik Australia Pty. Ltd., Thomastown, Australia) to fix and fill the gaps between the pinna simulator and the HATS body. The masses of the pinna simulators were 42 g for A40, 44 g for A20, 48 g for A10, and 49 g for the original HATS pinna.
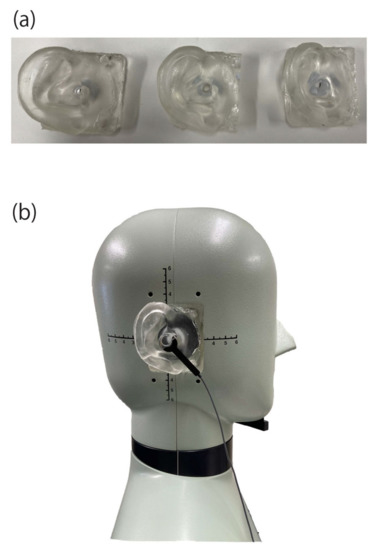
Figure 2.
(a) Developed pinna simulators (A10, A20, and A40 from the left) and (b) embedded pinna simulator in the HATS and position of the cartilage conduction transducer at the entrance of the external auditory canal.
2.2. Cartilage Conduction Transducer
As in previous studies, we used an annular transducer to induce cartilage conduction [3,4,5,6,7,8,17,18]. The device comprises an acrylic ring (external diameter: 16 mm; internal diameter: 8 mm) with an air vent to cancel the effects of occlusions and enable cartilage-conduction sound in the air canal to be recorded unmodified. The transducer is composed of a piezoelectric bimorph covered with elastic material. Although some resonance peaks have been observed in the vibrational output, the spectral characteristics are generally flat in the frequency range above 1 kHz [6]. The cartilage conduction transducer was fixed at the entrance of the external auditory canal between the concha wall and tragus, as shown in Figure 2b, so that the application pressure was less than 0.5 N.
2.3. Measurement Procedures
The input signal for the transducer was a pure-tone train with a frequency ranging from 125 Hz to 16 kHz in 1/12 octave steps. The tones were 1 s in duration and were each followed by a 0.5 s silent interval. The input level was 1.0 V. The SPLs were determined according to the spectral peaks at the corresponding pure-tone frequencies.
The pure tones recorded by the built-in ear simulator (half-inch microphone with an acoustic coupler approximating overall acoustic impedance of normal ear) in the HATS were calibrated using a conditional amplifier (NEXUS; Brüel & Kjaer, Naerum, Denmark). The data were digitized with a sampling rate of 44.1 kHz and a 16-bit resolution via an AD-DA converter (Fireface UCX, RME, Haimhausen, Germany), and then stored on a PC (MacBookPro, Apple, Cupertino, CA, USA). The sound recordings were conducted in a soundproof chamber with background noise that was less than 30 dB.
In the following Results section, we compared the SPLs obtained by these simulators with the SPL actually measured by three human participants in our previous research [6]. In that research, the input signals and the transducer were the same as in this study, although the outputs were measured by inserting a probe microphone (type 4182; Brüel & Kjaer, Naerum, Denmark) into the participants’ external auditory canals. We repeated the above methods three times for three participants (one male and two females, ages: 31–40) who did not have diseases of the outer or middle ear. To evaluate the performance of the simulators, the averaged errors between the simulators and humans were calculated from the output differences in response to the input pure tones.
3. Results
Figure 3 shows the SPLs obtained for the HATS according to the hardness of the pinna simulators and the SPLs obtained for human ears. To ensure the repeatability of our method, we attached the transducers to the HATS and removed them three times, as shown by solid lines (the averaged values) in the figure. The dashed lines indicate the average values from three human participants in a previous study [6].
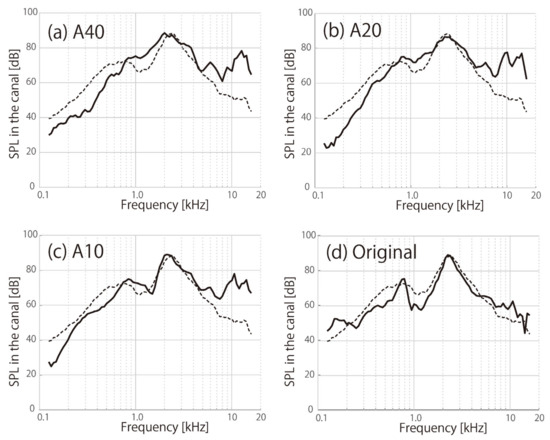
Figure 3.
Simulated cartilage-conduction sound obtained by the HATS with different pinna simulators: (a) A40, (b) A20, (c) A10, and (d) original silicon-rubber-based HATS pinna simulator (solid line). The dashed lines indicate the measured cartilage-conduction sound obtained from the participants. The spectral shapes of A10 and A20 became more similar to those obtained from the participants.
In the human cartilage-conduction sound data (dashed line), the spectral peak around 2.5 kHz is caused by resonance in the external auditory canal, while the peak around 800 Hz is specifically associated with cartilage conduction [6]. Although the lower peak was too sharp for the original HATS pinna, as shown in Figure 3d, the urethane pinna simulators in the present study produced close spectral shapes that were lower than 1 kHz. Particularly, when the hardness of the pinna simulator approached that of human aural cartilage (A10 to A20), the spectral shapes became more similar to the dashed line representing the human data (Figure 3b,c). In contrast, for frequencies higher than 5 kHz, we observed large differences in cartilage conduction between the HATS and human measurements, regardless of the pinna hardness. The averaged errors between the conduction sounds generated by the simulator (solid line) and human cartilage (dashed line) were 6.9 dB for A40, 6.4 dB for A20, 4.8 dB for A10, and 4.8 dB for the original HATS pinna in the frequency range below 5 kHz. Although the original HATS pinna showed a different spectral shape, the averaged error was close to the A10.
4. Discussion
Consistent with previous findings [18], the cartilage-conduction peak (around 800 Hz) of the pinna simulator approached that of human aural cartilage when the hardness of the pinna simulator was A20 or A10 (Figure 3). Unlike the previous simulation study that used a polyurethane pipe [17], the resonance peak in the external auditory canal (around 2.5 kHz) in the present study was closer to that of a human ear because of the more precise pinna shape (see Figure 1b). The frequencies of the HATS at the maximum peak below 1 kHz were 1 kHz for A40, 841 Hz for A20, and 841 Hz for A10, while that for a human ear was 749 Hz. In a previous study [18], these frequencies were 1414 Hz for A40, 1 kHz for A20, and 561 Hz for A10. The previous [18] and current pinna simulators were made out of silicon rubber and polyurethane resin, respectively, so dissimilarities in the physical properties might have created the differences in peak frequencies. If the cartilage-conduction peak is shifted toward the lower-frequency range (Figure 3c), the outputs from the pinna simulators and real ears can be more closely approximated for sounds below this peak.
The durometer value can be applied to Young’s modulus using the following equation:
where E is Young’s modulus [Mpa] and S is a type A durometer hardness [19]. This equation is considered a good first-order approximation of Young’s modulus for type A hardness, especially from A20 to A80. Based on this equation, the Young’s moduli of the pinna simulators were 1.69 Mpa for A40, 0.73 Mpa for A20, 0.41 Mpa for A10, and 0.28 Mpa for the original HATS pinna. Although Young’s moduli are often measured in research on auricular reconstructions, the values differ according to the measurement equipment and procedure. For cadaveric auricular cartilage dissected from the skin and fascia, the measured Young’s moduli were 5.02 Mpa in [20] and 1.41 to 2.08 Mpa in [21]. To simulate sound transmission numerically through an ear-filling earplug, Weinmann et al. assigned Young’s moduli to each type of tissue that makes up the pinna: 0.65 Mpa for skin, 0.001 Mpa for fat, 0.79 Mpa for muscle, 13.6 Mpa for bone, and 1.9 Mpa for cartilage [22]. According to this classification, the A20 and A10 correspond to the skin or muscle. In the previous study, the hardness of human aural cartilage was measured using a durometer (GS-719N; Teclock, Nagano, Japan) [18]. The pointed cylinder of the durometer was pressed against the ear tragus and was allowed to inflect the tragus during the measurement. In this case, the pointed cylinder directly contacted the skin over the cartilage, and so the measured hardness might be lower than the hardness of the singular aural cartilage. In hardness measurements that limited the displacement of the pinna, the hardness of the ear helix ranged from A25 to A55 according to individual differences [23]. The numerical simulations conducted by Weinman et al. revealed that the vibrations at the points of the pinna might be propagated via the surface of the skin [22]. However, stimulation at the ear lobe (adipose tissue) did not effectively propagate vibrations on the pinna, even though the ear lobe is also covered by skin [3]. Furthermore, vibrations were not propagated through the osseous part of the external auditory canal even though this bone is covered by skin [8]. According to these data, the layer construction of the skin and the aural cartilage appear to facilitate vibrational propagation.
For frequencies higher than 5 kHz, the pinna simulators showed a large gap with respect to the output of the human ear, regardless of the material hardness (Figure 3a–c). Although such a high-frequency range is not so essential for the output evaluation of a hearing aid aimed at speech recognition [24,25], the gap cannot be ignored for listening to music via the cartilage-conduction devices. The original HATS pinna had a concavo-convex surface on the base where it attached to the body of the HATS. In contrast, our new pinna simulators had a flat surface on the base. As a result, there was a narrow clearance with respect to the HATS device, although we tried to fill this space with rubber cement. Resonance in the remaining cavity might explain the difference in the output between the pinna simulator and real human ears. In further study, we hope to replicate the concavo-convex surface using 3D-printing technology for the purpose of filling the unnecessary cavities, and we will take effect beginning with measuring the output of the current commercial-release cartilage-conduction devices.
5. Conclusions
We embedded pinna simulators with three different hardness levels into the HATS and compared the outputs with those of human ears for the purpose of calibrating cartilage-conduction sounds. The spectral shapes of the simulator outputs more closely approximated those of human ears in the frequency lower than 5 kHz as the hardness decreased from A40 to A10. The hardness value of the simulator (A20 or A10) corresponded to that of when a durometer is pressed onto the ear tragus of a human ear, although anatomically removed aural cartilage is harder. While an ideal pinna simulator might have a layer structure that simulates the soft skin and hard aural cartilage of the human ear, a pinna simulator with a uniform structure is more easily manufactured. The ideal design must approximate the output of human ears via a careful selection of materials, hardness, and mass. In addition, we expect that the excessively emphasized SPL in the higher frequency than 5 kHz will be managed by designing the concavo-convex surface on the base of the simulators.
Author Contributions
The conceptualization, methodology, data analysis, and original draft of this study were developed by R.S., and the discussion and subsequent conclusion were supervised by H.H. and T.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a Grant-in-Aid for Science Research (B) from the Japan Society for the Promotion of Science (18H03560).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Masaki Kawashima (Exseal, Co., Ltd.) for making the pinna simulators. We thank R.W. Haase and Sydney Koke, from the Edanz Group (https://en-author-services.edanzgroup.com/, accessed on 9 November 2022) for editing a draft of this manuscript. We also thank the Elsevier Permission Granting Team for permitting us to reprint the figure.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hosoi, H. Receiver. Japanese Patent Application Number 166644, 4 June 2004. [Google Scholar]
- Hosoi, H. Approach in the use of cartilage conduction speaker. Japanese Patent Number 4541111, 17 November 2004. [Google Scholar]
- Shimokura, R.; Hosoi, H.; Nishimura, T.; Yamanaka, T. Aural cartilage vibration and sound measured in the external auditory canal for several transducer positions. J. Temp. Des. Arch. Env. 2014, 12, 137–143. [Google Scholar]
- Shimokura, R.; Hosoi, H.; Iwakura, T.; Nishimura, T.; Matsui, T. Development of monaural and binaural behind-the-ear cartilage conduction hearing aids. Appl. Acoust. 2013, 74, 1234–1240. [Google Scholar] [CrossRef]
- Nishimura, T.; Hosoi, H.; Sugiuchi, T.; Matsumoto, N.; Nishiyama, T.; Takano, K.; Sugimoto, S.; Yazama, H.; Sato, T.; Komori, M. Cartilage conduction hearing aid fitting in clinical practice. J. Am. Acad. Audiol. 2021, 32, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Shimokura, R.; Hosoi, H.; Nishimura, T.; Yamanaka, T.; Levitt, H. Cartilage conduction hearing. J. Acoust. Soc. Am. 2014, 135, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Hosoi, H.; Saito, O.; Miyamae, R.; Shimokura, R.; Matsui, T.; Yamanaka, T.; Levitt, H. Is cartilage conduction classified into air or bone conduction? Laryngoscope 2014, 124, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Hosoi, H.; Saito, O.; Miyamae, R.; Shimokura, R.; Yamanaka, T.; Kitahara, T.; Levitt, H. Cartilage conduction is characterized by vibrations of the cartilaginous portion of the ear canal. PLoS ONE 2015, 10, e0120135. [Google Scholar] [CrossRef]
- Hosoi, H.; Nishimura, T.; Shimokura, R.; Kitahara, T. Cartilage conduction as the third pathway for sound transmission. Auris Nasus Larynx 2019, 46, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.rionet.jp/product/series/ccha/ (accessed on 9 November 2022). (In Japanese).
- Available online: https://www.audio-technica.co.jp/product/ATH-CC500BT (accessed on 9 November 2022). (In Japanese).
- ISO 389-2; Acoustics—Reference zero for the calibration of audiometric equipment—Part 2: Reference equivalent threshold sound pressure levels for pure tones and insert earphones. International Organization for Standardization: Geneva, Switzerland, 1994.
- ISO 389-3; Acoustics—Reference zero for the calibration of audiometric equipment—Part 3: Reference equivalent threshold force levels for pure tones and bone vibrators. International Organization for Standardization: Geneva, Switzerland, 1994.
- IEC 60318-6 ; Electroacoustics—Simulations of human head and ear—Part 6: Mechanical coupler for the measurement on bone vibrators. International Electrotechnical Commission: Geneva, Switzerland, 2007.
- IEC 60318-7; Electroacoustics—Simulations of human head and ear—Part 7: Head and torso simulator for the measurement of hearing aids. International Electrotechnical Commission: Geneva, Switzerland, 2011.
- IEC 60318-4; Electroacoustics—Simulations of human head and ear—Part 4: Occluded-ear simulator for the measurement of earphones coupled to the ear by means of ear inserts. International Electrotechnical Commission: Geneva, Switzerland, 2010.
- Shimokura, R.; Hosoi, H.; Nishimura, T.; Iwakura, T.; Yamanaka, T. Simulating cartilage conduction sound to estimate the sound pressure level in the external auditory canal. J. Sound Vib. 2015, 335, 261–268. [Google Scholar] [CrossRef]
- Shimokura, R.; Nishimura, T.; Hosoi, H. Vibrational and acoustical characteristics of ear pinna simulators that differ in hardness. Audiol. Res. 2021, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Gent, A.N. On the relation between indentation hardness and Young’s modulus. Inst. Rubber Ind. Trans. 1958, 34, 46–57. [Google Scholar] [CrossRef]
- Griffin, M.F.; Premakumar, Y.; Seifalian, A.M.; Szarko, M.; Butler, P.E.M. Biomechanical characterization of human auricular cartilages; Implications for tissue engineering. Ann. Biomed. Eng. 2016, 44, 3460–3467. [Google Scholar] [CrossRef] [PubMed]
- Nayyer, L.; Birchall, M.; Seifalian, A.M.; Jell, G. Design and development of nanocomposite scaffolds for auricular reconstruction. Nanomedicine 2014, 10, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Tamura, A.; Irwansyah; Otsuka, S.; Nakagawa, S. Effects of morphology and hardness of the pinna and gender on detection threshold of cartilage conduction. Proc. Autumn Meet. Acoust. Soc. Jpn. 2022, 11, 943–944. (In Japanese) [Google Scholar]
- Blondé-Weinmann, C.; Joubaud, T.; Zimpfer, V.; Hamery, P.; Roth, S. Numerical and experimental investigation of the sound transmission delay from a skin vibration to the occluded ear canal. J. Sound Vib. 2023, 542, 117345. [Google Scholar] [CrossRef]
- Morimoto, C.; Nishimura, T.; Hosoi, H.; Saito, O.; Fukuda, F.; Shimokura, R.; Yamanaka, T. Sound transmission of cartilage conduction in the ear with fibrotic aural atresia. J. Rehabil. Res. Dev. 2014, 51, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Miyamae, R.; Nishimura, T.; Hosoi, H.; Saito, O.; Shimokura, R.; Yamanaka, T.; Kitahara, T. Perception of speech in cartilage conduction. Auris Nasus Larynx 2017, 44, 26–32. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).