Combined Effects of Cold Treatment and Phosphine in Drosophila suzukii (Diptera: Drosophilidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Fumigant and Grapes
2.3. Cold-Temperature Treatment in a 12 L Desiccator
2.4. Fumigation Experiments in a 12 L Desiccator
2.5. Combined PH3 Fumigation and Cold-Temperature Treatment
2.6. Gas Concentration and Sorption Measurement
2.7. Phytotoxicity Evaluation in Grapes
2.8. Statistical Analysis
3. Results
3.1. Effects of Cold-Temperature Treatment
3.2. Fumigation Activity of PH3 in a 12 L Desiccator
3.3. Effects of the Combined Treatment
3.4. Evaluation of the PH3 Sorption Rate in Grapes
3.5. Effects of the Combined Treatment on Grapes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitsui, H.; Takahashi, K.H.; Kimura, M.T. Spatial Distributions and Clutch Sizes of Drosophila Species Ovipositing on Cherry Fruits of Different Stages. Popul. Ecol. 2006, 48, 233–237. [Google Scholar] [CrossRef]
- Walsh, D.B.; Bolda, M.P.; Goodhue, R.E.; Dreves, A.J.; Lee, J.; Bruck, D.J.; Walton, V.M.; O’Neal, S.D.; Zalom, F.G. Drosophila suzukii (Diptera: Drosophilidae): Invasive Pest of Ripening Soft Fruit Expanding Its Geographic Range and Damage Potential. J. Integr. Pest Manag. 2011, 2, G1–G7. [Google Scholar] [CrossRef]
- Calabria, G.; Máca, J.; Bächli, G.; Serra, L.; Pascual, M. First Records of the Potential Pest Species Drosophila suzukii (Diptera: Drosophilidae) in Europe: First Record of Drosophila suzukii in Europe. J. Appl. Entomol. 2012, 136, 139–147. [Google Scholar] [CrossRef]
- Hauser, M. A Historic Account of the Invasion of Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) in the Continental United States, with Remarks on Their Identification. Pest Manag. Sci. 2011, 67, 1352–1357. [Google Scholar] [CrossRef] [PubMed]
- Biondi, A.; Traugott, M.; Desneux, N. Special Issue on Drosophila suzukii: From Global Invasion to Sustainable Control. J. Pest Sci. 2016, 89, 603–604. [Google Scholar] [CrossRef]
- Bell, C.H. Fumigation in the 21st Century. Crop Prot. 2000, 19, 563–569. [Google Scholar] [CrossRef]
- Ren, Y.; Mahon, D. Fumigation trials on the application of ethyl formate to wheat, split faba beans and sorghum in small metal bins. J. Stored Prod. Res. 2006, 42, 277–289. [Google Scholar] [CrossRef]
- Bond, E.J. Manual of Fumigation for Insect Control; FAO Plant production and Protection Paper No 54; Food and Agriculture Organization of the United Nations: Rome, Italy, 1984; pp. 103–113. [Google Scholar]
- UNEP (United Nations Environment Programme). Handbook for the Montreal Protocol on Substances that Deplete the Ozone Layer; UNEP/Earthprint: Nairobi, Kenya, 2006; pp. 10–11. [Google Scholar]
- Ducom, P.J.F. The return of the fumigants. In Proceedings of the Ninth International Working Conference on Stored Product Protection, Campinas, Brazil, 15–18 October 2006; pp. 510–516. [Google Scholar]
- Chaudhry, M.Q. Phosphine resistance. Pestic. Outlook 2000, 11, 88–91. [Google Scholar] [CrossRef]
- Chaudhry, M.Q.; Bell, H.A.; Savvidou, N.; MacNicoll, A.D. Effect of Low Temperatures on the Rate of Respiration and Uptake of Phosphine in Different Life Stages of the Cigarette Beetle Lasioderma serricorne (F.). J. Stored Prod. Res. 2004, 40, 125–134. [Google Scholar] [CrossRef]
- Kaur, R.; Nayak, M.K. Developing Effective Fumigation Protocols to Manage Strongly Phosphine-Resistant Cryptolestes ferrugineus (Stephens) (Coleoptera: Laemophloeidae): Fumigation Protocols for Cryptolestes ferrugineus. Pest Manag. Sci. 2015, 71, 1297–1302. [Google Scholar] [CrossRef]
- Rout, G.; Tripathy, H.M.; Biswal, L. Results of Application of Aluminum Phosphide Pellets to Rice Weevils in an Open Bin and to Angoumois Grain Moths Under Air-Tight Cond. Econ. Entomol. 1969, 62, 715–717. [Google Scholar] [CrossRef]
- Rajendran, S.; Gunasekaran, N. The Response of Phosphine-Resistant Lesser Grain Borer Rhyzopertha dominica and Rice Weevil Sitophilus oryzae in Mixed-Age Cultures to Varying Concentrations of Phosphine. Pest Manag. Sci. 2002, 58, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Phillips, T.W.; Thoms, E.M.; DeMark, J.; Walse, S. Fumigation. In Stored Product Protection; Hagstrum, D.W., Phillips, T.W., Cuperus, G., Eds.; Kansas State University: Manhattan, KS, USA, 2012; pp. 157–177. [Google Scholar]
- Liu, Y.B. Low Temperature Phosphine Fumigation for Postharvest Control of Western Flower Thrips (Thysanoptera: Thripidae) on Lettuce, Broccoli, Asparagus, and Strawberry. J. Econ. Entomol. 2008, 101, 1786–1791. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, L.E.; Page-Weir, N.E.M.; Chhagan, A.; Brash, D.W.; Klementz, D.; Bycroft, B.L.; Woolf, A.B. Phosphine fumigation to disinfest kiwifruit. N. Zealand Plant Prot. 2012, 65, 35–43. [Google Scholar] [CrossRef]
- Li, L.; Liu, T.; Li, B.; Zhang, F.; Dong, S.; Wang, Y. Toxicity of Phosphine Fumigation Against Bactrocera tau at Low Temperature. J. Econ. Entomol. 2014, 107, 601–605. [Google Scholar] [CrossRef]
- Liu, T.; Li, L.; Zhang, F.; Gong, S.; Li, T.; Zhan, G.; Wang, Y. Effect of Low-Temperature Phosphine Fumigation on the Survival of Bactrocera Correcta (Diptera: Tephritidae). J. Econ. Entomol. 2015, 108, 1624–1629. [Google Scholar] [CrossRef]
- Benschoter, C.A. Low-Temperature Storage as a Quarantine Treatment for the Caribbean Fruit Fly (Diptera: Tephritidae) in Florida Citrus. J. Econ. Entomol. 1984, 77, 1233–1235. [Google Scholar] [CrossRef]
- Burikam, I.; Sarnthoy, O.; Charernsom, K.; Kanno, T.; Homma, H. Cold Temperature Treatment for Mangosteens Infested with the Oriental Fruit Fly (Diptera: Tephritidae). J. Econ. Entomol. 1992, 85, 2298–2301. [Google Scholar] [CrossRef]
- Sharp, J.L. Heat and Cold Treatments for Postharvest Quarantine Disinfestation of Fruit Flies (Diptera: Tephritidae) and Other Quarantine Pests. Fla. Entomol. 1993, 76, 212–218. [Google Scholar] [CrossRef]
- Kawakami, F. Current research of alternatives to methyl bromide and its reduction in Japanese plant quarantine. Res. Bull. Plant Prot. Serv. Jpn. 1999, 35, 109–120. [Google Scholar]
- Enriquez, T.; Colinet, H. Cold Acclimation Triggers Lipidomic and Metabolic Adjustments in the Spotted Wing Drosophila Drosophila suzukii (Matsumara). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 316, R751–R763. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, C.; Moharramipour, S.; Asghar Talebi, A. Effects of Cold Acclimation, Cooling Rate and Heat Stress on Cold Tolerance of the Potato Tuber Moth Phthorimaea Operculella (Lepidoptera: Gelechiidae). Eur. J. Entomol. 2014, 111, 487–494. [Google Scholar] [CrossRef]
- Jakobs, R.; Ahmadi, B.; Houben, S.; Gariepy, T.D.; Sinclair, B.J. Cold Tolerance of Third-Instar Drosophila Suzukii Larvae. J. Insect Physiol. 2017, 96, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K.E.; Sinclair, B.J. Repeated Stress Exposure Results in a Survival–Reproduction Trade-off in Drosophila melanogaster. Proc. R. Soc. B Biol. Sci. 2010, 277, 963–969. [Google Scholar] [CrossRef]
- Martini, X.; Malfa, K.; Stockton, D.; Rivera, M.J. Cold Acclimation Increases Asian Citrus Psyllid Diaphorina citri (Hemiptera: Liviidae) Survival during Exposure to Freezing Temperatures. Insect Sci. 2022, 29, 531–538. [Google Scholar] [CrossRef]
- Weller, G.L.; Morton, R. Fumigation with Carbonyl Sulfide: A Model for the Interaction of Concentration, Time and Temperature. J. Stored Prod. Res. 2001, 37, 383–398. [Google Scholar] [CrossRef]
- Armstrong, J.W.; Whitehand, L.C. Effects of Methyl Bromide Concentration, Fumigation Time, and Fumigation Temperature on Mediterranean and Oriental Fruit Fly (Diptera: Tephritidae) Egg and Larval Survival. J. Econ. Entomol. 2005, 98, 1116–1125. [Google Scholar] [CrossRef]
- Barak, A.V.; Wang, Y.; Zhan, G.; Wu, Y.; Xu, L.; Huang, Q. Sulfuryl Fluoride as a Quarantine Treatment for Anoplophora glabripennis (Coleoptera: Cerambycidae) in Regulated Wood Packing Material. J. Econ. Entomol. 2006, 99, 1628–1635. [Google Scholar] [CrossRef]
- Abbott, W.S. A method for computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- AFHB/ACIAR. Suggested recommendations for the fumigation of grain in ASEAN region. In Principles and General Practice, Part 1; ASEAN Food Handling Bureau: Kuala Lumpur, Malaysia, 1989; p. 139. [Google Scholar]
- SAS Institute. SAS Institute SAS User’s Guide, Statistics Version 9, 1st ed.; SAS Institute: Cary, NC, USA, 2009. [Google Scholar]
- Walse, S.S.; Cha, D.H.; Lee, B.H.; Follett, P.A. Postharvest quarantine treatments for Drosophila suzukii in fresh fruit. In Drosophila suzukii Management; Garcia, F.R.M., Ed.; Springer: Cham, Switzerland, 2020; pp. 255–267. [Google Scholar]
- Tait, G.; Mermer, S.; Stockton, D.; Lee, J.; Avosani, S.; Abrieux, A.; Anfora, G.; Beers, E.; Biondi, A.; Burrack, H.; et al. Drosophila suzukii (Diptera: Drosophilidae): A Decade of Research Towards a Sustainable Integrated Pest Management Program. J. Econ. Entomol. 2021, 114, 1950–1974. [Google Scholar] [CrossRef]
- Jeon, J.C.; Kim, H.K.; Koo, H.N.; Kim, B.S.; Yang, J.O.; Kim, G.H. Synergistic Effect of Cold Treatment Combined with Ethyl Formate Fumigation against Drosophila Suzukii (Diptera: Drosophilidae). Insects 2022, 13, 664. [Google Scholar] [CrossRef] [PubMed]
- Dalton, D.T.; Walton, V.M.; Shearer, P.W.; Walsh, D.B.; Caprile, J.; Isaacs, R. Laboratory Survival of Drosophila suzukii under Simulated Winter Conditions of the Pacific Northwest and Seasonal Field Trapping in Five Primary Regions of Small and Stone Fruit Production in the United States. Pest Manag. Sci. 2011, 67, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Follett, P.A.; Swedman, A.; Price, D.K. Postharvest Irradiation Treatment for Quarantine Control of Drosophila Suzukii (Diptera: Drosophilidae) in Fresh Commodities. J. Econ. Entomol. 2014, 107, 964–969. [Google Scholar] [CrossRef] [PubMed]
- Ryan, G.D.; Emiljanowicz, L.; Wilkinson, F.; Kornya, M.; Newman, J.A. Thermal Tolerances of the Spotted-Wing Drosophila Drosophila suzukii (Diptera: Drosophilidae). J. Econ. Entomol. 2016, 109, 746–752. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Park, C.G. X-Ray Radiation and Developmental Inhibition of Drosophila suzukii (Matsumura) (Diptera: Drosophilidae). Int. J. Radiat. Biol. 2016, 92, 849–854. [Google Scholar] [CrossRef]
- Enriquez, T.; Colinet, H. Basal Tolerance to Heat and Cold Exposure of the Spotted Wing Drosophila, Drosophila Suzukii. PeerJ 2017, 5, e3112. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, J.S.; Jeong, J.S.; Choi, D.S.; Park, J.; Kim, I. Phytosanitary Cold Treatment of Spotted-Wing Drosophila, Drosophila suzukii (Diptera: Drosophilidae) in ‘Campbell Early’ Grape. J. Econ. Entomol. 2018, 111, 1638–1643. [Google Scholar] [CrossRef]
- Gutierrez-Palomares, V.M.; Cibrian-Tovar, J.; Alatorre-Rosas, R.; Quezada-Salinas, A. Effect of Irradiation on Quality and Fertility Parameters of Drosophila suzukii in Mexico. Southwest. Entomol. 2019, 44, 617–626. [Google Scholar] [CrossRef]
- Cho, S.W.; Kim, H.K.; Kim, B.S.; Yang, J.O.; Kim, G.H. Combinatory Effect of Ethyl Formate and Phosphine Fumigation on Pseudococcus longispinus and P. orchidicola (Hemiptera: Pseudococcidae) Mortality and Phytotoxicity to 13 Foliage Nursery Plants. J. Asia-Pac. Entomol. 2020, 23, 152–158. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, H.K.; Kyung, Y.; Park, G.H.; Lee, B.-H.; Yang, J.O.; Koo, H.N.; Kim, G.H. Fumigation Activity of Ethyl Formate and Phosphine Against Tetranychus urticae (Acari: Tetranychidae) on Imported Sweet Pumpkin. J. Econ. Entomol. 2018, 111, 1625–1632. [Google Scholar] [CrossRef]
- Kim, B.S.; Choi, J.E.; Choi, D.S.; Yang, J.O. Efficacy and Phytotoxicity Assessment of Successive Application of Methyl Bromide and Cold Treatment on Export Strawberry Fruits. Insects 2021, 12, 990. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.H.; Park, C.G.; Lee, B.H.; Zarders, D.R.; Roh, G.H.; Kendra, P.E.; Cha, D.H. Ethyl Formate Fumigation and Ethyl Formate plus Cold Treatment Combination as Potential Phytosanitary Quarantine Treatments of Drosophila suzukii in Blueberries. J. Asia-Pac. Entomol. 2021, 24, 129–135. [Google Scholar] [CrossRef]
- Zaitoon, A.; Jabeen, A.; Ahenkorah, C.; Scott-Dupree, C.; Lim, L.T. In-Package Fumigation of Blueberries Using Ethyl Formate: Effects on Spotted-Wing Drosophila (Drosophila suzukii Matsumura) Mortality and Fruit Quality. Food Packag. Shelf Life 2021, 30, 100717. [Google Scholar] [CrossRef]
- Aly, M.F.; Kraus, D.A.; Burrack, H.J. Effects of postharvest cold storage on the development and survival of immature Drosophila suzukii (Diptera: Drosophilidae) in artificial diet and fruit. J. Econ. Entomol. 2017, 110, 87–93. [Google Scholar] [PubMed]
- Kraft, L.J.; Yeh, D.A.; Gómez, M.I.; Burrack, H.J. Determining the Effect of Postharvest Cold Storage Treatment on the Survival of Immature Drosophila suzukii (Diptera: Drosophilidae) in Small Fruits. J. Econ. Entomol. 2020, 113, 2427–2435. [Google Scholar] [CrossRef] [PubMed]
- Vanitha, R.P.; Rajashekar, Y.; Begum, K.; Chandrappa, L.B.; Rajendran, S. The Relation between Phosphine Sorption and Terminal Gas Concentrations in Successful Fumigation of Food Commodities. Pest Manag. Sci. 2007, 63, 96–103. [Google Scholar] [CrossRef]
- Ramadan, G.R.M.; Zhu, K.Y.; Abdelgaleil, S.A.M.; Shawir, M.S.; El-Bakary, A.S.; Edde, P.A.; Phillips, T.W. Ethanedinitrile as a Fumigant for Lasioderma serricorne (Coleoptera: Anobiidae), and Rhyzopertha dominica (Coleoptera: Bostrichidae): Toxicity and Mode of Action. J. Econ. Entomol. 2020, 113, 1519–1527. [Google Scholar] [CrossRef]
- Kyung, Y.; Kim, H.K.; Lee, J.S.; Kim, B.S.; Yang, J.O.; Lee, B.H.; Kim, G.H. Efficacy and phytotoxicity of phosphine as fumigants for Frankliniella occidentalis (Thysanoptera: Thripidae) on asparagus. J. Econ. Entomol. 2018, 111, 2644–2651. [Google Scholar] [CrossRef]
- Kyung, Y.; Kim, H.K.; Cho, S.W.; Kim, B.-S.; Yang, J.-O.; Koo, H.-N.; Kim, G.-H. Comparison of the Efficacy and Phytotoxicity of Phosphine and Ethyl Formate for Controlling Pseudococcus longispinus (Hemiptera: Pseudococcidae) and Pseudococcus orchidicola on Imported Foliage Nursery Plants. J. Econ. Entomol. 2019, 112, 2149–2156. [Google Scholar] [CrossRef]


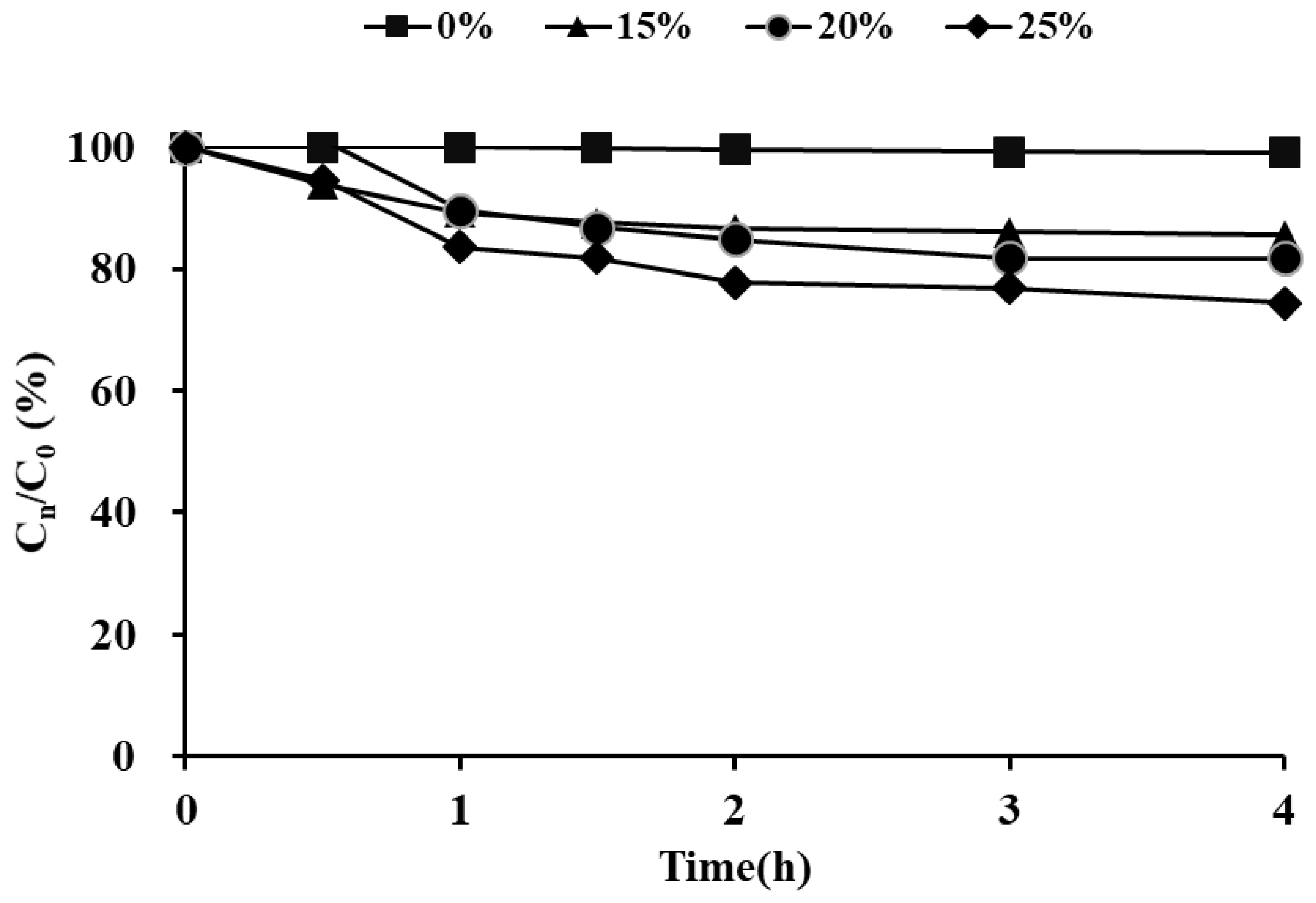
| Stage | n | LCT50 a (mg·h/L) (95% CL b) | TR c | LCT99 (mg·h/L) (95% CL) | TR | Slope ± SE | df | ⅹ2 |
|---|---|---|---|---|---|---|---|---|
| Egg | 360 | 0.41 (0.37–0.44) | 8.91 | 1.76 (1.39–2.48) | 20 | 3.64 ± 0.36 | 2 | 104.87 |
| Larva | 450 | 0.33 (0.31–0.35) | 7.17 | 1.14 (0.97–1.4) | 12.95 | 4.34 ± 0.32 | 3 | 162.88 |
| Pupa | 720 | 4.32 (3.90–4.77) | 93.91 | 53.20 (37.11–87.48) | 604.55 | 2.13± 0.18 | 6 | 142.16 |
| Adult | 378 | 0.046 (0.044–0.048) | 1 | 0.088 (0.081–0.100) | 1 | 8.22 ± 0.68 | 3 | 145.08 |
| Temp. (°C) | n | LCT50 (mg·h/L) (95% CL a) | SR b | LCT99 (mg·h/L) (95% CL) | SR | Slope ± SE | df | ⅹ2 |
|---|---|---|---|---|---|---|---|---|
| RT c | 540 | 4.80 (4.29–5.40) | 1 | 48.17 (33.52–79.95) | 1 | 2.32 ± 0.20 | 4 | 130.05 |
| 1 | 450 | 3.32 (3.06–3.57) | 1.45 | 10.49 (8.98–12.99) | 4.59 | 4.66 ± 0.41 | 3 | 128.33 |
| 5 | 450 | 4.54 (4.20–4.90) | 1.06 | 18.02 (14.94–23.16) | 2.67 | 3.89 ± 0.31 | 3 | 161.69 |
| Stage | Location | n | Mortality | CT (mg·h/L) |
|---|---|---|---|---|
| (Mean ± SE) | ||||
| Egg | Top | 270 | 100.0 ± 0.0a a | 10.58 |
| Middle | 270 | 100.0 ± 0.0a | ||
| Bottom | 270 | 100.0 ± 0.0a | ||
| Control | 270 | 8.5 ± 1.0b | ||
| Larva | Top | 369 | 100.0 ± 0.0a | |
| Middle | 384 | 100.0 ± 0.0a | ||
| Bottom | 393 | 100.0 ± 0.0a | ||
| Control | 394 | 3.6 ± 0.7b | ||
| Pupa | Top | 450 | 100.0 ± 0.0a | |
| Middle | 450 | 100.0 ± 0.0a | ||
| Bottom | 450 | 100.0 ± 0.0a | ||
| Control | 450 | 4.2 ± 0.8b | ||
| Adult | Top | 177 | 100.0 ± 0.0a | |
| Middle | 195 | 100.0 ± 0.0a | ||
| Bottom | 181 | 100.0 ± 0.0a | ||
| Control | 285 | 1.8 ± 0.8a |
| DAT a | Treatment | Weight Loss (%) | Berry Abscission (%) | Decay Rate (%) | Sugar Content (%, brix) | Mean Surface Color b | ||
|---|---|---|---|---|---|---|---|---|
| L | a | b | ||||||
| 3 | Control | 1.5 ± 0.1 c | 0.5 ± 0.3 | 0.0 ± 0.0 | 15.9 ± 0.1 | 21.5 ± 0.6 | −0.4 ± 0.1 | 4.9 ± 0.2 |
| Combination treatment | 1.8 ± 0.2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 16.0 ± 0.2 | 21.7 ± 0.6 | −0.4 ± 0.1 | 4.8 ± 0.1 | |
| p c | 0.156 | 0.172 | - | 0.662 | 0.804 | 0.825 | 0.534 | |
| 7 | Control | 2.4 ± 0.2 | 1.4 ± 0.7 | 0.3 ± 0.2 | 15.5 ± 0.3 | 20.9 ± 0.6 | −0.3 ± 0.2 | 4.8 ± 0.3 |
| Combination treatment | 2.5 ± 0.2 | 0.5 ± 0.3 | 0.3 ± 0.3 | 15.9 ± 0.3 | 21.1 ± 0.6 | −0.5 ± 0.2 | 4.7 ± 0.2 | |
| p | 0.480 | 0.338 | 0.850 | 0.375 | 0.779 | 0.499 | 0.813 | |
| 10 | Control | 3.1 ± 0.3 | 2.3 ± 0.8 | 0.6 ± 0.3 | 15.9 ± 0.3 | 20.9 ± 0.4 | −0.6 ± 0.1 | 4.9 ± 0.1 |
| Combination treatment | 3.3 ± 0.2 | 1.1 ± 0.6 | 0.5 ± 0.3 | 16.2 ± 0.2 | 21.1 ± 0.3 | −0.6 ± 0.1 | 5.0 ± 0.2 | |
| p | 0.700 | 0.312 | 0.801 | 0.454 | 0.702 | 0.743 | 0.408 | |
| 14 | Control | 3.9 ± 0.3 | 2.5 ± 0.8 | 0.7 ± 0.3 | 15.9 ± 0.2 | 22.2 ± 0.4 | −0.6 ± 0.1 | 4.7 ± 0.2 |
| Combination treatment | 4.0 ± 0.2 | 1.1 ± 0.6 | 1.0 ± 0.5 | 16.0 ± 0.2 | 22.1 ± 0.2 | −0.4 ± 0.1 | 4.7 ± 0.1 | |
| p | 0.715 | 0.274 | 0.534 | 0.620 | 0.824 | 0.255 | 0.761 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seok, S.-J.; Kim, H.K.; Koo, H.-N.; Kim, G.-H. Combined Effects of Cold Treatment and Phosphine in Drosophila suzukii (Diptera: Drosophilidae). Appl. Sci. 2022, 12, 12531. https://doi.org/10.3390/app122412531
Seok S-J, Kim HK, Koo H-N, Kim G-H. Combined Effects of Cold Treatment and Phosphine in Drosophila suzukii (Diptera: Drosophilidae). Applied Sciences. 2022; 12(24):12531. https://doi.org/10.3390/app122412531
Chicago/Turabian StyleSeok, Seung-Ju, Hyun Kyung Kim, Hyun-Na Koo, and Gil-Hah Kim. 2022. "Combined Effects of Cold Treatment and Phosphine in Drosophila suzukii (Diptera: Drosophilidae)" Applied Sciences 12, no. 24: 12531. https://doi.org/10.3390/app122412531
APA StyleSeok, S.-J., Kim, H. K., Koo, H.-N., & Kim, G.-H. (2022). Combined Effects of Cold Treatment and Phosphine in Drosophila suzukii (Diptera: Drosophilidae). Applied Sciences, 12(24), 12531. https://doi.org/10.3390/app122412531




