Featured Application
This systematic review compiles experimental features and results of cadmium exposure in lactating mice and rats. It provides other researchers with valuable technical guidance in selection of dose/concentration, administration route, exposure time, litter normalization, and lactation days for their future investigations.
Abstract
Cadmium (Cd) is a widely spread pollutant in the environment and its identification in human breast milk has caused concern. Children are particularly vulnerable, since their detoxification mechanism is not fully developed and their organs still being formed. Human- and animal-based studies demonstrate health issues and adverse pregnancy outcomes related to prenatal and postnatal Cd exposure. However, investigations of the effects, mechanisms, and treatments are still required. Thus, this systematic review compiled studies of Cd effects on lactating mice and rats focusing on experimental features and reported effects. The search was performed in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA), using PubMed, SciELO, and ScienceDirect databases. After identification, screening, and inclusion process 52 research articles were recovered and data, such as animal strain, metal compound, dose or concentration, administration route, exposure time, litter size normalization, lactation days, organs or samples examined, and effects reported were organized in tables. Three general schemes of Cd exposure on dams were identified: pregnancy, lactation, or pregnancy-lactation. The effects evaluated included neurotoxicity, sexual maturation, biochemical parameters, and Cd transfer and retention. Experimental features most common were Cd exposure during the pregnancy–lactation scheme and Cd administration by drinking water, while the most evaluated effect on offspring was Cd-neurotoxicity.
1. Introduction
Cadmium (Cd) is a heavy metal of considerable toxicity, that is widely spread in the environment. Cd concentrations have increased in soils, sediments, and water as a result of industrialization and mining activity [1]. The World Health Organization (WHO) recommends that Cd in breast milk should not be at concentrations higher than 1 µg/L [2]. However, reports of Cd measured in breast milk of lactating mothers from Iran (8.01 µg/L), Saudi Arabia (1.73 µg/L), Turkey (2.8 µg/L), India (1.95 µg/L), Mexico (1.31 µg/L), and Poland (2.11 µg/L) revealed concentrations higher than those indicated by the WHO [3]. Breast milk ensures proper nutrition and development in infants and contributes to maternal health [4], and the WHO, as well as the United Nations Children’s Fund (UNICEF), suggest exclusive breastfeeding newborns for the first six months and encourage continuing lactation for two years or more [5]. Children are particularly vulnerable to different inorganic elements because their detoxification mechanism is not fully developed, and their organs are in formation [6]. Cd is eliminated from organisms principally by the kidneys [7], but newborns present low glomerular filtration and diminished renal tubular secretion, as these only increase and mature after one year of life. In addition, the developing organism experiences processes, such as cell growth, differentiation, and morphogenesis, which may result in adverse developmental effects if they are altered [8]. Human- and animal-based studies demonstrate serious health issues and adverse pregnancy outcomes related to prenatal and postnatal Cd exposure, such as malformations, decrease in fetal growth rate, low birth weight, mortality, and diseases in childhood or later life. However, the mechanisms of Cd-induced toxicity remain unclear [9] and further studies related to mechanisms and effects, as well as possible treatments to decrease toxicity, are required.
Thus, this systematic review aimed to examine studies of Cd exposure in lactating mice and rats, focusing on the experimental features and the determined effects. Data included were the animal strain, metal compound, dose or concentration, administration route, exposure time, litter size normalization, lactation days, organs or samples examined, and reported effects.
2. Materials and Methods
2.1. Study Design
This review was performed in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) [10]. The research question was: “What experimental conditions have been applied in the study of Cd effects on lactating mice and rats, and what were the results obtained?”. Therefore, the Population, Exposure, Control and Outcomes (PECO) framework [11] established in this work was:
- Population: Mice and/or rats in lactating period
- Exposure: Cd administered to dams and lactating animals exposed by breastfeeding
- Control: Animal not exposed to Cd
- Outcomes: Effects on litters exposed to Cd by breastfeeding
2.2. Search Strategy and Selection Process
The search was conducted from March to July 2022, in PubMed, SciELO, and ScienceDirect using the following keywords and Boolean operators: (cadmium or Cd) and (breastfeeding or “breast milk” or lactation) and (mouse or mice or rat or rodent). The article type was selected as “Research articles” in ScienceDirect, “English” and “Spanish” languages were selected in PubMed, and the publication year was not specified in any database. After screening the titles and abstracts, research articles were selected in accordance with inclusion and exclusion criteria.
Inclusion and Exclusion Criteria
Inclusion criteria: (1) studies performed in lactating mice or rats exposed to Cd, (2) reports of dams exposed to Cd during pregnancy and/or lactation, (3) evaluation of the effects on offspring exposed to Cd through breastfeeding, (4) research articles written in Spanish or English with full text available.
Exclusion criteria: (1) studies not conducted on mice or rats, (2) works not related to Cd or breastfeeding, (3) reports of Cd exposure to Cd without previous controlled administration of Cd administration, (4) Cd dosage to litters by milk formulas, (5) effects of Cd evaluated only during gestation without allowing for birth and lactation, (6) reports of Cd effects focused only on dams without analyzing the offspring (7) studies of metal mixtures or Cd administered in combination with other toxic elements, and (8) articles other than research articles.
2.3. Data Extraction and Results Synthesis
Experimental features were obtained from material and methods and the most relevant findings were noted. The information recovered was organized according to the described Cd exposure scheme and the reported effects were summarized. Experimental features included animal model, Cd compound, dose or concentration, administration route, exposure time, lactation time, litter size standardization, and samples examined. Since the effect of Cd on offspring was evaluated in various organs, the results were classified according to the reported observations: (1) neurotoxic effects, (2) sexual maturation, (3) biochemical parameters, (4) Cd transfer and retention, and (5) other effects.
3. Results and Discussion
3.1. Selected Studies
Although, toxic effects of Cd have been reported in humans and animals, the analysis of Cd mechanisms, long-term consequences, and possible treatments that ameliorate Cd effects are required. Thus, our review aimed to systematically evaluate the experimental features described in papers reporting on the Cd exposure of lactating mice or rats. The flow diagram presented in Figure 1 describes the identification, screening, and inclusion process preformed in this review. From 8560 records obtained in PubMed, Scielo and ScienceDirect databases, 52 research articles were included, while 8508 records were excluded by automated filters or manually after screening titles and abstracts.
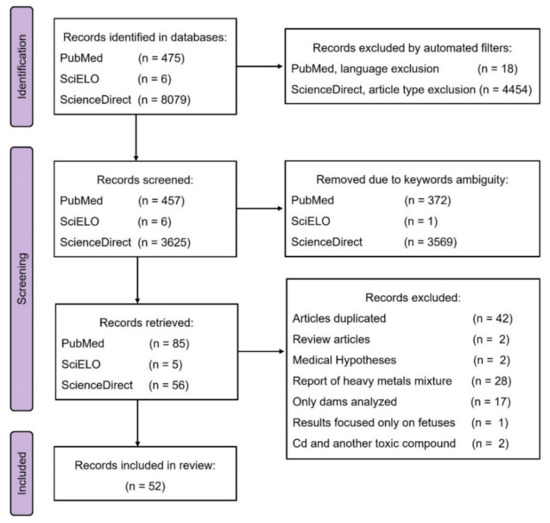
Figure 1.
Flow diagram.
3.2. Cd Exposure Schemes
From the analyzed papers three Cd exposure schemes were identified in the research articles: Cd exposure during pregnancy, during lactation, and during pregnancy–lactation periods. Cd exposure schemes are represented in Figure 2. Works reporting Cd exposure during pregnancy administered Cd from gestational day zero (GD 0) to the parturition day (PD). Some authors started Cd exposure days before mating (DBM) and continued until the PD (Figure 2a). Cd exposure during the lactation period began after the PD and concluded on the weaning day (WD), and most of the authors agreed on postnatal day 21 (PND 21) as the day of weaning (Figure 2b). The pregnancy–lactation scheme of Cd exposure included both periods. Cd administration started from GD 0 or DBM and continued during gestation and lactation until WD. Some authors even followed the Cd exposure in offspring after the WD until adulthood (Figure 2c). Since the Cd exposure schemes were different in the papers reviewed, the experimental features and evaluated Cd effects were organized according to the exposure scheme.
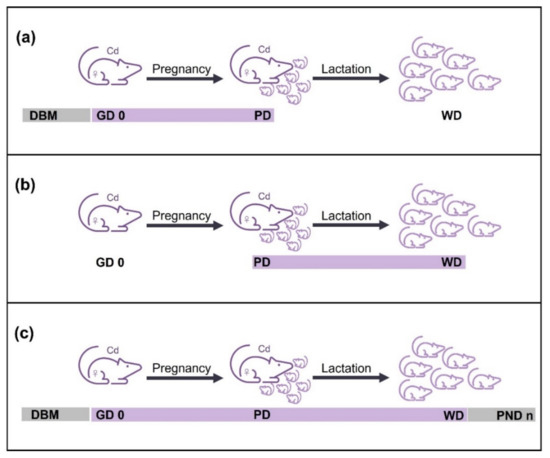
Figure 2.
Cd exposure schemes. (a) Administration of Cd during pregnancy period, from days before mating (DBM) or gestation day zero (GD 0) to the parturition day (PD). (b) Cd exposure during lactation period, from PD to the weaning day (WD). (c) Scheme of Cd exposure during pregnancy–lactation, from DBM or GD 0 to WD or until offspring reached adulthood (PND n). Purple line represents Cd administration described by most of the authors, whereas gray line indicates Cd exposure mentioned by some authors.
3.2.1. Cd Exposure of Dams during Pregnancy Period
From the 52 records included in this review, seven described Cd exposure of dams only during pregnancy (Figure 2a). All the reports were performed on rats and published from 1981 to 2013. The experimental features, summarized in Table 1, show differences in the administration route, dose or concentration, exposure time and effects determined. The intragastrical route was mostly reported (3/7), compared with intraperitoneal (1/7), intravenous (1/7), drinking water and inhalation chamber (1/7). The Cd-concentrations reported were 50 mg CdCl2/L by drinking water and 0.02 mg CdO/m3 by inhalation chamber, which were the highest and lowest concentrations of Cd exposure during pregnancy, respectively. The highest Cd dose was 20 mg/kg body weight (b.w.) intragastrically and the lowest was 1 mg/kg b.w. intravenously. Cd was administered as a single dose, as several doses in different pregnancy days, or daily during the gestation period. The reports of daily Cd administration varied the time of onset and termination of exposure. Most of the authors described dams being allowed to breastfeed their litters and then the Cd effects were evaluated in the offspring. Only Saillenfait et al. [12] conducted the cross-fostering strategy, whereby the litters of the Cd exposed dams were exchanged with those of the control groups at birth, enabling surrogate parenting (Figure 3). Litter normalization was described by most of the authors (6/7) and the effects on offspring were evaluated before WD, at WD or until adulthood (PND 84, 90, 120 or 180). Slyuzova et al. [13] included in their work an experimental group of Cd exposure during lactation, while the works of Liapi et al. [14] and Dési et al. [15] comprised experimental groups exposed to Cd during a pregnancy–lactation scheme. The Cd effects related to these experimental groups are mentioned in Section 3.2.2 and Section 3.2.3, respectively.
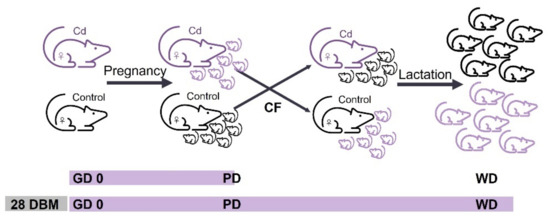
Figure 3.
Cross-fostering strategy. Litters of dams exposed to Cd were exchanged at birth with those of the unexposed groups, enabling surrogate parenting. Cross-fostering (CF) was reported for the pregnancy Cd exposure scheme, from gestation day zero (GD 0) to parturition day, and for the pregnancy–lactation scheme from 28 days before mating (DBM) or GD 0 to the weaning day (WD).
Effects of Cd Exposure during Pregnancy Period
Cd administration during pregnancy particularly impacts prenatal development. The effects determined in the offspring that were exposed to Cd gestationally included neurological, biochemical, and sexual modifications. Liapi et al. [14], Dési et al. [15], and Barański [16] observed neurological effects. Liapi et al. described an increased activity of brain enzymes (acetylcholinesterase and Na+, K+-ATPase) in newborns that was ameliorated after Cd-free lactation. Dési et al. and Barański performed behavioral tests and concluded that Cd alters electrophysiological functions at PND 84 and reduces exploratory activity at PND 90, respectively. Although, Dési et al. and Barański reported different administration routes (intragastric and inhalation chamber, respectively) as well as dose/concentration of Cd, both papers described a neurological Cd effect on offspring in adulthood. The dams were administered with Cd by inhalation chamber for 160 days, while dams exposed intragastrically were administered Cd over 10 days. Thus, the exposure time of dams could influence the effect observed in their offspring. On the other hand, Saillenfait et al. [12] determined renal alkaline phosphatase activity as an indicator of renal damage in cross-fostered groups. Pups fed by their biological mothers exposed to Cd exhibit diminished alkaline phosphatase activity compared to pups exposed to Cd gestationally but fed by unexposed mothers. Thus, they concluded that renal damage is not induced by pre-natal exposure alone. In addition, Saillenfait et al. evaluated the accumulation of Cd by the Cd isotope (109Cd). Interestingly, 109Cd was identified in the liver of newborns exposed gestationally and in the gastrointestinal tract evaluated at PND 12 of pups breastfed by their Cd-exposed mothers, suggesting that 109Cd is transferred by gestation and lactation periods.

Table 1.
Cd exposure during pregnancy period. Data ordered by dose/concentration of Cd administered to dams.
Table 1.
Cd exposure during pregnancy period. Data ordered by dose/concentration of Cd administered to dams.
| Strain (n) Dose/Concentration, Admin Route Exposure Period Litter (n), Lactation Days | Collection Time Organ Analyzed | Results | Year Author |
|---|---|---|---|
| Wistar rats (n = 6) 50 mg CdCl2/L, d.w. gestation (n = 6), 21 | PD and PND 21 Brain | Increased acetylcholinesterase and Na+, K+-ATPase activities in the newborn rat brain that were ameliorated through a Cd-free lactation. | 2013 Liapi et al. ‡ [14] |
| Wistar rats (n = 12) 20 mg CdCl2/kg bw, i.g GD 6 to GD 14 (n = 8), 21 | GD 20 Fetuses PND 1 to 180 Physical and behavioral parameters | External malformations in fetuses at GD 20 Improved testes descent, delayed vaginal opening, and modified male and female sexual behavior | 2004 Salvatori et al. [17] |
| Wistar rats (---) 14, 7, and 3.5 mg CdCl2/kg bw, i.g. GD 5 to GD 15 (n = 8), 28 | PND 84 Behavioral and neurotoxicological analysis | Cd dose dependently altered the spontaneous and evoked electrophysiological functions | 1998 Dési et al. ‡ [15] |
| Sprague-Dawley rats (n = 6–7) 2.5 mg 109CdCl2/kg bw, i.p. GD 8, 10, 12 and 14 (n = 10), 12, CF | PND 3, and 12 Kidney, liver, and gastrointestinal tract | Decreases renal alkaline phosphatase activity determined in pups fed by their biological mothers exposed to Cd. 109Cd in liver at birth and in the gastrointestinal tract at PND 12 of newborns fed by their mothers. Prenatal Cd exposure cannot induce alone kidney damage | 1992 Saillenfait et al. [12] |
| Rats (---) 2 mg Cd(NO3)2/kg bw, i.g. GD 6 to GD 15 (n = 10), --- | PND 120 Blood | At PND 120 increased plasma and erythrocyte levels of MDA, reduced glutathione, activities of glutathione-S-transferase, glutathione reductase, and γ-glutamyl transferase. Effects less pronounced than lactation exposure. | 2008 Slyuzova et al. ‡ [13] |
| Wistar rats (---) 1 mg CdCl2/kg bw, i.v. GD 18 (n = 8), 24 | PND 2, 9, and 24 Blood, liver, brain, kidneys, spleen, pancreas, stomach, duodenum, and femur | Decreased Zn in liver and delayed Zn deposition in bone. Probably Cd increases the demand of Zn in the newborn. | 1981 Bakka et al. [18] |
| Wistar rats (---) 0.16 and 0.02 mg CdO/m3, 5 h daily, i.c. 140 DBM to GD 20 (---), 21 | PND 90 Behavioral tests | Reduction of exploratory motor activity. Central nervous system dysfunction. | 1984 Barański [16] |
---, data not mentioned; ‡, work mentioned in another Cd exposure scheme; CF, cross-fostering; DBM, days before mating; GD, gestational day; MDA, malondialdehyde; PND, postnatal day. Admin route, administration route; bw, body weight; d.w., drinking water; i.c., inhalation chamber; i.g., intragastric; i.p., intraperitoneal; i.v., intravenous.
3.2.2. Cd Exposure of Dams during Lactation Period
Eleven records analyzed Cd effects on offspring breastfed by dams exposed to Cd during the lactation days (Figure 2b). The research articles were published from 1997 to 2016 and most of the experiments were performed in rats (10/11). Although, experimental features in Table 2 show differences in the administration route, concentration/dose, exposure time, and effects some papers reported identical features, so these papers were grouped in Table 2. Cd in drinking water was the most reported administration route (8/11), then intravenous (2/11), intragastrical (1/11), and intraperitoneal (1/11). Since Cd administration through the ad libitum drinking water was reported as a concentration, some authors estimated the dose consumed per dam by calculation of water consumed daily. Grawé et al. [19,20] and Andersson et al. [21] described both the Cd concentration in the drinking water and estimated Cd dose. However, several authors, such as Friedrichi et al. [22], Ribas et al. [23], Picoli et al. [24,25], and Pillet et al. [26], did not mention having estimated water consumption. In the lactation exposure scheme the highest Cd concentration was 300 mg/L and the lowest was 5 mg/L, while the highest dose was 4.8 mg/kg b.w. administered through drinking water and the lowest was 8.8 µg/kg b.w. intravenous. Cd was administered as a single dose or daily during lactation and most authors described the standardization of litters at birth. All the authors reported that suckling pups were fed by their biological mothers.

Table 2.
Cd exposure during lactation period. Data ordered by dose/concentration of Cd administered to dams.
Effects of Cd Exposure during Lactation Period
Dams exposed to Cd only during lactation transferred the heavy metal to their progeny by breastfeeding. Thus, this scheme ensured that the effect observed in offspring was caused by the Cd in milk, avoiding effects generated by gestational Cd exposure. In this scheme, neurological effects in suckling pups, such as increased motor activity [19], disturbances in the serotonergic system [21], and impairment in memory [27], were described at low Cd doses (4.8, 0.9 and 1.2 mg Cd/kg b.w., respectively). Increased motor activity was observed in offspring at PND 29 after 17 days of Cd exposure, disturbances in the serotonergic system was described at PND 51 after 19 Cd exposure days, and memory impairment was determined at PND 100 after seven days of Cd exposure. Interestingly, a low dose (1.2 mg Cd/kg b.w.) administered for seven days caused neurological effects on offspring at 100 days after birth, suggesting that Cd exposure only during lactation is sufficient to produce alterations in neuronal functions. In addition, biochemical effects were determined in offspring at PND 19 and 120 after Cd exposure of dams for 17 and 10 days, respectively. Results show fatty acid metabolism altered in brain [20] and increased oxidative stress evaluated by malondialdehyde determination in plasma and erythrocytes [13]. Slyuzova et al. evaluated oxidative stress in both pregnancy and lactation scheme. After results comparison, they concluded that Cd-effects in plasma and erythrocytes were pronounced in lactation exposure than in pregnancy, suggesting that only lactation exposure can induce effects on the offspring.
Other effects such as morphological changes in the mouth of the suckling pups [22,23,24,25] and decrease in body, kidney, and spleen weights of female offspring [26] were evaluated. Changes in mouth tissue were described in four papers that coincided in the experimental features such as Cd concentration of 300 mg/L administered to Wistar rats in drinking water from PD to PND 21, whereas decrease in body and organs weights were reported at lower Cd concentration (10 mg/L) in drinking water. Accumulation of Cd in kidneys of suckling pups [19,28], and immunotoxic effects were also reported [26].Although, Grawé et al. [19] and Grawé and Oskarsson [28] agree that Cd accumulates in kidney, they described different experimental features. While Grawé et al. administered Cd in drinking water at 25 mg/L (equivalent to 4.8 mg/k b.w.) for 17 days, Grawé and Oskarsson exposed dams to 109Cd 300 µg/kg b.w. intravenously for 14 days. The Cd accumulation in kidney evaluated in offspring at PND 29 and 16 suggests that Cd even at low doses is transferred by breastfeeding to the offspring from the beginning of the lactation period until weaning. All the effects observed in lactation exposure scheme confirm that Cd is transferred by breast milk from dams to pups, highlighting the importance of studying the mechanisms and effects of Cd exposure at low and high doses on infants.
3.2.3. Cd Exposure of Dams during Pregnancy–Lactation Period
From 52 records included in this review, 37 described Cd exposure during pregnancy and lactation. The research articles published from 1979 to 2022 mostly report experiments in rats (29/37) than in mice (8/37).Experimental data and results summarized in Table 3 show differences in experimental features. Nevertheless, some reports mentioned identical strain, concentration/dose, exposure time, etc. Thus, they were grouped within the Table 3. Cd in drinking water was the most mentioned route of administration (23/37), followed by intragastric via (11/37), and Cd administered in food (3/37). Some authors such as Feng et al. [29], Antonio et al. [30,31], Gupta and Shukla [32], Luo et al. [33], and Zhang et al. [34] reported Cd concentration in drinking water and mentioned the Cd doses estimated from daily water consumption, while Li et al. [1], Kostial et al. [35], Ronco et al. [36], Gupta and Shukla [37], and Young et al. [38] described Cd concentration in drinking water and daily water consumption but they do not mentioned the estimated dose. In this case, we calculated the Cd dose by multiplying the water consumption by the Cd concentration. On the other hand, authors such as Kostial et al. [39], Liapi et al. [14], Stolakis et al. [40], Mikolic et al. [41], Yargiçoğlu et al. [42], Zhao et al. [43], Honda et al. [44], Santana et al. [45], Petrochelli et al. [46], Ishitobi and Watanabe [47], and Brako et al. [48] only reported the concentration of Cd in drinking water without estimating either dose or water consumption. The highest Cd concentration described was 100 mg/L from GD 0 to PND 21 [39] and the lowest was 0.15 µg/L from GD 0 to PND 11 [48] both administered in drinking water, whereas the highest dose was 68 mg Cd/kg b.w. and the lowest 1.089 µg Cd/kg b.w. both estimated from water consumption. Although, pregnancy-lactation scheme consists of administering Cd during both periods (Figure 2c), Table 3 indicates that exposure times were different. From 37 papers included in this scheme 18 reported a Cd exposure from GD 0 to PND 21, four articles mention exposed dams from GD 0 to PND 11 or 1–5 days before, seven papers described the onset of Cd exposure several DBM ending at PND 21, five begin DBM and end on PND 15, 28 or 35, and three papers reported a varied exposure time. Cross-fostering was reported by two authors, Mikolić et al. [41] and Zhao et al. [43], but describing different experimental features. While Mikolić et al. administered 50 mg Cd/L to rat dams in drinking water from 28 DBM to PND 21, Zhao et al. exposed mouse dams to 10 mg Cd/L from GD 0 to PND21. Effects of Cd exposure during Pregnancy-Lactation Period.
Whereas Cd exposure schedules during pregnancy and lactation induce separately effects on offspring related to Cd transfer gestationally and by breast milk respectively, pregnancy-lactation scheme combines both exposures generating effects related to perinatal exposure. Probably, this scheme simulates perinatal Cd exposure in humans more closely since pregnant women can be exposed before and after pregnancy and considering that Cd presents a half-life of 17–30 years in human body [49]. The effects evaluated in pregnancy-lactation scheme includes neurotoxicity, changes in sexual maturation, alterations in biochemical parameters, and Cd transfer and retention. From 37 papers included in this exposure-scheme 15 studied neurological Cd-effects, 13 were related to changes in sexual maturation, 5 reported biochemical effects and 4 evaluated Cd transfer or retention. Analyses of brain tissue and/or behavioral tests conducted on offspring demonstrate that Cd exposure during pregnancy-lactation period altered chemical and enzyme activities in the brains of the offspring. Cd administered in drinking water at 50 mg/L from GD 0 to PND 21 altered acetylcholinesterase, Na+-, K+- [14,40], and Mg2+-ATPase activities [40], while 10 mg Cd/L (estimated as 1 mg/kg bw) by identical administration via decreased serotonin, dopamine, 3,4-dihydroxyphenylacetic acid [31], glutamate, and γ-aminobutyric acid [30] in different brain zones. This suggest that a Cd concentration five times lower than 50 mg/L also generates also alterations in neurotransmitters content. Both concentrations were administered by other authors using drinking water, they observed structural alterations in brain, such as synapses and neurites in the hippocampus being destroyed [29], sparse arrangement of cells in the hippocampal area, as well as changes in the expression of γ-aminobutyric acid receptor subunits, cognitive deficit [29,43], impairment of memory, altered exploratory activities, and increased escape latency [43]. Altered exploratory activities was also reported at lower Cd doses (14, 7 and 3.5 mg/kg bw intragastrically) [50] accompanied by changes in spontaneous/evoked physiological functions [15,50]. Cd modified cocaine [51,52] and morphine [53] sensitization, and studies of oxidative stress in the brain showed an increase in lipid peroxidation [42], and an increment of thiobarbituric acid reactive substances and elevated activity of superoxide dismutase, glutathione peroxidase, catalase, and glutathione reductase [37]. Overall, the results suggested that maternal Cd exposure could lead to nervous system dysfunction. On the other hand, Cd caused sexual alterations. In male offspring were observed testicular development disorder, decrease in serum testosterone [54], steroid hormones and steroidogenic enzymes, low relative testis weight, disrupted Leydig cells development [55], sperm quality impairment, and increased apoptosis in testis [46,56]. Histologic studies revealed stromal inflammatory foci and multifocal inflammation increased in ventral prostates [45], and in testis alteration of spermatogenic epithelial [34], and changes in genetic regulation of cytoskeleton related proteins (CORO1A, cofilin 1 and profilin) [57]. Male pups presented increased body weight, reduced anogenital index, and delayed physical and reflex development [58]. In female offspring, authors reported alterations in ovarian granulosa cells, such as lipid droplets smaller than normal and down-regulation of adipose differentiation-related protein, that resulted in a high level of progesterone in serum [59]. Additionally, Cd increased the biosynthesis of steroid hormones by activation of cAMP/PKA pathway and up-regulated steroidogenesis related proteins increasing the levels of steroid hormones and contributing to early puberty onset and promotion of differentiation and maturation of follicles [60]. In contrast, other authors observed impairment in sexual development [33], vaginal opening delay [1,47], irregular estrous cycle [47], inhibition of follicular development, reduction of serum estradiol, and down-regulation of mRNA of genes related to steroidogenesis [1]. The lowest dose of 100 µg/kg b.w. administered in drinking water and estimated from Cd concentration (0.1 mg/L) and water consumption induced alterations in spermatogenic epithelial of testis [34], that compared to the highest dose of 20 mg Cd/kg b.w. administered intragastrically demonstrates that Cd-effects on sexual organs can be provoked even at minimal doses.
Biochemical effects determined after pregnancy-lactation exposure included hematotoxic effects, diminished Zn and Fe [41], liver injury related to nonalcoholic fatty liver disease development [38], changes in insulin secretion [61], and altered heart morphology accompanied by reduced endothelium-dependent reactivity and increased hem-oxygenase 1 in aortic rings [36]. While the Cd transfer/accumulation studies show that 109Cd in pups at birth was less than 1% of the total 109Cd transferred, percentage tripled during lactation being the 94% sequestered in the gastrointestinal tract of suckling pups [62]. Thus, is confirmed that Cd exposure during lactation is more important than prenatal exposure for pups [35]. Since biochemical parameter evaluated were different, it is difficult to compare and discuss the results obtained. However, it is important to highlight that low Cd-doses (1.2 and 0.5 mg/kg) generated important alterations such as liver injury and changes in insulin secretion.

Table 3.
Cd exposure during pregnancy–lactation period. Experimental data are ordered by dose/concentration of Cd.
Table 3.
Cd exposure during pregnancy–lactation period. Experimental data are ordered by dose/concentration of Cd.
| Strain (n) Dose/Concentration, Admin Route Exposure Period Litter (n), Lactation Days | Collection Time Organ Analyzed | Results | Year Author |
|---|---|---|---|
| Albino rats (n = 12) 100 mg CdCl2/L, d.w. GD 0 to PND 21 (n = 6), 21 | PND 7 and 126 Blood, liver, kidneys, and gut | Pups retained higher Cd-levels in blood, carcass, and gut at PND 7 than at PND 126. | 1979 Kostial et al. [39] |
| ICR mice (n = 10) 100 and 10 mg CdCl2/L, d.w. (23.7, 26.9, and 68.2 mg Cd/kg bw for 100 mg/L at DBM, GDs, and PNDs) ** (3.2, 3.4, and 9.3 mg Cd/kg bw for 10 mg/L at DBM, GDs, and PNDs) ** 28 DBM to PND 21 (n = 10), 21 | PND 21, 35 and 56 Blood and ovaries | Vaginal opening delay, irregular estrous cycle, inhibition of follicular development. The level of serum estradiol was reduced, and the mRNA levels of genes related to steroidogenesis were downregulated. | 2022 Li et al. [1] |
| Wistar rats (n = 6) 50 mg CdCl2/L, d.w. GD 0 to PND 21 (n = 6), 21 | PD and PND 21 Brain | Decrease in acetylcholinesterase activity. | 2013 Liapi et al. ‡ [14] |
| PND 21 Brain | Cd exposure modified the activities of acetylcholinesterase and Na+, K+-ATPase in the frontal cortex and cerebellum, and increased Mg2+ -ATPase activity in the hippocampus. | 2013 Stolakis et al. [40] | |
| Wistar rats (n = 64) 50 mg CdCl2/L, d.w. 28 DBM to PND 21 (n = 6), 21, CF | PD, PND 21, and PND 49 Blood, liver, kidney, and brain | Gestational plus lactational Cd exposure decreased Fe and Zn levels and caused hematotoxic effects more pronouncedly than exposure during either gestational or lactational period alone. | 2016 Mikolic et al. [41] |
| Rats (---) 50 mg CdCl2/L, d.w. (4.8, and 5.7 mg Cd/kg bw for DBM and pregnancy) ** 28 DBM to PND 21 (n = 6), 21 | PND 11, 21, 49, and 55 Liver and kidney | Between PND 11 and 21 Cd deposition increased in liver and kidney. Cd exposure during lactation was more important for pups than prenatal exposure. | 1993 Kostial et al. [35] |
| Sprague-Dawley rats (n = 8) 50 mg CdCl2/kg diet, f.i. 30 DBM to PND 15 (n = 10), 21 | PND 21 Blood, kidney, liver, and brain | Cocaine sensitization was attenuated in rats perinatally exposed to Cd. | 2003 Smith and Nation [51] |
| Sprague-Dawley rats (---) 50 and 10 mg CdCl2/L, d.w. (5 and 1 mg Cd/kg bw) * GD 0 to PND 21 (---), 21 | PND 21, 35, and 56 Brain | The synapses and neurites in the hippocampus were destroyed by high Cd exposure. Cognitive behavior deficit lasting from childhood to adulthood. Cd affects Cornin-1a pathway. | 2019 Feng et al. [29] |
| Sprague-Dawley rats (---) 50 and 25 mg CdCl2/kg diet, f.i. 30 DBM to PND 15 (n = 10), 21 | PND 1 and 21 Blood | Developmental Cd exposure attenuated the development/expression of morphine sensitization. Suppressive effect of an antagonist of the dopamine D2 receptor was decreased. | 2002 Smith et al. [53] |
| Wistar rats (n = 8) 30 mg CdCl2/L, d.w. (2.2 mg Cd/kg bw) ** GD 0 to PND 21 (n = 10), 21 | PND 21 Blood, liver, and kidney PND 60 Aortas | Echocardiography showed altered heart morphology. In aortic rings (PND 60) reduced endothelium-dependent reactivity and increased HO-1 (greater in females than males). VCAM-1 was lower in adult females than males | 2011 Ronco et al. [36] |
| Druckrey rats (n = 20) 20 mg Cd(CH3COO)2/L, d.w. (2.8 mg Cd/kg bw) ** GD 0 to PND 21 (n = 8), 21 | PND 1 7, 14 and 21 Brain | Cd increased thiobarbituric acid reactive substances and evoked a marked variation in reduced glutathione levels. Brain total sulphydryls were increased at PND 1. The activities of SOD and GPx were increased at PND 1 but inhibited at PND 7, 14 and 21. Brain catalase and glutathione reductase were elevated. | 1995 Gupta and Shukla [37] |
| Wistar rats (n = 16) 20 and 10 mg CdCl2/kg bw, i.g. GD 18 and PD to PND 7 (n = 8), 21 | PND 100 Blood, testes, epididymis, seminal vesicle, and ventral prostate | Cd exposure in utero and through lactation affected sperm quality and increased rate of cell death in testis. | 2012 Couto-Moraes et al. [56] |
| GD 18 to PND 7 or PND 21 | Physical and reflex development test | Cd increased bw, reduced the anogenital index and delayed physical and reflexes development. Perinatal Cd exposure promoted changes in the development of male rat offspring, reprogramming the pup’s development. | 2010 Couto-Moraes et al. [58] |
| Swiss albino rats (n = 8) 15 mg CdCl2/L, d.w. GD 0 to PND 22 (n = 15), 22 | PND 60 Blood, kidney, and brain | Pre- and post-natal Cd exposure caused a significant increase of lipid peroxidation in the brain. | 1997 Yargiçoğlu et al. [42] |
| Wistar rats (---) 14, 7, and 3.5 mg CdCl2/kg bw, i.g. GD 5 to GD 15 and PND2 to PND 28 (n = 8), 28 Males treated until PND 84 | PND 84 Behavioral and neurotoxicological analysis | Alterations in spontaneous and evoked electrophysiological functions, decreased horizontal and vertical exploratory activity and diminished exploration frequency of the open-field center. Low-level pre- and post-natal Cd exposure affected the bioelectrical and higher order functions of the nervous system. | 1998 Dési et al. ‡ [15] |
| Wistar rats (n = 10) 14, 7, and 3.5 mg CdCl2/kg bw, i.g 49 DBM to PND 28 (n = 8), 28 | PND 84 Behavioral investigations | Changes in vertical exploration activity and increased exploration of an open field center. Spontaneous and evoked electrophysiological variables showed dose- and generation-dependent variations, signaling a change in neural functions. | 1997 Nagymajtényi, et al. [50] |
| C57BL/6 mice (n = 10) 10 mg CdCl2/L, d.w. GD 0 to PND 21 (---), 21, CF | PND 21, 35, 49, and 84 Blood and brain | At birth, Cd serum levels were increased in exposed group. Serum estradiol of female offspring was decreased at PND 49. Histopathological results showed a sparse arrangement of cells in hippocampal area. Prolonged scape latency and exploring time were shown at PND 35 and 49. Learning and memory were affected, especially in female offspring, through changed structure in the hippocampal area and protein expression of γ-aminobutyric acid receptor subtype subunits. | 2018 Zhao et al. [43] |
| C57BL/6J mice (---) 10 mg CdCl2/L, d.w. GD 0 to PND 10 (---), 10 | PND 10 Brain | Transferrin receptor was upregulated in the neonatal brain | 2013 Honda et al. [44] |
| Wistar rats (n = 4–10) 10 mg Cd(CH3CO2)2/L, d.w. GD 0 to PND 21 (n = 8–12), 21 (1.12 and 2.41 mg Cd/kg bw for GDs and PNDs, respectively) * | PND 90 Blood | Male offspring at PND 90 presented no differences in testosterone levels, cell proliferation and apoptosis indexes compared with control group, but stromal inflammatory foci and multifocal inflammation increased in ventral prostates of treated group. | 2016 Santana et al. [45] |
| PND 90 Blood, testes, epididymis, vas deferens, ventral prostate, and seminal vesicle | Cd exposure affected sperm quality (morphology and motility) and increased apoptosis in testis. | 2012 Petrochelli et al. [46] | |
| PND 21 Blood and brain | Cd showed neurochemical disturbances on serotoninergic and aminoacidergic systems during development. Hippocampal levels of serotonin and 5-hydroxyindolacetic acid were significantly reduced but dopamine content was maintained. Glutamate concentration decreased in hypothalamus and increased in hippocampus, while γ-aminobutiric acid decreased only in cerebral cortex | 2010 Antonio et al. [30] | |
| Wistar rats (n = 4) 10 mg Cd(CH3CO2)2/L, d.w. (1.13 mg Cd/kg bw) * GD 0 to PND 5 (n = 10–15), 5 | PD or PND 5 Brain | Cd increased the 5-hydroxytryptamine and 5-hydroxyindoleacetic acid content in all areas of the brain and decreased the dopamine and 3,4-dihydroxyphenylacetic acid levels in mesencephalon. Decrease in brain nuclei acids was observed at PND 5. | 1998 Antonio et al. [31] |
| Druckrey rats (---) 10 mg Cd(CH3COO)2·2H2O/L, d.w. (0.97–1.44 mg/kg bw) * GD 0 to PND 21 (n = 8), 21 Pups received 10 ppm, d.w. PND 21 to PND 45 | PND 15, 21, 30, and 45 Brain | Brain lipids and cholesterol were reduced at PND 15, 21, 30 and 45. Zn and Cu levels were reduced in brains. Cd may produce central nervous system dysfunctions. | 1996 Gupta and Shukla [32] |
| C57BL/6J Jcl mice (n = 6) 10, and 1 mg CdCl2/L, d.w. GD 0 to PND 10 (---), 21 | PND 10 and 21 Brain, kidney, and liver PND 50 Vaginal smears | Cd levels in brain were higher at birth than in control group, while Cd in kidney and liver was increased at PND 10. Zn was elevated in kidney and liver at birth, while hepatic Cu was diminished at birth and PND 10. Female offspring presented delay in the timing of vaginal opening and had perturbed estrous cycles. | 2005 Ishitobi and Watanabe [47] |
| Sprague-Dawley rats (n = 10) 10, 5, and 2 mg CdCl2/L, d.w. (0.96, 0.44, and 0.089 mg Cd/kg bw) * GD 0 to PND 21 (---), 21 | PND 63 and 77 Vaginal smears PND 84 Blood, brain, thyroid, heart, liver, spleen, lung, kidney adrenals, seminal vesicle, prostate, testes, epididymis, and ovaries | There were no adverse effects on the physical and sexual development in the pups, except to delay the development of offspring. The relative weights of livers and kidneys in the adult female offspring were decreased after exposure to 10 ppm Cd. | 2015 Luo et al. [33] |
| Sprague-Dawley rats (n = 10) 8, 2, and 0.5 mg CdCl2/kg bw/day, i.g. GD 0 to PND 21, F1-F2 (---), 21 | PND 21 and 56 Blood and testes | Testicular development disorder and decrease in serum testosterone was determined in F1, but testosterone increase was observed in F2. Cd caused male reproductive problems in a multigenerational manner. The protein expression for testicular steroidogenic factor 1 and steroidogenic enzymes at PND 21 and 56 had different patterns in F1 and F2 rats. | 2020 Huang et al. [54] |
| Sprague-Dawley rats (---) 5 and 1 mg CdCl2/kg bw, i.g. GD 0 to PND 21 (n = 12), 21 | PND 21, 35, and 56 Blood and testes | CORO1A and cofilin 1 were up-regulated, while profilin 1 was down-regulated in the testis of maternal Cd-exposed male offspring. | 2022 Wang et al. [57] |
| PND 21, 35, and 56 Ovarian granulosa cells and serum | In the ovarian granulosa cells of female offspring exposed to Cd the lipid droplets were smaller than normal; ADRP was down-regulated, accompanied by decrease in PLCβ2 and PKCα. The HSL phosphorylation was increased and StAR and CYP11A1 were up-regulated. This series of events resulted in a high level of progesterone in serum. | 2020 Liu et al. [59] | |
| PND 21, 35, and 56 Blood and testes | Decreased relative testis weight and steroid hormone levels, disrupted Leydig cell development, increased SRD5α expression, inhibited activation of the cAMP/PKA signaling pathway and down-regulated steroidogenic enzymes. | 2018 Tian et al. [55] | |
| PND 21, 35, and 56 Blood, ovaries, and uterus F1-F2 | Increased biosynthesis of steroid hormones by activation of cAMP/PKA pathway, and up-regulated steroidogenesis related proteins, such as StAR, CYP11A1, 3β-HSD and CYP19A1. Elevated levels of steroid hormones contributed to early puberty onset and promoted differentiation and maturation of follicles in female offspring (F1). In the ovaries of F2 female rats the levels of CYP11A1 and CYP19A1 were also high, accompanied by increased serum progesterone. Hormonal changes induced by Cd exposure in utero might have a lasting effect beyond the first generation. | 2018 Li et al. [60] | |
| C57BL/J mice 5 and 0.5 mg CdCl2/L, d.w. (1.23 and 0.1 mg Cd/kg bw) ** 196 DBM to PND 21 21 days | PND 189 Blood and liver | Cd exacerbated liver injury and lipid deposition associated with a high fat diet, contributing to nonalcoholic fatty liver disease development. | 2022 Young et al. [38] |
| Wistar rats (n = 5) 5, 2, and 1 mg CdCl2/kg bw/day, i.g. 21 DBM to PND 28 (---), 28 | PND 1, and 28 Blood, liver, and kidney | At PND 28 Cd in kidney was higher than in liver. Uterine MT increased with Cd accumulation. Probably, MT in placenta and uterus did not play a significant role preventing Cd transport to the fetus. | 2012 Nakamura et al. [63] |
| Sprague-Dawley rats (---) 5 mg CdCl2, i.g. 30 DBM to PND 21 (n = 8), 21 | PND 3 Blood and ingested milk | Cd exposure during early life affect cocaine sensitivity. | 2004 Cardon et al. [52] |
| CF1 mice (n = 10) 5 mg CdCl2/kg diet, f.i. 139 DBM to PND 21 (---), 21 | PD, PND 7, 14, and 21 Whole body | At birth, 109Cd in pups was less than 1% of the total 109Cd transferred during the full reproductive period. During lactation, 109Cd levels tripled with each seven days interval. Approximately 94% of the total 109Cd in pups’ bodies was sequestered in the gastrointestinal tract in PND 21. 109Cd transfer to pups was about 30% increased for multiparous versus uniparous females. Transfer was not significantly affected by nutrient quality of the dams’ diet. | 1993 Whelton et al. [62] |
| C57BL/6J mice (n = 10) 0.1, 0.01, 0.001 mg CdCl2/L, d.w. (100.12, 10.03, and 1.089 µg Cd/kg bw) * 30, 90, or 150 DBM to PND 21 (---), 21 Pups received Cd until PND 70 | PND 70 Blood, brain, liver, kidney, seminal vesicle, prostate, testis, epididymis, uterus, and ovary | Cd induced alteration of spermatogenic epithelial staging in testis (reproductive toxicity) and anxiety (neurotoxicity) in male offspring. The levels of total protein, globulins, total bile acid and direct bilirubin were altered. | 2019 Zhang et al. [34] |
| Wistar rats (n = 15–17) 0.5, and 0.05 mg CdCl2/kg bw/day, d.w. 21 DBM to MD and GD 0 to PND 35 (n = 8), 21 | PND 21, 26, and 60 Blood and kidney PND 60 Urine PND 14 milk * | Maternal Cd exposure led to significant amounts of Cd in the liver and kidney of pups. In pups, insulin secretion was transiently affected by Cd exposure. | 2019 Jacquet et al. [61] |
| 129/SvJ MT1,2KO mice (n = 9) 0.15 µg Cd/L (74 µCi), 109CdCl2, d.w. GD 0 to PND 11 (n = 5), 11 | PND 11 Stomach, intestine, and feces | Placental 109Cd were higher in MT1,2KO dams than normal control. MT had no effect on the amount of 109Cd transferred to pups via milk, 85–90% of total pup 109Cd was recovered in gastrointestinal tracts. Specific sequestration of 109Cd by both maternal and neonatal intestinal tract does not require MT. | 2003 Brako et al. [48] |
*, dosage reported by the authors based on daily water consumption; **, dosage estimated by the review authors based on daily water consumption reported in the research article; ---, data not mentioned; ‡, work mentioned in another Cd exposure scheme; 3β-HSD, 3-β-hydroxysteroid dehydrogenase; ADRP, adipose differentiation-related protein; cAMP/PKA, cyclic adenosine monophosphate/protein kinase A; CF, cross-fostering; CYP19A1, aromatase cytochrome P450; CYP11A1, cholesterol side-chain cleavage enzyme; DBM, days before mating; F1-F2, first and second filial generations treated; GD, gestational day; GPx, glutathione peroxidase; GST, glutathione-S-transferase; HO-1, hem-oxygenase 1; HSL, hormone-sensitive lipase; MD, mating day; MDA, malondialdehyde; MT, metallothionein; MT1,2KO, metallothionein-knockout; PKCα, protein kinase C α-type; PLCβ2, 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase β-2; PND, postnatal day; SOD, superoxide dismutase; SRD5α, 5α-reductase1; StAR, steroidogenic acute regulation protein; VCAM-1, vascular cell adhesion molecule 1. Admin route, administration route; bw, body weight; d.w., drinking water; f.i., food intake; i.g., intragastric.
3.3. Experimental Features
Since each reviewed article aimed to study a particular Cd effect on offspring, it was expected that experimental conditions would be different from each other. Even when analyzing administration routes and effects evaluated for each exposure scheme separately, experimental differences were identified (Figure S1). Figure 4 describes the percentage of papers reporting each Cd exposure scheme, the proportion of works evaluating each effect, and the frequency of articles mentioning each administration route. In general, from 52 records included in this review the most reported Cd exposure scheme was pregnancy-lactation (67.27%), while neurotoxicity was the most evaluated effect (36.54%). Although, Cd in drinking water does not ensure that all dams consume the same dose of metal, it was the most reported administration route independently of the effect evaluated (Figure 4c). Some authors estimated the dose administered in drinking water by measuring the water consumption for each dam. This facilitates to know the approximate dose to compare with other experiments in which another route or dose were used. On the other hand, strategies such as cross-fostering was underreported with only three papers mentioning this strategy. It is important note that differences in experimental features difficult compare and discuss the effects observed. However, the Cd-doses and their effects resumed in Table 1, Table 2 and Table 3, show that even at lowest Cd dose adverse effects are generated. The experimental features described for each author are ordered in this review allowing other researchers to select the ideal conditions and exposure-scheme for studying the effects of perinatal Cd exposure.
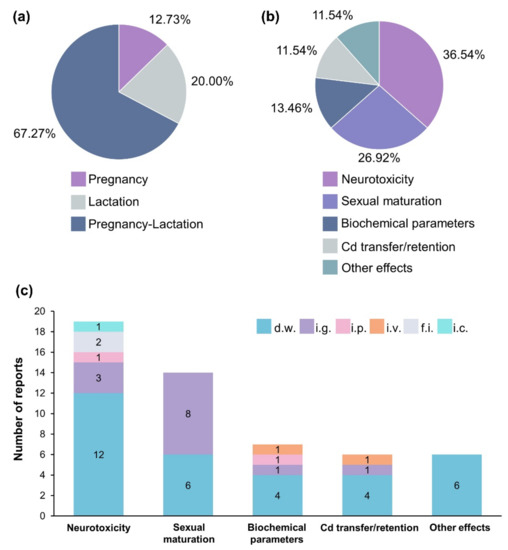
Figure 4.
Cd exposure scheme, effects evaluated and administration routes. (a) Percentage of works reporting pregnancy, lactation, or pregnancy–lactation exposure. (b) Percentage of reports evaluating different Cd effects. (c) Number of reports that described each Cd administration route. D.w., drinking water; i.g., intragastrical; i.p., intraperitoneal; i.v., intravenous; f.i., food intake; i.c., inhalation chamber.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app122211412/s1, Figure S1: Administration route, Cd-exposure scheme, and effects evaluated. Graphs represent the frequency of papers reporting each administration route during pregnancy, lactation, or pregnancy-lactation scheme, as well as the effect evaluated. D.w., drinking water; i.g., intragastrical; i.p., intraperitoneal; i.v., intravenous; f.i., food intake; i.c., inhalation chamber.
Author Contributions
Investigation, methodology, formal analysis, data curation, and original draft preparation, X.A.-P. and J.B.-B.; validation, visualization, and writing review and editing, A.L.-L.; visualization, supervision, writing review and editing, and project administration, J.A.F.d.l.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
Postdoctoral fellow X.A.-P., appreciates the scholarship awarded by the Consejo Nacional de Ciencia y Tecnología (CONACYT) and the support of Universidad Autónoma de Zacatecas (UAZ).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, C.; Wang, B.; Lu, X.; Huang, Y.; Wang, H.; Xu, D.; Zhang, J. Maternal exposure to cadmium from puberty through lactation induces abnormal reproductive development in female offspring. Ecotoxicol. Environ. Saf. 2022, 242, 113927. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; International Atomic Energy Agency. Minor and Trace Elements in Breast Milk: Report of a Joint WHO/IAEA Collaborative Study; World Health Organization: Geneva, Switzerland, 1989. [Google Scholar]
- Khanjani, N.; Jafari, M.; Ahmadi Mousavi, E. Breast milk contamination with lead and cadmium and its related factors in Kerman, Iran. J. Environ. Health Sci. Eng. 2018, 16, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Herrera, S.R.; Vincent, K.L.; Poole, A.; Olson, G.; Patrikeev, I.; Saada, J.; Gamble, P.; Motamedi, M.; Saade, G.R.; Stuebe, A.M.; et al. Long-Term Effect of Lactation on Maternal Cardiovascular Function and Adiposity in a Murine Model. Am. J. Perinatol. 2019, 36, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Tinia Hasianna, S.; Gunadi, J.W.; Rohmawaty, E.; Lesmana, R. Potential role of β-carotene-modulated autophagy in puerperal breast inflammation (Review). Biomed. Rep. 2022, 17, 75. [Google Scholar] [CrossRef] [PubMed]
- Motas, M.; Jiménez, S.; Oliva, J.; Cámara, M.; Pérez-Cárceles, M.D. Heavy Metals and Trace Elements in Human Breast Milk from Industrial/Mining and Agricultural Zones of Southeastern Spain. Int. J. Environ. Res. Public Health 2021, 18, 9289. [Google Scholar] [CrossRef]
- World Health Organization; International Programme on Chemical Safety. Cadmium: Environmental Aspects/Published under the Joint Sponsorship of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization; World Health Organization: Geneva, Switzerland, 1992. [Google Scholar]
- Scheuplein, R.; Charnley, G.; Dourson, M. Differential Sensitivity of Children and Adults to Chemical Toxicity: I. Biological Basis. Regul. Toxicol. Pharmacol. 2002, 35, 429–447. [Google Scholar] [CrossRef]
- Chandravanshi, L.; Shiv, K.; Kumar, S. Developmental toxicity of cadmium in infants and children: A review. Env. Anal. Health Toxicol. 2021, 36, e2021003. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Morgan, R.L.; Whaley, P.; Thayer, K.A.; Schünemann, H.J. Identifying the PECO: A framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ. Int. 2018, 121, 1027–1031. [Google Scholar] [CrossRef]
- Saillenfait, A.M.; Payan, J.P.; Ban, M.; de Ceaurriz, J. Indirect and lactation-associated changes in renal alkaline phosphatase of newborn rats prenatally exposed to cadmium chloride. J. Appl. Toxicol. 1992, 12, 205–210. [Google Scholar] [CrossRef]
- Slyuzova, O.V.; Stepanova, E.V.; Temraleeva, A.D.; Kireev, R.A.; Ignatov, V.V. Effects of prenatal and neonatal cadmium intoxication on the intensity of lipid peroxidation and activity of glutathione system in progeny of albino rats. Bull. Exp. Biol. Med. 2008, 146, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Liapi, C.; Stolakis, V.; Zarros, A.; Zissis, K.M.; Botis, J.; Al-Humadi, H.; Tsakiris, S. Gestational exposure to cadmium alters crucial offspring rat brain enzyme activities: The role of cadmium-free lactation. Environ. Toxicol. Pharm. 2013, 36, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Dési, I.; Nagymajtényi, L.; Schulz, H. Behavioural and neurotoxicological changes caused by cadmium treatment of rats during development. J. Appl. Toxicol. 1998, 18, 63–70. [Google Scholar] [CrossRef]
- Barański, B. Behavioral alterations in offspring of female rats repeatedly exposed to cadmium oxide by inhalation. Toxicol. Lett. 1984, 22, 53–61. [Google Scholar] [CrossRef]
- Salvatori, F.; Talassi, C.B.; Salzgeber, S.A.; Spinosa, H.S.; Bernardi, M.M. Embryotoxic and long-term effects of cadmium exposure during embryogenesis in rats. Neurotoxicol. Teratol. 2004, 26, 673–680. [Google Scholar] [CrossRef]
- Bakka, A.; Samarawickrama, G.P.; Webb, M. Metabolism of zinc and copper in the neonate: Effect of cadmium administration during late gestation in the rat on the zinc and copper metabolism of the newborn. Chem. Biol. Interact. 1981, 34, 161–171. [Google Scholar] [CrossRef]
- Grawé, K.P.; Teiling-Gårdlund, A.; Jalkesten, E.; Oskarsson, A. Increased spontaneous motor activity in offspring after maternal cadmium exposure during lactation. Environ. Toxicol. Pharmacol. 2004, 17, 35–43. [Google Scholar] [CrossRef]
- Grawé, K.P.; Pickova, J.; Dutta, P.C.; Oskarsson, A. Fatty acid alterations in liver and milk of cadmium exposed rats and in brain of their suckling offspring. Toxicol. Lett. 2004, 148, 73–82. [Google Scholar] [CrossRef]
- Andersson, H.; Petersson-Grawé, K.; Lindqvist, E.; Luthman, J.; Oskarsson, A.; Olson, L. Low-level cadmium exposure of lactating rats causes alterations in brain serotonin levels in the offspring. Neurotoxicol. Teratol. 1997, 19, 105–115. [Google Scholar] [CrossRef]
- Friedrichi, C.; Lopes, R.A.; Sala, M.A.; Felippini, A.L.d.C.; Issa, J.P.M.; Watanabe, I.-S.; Lopes, T.R.V.P. Efectos del Cadmio Sobre las Glándulas Salivales de Rata, Durante la Lactancia. Int. J. Morphol. 2009, 27, 1129–1137. [Google Scholar] [CrossRef]
- Ribas, P.; Lopes, R.A.; Sala, M.A.; Ribas, L.M.R.; de Mattos, M.d.G.C.; Semprini, M.; Watanabe, I.-S.; Regalo, S.C.H. Effect of Cadmium on Rat Maxillary Molar Junctional Epithelium During Lactation: Efecto Del Cadmio Sobre El Epitelio De La Zona De Unión Maxilo-Molar De Ratas Durante La Lactancia. Int. J. Morphol. 2004, 22, 257–262. [Google Scholar] [CrossRef][Green Version]
- Picoli, L.C.; Watanabe, I.S.; Lopes, R.A.; Sala, M.A.; Picoli, F. Effect of cadmium on the floor of the mouth on rats during lactation. Braz. Oral. Res. 2004, 18, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Picoli, L.C.; Watanabe, I.-S.; Lopes, R.A.; Sala, M.A.; Picoli, F. Efectos del cadmio en la mucosa yugal de la rata durante la lactancia. Estudio morfológico e histométrico. Int. J. Morphol. 2003, 21, 191–198. [Google Scholar] [CrossRef]
- Pillet, S.; Rooney, A.A.; Bouquegneau, J.M.; Cyr, D.G.; Fournier, M. Sex-specific effects of neonatal exposures to low levels of cadmium through maternal milk on development and immune functions of juvenile and adult rats. Toxicology 2005, 209, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Kar, R.; Galav, V.; Mehta, A.K.; Bhattacharya, S.K.; Mediratta, P.K.; Banerjee, B.D. Cadmium exposure during lactation causes learning and memory-impairment in F1 generation mice: Amelioration by quercetin. Drug Chem. Toxicol. 2016, 39, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Grawé, K.P.; Oskarsson, A. Cadmium in milk and mammary gland in rats and mice. Arch. Toxicol. 2000, 73, 519–527. [Google Scholar] [CrossRef]
- Feng, J.; Chen, S.; Wang, Y.; Liu, Q.; Yang, M.; Li, X.; Nie, C.; Qin, J.; Chen, H.; Yuan, X.; et al. Maternal exposure to cadmium impairs cognitive development of male offspring by targeting the Coronin-1a signaling pathway. Chemosphere 2019, 225, 765–774. [Google Scholar] [CrossRef]
- Antonio, M.T.; Peinado, V.; González, J.C.; Leret, M.L. Effects of maternal cadmium administration on development of monoaminergic, GABAergic and glutamatergic systems. Environ. Toxicol Pharm. 2010, 29, 87–90. [Google Scholar] [CrossRef]
- Antonio, M.T.; Benito, M.J.; Leret, M.L.; Corpas, I. Gestational administration of cadmium alters the neurotransmitter levels in newborn rat brains. J. Appl. Toxicol. 1998, 18, 83–88. [Google Scholar] [CrossRef]
- Gupta, A.; Shukla, G.S. Ontogenic profile of brain lipids following perinatal exposure to cadmium. J. Appl. Toxicol. 1996, 16, 227–233. [Google Scholar] [CrossRef]
- Luo, X.; Li, L.; Ma, M.; Li, R. Effects of low-dose cadmium exposure during gestation and lactation on development and reproduction in rats. Environ. Sci. Pollut. Res. Int. 2015, 22, 10569–10579. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Gao, X.; Luo, X.; Li, L.; Ma, M.; Zhu, Y.; Zhao, L.; Li, R. The effects of long-term exposure to low doses of cadmium on the health of the next generation of mice. Chem. Biol. Interact. 2019, 312, 108792. [Google Scholar] [CrossRef] [PubMed]
- Kostial, K.; Blanusa, M.; Schönwald, N.; Arezina, R.; Piasek, M.; Jones, M.M.; Singh, P.K. Organ cadmium deposits in orally exposed female rats and their pups and the depleting efficiency of sodium N-(4-methoxybenzyl)-D-glucamine-N-carbodithioate monohydrate (MeOBDCG). J. Appl. Toxicol. 1993, 13, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Ronco, A.M.; Montenegro, M.; Castillo, P.; Urrutia, M.; Saez, D.; Hirsch, S.; Zepeda, R.; Llanos, M.N. Maternal exposure to cadmium during gestation perturbs the vascular system of the adult rat offspring. Toxicol. Appl. Pharmacol. 2011, 251, 137–145. [Google Scholar] [CrossRef]
- Gupta, A.; Shukla, G.S. Development of brain free radical scavenging system and lipid peroxidation under the influence of gestational and lactational cadmium exposure. Hum. Exp. Toxicol. 1995, 14, 428–433. [Google Scholar] [CrossRef]
- Young, J.L.; Cave, M.C.; Xu, Q.; Kong, M.; Xu, J.; Lin, Q.; Tan, Y.; Cai, L. Whole life exposure to low dose cadmium alters diet-induced NAFLD. Toxicol. Appl. Pharmacol. 2022, 436, 115855. [Google Scholar] [CrossRef]
- Kostial, K.; Kello, D.; Blanusa, M.; Maljković, T.; Rabar, I. Influence of some factors on cadmium pharmacokinetics and toxicity. Environ. Health Perspect 1979, 28, 89–95. [Google Scholar] [CrossRef]
- Stolakis, V.; Tsakiris, S.; Kalafatakis, K.; Zarros, A.; Skandali, N.; Gkanti, V.; Kyriakaki, A.; Liapi, C. Developmental neurotoxicity of cadmium on enzyme activities of crucial offspring rat brain regions. Biometals 2013, 26, 1013–1021. [Google Scholar] [CrossRef]
- Mikolić, A.; Schönwald, N.; Piasek, M. Cadmium, iron and zinc interaction and hematological parameters in rat dams and their offspring. J. Trace Elem. Med. Biol. 2016, 38, 108–116. [Google Scholar] [CrossRef]
- Yargiçoğlu, P.R.; Ağar, A.; Oğuz, Y.; izgüt-Uysal, V.N.; Sentürk, Ü.K.; Öner, G. The effect of developmental exposure to cadmium (Cd) on visual evoked potentials (VEPs) and lipid peroxidation. NeuroToxicol. Teratol. 1997, 19, 213–219. [Google Scholar] [CrossRef]
- Zhao, Q.; Gao, L.; Liu, Q.; Cao, Y.; He, Y.; Hu, A.; Chen, W.; Cao, J.; Hu, C.; Li, L.; et al. Impairment of learning and memory of mice offspring at puberty, young adulthood, and adulthood by low-dose Cd exposure during pregnancy and lactation via GABA(A)R α5 and δ subunits. Ecotoxicol Env. Saf. 2018, 166, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Honda, A.; Watanabe, C.; Yoshida, M.; Nagase, H.; Satoh, M. Microarray analysis of neonatal brain exposed to cadmium during gestation and lactation. J. Toxicol. Sci. 2013, 38, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Santana, V.P.; Salles, É.S.; Correa, D.E.; Gonçalves, B.F.; Campos, S.G.; Justulin, L.A.; Godinho, A.F.; Scarano, W.R. Long-term effects of perinatal exposure to low doses of cadmium on the prostate of adult male rats. Int. J. Exp. Pathol. 2016, 97, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Petrochelli Banzato, T.; Godinho, A.F.; da Silva Zacarin, E.C.; Perobelli, J.E.; Dal Bianco Fernandez, C.; Favareto, A.P.; De Grava Kempinas, W. Sperm quality in adult male rats exposed to cadmium in utero and lactation. J. Toxicol. Env. Health A 2012, 75, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Ishitobi, H.; Watanabe, C. Effects of low-dose perinatal cadmium exposure on tissue zinc and copper concentrations in neonatal mice and on the reproductive development of female offspring. Toxicol. Lett. 2005, 159, 38–46. [Google Scholar] [CrossRef]
- Brako, E.E.; Wilson, A.K.; Jonah, M.M.; Blum, C.A.; Cerny, E.A.; Williams, K.L.; Bhattacharyya, M.H. Cadmium pathways during gestation and lactation in control versus metallothoinein 1,2-knockout mice. Toxicol. Sci. 2003, 71, 154–163. [Google Scholar] [CrossRef]
- Fatima, G.; Raza, A.M.; Hadi, N.; Nigam, N.; Mahdi, A.A. Cadmium in Human Diseases: It’s More than Just a Mere Metal. Indian J. Clin. Biochem. 2019, 34, 371–378. [Google Scholar] [CrossRef]
- Nagymajtényi, L.; Schulz, H.; Dési, I. Behavioural and functional neurotoxicological changes caused by cadmium in a three-generational study in rats. Hum. Exp. Toxicol. 1997, 16, 691–699. [Google Scholar] [CrossRef]
- Smith, K.R.; Nation, J.R. Developmental exposure to cadmium alters responsiveness to cocaine in the rat. Drug Alcohol Depend 2003, 72, 1–11. [Google Scholar] [CrossRef]
- Cardon, A.L.; Rocha, A.; Valles, R.; Bratton, G.R.; Nation, J.R. Exposure to Cadmium During Gestation and Lactation Decreases Cocaine Self-Administration in Rats. Neuro Toxicol. 2004, 25, 869–875. [Google Scholar] [CrossRef]
- Smith, K.R.; Nation, J.R.; Bratton, G.R. The effects of developmental cadmium exposure on morphine sensitization and challenge with selective D1 and D2 antagonists. Pharmacol. Biochem. Behav. 2002, 72, 581–590. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, J.; Li, H.; Wang, W.; Li, Y.; Yang, X.; Zheng, N.; Liu, Q.; Zhang, Q.; Zhang, W.; et al. Cadmium exposure during prenatal development causes testosterone disruption in multigeneration via SF-1 signaling in rats. Food Chem. Toxicol. 2020, 135, 110897. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Chen, S.; Leng, Y.; Li, T.; Li, Z.; Chen, H.; Zhang, Q. Exposure to cadmium during gestation and lactation affects development and function of Leydig cells in male offspring. Env. Toxicol. 2018, 33, 351–360. [Google Scholar] [CrossRef]
- Couto-Moraes, R.; Felício, L.F.; de Oliveira, C.A.; Bernardi, M.M. Post-partum testosterone administration partially reverses the effects of perinatal cadmium exposure on sexual behavior in rats. Psychol. Neurosci. 2012, 5, 221–229. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.; Li, H.; Liang, Y.; Mai, W.; Liu, C.; Chen, H.; Huang, Y.; Zhang, Q. CORO1A regulates lipoprotein uptake in Leydig cells exposed to cadmium. Ecotoxicol. Environ. Saf. 2022, 232, 113255. [Google Scholar] [CrossRef] [PubMed]
- Couto-Moraes, R.; Felicio, L.F.; Bernardi, M.M. Post-partum testosterone administration does not reverse the effects of perinatal exposure to cadmium on rat offspring development. J. Appl. Toxicol. 2010, 30, 233–241. [Google Scholar] [CrossRef]
- Liu, Q.; Liang, Y.; Gao, N.; Gao, J.; Wang, Y.; Li, X.; Qin, J.; Xiang, Q.; Wu, X.; Chen, H.; et al. Regulation of lipid droplets via the PLCβ2-PKCα-ADRP pathway in granulosa cells exposed to cadmium. Env. Pollut. 2020, 267, 115541. [Google Scholar] [CrossRef]
- Li, Z.; Li, T.; Leng, Y.; Chen, S.; Liu, Q.; Feng, J.; Chen, H.; Huang, Y.; Zhang, Q. Hormonal changes and folliculogenesis in female offspring of rats exposed to cadmium during gestation and lactation. Env. Pollut. 2018, 238, 336–347. [Google Scholar] [CrossRef]
- Jacquet, A.; Barbeau, D.; Arnaud, J.; Hijazi, S.; Hazane-Puch, F.; Lamarche, F.; Quiclet, C.; Couturier, K.; Fontaine, E.; Moulis, J.-M.; et al. Impact of maternal low-level cadmium exposure on glucose and lipid metabolism of the litter at different ages after weaning. Chemosphere 2019, 219, 109–121. [Google Scholar] [CrossRef]
- Whelton, B.D.; Toomey, J.M.; Bhattacharyya, M.H. Cadmium-109 metabolism in mice. IV. Diet versus maternal stores as a source of cadmium transfer to mouse fetuses and pups during gestation and lactation. J. Toxicol. Env. Health 1993, 40, 531–546. [Google Scholar] [CrossRef]
- Nakamura, Y.; Ohba, K.; Suzuki, K.; Ohta, H. Health effects of low-level cadmium intake and the role of metallothionein on cadmium transport from mother rats to fetus. J. Toxicol. Sci. 2012, 37, 149–156. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).