Electroporation in Clinical Applications—The Potential of Gene Electrotransfer and Electrochemotherapy
Abstract
1. Introduction
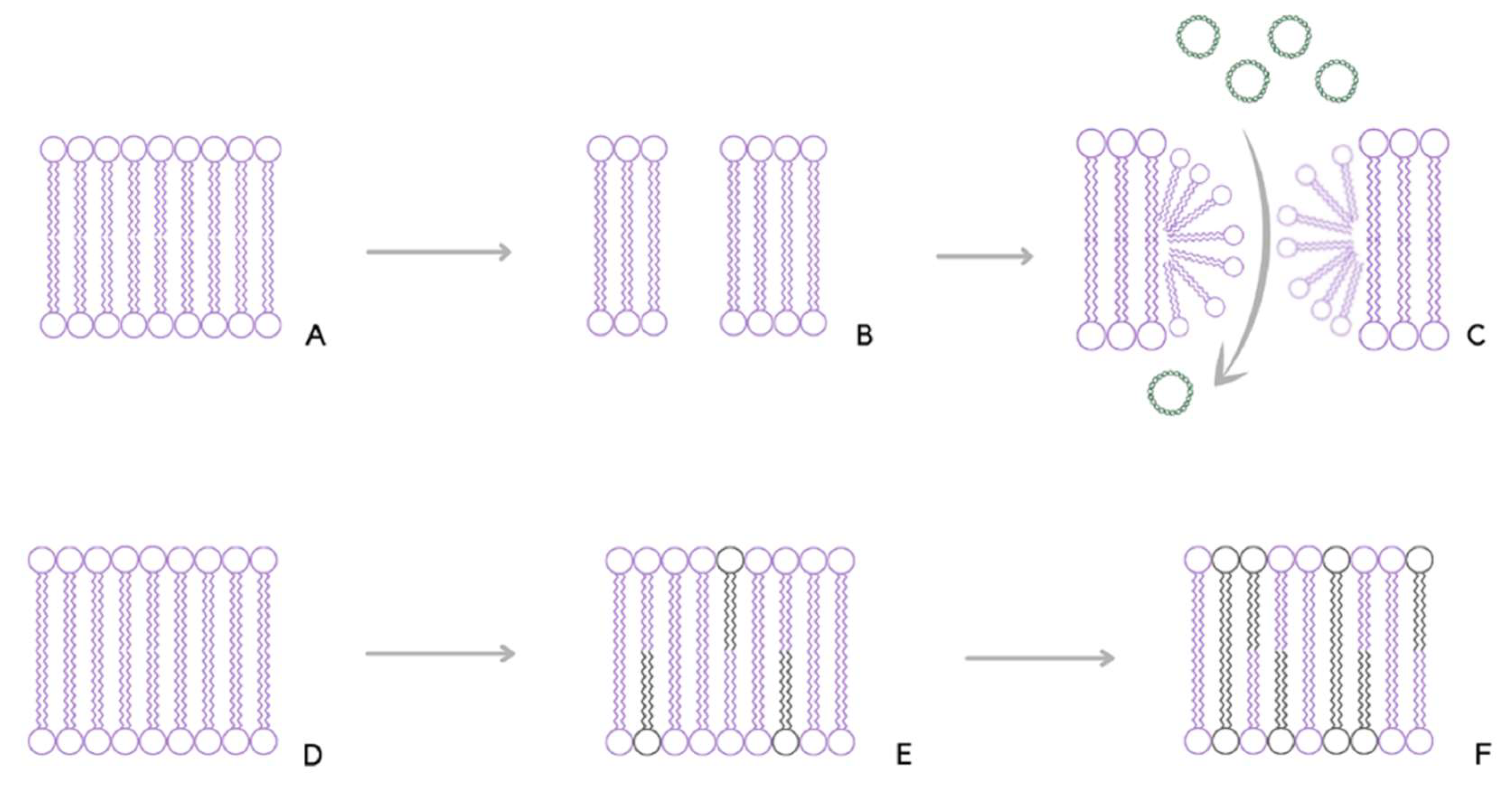
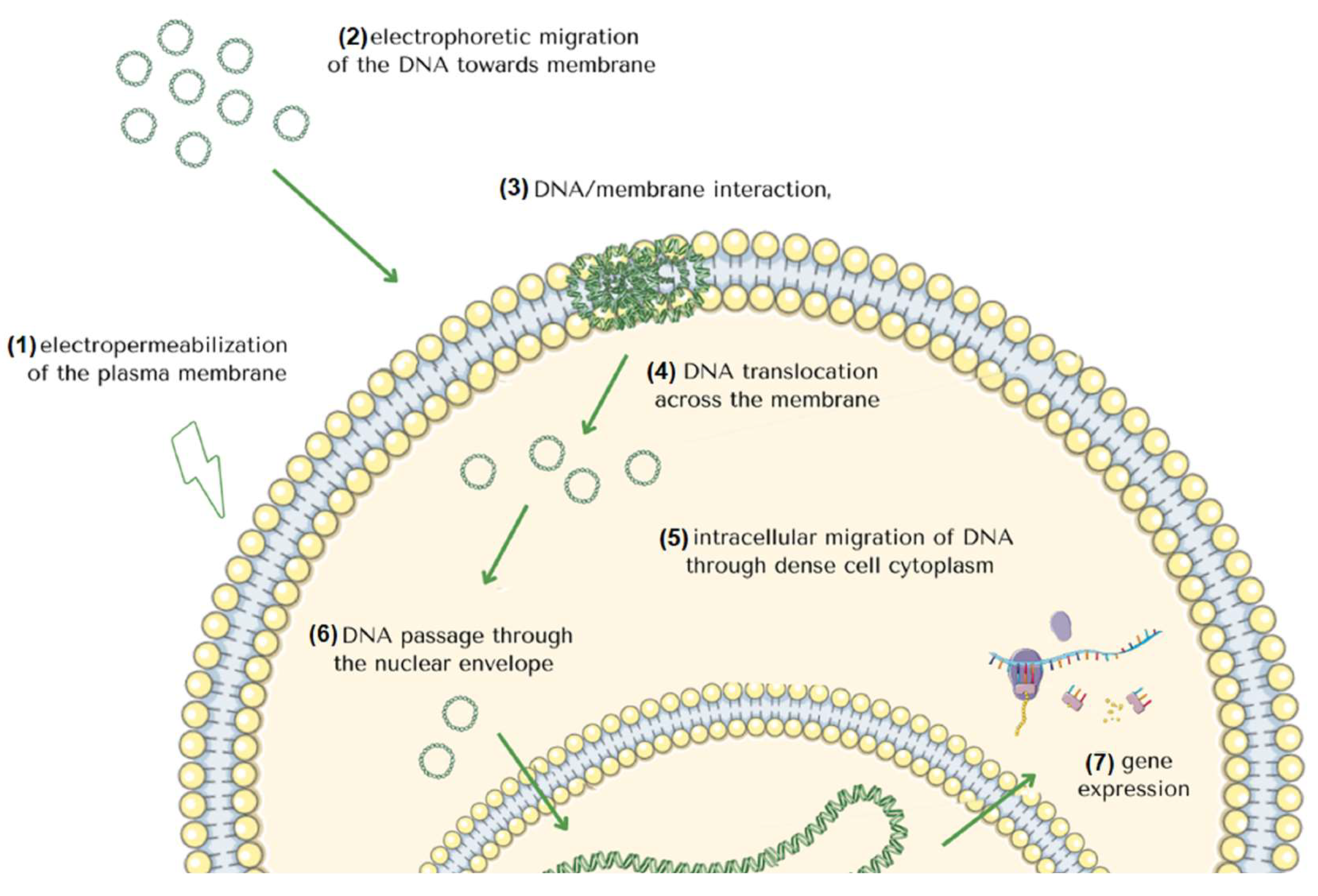
2. Molecular Mechanism of Gene Electrotransfer
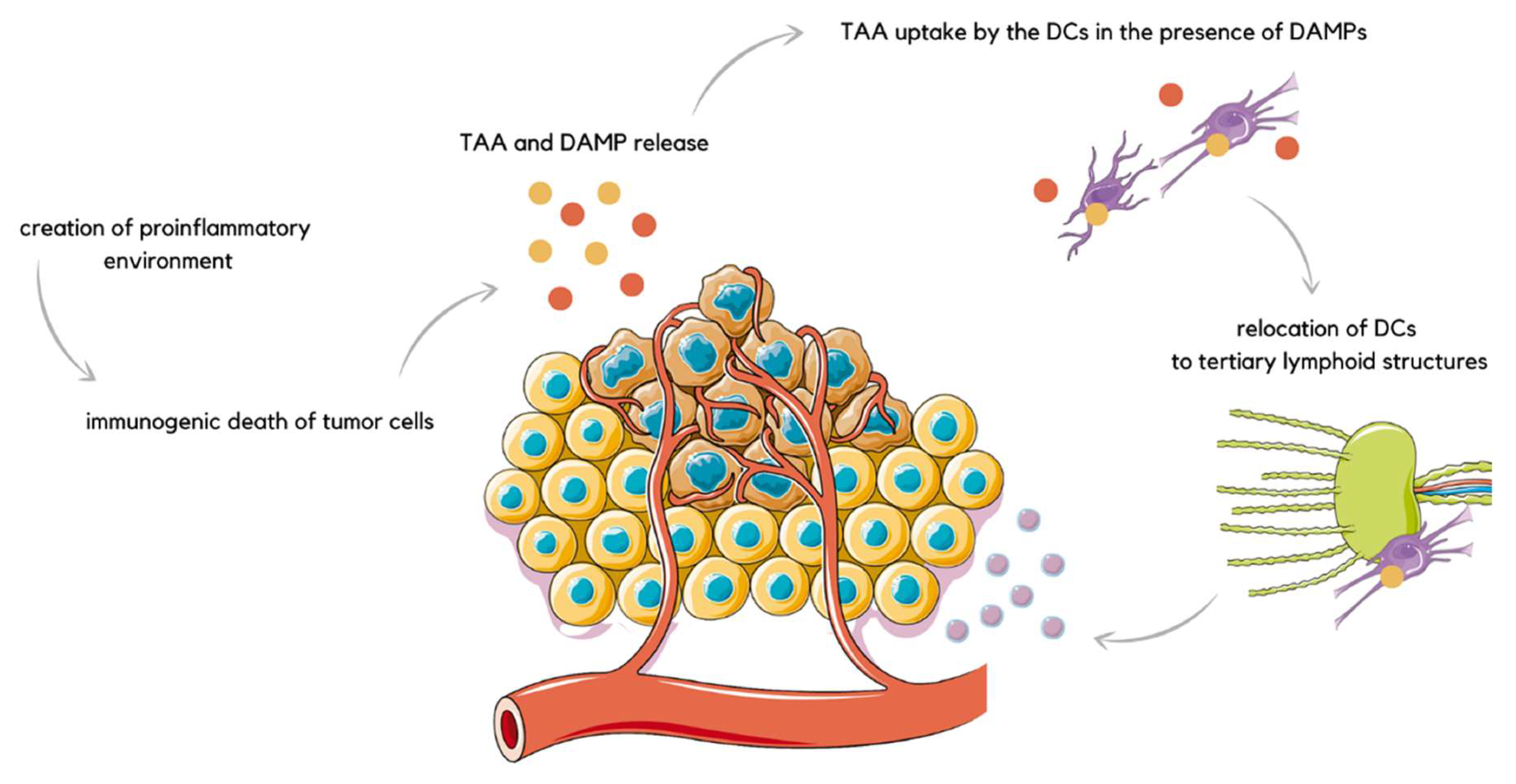
3. EP in Immunogen Electrotransfer
4. Preclinical Studies
5. Clinical Studies
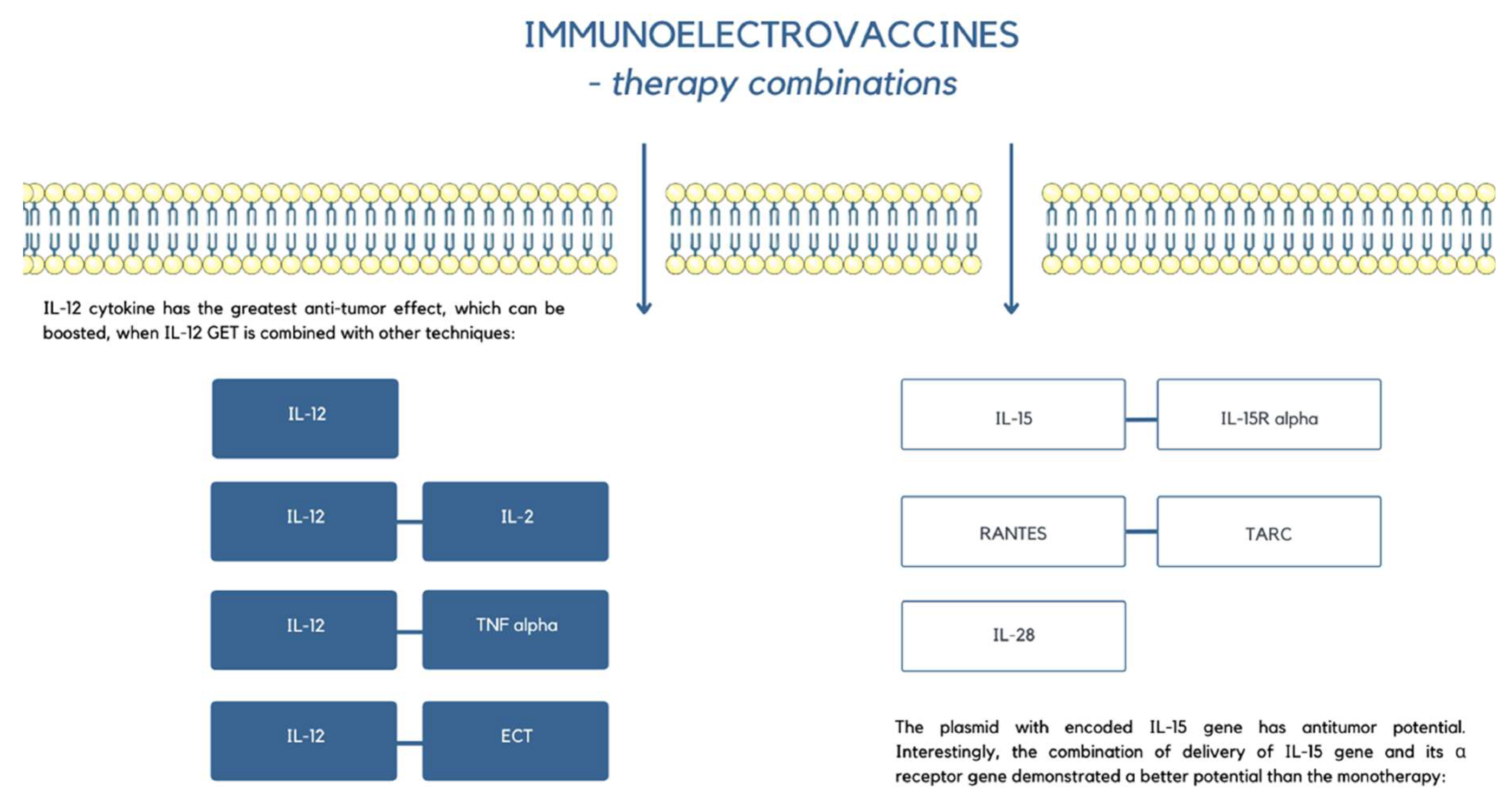
6. EP in Electrovaccines
7. Future Perspectives
8. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Nigam, S.K. What do drug transporters really do? Nat. Rev. Drug Discov. 2015, 14, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Bosshart, P.D.; Fotiadis, D. Secondary Active Transporters. Subcell. Biochem. 2019, 92, 275–299. [Google Scholar] [PubMed]
- Young, J.L.; Dean, D.A. Electroporation-Mediated Gene Delivery. Adv. Genet. 2015, 89, 49. [Google Scholar] [PubMed]
- Stewart, M.P.; Langer, R.; Jensen, K.F. Intracellular delivery by membrane disruption: Mechanisms, strategies, and concepts. Chem. Rev. 2018, 118, 7409–7531. [Google Scholar] [CrossRef]
- Weaver, J.C. Electroporation theory. Concepts and mechanisms. Methods Mol. Biol. 1995, 55, 3–28. [Google Scholar]
- Chu, G.; Hayakawa, H.; Berg, P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987, 15, 1311–1326. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. DNA transfection by electroporation. Cold Spring Harb. Protoc. 2019, 2019, 559–563. [Google Scholar] [CrossRef]
- Drinkwater, N.R.; Klinedinst, D.K. Chemically induced mutagenesis in a shuttle vector with a low-background mutant frequency. Proc. Natl. Acad. Sci. USA 1986, 83, 3402–3406. [Google Scholar] [CrossRef]
- Golberg, A.; Rubinsky, B. The effect of electroporation type pulsed electric fields on DNA in aqueous solution. Technol. Cancer Res. Treat. 2010, 9, 423–430. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Electroporation. Cold Spring Harb. Protoc. 2019, 2019, 519–525. [Google Scholar] [CrossRef]
- Pliquett, U.; Joshi, R.P.; Sridhara, V.; Schoenbach, K.H. High electrical field effects on cell membranes. Bioelectrochemistry 2007, 70, 275–282. [Google Scholar] [CrossRef]
- Kotnik, T.; Rems, L.; Tarek, M.; Miklavčič, D. Membrane Electroporation and Electropermeabilization: Mechanisms and Models. Annu. Rev. Biophys. 2019, 48, 63–91. [Google Scholar] [CrossRef] [PubMed]
- Freeman, S.A.; Wang, M.A.; Weaver, J.C. Theory of electroporation of planar bilayer membranes: Predictions of the aqueous area, change in capacitance, and pore-pore separation. Biophys. J. 1994, 67, 42–56. [Google Scholar] [CrossRef]
- Tarek, M. Membrane Electroporation: A Molecular Dynamics Simulation. Biophys. J. 2005, 88, 4045–4053. [Google Scholar] [CrossRef]
- DeBruin, K.A.; Krassowska, W. Electroporation and shock-induced transmembrane potential in a cardiac fiber during defibrillation strength shocks. Ann. Biomed. Eng. 1998, 26, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Kavian, O.; Leguèbe, M.; Poignard, C.; Weynans, L. “Classical” Electropermeabilization Modeling at the Cell Scale. J. Math. Biol. 2014, 68, 235–265. [Google Scholar] [CrossRef] [PubMed]
- Rosenheck, K.; Lindner, P.; Pecht, I. Effect of electric fields on light-scattering and fluorescence of chromaffin granules. J. Membr. Biol. 1975, 20, 1–12. [Google Scholar] [CrossRef]
- Leguèbe, M.; Silve, A.; Mir, L.M.; Poignard, C. Conducting and permeable states of cell membrane submitted to high voltage pulses: Mathematical and numerical studies validated by the experiments. J. Theor. Biol. 2014, 360, 83–94. [Google Scholar] [CrossRef]
- Lindner, P.; Neumann, E.; Rosenheck, K. Kinetics of permeability changes induced by electric impulses in chromaffin granules. J. Membr. Biol. 1977, 32, 231–254. [Google Scholar] [CrossRef]
- Zimmermann, U. Electric field-mediated fusion and related electrical phenomena. Biochim. Biophys. Acta—Rev. Biomembr. 1982, 694, 227–277. [Google Scholar] [CrossRef]
- Chafai, D.E.; Vostárek, F.; Dráberová, E.; Havelka, D.; Arnaud-Cormos, D.; Leveque, P.; Janáček, J.; Kubínová, L.; Cifra, M.; Dráber, P. Microtubule Cytoskeleton Remodeling by Nanosecond Pulsed Electric Fields. Adv. Biosyst. 2020, 4, e2000070. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Rols, M.P.; Teissie, J. Phosphorus-31 NMR analysis of membrane phospholipid organization in viable, reversibly electropermeabilized Chinese hamster ovary cells. Biochemistry 2002, 27, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Varnai, R.; Koskinen, L.; Mäntylä, L.; Szabo, I.; FitzGerald, L.; Sipeky, C. Pharmacogenomic Biomarkers in Docetaxel Treatment of Prostate Cancer: From Discovery to Implementation. Genes 2019, 10, 599. [Google Scholar] [CrossRef] [PubMed]
- Vernier, P.T.; Ziegler, M.J. Nanosecond field alignment of head group and water dipoles in electroporating phospholipid bilayers. J. Phys. Chem. B 2007, 111, 12993–12996. [Google Scholar] [CrossRef]
- Teissie, J.; Golzio, M.; Rols, M.P. Mechanisms of cell membrane electropermeabilization: A minireview of our present (lack of?) knowledge. Biochim. Biophys. Acta—Gen. Subj. 2005, 1724, 270–280. [Google Scholar] [CrossRef]
- Batista Napotnik, T.; Polajžer, T.; Miklavčič, D. Cell death due to electroporation—A review. Bioelectrochemistry 2021, 141, 107871. [Google Scholar] [CrossRef]
- Deipolyi, A.R.; Golberg, A.; Yarmush, M.L.; Arellano, R.S.; Oklu, R. Irreversible electroporation: Evolution of a laboratory technique in interventional oncology. Diagn. Interv. Radiol. 2014, 20, 147–154. [Google Scholar] [CrossRef]
- Rubinsky, B. Irreversible electroporation in medicine. Technol. Cancer Res. Treat. 2007, 6, 255–259. [Google Scholar] [CrossRef]
- Al-Sakere, B.; Bernat, C.; André, F.; Connault, E.; Opolon, P.; Davalos, R.V.; Mir, L.M. A study of the immunological response to tumor ablation with irreversible electroporation. Technol. Cancer Res. Treat. 2007, 6, 301–305. [Google Scholar] [CrossRef]
- Probst, U.; Fuhrmann, I.; Beyer, L.; Wiggermann, P. Electrochemotherapy as a new modality in interventional oncology: A review. Technol. Cancer Res. Treat. 2018, 17, 1533033818785329. [Google Scholar] [CrossRef]
- Hendel, K.; Jemec, G.B.E.; Haedersdal, M.; Wiegell, S.R. Electrochemotherapy with bleomycin for basal cell carcinomas: A systematic review. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 2208–2215. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, N.; Mowatt, D.; Sykes, A.J. Electrochemotherapy and Ablative Therapies in Non-melanoma Skin Cancer. Clin. Oncol. (R. Coll. Radiol). 2019, 31, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Miklavčič, D.; Mali, B.; Kos, B.; Heller, R.; Serša, G. Electrochemotherapy: From the drawing board into medical practice. Biomed. Eng. Online 2014, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Fattori, E.; La Monica, N.; Ciliberto, G.; Toniatti, C. Electro-gene-transfer: A new approach for muscle gene delivery. Somat. Cell Mol. Genet. 2002, 27, 75–83. [Google Scholar] [CrossRef]
- Shi, J.; Ma, Y.; Zhu, J.; Chen, Y.; Sun, Y.; Yao, Y.; Yang, Z.; Xie, J. A Review on Electroporation-Based Intracellular Delivery. Molecules 2018, 23, 3044. [Google Scholar] [CrossRef]
- Lambricht, L.; Lopes, A.; Kos, S.; Sersa, G.; Préat, V.; Vandermeulen, G. Clinical potential of electroporation for gene therapy and DNA vaccine delivery. Expert Opin. Drug Deliv. 2015, 13, 295–310. [Google Scholar] [CrossRef]
- Pelofy, S.; Teissié, J.; Golzio, M.; Chabot, S. Chemically modified oligonucleotide-increased stability negatively correlates with its efficacy despite efficient electrotransfer. J. Membr. Biol. 2012, 245, 565–571. [Google Scholar] [CrossRef]
- Orio, J.; Coustets, M.; Mauroy, C.; Teissie, J. Electric field orientation for gene delivery using high-voltage and low-voltage pulses. J. Membr. Biol. 2012, 245, 661–666. [Google Scholar] [CrossRef]
- Baliga, U.K.; Dean, D.A. Pulmonary gene delivery—Realities and possibilities. Exp. Biol. Med. 2021, 246, 260. [Google Scholar] [CrossRef]
- Venkatesh, A.; Ma, S.; Langellotto, F.; Gao, G.; Punzo, C. Retinal gene delivery by rAAV and DNA electroporation. Curr. Protoc. Microbiol. 2013. [Google Scholar] [CrossRef]
- Dudek, A.M.; Porteus, M.H. Answered and Unanswered Questions in Early-Stage Viral Vector Transduction Biology and Innate Primary Cell Toxicity for Ex-Vivo Gene Editing. Front. Immunol. 2021, 12, 660302. [Google Scholar] [CrossRef] [PubMed]
- Mingozzi, F.; High, K.A. Immune responses to AAV vectors: Overcoming barriers to successful gene therapy. Blood 2013, 122, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Ertl, H.C.J. Immunogenicity and toxicity of AAV gene therapy. Front. Immunol. 2022, 13, 975803. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.H.; Kritz, A.; Work, L.M.; Nicklin, S.A. Cell-selective viral gene delivery vectors for the vasculature. Exp. Physiol. 2005, 90, 27–31. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Satterle, A.; Wu, Q.; Wang, J.; Liu, F. Gene transfer to skeletal muscle by site-specific delivery of electroporation and ultrasound. Biochem. Biophys. Res. Commun. 2012, 424, 203–207. [Google Scholar] [CrossRef]
- Rosazza, C.; Haberl Meglic, S.; Zumbusch, A.; Rols, M.-P.; Miklavcic, D. Gene Electrotransfer: A Mechanistic Perspective. Curr. Gene Ther. 2016, 16, 98–129. [Google Scholar] [CrossRef]
- Escoffre, J.M.; Mauroy, C.; Portet, T.; Wasungu, L.; Rosazza, C.; Gilbart, Y.; Mallet, L.; Bellard, E.; Golzio, M.; Rols, M.P.; et al. Gene electrotransfer: From biophysical mechanisms to in vivo applications: Part 1- Biophysical mechanisms. Biophys. Rev. 2009, 1, 177–184. [Google Scholar] [CrossRef]
- Šatkauskas, S.; Ruzgys, P.; Venslauskas, M.S. Towards the mechanisms for efficient gene transfer into cells and tissues by means of cell electroporation. Expert Opin. Biol. Ther. 2012, 12, 275–286. [Google Scholar] [CrossRef]
- Kandušer, M.; Miklavčič, D.; Pavlin, M. Mechanisms involved in gene electrotransfer using high- and low-voltage pulses—An in vitro study. Bioelectrochemistry 2009, 74, 265–271. [Google Scholar] [CrossRef]
- André, F.M.; Gehl, J.; Sersa, G.; Préat, V.; Hojman, P.; Eriksen, J.; Golzio, M.; Cemazar, M.; Pavselj, N.; Rols, M.-P.; et al. Efficiency of high- and low-voltage pulse combinations for gene electrotransfer in muscle, liver, tumor, and skin. Hum. Gene Ther. 2008, 19, 1261–1271. [Google Scholar] [CrossRef]
- Cemazar, M.; Golzio, M.; Sersa, G.; Hojman, P.; Kranjc, S.; Mesojednik, S.; Rols, M.-P.; Teissie, J. Control by pulse parameters of DNA electrotransfer into solid tumors in mice. Gene Ther. 2009, 16, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Rosazza, C.; Deschout, H.; Buntz, A.; Braeckmans, K.; Rols, M.P.; Zumbusch, A. Endocytosis and Endosomal Trafficking of DNA After Gene Electrotransfer In Vitro. Mol. Ther. Nucleic Acids 2016, 5, e286. [Google Scholar] [CrossRef] [PubMed]
- Pavlin, M.; Pucihar, G.; Kandušer, M. The role of electrically stimulated endocytosis in gene electrotransfer. Bioelectrochemistry 2012, 83, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Trotovšek, B.; Djokić, M.; Čemažar, M.; Serša, G. New era of electrochemotherapy in treatment of liver tumors in conjunction with immunotherapies. World J. Gastroenterol. 2021, 27, 8216. [Google Scholar] [CrossRef]
- Remic, T.; Sersa, G.; Ursic, K.; Cemazar, M.; Kamensek, U. Development of Tumor Cell-Based Vaccine with IL-12 Gene Electrotransfer as Adjuvant. Vaccines 2020, 8, 111. [Google Scholar] [CrossRef]
- Kamensek, U.; Ursic, K.; Markelc, B.; Cemazar, M.; Setrajcic Dragos, V.; Sersa, G. Mutational burden, MHC-I expression and immune infiltration as limiting factors for in situ vaccination by TNFα and IL-12 gene electrotransfer. Bioelectrochemistry 2021, 140, 107831. [Google Scholar] [CrossRef]
- Yang, B.; Jeang, J.; Yang, A.; Wu, T.C.; Hung, C.F. DNA vaccine for cancer immunotherapy. Hum. Vaccin. Immunother. 2014, 10, 3153. [Google Scholar] [CrossRef]
- Sersa, G.; Teissie, J.; Cemazar, M.; Signori, E.; Kamensek, U.; Marshall, G.; Miklavcic, D. Electrochemotherapy of tumors as in situ vaccination boosted by immunogene electrotransfer. Cancer Immunol. Immunother. 2015, 64, 1315. [Google Scholar] [CrossRef]
- Cha, E.; Daud, A. Plasmid IL-12 electroporation in melanoma. Hum. Vaccin. Immunother. 2012, 8, 1734. [Google Scholar] [CrossRef]
- Lucas, M.L.; Heller, L.; Coppola, D.; Heller, R. IL-12 plasmid delivery by in vivo electroporation for the successful treatment of established subcutaneous B16.F10 melanoma. Mol. Ther. 2002, 5, 668–675. [Google Scholar] [CrossRef]
- Ursic, K.; Kos, S.; Kamensek, U.; Cemazar, M.; Miceska, S.; Markelc, B.; Bucek, S.; Staresinic, B.; Kloboves Prevodnik, V.; Heller, R.; et al. Potentiation of electrochemotherapy effectiveness by immunostimulation with IL-12 gene electrotransfer in mice is dependent on tumor immune status. J. Control. Release 2021, 332, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Lampreht Tratar, U.; Loiacono, L.; Cemazar, M.; Kamensek, U.; Fazio, V.M.; Sersa, G.; Signori, E. Gene Electrotransfer of Plasmid-Encoding IL-12 Recruits the M1 Macrophages and Antigen-Presenting Cells Inducing the Eradication of Aggressive B16F10 Murine Melanoma. Mediators Inflamm. 2017, 2017, 5285890. [Google Scholar] [CrossRef] [PubMed]
- Miklavcic, D.; Davalos, R.V. Electrochemotherapy (ECT) and irreversible electroporation (IRE)-advanced techniques for treating deep-seated tumors based on electroporation. Biomed. Eng. Online 2015, 14 (Suppl. S3), I1. [Google Scholar] [CrossRef]
- Jaroszeski, M.J.; Dang, V.; Pottinger, C.; Hickey, J.; Gilbert, R.; Heller, R. Toxicity of anticancer agents mediated by electroporation in vitro. Anticancer. Drugs 2000, 11, 201–208. [Google Scholar] [CrossRef]
- Fiorentzis, M.; Kalirai, H.; Katopodis, P.; Seitz, B.; Viestenz, A.; Coupland, S.E. Electrochemotherapy with bleomycin and cisplatin enhances cytotoxicity in primary and metastatic uveal melanoma cell lines in vitro. Neoplasma 2018, 65, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Girelli, R.; Prejanò, S.; Cataldo, I.; Corbo, V.; Martini, L.; Scarpa, A.; Claudio, B. Feasibility and safety of electrochemotherapy (ECT) in the pancreas: A pre-clinical investigation. Radiol. Oncol. 2015, 49, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Scuderi, M.; Rebersek, M.; Miklavcic, D.; Dermol-Cerne, J. The use of high-frequency short bipolar pulses in cisplatin electrochemotherapy in vitro. Radiol. Oncol. 2019, 53, 194–205. [Google Scholar] [CrossRef]
- Komel, T.; Bosnjak, M.; Kranjc Brezar, S.; De Robertis, M.; Mastrodonato, M.; Scillitani, G.; Pesole, G.; Signori, E.; Sersa, G.; Cemazar, M. Gene electrotransfer of IL-2 and IL-12 plasmids effectively eradicated murine B16.F10 melanoma. Bioelectrochemistry 2021, 141, 107843. [Google Scholar] [CrossRef]
- Vandermeulen, G.; Marie, C.; Scherman, D.; Préat, V. New generation of plasmid backbones devoid of antibiotic resistance marker for gene therapy trials. Mol. Ther. 2011, 19, 1942–1949. [Google Scholar] [CrossRef]
- Kos, S.; Bosnjak, M.; Jesenko, T.; Markelc, B.; Kamensek, U.; Znidar, K.; Matkovic, U.; Rencelj, A.; Sersa, G.; Hudej, R.; et al. Non-Clinical In Vitro Evaluation of Antibiotic Resistance Gene-Free Plasmids Encoding Human or Murine IL-12 Intended for First-in-Human Clinical Study. Pharmaceutics 2021, 13, 1739. [Google Scholar] [CrossRef]
- Kamensek, U.; Rencelj, A.; Jesenko, T.; Remic, T.; Sersa, G.; Cemazar, M. Maintenance and gene electrotransfer efficiency of antibiotic resistance gene-free plasmids encoding mouse, canine and human interleukin-12 orthologues. Heliyon 2022, 8, e08879. [Google Scholar] [CrossRef] [PubMed]
- Kamensek, U.; Sersa, G.; Cemazar, M. Evaluation of p21 promoter for interleukin 12 radiation induced transcriptional targeting in a mouse tumor model. Mol. Cancer 2013, 12, 136. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, B.; Cruz, Y.L.; Coppola, D.; Heller, R. Intradermal delivery of plasmid VEGF(165) by electroporation promotes wound healing. Mol. Ther. 2009, 17, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Steinstraesser, L.; Lam, M.C.; Jacobsen, F.; Porporato, P.E.; Chereddy, K.K.; Becerikli, M.; Stricker, I.; Hancock, R.E.; Lehnhardt, M.; Sonveaux, P.; et al. Skin electroporation of a plasmid encoding hCAP-18/LL-37 host defense peptide promotes wound healing. Mol. Ther. 2014, 22, 734–742. [Google Scholar] [CrossRef]
- Marshall, W.G.; Boone, B.A.; Burgos, J.D.; Gografe, S.I.; Baldwin, M.K.; Danielson, M.L.; Larson, M.J.; Caretto, D.R.; Cruz, Y.; Ferraro, B.; et al. Electroporation-mediated delivery of a naked DNA plasmid expressing VEGF to the porcine heart enhances protein expression. Gene Ther. 2009, 17, 419–423. [Google Scholar] [CrossRef]
- Ayuni, E.L.; Gazdhar, A.; Giraud, M.N.; Kadner, A.; Gugger, M.; Cecchini, M.; Caus, T.; Carrel, T.P.; Schmid, R.A.; Tevaearai, H.T. In Vivo Electroporation Mediated Gene Delivery to the Beating Heart. PLoS ONE 2011, 5, e14467. [Google Scholar] [CrossRef]
- Hargrave, B.; Downey, H.; Strange, R.; Murray, L.; Cinnamond, C.; Lundberg, C.; Israel, A.; Chen, Y.-J.; Marshall, W.; Heller, R. Electroporation-mediated gene transfer directly to the swine heart. Gene Ther. 2013, 20, 151–157. [Google Scholar] [CrossRef]
- Dean, D.A.; Machado-Aranda, D.; Blair-Parks, K.; Yeldandi, A.V.; Young, J.L. Electroporation as a method for high-level nonviral gene transfer to the lung. Gene Ther. 2003, 10, 1608–1615. [Google Scholar] [CrossRef]
- Gazdhar, A.; Temuri, A.; Knudsen, L.; Gugger, M.; Schmid, R.A.; Ochs, M.; Geiser, T. Targeted gene transfer of hepatocyte growth factor to alveolar type II epithelial cells reduces lung fibrosis in rats. Hum. Gene Ther. 2013, 24, 105–116. [Google Scholar] [CrossRef]
- da Luz, J.C.D.S.; Antunes, F.; Clavijo-Salomon, M.A.; Signori, E.; Tessarollo, N.G.; Strauss, B.E. Clinical Applications and Immunological Aspects of Electroporation-Based Therapies. Vaccines 2021, 9, 727. [Google Scholar] [CrossRef]
- Greaney, S.K.; Algazi, A.P.; Tsai, K.K.; Takamura, K.T.; Chen, L.; Twitty, C.G.; Zhang, L.; Paciorek, A.; Pierce, R.H.; Le, M.H.; et al. Intratumoral plasmid IL-12 electroporation therapy in advanced melanoma patients induces systemic and intratumoral T cell responses: T cell responses induced by plasmid IL-12 electroporation. Cancer Immunol. Res. 2020, 8, 246. [Google Scholar] [CrossRef]
- Shi, G.; Edelblute, C.; Arpag, S.; Lundberg, C.; Heller, R. IL-12 Gene Electrotransfer Triggers a Change in Immune Response within Mouse Tumors. Cancers 2018, 10, 498. [Google Scholar] [CrossRef]
- Pasquet, L.; Bellard, E.; Chabot, S.; Markelc, B.; Rols, M.P.; Teissie, J.; Golzio, M. Pre-clinical investigation of the synergy effect of interleukin-12 gene-electro-transfer during partially irreversible electropermeabilization against melanoma. J. Immunother. Cancer 2019, 7, 161. [Google Scholar] [CrossRef]
- Lucas, M.L.; Heller, R. IL-12 gene therapy using an electrically mediated nonviral approach reduces metastatic growth of melanoma. DNA Cell Biol. 2003, 22, 755–763. [Google Scholar] [CrossRef]
- Pavlin, D.; Cemazar, M.; Kamensek, U.; Tozon, N.; Pogacnik, A.; Sersa, G. Local and systemic antitumor effect of intratumoral and peritumoral IL-12 electrogene therapy on murine sarcoma. Cancer Biol. Ther. 2009, 8, 2114–2122. [Google Scholar] [CrossRef]
- Sedlar, A.; Dolinsek, T.; Markelc, B.; Prosen, L.; Kranjc, S.; Bosnjak, M.; Blagus, T.; Cemazar, M.; Sersa, G. Potentiation of electrochemotherapy by intramuscular IL-12 gene electrotransfer in murine sarcoma and carcinoma with different immunogenicity. Radiol. Oncol. 2012, 46, 302. [Google Scholar] [CrossRef]
- Kamensek, U.; Cemazar, M.; Lampreht Tratar, U.; Ursic, K.; Sersa, G. Antitumor in situ vaccination effect of TNFα and IL-12 plasmid DNA electrotransfer in a murine melanoma model. Cancer Immunol. Immunother. 2018, 67, 785–795. [Google Scholar] [CrossRef]
- Chuang, T.F.; Lee, S.C.; Liao, K.W.; Hsiao, Y.W.; Lo, C.H.; Chiang, B.L.; Lin, X.Z.; Tao, M.H.; Chu, R.M. Electroporation-mediated IL-12 gene therapy in a transplantable canine cancer model. Int. J. Cancer 2009, 125, 698–707. [Google Scholar] [CrossRef]
- Cemazar, M.; Ambrozic Avgustin, J.; Pavlin, D.; Sersa, G.; Poli, A.; Krhac Levacic, A.; Tesic, N.; Lampreht Tratar, U.; Rak, M.; Tozon, N. Efficacy and safety of electrochemotherapy combined with peritumoral IL-12 gene electrotransfer of canine mast cell tumours. Vet. Comp. Oncol. 2017, 15, 641–654. [Google Scholar] [CrossRef]
- Daud, A.I.; DeConti, R.C.; Andrews, S.; Urbas, P.; Riker, A.I.; Sondak, V.K.; Munster, P.N.; Sullivan, D.M.; Ugen, K.E.; Messina, J.L.; et al. Phase I Trial of Interleukin-12 Plasmid Electroporation in Patients With Metastatic Melanoma. J. Clin. Oncol. 2008, 26, 5896. [Google Scholar] [CrossRef]
- Ugen, K.E.; Kutzler, M.A.; Marrero, B.; Westover, J.; Coppola, D.; Weiner, D.B.; Heller, R. Regression of subcutaneous B16 melanoma tumors after intratumoral delivery of an IL-15-expressing plasmid followed by in vivo electroporation. Cancer Gene Ther. 2006, 13, 969. [Google Scholar] [CrossRef]
- Shirley, S.A.; Lundberg, C.G.; Heller, R. Electrotransfer of IL-15/IL-15Rα Complex for the Treatment of Established Melanoma. Cancers 2020, 12, 3072. [Google Scholar] [CrossRef]
- Bozic, T.; Sersa, G.; Kranjc Brezar, S.; Cemazar, M.; Markelc, B. Gene electrotransfer of proinflammatory chemokines CCL5 and CCL17 as a novel approach of modifying cytokine expression profile in the tumor microenvironment. Bioelectrochemistry 2021, 140, 107795. [Google Scholar] [CrossRef]
- Shah, K.; Connolly, R.J.; Chapman, T.; Jaroszeski, M.J.; Ugen, K.E. Electrogenetherapy of B16.F10 murine melanoma tumors with an interleukin-28 expressing DNA plasmid. Hum. Vaccin. Immunother. 2012, 8, 1722. [Google Scholar] [CrossRef][Green Version]
- Ferioli, M.; Lancellotta, V.; Perrone, A.M.; Arcelli, A.; Galuppi, A.; Strigari, L.; Buwenge, M.; De Terlizzi, F.; Cammelli, S.; Iezzi, R.; et al. Electrochemotherapy of skin metastases from malignant melanoma: A PRISMA-compliant systematic review. Clin. Exp. Metastasis 2022, 39, 743–755. [Google Scholar] [CrossRef]
- Mir-Bonafé, J.M.; Vilalta, A.; Alarcón, I.; Carrera, C.; Puig, S.; Malvehy, J.; Rull, R.; Bennàssar, A. Electrochemotherapy in the treatment of melanoma skin metastases: A report on 31 cases. Actas Dermosifiliogr. 2015, 106, 285–291. [Google Scholar] [CrossRef]
- Goggins, C.A.; Khachemoune, A. The use of electrochemotherapy in combination with immunotherapy in the treatment of metastatic melanoma: A focused review. Int. J. Dermatol. 2019, 58, 865–870. [Google Scholar] [CrossRef]
- Broderick, K.E.; Humeau, L.M. Electroporation-enhanced delivery of nucleic acid vaccines. Expert Rev. Vaccines 2015, 14, 195–204. [Google Scholar] [CrossRef]
- Sardesai, N.Y.; Weiner, D.B. Electroporation delivery of DNA vaccines: Prospects for success. Curr. Opin. Immunol. 2011, 23, 421–429. [Google Scholar] [CrossRef]
- Hojman, P.; Spanggaard, I.; Olsen, C.H.; Gehl, J.; Gissel, H. Calcium Electrotransfer for Termination of Transgene Expression in Muscle. Hum. Gene Ther. 2011, 22, 753–760. [Google Scholar] [CrossRef]
- Pagant, S.; Liberatore, R.A. In Vivo Electroporation of Plasmid DNA: A Promising Strategy for Rapid, Inexpensive, and Flexible Delivery of Anti-Viral Monoclonal Antibodies. Pharmaceutics 2021, 13, 1882. [Google Scholar] [CrossRef]
- Gothelf, A.; Gehl, J. What you always needed to know about electroporation based DNA vaccines. Hum. Vaccin. Immunother. 2012, 8, 1694. [Google Scholar] [CrossRef]
- Bodles-Brakhop, A.M.; Heller, R.; Draghia-Akli, R. Electroporation for the delivery of DNA-based vaccines and immunotherapeutics: Current clinical developments. Mol. Ther. 2009, 17, 585–592. [Google Scholar] [CrossRef]
- Frelin, L.; Braß, A.; Ahlén, G.; Brenndörfer, E.D.; Chen, M.; Sällberg, M. Electroporation: A promising method for the nonviral delivery of DNA vaccines in humans? Drug News Perspect. 2010, 23, 647–653. [Google Scholar] [CrossRef]
- Kutzler, M.A.; Weiner, D.B. DNA vaccines: Ready for prime time? Nat. Rev. Genet. 2008, 9, 776–788. [Google Scholar] [CrossRef]
- Khan, A.S.; Broderick, K.E.; Sardesai, N.Y. Clinical development of intramuscular electroporation: Providing a “boost” for DNA vaccines. Methods Mol. Biol. 2014, 1121, 279–289. [Google Scholar]
- Dunki-Jacobs, E.M.; Philips, P.; Martin, R.C.G. Evaluation of thermal injury to liver, pancreas and kidney during irreversible electroporation in an in vivo experimental model. Br. J. Surg. 2014, 101, 1113–1121. [Google Scholar] [CrossRef]
- Heller, L.; Pottinger, C.; Jaroszeski, M.J.; Gilbert, R.; Heller, R. In vivo electroporation of plasmids encoding GM-CSF or interleukin-2 into existing B16 melanomas combined with electrochemotherapy induces long-term antitumour immunity. Melanoma Res. 2000, 10, 577–583. [Google Scholar] [CrossRef]
- El-Kamary, S.S.; Billington, M.; Deitz, S.; Colby, E.; Rhinehart, H.; Wu, Y.; Blackwelder, W.; Edelman, R.; Lee, A.; King, A. Safety and tolerability of the Easy VaxTM clinical epidermal electroporation system in healthy adults. Mol. Ther. 2012, 20, 214–220. [Google Scholar] [CrossRef]
- Kichaev, G.; Mendoza, J.M.; Amante, D.; Smith, T.R.F.; McCoy, J.R.; Sardesai, N.Y.; Broderick, K.E. Electroporation mediated DNA vaccination directly to a mucosal surface results in improved immune responses. Hum. Vaccines Immunother. 2013, 9, 2041–2048. [Google Scholar] [CrossRef]
- Linnert, M.; Gehl, J. Bleomycin treatment of brain tumors: An evaluation. Anticancer. Drugs 2009, 20, 157–164. [Google Scholar] [CrossRef]
- Ding, X.-F.; Ma, D.-L.; Zhang, Q.; Peng, W.; Fan, M.; Suo, W. Progress of in vivo electroporation in the rodent brain. Curr. Gene Ther. 2014, 14, 211–217. [Google Scholar] [CrossRef]
- Lin, F.; Shen, X.; Kichaev, G.; Mendoza, J.M.; Yang, M.; Armendi, P.; Yan, J.; Kobinger, G.P.; Bello, A.; Khan, A.S.; et al. Optimization of electroporation-enhanced intradermal delivery of DNA vaccine using a minimally invasive surface device. Hum. Gene Ther. Methods 2012, 23, 157–168. [Google Scholar] [CrossRef]
- Ferraro, B.; Talbott, K.T.; Balakrishnan, A.; Cisper, N.; Morrow, M.P.; Hutnick, N.A.; Myles, D.J.; Shedlock, D.J.; Obeng-Adjei, N.; Yan, J.; et al. Inducing humoral and cellular responses to multiple sporozoite and liver-stage malaria antigens using exogenous plasmid DNA. Infect. Immun. 2013, 81, 3709–3720. [Google Scholar] [CrossRef]
- Jalah, R.; Kulkarni, V.; Patel, V.; Rosati, M.; Alicea, C.; Bear, J.; Yu, L.; Guan, Y.; Shen, X.; Tomaras, G.D.; et al. DNA and protein co-immunization improves the magnitude and longevity of humoral immune responses in macaques. PLoS ONE 2014, 9, e91550. [Google Scholar]
- Bråve, A.; Gudmundsdotter, L.; Sandström, E.; Haller, B.K.; Hallengärd, D.; Maltais, A.K.; King, A.D.; Stout, R.R.; Blomberg, P.; Höglund, U.; et al. Biodistribution, persistence and lack of integration of a multigene HIV vaccine delivered by needle-free intradermal injection and electroporation. Vaccine 2010, 28, 8203–8209. [Google Scholar] [CrossRef]
- Yan, J.; Corbitt, N.; Pankhong, P.; Shin, T.; Khan, A.; Sardesai, N.Y.; Weiner, D.B. Immunogenicity of a novel engineered HIV-1 clade C synthetic consensus-based envelope DNA vaccine. Vaccine 2011, 29, 7173–7181. [Google Scholar] [CrossRef][Green Version]
- Dupuy, L.C.; Schmaljohn, C.S. DNA vaccines for biodefense. Expert Rev. Vaccines 2009, 8, 1739–1754. [Google Scholar] [CrossRef]
- Grant-Klein, R.J.; Van Deusen, N.M.; Badger, C.V.; Hannaman, D.; Dupuy, L.C.; Schmaljohn, C.S. A multiagent filovirus DNA vaccine delivered by intramuscular electroporation completely protects mice from ebola and Marburg virus challenge. Hum. Vaccin. Immunother. 2012, 8, 1703–1706. [Google Scholar] [CrossRef]
- Laddy, D.J.; Yan, J.; Khan, A.S.; Andersen, H.; Cohn, A.; Greenhouse, J.; Lewis, M.; Manischewitz, J.; King, L.R.; Golding, H.; et al. Electroporation of synthetic DNA antigens offers protection in nonhuman primates challenged with highly pathogenic avian influenza virus. J. Virol. 2009, 83, 4624–4630. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Peng, B.; Deng, H.; Chen, G.; Yang, F.; Shao, M.; Lu, H.; Li, Y.; Peng, J.; Xu, L.; et al. Anti-HBV immune responses in rhesus macaques elicited by electroporation mediated DNA vaccination. Vaccine 2006, 24, 897–903. [Google Scholar] [CrossRef]
- Abbasi, J. COVID-19 and mRNA Vaccines—First Large Test for a New Approach. JAMA 2020, 324, 1125–1127. [Google Scholar] [CrossRef]
- Laczkó, D.; Hogan, M.J.; Toulmin, S.A.; Hicks, P.; Lederer, K.; Gaudette, B.T.; Castaño, D.; Amanat, F.; Muramatsu, H.; Oguin, T.H., 3rd; et al. A Single Immunization with Nucleoside-Modified mRNA Vaccines Elicits Strong Cellular and Humoral Immune Responses against SARS-CoV-2 in Mice. Immunity 2020, 53, 724–732.e7. [Google Scholar]
- Lu, J.; Lu, G.; Tan, S.; Xia, J.; Xiong, H.; Yu, X.; Qi, Q.; Yu, X.; Li, L.; Yu, H.; et al. A COVID-19 mRNA vaccine encoding SARS-CoV-2 virus-like particles induces a strong antiviral-like immune response in mice. Cell Res. 2020, 30, 936–939. [Google Scholar] [CrossRef]
- Zhang, N.-N.; Li, X.-F.; Deng, Y.-Q.; Zhao, H.; Huang, Y.-J.; Yang, G.; Huang, W.-J.; Gao, P.; Zhou, C.; Zhang, R.-R.; et al. A Thermostable mRNA Vaccine against COVID-19. Cell 2020, 182, 1271–1283.e16. [Google Scholar] [CrossRef]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020, 586, 589–593. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
- Gehl, J.; Sersa, G.; Matthiessen, L.W.; Muir, T.; Soden, D.; Occhini, A.; Quaglino, P.; Curatolo, P.; Campana, L.G.; Kunte, C.; et al. Updated standard operating procedures for electrochemotherapy of cutaneous tumours and skin metastases. Acta Oncol. 2018, 57, 874–882. [Google Scholar] [CrossRef]
- Cemazar, M.; Sersa, G. Recent Advances in Electrochemotherapy. Bioelectricity 2019, 1, 204. [Google Scholar] [CrossRef]
- Miklavčič, D.; Serša, G.; Brecelj, E.; Gehl, J.; Soden, D.; Bianchi, G.; Ruggieri, P.; Rossi, C.R.; Campana, L.G.; Jarm, T. Electrochemotherapy: Technological advancements for efficient electroporation-based treatment of internal tumors. Med. Biol. Eng. Comput. 2012, 50, 1213–1225. [Google Scholar] [CrossRef]
- Esmaeili, N.; Friebe, M. Electrochemotherapy: A Review of Current Status, Alternative IGP Approaches, and Future Perspectives. J. Healthc. Eng. 2019, 2019, 2784516. [Google Scholar] [CrossRef]
- Mali, B.; Gorjup, V.; Edhemovic, I.; Brecelj, E.; Cemazar, M.; Sersa, G.; Strazisar, B.; Miklavcic, D.; Jarm, T. Electrochemotherapy of colorectal liver metastases—An observational study of its effects on the electrocardiogram. Biomed. Eng. Online 2015, 14 (Suppl. S3), S5. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Rassu, G.; Gavini, E.; Sorrenti, M.; Catenacci, L.; Torre, M.L.; Perteghella, S.; Ansaloni, L.; Maestri, M.; Giunchedi, P. Electrochemotherapy of Deep-Seated Tumors: State of Art and Perspectives as Possible “EPR Effect Enhancer” to Improve Cancer Nanomedicine Efficacy. Cancers 2021, 13, 4437. [Google Scholar] [CrossRef]
- Kozak, O.; Hać, S.; Pieńkowska, J.; Studniarek, M. Benefitial role of electrochemotherapy in locally advanced pancreatic cancer-radiological perspective. Polish J. Radiol. 2022, 87, 30–42. [Google Scholar] [CrossRef]
- Savic, L.J.; Chapiro, J.; Hamm, B.; Gebauer, B.; Collettini, F. Irreversible Electroporation in Interventional Oncology: Where We Stand and Where We Go. Rofo 2016, 188, 735–745. [Google Scholar] [CrossRef]
- Frandsen, S.K.; Vissing, M.; Gehl, J. A comprehensive review of calcium electroporation—A novel cancer treatment modality. Cancers 2020, 12, 290. [Google Scholar] [CrossRef]
- Frandsen, S.K.; Gehl, J. A Review on Differences in Effects on Normal and Malignant Cells and Tissues to Electroporation-Based Therapies: A Focus on Calcium Electroporation. Technol. Cancer Res. Treat. 2018, 17, 1533033818788077. [Google Scholar] [CrossRef]
- Lee, S.H.; Danishmalik, S.N.; Sin, J.I. DNA vaccines, electroporation and their applications in cancer treatment. Hum. Vaccin. Immunother. 2015, 11, 1889–1900. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakoczy, K.; Kisielewska, M.; Sędzik, M.; Jonderko, L.; Celińska, J.; Sauer, N.; Szlasa, W.; Saczko, J.; Novickij, V.; Kulbacka, J. Electroporation in Clinical Applications—The Potential of Gene Electrotransfer and Electrochemotherapy. Appl. Sci. 2022, 12, 10821. https://doi.org/10.3390/app122110821
Rakoczy K, Kisielewska M, Sędzik M, Jonderko L, Celińska J, Sauer N, Szlasa W, Saczko J, Novickij V, Kulbacka J. Electroporation in Clinical Applications—The Potential of Gene Electrotransfer and Electrochemotherapy. Applied Sciences. 2022; 12(21):10821. https://doi.org/10.3390/app122110821
Chicago/Turabian StyleRakoczy, Katarzyna, Monika Kisielewska, Mikołaj Sędzik, Laura Jonderko, Julia Celińska, Natalia Sauer, Wojciech Szlasa, Jolanta Saczko, Vitalij Novickij, and Julita Kulbacka. 2022. "Electroporation in Clinical Applications—The Potential of Gene Electrotransfer and Electrochemotherapy" Applied Sciences 12, no. 21: 10821. https://doi.org/10.3390/app122110821
APA StyleRakoczy, K., Kisielewska, M., Sędzik, M., Jonderko, L., Celińska, J., Sauer, N., Szlasa, W., Saczko, J., Novickij, V., & Kulbacka, J. (2022). Electroporation in Clinical Applications—The Potential of Gene Electrotransfer and Electrochemotherapy. Applied Sciences, 12(21), 10821. https://doi.org/10.3390/app122110821