Abstract
Muscle regeneration after a traumatic injury can take an excessively long period of time. The purpose of this study is to assess whether the action of percutaneous needle electrolysis (PNE) accelerates muscle regeneration in cases of partial muscle injuries. The gastrocnemius muscle from adult Swiss male mice was inoculated with bupivacaine. The PNE protocol was applied 48 h after treatment with bupivacaine. Immunofluorescence techniques were performed 72 h after treatment with bupivacaine to evaluate the synaptic contacts. The end plate noise was recorded by electromyography after treatment with bupivacaine. Bupivacaine induced a local injury in muscles, axons were retracted, and the endplate noise decreased at 72 h, while the endplate noise increased in the injured limb where PNE had been applied. Seven days later, the functional values were the same as the controls and they were maintained for 10 days. The endplate noise was significantly greater on the limb treated with the electric current when compared to the limb receiving only bupivacaine, indicating that the use of galvanic current facilitated muscle regeneration at least from a functional point of view. The application of PNE during muscle regeneration in an animal model reduces the recovery time of the damaged muscle tissue.
1. Introduction
Modern medicine has experienced continual advancements in percutaneous galvanic current delivery for musculoskeletal injuries [1,2]. The clinical use of the same comprises oncological treatments [3], known as electrolytic ablation, and chronic tendinitis [4,5], known as percutaneous needle electrolysis (PNE). The clinical use of electric currents has also been found to accelerate muscle regeneration in humans [6].
Additionally, numerous in vivo as well as ex vivo studies have shown that cells can respond directionally to the application of electrical fields. This phenomenon is known as electrotaxis or galvanotaxis (reviewed by Cortese et al. [7]). Vascular endothelial cells also respond to the phenomenon of electrotaxis, thereby enhancing angiogenesis [8]. Additionally, cells involved in inflammatory or immune responses, such as lymphocytes [9] or macrophages [10], are also attracted to electrical currents. Furthermore, the migration mediated by electric currents involves cAMP and the G protein [11,12,13]. Generally, cAMP and G proteins trigger many other events of cell signal transduction [14,15]. Therefore, electric currents can activate many specific cellular capacities.
Abat et al. [16] assessed the effect of the application of PNE on a model of muscle lesions in rat by injecting intramuscular notexin. A small amount of intramuscular notexin caused a lesion of the whole muscle. The main results observed were that the application of a galvanic current induced a decrease in pro-inflammatory mediators (TNF-α and IL-1β) and an increased expression of anti-inflammatory proteins (PPAR-γ), as well as an increase in both the vascular endothelial growth factor (VEGF) and the VEGF–R1 receptor. More recently, Jorda et al. [17] replicated the experiment of Abat et al., confirming that when applying PNE after injury, an increase in anti-inflammatory mediators and a reduction in pro-inflammatory mediators were observed. These authors reported that an overall promoted muscle regeneration was observed. Notexin creates a generalized lesion of the treated muscle. Plant et al. [18] conducted a comparative study between the two myolytic agents; notexin and bupivacaine. The notexin caused a greater muscle injury and was more difficult to regenerate than with the bupicavaine. All previous studies used notexin to cause extensive muscle injury. However, muscle lesions in humans are usually localized and rarely extensive. In this study, bupivacaine has been used in order to produce very localized muscle injuries.
The neuromuscular synapse releases vesicles of acetylcholine as a neurotransmitter. These vesicles can be released massively, generating an action potential in the muscle fiber, or spontaneously. In spontaneous neurotransmission, isolated vesicles are released and stochastically distributed over time [19]. An electromyographic-recording needle can record the spontaneous release of vesicles from several dozen muscle fibers [20]. Electromyography devices usually incorporate a transducer that converts the electrical record into sound. Finally, the electromyographic record of spontaneous neuromuscular release is identified as a characteristic “endplate noise” [20,21]. No previous study has evaluated the restoration of neurotransmission after muscle injury. In this study, endplate noise was recorded as an indicator of the fully functional recovery of muscle fibers.
The purpose of this study was to determine the action of percutaneous needle electrolysis in muscle regeneration, by endplate noise recording, and functional reinnervation in an animal model based on bupivacaine-induced localized muscle injury.
2. Materials and Methods
2.1. Ethical Approval
The experiments on mice were carried out in accordance with the U.K. Animals (Scientific Procedures) Act, 1986, and in accordance with the guidelines of the European Community’s Council Directive (2010/63/EU) and the Spanish Royal Decree 53/2013 for the humane treatment of laboratory animals. The Animal Research Committee of the Universitat Rovira i Virgili (Reference number: 11337) reviewed and approved all experiments on animals. The experiments were performed 45 to 50 days post-natal on young adult Swiss male mice (Charles River, L’Arbresle, France). Mice were habituated to the facility for at least 1 week prior to studies and were housed in groups of four, with sawdust bedding and ad libitum access to water and food throughout. The animals’ room was maintained at a temperature of 22 ± 2 °C, a relative humidity of 50 ± 10%, and a 12-h light/dark automatic light cycle.
2.2. Experimental Groups
For the control series of the muscle injury, three groups were formed with three mice in each group. After BPV was injected into the right limb and the same volume of saline was injected into the left limb (control), electromyography assessments of both limbs were performed at 72 h and 7 and 10 days (see Figure 1a).
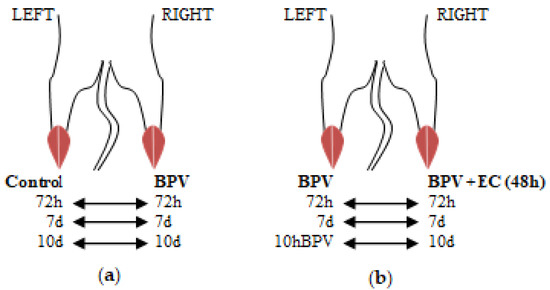
Figure 1.
Electromyography: experimental design. This figure displays how the experimental and control groups for electromyography recordings were created. For each animal, the control limb was compared to the experimental limb. (a) After BPV was injected into the right limb and the same volume of saline was injected into the left limb (control), electromyography assessments of both limbs were performed at 72 h and 7 and 10 days. (b) Electromyography of both limbs was performed: left limb BPV, right limb BPV + PNE at 72 h and 7 and 10 days. Forty-eight hours after the application of BPV, PNE was applied. The PNE protocol was only administered in the right limb of each animal. Thereafter, electromyography of both limbs was performed (left limb BPV; right limb BPV + PNE) at 72 h and 7 and 10 days. BPV, bupivacaine. EC, electrical currents.
Regarding the experimental series for the currents, three groups were formed with three mice each, and BPV was used to treat the two limbs of each animal. Forty-eight hours after the application of BPV, the destruction of the affected muscle fibers commence, followed by the inflammatory reaction [22]. PNE was applied at this time. A needle was longitudinally inserted into the muscle package; however, the PNE protocol was only administered in the right limb of each animal. Thereafter, electromyography of both limbs was performed (left limb BPV; right limb BPV+PNE) at 72 h and 7 and 10 days (see Figure 1b).
2.3. Experimental Protocols: Focal Muscle Injury and Percutaneous Needle Electrolysis
The muscle injury in the gastrocnemius was provoked in the posterior muscle package of the limb using an intramuscular administration of three injections of bupivacaine (BPV) on the midline of the distal third, middle third, and proximal third of the posterior muscle package (bupivacaine hydrochloride monohydrate, Sigma Aldrich, Madrid, Spain; the working solution was made up at 0.75% in saline solution at 0.9% at pH 6.5). The volume injected in each area was 0.02 mL, and the process was repeated 24 h after the first administration (total volume administrated was 0.12 mL). The same procedure and volume were applied in the control limb by administering 0.9% NaCl (saline solution). The gastrocnemius muscles were used for electromyographic recordings.
Single and highly localized subcutaneous infiltrations of BPV were performed in the cranial portion of the LAL muscle. As with the gastrocnemius muscle, these were limited to the middle third of the muscle. In this scenario, the injected volume was 0.02 mL in a single point, and the process was repeated 24 h after the first administration (total administrated volume was 0.04 mL). In this muscle, electric currents were not applied. The aim was to evaluate the effectiveness of focal BPV treatment.
For the intramuscular treatment administration of Microlance, a needle measuring 0.3 × 13 mm was used. For all injections, the depth of administration was controlled using a depth limiter placed on the external part of the needle cannula.
The protocol for PNE used during this study was 1.5 mA for five seconds with three repetitions. This was administered by inserting the needle longitudinally into the muscle package on the back of the right leg (anode), which had been inoculated with BPV 48 h beforehand. The cathode was subcutaneously inserted into the lumbosacral area of the mouse. The needles used were Physio Invasiva® Needles, 0.30 × 30 mm (PRIM, Madrid, Spain). The device generating electrical currents was a Physio Invasiva® 2.0 (PRIM, Madrid, Spain).
2.4. Endplate Noise Recordings
The needle EMG (nEMG) recordings were obtained from an anesthetized animal at a controlled room temperature of between 22 and 25.8 °C. The muscle used for this study was the gastrocnemius because of its ease of access and suitability. Recordings were obtained with an electromyography system (Medelec Mystro plus, GR20, Teca Medelec, London, UK) using a monopolar EMG needle (Natus Manufacturing Limited, Galway, Ireland). The needle was slowly inserted into the muscle and, once inside, it was moved in order to enable recording in all directions. The muscle was divided into twelve areas to cover both the entire muscle and avoid recording the same endplate noise twice. The recording needle was introduced into the gastrocnemius until an audible change was heard. The electromyography screen was then studied, and if it was correct (without an alternating current, artifacts, etc.), the endplate noise was recorded. The number of areas with endplate noise (maximum twelve) was recorded. During the endplate noise recording, the frequency was studied as the number of potentials per second that appeared, expressed in Hz.
The first record was made in the right gastrocnemius (control record). The left gastrocnemius was then recorded (experimental record). In order not to repeat or omit any area, a systematic order of the areas were followed, whereby the registration procedure commenced from the distal to the proximal end. In each area, the recording needle was inserted slowly and without making sudden movements and once placed, small turns were made, within each of the twelve areas, to locate any recordable signal. A zone was considered to have plaque noise when it appeared and was sustained for 5 s.
The gastrocnemius muscles were partially injured after the 12 insertions of the electromyographic recording electrode, so they could not be used for the morphological techniques (methylene blue and immunofluorescence). In order to optimize the mice, the morphological techniques were performed in the LAL muscle.
2.5. Sample Collection and Histology
At the end of the experiment, animals were deeply anaesthetized with isoflurane before being euthanized by exsanguination. The levator auris longus (LAL) was excised and dissected on a Sylgard-coated Petri dish containing normal Ringer solution continuously bubbled with 95% O2/5% CO2. The LAL muscles were used for methylene blue staining and immunological labeling.
2.6. Methylene Blue
Whole LAL muscles were removed and exposed to 1% methylene blue (Sigma–Aldrich, Steinheim, Germany) dissolved in 1% borax for two minutes. Subsequently, the samples were washed with distilled water for three periods of two minutes each. Finally, dehydration procedures and mounting with epoxy resin were performed.
2.7. Immunohistochemistry
Whole LAL muscles were removed and fixed in 4% paraformaldehyde in PBS (pH 7.4) for 45 min at room temperature (~22 °C). The LALs were double labeled for axons with fluorescein isothiocyanate (FITC)-conjugated antibodies against 200-kD neurofilament protein monoclonal antibodies (Cat# N2912, Sigma–Aldrich, Steinheim, Germany; 1:500 in 1% BSA) and post-synaptic nicotinic acetylcholine receptors with Tetramethyl Rhodamine Isothiocyanate (TRITC)-α-bungarotoxin (Molecular Probes, Eugene, OR, USA; Cat# T1175). The muscles were mounted in Mowiol with p-phenylenediamine (Sigma–Aldrich, Steinheim, Germany).
2.8. Statistical Procedure
Values are expressed as the mean ± SEM. The values are expressed as the “percentage of change”. This is defined as: [experimental value/control value] × 100. We used the two-tailed Welch’s t-test for unpaired values because our variances were not equal. This test was chosen as it is more conservative than the ordinary t-test. Differences were considered significant at p < 0.05.
3. Results
Bupivacaine has been shown to have a specific myotoxic effect and its injection into the muscle tissue results in rapid degeneration of muscle cells followed by regeneration with no damage done to the intramuscular nerves. In this study, this lesion was limited to a reduced area. This study attempts to determine whether the intramuscular application of galvanic electric current can lead to an improvement in the sequence of neuromuscular regeneration.
3.1. Muscular Damage
As previously described [22], a large inflammatory reaction was visible during the first 72 h after BPV treatment. Comparing the usual control image shown in Figure 2a with the image of the muscle treated with BPV in Figure 2b: the muscle fibers disappeared, and a significant inflammatory infiltrate was observed. The inflammatory reaction included excess fluid that diluted the dye. After treatment, this inflammatory reaction disappeared on the seventh day. However, there was an excess of connective tissue that blurred the contour of the muscle fibers and increased the intensity of coloration (Figure 2c). As documented in the methods section, a specific protocol was performed to achieve only the localized lesions. This was confirmed with methylene blue.
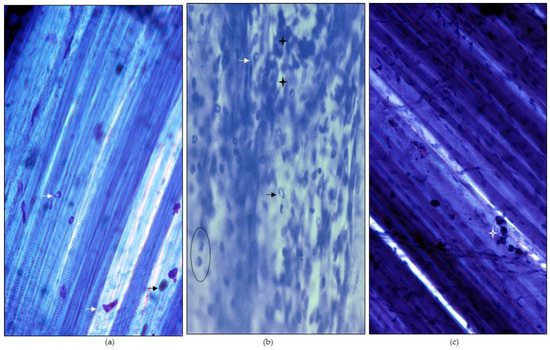
Figure 2.
Muscular injury. The samples (LAL) were stained with methylene blue. (a) Normal image of the LAL muscle stained with methylene blue. The muscle fibers can be clearly distinguished. Some mast cells (arrow heads) are also commonly seen. Mast cell granules stain better than nuclei and a clear central part is present. (b) Bupivacaine provokes a very clear muscle lesion that is visible at 72 h. Muscle fibers cannot be seen because they are degenerated. In addition to mast cells (arrowheads), there are areas with abundant inflammatory cells (stars). Macrophages are easily identifiable (within a black circle). (c) After 7 days of exposing the muscle to bupivacaine, a fully regenerated muscle can be seen. The muscle fibers can be clearly seen again. Some residual inflammatory cells (star) can be seen. Initial magnification, 100×.
3.2. Axonal Retractions Induced by Bupivacaine
After a period of 72 h and after the subcutaneous inoculation with BPV on the area of the LAL muscles, these muscles were extracted and processed for immunofluorecence. When a muscle fiber is injured with BPV, the axons that innervate the same become transiently retracted. Thus, from an image of fine axonal ramifications (Figure 3A) on a well-structured postsynaptic component (Figure 3A’) belonging to a normal synaptic contact, an image of completely retracted axons, may be obtained, such as that shown in Figure 3B. In these axons, no axonal ramification can be identified and the complete axoplasmatic volume is proximally accumulated, leading to a greater width. Concurrently, these dramatically retracted axons displayed a postsynaptic component with poorly defined limits, such as that shown in Figure 3B’.
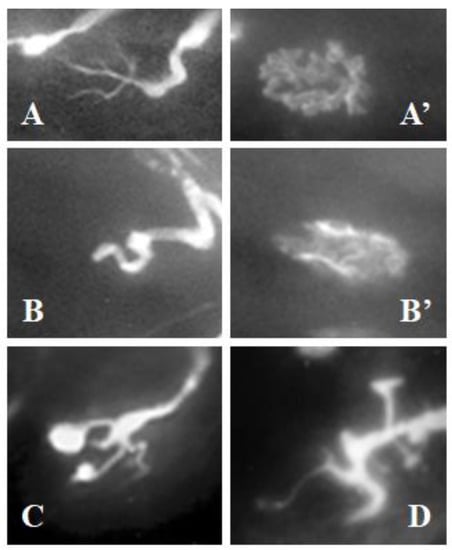
Figure 3.
Neuromuscular junctions. The neurofilament (axon) was labeled with fluorescein (A–D) and post-synaptic receptors with α-bungarotoxin rhodaminated (A’,B’). (A) normal axonal ramification of a neuromuscular junction located over a healthy muscle fiber. (A’) normal postsynaptic component corresponding to the axon displayed in (A). (B) completely retracted axon; note that no axonal ramification can be identified, but it is notably thickened. (B’) postsynaptic-destructured component corresponding to the axon displayed in (B). (C) axon displaying some of its branches with distal bulbs that are characteristic of axonal retraction. (D) axon showing some of its branches notably thickened, characteristic of axonal retraction. The images shown in (B–D) correspond to LAL muscles after 72 h of injecting bupivacaine. Initial enlargement is 200× in all images.
However, not all the axons of the area treated with BPV were completely retracted. It was common to find axonal ramifications with some of the branches having distal bulbs (Figure 3C) or with only some of the branches being notably thickened (Figure 3D). Analysis of the damaged areas showed that all synaptic contacts reported that at least one axonal branch retracted.
3.3. Endplate Noise Recording in Muscles Injured with Bupivacaine
Special care was taken to achieve only a partial lesion of the gastrocnemius muscles. The first record was made in the right gastrocnemius (control record, untreated). The left gastrocnemius was then recorded (experimental record, treated with BPV). For the assessment performed 72 h after the administration of BPV, a decrease of up to half of the number of areas with endplate noise was observed (Control: 3.0 ± 0.01; BPV: 1.33 ± 0.33; % of variation compared to the control: 49.89% ± 9.42, n = 36 muscular areas from three mice; p = 0.002; Figure 4a). Thereafter, at seven days, there were 25% fewer areas with endplate noise (Control: 8.0 ± 0.01; BPV: 5.67 ± 0.33; % of variation compared to the control: 75.00% ± 7.22, n = 36 muscular areas from three mice; p < 0.001; Figure 4a). Seeing that the electromyography values were still not normalized, experiments were performed 10 days post-treatment with BPV. However, at 10 days, the muscles had still not completely recovered (Control: 7.33 ± 0.88; BPV: 6.00 ± 0.58), as they still presented 20% fewer areas with endplate noise (% of variation compared to the control: 82.13% ± 3.57, n = 36 muscular areas from three mice; p = 0.022; Figure 4a).
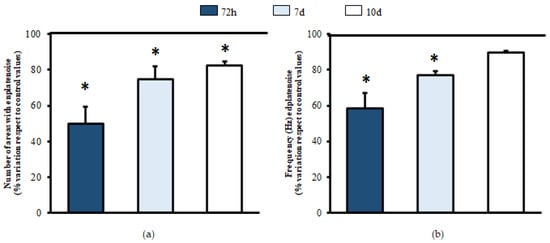
Figure 4.
Electromyography of muscles injected with bupivacaine. Comparative recordings were performed between the gastrocnemius treated with saline solution (left, control) compared to the one injected with BPV (right, experimental) of each animal. At 72 h and 7 and 10 days, needle electromyography was performed. The number of areas presenting endplate noise was evaluated (a). During the endplate noise recording, the frequency was studied as the number of potentials that appeared per second, expressed in Hz (b). For each column, n = 36 muscular areas from 3 animals. Values are expressed as the mean ± SEM. * p < 0.05 compared to control values.
In the analysis of the frequency of the endplate noise, recording 72 h after the administration of BPV (control: 92.85 ± 3.07; BPV: 54.08 ± 8.21), a decrease of 40% was observed compared to the control limb (% of variation compared to the control:58.38% ± 8.86, n = 36 muscular areas from three mice; p = 0.013; Figure 4b). Regarding the assessment performed at seven days, there was still 23% less frequency of endplate noise (control: 123.23 ± 6.87; BPV: 94.64 ± 8.89; % of variation compared to the control: 76.87 ± 2.25, n = 36 muscular areas from three mice; p = 0.002; Figure 4b). However, at 10 days after inoculation with BPV, the control values of endplate noise were achieved (control: 136.29 ± 3.91; BPV: 122.02 ± 1.40; % of variation compared to control: 89.52% ± 1.26, n = 36 muscular areas from three mice; Figure 4b).
3.4. Muscles Partially Lesioned with BPV and Treated with Percutaneous Needle Electrolysis
Two days after the inoculation of BPV, the PNE protocol was applied (1.5 mA for five seconds with three repetitions [1]). The electrographic recordings were performed at 72 h and 7 and 10 days after inoculation with BPV. The first record was made in the right gastrocnemius (control record, treated only with BPV). The left gastrocnemius was then recorded (experimental record, treated with BPV and PNE).
The number of areas with endplate noise recording at 72 h (1.33 ± 0.33; Figure 5a) shows that the limb treated with PNE (2.67 ± 0.33) led to an important increase in the same (≈130%) when compared to the contralateral untreated limb (%variation compared to the control: 233.33% ± 5.34, n = 36 muscular areas from three mice; p = 0.002; Figure 5a). However, at seven days, the values of the limb treated with EC (6.00 ± 0.01) were identical to those of the untreated limb (5.67 ± 0.33; % of variation compared to the untreated limb: 106.67% ± 6.90, n = 36 muscular areas from three mice; p = 0.157; Figure 5a). Similarly on day 10, a slight, but significant improvement was detected in the number of areas with endplate noise (6.00 ± 0.58 vs. 7.33 ± 0.33; % of variation compared to the untreated limb: 125.55% ± 7.47, n = 36 muscular areas from three mice; p = 0.007; Figure 5a).
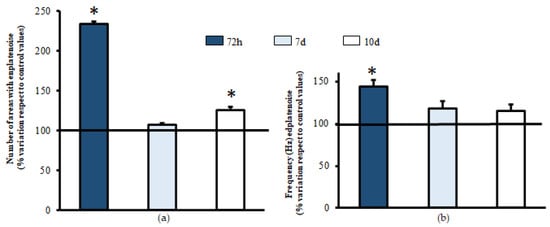
Figure 5.
Action of electrical currents in muscles treated with bupivacaine. Intramuscular injections of bupivacaine were performed in both gastrocnemius muscles of each animal. After 48 h, the PNE protocol was applied (1.5 mA for 5 s, 3 repetitions). At 72 h and 7 and 10 days after inoculation with BPV, needle electromyography was performed. Comparative recordings were performed between the limb untreated with electric currents (left, control) compared to the treated limb (right, experimental) for each animal. The number of areas presenting endplate noise was evaluated (a). During the endplate noise recording, the frequency was studied as the number of potentials that appeared per second, expressed in Hz (b). For each column, n = 36 muscular areas from 3 animals. Values are expressed as the mean ± SEM. * p < 0.05 compared to the control values.
Similarly, for the results regarding the number of areas, the frequency of endplate noise events was also found to be elevated at 72 h after treatment with PNE (54.08 ± 8.21 vs. 75.37 ± 6.73; % of variation compared to the untreated limb: 143.80% ± 6.14, n = 36 muscular areas from three mice; p = 0.022; Figure 5b). Furthermore, in areas with endplate noise, a comparison before (94.64 ± 8.89) and after (111.18 ± 7.99) the PNE protocol was applied, which showed that this advantage was no longer evident at seven days (% of variation compared to the untreated limb: 117.74% ± 8.44, n = 36 muscular areas from three mice; p = 0.061; Figure 5b), and this was maintained at 10 days (120.22 ± 5.41 vs. 138.69 ± 6.04; % of variation compared to the untreated limb: 115.37% ± 7.37, n = 36 muscular areas from three mice; p = 0.057; Figure 5b).
These results suggest that PNE increases the number of synaptic contacts in the first 72 h after injury with BPV.
4. Discussion
This study performed a controlled experimental partial muscle injury. We found that, in lesioned areas, an axonal retraction occurred, which justifies the decreased spontaneous neurotransmission. Furthermore, the application of PNE improved the functional recovery of the injured muscle tissue.
4.1. Muscular Damage by Bupivacaine
Initially, a highly localized, easily reproducible lesion was achieved. Bupivacaine was chosen because it is described as a contact myolytic agent [23]. Toxins such as notexin have been more recently used [16], but nevertheless, those toxins have been proven to cause more extensive and serious injury than bupivacaine [17].
The myotoxic effects of BPV have been known since the 1980s. The works by Tomas i Ferrer et al. [22] as well as Benoit and Belt [23] have demonstrated that intramuscular treatment produces a degeneration of the muscle tissue without affecting the nerves or the intramuscular vessel, although it does provoke a retraction of the axon in the neuromuscular junction due to the destruction of muscle fibers within 24 and 48 h. In the present study, we performed a controlled lesion, which was observable with methylene blue.
The axonal retraction secondary to the lesion of the muscle fibers is associated with the decrease in the number of areas and the reduction in the frequency of the endplate noise in the electromyography recordings obtained in the present study. As there were fewer functional axonal branches, the probability of ACh release was reduced, with a reduction in all associated parameters.
The axonal branches remained in the area affected by BPV until the myotube phase, which is when they completely reoccupied the synaptic contacts. This occurred between days five and seven [22]. Furthermore, Plant et al. [18] observed that the greatest degeneration of muscle tissue treated with BPV occurred within 48–72 h. There was also a correlation between the tissue degeneration observed with histological tissues and the functional study in which the contractile properties were assessed. During both periods, a partial degeneration of the tissue was recorded, which, on a functional level, represented a decrease in the capacity to generate isometric strength. In the present study, the electromyographic recording at 72 h showed a decrease in the number of areas with endplate noise as well as a decreased frequency. This is compatible with findings reported by Plant [18]. These authors found a recovery of the treated tissues in the recordings performed on days 7 and 10, leading to an increase in the isometric strength.
Thus, by assuming a functional recovery of acetylcholine release, it should be possible to electromyographically record a recovery of endplate noise to control values. However, in the present study, on day 7, the initial values were still not obtained, and only in a number of areas were these values observed on day 10. Therefore, although morphological recovery of the synaptic contacts was shown, they were not yet completely operational.
4.2. Muscles Partially Injured with BPV and Treated with Percutaneous Needle Electrolysis
Electrical currents are beginning to be used to treat patients with muscle injuries in order to accelerate regeneration. Thus, for example, Hollis and McClure [6] described the case of a muscle lesion of the tibialis anterior secondary to a reparatory surgery of the ankle which, after several weeks, recovered rapidly by applying electrical currents.
In previous works, Guo and coworkers [24] and Fujiya et al. [25] stated that electro stimulation protocols with microcurrents enhanced the recovery of muscle tissue during the regeneration phase of muscles damaged with a cardiotoxin. These authors described an increase in the transcription box protein 7 (PAX-7) in the muscle treated with micro currents. PAX 7 is expressed in the activated satellite cells. These cells proliferate and differentiate to mature fibers and replace those damaged in situations of muscle injury [26]. The mechanisms described by those previous works may also be implicated in the faster regeneration observed in samples exposed to galvanic current in the present study.
There appears to be proof that electrical currents applied to lesioned muscles induce a decrease in pro-inflammatory mediators (TNF-α and IL-1β), as well as an increased expression of anti-inflammatory proteins (PPAR-γ) and an increase in vascular endothelial growth factor (VEGF) and its VEGF-R1 receptor [16]. Recently, the team led by Soraya L Valles [17] replicated the study by Abat et al., finding similar results but with more mediators. For example, the galvanic current prevented the increase of proinflammatory mediators, such as the cytokine IL-6 and the chemokines CCL3, CCL4 and CCL5. In addition, they described a decrease in the expression of CCR8 and NF-κB after treatment with galvanic current. In contrast, an increase in the anti-inflammatory mediators IL-10 and IL-13 after galvanic current treatment can aid in recovery from notexin-induced damage. All of these results indicate that galvanic currents can improve muscle regeneration. Therefore, these currents have an anti-inflammatory action. However, they facilitate the revascularization of the lesioned area. In addition, it is important to note that the endothelial cells respond to electrotaxis, thereby generating angiogenesis [8]. In parallel, electrotaxis phenomena have been linked to cells involved in inflammatory or immune responses [9,23]. Muscle regeneration after a focal lesion was described many years ago [26,27]. First, an inflammatory reaction occurs, which produces a “flushing out” of the necrotic waste in the lesioned area. Thereafter, the satellite cells that are activated proliferate and fuse to create a myotube. Finally, the myotubes synthesize the actin and myosin myofilaments that form sarcomeres to fuse with those previously existing. Efficient muscle regeneration requires appropriate muscle irrigation [28], and electrical currents may facilitate this irrigation [8]. Furthermore, an effective inflammatory reaction facilitates complete muscle regeneration [2,27]. Electric currents facilitate the inflammatory reaction and muscle irrigation, thereby facilitating muscle regeneration. Our findings revealed that electromyographic recovery of endplate noise in muscles treated percutaneously with galvanic current is relatively quick (72 h), coinciding with the inflammatory reaction.
4.3. New Therapies in Muscular Regeneration
In recent decades, novel strategies have been developed to improve muscle regeneration. For example, the use of bone marrow mesenchymal stem cells (BM-MSCs) (see review by [29]). Thus, BM-MSCs could promote regeneration of musculoskeletal tissue and activate myogenic differentiation of satellite cells. Furthermore, BM-MSCs also promote nerve regeneration in case the injury also has neural consequences [30]. However, these strategies are often used for severe and substance-loss injuries. In injuries without loss of substance, such as sports injuries, there is not much progress. Cryotherapy, possibly one of the oldest non-pharmacological therapies in muscle regeneration [31], continues to be used successfully. In these years, several variations have been introduced in cryotherapy, varying the exposure to cold only of the affected limb or the entire body, duration of treatment, time of application post-injury, etc. [32]). In contrast, microwave heating has been applied with relative success [33]. Platelet-rich plasma injections have also been tested but showed no effect compared to the control [34,35]. Extracorporeal shock wave therapy can accelerate regeneration after acute skeletal muscle injury [36], but it requires many applications over several weeks. The PNE therapy that is evaluated in this study is applied in a much localized and effective way in the injured area. Its main advantage lies precisely on the fact that it is quick and easy to apply for an expert professional. In addition, the infrastructure needed is portable and minimal. Moreover, PNE can also be applied in combination with others such as diathermia or more complex therapies such as BM-MSC and even those that are pharmacological based on the professional’s choice.
4.4. Limitations of the Work
The use of mice in translational science has several limitations. Unlike athletes, the state of well-being of mice can only be known indirectly. Thus, a mild annoyance that may be critical for an athlete may be missed in a mouse study.
The significant size difference between the muscles of mice and athletes may also have implications. Percutaneous electrolysis in the muscles of mice can easily encompass the entire muscle treated with a single central insertion. However, in the case of athletes, it is essential to use ultrasound to insert the needle into the injured area.
In summary, the histological and functional evidence observed in this study demonstrates that the application of PNE during muscle regeneration in an animal model of muscle injury reduces the recovery time of the damaged muscle tissue.
Author Contributions
Conceptualization, F.M.-M., F.V.-G. and M.M.S.; Methodology, R.M. and M.B.; Formal Analysis, R.M. and M.B.; Investigation, F.M.-M. and M.M.S.; Data Curation, M.B., R.M. and F.V.-G.; Writing—Original Draft Preparation, Writing—Review & Editing and Approved final version of manuscript, F.V.-G., R.M., M.B., F.M.-M. and M.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Animal Research Committee of the Universitat Rovira i Virgili (Reference number: 11337; Date of approval: 01/12/2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the Results section.
Acknowledgments
The authors would like to thank PRIM Physio for supplying the Physio Invasiva® 2.0 device used to generate the electric currents.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Valera-Garrido, F.; Minaya-Muñoz, F. Fisioterapia Invasiva, 2nd ed.; Elsevier España SL: Barcelona, Spain, 2016. [Google Scholar]
- Molsberger, A.; McCaig, C.D. Percutaneous direct current stimulation—A new electroceutical solution for severe neurological pain and soft tissue injuries. Med. Devices 2018, 11, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Cen, C.; Chen, X. The Electrode Modality Development in Pulsed Electric Field Treatment Facilitates Biocellular Mechanism Study and Improves Cancer Ablation Efficacy. J. Healthc. Eng. 2017, 2017, 3624613. [Google Scholar] [CrossRef] [PubMed]
- Burssens, P.; Forsyth, R.; Steyaert, A.; Van Ovost, E.; Praet, M.; Verdonk, R. Influence of burst TENS stimulation on collagen formation after Achilles tendon suture in man. A histological evaluation with Movat’s pentachrome stain. Acta Orthop. Belg. 2005, 71, 342–346. [Google Scholar] [PubMed]
- Valera-Garrido, F.; Minaya-Muñoz, F.; Medina-Mirapeix, F. Ultrasound-guided percutaneous needle electrolysis in chronic lateral epicondylitis: Short-term and long-term results. Acupunct. Med. 2014, 326, 446–454. [Google Scholar] [CrossRef]
- Hollis, S.; McClure, P. Intramuscular Electrical Stimulation for Muscle Activation of the Tibialis Anterior after Surgical Repair: A Case Report. J. Orthop. Sports Phys. Ther. 2017, 47, 965–969. [Google Scholar] [CrossRef]
- Cortese, B.; Palamà, I.E.; D’Amone, S.; Gigli, G. Influence of electrotaxis on cell behaviour. Integr. Biol. 2014, 6, 817–830. [Google Scholar] [CrossRef]
- Papetti, M.; Herman, I.M. Mechanisms of normal and tumor-derived angiogenesis. Am. J. Physiol. Cell Physiol. 2002, 282, C947–C970. [Google Scholar] [CrossRef]
- Lin, F.; Baldessari, F.; Gyenge, C.G.; Sato, T.; Chambers, R.D.; Santiago, J.G.; Butcher, E.C. Lymphocyte electrotaxis in vitro and in vivo. J. Immunol. 2008, 181, 2465–2471. [Google Scholar] [CrossRef]
- Sun, Y.S.; Peng, S.W.; Cheng, J.Y. In vitro electrical-stimulated wound-healing chip for studying electric field-assisted wound-healing process. Biomicrofluidics 2012, 6, 34117. [Google Scholar] [CrossRef]
- Allen, G.M.; Mogilner, A.; Theriot, J.A. Electrophoresis of cellular membrane components creates the directional cue guiding keratocyte galvanotaxis. Curr. Biol. 2013, 23, 560–568. [Google Scholar] [CrossRef]
- Miao, Y.; Bhattacharya, S.; Edwards, M.; Cai, H.; Inoue, T.; Iglesias, P.A.; Devreotes, P.N. Altering the threshold of an excitable signal transduction network changes cell migratory modes. Nat. Cell Biol. 2017, 19, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Song, B.; Pu, J.; Wada, T.; Reid, B.; Tai, G.; Wang, F.; Guo, A.; Walczysko, P.; Gu, Y.; et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature 2006, 442, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, V.V.; Gurevich, E.V. GPCR Signaling Regulation: The Role of GRKs and Arrestins. Front. Pharmacol. 2019, 10, 125. [Google Scholar] [CrossRef] [PubMed]
- Reyes, P.; Brown, K.N. Physiology, Cellular Messengers; Stat Pearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Abat, F.; Valles, S.L.; Gelber, P.E.; Polidori, F.; Jorda, A.; García-Herreros, S.; Monllau, J.C.; Sanchez-Ibáñez, J.M. An experimental study of muscular injury repair in a mouse model of notexin-induced lesion with EPI® technique. BMC Sports Sci. Med. Rehabil. 2015, 7, 7. [Google Scholar] [CrossRef]
- Jorda, A.; Campos-Campos, J.; Aldasoro, C.; Campo-Palacio, I.; Alvarez-Gamez, K.; Blasco, M.C.; Aldasoro, M.; Valles, S.L. Protective Action of Percutaneous Intratissular Electrolysis Technique on the Muscle Damage Induced by Notexin in Rats. Res. Sq. 2022, in press. [Google Scholar] [CrossRef]
- Plant, D.R.; Colarossi, F.E.; Lynch, G.S. Notexin causes greater myotoxic damage and slower functional repair in mouse skeletal muscles than bupivacaine. Muscle Nerve 2006, 34, 577–585. [Google Scholar] [CrossRef]
- Liley, A.W. An investigation of spontaneous activity at the neuromuscular junction of the rat. J. Physiol. 1956, 132, 650–666. [Google Scholar] [CrossRef]
- Kimura, J. Electrodiagnosis in diseases of nerve and muscle: Principles and practice. In Techniques to Assess Muscle Function; Kimura, J., Ed.; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Wiederholt, W.C. “End-plate noise” in electromyography. Neurology 1970, 20, 214–224. [Google Scholar] [CrossRef]
- Tomas i Ferré, J.; Fenoll i Brunet, R.; Santafé, M.; Mayayo, E. Changes in motor nerve terminals during bupivacaine-induced postsynaptic deprivation. J. Anat. 1989, 162, 225–234. [Google Scholar] [CrossRef]
- Benoit, P.W.; Belt, W.D. Destruction and regeneration of skeletal muscle after treatment with a local anaesthetic, bupivacaine (Marcaine). J. Anat. 1970, 107, 547–556. [Google Scholar]
- Guo, B.S.; Cheung, K.K.; Yeung, S.S.; Zhang, B.T.; Yeung, E.W. Electrical stimulation influences satellite cell proliferation and apoptosis in unloading-induced muscle atrophy in mice. PLoS ONE 2012, 7, e30348. [Google Scholar] [CrossRef] [PubMed]
- Fujiya, H.; Ogura, Y.; Ohno, Y.; Goto, A.; Nakamura, A.; Ohashi, K.; Uematsu, D.; Aoki, H.; Musha, H.; Goto, K. Microcurrent electrical neuromuscular stimulation facilitates regeneration of injured skeletal muscle in mice. J. Sports Sci. Med. 2015, 14, 297–303. [Google Scholar] [PubMed]
- Mauro, A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 1961, 9, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Reznik, M. Current Concepts of Skeletal Muscle Regeneration; Williams & Wilkins: Baltimore, MD, USA, 1973. [Google Scholar]
- Turner, H.J.; Badylak, S.F. Regeneration of skeletal muscle. Cell Tissue Res. 2012, 347, 759–774. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wang, D.R.; Guo, Y.C.; Liu, J.Y.; Pan, J. The application of bone marrow mesenchymal stem cells and biomaterials in skeletal muscle regeneration. Regen. Ther. 2020, 15, 285–294. [Google Scholar] [CrossRef]
- Kubiak, C.A.; Grochmal, J.; Kung, T.A.; Cederna, P.S.; Midha, R.; Kemp, S.W.P. Stem-cell-based therapies to enhance peripheral nerve regeneration. Muscle Nerve 2020, 61, 449–459. [Google Scholar] [CrossRef]
- Michlovitz, S.L. Thermal Agents in Rehabilitation; Davis Company: Boston, MA, USA, 1990. [Google Scholar]
- Kwiecien, S.Y.; McHugh, M.P.; Howatson, G. Don’t Lose Your Cool with Cryotherapy: The Application of Phase Change Material for Prolonged Cooling in Athletic Recovery and Beyond. Front. Sports Act. Living 2020, 2, 118. [Google Scholar] [CrossRef]
- McGorm, H.; Roberts, L.A.; Coombes, J.S.; Peake, J.M. Turning Up the Heat: An Evaluation of the Evidence for Heating to Promote Exercise Recovery, Muscle Rehabilitation and Adaptation. Sports Med. 2018, 48, 1311–1328. [Google Scholar] [CrossRef]
- Reurink, G.; Goudswaard, G.J.; Moen, M.H.; Weir, A.; Verhaar, J.A.N.; Bierma-Zeinstra, S.M.A.; Maas, M.; Tol, J.L. Platelet-rich plasma injections in acute muscle injury. N. Engl. J. Med. 2014, 370, 2546–2547. [Google Scholar] [CrossRef]
- Bayer, M.L.; Magnusson, S.P.; Kjaer, M.; Tendon Research Group Bispebjerg. Early versus Delayed Rehabilitation after Acute Muscle Injury. N. Engl. J. Med. 2017, 377, 1300–1301. [Google Scholar] [CrossRef]
- Crupnik, J.; Silveti, S.; Wajnstein, N.; Rolon, A.; Vollhardt, A.; Stiller, P.; Schmitz, C. Is radial extracorporeal shock wave therapy combined with a specific rehabilitation program (rESWT + RP) more effective than sham-rESWT + RP for acute hamstring muscle complex injury type 3b in athletes? Study protocol for a prospective, randomized, double-blind, sham-controlled single centre trial. J. Orthop. Surg. Res. 2019, 14, 234. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).