Abstract
Abietane diterpenoids (e.g., carnosic acid, aethiopinone, 1-oxoaethiopinone, salvipisone, and ferruginol) synthesized in the roots of several Salvia species have proved to have promising biological activities, but their use on a large scale is limited by the very low content extracted from in vivo roots. In this review, we summarized our efforts and the achieved results aimed at optimizing the synthesis of these diterpenes in Salvia sclarea hairy roots by either elicitation or by modifying the expression of genes encoding enzymes of the MEP-pathway, the biosynthetic route from which they derive. Stable S. sclarea hairy roots (HRs) were treated with methyl jasmonate or coronatine, or genetically engineered, by tuning the expression of genes controlling enzymatic rate-limiting steps (DXS, DXR, GGPPS, CPPS alone or in combination), by silencing of the Ent-CPPS gene, encoding an enzyme acting at gibberellin lateral competitive route or by coordinate up-regulation of biosynthetic genes mediated by transcription factors (WRKY and MYC2). Altogether, these different approaches successfully increased the amount of abietane diterpenes in S. sclarea HRs from to 2 to 30 times over the content found in the control HR line.
1. Introduction
Medicinal plants are largely used as a source of natural remedies for preventing or combating chronic diseases. In addition, the identification of bioactive plant-derived molecules and the uncovering of their molecular targets provide an enormous opportunity for new drug development. The majority of the bioactive plant compounds are secondary metabolites, with low molecular weights (<1000 Da), which are synthesized in plants to promote their adaptation to both abiotic and biotic stresses, protection against herbivores and phytopathogens, and as attraction for pollinators and the dispersion of seeds mediated by animals. It is widely reported that the content of most of the bioactive secondary metabolites in wild or cultivated medicinal plants is generally very low (less than 1% dry weight) and variable, dependent on the physiological and developmental stages [1].
In addition, the complex chemical structures of medicinal plants make total chemical syntheses highly unrealistic. Although pharmaceutical companies have optimized the extraction of bioactive compounds or of their precursors from plants and developed semisynthetic processes, valuable plant-derived drugs, currently used in the clinic, usually have high costs of production. Plant biotechnology offers a sustainable method for the bioproduction of plant secondary metabolites using plant in vitro systems. However, there are still many challenges to overcome to enhance the production of these metabolites from plant in vitro systems and establish a sustainable large-scale biotechnological process [2].
Plant terpenoids constitute one of the most functionally and structurally diverse group of plant secondary metabolites thus far described, consisting of more than 80,000 different compounds, with diverse biological functions in the plant kingdom and for human health [3]. Many studies have been focused on elucidating the isoprenoid pathways, identifying the enzymatic steps, their genetic control, and uncovering potential enzymatic bottlenecks, with the final aim being metabolic bioengineering or refactoring a plant’s natural product biosynthetic pathways in microorganisms [4].
Most plant isoprenoids, including also diterpenes, derive from two common C5 precursors, IPP and its isomer DMAPP, through two distinct pathways: the well-studied mevalonate (MVA) pathway, which predominates in cytosol, and the more recently unveiled deoxyxylulose 5-phosphate/2-C-methyl-D-erythritol 4-phosphate (DXP/MEP) mevalonate-independent pathway, localized in the plastids (Figure 1). Briefly, the condensation of pyruvate and glyceraldehyde 3-phosphate (G3P) produces the 1-deoxy-D-xylulose 5-phosphate (DXP), catalyzed by the 1-deoxy-D-xylulose 5-phosphate synthase (DXS), the first committed step in the plastidial MEP-pathway. DXP is then reorganized and reduced to 2-C-methyl-D-erythritol 4-phosphate (MEP), by 1-deoxy-D-xylulose 5- phosphate reductoisomerase (DXR). MEP is then cyclized to the intermediate methylerythritol 2,4-cyclodiphosphate (ME-cPP), through the action of three consecutive enzymatic steps, involving a cytidylation step (by 4-Diphosphocytidyl-2-C-methyl-D-erythritol synthase, CMS), an ATP-dependent phosphorylation (by the CMK 4-(cytidine 5′-diphospho)-2-C-methyl-D-erythritol kinase), and a cyclization step (by the 2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase, MDS). The final two enzymatic steps of the MEP-pathway consist in the synthesis of the hydroxymethylbutenyl 4-diphosphate (HMBPP) from ME-cPP, catalyzed by the hydroxymethylbutenyl 4-diphosphate synthase (HDS) and the conversion of HMBPP into a 5:1 mixture of IPP and its isomer DMAPP, by the IPP and DMAPP synthase (IDS). According to the number of C5 units of IPP and DMAPP, different isoprenoids are formed: in particular, diterpenes contain four C5 units, characterized by a large diversity in their chemical structure, with more than 18,000 structures thus far described [5]. Basically, diterpenoids derive from three enzymatic steps: (i) condensation of four units of IPP and formation of the universal precursor GGPP (Geranyl geranyl diphosphate); (ii) the formation of very diversified chemical skeletons, catalyzed by different classes of diterpene synthases (diTPSs) [6], and (iii) the subsequent modification of the diterpene skeletons, catalyzed by cytochrome P450s (P450s or CYPs) [5], yielding a wide variety of complex chemical structures, including acyclic bi-tri- and tetra-cyclic compounds. The Salvia species are rich in tricyclic diterpenoids, and more than 400 diterpenoids with different abietane skeletons have been isolated from Salvia species [7].
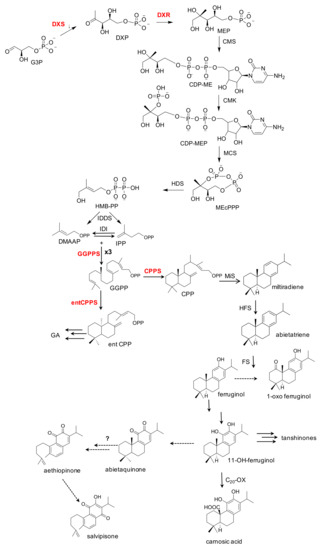
Figure 1.
Simplified MEP-derived biosynthetic route of abietane diterpenes and other isoprenoids in Lamiaceae species. The main enzymatic steps, intermediates, and final products are indicated. Abbreviations: MEP, 2-C-methyl-D-erythriol-4P; DXS, deoxyxylulose 5-phosphate synthase; DXR, deoxyxylulose 5-phosphate reductoisomerase; CMS, 4-diphosphocytidyl-methylerythritol synthase; CMK, 4-diphosphocytidyl-methylerythritol kinase; MCS, methylerythritol 2,4-cyclodiphosphate synthase; HDS, hydroxymethylbutenyl 4-diphosphate synthase; HDR, hydroxymethylbutenyl 4 diphosphate reductase; GPPS, GPP synthase; IDI, isopentenyl diphosphate isomerase; CPPS, CPP synthase; IPP, isopentenyl pyrophosphate; DMAPP, dimethylallyl pyrophosphate; GPP, geranyl pyrophosphate; GGPP, geranylgeranyl pyrophosphate; CPP, copalyl diphosphate; entCPP, ent-copalyldiphosphate; GAs, Gibberellins; entCPPS, ent-copalyldiphosphate synthase; MiS, miltiradiene synthase; HFS, hydroxy-ferruginol synthase; C20-Ox, C20-oxidase. The terpene synthases CPPS and MiS cyclize GGPP to miltiradiene, which is converted spontaneously by oxidization to abietatriene. A HFS, a cytochrome P450 monooxygenases, oxidizes abietatriene to ferruginol and 11-hydroxyferruginol, through two subsequent oxidation steps. A further oxidation of 11-hydroxyferruginol position at the C20, by a C20-oxidase, produces carnosic acid. A possible biosynthetic route of aethiopinone, 1-oxo-aethiopinone and salvipisone is also shown. Dotted lines indicated unknown enzymatic steps. In red are indicated the biosynthetic genes whose expression has been modified by metabolic engineering in S. sclarea hairy roots.
Different biological properties for most plant diterpenoids have been reported, including antitumor [8,9], cytotoxic, antibacterial [10], antiplasmodial [11,12], leishmanicidal, gastroprotection, molluscicidal [13], and antifungal activities [14]. Some diterpenoids are currently used in the clinical practice, such as paclitaxel, ginkgolide, oridonin, tanshinones, and triptolide [15].
In this review, we present an integrated overview of the possible avenues for enhancing the biosynthesis of abietane diterpenes in Salvia sclarea hairy roots by either elicitation or metabolic engineering of the MEP pathway from which they derive.
2. Methodologies
This review is based on the main results published by our group on metabolic engineering of abietane diterpenes in S. sclarea, compared with publically available data on this specific topic in S. sclarea and other medicinal or crop plants. A bibliographic search was launched by using the keywords “Salvia sclarea”, “elicitation”, “metabolic engineering”, “abietane diterpenes”, “hairy roots”, which yielded a total of 24,949 results, divided as follows: Salvia sclarea: 164 results; abietane diterpenes: 3152; hairy roots: 2093; plant metabolic engineering: 7931; the plant elicitation: 10,909 results. The search field was further restricted considering these same keywords coupled to “Salvia sclarea” keyword and considering also a search for the therapeutic relevance, the bioactivity, and the anti-tumor properties of abietane diterpenes. The use of these criteria permitted to have a collection of 1230 articles, published until June 2022. Non-relevant articles were excluded, and relevant studies were considered for writing the manuscript. The selection criteria of the collected articles are schematized in Figure 2.
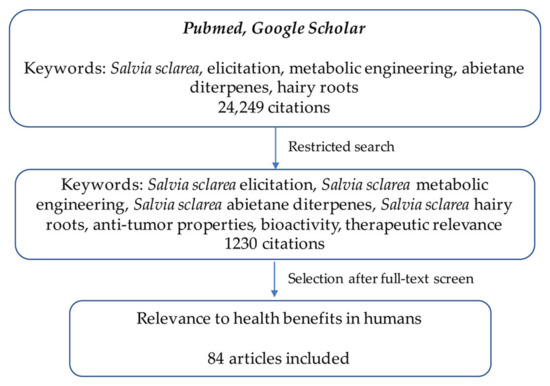
Figure 2.
Diagram representing bibliographic research criteria used for this review.
3. S. sclarea Roots Contain Bioactive Abietane Diterpenes
Salvia sclarea (clary sage) is a biennial or perennial plant belonging to the Lamiaceae family, typical of the north of the Mediterranean, central Asia, and some areas of North Africa. It is well known for the extraction of sclareol produced in the flower calyces, which is used for the semi-synthesis of the ambroxide, a well-prized perfume ingredient. This compound derives by the cyclization of GGPP by a Labd-13-en-8-ol diphosphate synthase (SsLPPS) to labda-13-en-8-ol diphosphate (LPP), which is subsequently converted into sclareol [16]. However, interesting additional abietane-quinone-type diterpenes are synthesized in the roots of S. sclarea, such as carnosic acid, aethiopinone, 1-oxoaethiopinone, salvipisone, and ferruginol, with known pharmacological properties, summarized in Table 1. Aethiopinone, salvipisone, 1-oxoaethiopinone, and ferruginol, from S. sclarea, showed bacteriostatic as well as bactericidal activities against different strains of Staphylococcus aureus and Staphylococcus epidermidis, through a synergistic action of salvipisone and aethiopinone with ß-lactam antibiotics [17]. Cytotoxic and antitumor activities in several human tumor cell lines have been reported for tricyclic diterpenoids [9,18,19], especially those containing quinone moiety, often present in several effective cancer chemotherapeutic agents [20]. Salvipisone and aethiopinone showed relatively high cytotoxicity against HL-60 and NALM-6 leukemia cells, by inducing a caspase-3-mediated apoptosis [19]. In addition, our studies have contributed to establish that aethiopinone, purified from S. sclarea hairy roots, has also cytotoxic different and anti-proliferative activities against other tumor cells, in particular against solid tumor cell lines, such as MCF7 (breast adenocarcinoma), HeLa (epithelial carcinoma), PC3 (prostate adenocarcinoma), and A375 (human melanoma), for which drug resistance is often reported [21], and with negligible effects in non-tumor cells [9]. Especially interesting is this anti-melanoma activity exerted by aethiopinone, since melanoma cells are intrinsically resistant to pharmacological anticancer treatment and pharmaceutical companies are searching for more active and stable drugs [21].

Table 1.
Abietane-quinone-type diterpenes and their reported biological activities in Lamiaceae species.
One drawback in using these interesting compounds as potential new anti-tumor drugs is their very low amount (<0.5% dry weight) in natural and cultivated plants, as reported frequently for most of the plant secondary metabolites [1]. Plant cell and tissue cultures have been largely considered an attractive possibility of extraction of plant bioactive secondary metabolites [22]. In particular, hairy root (HR) technology has been reported to be an efficient system for producing secondary metabolites, especially for those which accumulate preferably in differentiated plant organs, such as roots [23]. A HR-based platform has been developed in our laboratory as a starting point to overcoming potential metabolic bottlenecks in the enzymatic reactions acting upstream or downstream of GGPP, and with the final aim to optimizing the biosynthesis of aethiopinone and other tricyclic abietane diterpenoids in S. sclarea, as summarized below.
It is worthy to note that several bioactive diterpenes produced in the roots of other Salvia species have been largely studied. For example, tanshinones, a class of lipophilic abietane diterpenes present in the roots of S. miltiorrhyza, known for their multiple therapeutic activities, have been deeply characterized, and their biosynthetic route from GGPP almost completely clarified [39].
Less is known on the enzymatic steps that from GGPP lead to the synthesis of abietane diterpenes synthesized in the roots of S. sclarea, which may hinder the possibility of genetically engineering genes encoding limiting enzymes with the final aim to increase the availability of substrates/intermediates/final products of this biosynthetic pathway. This is a quite relevant aspect not only for a cost-effective scale-up of the production of this class of compounds, but even for a deeper understanding of their cellular targets and pharmacological characterization.
As reported in the following paragraphs, the establishment of stable HR lines of S. sclarea, combined with complementary strategies of metabolic engineering and elicitation, has contributed for the first time to establish that the biosynthesis of aethiopinone proceeds through the cyclization of GGPP to CPP mediated by the enzyme CPPS. We have also demonstrated that not only the availability of GGPP, as already reported for several different plant diterpenes, but also of CPP is quite critical to enhance the accumulation of aethiopinone and other abietane-type diterpenes in S. sclarea HRs [40].
4. A Hairy Root Platform for the Production of Bioactive Abietane Diterpenes
HRs are adventitious roots obtained at plant-wounded sites by the infection of Rhizobium rhizogenes (previously denominated Agrobacterium rhizogenes), a Gram-negative soil bacterium. HRs are differentiated organs, which grow under hormone-free culture conditions, and are considered a valuable and stable source of plant bioactive compounds more than plant cell cultures, which are frequently biochemically variable and unable to produce sufficient quantities of bioactive secondary metabolites, [23].
Stable massive cultures of S. sclarea HRs were obtained in our laboratory by customizing the growth media and conditions [9], in which the metabolic flux was modified toward a higher synthesis of aethiopinone and other abietane diterpenoids, as schematized in Figure 3.
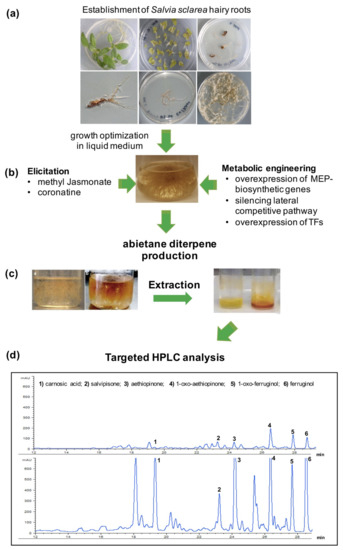
Figure 3.
Enhancing abietane diterpene content of S. sclarea by coupling hairy root technology and different elicitation or metabolic engineering approaches. (a) Stable hairy root cultures obtained by transformation with R. rhizogenes; (b) elicitation with methyl jasmonate or coronatine and different metabolic engineering approaches used to increase the accumulation of abietane diterpenes, as phenotypically visible from the red color of the hairy roots and the methanol extracts; (c) representative HPLC-DAD chromatograms of the main abietane diterpenes identified in control S. sclarea HRs (upper panel) and HRs elicited with MeJA for 1 week (lower panel) (d). The photos and the chromatograms are original and used by the authors for this review.
5. Boosting the Biosynthesis of Abietane Diterpenes in S. sclarea HRs by Elicitation and Metabolic Engineering
In the last decade, our efforts were focused on trying to elucidate the metabolic pathway of abietane diterpenes in S. sclarea and to identify potential limiting enzymatic reactions and precursors/intermediates that might affect their accumulation in roots. This information was gathered by designing complementary strategies of elicitation and metabolic engineering to modify the expression of genes encoding known, unknown limiting or competitive enzymatic reactions of the MEP-pathway to boost the metabolic flux toward a higher synthesis of aethiopinone and other abietane diterpenoids, as recapitulated in Table 2.

Table 2.
A summary of the effects on the abietane diterpene accumulation in Salvia sclarea HRs obtained by designing different elicitation and metabolic engineering approaches.
5.1. Elicitation with Methyl Jasmonate and Its Analogue Coronatine
Elicitors are biotic or abiotic agents which trigger signal cascades inducing tolerance and immune response in plants. They have been widely applied to stimulate the synthesis of secondary metabolites in HRs as well as in plant cell culture of different plants [43,44]. Jasmonic acid (JA) and its structural analogue coronatine (Cor) have been extensively reported as elicitors of the MEP-pathway. Both JA or Cor, which structurally mimics JA-isoleucine (JA-Ile), the bioactive JA, bind the CORONATINE INSENSITIVE 1 (COI1), to activate JA signaling and the expression of JA-induced genes. Cor, however, is able to induce other defense pathways in an independent fashion from the JA signaling [45].
The COI1 dependent perception and signaling pathway is regulated by the transcription factor MYC2 that acts as activator and repressor of JA-responsive gene expression in Arabidopsis [46]. It is known that MYC2 is repressed by the JAZ proteins, which bind the co-repressor TOPLESS (TPL) protein, directly through the EAR (Ethylene Response Factor-Associated Amphifilic Repression) motif or indirectly through an additional repressor, the NINJA (Novel Interactor of JAZ) protein. The histone acetylases HDA6 and HDA19 are then recruited to form a closed complex inhibiting JA response (Figure 4a). Under a high level of JA-Ile (caused by stress conditions) or elicitation with Cor, JA-Ile or Cor are transported into the nucleus, where they interact with the F-box protein COI1 within the SCF E3 ubiquitin ligase (Skp–Cullin–F-box-type) and form the SCF-COI1 complex. This complex recruits the JAZ repressor proteins, bound to the promoter of MYC2, and sends them to degradation via the 26S proteasome. Released MYC2 binds the subunit 25 of Mediator complex (MED25) and induces the transcription of early JA-responsive genes [46], as well as genes involved in the plant defense mechanisms, including also genes encoding enzymes of different secondary metabolites (Figure 4b) [47].
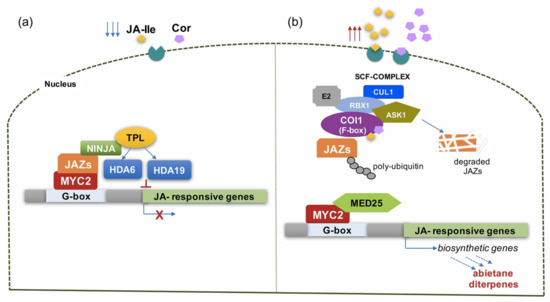
Figure 4.
A Schematic representation of the MJ or Cor signal perception and transduction mediated by the MYC2 transcription factor, based on the current knowledge available in literature (original figure). A detailed description of MYC2-mediated signaling and transduction in normal conditions (low level of JA-Ile or Cor) (a) or in stress or elicitation conditions (high level of JA-Ile or Cor) (b) is reported in the text. Abbreviations: JA-Ile, jasmonoyl isoleucine; Cor, coronatine; JAZ, jasmonate ZIM domain; NINJA, novel interactor of JAZ; TPL, topless; HDA6, HDA19, histone deacetylase 6, 19; ASK1, Arabidopsis SKP1 (S-phase kinase-associated protein (1) homologue; CUL, CULLIN; E2, ubiquitin-conjugating enzyme; MYC2, bHLH zip transcription factor; RBX, RING-H2 protein; SCF-complex, complex consisting of Skp1, Cullin-1, and F-box protein; Ub, ubiquitin; COI1, coronatine insensitive 1; MED25, mediator 25.
Elicitation of S. sclarea HRs with Methyl-jasmonate (MJ) or Cor has been very informative for us to pinpoint biosynthetic genes of MEP-pathway that might limit the accumulation of aethiopinone and other bioactive diterpenoids in S. sclarea HRs, as reported below.
5.1.1. MJ-Induced Accumulation of Bioactive Abietane Diterpenes in S. sclarea Is Due to the Transcriptional Regulation of Genes of the MEP-Pathway
It is well documented that the JA-activated biosynthesis of different terpenoids [47], is due to transcriptional up-regulation of genes belonging to either the MEP-pathway or the MVA-pathway [48,49,50].
We found that also in S. sclarea HRs, MJ elicitation was able to enhance the transcriptional levels of several biosynthetic genes of the plastidial MEP-dependent pathway, such as SsDXS, SsDXR, SsCMK, SsMCS, SsHDS, SsHPR, SsGGPPS, and SsCPPS genes [51]. The most induced transcript by MJ was SsDXS (60-fold increase), the enzyme acting up-stream the MEP-derived pathway. Since it is well known that this enzymatic reaction is limiting the accumulation of different isoprenoids in many plant species, increasing the DXS activity by metabolic engineering has been successful to enhance the content of terpenoids in different plant species and prokaryotic cells [52]. The SsGGPPS gene encoding the synthase involved in the condensation of IPP and DMAPP to GGPP, the common precursor of many terpenes of plastidial origin, was also transcriptionally activated by MJ (20-fold increase) in S. sclarea HRs as well as the SsCPPS gene (60-fold increase). This is indirect evidence that abietane-type diterpenes in S. sclarea are produced from the conversion of GGPP via a copalyl-diphosphate (CPP) intermediate, by using this CPP synthase, as widely reported for other plant diterpenoids [53,54,55]. This MJ-coordinated expression of multiple genes of the MEP-pathway induced a significant increase of the abietane diterpene content in S. sclarea HRs, as phenotypically visible from the red color of the MJ-elicited HRs (Figure 3). Compared to control HRs (14.50 ± 0.31 mg L−1), MJ elicitation (100 µM) for 7 days induced an approximatively 20-fold enhancement in the total abietane diterpene content (304.01 ± 2.21 mg L−1). A long-term elicitation (4 weeks) with MJ also increased the content of total abietane diterpenoids, but it was associated with a severe HR growth inhibition, which penalized the final yield of total abietane diterpenes (210.74 ± 1.26 mg L−1) (Figure 5) [51].
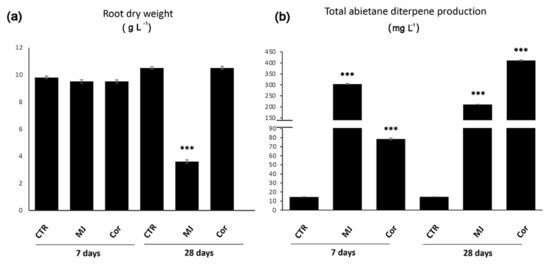
Figure 5.
Effects of MJ or Cor elicitation (7 or 28 days) on the final biomass (a) and on the total abietane diterpene production in S. sclarea hairy roots (b). The asterisks denote a significant difference between elicited and control hairy roots (p < 0.001) according to Student’s t test. Data are from Vaccaro et al. 2017.
This set of data are consistent with those reported in Salvia milthiorriza HRs elicited with MJ, which triggered the accumulation of tanshinone and phenolic acids [56], due also to the up-regulation of biosynthetic genes [57]. MJ has been also proved to induce simultaneously the transcription of most of the biosynthetic genes in specific pathways of other plant species, as reported for the TIA (terpenoid-indole-alkaloid) in C. roseus [58], for nicotine in N. tabacum [59], and for artemisinin in A. annua [60]. This MJ-concerted transcriptional activation of many genes encoding enzymes of the MEP-pathway has prompted numerous studies for identifying “master” regulators of this pathway. Different families of plant transcription factors have been identified as able to regulate the biosynthesis of terpenoids [61], as discussed in the Section 5.2.3.
5.1.2. Coronatine as Alternative Elicitor of the Synthesis of Abietane Diterpenes
Coronatine (Cor) is a structural analogue of the bioactive conjugate of the JA-leu, the active intracellular form of JA, capable of mediating various aspects of bacterial virulence. It causes the stomata to reopen to facilitate bacterial invasion, their growth in the apoplast, and the induction of disease symptoms [62]. There are several features that make Cor preferable to MJ as elicitor agents in plant cell/HR cultures: it is a more stable molecule, and does not need to be chemically converted to JA-Ile to bind the COI1 receptor [63]. S. sclarea HRs elicited continuously for 7 or 28 days with Cor (0.1 µM) showed a significant transcriptional activation of different genes encoding biosynthetic enzymes of the MEP-pathway, although to a lesser extent compared to the MJ treatment. However, similarly to MJ treatment, the transcriptional gene activation was transient, peaking at 24 h from the beginning of the Cor elicitation [51]. Consistent with this coordinated transcription of biosynthetic genes, the total amount of abietane diterpenes was significantly enhanced in Cor-elicited S. sclarea HRs. After 7 days compared to the untreated HRs (14.50 ± 0.31 mg L−1), a significant increase was observed in total abietane diterpene production (78.56 ± 1.12 mg L−1, approximatively a 5-fold increase). After 28 days of Cor elicitation, the content of aethiopinone in S. sclarea HRs increased further (103.32 ± 2.10 mg L−1, approximatively a 24-fold increase over the basal content of control hairy roots (4.40 ± 0.13 mg L−1). Very low concentrations (0.1 µM) of Cor for 28 days allowed to extract 410.97 ± 2.50 mg L−1 of abietane diterpenes, determining a 28-fold increase compared to the content of unelicited HRs, significantly higher than the increase observed after a long-term elicitation with MJ (Figure 5b). This was due mainly to a negligible negative effect caused by Cor treatment on the final HR biomass (Figure 5a) [51].
5.2. Metabolic Engineering to Enrich the Content of Abietane Diterpenes in S. sclarea Hairy Roots
Plant metabolic engineering is a powerful strategy to modify metabolic routes aimed at increasing the synthesis of a specific bioactive compound, by either genetic engineering of endogenous genes, with the final aim to increase or divert the metabolic flux towards desired/undesired products, respectively. Another application is to generate plants able to synthesize novel compounds through transformation with genes from other plants/organisms [64].
Our previous elicitation studies evidenced a strong correlation between the accumulation of abietane diterpenes and the level of expression of several biosynthetic genes of the MEP-pathway [51]. In the next section, we summarize the different approaches we have used and the results achieved in boosting the synthesis of abietane diterpenes in S. sclarea HRs by: i) tuning the expression of biosynthetic genes that control rate-limiting enzymatic steps (DXS, DXR, GGPPS, CPPS alone or in combination); ii) RNAi silencing of a gene acting at the lateral competitive route of gibberellin (Ent-CPPS); iii) overexpressing different transcription factors (WRKYs and MYC2) involved in the coordinated regulation of several genes of the MEP-pathway.
5.2.1. Overexpression of DXS and DXR Genes, encoding the First Two Enzymes Acting Up-Stream the MEP-Pathway
As reported previously, Deoxyxylulose 5-phosphate synthase (DXS), the first enzymatic step of the MEP-pathway, has been often reported as one of the limiting enzymes of the up-stream pathway to GGPP, by influencing the supply of IPP and its isomer DMAPP (extensively and recently reviewed by [65]). Experimental evidence has indicated that also the activity of DXP reducto-isomerase (DXR), the second enzymatic step, is limiting the biosynthesis of several plant isoprenoids [66]. The expression of the DXS and DXR genes has been modified in different plant species to enhance successfully the synthesis of different isoprenoids, although the reported results are not always consistent in different plant species [67,68,69]. In our earlier studies, we have demonstrated that constitutive overexpression of the heterologous A. thaliana AtDXS or AtDXR genes is able to enhance the content of bioactive abietane-type diterpenoids in S. sclarea HRs [9]. As reported in Table 2, overexpression of the DXS protein triggered a 2-fold increase in aethiopinone content compared to the control HR lines. However, high levels of the exogenous DXS protein caused a negative pleotropic effect on the HR growth, probably due to a general competition with the basal protein synthesis or to the modification of the content of other MEP-derived phytohormones (cytokines, gibberellin or ABA), which might interfere with normal HR growth and development. Interestingly, the overexpression of the AtDXR gene appeared to be slightly more efficient in enhancing the synthesis of abietane-type diterpenes in S. sclarea HRs (a 3-fold increase compared to the control HR line), coupled to no detrimental effects on HR growth [9]. Overexpressing both genes in S. miltiorrhiza HRs has improved the accumulation of tanshinones by [68] as well as of other terpenoids in different plant species [69].
In a parallel study, we also tested whether overexpression of DXS and DXR genes of cyanobacterial origin might offer some biotechnological advantages in increasing the synthesis of bioactive diterpenes in S. sclarea HRs. Indeed, the introduction in a plant genome of heterologous genes by genetic transformation might avoid potential gene silencing or co-suppression events, often reported when overexpressing homologous genes, and, at the same time, ensuring a possible greater stability and activity of the two enzymes in a plastidial environment. Orthologous genes, amplified by the genomic DNA of Synechocystis sp PCC6803, were overexpressed in S. sclarea HRs, by targeting them to the chloroplast by the rbc plastid transit peptide [41]. An increased accumulation of bioactive diterpenes was obtained, and, interestingly, the overexpression of the bacterial DXR gene triggered a 5-fold accumulation of aethiopinone, significantly greater than the increase obtained by overexpressing the plant AtDXR gene (a 3-fold increase).
Altogether, these data corroborate the consolidated notion that DXR and DXS are limiting enzymatic steps of the MEP-pathway in several plant species, possibly directing the metabolic flux toward a higher availability of IPP and DMAPP. However, these two enzymes only partially explain the robust increase in abietane diterpenes we found in elicited HRs by MJ and Cor. Therefore, we focused our further studies on establishing other potential enzymatic bottlenecks in the MEP-pathway that might limit their accumulation in S. sclarea HRs.
5.2.2. GGPP Availability Limits the Accumulation of Abietane Diterpenes in S. sclarea HRs
GGPP is the universal isoprenoid precursor and it has been postulated that modifying its distribution among the different downstream metabolic branches of the plastidial MEP-pathway might direct the metabolic flux towards a higher biosynthesis of a targeted end product [70]. We proved that this is also true for an enhanced abietane diterpene accumulation in S. sclarea HRs, by chemically inhibiting the competitive gibberellin (GA) route with the 2-chloroethyl-N,N,N-trimethyl-ammonium chloride (CCC), a known inhibitor of entCPPS, the first enzymatic step, that from GGPP leads to gibberellins (GA) biosynthesis, or by RNAi-mediated silencing of the ent-CPPS gene (Figure 3). The block of this competitive metabolic pathway enhanced the content of all analyzed diterpenes (carnosic acid, ferruginol, 1-oxo-ferruginol, salvipisone, aethiopinone, and 1-oxo-aethiopinone) in CCC-treated HRs compared to the control HRs, with the most relevant increase in the content of aethiopinone (a 7-fold increase). RNAi-mediated silencing of the entCPPS gene confirmed that blocking this lateral GA route from GGPP is an efficient strategy to increase the abietane diterpene content in S. sclarea HRs [40] (Table 2). These data indirectly demonstrated that the GGPP pool is limiting in the metabolic route to abietane diterpenes, as already suggested by the significant correlation between the expression level of the GGPPS gene and the abietane diterpene content we have found in S. sclarea-elicited HRs [51]. These findings also pointed to the GGPPS enzyme as a potential target for increasing the biosynthesis of abietane diterpenes in S. sclarea HRs. By assuming that the IPP and DMAPP, the immediate GGPP precursors, would not be limiting, the cDNA full-length SsGGPPS gene, including its own plastid transit peptide, was constitutively overexpressed in S. sclarea HRs. Higher transcript levels of the SsGGPPS gene significantly increased the content of aethiopinone (7.80 ± 0.71 mg g−1 dw), salvipisone (5.57 ± 0.78 mg g−1 dw), and ferruginol (8.51 ± 0.35 mg g−1 dw), which was approximately 8, 7, and 28 times, respectively, higher than that in the control HR line (0.99 ± 0.08 mg g−1 dw). Altogether, these results indicate indirectly that the GGPP pool in S. sclarea HRs might limit the biosynthesis of abietane diterpenes, and that GGPPS overexpression is an efficient strategy to increase the overall content of this class of compounds in S. sclarea HRs [40].
5.2.3. CPP Is a Precursor of Abietane Diterpenes in S. sclarea and Overexpression of SsCPPS Also Enhances Their Content
The biosynthetic pathway of the abietane diterpenes synthesized in S. sclarea roots is poorly understood, although their chemical structures were established more than two decades ago [38,71]. For instance, little is known on the first GGPP cyclization step operated by diterpene synthases (diTPSs) in S. sclarea roots to yield abietane diterpenes. It is well documented that in conifers/gymnosperms, the formation of diterpenes requires an initial double cyclization of GGPP into (+)-copalyl diphosphate [(+)-CPP], operated by the class II active site of the abietadiene synthase, a bifunctional diTPS. The (+)-CPP intermediate is then modified by the class I diTPS active site and is subjected to a second cyclization enzymatic step, followed by structural rearrangements via intermediate carbocations [54]. In Lamiaceae species, it has been reported that the synthesis of labdane-related diterpenes also proceeds through the cyclization of GGPP to CPP, catalyzed by the copalyl diphosphate synthase (CPPS), a class II diTPS [72]. The following enzymatic reaction in the biosynthesis of this group of diterpenes is due to the action of a kaurene synthase-like enzyme, the miltiradiene synthase (MiS), which, through a spontaneous oxidation reaction, transforms CPP to miltiradiene.
A first indirect indication of the potential involvement of the CPPS in the cyclization of GGPP to CPP in the biosynthesis of S. sclarea abietane diterpenes was provided by the high correlation we found between the expression level of the CPPS gene and the abietane diterpene content in S. sclarea HRs [51]. These findings were further confirmed by the SsCPPS overexpression, which boosted a significant 20-fold increase in the aethiopinone content (10.44 ± 0.21 mg g−1 dw) compared to the control HR lines (0.5 ± 0.03 mg g−1 dw). Interestingly, the content of ferruginol (11.50 ± 0.38 mg g−1 dw) and salvipisone (6.65 ± 0.72 mg g−1 dw) was also enhanced by 30 or 9 times, respectively, by overexpressing the CPPS gene in S. sclarea HRs [40].
To the best of our knowledge, this is the first evidence that aethiopinone in S. sclarea might be synthesized from GGPP to CPP by a CPPS, which occurs for other abietane diterpenes [73,74]. In addition, the availability of the CPP might limit the synthesis of abietane diterpenes in S. sclarea, since we have proved for the first time that the CPPS overexpression overcomes this metabolic bottleneck.
5.2.4. Co-Expression of GGPPS and CPPS Genes
Despite the success obtained by overexpressing single genes, frequently, the final amount of a targeted metabolite is limited by multiple enzymatic activities. Therefore, the concerted regulation of two or multiple genes would be more appropriate to significantly obtain higher content of a desired compound. Co-expression of rate-limiting biosynthetic genes has been successfully applied to boost the synthesis of a variety of high-value plant-derived compounds [69,75]. Considering the significant increase in aethiopinone content obtained by overexpression of GGPPS and CPPS genes individually, these two biosynthetic genes were co-expressed in S. sclarea HRs [40]. This “push and pull” strategy induced an increase in the aethiopinone content by 6 times, against the 8- and 10-fold increase obtained by overexpressing the two genes singularly (Table 2). This might be probably caused by an unbalanced simultaneous high level of GGPPS and CPPS transcripts, and an impaired ratio of the levels of the two encoding enzymes and/or relative substrates. To better understand these unexpected results, it would be useful to determine the relative level of GGPPS and CPPS proteins and their relative enzymatic activities in order to identify overexpressing HR lines with a balanced level of both enzymes, which could affect additively or synergistically the final yield of aethiopinone and other bioactive diterpenes in S. sclarea HRs [40].
5.2.5. Orchestrating the Expression of Multiple Biosynthetic Genes of the MEP-Pathway by TFs
Transcription factors (TFs) provide an attractive alternative for modifying a metabolic flux in plants since they are able to activate in a coordinated manner the transcription of multiple biosynthetic pathway genes. It is widely reported that TFs may act alone or in combination with other regulators to activate and/or to inhibit/de-repress gene transcription [76]. A bioinformatic analysis of A. thaliana genes encoding enzymes of the MEP-pathway revealed that the promoter of many of these genes contains the W-box (TTGAC), the known binding site of WRKY TFs, and the G-box (CACGT), the binding domain of MYC2, a TF known to be regulated by MJ (Figure 4). The WRKY TFs, members of a plant-specific TF family, characterized by a conserved peptide motif WRKYGQK and a zinc finger domain, play different biological roles and have a prominent involvement in controlling the plant response to biotic and abiotic constraints [77,78]. Among the different WRKY TFs thus far identified, we focused our attention on AtWRK18 and AtWRK40, since we identified in their promoter region multiple conserved methyl jasmonate responsive elements (MJRE), localized at −800 bp from the TSS, and confirmed their transcriptional activation in MJ-elicited Arabidopsis plantlets [42]. The overexpression of AtWRKY18, AtWRKY40 genes in S. sclarea HRs positively activated the transcription of different biosynthetic genes of the plastidial MEP-derived pathway. In particular, S. sclarea genes DXS, DXR, HDS, GPPS, and CPPS were up-regulated by overexpression of AtWRKY18 and AtMYC2, while AtWRKY40 preferentially activated the transcription of the DXS and CPPS genes. Targeted metabolic profiling of overexpressing WRKY HR lines revealed that the simultaneous up-regulation of the biosynthetic genes correlated to an increased content of abietane diterpenes compared to control line (a fold-increase >4 and >2 in the AtWRKY40 and AtWRK18 overexpressing lines, respectively), especially in the production of salvipisone and aethiopinone [42]. This is one of the first published evidence on the involvement of WRKY TFs in the specific metabolic pathway of abietane diterpenes in S. sclarea, which was confirmed also for the biosynthesis of tanshinones in S. miltiorrhyza [79].
We also overexpressed in S. sclarea HRs the AtMYC2 TF, which contains a basic helix-loop-helix (bHLH) domain, and, as already discussed, is a master regulator of the JA signaling pathway and also controls secondary metabolism in plants [80,81,82] (Figure 4). AtMYC2 overexpression was able to enhance the transcript levels of several biosynthetic genes of the MEP-derived pathway [42]. This coordinated up-regulation resulted in a significant accumulation of the total abietane diterpene content (a fold-increase > 5) (Table 2), which caused however, a considerable growth inhibition of the overexpressing HRs [42], due possibly to the multiple roles of MYC2 in JA signal transduction. As mentioned earlier, a possible unexpected modification of the level of the synthesis of phytohormones or chlorophyll derived from the MEP-pathway might also explain partially the growth impairment of the MYC2 overexpressing HR lines. At any rate, the MYC2- or WRKY-dependent increased content of abietane diterpenes was lower than that obtained by MJ or Cor elicitation, suggesting that it is more likely a combinatorial role for different TFs, belonging to the same TF group or to different families, in the transcriptional regulation of biosynthetic genes of JA-mediated metabolic pathways [42].
6. Conclusions and Perspectives
Our results demonstrate that the combined application of massive HR culture and metabolic engineering and/or elicitation strategies can contribute to bypass metabolic constraints in the MEP-pathway and improve the accumulation of bioactive abietane diterpenes in S. sclarea, and they might be successful applied as well as in other Salvia species. We are, however, still very far from a full comprehension of the molecular players involved in the regulation of the biosynthesis of abietane diterpenes in the roots of S. sclarea. Enzymatic steps downstream CPP to aethiopinone have not been elucidated yet and we are only at the beginning of understanding the complex TF network controlling positively or negatively this pathway.
In general terms, it is now evident that the regulation of plant secondary metabolism is not simply an on/off switch of single or multiple genes. New emerging technologies and the disclosure of novel molecular mechanisms regulating the plant secondary metabolism will give a great impulse to basic and translational research in this field, such as:
- i.
- the possibility of engineering enzymes, removing catalytic constraints in key enzymes or knocking repressors, by CRISPRS-Cas9 genome editing (as reviewed in [83];
- ii.
- many secondary metabolites are synthesized/accumulated in response to a plethora of abiotic and biotic stress. Epigenetic control of plant stress response is well documented, but the role of epigenetics in regulating the secondary metabolism has so far been largely overlooked [84].
It is expected that in the near future, the novel knowledge globally gathered on the regulation of plant secondary metabolism will lead to biotechnological innovations for the production of molecules of plant origin with high added value, from massive cell and tissue cultures or by reconstructing metabolic pathways in heterologous systems, according to the principles of sustainability, economy, and standardization required by the pharmaceutical industry and the market.
Author Contributions
A.L. and M.A. conceived and structured this review paper, based on experimental results designed and carried out by A.L., M.A., M.V., A.A. and N.D.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Marchev, A.S.; Yordanova, Z.P.; Georgiev, M.I. Green (cell) factories for advanced production of plant secondary metabolites. Crit. Rev. Biotechnol. 2020, 40, 443–458. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Tetali, S.D. Terpenes and isoprenoids: A wealth of compounds for global use. Planta 2019, 249, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Courdavault, V.; O’Connor, S.E.; Jensen, M.K.; Papon, N. Metabolic engineering for plant natural products biosynthesis: New procedures, concrete achievements and remaining limits. Nat. Prod. Rep. 2021, 38, 2145–2153. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Liu, X.; Tian, M.; Ma, Y.; Jin, B.; Gao, W.; Cui, G.; Guo, J.; Huang, L. Recent progress and new perspectives for diterpenoid biosynthesis in medicinal plants. Med. Res. Rev. 2021, 41, 2971–2997. [Google Scholar] [CrossRef]
- Johnson, S.R.; Bhat, W.W.; Bibik, J.; Turmo, A.; Hamberger, B.; Evolutionary Mint Genomics Consortium; Hamberger, B. A database-driven approach identifies additional diterpene synthase activities in the mint family (Lamiaceae). J. Biol. Chem. 2019, 294, 1349–1362. [Google Scholar] [CrossRef] [Green Version]
- Bisio, A.; Pedrelli, F.; D’Ambola, M.; Labanca, F.; Schito, A.M.; Govaerts, R.; De Tommasi, N.; Milella, L. Quinone Diterpenes from Salvia Species: Chemistry, Botany, and Biological Activity. Phytochem. Rev. 2019, 18, 665–842. [Google Scholar] [CrossRef]
- Devappa, R.K.; Makkar, H.P.S.; Becker, K. Jatropha Toxicity—A Review. J. Toxicol. Environ. Health Part B 2010, 13, 476–507. [Google Scholar] [CrossRef]
- Vaccaro, M.; Malafronte, N.; Alfieri, M.; De Tommasi, N.; Leone, A. Enhanced biosynthesis of bioactive abietane diterpenes by overexpressing AtDXS or AtDXR genes in Salvia sclarea hairy roots. Plant Cell Tissue Organ Cult. (PCTOC) 2014, 119, 65–77. [Google Scholar] [CrossRef]
- Aiyelaagbe, O.O.; Adesogan, K.; Ekundayo, O.; Gloer, J.B. Antibacterial diterpenoids from Jatropha podagrica Hook. Phytochemistry 2007, 68, 2420–2425. [Google Scholar] [CrossRef]
- Clarkson, C.; Musonda, C.C.; Chibale, K.; Campbell, W.E.; Smith, P. Synthesis of totarol amino alcohol derivatives and their antiplasmodial activity and cytotoxicity. Bioorganic Med. Chem. 2003, 11, 4417–4422. [Google Scholar] [CrossRef]
- Sutthivaiyakit, S.; Mongkolvisut, W.; Ponsitipiboon, P.; Prabpai, S.; Kongsaeree, P.; Ruchirawat, S.; Mahidol, C. A novel 8,9-seco-rhamnofolane and a new rhamnofolane endoperoxide from Jatropha integerrima roots. Tetrahedron Lett. 2003, 44, 3637–3640. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; Razmilic, I.; Sauvain, M.; Moretti, C.; Munoz, V.; Ruiz, E.; Balanza, E.; Fournet, A. Antiprotozoal Activity of Jatrogrossidione from Jatropha Grossidentata and Jatrophone from Jatropha Isabellii. Phytother. Res. 1996, 10, 375–378. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; Tsichritzis, F.; Jakupovic, J. Diterpenes and a lignan from Jatropha grossidentata. Phytochemistry 1992, 31, 1731–1735. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, S. Overview of Medicinally Important Diterpenoids Derived from Plastids. Mini-Rev. Med. Chem. 2017, 17, 988–1001. [Google Scholar] [CrossRef] [PubMed]
- Caniard, A.; Zerbe, P.; Legrand, S.; Cohade, A.; Valot, N.; Magnard, J.-L.; Bohlmann, J.; Legendre, L. Discovery and Functional Characterization of Two Diterpene Synthases for Sclareol Biosynthesis in Salvia sclarea (L.) and Their Relevance for Perfume Manufacture. BMC Plant Biol. 2012, 12, 119. [Google Scholar] [CrossRef] [Green Version]
- Walencka, E.; Rozalska, S.; Wysokinska, H.; Rozalski, M.; Kuzma, L.; Rozalska, B. Salvipisone and Aethiopinone from Salvia sclarea Hairy Roots Modulate Staphylococcal Antibiotic Resistance and Express Anti-Biofilm Activity. Planta Med. 2007, 73, 545–551. [Google Scholar] [CrossRef]
- Hernández-Pérez, M.; Rabanal, R.M.; de la Torre, M.C.; Rodríguez, B. Analgesic, Anti-Inflammatory, Antipyretic and Haematological Effects of Aethiopinone, an o-Naphthoquinone Diterpenoid from Salvia Aethiopis Roots and Two Hemisynthetic Derivatives. Planta Med. 1995, 61, 505–509. [Google Scholar] [CrossRef]
- Różalski, M.; Kuźma, Ł.; Wysokińska, H.; Krajewska, U. Cytotoxic and Proapoptotic Activity of Diterpenoids from in vitro Cultivated Salvia sclarea Roots. Studies on the Leukemia Cell Lines. Z. Nat. C 2006, 61, 483–488. [Google Scholar] [CrossRef] [Green Version]
- Pereyra, C.E.; Dantas, R.F.; Ferreira, S.; Gomes, L.P.; Silva-Jr, F.P. The diverse mechanisms and anticancer potential of naphthoquinones. Cancer Cell Int. 2019, 19, 207. [Google Scholar] [CrossRef] [Green Version]
- Winder, M.; Virós, A. Mechanisms of drug resistance in melanoma BT. In Mechanisms of Drug Resistance in Cancer Therapy; Mandalà, M., Romano, E., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 91–108. ISBN 978-3-030-10507-5. [Google Scholar]
- Wu, T.; Kerbler, S.M.; Fernie, A.R.; Zhang, Y. Plant cell cultures as heterologous bio-factories for secondary metabolite production. Plant Commun. 2021, 2, 100235. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Valdes, N.; Häkkinen, S.T.; Lemasson, C.; Guillet, M.; Oksman-Caldentey, K.-M.; Ritala, A.; Cardon, F. Hairy Root Cultures—A Versatile Tool with Multiple Applications. Front. Plant Sci. 2020, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.H.; Firuzi, O.; Jassbi, A.R. Diterpenoids from Roots of Salvia lachnocalyx; In-silico and In-vitro Toxicity against Human Cancer Cell Lines. Iran. J. Pharm. Res. IJPR 2020, 19, 85–94. [Google Scholar] [CrossRef]
- Benrezzouk, R.; Terencio, M.; Ferrandiz, M.; Hernandez-Perez, M.; Rabanal, R.; Alcaraz, M. Inhibition of 5-lipoxygenase activity by the natural anti-inflammatory compound aethiopinone. Agents Actions 2001, 50, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Trudler, D.; Oh, C.-K.; Lipton, S.A. Potential Therapeutic Use of the Rosemary Diterpene Carnosic Acid for Alzheimer’s Disease, Parkinson’s Disease, and Long-COVID through NRF2 Activation to Counteract the NLRP3 Inflammasome. Antioxidants 2022, 11, 124. [Google Scholar] [CrossRef]
- Wei, K.; Louis, H.; Emori, W.; Idante, P.S.; Agwamba, E.C.; Cheng, C.-R.; Eno, E.A.; Unimuke, T.O. Antispasmodic Activity of Carnosic Acid Extracted from Rosmarinus Officinalis: Isolation, Spectroscopic Characterization, DFT Studies, and in Sili-co Molecular Docking Investigations. J. Mol. Struct. 2022, 1260, 132795. [Google Scholar] [CrossRef]
- Irtegun Kandemir, S.; Fidan, H.S.; Yener, I.; Mete, N.; Ertas, A.; Topcu, G.; Kolak, U. Investigation of Cytotoxic and Apoptotic Effects of 63 Compounds Obtained from Salvia Species: Promising Anticancer Agents. J. Food Biochem. 2022, e14226. [Google Scholar] [CrossRef] [PubMed]
- Min, F.; Liu, X.; Li, Y.; Dong, M.; Qu, Y.; Liu, W. Carnosic Acid Suppresses the Development of Oral Squamous Cell Carcinoma via Mitochondrial-Mediated Apoptosis. Front. Oncol. 2021, 11, 760861. [Google Scholar] [CrossRef]
- Jiang, S.; Qiu, Y.; Wang, Z.; Ji, Y.; Zhang, X.; Yan, X.; Zhan, Z. Carnosic Acid Induces Antiproliferation and Anti-Metastatic Property of Esophageal Cancer Cells via MAPK Signaling Pathways. J. Oncol. 2021, 2021, 4451533. [Google Scholar] [CrossRef]
- Ossikbayeva, S.; Khanin, M.; Sharoni, Y.; Trachtenberg, A.; Tuleukhanov, S.; Sensenig, R.; Rom, S.; Danilenko, M.; Orynbayeva, Z. Curcumin and Carnosic Acid Cooperate to Inhibit Proliferation and Alter Mitochondrial Function of Metastatic Prostate Cancer Cells. Antioxidants 2021, 10, 1591. [Google Scholar] [CrossRef]
- Kolak, U.S.; Arı, Ş.; Birman, H.; Hasançebi, S.; Ulubelen, A. Cardioactive Diterpenoids from the Roots of Salvia amplexicaulis. Planta Med. 2001, 67, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Ulubelen, A.; Birman, H.; Öksüz, S.; Topçu, G.; Kolak, U.; Barla, A.; Voelter, W. Cardioactive Diterpenes from the Roots of Salvia eriophora. Planta Med. 2002, 68, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cao, J.; Wang, X.; Zhang, Y.; Sun, Q.; Jiang, Y.; Yao, J.; Li, C.; Wang, Y.; Wang, W. Ferruginol Restores SIRT1-PGC-1α-Mediated Mitochondrial Biogenesis and Fatty Acid Oxidation for the Treatment of DOX-Induced Cardiotoxicity. Front. Pharmacol. 2021, 12, 773834. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, G.; Ding, D.; Li, F.; Zhao, X.; Wang, J.; Yang, Y. Ferruginol Prevents Degeneration of Dopaminergic Neurons by Enhancing Clearance of α-Synuclein in Neuronal Cells. Fitoterapia 2022, 156, 105066. [Google Scholar] [CrossRef]
- Luo, G.; Zhou, J.; Li, G.; Hu, N.; Xia, X.; Zhou, H. Ferruginol Diterpenoid Selectively Inhibits Human Thyroid Cancer Growth by Inducing Mitochondrial Dependent Apoptosis, Endogenous Reactive Oxygen Species (ROS) Production, Mitochondrial Membrane Potential Loss and Suppression of Mitogen-Activated Protein. Med. Sci. Monit. 2019, 25, 2935–2942. [Google Scholar] [CrossRef]
- Ho, S.-T.; Tung, Y.-T.; Kuo, Y.-H.; Lin, C.-C.; Wu, J.-H. Ferruginol Inhibits Non–Small Cell Lung Cancer Growth by Inducing Caspase-Associated Apoptosis. Integr. Cancer Ther. 2015, 14, 86–97. [Google Scholar] [CrossRef]
- Kuźma, L.; Bruchajzer, E.; Wysokińska, H. Diterpenoid Production in Hairy Root Culture of Salvia sclarea L. Z. Nat. C J. Biosci. 2008, 63, 621–624. [Google Scholar] [CrossRef]
- Ma, X.-H.; Ma, Y.; Tang, J.-F.; He, Y.-L.; Liu, Y.-C.; Ma, X.-J.; Shen, Y.; Cui, G.-H.; Lin, H.-X.; Rong, Q.-X.; et al. The Biosyn-thetic Pathways of Tanshinones and Phenolic Acids in Salvia Miltiorrhiza. Molecules 2015, 20, 16235–16254. [Google Scholar] [CrossRef] [Green Version]
- Vaccaro, M.C.; Alfieri, M.; De Tommasi, N.; Moses, T.; Goossens, A.; Leone, A. Boosting the Synthesis of Pharmaceutically Active Abietane Diterpenes in S. sclarea Hairy Roots by Engineering the GGPPS and CPPS Genes. Front. Plant Sci. 2020, 11, 924. [Google Scholar] [CrossRef]
- Vaccaro, M.; Bernal, V.O.; Malafronte, N.; De Tommasi, N.; Leone, A. High Yield of Bioactive Abietane Diterpenes in Salvia sclarea Hairy Roots by Overexpressing Cyanobacterial DXS or DXR Genes. Planta Med. 2019, 85, 973–980. [Google Scholar] [CrossRef] [Green Version]
- Alfieri, M.; Vaccaro, M.C.; Cappetta, E.; Ambrosone, A.; De Tommasi, N.; Leone, A. Coactivation of MEP-biosynthetic genes and accumulation of abietane diterpenes in Salvia sclarea by heterologous expression of WRKY and MYC2 transcription factors. Sci. Rep. 2018, 8, 11009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an Effective Strategy for the Biotechnological Production of Bioactive High-Added Value Compounds in Plant Cell Factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef] [PubMed]
- Halder, M.; Sarkar, S.; Jha, S. Elicitation: A biotechnological tool for enhanced production of secondary metabolites in hairy root cultures. Eng. Life Sci. 2019, 19, 880–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, X.; Jin, L.; Shimada, M.; Kim, M.G.; Mackey, D. The phytotoxin coronatine is a multifunctional component of the virulence armament of Pseudomonas syringae. Planta 2014, 240, 1149–1165. [Google Scholar] [CrossRef] [Green Version]
- Durand, A.N.; Pauwels, L.; Goossens, A. The Ubiquitin System and Jasmonate Signaling. Plants 2016, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Ho, T.-T.; Murthy, H.N.; Park, S.-Y. Methyl Jasmonate Induced Oxidative Stress and Accumulation of Secondary Metabolites in Plant Cell and Organ Cultures. Int. J. Mol. Sci. 2020, 21, 716. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Wang, H.; Yang, J.; Deng, K.; Wang, T. RNA sequencing on Amomum villosum Lour. induced by MeJA identifies the genes of WRKY and terpene synthases involved in terpene biosynthesis. Genome 2018, 61, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Hampel, D.; Mosandl, A.A.; Wüst, M. Induction of de Novo Volatile Terpene Biosynthesis via Cytosolic and Plastidial Pathways by Methyl Jasmonate in Foliage of Vitis vinifera L. J. Agric. Food Chem. 2005, 53, 2652–2657. [Google Scholar] [CrossRef]
- Martin, D.M.; Gershenzon, J.; Bohlmann, J. Induction of Volatile Terpene Biosynthesis and Diurnal Emission by Methyl Jasmonate in Foliage of Norway Spruce. Plant Physiol. 2003, 132, 1586–1599. [Google Scholar] [CrossRef] [Green Version]
- Vaccaro, M.C.; Mariaevelina, A.; Malafronte, N.; De Tommasi, N.; Leone, A. Increasing the Synthesis of Bioactive Abietane Diterpenes in Salvia sclarea Hairy Roots by Elicited Transcriptional Reprogramming. Plant Cell Rep. 2017, 36, 375–386. [Google Scholar] [CrossRef]
- Wang, Q.; Quan, S.; Xiao, H. Towards efficient terpenoid biosynthesis: Manipulating IPP and DMAPP supply. Bioresour. Bioprocess. 2019, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Shi, M.; Luo, X.; Ju, G.; Li, L.; Huang, S.; Zhang, T.; Wang, H.; Kai, G. Enhanced Diterpene Tanshinone Accumulation and Bioactivity of Transgenic Salvia miltiorrhiza Hairy Roots by Pathway Engineering. J. Agric. Food Chem. 2016, 64, 2523–2530. [Google Scholar] [CrossRef] [PubMed]
- Scheler, U.; Brandt, W.; Porzel, A.; Rothe, K.; Manzano, D.; Božić, D.; Papaefthimiou, D.; Balcke, G.U.; Henning, A.; Lohse, S.; et al. Elucidation of the biosynthesis of carnosic acid and its reconstitution in yeast. Nat. Commun. 2016, 7, 12942. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.J. Two rings in them all: The labdane-related diterpenoids. Nat. Prod. Rep. 2010, 27, 1521–1530. [Google Scholar] [CrossRef] [Green Version]
- Xing, B.; Yang, D.; Liu, L.; Han, R.; Sun, Y.; Liang, Z. Phenolic acid production is more effectively enhanced than tanshinone production by methyl jasmonate in Salvia miltiorrhiza hairy roots. Plant Cell Tissue Organ Cult. (PCTOC) 2018, 134, 119–129. [Google Scholar] [CrossRef]
- Zhou, W.; Huang, Q.; Wu, X.; Zhou, Z.; Ding, M.; Shi, M.; Huang, F.; Li, S.; Wang, Y.; Kai, G. Comprehensive transcriptome profiling of Salvia miltiorrhiza for discovery of genes associated with the biosynthesis of tanshinones and phenolic acids. Sci. Rep. 2017, 7, 10554. [Google Scholar] [CrossRef] [Green Version]
- Akhgari, A.; Laakso, I.; Maaheimo, H.; Choi, Y.H.; Seppänen-Laakso, T.; Oksman-Caldentey, K.-M.; Rischer, H. Methyljasmonate Elicitation Increases Terpenoid Indole Alkaloid Accumulation in Rhazya stricta Hairy Root Cultures. Plants 2019, 8, 534. [Google Scholar] [CrossRef] [Green Version]
- Shoji, T.; Kajikawa, M.; Hashimoto, T. Clustered Transcription Factor Genes Regulate Nicotine Biosynthesis in Tobacco. Plant Cell 2010, 22, 3390–3409. [Google Scholar] [CrossRef] [Green Version]
- Xiang, L.; Zhu, S.; Zhao, T.; Zhang, M.; Liu, W.; Chen, M.; Lan, X.; Liao, Z. Enhancement of artemisinin content and relative expression of genes of artemisinin biosynthesis in Artemisia annua by exogenous MeJA treatment. Plant Growth Regul. 2015, 75, 435–441. [Google Scholar] [CrossRef]
- Lu, X.; Tang, K.; Li, P. Plant Metabolic Engineering Strategies for the Production of Pharmaceutical Terpenoids. Front. Plant Sci. 2016, 7, 1647. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Wang, Y.; Xing, J.; Zhang, Y.; Duan, L.; Zhang, M.; Li, Z. Coronatine Modulated the Generation of Reactive Oxygen Species for Regulating the Water Loss Rate in the Detaching Maize Seedlings. Agriculture 2021, 11, 685. [Google Scholar] [CrossRef]
- Singh, P.; Arif, Y.; Siddiqui, H.; Hayat, S. Jasmonate: A versatile messenger in plants. In Jasmonates and Salicylates Signaling in Plants; Aftab, T., Yusuf, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 129–158. ISBN 978-3-030-75805-9. [Google Scholar]
- Barone, R.P.; Knittel, D.K.; Ooka, J.K.; Porter, L.N.; Smith, N.T.; Owens, D.K. The production of plant natural products beneficial to humanity by metabolic engineering. Curr. Plant Biol. 2020, 24, 100121. [Google Scholar] [CrossRef]
- Tian, S.; Wang, D.; Yang, L.; Zhang, Z.; Liu, Y. A systematic review of 1-Deoxy-D-xylulose-5-phosphate synthase in terpenoid biosynthesis in plants. Plant Growth Regul. 2021, 96, 221–235. [Google Scholar] [CrossRef]
- Carretero-Paulet, L.; Cairó, A.; Botella-Pavía, P.; Besumbes, O.; Campos, N.; Boronat, A.; Rodríguez-Concepción, M. Enhanced flux through the methylerythritol 4-phosphate pathway in Arabidopsis plants overexpressing deoxyxylulose 5-phosphate reductoisomerase. Plant Mol. Biol. 2006, 62, 683–695. [Google Scholar] [CrossRef]
- Estévez, J.M.; Cantero, A.; Reindl, A.; Reichler, S.; León, P. 1-Deoxy-d-xylulose-5-phosphate Synthase, a Limiting Enzyme for Plastidic Isoprenoid Biosynthesis in Plants. J. Biol. Chem. 2001, 276, 22901–22909. [Google Scholar] [CrossRef] [Green Version]
- Kai, G.; Xu, H.; Zhou, C.; Liao, P.; Xiao, J.; Luo, X.; You, L.; Zhang, L. Metabolic engineering tanshinone biosynthetic pathway in Salvia miltiorrhiza hairy root cultures. Metab. Eng. 2011, 13, 319–327. [Google Scholar] [CrossRef]
- Peebles, C.A.; Sander, G.W.; Hughes, E.H.; Peacock, R.; Shanks, J.V.; San, K.-Y. The expression of 1-deoxy-d-xylulose synthase and geraniol-10-hydroxylase or anthranilate synthase increases terpenoid indole alkaloid accumulation in Catharanthus roseus hairy roots. Metab. Eng. 2011, 13, 234–240. [Google Scholar] [CrossRef]
- Vranová, E.; Coman, D.; Gruissem, W. Network Analysis of the MVA and MEP Pathways for Isoprenoid Synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef]
- Boya, M.T.; Valverde, S. An orthoquinone isolated from Salvia aethiopis. Phytochemistry 1981, 20, 1367–1368. [Google Scholar] [CrossRef]
- Zi, J.; Peters, R.J. Characterization of CYP76AH4 clarifies phenolic diterpenoid biosynthesis in the Lamiaceae. Org. Biomol. Chem. 2013, 11, 7650–7652. [Google Scholar] [CrossRef] [Green Version]
- Božić, D.; Papaefthimiou, D.; Brückner, K.; De Vos, R.C.H.; Tsoleridis, C.A.; Katsarou, D.; Papanikolaou, A.; Pateraki, I.; Chatzopoulou, F.M.; Dimitriadou, E.; et al. Towards Elucidating Carnosic Acid Biosynthesis in Lamiaceae: Functional Characterization of the Three First Steps of the Pathway in Salvia Fruticosa and Rosmarinus Officinalis. PLoS ONE 2015, 10, e0124106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, P.; Tong, Y.; Cheng, Q.; Hu, Y.; Zhang, M.; Yang, J.; Teng, Z.; Gao, W.; Huang, L. Functional characterization of ent-copalyl diphosphate synthase, kaurene synthase and kaurene oxidase in the Salvia miltiorrhiza gibberellin biosynthetic pathway. Sci. Rep. 2016, 6, 23057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, L.; Ni, X.; Ji, Q.; Teng, X.; Yang, Y.; Wu, C.; Zekria, D.; Zhang, D.; Kai, G. Co-overexpression of geraniol-10-hydroxylase and strictosidine synthase improves anti-cancer drug camptothecin accumulation in Ophiorrhiza pumila. Sci. Rep. 2015, 5, 08227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strader, L.; Weijers, D.; Wagner, D. Plant transcription factors—Being in the right place with the right company. Curr. Opin. Plant Biol. 2022, 65, 102136. [Google Scholar] [CrossRef]
- Wani, S.H.; Anand, S.; Singh, B.; Bohra, A.; Joshi, R. WRKY transcription factors and plant defense responses: Latest discoveries and future prospects. Plant Cell Rep. 2021, 40, 1071–1085. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Pang, S.; Lu, Z.; Jin, B. Function and Mechanism of WRKY Transcription Factors in Abiotic Stress Responses of Plants. Plants 2020, 9, 1515. [Google Scholar] [CrossRef]
- Yu, H.; Guo, W.; Yang, D.; Hou, Z.; Liang, Z. Transcriptional Profiles of SmWRKY Family Genes and Their Putative Roles in the Biosynthesis of Tanshinone and Phenolic Acids in Salvia miltiorrhiza. Int. J. Mol. Sci. 2018, 19, 1593. [Google Scholar] [CrossRef] [Green Version]
- Kazan, K.; Manners, J.M. MYC2: The Master in Action. Mol. Plant 2013, 6, 686–703. [Google Scholar] [CrossRef] [Green Version]
- Goossens, J.; Fernández-Calvo, P.; Schweizer, F.; Goossens, A. Jasmonates: Signal transduction components and their roles in environmental stress responses. Plant Mol. Biol. 2016, 91, 673–689. [Google Scholar] [CrossRef]
- De Geyter, N.; Gholami, A.; Goormachtig, S.; Goossens, A. Transcriptional machineries in jasmonate-elicited plant secondary metabolism. Trends Plant Sci. 2012, 17, 349–359. [Google Scholar] [CrossRef]
- Sabzehzari, M.; Zeinali, M.; Naghavi, M.R. CRISPR-based metabolic editing: Next-generation metabolic engineering in plants. Gene 2020, 759, 144993. [Google Scholar] [CrossRef] [PubMed]
- Selma, S.; Orzáez, D. Perspectives for epigenetic editing in crops. Transgenic Res. 2021, 30, 381–400. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).