Abstract
In this study, the impact of the apiculate yeast Hanseniaspora occidentalis as a co-partner with Saccharomyces cerevisiae was investigated in a sequential-type mixed-culture fermentation of Muscaris grape must. As with other fermentation trials using Hanseniaspora strains, a significant increase in ethyl acetate was observed, but most intriguing was the almost complete abolition of malic acid (from 2.0 g/L to 0.1 g/L) in the wine. Compared to the pure S. cerevisiae inoculum, there was also a marked increase in the concentrations of the other acetate esters. Modulation of some of the varietal elements, such as rose oxide, was also observed. This work shows the promising use of H. occidentalis in a mixed-culture must fermentation, especially in the acid modulation of fruit juice matrices.
1. Introduction
Species belonging to the genus Hanseniaspora are important yeast in food and beverage production, as they are common isolates on ripe fruits. This is especially the case in viticulture where they are often the most dominant yeast isolated from mature grape berries [1]. This implies that they would play an important role at the onset of must fermentations and could affect the nutrient availability for fermenting yeast such as Saccharomyces cerevisiae but could also make meaningful contributions to the final wine aroma composition. A typical feature of Hanseniaspora activity within a fermentation is an increase in ethyl acetate levels that imparts a nail polish-like aroma, which is the main reason this genus is considered a contaminant when it persists within a fermentation [2]. Most Hanseniaspora spp. are not considered good fermenters, and their numbers drop significantly in the first couple of days in both inoculated and spontaneous fermentations [3,4]. Although not well understood, the current thinking is that so-called “killer factors”, and not increasing alcohol levels produced by Saccharomyces cerevisiae strains, are responsible for the rapid decline in the Hanseniaspora population [5,6].
In order to diversify the flavor profiles of wines, the deliberate use of non-Saccharomyces strains (NSYs), including members of the Hanseniaspora genus, has enjoyed tremendous interest in the past two decades [7]. This culminated in the development of many commercial non-Saccharomyces starter cultures [8]. Within the Hanseniaspora genus, two species in particular, namely, Hanseniaspora osmophila and Hanseniaspora vineae, have been extensively assessed as potential co-partners with S. cerevisiae in fermented beverages [9,10,11,12,13,14,15,16,17,18]. Although H. osmophila and H. vineae additions also tend to lead to higher levels of ethyl acetate and acetic acid in the final product, an outstanding characteristic is the substantial increase in benzenoids, like phenylethanol and phenethyl acetate levels, which impart a floral or honey aroma to a beverage.
Papers published in the past couple of years, exploring the genomic content of Hanseniaspora, underline the growing interest in this genus [19,20]. An instructive survey on the genomic make-up of the Hanseniaspora genus divided species into two lineages based on gene loss frequency and their evolutionary rate at their stem branch, namely the “fast-evolving” lineage, which includes the two most commonly isolated Hanseniaspora spp. from viticultural settings, namely, Hanseniaspora uvarum and Hanseniaspora guilliermondii, and the “slow-evolving” lineage [21]. H. osmophila and H. vineae are both grouped into the slow-evolving lineage along with another species, namely, H. occidentalis. Although many trials were conducted evaluating both H. osmophila and H. vineae in must fermentations, little is known on how the intentional use of H. occidentalis would affect the final wine composition. H. occidentalis is also a common isolate in vitivinicultural settings, including on fruit flies that often populate vineyards [1,22]. In this study, we explore the impact of H. occidentalis as a co-partner for S. cerevisiae in the fermentation of a terpene-rich white grape variety, Muscaris.
2. Materials and Methods
2.1. Strains Used
The S. cerevisiae wine strain Viniferm Revelacíon (Agrovin S.A., Alcázar de San Juan, Spain) was used in the study. A Hanseniaspora occidentalis 211 strain (from the Geisenheim yeast breeding culture collection) was initially identified using Fourier-transform infrared spectroscopy [23] and subsequently verified using Sanger sequencing of the D1D2 domain of the 28S rRNA subunit and the internal transcribed spacer region (sequencing conducted by Starseq GmbH, Mainz, Germany).
2.2. Must Fermentation
Muscaris grape must from the 2020 vintage was used as starting material. It was pasteurized by heating the must at 85 °C for 45 s, followed by a 10 min heating at 76 °C degrees. It had a total sugar content of 243.0 g/L (116.8 g/L glucose and 126.2 g/L fructose). The malic acid concentration was 2.0 g/L. Its free primary amino acid content, determined using the o-phthaldialdehyde (NOPA) method, was 50.7 mg/L. Its free ammonium content, as measured using the Rapid Ammonium kit from Megazyme (Bray, Ireland), was 49.0 mg/L, which amounted to a yeast available nitrogen (YAN) content of 99.7 mg/L. No additional nutrient sources were added to the must. The H. occidentalis strain was cultured overnight at 30 °C in YEPD media (10 g/L yeast extract, 20 g/L peptone, 20 g/L glucose), spun down, and resuspended in phosphate buffered saline. A concentration of 1 × 107 cells/mL (as estimated by a haemocytometer) was used to inoculate 100 mL of Muscaris must. The fermentation vessels were 100 mL Schott Duran® bottles and were only covered with aluminum foil for the first three days (prior to S. cerevisiae addition) and incubated at 22 °C. This is to allow for standard air flow during the first three days, as H. occidentalis displays minimal fermentative activity under strict anaerobic conditions. A S. cerevisiae wine strain, Viniferm Revelacíon (Agrovin S.A., Alcázar de San Juan, Spain), was added after three days of incubation at a cell concentration of 1 × 106 cells/mL, and the Schott Duran® bottles were sealed with airlocks filled with approximately 3 mL of water. Bottles were weighed daily, and once no weight loss was recorded over a period of one day, the fermentation was deemed finished, and samples were prepared for gas-chromatography-mass spectrometry (GC-MS), high-performance liquid chromatography (HPLC), and spectrophotometric analyses. The same must was also used for a subsequent fermentation using only H. occidentalis, with additions of different concentrations of malic acid (6.2, 14.4, and 17.1 g/L). Fermentations were incubated as described above without adding airlocks, and samples were taken on days 3 and 5 and used for HPLC analysis.
2.3. Analysis of the Wines
The composition of the wines was analyzed following the standard operating procedures established by the analysis team at the Department of Microbiology and Biochemistry, Geisenheim University. For the analysis of the initial and residual sugars, the final ethanol and glycerol concentrations, and the major organic acids of the fermentation samples, high-performance liquid chromatography was employed with a method described previously [24]. The HPLC analysis was done with a 1260 Infinity II LC system (Agilent Technologies GmbH, Waldbronn, Germany), and the flow rate was 0.5 mL/min. The concentrations of commonly-found terpenes and norisoprenoids in wine, as well as the major higher alcohols, medium-chain fatty acids, ethyl esters, and acetate esters, were determined using headspace gas chromatography coupled with mass spectrometry, as described previously [25,26,27]. For determining H2S in the wines, headspace gas chromatography coupled with pulsed-flame photometric detection was implemented using a method described previously [26]. Acetaldehyde content was measured using the Acetaldehyde Assay Kit from Megazyme (Bray, Ireland). An Evolution 220 spectrophotometer (Thermo Scientific, Dreieich, Germany) was used in determining the acetaldehyde concentrations. A Multical pH 526 pH/mV-meter (WTW, Weilheim, Germany) was used to determine the pH of the final wines. The pH meter was calibrated at pH 4.0, 7.0, and 10.0.
2.4. Statistical Analysis
The fermentations were conducted in triplicate (n = 3). Statistical analyses and graphical representations were computed using R version 4.0.3 [28]. Mean values and standard deviation were calculated. For all the tests, a significancy threshold of α = 0.05 was chosen. The assumption of homogeneity of variances was tested with the Levene test from the car package [29]. Group means were tested with a two-tailed t-test under the null hypothesis of no significant differences. For heteroscedastic observations, the Wilcoxon rank-sum test was preferred.
3. Results and Discussion
As expected, a pure inoculation of H. occidentalis did not result in a complete fermentation of the Muscaris must, as approximately 188 ± 4 g/L of total sugar were still present at the end of the fermentation. Interestingly, only 4 g/L of fructose was utilized during the fermentation duration of 25 days by the pure inoculation of H. occidentalis. A previous study also showed H. occidentalis had a higher consumption rate of glucose to fructose (in minimal media), but not to the same extent as shown here [30]. This could allude to strain heterogeneity regarding sugar preference.
Based on the weight-loss curves (Figure 1), inoculation of H. occidentalis had little to no effect on the fermentative behavior of the Revelacíon wine yeast. This is in concurrence with other mixed-culture fermentations using Hanseniaspora spp., where no noteworthy effect on the fermentative capability of S. cerevisiae was observed [31].
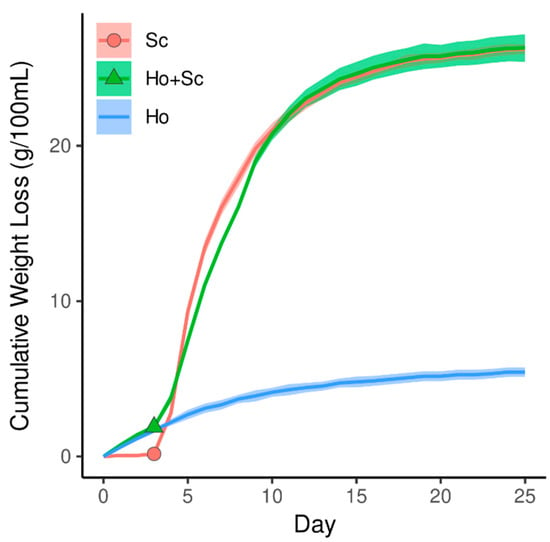
Figure 1.
Cumulative weight loss of the Hanseniaspora occidentalis-initiated fermentations. After three days, Saccharomyces cerevisiae was added to three bottles inoculated with H. occidentalis and three uninoculated as indicated by points on the weight-loss curve. Continuous lines represent the mean value of three fermentation replicates, and the shaded area represents their standard deviation.
With the analysis of the chemical composition of the Muscaris wine, clear differences were seen with the addition of H. occidentalis (Table 1, Table 2 and Table 3). Table 1 shows the main parameters of the wines and, interestingly, an almost complete consumption of the malic acid was observed with the addition of H. occidentalis. A slight but significant increase in acetic acid levels was also measured—a feature of Hanseniaspora additions. The modulations of these acid components could explain the significant deacidification of the wine.

Table 1.
Parameters of the wines after fermentation.

Table 2.
Quantitative aroma composition of the wines.

Table 3.
Terpene and norisoprenoid composition of the wines.
Table 2 shows the main aroma constituents of the wines. A three-fold increase in ethyl acetate was observed with the addition of H. occidentalis, which is 25-times more than its odor threshold of 12.3 mg/L, as determined in red wines [27].
The fruity esters, namely isoamyl acetate and active amyl acetate, were higher in the H. occidentalis-initiated samples, yet their amounts are below the significance threshold of the t-test, likely due to a large variance in the measured concentrations. As with H. osmophila and H. vineae-initiated fermentations, an increase in 2-phenylethyl acetate was measured. An interesting observation was the two-fold increase in the ethyl ester ethyl propionate, an aroma compound imparting a pineapple-like aroma, although none of the other ethyl esters were significantly improved.
Muscaris is a fungal-resistant white grape variety relative of the Muscat family of grapes known to be rich in terpenes. As all members of the Hanseniaspora genus are capable of growing on cellobiose [32], suggestive of β-glucosidase action, their use in a terpene-rich must could enable more release of terpenes bound to sugar moieties. The addition of H. occidentalis did lead to a significant increase of both species of rose-oxide (Table 3), but no other terpenes were meaningfully affected. Whether the increase in rose-oxide was caused by releasing action or by the modification of non-sugar precursors remains unclear [33].
In a subsequent fermentation, we tested the ability of H. occidentalis to consume malic acid at different starting concentrations. H. occidentalis was inoculated with different versions of the same must with increased amounts of malic acid (2.0–17.1 g/L), and the consumption of this acid was assessed after three and five days (Figure 2). H. occidentalis removes the majority of malic acid after three days of fermentation: in the lower starting concentration of 2.0 g/L, 88% was removed from the must. From the must containing 17.1 g/L of malic acid, H. occidentalis consumed 65% and 73% after three and five days, respectively.
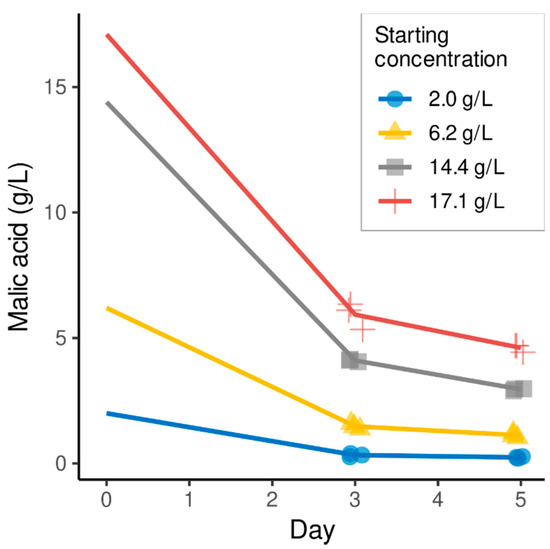
Figure 2.
Short Muscaris must fermentations inoculated with H. occidentalis containing additions of malic acid. Malic acid concentrations were determined after three and five days of incubation.
Development of acidity as the grape berry matures is an involved process further complicated by rapid climate change, leading to unpredictable yields and thus unexpected acid profiles of must [34]. It is well-known that grapes harvested from cooler climates tend to accumulate more malic acid [35], imparting a tartness to a wine not often desired by consumers. To remove excess malic acid from wine, winemakers generally employ three methods: (i) physical methods which include blending and/or amelioration; (ii) chemical methods by adding bicarbonates; or (iii) biological methods by adding yeast or bacteria [36,37]. This is because wine S. cerevisiae strains lack a dedicated malate transporter and, with its malic enzyme being located in the mitochondria, cannot effectively remove malic acid from must or wine matrices [38]. Lactic acid bacteria, especially Oenococcus oeni, are widely used for converting malic acid to lactic acid, known as malolactic fermentation [39]. Despite their widescale use, it should be noted that lactic acid bacteria are chiefly responsible for the increased biogenic amines levels in wine, which can have several adverse impacts on human health [40]. Schizosaccharomyces pombe or Zygosaccharomyces spp. are shown in literature to effectively remove malic acid due to malo-ethanolic fermentation [41,42]. A genetically modified S. cerevisiae containing the malate transporter of S. pombe and the malic enzyme of O. oeni was shown to be effective in conducting malolactic fermentation without the addition of lactic acid bacteria [43]. Commercial applications of the abovementioned yeasts are still limited, due to the excess production of acetaldehyde and acetic acid for both non-Saccharomyces yeast species and the limitations of wide-spread use of genetically modified yeast in winemaking.
This work is, to our knowledge, the first report of an apiculate yeast showing meaningful consumption of malic acid in an oenological setting, and it warrants further exploration. Apart from wine, this feature of H. occidentalis could be useful for the fermentations of other fruit musts where the malic acid content is much higher, such as in apple (ranging from 3–7 g/L) or sweet cherries (which can reach 15 g/L of malic acid) [44]. Further work should include exploring strategies that could mitigate the ethyl acetate production by this species. It has been shown that a simultaneous inoculation of Hanseniaspora uvarum and S. cerevisiae co-partners instead of a sequential inoculation could lead to a dramatically different aroma profile, including less ethyl acetate [45]. It has been shown that S. cerevisiae strains respond very differently to H. uvarum in a must fermentation, resulting in distinctive aroma profiles [6], thereby implying that exploring different combinations of wine strains of S. cerevisiae with H. occidentalis might lead to an ideal pairing. It would also be useful to evaluate intraspecies variation of H. occidentalis, specifically regarding its acid modulation and ester production.
As more genomic information becomes available on this species, identifying the genetic determinants driving its interesting oenological features (i.e., its malic acid metabolism and acetate ester production) could lead the way in providing insights on the biology of this unexplored apiculate yeast.
Author Contributions
Conceptualization, N.v.W. and C.v.W.; methodology, N.v.W.; formal analysis, S.S., B.B., S.B., S.F. and H.S.; investigation, N.v.W.; resources, I.S.P., D.R. and C.v.W.; data curation, S.S.; writing—original draft preparation, N.v.W.; writing—review and editing, N.v.W., S.S., I.S.P., D.R. and C.v.W.; visualization, S.S.; supervision, I.S.P., D.R. and C.v.W.; project administration, C.v.W.; funding acquisition, C.v.W. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank Geisenheim University and Macquarie University for co-funding of this project and the research fellowship of N.v.W. The authors also thank the Hesse State Ministry of Higher Education, Research and the Arts for the financial support within the Doctoral Platform Geisenheim–Gießen–Marburg (https://www.promotionsplattform-ggm.de/research-projects/joint-projects/current-projects—accessed on 12 May 2022) and the Hesse initiative for scientific and economic excellence (LOEWE) in the framework of AROMAplus (https://www.hs-geisenheim.de/aromaplus/—accessed on 12 May 2022). I.S.P. is a team member of the Macquarie-led national Centre of Excellence in Synthetic Biology funded by the Australian Government thorough its agency, the Australian Research Council.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
Kerstin Zimmer, Georgia Tylaki, and Khavar Feyzullayeva (Hochschule Geisenheim University) are thanked for their technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Drumonde-Neves, J.; Fernandes, T.; Lima, T.; Pais, C.; Franco-Duarte, R. Learning from 80 years of studies: A comprehensive catalogue of non-Saccharomyces yeasts associated with viticulture and winemaking. FEMS Yeast Res. 2021, 21, foab017. [Google Scholar] [CrossRef] [PubMed]
- Malfeito-Ferreira, M. Yeasts and wine off-flavours: A technological perspective. Ann. Microbiol. 2011, 61, 95–102. [Google Scholar] [CrossRef]
- Mendoza, L.M.; De Nadra, M.C.M.; Farías, M.E. Kinetics and metabolic behavior of a composite culture of Kloeckera apiculata and Saccharomyces cerevisiae wine related strains. Biotechnol. Lett. 2007, 29, 1057–1063. [Google Scholar] [CrossRef]
- Egli, C.M.; Edinger, W.D.; Mitrakul, C.M.; Henick-Kling, T. Dynamics of indigenous and inoculated yeast populations and their effect on the sensory character of Riesling and Chardonnay wines. J. Appl. Microbiol. 1998, 85, 779–789. [Google Scholar] [CrossRef]
- Wang, C.; Mas, A.; Esteve-Zarzoso, B. The interaction between Saccharomyces cerevisiae and non-Saccharomyces yeast during alcoholic fermentation is species and strain specific. Front. Microbiol. 2016, 7, 502. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, K.; Xu, Y.; Mei, W.; Tao, Y. Biomass suppression of Hanseniaspora uvarum by killer Saccharomyces cerevisiae highly increased fruity esters in mixed culture fermentation. LWT 2020, 132, 109839. [Google Scholar] [CrossRef]
- van Wyk, N.; Grossmann, M.; Wendland, J.; von Wallbrunn, C.; Pretorius, I.S. The whiff of wine yeast innovation: Strategies for enhancing aroma production by yeast during wine fermentation. J. Agric. Food Chem. 2019, 67, 13496–13505. [Google Scholar] [CrossRef]
- van Wyk, N.; von Wallbrunn, C.; Swiegers, J.H.; Pretorius, I.S. Biotechnology of wine yeasts. In Encyclopedia of Mycology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 34–44. ISBN 9781498740814. [Google Scholar]
- Viana, F.; Belloch, C.; Vallés, S.; Manzanares, P. Monitoring a mixed starter of Hanseniaspora vineae-Saccharomyces cerevisiae in natural must: Impact on 2-phenylethyl acetate production. Int. J. Food Microbiol. 2011, 151, 235–240. [Google Scholar] [CrossRef]
- Viana, F.; Gil, J.V.; Vallés, S.; Manzanares, P. Increasing the levels of 2-phenylethyl acetate in wine through the use of a mixed culture of Hanseniaspora osmophila and Saccharomyces cerevisiae. Int. J. Food Microbiol. 2009, 135, 68–74. [Google Scholar] [CrossRef]
- Granchi, L.; Ganucci, D.; Messini, A.; Vincenzini, M. Oenological properties of Hanseniaspora osmophila and Kloeckera corticis from wines produced by spontaneous fermentations of normal and dried grapes. FEMS Yeast Res. 2002, 2, 403–407. [Google Scholar] [CrossRef][Green Version]
- Wei, J.; Zhang, Y.; Qiu, Y.; Guo, H.; Ju, H.; Wang, Y.; Yuan, Y.; Yue, T. Chemical composition, sensorial properties, and aroma-active compounds of ciders fermented with Hanseniaspora osmophila and Torulaspora quercuum in co- and sequential fermentations. Food Chem. 2020, 306, 125623. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, B.; Zambelli, P.; Vigentini, I.; Bauer, F.F.; Setati, M.E. Investigating the effect of selected non-Saccharomyces species on wine ecosystem function and major volatiles. Front. Bioeng. Biotechnol. 2018, 6, 169. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, L.M.; Vega-Lopez, G.A.; Fernández de Ullivarri, M.; Raya, R.R. Population and oenological characteristics of non-Saccharomyces yeasts associated with grapes of Northwestern Argentina. Arch. Microbiol. 2019, 201, 235–244. [Google Scholar] [CrossRef] [PubMed]
- González-Robles, I.W.; Estarrón-Espinosa, M.; Díaz-Montaño, D.M. Fermentative capabilities and volatile compounds produced by Kloeckera/Hanseniaspora and Saccharomyces yeast strains in pure and mixed cultures during Agave tequilana juice fermentation. Antonie Van Leeuwenhoek 2015, 108, 525–536. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Aponte, M.; Piombino, P.; Lisanti, M.T.; Moio, L.; Ercolini, D.; Blaiotta, G. Influence of microbial communities on the chemical and sensory features of Falanghina sweet passito wines. Food Res. Int. 2019, 120, 740–747. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, D.; Duan, C.; Yan, G. Synergistic effect enhances 2-phenylethyl acetate production in the mixed fermentation of Hanseniaspora vineae and Saccharomyces cerevisiae. Process Biochem. 2020, 90, 44–49. [Google Scholar] [CrossRef]
- Olivera, V.; Boido, E.; Dellacassa, E. Wine aroma characterization of the two main fermentation yeast species of the apiculate genus Hanseniaspora. Fermentation 2021, 7, 162. [Google Scholar] [CrossRef]
- Sternes, P.R.; Lee, D.; Kutyna, D.R.; Borneman, A.R. Genome sequences of three species of Hanseniaspora isolated from spontaneous wine fermentations. Genome Announc. 2016, 4, e01287-16. [Google Scholar] [CrossRef]
- Saubin, M.; Devillers, H.; Proust, L.; Brier, C.; Grondin, C.; Pradal, M.; Legras, J.L.; Neuvéglise, C. Investigation of genetic relationships between Hanseniaspora species found in grape musts revealed interspecific hybrids with dynamic genome structures. Front. Microbiol. 2020, 10, 2960. [Google Scholar] [CrossRef]
- Steenwyk, J.L.; Opulente, D.A.; Kominek, J.; Shen, X.; Zhou, X.; Labella, A.L.; Bradley, N.P.; Eichman, B.F.; Libkind, D.; Devirgilio, J.; et al. Extensive loss of cell-cycle and DNA repair genes in an ancient lineage of bipolar budding yeasts. PLoS Biol. 2019, 1, e3000255. [Google Scholar] [CrossRef]
- Hoang, D.; Kopp, A.; Chandler, J.A. Interactions between Drosophila and its natural yeast symbionts-Is Saccharomyces cerevisiae a good model for studying the fly-yeast relationship? PeerJ 2015, 3, e1116. [Google Scholar] [CrossRef]
- Grangeteau, C.; Gerhards, D.; Rousseaux, S.; von Wallbrunn, C.; Alexandre, H.; Guilloux-Benatier, M. Diversity of yeast strains of the genus Hanseniaspora in the winery environment: What is their involvement in grape must fermentation? Food Microbiol. 2015, 50, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Mecca, D.; Benito, S.; Beisert, B.; Brezina, S.; Fritsch, S.; Semmler, H.; Rauhut, D. Influence of nutrient supplementation on Torulaspora delbrueckii wine fermentation aroma. Fermentation 2020, 6, 35. [Google Scholar] [CrossRef]
- Brandt, M. The Influence of Abiotic Factors on the Composition of Berries, Juice and Wine in Vitis vinifera L. cv. Riesling. Ph.D. Thesis, Hochschule Geisenheim, Geisenheim, Germany, 2021. [Google Scholar]
- Jung, R.; Kumar, K.; Patz, C.; Rauhut, D.; Tarasov, A.; Schuessler, C. Influence of transport temperature profiles on wine quality. Food Packag. Shelf Life 2021, 29, 100706. [Google Scholar] [CrossRef]
- Scansani, S.; van Wyk, N.; Nader, K.B.; Beisert, B.; Brezina, S.; Fritsch, S.; Semmler, H.; Pasch, L.; Pretorius, I.S.; von Wallbrunn, C.; et al. The film-forming Pichia spp. in a winemaker’s toolbox: A simple isolation procedure and their performance in a mixed-culture fermentation of Vitis vinifera L. cv. Gewürztraminer must. Int. J. Food Microbiol. 2022, 365, 109549. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 12 May 2022).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Ciani, M.; Fatichenti, F. Selective sugar consumption by apiculate yeasts. Lett. Appl. Microbiol. 1999, 28, 203–206. [Google Scholar] [CrossRef]
- Rossouw, D.; Bauer, F.F. Exploring the phenotypic space of non-Saccharomyces wine yeast biodiversity. Food Microbiol. 2016, 55, 32–46. [Google Scholar] [CrossRef]
- Čadež, N.; Smith, M.T. Hanseniaspora Zikes (1912); Elsevier B.V.: Amsterdam, The Netherlands, 2011; Volume 2, ISBN 9780444521491. [Google Scholar]
- Koslitz, S.; Renaud, L.; Kohler, M.; Wüst, M. Stereoselective formation of the varietal aroma compound rose oxide during alcoholic fermentation. J. Agric. Food Chem. 2008, 56, 1371–1375. [Google Scholar] [CrossRef]
- Mira de Orduña, R. Climate change associated effects on grape and wine quality and production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A.; Satora, P.; Sroka, P.; Gojniczek, I. Chemical composition of cool-climate grapes and enological parameters of cool-climate wines. Fruits 2014, 69, 75–86. [Google Scholar] [CrossRef]
- Vilela, A. Biological demalication and deacetification of musts and wines: Can wine yeasts make the wine taste better? Fermentation 2017, 3, 51. [Google Scholar] [CrossRef]
- Volschenk, H.; van Vuuren, H.J.J.; Viljoen-Bloom, M. Malic acid in wine: Origin, function and metabolism during vinification. S. Afr. J. Enol. Vitic. 2006, 27, 123–136. [Google Scholar] [CrossRef]
- Main, G.L.; Threlfall, R.T.; Morris, J.R. Reduction of malic acid in wine using natural and genetically enhanced microorganisms. Am. J. Enol. Vitic. 2007, 3, 341–345. [Google Scholar]
- Knoll, C.; Fritsch, S.; Schnell, S.; Grossmann, M.; Rauhut, D.; Du Toit, M. Influence of pH and ethanol on malolactic fermentation and volatile aroma compound composition in white wines. LWT Food Sci. Technol. 2011, 44, 2077–2086. [Google Scholar] [CrossRef]
- Barbieri, F.; Montanari, C.; Gardini, F.; Tabanelli, G. Biogenic amine production by lactic acid bacteria: A review. Foods 2019, 8, 17. [Google Scholar] [CrossRef]
- Scansani, S.; Rauhut, D.; Brezina, S.; Semmler, H.; Benito, S. The impact of chitosan on the chemical composition of wines fermented with Schizosaccharomyces pombe and Saccharomyces cerevisiae. Foods 2020, 9, 1423. [Google Scholar] [CrossRef]
- Cioch-Skoneczny, M.; Grabowski, M.; Satora, P.; Skoneczny, S.; Klimczak, K. The use of yeast mixed cultures for deacidification and improvement of the composition of cold climate grape wines. Molecules 2021, 26, 2628. [Google Scholar] [CrossRef]
- Volschenk, H.; Viljoen-Bloom, M.; Subden, R.E.; Van Vuuren, H.J.J. Malo-ethanolic fermentation in grape must by recombinant strains of Saccharomyces cerevisiae. Yeast 2001, 18, 963–970. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; Liu, H.; Liu, J.; Jiao, Z. Profiles of sugar and organic acid of fruit juices: A comparative study and implication for authentication. J. Food Qual. 2020, 2020, 7236534. [Google Scholar] [CrossRef]
- Hu, K.; Jin, G.J.; Xu, Y.H.; Tao, Y.S. Wine aroma response to different participation of selected Hanseniaspora uvarum in mixed fermentation with Saccharomyces cerevisiae. Food Res. Int. 2018, 108, 119–127. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).