Featured Application
This work could lay the foundation for future hop breeding research; moreover, the results of this research answers to the brewers’ question of how territory, in this case the Corsican territory, influences aromatic and morphological characters of cultivars.
Abstract
Hops (Humulus lupulus L.) is a species that grows spontaneously in Corsica, but the characterization of this species in this territory has not yet been investigated. The main objectives of this study are to explore the features of wild hops from Corsica and to determine the effect of the island terroir on some cultivars in the first year of growth. A multidisciplinary approach consisting of the genetic analysis, morphological comparison and chemical characterization of essential oils was carried out on four wild Corsican hops and three hop cultivars grown in Tettnang, Germany and Corsica, France. The morphological and GC-MS analysis of Corsican wild hops, set cluster coastal samples apart from the one far from the coast. This dissimilarity is supported by the SSR analysis by two of the three coastal accessions. The genetics demonstrate a proximity between the European noble cultivar Tettnanger and the mountain Corsican wild hop from Corte. The morphological comparison between German hops cultivated in Tettnang and in Corsican soil, and the GC-MS characterization of their essential oils’ chemical profiles, show different features between year 0 and year +1 for each sample. This multidisciplinary approach highlights an acclimatization of hop cultivars to the Corsican terroir one year after planting.
1. Introduction
Hops (Humulus lupulus L.) have been used in beer brewing since the 13th century for their aroma, bitterness, and preservative properties. The volatile compounds of hops and the chemical variability of essential oils have generated interest in the brewery industry. Several studies have focused on their chemical characterization by GC-MS analysis. Usually, the main constituents are myrcene, humulene and caryophyllene [1,2,3,4,5,6]. To our knowledge there are five botanical varieties of hops (var. lupulus, -cordifolius, -neomexicanus, -pubescens and -lupuloides) [7]. So far, only var. lupulus grows spontaneously in Europe. These botanical varieties differ in their chemical composition, genetics, and morphology. For plants, and specifically for hop genotypes differentiation, it is possible to use morphological, chemical and or genetic markers [8,9,10,11,12,13]. The use of morphological or chemical markers alone make the differentiation among the studied genotypes not simple because of the small differences often present within the species, and because morphological and chemical characters are often dependent on biotic and abiotic factors and on plant age [14,15,16,17,18,19]. Several studies differentiate hop varieties by observing the ratio between antioxidants and aromatic compounds; Kralj and coworkers [4] observed that the α humulene/β caryophyllene ratio is a varietal trait used as a marker because it is independent from ripeness and storage, and it is characteristic of each variety. Instead, the genetic study allows the differentiation among genotypes that could present the same morphological or chemical characters, and allows the recognition and discrimination of genotypes grown in different conditions or plant age; DNA analyses are able to reveal variations in genomic sequence with frequencies over 1% among individuals of the same species [20]. Among the molecular markers, SSRs are relevant and effective tools to determine genetic differences within plant genotypes [20,21,22,23,24,25,26,27,28]. Patzak and collaborators [23] used nine SSR makers and three STS to genetically characterize 136 hop genotypes from Czech Republic, France, Switzerland, the Caucasus Region, Canada, the U.S.A. and five world cultivars, highlighting the genetic differences between American and European genotypes. In the study of Rodolfi and coworkers [27], nine SSR markers were successfully used to obtain the genetic structure of a population of 123 Italian wild hop samples and commercial cultivars, highlighting, in particular, the marked genetic differences between wild and cultivated hops. In Corsica, the characterization of the wild hop biodiversity was never studied before, and it is a priority for a sector, such as brewing in the continuous expansion and interest in peculiar new varieties. The most widely cultivated varieties of the current crops were selected using conventional methods [29], and the level of biodiversity of the starting material is the base for a successful plant selection. In hops, wild material was usually used for the most important varieties cultivated today, providing resistance to diseases or high percentages of alpha acids and peculiar aromas [30]. Hops, around the globe, is distributed between the 35 and 55° parallel [31], and in Corsica (43° parallel North), wild hop is naturally present in riverbeds, such as the Restonica, in river mouths near Ajaccio and Bastia, and in northern sandy clay soils. Corsica could be a new place for hop cultivation. All the environmental and territory effects on crop or the final product, biotic and abiotic factors, including climate, soil composition, living environment ecosystem, cultivar, but also human factors, such as agronomic practices, constitute the terroir concept [32]. A study on the influence of territory on hop field performances, made by Rodolfi and collaborators [27], evaluated the different performances of Cascade cultivar growth in 13 growing areas in Italy, Slovenia, Germany, Michigan and Ohio, with particular attention given to the Italian territory; the results showed important differences in the quality of this cultivar, concerning bitter acids, antioxidants and aroma. Differences in bitter acids and essential oil content were reported for hops grown very far from each other, but also (in particular in Italy) for hops grown at relatively small distances, due to the peculiar conformation of the territory. Moreover, Van Holle and collaborators [17] studied the differences between Amarillo hops grown in Washington State and Idaho (U.S.A.), highlighting differences, especially in the quality and quantity of essential oils; the monovarietal beers produced with the studied hops showed differences in flavors associated with the provenience of hops.
In this study, two aims were addressed: (i) to evaluate the morphological, genetic and aromatic characteristics of wild Corsican hops, in order to characterize new sources of genetic material for breeding purposes and (ii) to evaluate the terroir adaptability/influence of/on Cascade, Smaragd, and Tettnanger cultivars by measuring aromatic and morphological differences when grown in a native territory (Tettnang) and in a new growing area (Corsica).
2. Materials and Methods
2.1. Plant Material
Corsican wild hops were found in different Corsican riverbeds and planted in an experimental field in Patrimonio (Corsica, France). Leaves sample were collected for the DNA analysis. Rizhomes of Cascade, Tettnanger and Smaragd cultivars were obtained from German grower (Locher-Hopfen-Tettnang, Missenhard, Germany), and planted in the experimental field. The hop cones of all the studied hops were picked two times: three months after planting and the following picking year.
To have the evidence of the varieties and to be sure to compare the same varieties, leaves samples of the German hops cultivated in Patrimonio and in Tettnang region were genetically analyzed. The climatic conditions of the Tettnang valley and the four studied Corsican areas have been gathered from the open database “climate-data.org” for which the results shown are averaged from 1999 to 2019, and from the database “meteoblue” for which the data are averaged from 2010 to 2020. The “Patrimonio” data can be considered common to “Oletta” due to the geographical proximity. During the growth period of hop from May to September, the Tettnang valley is characterized by its very high rain content (90–130 mm/month; av: 114 mm/month), low temperatures (13–18 °C; av: 15.8 °C), strong south-west to north-east winds (5–50 km/h) and a low solar exposition (4–7 days/month; av: 5.6 days/month). For the same period, the Corsican planting site (Patrimonio) and Oletta are characterized by their low rain content (10–54 mm/month; av: 30 mm/month), high temperatures (16–24 °C; av: 21.2 °C), strong south-east to north-west winds (5–50 km/h) and a very high solar exposition (10–20 days/month; av: 14.2 days/month). Ajaccio is characterized by its low rain content (15–55 mm/month; av: 30 mm/month), high temperatures (17–24 °C; av: 21.4 °C), medium west-south-west to east-north-east winds (5–19 km/h) and a high solar exposition (5–14 days/month; av: 9 days/month). Corte is characterized by its medium rain content (15–60 mm/month; av: 38 mm/month), medium temperatures (15–21 °C; av: 18.6 °C), weak north to south winds (0–38 km/h) and a high solar exposition (7–16 days/month; av: 11.4 days/month).
In the first part of this study, wild hops (Table 1), located in Ajaccio, Corte, Patrimonio and Oletta, were characterized by a morphological, genetic and chemical approach.

Table 1.
List of the four wild hops and characteristics of their provenance.
In the second part of the study were compared three hops cultivars: Cascade, Smaragd and Tettnanger. In this case, the hops have been identified with the commercial name of the cultivar followed by the geographical origin, for example, Cascade Tettnang, Cascade Corsica, etc. The geographical characteristics of the two growing sites (Tettnang valley, Tettnang, Germany; Patrimonio, Corsica, France) are shown in Table 2.

Table 2.
Geographical indication and characteristics of the two growing sites.
2.2. SSR Analysis
Genomic DNA of the 10 samples (Table 1 and Table 2) was extracted from (i) young leaves (L) collected from young Corsican wild hops and from (ii) Cascade, Tettnanger and Smaragd cvs grown in the Tettnang valley and in Corsica in order to highlight the terroir modification or retention of their genome. The samples, after immersion in liquid nitrogen, were stored at −80 °C and then lyophilized until DNA extraction. Genomic DNA was extracted following the CTAB protocol [33].
For DNA amplification, six SSR primer sets were used, which had shown a high discriminating power [34] (Table S1).
The PCR amplification was performed in a 25 μL volume containing: 1x Reaction Buffer (Biotools, B&M Labs, S.A., Madrid, Spain), 1.5 mM MgCl2 (Biotools, B&M Labs, S.A., Madrid, Spain), 0.2 mM dNTPs (Amersham Biosciences, Piscataway, NJ, USA), 0.2 μM primer (MWG Biotech, Ebersberg, Germany), 20 ng genomic DNA and 0.6 U of Taq polymerase (Biotools, B&M Labs, S.A., Madrid, Spain). For primer HlACA3 and HlGA23, the MgCl2 concentration was 2.5 mM to obtain a better quality of amplification.
The PCR amplifications were performed in thermal cycler MJ PCT 100 Research (Watertown, MA, USA), using the following amplification protocol: programming a first step at 95 °C for 5 min followed by 30 cycles of 45″ at 94 °C, 30″ at the specific annealing temperature for each couple of primers, and 90″ at 72 °C, for denaturation, annealing and primer extension, respectively; then a final extension at 72 °C for 8 min.
The amplification products were separated with a CEQ 2000 Genetic Analysis System (Beckman Coulter, Inc., Brea, CA, USA) sequencer on acrylamide gel CEQ Separation Gel LPA-1 (Beckman Coulter, Inc., Brea, CA, USA). A marker CEQ DNA Size Standard kit 400 (Beckman Coulter, Inc., Brea, CA, USA) was used to estimate the approximate molecular weight of the amplified products. One of the two PCR primers in each reaction was end-labelled with a fluorescent dye (Cy5, MWG-Biotech, Ebersberg, Germany). The analysis was performed in triplicate.
2.3. Morphological Comparison
Many parameters were observed and measured at different times of maturity, following the UPOV (International Union for the Protection Of new Varieties of plants) recommendations (Table S2). These observations were made directly on field or in riverbeds on thirty plants for each sampled hop. The hop plants were observed and compared to literature standards (UPOV) during growing from May to August in two growing seasons (2019 and 2020). The first growth season was named “year 0” and the second one, “year +1”.
2.4. Essential Oils Chemical Characterization
Three hydrodistillations for each accession were performed on hop cones in a Clevenger apparatus for four hours, as recommended by the European Pharmacopoeia [35]. The essential oils were collected and then analyzed by GC-FID and GC-MS.
These analyses were carried out using a PerkinElmer Autosystem GC apparatus equipped with a dual flame ionization detection (FID) and fused-silica capillary columns (60 m × 0.22 mm i.d., film thickness 0.25 μm) with different stationary phases: Rtx-1 (polydimethylsiloxane) and Rtx-Wax (polyethylene glycol). The oven temperature program was from 60 to 230 °C at 2 °C/min and then was held isothermally (30 min). The carrier gas was helium (1 mL/min). Injector and detector temperatures were held at 280 °C. Split injection was performed with a fractional ratio of 1:80. The injected oil volume was 0.1 μL. The relative percentage of the oil constituents was calculated from the GC peak areas without using correction factors.
The oils were analyzed with a PerkinElmer TurboMass detector (quadrupole), coupled in line to a PerkinElmer Autosystem XL equipped with two fused-silica capillary columns (60 m × 0.22 mm i.d., film thickness 0.25 μm), Rtx-1 (polydimethylsiloxane) and Rtx-Wax (polyethylene glycol). The other GC conditions were the same as described above. The ion source temperature was 150 °C, the energy ionization was 70 eV and the electron ionization (EI) mass spectra were acquired over the mass range of 35–350 Da. The volume of oil injected was 0.1 μL. The main volatile compounds were identified on the basis of their mass spectra compared with the reference mass spectra libraries (WILEY275, NBS75K, Adams 2001) and of their calculated retention indexes (RIs) through the application of the Kovats index (KI) formula compared with those reported in the literature and linear retention index (LRI). When it was not possible to find the KI in the literature, a tentative identification was obtained by matching with mass spectra libraries data: a match quality of 98% minimum was used as a criterion. The gas-chromatographic signals were manually integrated, and the resulting peak areas were compared with the total sum of area and expressed in percentage.
2.5. Data Analysis
The nuclear DNA fragments, resulted by the DNA amplification with the SSR markers, were sized by using a conservative binning approach [36] through the statistical R software (R Development Core Team 2005), which takes into account the type of replicate and compensates for the limits of the fragment resolution. The differences among the examined hops were evaluated through the genetic similarity matrix using Euclidean distance [25,37,38]. The cluster analysis and construction of the dendrogram relative to genetic distances were obtained by using the unweighted pair–group method with arithmetic mean (UPGMA) algorithm, with Genetix [39] and XLSTAT 2009 software (AddinsoftTM 1995–2009). The univariate clustering analysis was performed using XLSTAT 2009 software (AddinsoftTM 1995–2009) to discriminate the genotypes.
A statistical analysis of the chemical composition of essential oils was performed using the opensource R studio software (version 1.2.5001, Factoextra package). The normalized data were put in a dissimilarity matrix using Euclidean distance. The hierarchical tree resulting of the matrix linkage was then graphically represented into a dendrogram.
3. Results and Discussion
3.1. SSR Analysis
From the genetic analysis, the four wild accessions were separated; the cultivars are recognized to be the same in Tettnang and Corsica and the genetic profile of the analyzed hops are reported in Table 3. The dissimilarity in the studied samples were highlighted and analyzed in the dendrogram resulted by the Cluster analysis (UPGMA) at Euclidean distance (Figure 1). The relationship between all the accessions is shown with a dissimilarity index from 0 to 76. The examined population is divided in two main clusters (76% of dissimilarity); the first cluster is composed of the accessions Patrimonio, Ajaccio, Cascade and Smaragd; the other two Corsican accessions, Corte and Oletta, fall in the second cluster with the cultivar Tettnanger (Figure 1). Corte accession is separated from those of Patrimonio and Ajaccio; this could be explained by their very different growing conditions (Table 1), such as distance from the coast (respectively away, very close, and very close), temperatures (respectively av: 18.6 °C, av: 21.2 °C, and av: 21.4 °C), salinity (respectively weak, high, and high), altitude (respectively 410 m, 10 m, and 5 m), winds (respectively weak from north to south, strong from south-east to north-west, and medium from west-south-west to east-north-east), and precipitations (respectively av: 38 mm/month, 30 mm/month, and 30 mm/month) (“climate-data.org” and “meteoblue” data). However, these peculiar growing conditions do not apply to Oletta. As no hop traceability data are available in Corsica, it is difficult to estimate when or how the plant was established on the island, nor if it was implanted from different cultivars. Currently, no origin or environmental condition has been found explaining the dissimilarity among the coastal hops’ genotypes. Another possibility is the autochthonous origin of Corsican germplasm; no data, however, are available to support this hypothesis.

Table 3.
Genetic profiles of ten hop samples (six cultivars and four wild hops).
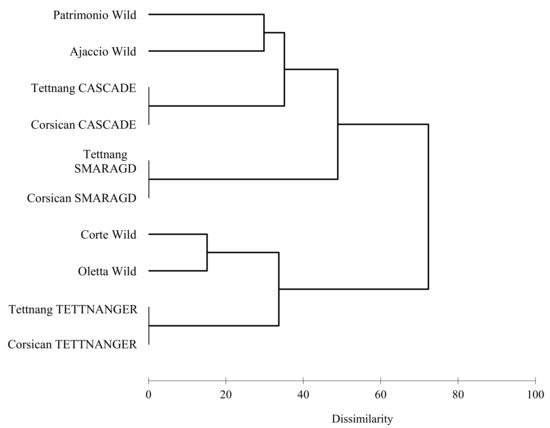
Figure 1.
UPGMA dendrogram based on Euclidean distance of cultivated and Corsican wild hops.
Tettnanger cultivar resulted closer to Corte and Oletta wild Corsican accessions (Figure 1). This result indicates the affinity of wild hops with some European cultivar selected from landraces. Other authors confirm this sentence [25,40]. Rodolfi and collaborators [25], in a population study on 123 hop genotypes, observed that Tettnanger and other European cultivars (Galena, East Kent Golding, Goldings, Challenger, H. Mittelfrüh) were clustered together with Italian wild accessions. This sentence is also in accord with Patzak and coworkers [38]; the authors assert that Tettnanger cultivar, selected by German landrace, have a close relationship with the wild European ancestors [40]. Smaragd and Cascade cultivar resulted in a separated cluster together with Patrimonio and Ajaccio accessions (Figure 1). The genetic similarity to the Corsican accessions could be due to Smaragd and Cascade origins: Smaragd was developed from the Hallertau variety that belongs to the wild German hop population, but Cascade possesses a complex genetic structure [25] and it has among its ancestors, Fuggle, an English hop variety selected from wild [21,40]. This similarity could also demonstrate the correlations between European ancestors and Corsican wild hops.
3.2. Morphological Comparison
Morphological characters of the four wild Corsican hops are described in Table 4.

Table 4.
Morphological description of wild hops from Corsica. In bold, the differences are highlighted.
From Table 4 it is possible to observe the absence of anthocyanin coloration in three wild hops from coastal areas of Corsica (Oletta, Patrimonio and Ajaccio). As Treutter et al. [41] explained, the absence of plant pathogens, such as Tetranychus urticae Koch, downy mildew, and powdery mildew, makes the biosynthesis of protective anthocyanins unnecessary. Therefore, the absence of these biotic stresses in the studied areas could explain the absence of anthocyanin coloration of the main shoot of these wild accessions. The side shoots of the coastal plants Oletta, Patrimonio, and Ajaccio are short, and the number of cones is low (Table 4). As exposed previously, these three Corsican areas are characterized by high temperatures, high solar exposition, medium to strong winds and low precipitations, leading to a severe hydric stress, especially during the hop growing period. Therefore, this observation supports the correlation between the size of the plant, the number of cones, and water stress exposed by Lisar and collaborators [42]. Moreover, even if salt sensitivity of hop plants has not yet been studied, Oletta, Patrimonio and Ajaccio accessions grow in the coastal area, and salt stress could influence the plants’ morphological traits [43].
Corte accession, instead, comes from a particular area of Corsica characterized by a humid microclimate caused by its weak winds, its medium precipitations and temperatures and the presence of two big rivers [44]. This leads to the presence, in Corte accessions, of a strong anthocyanin coloration of the main shoot (Table 4). This humid microclimate is perfect for the growth of powdery and downy mildew. Anthocyanins are supposed to have a role in the plant resistance to pathogens and herbivores, but their biosynthesis requires energy [42] at the expense of the biosynthesis of prenylated compounds, such as xanthohumol [45]; this theory is supported by the anthocyanin coloration of the main shoots of wild hops from Corte. In the same way, the major length of the side shoots and the higher number of cones in wild hops from Corte compared to the other wild hops (Table 4) prove that these characteristics can be influenced by water stress (less present in Corte area), as a response to preserve the vital parts of the plant [43]. These observations seem to corroborate the previously enounced fact that the peculiar growing conditions of the Corte accessions lead to differentiations, both genetic and morphological.
The morphological comparison between the Tettnang cultivars of hops and their cuttings planted in Corsica is described in Table 5. It is possible to observe that in the first year after planting, the morphological characters of the cultivars remain the same in Corsica and in Tettnang. It is instead very interesting to observe that morphological modifications occurred from the year +1 after planting. The first important change occurred to the main shoots of all cultivars (Table 5), which lost their anthocyanin coloration. The presence of Tetranychus urticae Koch, downy mildew, and powdery mildew in the Tettnang area [46] could explain the coloration of the main shoot in Tettnang samples as a result of the synthesis of anthocyanins responding to stress (Table 5) [47]. Therefore, the absence of these attacks on the coastal areas of Corsica could explain the anthocyanins disappearing a year after transplantation. Salt stress is often related to an increase in anthocyanin content, but Akula and Ravishankar [43] reported that salt stress in salt sensitive species could decrease anthocyanins level, as may have happened in the studied plants. Another observable change in the cultivars occurred after one year in the Corsican territory: the shortening of side shoots (Table 5). Moreover, in Table 5, it is highlighted the reduced number of cones in the side shoot in hops cultivated in Corsica. These last two modifications are probably symptoms of plant stress, especially water, wind and salt stress [43], that affects most plants in the northwest of Corsica. Evidence of the modification of plant character caused by the zone of cultivation is also the picking time. Not all the cultivars react in the same way, but Cascade shows anticipation in picking maturity (Table 5). It is well known that the maturity of cones depends on their percentage of water loss. This anticipation could therefore be explained by the dry climate of the new growing site, leading to a faster loss of water. Moreover, the early production of cones could be a response to plant stress; plants often react to stress by shortening flowering and maturation of fruit, in order to produce seeds for the survival of the species [48]. These data are very important for growers to let them know how hop cultivars react to a new growing condition.

Table 5.
Morphological description of hop cultivars (Tettnanger, Smaragd and Cascade) grown in Tettnang and in Corsica. In bold are highlighted the morphological modifications.
3.3. Essential Oils Chemical Characterizations
The essential oils chemical profiles of the German and Corsican hops were characterized. The GC/MS analysis shows a similarity between the wild hops from Corsica (Table 6), but the abundance of the four compounds shows variation, dependent on the location: myrcene, (E)-β-farnesene, α-humulene, and α-selinene.

Table 6.
Essential oils composition of the wild Corsican hops by GC/MS.
The essential oil chemical profiles of Ajaccio, Oletta and Patrimonio are very similar. They contain respectively 20.1, 19.0, 19.1% of myrcene (characterized by spicy and balsamic herbal notes), 2.0, 2.5, 2.8% of (E)-β-farnesene (citrus herbal notes), 18.3, 15.2, 24.9% of α-humulene (woody notes), and 9.3, 10.1, 7.7% of α-selinene (herbal notes), showing especially a spicy citrus and woody character (Table 6). Instead, the essential oil of the Corte hop contains 6.7% of myrcene, 0.1% of (E)-β-farnesene, 5.4% of α-humulene, and 25.9% of α-selinene, thus it is characterized prevalently by herbal notes. This clustering of the Ajaccio, Oletta, and Patrimonio essential oils (Figure 2) could be explained by the specific environment conditions inherent to the coastal areas of Corsica, especially high temperatures, low rain content, medium to strong winds, and high salinity of soils (Table 1). The differentiation of the essential oil of Corte hops, grown in altitude and far from the coast, corroborates this view. The high presence of selinene in Corte genotypes, characterized by a humid environment, is in accord with the study of Patzak and coworkers [50], where European hops were found to be characterized by higher amounts of selinene compared to American hops. Moreover, this result is also in accord with the study of Mongelli and collaborators [51] on Italian wild and cultivated hops, where a correlation was found between high selinene presence and wild Italian hops.
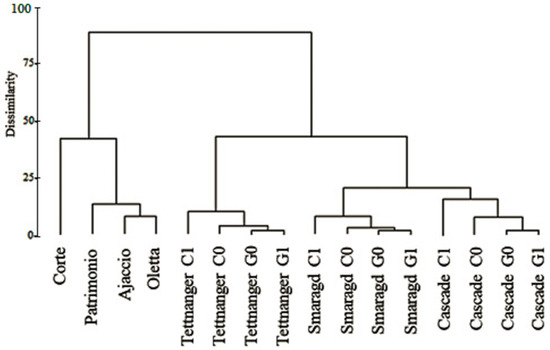
Figure 2.
Dendrogram of German (G) and Corsican (C) hops essential oils. The numbers indicate the year of the plant age (0 = first growing season; 1 = second growing season).
The GC/MS analysis of the Tettnang cultivars and those transplanted in Corsica is shown in Appendix A. It reveals an evolution of the abundance, especially of four compounds from year 0 to year +1 after transplantation: β-pinene, α-humulene, zingiberene, and linalool.
The amount of these four compounds evolved in the same way for the three German hops grown in Corsica. The abundance of β-pinene (resinous and piney aroma) and linalool (floral, citrus, sweet notes) decrease after planting, respectively from 2.0% to 0.9%, and 1.0% to 0.3% of the essential oils for the Cascade hops; from 1.5% to 1.2%, and 1.5% to 0.5% for the Smaragd hops; and from 1.3% to 0.7%, and 1.5% to 0.4% for the Tettnanger hops. The abundance of α-humulene (woody notes), and zingiberene (spicy notes) increase after planting, respectively from 9.2% to 13.7%, and <0.1% to 5.1% of the essential oils for the Cascade hops; from 16.2% to 18.2%, and <0.1% to 7.1% for the Smaragd hops; and from 8.2% to 10.2%, and <0.1% to 7.9% for the Tettnanger hops. Similar results were found in a study [52] on the evaluation of the quality parameters of Cascade to the Sardinian environment; in the chemical composition of Sardinian Cascade oil was observed a low content in β-pinene and linalool, and a high percentage of α-humulene, especially in the samples coming from the two coastal areas (Alghero and Orosei). As Sardinia is an island characterized by geographic and pedoclimatic conditions similar to Corsica, it is possible to assume a comparable oil composition and an analogous performance of the cultivars. This trend in the aromatic compound development highlights a modulation of the German hops to the Corsican coastal terroir and its environmental conditions. The increase in zingiberene, characterized by strong organoleptic properties and efficiency in hop aggressor repellence [53], is a beneficial consequence of the German hops’ acclimatization. Moreover, the comparison of Table 6 and Appendix A shows that the abundance of these four compounds tends toward that of the hops growing wild in coastal areas of Corsica (on average 0.3% for the β-pinene, 19.5% for the α-humulene, 9.7% for the zingiberene, and 0.3% for the linalool). These results are supported by the dendrogram of the essential oil characterizations (Figure 2).
Data of essential oils of German and Corsican (cultivars and wild) hops were used in the cluster analysis to characterize the hop samples (Figure 2). The resulted dendrogram indicates the presence of two clusters with 85% dissimilarity. The first cluster is characterized by the sole presence of wild Corsican hops, while the second cluster contains the studied cultivars. These data, according to a previous study on wild hops [24], demonstrated the discrimination power of the aromatic profile of hops. At the same time, these results are in partial disagreement with another study [13] where, among 75 Portuguese native hops studied, similarities were found between the aromatic profiles of 11 wild hops and 4 cultivars. As our results suggest, the essential oils of the accessions located in coastal areas of the island (Patrimonio, Ajaccio, Oletta) are similar (13% dissimilarity), unlike the one located in Corte far from the coast (40% dissimilarity). This discrimination of the Corte essential oil supports the genetic and the morphological analyses. As explained before, it is apparent that this isolation is caused by the peculiar environmental conditions of the area of Corte. In Figure 2, the cluster analysis shows one homogeneous cluster for each cultivar. While the essential oils of Smaragd and Cascade are close (20% dissimilarity), Tettnanger’s oil presents more than 40% of dissimilarity with the other German hops. This difference corroborates the observations from the genetic analysis and can be explained by the peculiar aromatic profile of Tettnanger, which is considered one of the few noble aromatic cultivars [54]. An interesting result can be observed on the clustering of the cultivars grown in Corsica compared with those grown in Germany. The results indicate that after one year of cultivation in Corsica, all three analyzed cultivars differ markedly (10% dissimilarity for Tettnanger, 8% for Smaragd, 15% for Cascade); the same cultivars grown in Germany show almost identical essential oil contents (3% dissimilarity). These observations show that the terroir and the climatic conditions play a fundamental role on the quality performance of these cultivars.
4. Conclusions
The analysis of wild Corsican hops highlights the presence of interesting genotypes both from the genetic and aromatic point of view, as they possess a unique genetic profile and peculiar aromatic bouquet. It is possible to observe the apparent epigenetic influence of the territory on wild hops, as they show differences in morphological characters and, in particular, the anthocyanin coloration of the main shoot, presumably linked to the pedoclimatic conditions. The observation of the influence of the Corsican territory on three hop cultivars, from the morphological and aromatic point of view, allow us to confirm the adaptability of the German hops to the Corsican terroir after planting. In this study, we can recognize that the acclimatization to the new environment of hop cultivars is not immediate, but it is observable almost one year after planting. The variation among the profiles of the studied hop varieties concerns the increase of zingiberene and modifications in the content of α-humulene, β-pinene and linalool. Moreover, we can observe that German cultivars established in Patrimonio present some common features in morphological characters with the wild hops from coastal areas of Corsica (Oletta, Patrimonio, Ajaccio). The obtained results are important for future Corsican hop growers and for brewers. The investigation demonstrates the key role of the origin of the hop cultivars that they use and the importance of knowing the characteristics that the territory gives to the hop in order to produce beers with the desired aromatic character.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/app11093756/s1.
Author Contributions
A.D.-W. contributed to the project development, performed the chemical and morphological analysis, and interpreted the results. M.R. contribute to the project development, performed the genetic analysis and interpreted the results. T.G. planned the project concept and contributed to the genetic analysis, statistical analysis and results interpretation. J.P. and J.C. contributed to the project development. A.D.-W., M.R., T.G., were involved in the first draft writing and final manuscript editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data presented in this study appear in the submitted article.
Acknowledgments
The German samples of Humulus lupulus L. were grown and harvested by Locher-Hopfen (Missenhardt 2, 88069 Tettnang, Germany). The planted cultivars were cultivated in the experimental hop garden of the Ribella brewery (Patrimonio, 20123 Corse, France). We would like to thank Ludwig Locher (Locher-Hopfen) and Pierre-François Maestracci (Ribella) for their support and contribution.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Essential oils composition of the German hops by GC/MS (Year 0 = first growing season; Year +1 = second growing season).
Table A1.
Essential oils composition of the German hops by GC/MS (Year 0 = first growing season; Year +1 = second growing season).
| Compounds | Cascade | Smaragd | Tettnanger | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tettnang | Corsica | Tettnang | Corsica | Tettnang | Corsica 2 | |||||||
| Year 0 | Year +1 | Year 0 | Year +1 | Year 0 | Year +1 | Year 0 | Year +1 | Year 0 | Year +1 | Year 0 | Year +1 | |
| Hydrocarbonates | ||||||||||||
| β-Pinene | 2.0 | 2.0 | 1.2 | 0.9 | 1.5 | 1.4 | 1.3 | 1.2 | 1.3 | 1.0 | 1.0 | 0.7 |
| Myrcene | 50.3 | 49.1 | 45.6 | 58.1 | 46.1 | 46.3 | 45.8 | 47.3 | 40.6 | 40.9 | 39.9 | 42.5 |
| p-Cymene | 0.3 | 0.1 | 0.2 | 0.3 | 1.1 | 1.1 | 1.1 | 1.1 | 0.7 | 0.7 | 0.8 | 0.7 |
| Limonene | 0.9 | 1.1 | 1.1 | 0.9 | 0.1 | 0.1 | 0.5 | 0.3 | 0.6 | 0.6 | 0.6 | 0.6 |
| α-Ylangene | 0.2 | 0.1 | 0.4 | 0.2 | 0.2 | 0.1 | 0.1 | 0.2 | 0.5 | 0.3 | 0.8 | 0.6 |
| α-Copaene | - | - | - | - | - | - | - | - | - | - | - | - |
| Isocaryophyllene | - | - | 0.1 | - | - | - | 0.4 | 0.2 | - | - | - | - |
| (E)-β-Caryophyllene | 4.8 | 4.3 | 7.7 | 6.4 | 6.1 | 6.0 | 7.2 | 6.5 | 5.7 | 4.7 | 6.1 | 5.5 |
| β-Copaene | - | - | 0.1 | 0.2 | 0.2 | 0.2 | 0.3 | 0.4 | 0.2 | 0.2 | - | 0.1 |
| (E)-α-Bergamotene | 0.4 | 0.7 | 0.3 | - | 0.3 | 0.2 | 0.4 | - | 0.8 | 0.9 | 0.9 | 0.7 |
| (E)-β-Farnesene | 6.8 | 6.6 | 8.2 | 3.9 | 4.1 | 4.5 | 3.9 | 4.0 | 20.2 | 20.8 | 21.2 | 19.3 |
| α-Humulene | 9.2 | 8.9 | 9.8 | 13.7 | 16.2 | 16.1 | 16.9 | 18.2 | 8.2 | 8.1 | 8.6 | 10.2 |
| 4.5-Di-epi-aristolochene | 0.1 | 0.5 | 0.1 | 0.6 | 0.1 | 0.1 | 0.2 | 0.4 | - | - | - | - |
| γ-Muurolene | 0.2 | 0.1 | 0.8 | 0.1 | 0.2 | 0.2 | 0.5 | 0.3 | 0.9 | 0.7 | 1.2 | 0.7 |
| γ-Himachalene | - | - | 0.1 | 0.1 | 0.2 | 0.4 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| β-Selinene | 1.2 | 1.3 | 1.5 | 1.2 | 5.1 | 5.9 | 4.9 | 5.1 | 0.6 | 0.7 | 0.7 | 0.5 |
| Zingiberene | - | - | 1.1 | 5.1 | - | - | 0.2 | 7.1 | - | - | 2.1 | 7.9 |
| Valencene | 1.1 | 0.8 | 2.1 | 1.2 | 0.5 | 0.3 | 0.7 | 0.5 | 0.1 | 0.1 | - | 0.1 |
| α-Selinene | 0.4 | 0.4 | 0.1 | 0.4 | 0.5 | 0.5 | 0.6 | 0.5 | 0.5 | 0.4 | 0.6 | 0.3 |
| (E.E) α-Farnesene | 0.5 | 0.4 | 0.1 | 0.1 | 0.1 | 0.3 | 0.1 | - | - | - | - | - |
| γ-Cadinene | 0.7 | 0.4 | 0.6 | 0.5 | 1.3 | 1.6 | 1.5 | 1.3 | 0.1 | 0.1 | 0.2 | 0.1 |
| δ-Cadinene | - | - | 1.2 | 0.9 | 0.1 | 0.1 | 0.9 | 0.1 | 0.1 | - | 0.1 | |
| γ-Bisabolene | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 |
| α-Cadinene | 0.3 | 0.1 | 0.4 | 0.2 | - | - | - | - | 0.2 | 0.2 | 0.4 | 0.2 |
| Alcohols | ||||||||||||
| Linalool | 1.0 | 1.4 | 0.4 | 0.3 | 1.5 | 1.4 | 1.0 | 0.5 | 1.5 | 1.2 | 1.1 | 0.4 |
| α-Terpineol | 0.1 | - | 0.1 | - | - | - | - | - | 0.1 | 0.1 | - | - |
| 8-caryolanol | 0.3 | 0.3 | 0.1 | 0.2 | 0.1 | 0.1 | - | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 |
| Viridiflorol | - | - | 0.2 | - | 1.0 | 1.2 | 1.2 | 1.0 | 1.3 | 1.7 | 0.9 | 0.9 |
| Humulol | - | - | 0.1 | 0.2 | - | - | - | 0.1 | - | - | - | - |
| Zingiberenol 1 | - | - | 0.2 | 0.5 | - | - | 0.2 | 0.9 | - | - | 0.5 | 1.2 |
| Zingiberenol 2 | 0.1 | 0.1 | 0.1 | - | 0.1 | 0.1 | 0.2 | 0.1 | - | - | - | - |
| α-Cadinol | 0.4 | 0.3 | 1.7 | 0.7 | 0.1 | 0.2 | 0.1 | 0.2 | 0.2 | 0.2 | 0.3 | 0.1 |
| Eudesm-11-en-4α-ol | 0.1 | 0.2 | 1.1 | 0.1 | - | - | - | - | 0.2 | - | 0.5 | 0.1 |
| α-Bisabolol | - | - | 0.1 | - | - | - | - | - | 0.1 | 0.1 | - | - |
| Ketones | ||||||||||||
| 2-nonanone | 0.1 | 0.2 | 0.1 | - | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.2 | - | - |
| 2-decanone | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.3 | 0.1 | 0.1 | 0.5 | 0.4 | 0.2 | 0.1 |
| 2-undecanone | 0.6 | 0.8 | 0.4 | 0.2 | 1.0 | 1.1 | 0.8 | 0.4 | 1.7 | 1.4 | 1.6 | 0.9 |
| 2-dodecanone | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| 2-tetradecanone | 0.2 | 0.1 | 0.1 | - | 0.3 | 0.3 | 0.5 | - | 0.1 | 0.1 | 0.2 | 0.1 |
| (Z) 2-pentadec-6-enone | - | - | - | - | 0.1 | 0.4 | - | - | 0.1 | 0.1 | - | - |
| Aldehydes | ||||||||||||
| Nonanal | 0.4 | 0.6 | 0.8 | 0.7 | 0.2 | 0.2 | 0.3 | 0.4 | - | - | - | - |
| Decanal | - | - | 0.1 | 0.1 | - | - | - | - | 0.1 | - | 0.1 | 0.1 |
| Geranial | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.4 | 0.3 | 0.1 | 0.2 | 0.1 | 0.1 |
| Esters | ||||||||||||
| 2-methylbutyl isobutyrate | 0.3 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | - | 0.1 | 0.1 | 0.1 | - | 0.1 |
| Methyl heptanoate | 1.8 | 1.5 | 0.1 | 0.1 | 0.3 | 0.1 | 0.4 | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 |
| Methyl 6-methylheptanoate | 0.2 | 0.5 | 0.2 | 0.1 | 0.5 | 0.6 | 0.8 | 0.5 | 0.6 | 0.4 | 0.7 | 0.7 |
| Methyl octanoate | 0.1 | 0.3 | 0.2 | 0.1 | 0.7 | 0.7 | 0.5 | 0.7 | 0.1 | - | 0.1 | 0.1 |
| Methyl nonanoate | 0.1 | 0.1 | 0.1 | 0.1 | - | - | - | - | 0.2 | 0.1 | 0.5 | 0.5 |
| Methyl 4-decenoate | 0.6 | 0.8 | 0.7 | 0.6 | 1.7 | 1.9 | 0.8 | 1.4 | 0.7 | 0.9 | 0.4 | 0.7 |
| Methyl geraniate | 0.3 | 0.1 | 0.4 | 0.6 | 0.5 | 0.4 | 0.4 | 0.7 | 0.2 | 0.2 | - | 0.1 |
| Other | ||||||||||||
| Caryophyllene oxide | - | - | 0.1 | 0.1 | - | - | - | - | 0.3 | 0.5 | - | 0.3 |
| Humulene epoxide II | - | - | 0.1 | 0.1 | - | - | - | - | - | - | - | - |
| Humulene epoxide III | 0.8 | 1.0 | 0.4 | 0.1 | - | - | - | - | 0.1 | 0.1 | 0.4 | 0.5 |
| Aromadendrene oxide | 0.1 | 0.1 | 0.1 | 0.1 | - | - | - | - | - | - | - | - |
| Hexadecanoic acid | 0.2 | 0.1 | 0.2 | 0.4 | 0.1 | 0.1 | 0.1 | 0.3 | 0.2 | 0.1 | 0.3 | 0.3 |
| Essential oil rate % | 1.0 | 1.0 | 1.0 | 1.0 | 1.2 | 1.2 | 1.9 | 1.2 | 0.3 | 0.3 | 0.3 | 0.4 |
Bold highlights the four components for which the contents seem to be influenced by transplantation.
References
- Cerenak, A.; Pavlovic, M.; Luskar, M.; Kosir, I. Characterization of slovenian hop (Humulus lupulus L.) varieties by analysis of essential oil. Hop Bull. 2011, 18, 27–32. [Google Scholar]
- Howard, G.A.; Slater, C.A. Evaluation of Hops VII. Composition of the Essential Oil of Hops. J. Inst. Brew. 1957, 63, 491–506. [Google Scholar] [CrossRef]
- Katsiotis, S.T.; Langezaal, C.R.; Scheffer, J.J.C.; Verpoorte, R. Comparative study of the essential oils from hops of various Humulus lupulus L. Cultivars. Flavour Fragr. J. 1989, 4, 187–191. [Google Scholar] [CrossRef]
- Kralj, D.; Zupanec, J.; Vasilj, D.; Kralj, S.; Pšeničnik, J. Variability of Essential Oils of Hops, Humulus lupulus L. J. Inst. Brew. 1991, 97, 197–206. [Google Scholar] [CrossRef]
- Nance, M.R.; Setzer, W.N. Volatile Components of Aroma Hops (Humulus lupulus L.) Commonly Used in Beer Brewing. J. Brew. Distilling 2011, 2, 16–22. [Google Scholar]
- Shellie, R.A.; Poynter, S.D.H.; Li, J.; Gathercole, J.L.; Whittock, S.P.; Koutoulis, A. Varietal characterization of hop (Humulus lupulus L.) by GC-MS analysis of hop cone extracts. J. Sep. Sci. 2009, 32, 3720–3725. [Google Scholar] [CrossRef]
- Small, E. A Numerical and Nomenclatural Analysis of Morpho-Geographic Taxa of Humulus. Syst. Bot. 1978, 3, 37–76. [Google Scholar] [CrossRef]
- Small, E. A numerical analysis of morpho-geographic groups of cultivars of Humulus lupulus based on samples of cones. Can. J. Bot. 1981, 59, 311–324. [Google Scholar] [CrossRef]
- Stevens, J.F.; Ivancic, M.; Hsu, V.L.; Deinzer, M.L. Prenylflavonoids from Humulus lupulus. Phytochemistry 1997, 44, 1575–1585. [Google Scholar] [CrossRef]
- Stevens, J.F.; Taylor, A.W.; Nickerson, G.B.; Ivancic, M.; Henning, J.; Haunold, A.; Deinzer, M.L. Prenylflavonoid variation in Humulus lupulus: Distribution and taxonomic significance of xanthogalenol and 4′-O-methylxanthohumol. Phytochemistry 2000, 53, 759–775. [Google Scholar] [CrossRef]
- Henning, J.A.; Steiner, J.J.; Hummer, K.E. Genetic Diversity among World Hop Accessions Grown in the USA. Crop Sci. 2004, 44, 411–417. [Google Scholar] [CrossRef]
- Štěrba, K.; Čejka, P.; Čulík, J.; Jurková, M.; Krofta, K.; Pavlovič, M.; Mikyška, A.; Olšovská, J. Determination of Linalool in Different Hop Varieties Using a New Method Based on Fluidized-Bed Extraction with Gas Chromatographic-Mass Spectrometric Detection. J. Am. Soc. Brew. Chem. 2015, 73, 151–158. [Google Scholar] [CrossRef]
- Martins, Z.; Machado, J.; Cunha, S.; Barata, A.; Ferreira, I. A chemometric approach to compare Portuguese native hops with worldwide commercial varieties. J. Chemom. 2020, 34. [Google Scholar] [CrossRef]
- Green, C.P. Use of a Chromatography Data System to Identify Varieties in Binary Mixtures of Hops. J. Inst. Brew. 1997, 103, 293–296. [Google Scholar] [CrossRef]
- Pluhackova, H.; Ehrenbergerova, J.; Kretek, P.; Kocourkova, B. Hop Essential Oils in the Selected Varieties from Differently Old Hop Yards. Kvasny Prumysl 2011, 57, 266–271. [Google Scholar] [CrossRef][Green Version]
- Matsui, H.; Inui, T.; Oka, K.; Fukui, N. The influence of pruning and harvest timing on hop aroma, cone appearance, and yield. Food Chem. 2016, 202, 15–22. [Google Scholar] [CrossRef]
- Van Holle, A.; Van Landschoot, A.; Roldán-Ruiz, I.; Naudts, D.; De Keukeleire, D. The brewing value of Amarillo hops (Humulus lupulus L.) grown in northwestern USA: A preliminary study of terroir significance. J. Inst. Brew. 2017, 123, 312–318. [Google Scholar] [CrossRef]
- Morcol, T.B.; Negrin, A.; Matthews, P.D.; Kennelly, E.J. Hop (Humulus lupulus L.) terroir has large effect on a glycosylated green leaf volatile but not on other aroma glycosides. Food Chem. 2020, 321, 126644. [Google Scholar] [CrossRef]
- Almeida, A.D.R.; Maciel, M.V.D.O.B.; Gandolpho, B.C.G.; Machado, M.H.; Teixeira, G.L.; Bertoldi, F.C.; Noronha, C.M.; Vitali, L.; Block, J.M.; Barreto, P.L.M. Brazilian Grown Cascade Hop (Humulus lupulus L.): LC-ESI-MS-MS and GC-MS Analysis of Chemical Composition and Antioxidant Activity of Extracts and Essential Oils. J. Am. Soc. Brew. Chem. 2020, 1–11. [Google Scholar] [CrossRef]
- Ganino, T.; Bartolini, G.; Fabbri, A. The classification of olive germplasm. J. Hortic. Sci. Biotechnol. 2006, 81, 319–334. [Google Scholar] [CrossRef]
- Jakse, J.; Satovic, Z.; Javornik, B. Microsatellite variability among wild and cultivated hops (Humulus lupulus L.). Genome 2004, 47. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Darby, P.; Javornik, B.; Pais, M.S.S.; Seigner, E.; Lutz, A.; Svoboda, P. Microsatellite DNA Analysis of Wild Hops, Humulus lupulus L. Genet. Resour. Crop. Evol. 2006, 53. [Google Scholar] [CrossRef]
- Patzak, J.; Nesvadba, V.; Henychová, A.; Krofta, K. Assessment of the genetic diversity of wild hops (Humulus lupulus L.) in Europe using chemical and molecular analyses. Biochem. Syst. Ecol. 2010, 38, 136–145. [Google Scholar] [CrossRef]
- Mongelli, A.; Rodolfi, M.; Ganino, T.; Marieschi, M.; Dall’Asta, C.; Bruni, R. Italian hop germplasm: Characterization of wild Humulus lupulus L. genotypes from Northern Italy by means of phytochemical, morphological traits and multivariate data analysis. Ind. Crop. Prod. 2015, 70, 16–27. [Google Scholar] [CrossRef]
- Rodolfi, M.; Silvanini, A.; Chiancone, B.; Marieschi, M.; Fabbri, A.; Bruni, R.; Ganino, T. Identification and genetic structure of wild Italian Humulus lupulus L. and comparison with European and American hop cultivars using nuclear microsatellite markers. Genet. Resour. Crop. Evol. 2018, 65, 1405–1422. [Google Scholar] [CrossRef]
- Patzak, J.; Henychová, A. Evaluation of genetic variability within actual hop (Humulus lupulus L.) cultivars by an enlarged set of molecular markers. Czech J. Genet. Plant Breed. 2018, 54, 86–91. [Google Scholar] [CrossRef]
- Rodolfi, M.; Chiancone, B.; Liberatore, C.M.; Fabbri, A.; Cirlini, M.; Ganino, T. Changes in chemical profile of Cascade hop cones according to the growing area. J. Sci. Food Agric. 2019, 99, 6011–6019. [Google Scholar] [CrossRef]
- Mafakheri, M.; Kordrostami, M.; Rahimi, M.; Matthews, P.D. Evaluating genetic diversity and structure of a wild hop (Humulus lupulus L.) germplasm using morphological and molecular characteristics. Euphytica 2020, 216, 58. [Google Scholar] [CrossRef]
- Bernath, J. Acta Horticulturae; International Society for Horticultural Science: Leuven, Belgium, 2002; pp. 115–128. [Google Scholar]
- Neve, R.A. Fungal diseases. In Hops; Neve, R.A., Ed.; Springer: Dordrecht, The Netherlands, 1991; pp. 137–173. [Google Scholar]
- DeLyser, D.Y.; Kasper, W.J. Hopped Beer: The Case for Cultivation. Econ. Bot. 1994, 48, 166–170. [Google Scholar] [CrossRef]
- Van Alfen, N.K. Encyclopedia of Agriculture and Food Systems; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Stajner, N.; Jakse, J.; Kozjak, P.; Javornik, B. The isolation and characterisation of microsatellites in hop (Humulus lupulus L.). Plant Sci. 2005, 168, 213–221. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, 3rd ed.; The Stationery Office Books: Strasbourg, France, 1997. [Google Scholar]
- Rabenold, P.P. DNA fingerprinting: An introduction. Trends Ecol. Evol. 1991, 6, 306. [Google Scholar] [CrossRef]
- Beghè, D.; Molano, J.F.G.; Fabbri, A.; Ganino, T. Olive biodiversity in Colombia: A molecular study of local germplasm. Sci. Hortic. 2015, 189, 122–131. [Google Scholar] [CrossRef]
- Ganino, T. Genetic Resources of Olea europaea L. In the Bologna Province (Italy): SSR Analysis and Identification of Local Germplasm; Firenze University Press: Florence, Italy, 2008; pp. 1000–1007. [Google Scholar]
- Belkhir, K. Genetix 4.05. Available online: https://kimura.univ-montp2.fr/genetix/ (accessed on 8 February 2021).
- Patzak, J.P.; Vrba, L.V.; Matoušek, J.M. New STS molecular markers for assessment of genetic diversity and DNA fingerprinting in hop (Humulus lupulus L.). Genome 2007, 50. [Google Scholar] [CrossRef]
- Treutter, D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006, 4, 147. [Google Scholar] [CrossRef]
- Lisar, S.Y.; Motafakkerazad, R.; Hossain, M.; Rahman, I.M.M. Water Stress in Plants: Causes, Effects and Responses; IntechOpen: London, UK, 2012; pp. 1–14. [Google Scholar]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Bénévent, E. La pluviosité de la Corse. Revue Géographie Alpine 1914, 2, 239–264. [Google Scholar] [CrossRef]
- Matoušek, J.; Kocábek, T.; Patzak, J.; Bříza, J.; Siglová, K.; Mishra, A.K.; Duraisamy, G.S.; Tỳcová, A.; Ono, E.; Krofta, K. The putative role of transcription factors from HlWRKY family in regulation of the final steps of prenylflavonid and bitter acids biosynthesis in hop (Humulus lupulus L.). Plant Mol. Biol. 2016, 92, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Weihrauch, F. A new monitoring approach for the two-spotted spider mite Tetranychus urticae Koch (Acari: Tetranychidae) in hop culture. J. Plant Dis. Prot. 2004, 111, 197–205. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, X.; Wei, W.; Fan, Z.; Huang, T.; Pan, X. Analysis of leaf morphology, secondary metabolites and proteins related to the resistance to Tetranychus cinnabarinus in cassava (Manihot esculenta Crantz). Sci. Rep. 2020, 10, 14197. [Google Scholar] [CrossRef]
- Polijakoff-Mayber, A. Morphological and Anatomical Changes in Plants as a Response to Salinity Stress. Plants Saline Environ. 1975, 97–117. [Google Scholar] [CrossRef]
- Joulain, D.; König, W.A. The Atlas of Spectral Data of Sesquiterpene Hydrocarbons; EB-Verlag: Hamburg, Germany, 1998. [Google Scholar]
- Patzak, J. Evaluation of genetic variability of wild hops (Humulus lupulus L.) in Canada and the Caucasus region by chemical and molecular methods. Genome 2010, 53, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Mongelli, A.; Rodolfi, M.; Ganino, T.; Marieschi, M.; Caligiani, A.; Dall’Asta, C.; Bruni, R. Are Humulus lupulus L. ecotypes and cultivars suitable for the cultivation of aromatic hop in Italy? A phytochemical approach. Ind. Crop. Prod. 2016, 83, 693–700. [Google Scholar] [CrossRef]
- Forteschi, M.; Porcu, M.C.; Fanari, M.; Zinellu, M.; Secchi, N.; Buiatti, S.; Passaghe, P.; Bertoli, S.; Pretti, L. Quality assessment of Cascade Hop (Humulus lupulus L.) grown in Sardinia. Eur. Food Res. Technol. 2019, 245, 863–871. [Google Scholar] [CrossRef]
- Maluf, W.R.; Campos, G.A.; Cardoso, M.D.G. Relationships between trichome types and spider mite (Tetranychus evansi) repellence in tomatoes with respect to foliar zingiberene contents. Euphytica 2001, 121, 73–80. [Google Scholar] [CrossRef]
- Alberts, L. Zatec, cradle of Saaz hops and commercial hop cultivation. Brew. Hist. 2020, 181, 43–50. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).