Paraben Compounds—Part II: An Overview of Advanced Oxidation Processes for Their Degradation
Abstract
1. Introduction
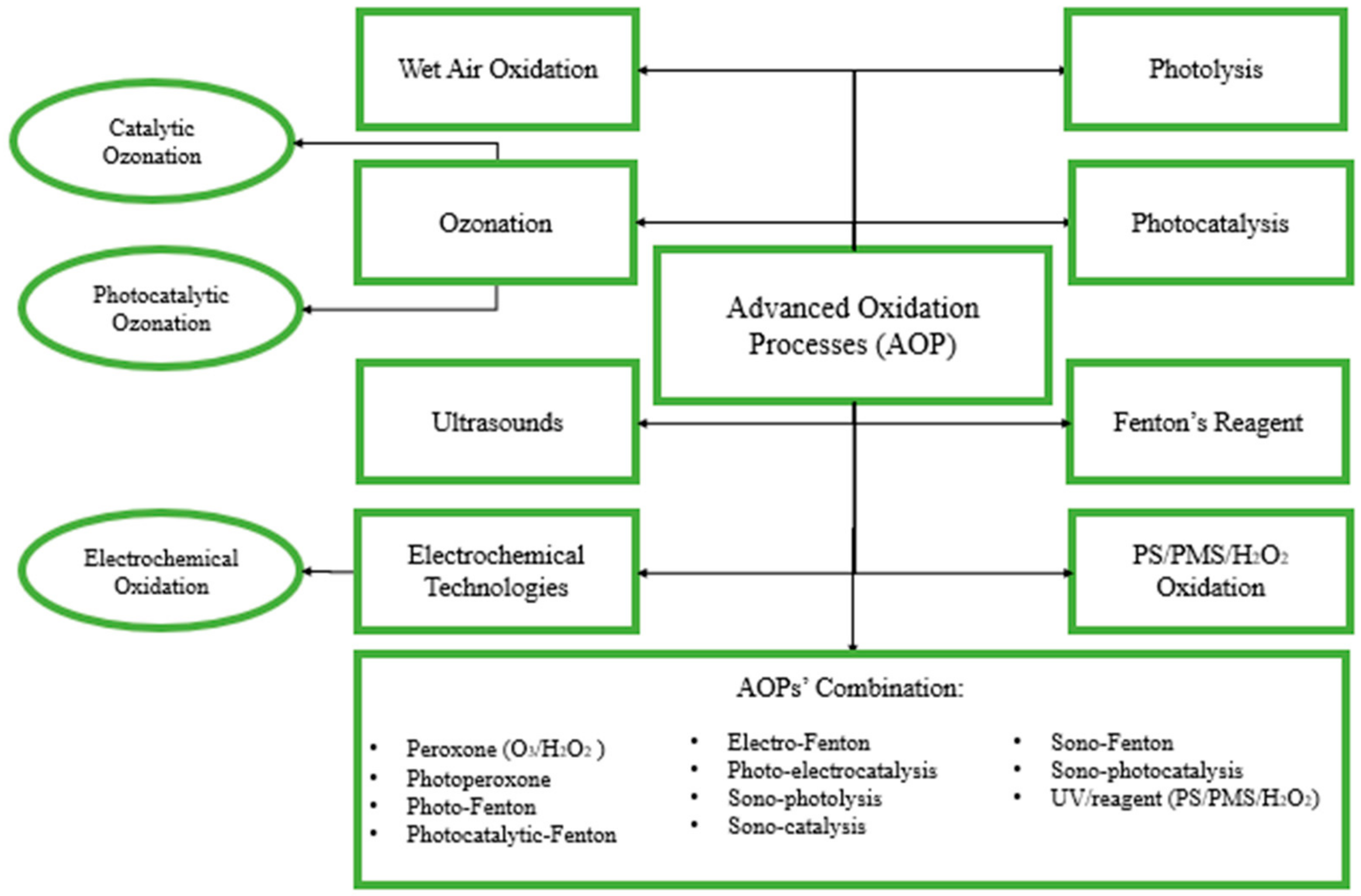
2. Advanced Oxidation Processes
2.1. Photolysis and Photocatalysis
2.2. Ozone Based Technologies
2.3. Fenton’s Reagent
2.4. Persulfate, Peroxymonosulfate, and Hydrogen Peroxide
2.5. Electrochemical Oxidation
2.6. Sonochemical Methods
3. AOP for Parabens Removal
3.1. Photolysis and Photocatalysis
3.1.1. Parabens Abatement by Photolysis
3.1.2. Parabens Abatement by Photocatalysis
3.2. Ozone-Based Technologies
3.2.1. Single Ozonation
3.2.2. Catalytic and Photocatalytic Ozonation
3.3. Fenton’s Process
3.4. PS, PMS, and H2O2 Oxidation
3.5. Electrochemical Technologies
3.6. Sono-Based Technologies
4. Toxicological Studies of Parabens Abatement
5. Treatment Costs
6. Future Perspectives
- Produce high-performance catalysts with lower costs;
- Dope and support catalyst to allow their reuse and the use of other types of radiation, such as solar radiation;
- Produce cheaper and more eco-friendly radiation sources;
- Test different types of reactors and operational parameters;
- Test different AOP technologies for the same effluent on a real scale;
- Study in depth the toxicological effects in different species and impacts in the environment;
- Study in depth the operational economics for different technologies.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WWF—World Wildlife. Water Scarcity. 2020. Available online: https://www.worldwildlife.org/threats/water-scarcity (accessed on 1 December 2020).
- Rizzo, L.; Gernjak, W.; Krzeminski, P.; Malato, S.; McArdell, C.S.; Perez, J.A.S.; Schaar, H.; Fatta-Kassinos, D. Best available technologies and treatment trains to address current challenges in urban wastewater reuse for irrigation of crops in EU countries. Sci. Total Environ. 2020, 710, 136312. [Google Scholar] [CrossRef]
- Vela, N.; Calín, M.; Yáñez-Gascón, M.J.; Garrido, I.; Pérez-Lucas, G.; Fenoll, J.; Navarro, S. Solar reclamation of wastewater effluent polluted with bisphenols, phthalates and parabens by photocatalytic treatment with TiO2/Na2S2O8 at pilot plant scale. Chemosphere 2018, 212, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Frontistis, Z.; Antonopoulou, M.; Petala, A.; Venieri, D.; Konstantinou, I.; Kondarides, D.I.; Mantzavinos, D. Photodegradation of ethylparaben using simulated solar radiation and Ag3PO4 photocatalyst. J. Hazard. Mater. 2017, 323, 478–488. [Google Scholar] [CrossRef]
- Esplugas, S.; Bila, D.M.; Krause, L.G.T.; Dezotti, M. Ozonation and advanced oxidation technologies to remove endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) in water effluents. J. Hazard. Mater. 2007, 149, 631–642. [Google Scholar] [CrossRef]
- Fernandes, R.A.; Sampaio, M.J.; Dražić, G.; Faria, J.L.; Silva, C.G. Efficient removal of parabens from real water matrices by a metal-free carbon nitride photocatalyst. Sci. Total Environ. 2020, 716, 135346. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ferronato, C.; Deng, N.; Wu, F.; Chovelon, J.-M. Photocatalytic degradation of methylparaben by TiO2: Multivariable experimental design and mechanism. Appl. Catal. B Environ. 2009, 88, 32–41. [Google Scholar] [CrossRef]
- Sousa, J.C.; Ribeiro, A.R.; Barbosa, M.O.; Ribeiro, C.; Tiritan, M.E.; Pereira, M.F.R.; Silva, A.M. Monitoring of the 17 EU Watch List contaminants of emerging concern in the Ave and the Sousa Rivers. Sci. Total Environ. 2019, 649, 1083–1095. [Google Scholar] [CrossRef]
- Lincho, J.; Martins, R.C.; Gomes, J. Paraben compounds—Part I: An overview of their characteristics, detection, and impacts. Appl. Sci. 2021, 11, 2307. [Google Scholar] [CrossRef]
- Jonkers, N.; Sousa, A.; Galante-Oliveira, S.; Barroso, C.M.; Kohler, H.-P.E.; Giger, W. Occurrence and sources of selected phenolic endocrine disruptors in Ria de Aveiro, Portugal. Environ. Sci. Pollut. Res. 2009, 17, 834–843. [Google Scholar] [CrossRef]
- Carmona, E.; Andreu, V.; Picó, Y. Occurrence of acidic pharmaceuticals and personal care products in Turia River Basin: From waste to drinking water. Sci. Total Environ. 2014, 484, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shi, Y.; Gao, L.; Liu, J.; Cai, Y. Occurrence and human exposure of parabens and their chlorinated derivatives in swimming pools. Environ. Sci. Pollut. Res. 2015, 22, 17987–17997. [Google Scholar] [CrossRef]
- Gibs, J.; Stackelberg, P.E.; Furlong, E.T.; Meyer, M.; Zaugg, S.D.; Lippincott, R.L. Persistence of pharmaceuticals and other organic compounds in chlorinated drinking water as a function of time. Sci. Total Environ. 2007, 373, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Gorga, M.; Petrovic, M.; Barceló, D. Multi-residue analytical method for the determination of endocrine disruptors and related compounds in river and waste water using dual column liquid chromatography switching system coupled to mass spectrometry. J. Chromatogr. A 2013, 1295, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Gao, L.; Shi, Y.; Wang, Y.; Liu, J.; Cai, Y. Spatial distribution, temporal variation and risks of parabens and their chlorinated derivatives in urban surface water in Beijing, China. Sci. Total Environ. 2016, 539, 262–270. [Google Scholar] [CrossRef]
- Ana, F.; Fonseca, A.P. Parabens paradoxes in cosmetic formulations: A review. Int. J. Med. Res. Pharm. Sci. 2016, 3, 1–11. [Google Scholar]
- Tavares, R.S.; Martins, F.C.; Oliveira, P.J.; Ramalho-Santos, J.; Peixoto, F.P. Parabens in male infertility—Is there a mitochondrial connection? Reprod. Toxicol. 2009, 27, 1–7. [Google Scholar] [CrossRef]
- Błędzka, D.; Gromadzińska, J.; Wąsowicz, W. Parabens. From environmental studies to human health. Environ. Int. 2014, 67, 27–42. [Google Scholar] [CrossRef]
- Terasaka, S.; Inoue, A.; Tanji, M.; Kiyama, R. Expression profiling of estrogen-responsive genes in breast cancer cells treated with alkylphenols, chlorinated phenols, parabens, or bis- and benzoylphenols for evaluation of estrogenic activity. Toxicol. Lett. 2006, 163, 130–141. [Google Scholar] [CrossRef]
- Chen, F.; Wang, F.; Li, Q.; Cao, C.; Zhang, X.; Ma, H.; Guo, Y. Effect of support (Degussa P25 TiO2, anatase TiO2, γ-Al2O3, and AlOOH) of Pt-based catalysts on the formaldehyde oxidation at room temperature. Catal. Commun. 2017, 99, 39–42. [Google Scholar] [CrossRef]
- Darbre, P.D.; Aljarrah, A.; Miller, W.R.; Coldham, N.G.; Sauer, M.J.; Pope, G.S. Concentrations of parabens in human breast tumours. J. Appl. Toxicol. 2004, 24, 5–13. [Google Scholar] [CrossRef]
- Peng, X.; Yu, Y.; Tang, C.; Tan, J.; Huang, Q.; Wang, Z. Occurrence of steroid estrogens, endocrine-disrupting phenols, and acid pharmaceutical residues in urban riverine water of the Pearl River Delta, South China. Sci. Total Environ. 2008, 397, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Cuerda-Correa, E.M.; Domínguez, J.R.; Muñoz-Peña, M.J.; González, T. Degradation of parabens in different aqueous matrices by several O3-derived advanced oxidation processes. Ind. Eng. Chem. Res. 2016, 55, 5161–5172. [Google Scholar] [CrossRef]
- Tay, K.S.; Rahman, N.A.; Bin Abas, M.R. Ozonation of parabens in aqueous solution: Kinetics and mechanism of degradation. Chemosphere 2010, 81, 1446–1453. [Google Scholar] [CrossRef]
- Tay, K.S.; Rahman, N.A.; Bin Abas, M.R. Kinetic studies of the degradation of parabens in aqueous solution by ozone oxidation. Environ. Chem. Lett. 2010, 8, 331–337. [Google Scholar] [CrossRef]
- Gomes, J.F.; Bednarczyk, K.; Gmurek, M.; Stelmachowski, M.; Zaleska-Medynska, A.; Bastos, F.C.; Quinta-Ferreira, M.E.; Costa, R.; Quinta-Ferreira, R.M.; Martins, R.C. Noble metal–TiO2 supported catalysts for the catalytic ozonation of parabens mixtures. Process. Saf. Environ. Prot. 2017, 111, 148–159. [Google Scholar] [CrossRef]
- Gomes, J.F.; Leal, I.; Bednarczyk, K.; Gmurek, M.; Stelmachowski, M.; Diak, M.; Quinta-Ferreira, M.E.; Costa, R.; Quinta-Ferreira, R.M.; Martins, R.C. Photocatalytic ozonation using doped TiO2 catalysts for the removal of parabens in water. Sci. Total Environ. 2017, 609, 329–340. [Google Scholar] [CrossRef]
- Asgari, E.; Esrafili, A.; Rostami, R.; Farzadkia, M. O3, O3/UV and O3/UV/ZnO for abatement of parabens in aqueous solutions: Effect of operational parameters and mineralization/biodegradability improvement. Process. Saf. Environ. Prot. 2019, 125, 238–250. [Google Scholar] [CrossRef]
- Martins, R.C.; Gmurek, M.; Rossi, A.F.; Corceiro, V.; Costa, R.; Quinta-Ferreira, M.E.; Ledakowicz, S.; Quinta-Ferreira, R.M. Application of Fenton oxidation to reduce the toxicity of mixed parabens. Water Sci. Technol. 2016, 74, 1867–1875. [Google Scholar] [CrossRef]
- Gmurek, M.; Gomes, J.F.; Martins, R.C.; Quinta-Ferreira, R.M. Comparison of radical-driven technologies applied for paraben mixture degradation: Mechanism, biodegradability, toxicity and cost assessment. Environ. Sci. Pollut. Res. 2019, 26, 37174–37192. [Google Scholar] [CrossRef] [PubMed]
- Orak, C.; Atalay, S.; Ersöz, G. Photocatalytic and photo-Fenton-like degradation of methylparaben on monolith-supported perovskite-type catalysts. Sep. Sci. Technol. 2017, 52, 1310–1320. [Google Scholar] [CrossRef]
- Lin, Y.; Ferronato, C.; Deng, N.; Chovelon, J.-M. Study of benzylparaben photocatalytic degradation by TiO2. Appl. Catal. B Environ. 2011, 104, 353–360. [Google Scholar] [CrossRef]
- Xiao, X.; Lu, M.; Nan, J.; Zuo, X.; Zhang, W.; Liu, S.; Wang, S. Rapid microwave synthesis of I-doped Bi4O5Br2 with significantly enhanced visible-light photocatalysis for degradation of multiple parabens. Appl. Catal. B Environ. 2017, 218, 398–408. [Google Scholar] [CrossRef]
- Kotzamanidi, S.; Frontistis, Z.; Binas, V.; Kiriakidis, G.; Mantzavinos, D. Solar photocatalytic degradation of propyl paraben in Al-doped TiO2 suspensions. Catal. Today 2018, 313, 148–154. [Google Scholar] [CrossRef]
- Gmurek, M.; Rossi, A.F.; Martins, R.C.; Quinta-Ferreira, R.M.; Ledakowicz, S. Photodegradation of single and mixture of parabens—Kinetic, by-products identification and cost-efficiency analysis. Chem. Eng. J. 2015, 276, 303–314. [Google Scholar] [CrossRef]
- Martins, A.S.; Nuñez, L.; Lanza, M.R.D.V. Enhanced photoelectrocatalytic performance of TiO2 nanotube array modified with WO3 applied to the degradation of the endocrine disruptor propyl paraben. J. Electroanal. Chem. 2017, 802, 33–39. [Google Scholar] [CrossRef]
- Daghrir, R.; Dimboukou-Mpira, A.; Seyhi, B.; Drogui, P. Photosonochemical degradation of butyl-paraben: Optimization, toxicity and kinetic studies. Sci. Total Environ. 2014, 490, 223–234. [Google Scholar] [CrossRef]
- Nikolaou, S.; Vakros, J.; Diamadopoulos, E.; Mantzavinos, D. Sonochemical degradation of propylparaben in the presence of agro-industrial biochar. J. Environ. Chem. Eng. 2020, 8, 104010. [Google Scholar] [CrossRef]
- Papadopoulos, C.; Frontistis, Z.; Antonopoulou, M.; Venieri, D.; Konstantinou, I.K.; Mantzavinos, D. Sonochemical degradation of ethyl paraben in environmental samples: Statistically important parameters determining kinetics, by-products and pathways. Ultrason. Sonochem. 2016, 31, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Paździor, K.; Bilińska, L.; Ledakowicz, S. A review of the existing and emerging technologies in the combination of AOPs and biological processes in industrial textile wastewater treatment. Chem. Eng. J. 2019, 376, 120597. [Google Scholar] [CrossRef]
- Boczkaj, G.; Fernandes, A. Wastewater treatment by means of advanced oxidation processes at basic pH conditions: A review. Chem. Eng. J. 2017, 320, 608–633. [Google Scholar] [CrossRef]
- Babu, D.S.; Srivastava, V.; Nidheesh, P.; Kumar, M.S. Detoxification of water and wastewater by advanced oxidation processes. Sci. Total Environ. 2019, 696, 133961. [Google Scholar] [CrossRef]
- Ge, J.; Zhang, Y.; Heo, Y.-J.; Park, S.-J. Advanced design and synthesis of composite photocatalysts for the remediation of wastewater: A review. Catalysts 2019, 9, 122. [Google Scholar] [CrossRef]
- Litter, M.I.; Quici, N. Photochemical advanced oxidation processes for water and wastewater treatment. Recent Pat. Eng. 2010, 4, 217–241. [Google Scholar] [CrossRef]
- M’Arimi, M.; Mecha, C.; Kiprop, A.; Ramkat, R. Recent trends in applications of advanced oxidation processes (AOPs) in bioenergy production: Review. Renew. Sustain. Energy Rev. 2020, 121, 109669. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Tufail, A.; Price, W.E.; Mohseni, M.; Pramanik, B.K.; Hai, F.I. A critical review of advanced oxidation processes for emerging trace organic contaminant degradation: Mechanisms, factors, degradation products, and effluent toxicity. J. Water Process. Eng. 2021, 40, 101778. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Heterogeneous photocatalysis: State of the art and present applications. Top. Catal. 2005, 34, 49–65. [Google Scholar] [CrossRef]
- Rueda-Marquez, J.J.; Levchuk, I.; Ibañez, P.F.; Sillanpää, M. A critical review on application of photocatalysis for toxicity reduction of real wastewaters. J. Clean. Prod. 2020, 258, 120694. [Google Scholar] [CrossRef]
- Vela, N.; Calín, M.; Yáñez-Gascón, M.J.; Garrido, I.; Pérez-Lucas, G.; Fenoll, J.; Navarro, S. Photocatalytic oxidation of six pesticides listed as endocrine disruptor chemicals from wastewater using two different TiO2 samples at pilot plant scale under sunlight irradiation. J. Photochem. Photobiol. A Chem. 2018, 353, 271–278. [Google Scholar] [CrossRef]
- Byrne, C.; Subramanian, G.; Pillai, S.C. Recent advances in photocatalysis for environmental applications. J. Environ. Chem. Eng. 2018, 6, 3531–3555. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef]
- Shan, A.Y.; Ghazi, T.I.M.; Rashid, S.A. Immobilisation of titanium dioxide onto supporting materials in heterogeneous photocatalysis: A review. Appl. Catal. A Gen. 2010, 389, 1–8. [Google Scholar] [CrossRef]
- Canle, M.; Pérez, M.I.F.; Santaballa, J.A. Photocatalyzed degradation/abatement of endocrine disruptors. Curr. Opin. Green Sustain. Chem. 2017, 6, 101–138. [Google Scholar] [CrossRef]
- Gomes, J.; Lincho, J.; Domingues, E.; Gmurek, M.; Mazierski, P.; Zaleska-Medynska, A.; Klimczuk, T.; Quinta-Ferreira, R.M.; Martins, R.C. TiO2 nanotube arrays-based reactor for photocatalytic oxidation of parabens mixtures in ultrapure water: Effects of photocatalyst properties, operational parameters and light source. Sci. Total Environ. 2019, 689, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Kaur, T.; Sraw, A.; Wanchoo, R.; Toor, A.P. Solar assisted degradation of carbendazim in water using clay beads immobilized with TiO2 & Fe doped TiO2. Sol. Energy 2018, 162, 45–56. [Google Scholar] [CrossRef]
- Kaur, T.; Sraw, A.; Toor, A.P.; Wanchoo, R. Utilization of solar energy for the degradation of carbendazim and propiconazole by Fe doped TiO2. Sol. Energy 2016, 125, 65–76. [Google Scholar] [CrossRef]
- Abramović, B.F.; Šojić, D.V.; Anderluh, V.B.; Abazović, N.D.; Čomor, M.I. Nitrogen-doped TiO2 suspensions in photocatalytic degradation of mecoprop and (4-chloro-2-methylphenoxy)acetic acid herbicides using various light sources. Desalination 2009, 244, 293–302. [Google Scholar] [CrossRef]
- Gągol, M.; Przyjazny, A.; Boczkaj, G. Wastewater treatment by means of advanced oxidation processes based on cavitation—A review. Chem. Eng. J. 2018, 338, 599–627. [Google Scholar] [CrossRef]
- Quiñones, D.H.; Álvarez, P.M.; Rey, A.; Beltrán, F.J. Removal of emerging contaminants from municipal WWTP secondary effluents by solar photocatalytic ozonation. A pilot-scale study. Sep. Purif. Technol. 2015, 149, 132–139. [Google Scholar] [CrossRef]
- Quiñones, D.; Rey, A.; Álvarez, P.; Beltrán, F.; Puma, G.L. Boron doped TiO2 catalysts for photocatalytic ozonation of aqueous mixtures of common pesticides: Diuron, o-phenylphenol, MCPA and terbuthylazine. Appl. Catal. B Environ. 2015, 178, 74–81. [Google Scholar] [CrossRef]
- Bertagna Silva, D.; Cruz-Alcalde, A.; Sans, C.; Giménez, J.; Esplugas, S. Performance and kinetic modelling of photolytic and photocatalytic ozonation for enhanced micropollutants removal in municipal wastewaters. Appl. Catal. B Environ. 2019, 249, 211–217. [Google Scholar] [CrossRef]
- Mehrjouei, M.; Müller, S.; Möller, D. A review on photocatalytic ozonation used for the treatment of water and wastewater. Chem. Eng. J. 2015, 263, 209–219. [Google Scholar] [CrossRef]
- Orge, C.; Faria, J.; Pereira, M. Photocatalytic ozonation of aniline with TiO2-carbon composite materials. J. Environ. Manag. 2017, 195, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-J.; Oh, D.; Lee, C.-S.; Gong, J.; Kim, J.; Chang, Y.-S. Manganese oxide nanorods as a robust Fenton-like catalyst at neutral pH: Crystal phase-dependent behavior. Catal. Today 2017, 282, 71–76. [Google Scholar] [CrossRef]
- Lee, H.-J.; Lee, H.; Lee, C. Degradation of diclofenac and carbamazepine by copper(II)-catalyzed dark and photo-assisted Fenton-like systems. Chem. Eng. J. 2014, 245, 258–264. [Google Scholar] [CrossRef]
- Prieto-Rodríguez, L.; Spasiano, D.; Oller, I.; Fernández-Calderero, I.; Agüera, A.; Malato, S. Solar photo-Fenton optimization for the treatment of MWTP effluents containing emerging contaminants. Catal. Today 2013, 209, 188–194. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.R.; Silva, A.M.T. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Couras, C.; Karim, A.V.; Nadais, H. A review of integrated advanced oxidation processes and biological processes for organic pollutant removal. Chem. Eng. Commun. 2021, 1–43. [Google Scholar] [CrossRef]
- Dhaka, S.; Kumar, R.; Lee, S.-H.; Kurade, M.B.; Jeon, B.-H. Degradation of ethyl paraben in aqueous medium using advanced oxidation processes: Efficiency evaluation of UV-C supported oxidants. J. Clean. Prod. 2018, 180, 505–513. [Google Scholar] [CrossRef]
- Dhaka, S.; Kumar, R.; Khan, M.A.; Paeng, K.-J.; Kurade, M.B.; Kim, S.-J.; Jeon, B.-H. Aqueous phase degradation of methyl paraben using UV-activated persulfate method. Chem. Eng. J. 2017, 321, 11–19. [Google Scholar] [CrossRef]
- Velegraki, T.; Hapeshi, E.; Fatta-Kassinos, D.; Poulios, I. Solar-induced heterogeneous photocatalytic degradation of methyl-paraben. Appl. Catal. B Environ. 2015, 178, 2–11. [Google Scholar] [CrossRef]
- Błędzka, D.; Gryglik, D.; Olak, M.; Gębicki, J.L.; Miller, J.S. Degradation of n-butylparaben and 4-tert-octylphenol in H2O2/UV system. Radiat. Phys. Chem. 2010, 79, 409–416. [Google Scholar] [CrossRef]
- Popiel, S.; Nalepa, T.; Dzierżak, D.; Stankiewicz, R.; Witkiewicz, Z. Rate of dibutylsulfide decomposition by ozonation and the O3/H2O2 advanced oxidation process. J. Hazard. Mater. 2009, 164, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Saritha, P.; Aparna, C.; Himabindu, V.; Anjaneyulu, Y. Comparison of various advanced oxidation processes for the degradation of 4-chloro-2 nitrophenol. J. Hazard. Mater. 2007, 149, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Zangeneh, H.; Zinatizadeh, A.; Feizy, M. A comparative study on the performance of different advanced oxidation processes (UV/O3/H2O2) treating linear alkyl benzene (LAB) production plant’s wastewater. J. Ind. Eng. Chem. 2014, 20, 1453–1461. [Google Scholar] [CrossRef]
- Siciliano, A.; Stillitano, M.A.; Limonti, C. Energetic valorization of wet olive mill wastes through a suitable integrated treatment: H2O2 with lime and anaerobic digestion. Sustainability 2016, 8, 1150. [Google Scholar] [CrossRef]
- Afify, A.S.; Mahmoud, M.A.; Emara, H.A.; Abdelkreem, K.I. Phenolic compounds and COD removal from olive mill wastewater by chemical and biological procedures. Aust. J. Basic. Appl. Sci. 2009, 3, 1087–1095. [Google Scholar]
- Martínez-Huitle, C.A.; Brillas, E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: A general review. Appl. Catal. B Environ. 2009, 87, 105–145. [Google Scholar] [CrossRef]
- Bosio, M.; Souza-Chaves, B.; Gomes, J.; Gmurek, M.; Martins, R.; Saggioro, E.; Dezotti, M.; Bassin, J.; Quinta-Ferreira, M. Electrochemical oxidation of paraben compounds and the effects of byproducts on neuronal activity. Energy Rep. 2020, 6, 903–908. [Google Scholar] [CrossRef]
- Feng, L.; Van Hullebusch, E.D.; Rodrigo, M.A.; Esposito, G.; Oturan, M.A. Removal of residual anti-inflammatory and analgesic pharmaceuticals from aqueous systems by electrochemical advanced oxidation processes. A review. Chem. Eng. J. 2013, 228, 944–964. [Google Scholar] [CrossRef]
- Frontistis, Z.; Antonopoulou, M.; Yazirdagi, M.; Kilinc, Z.; Konstantinou, I.; Katsaounis, A.; Mantzavinos, D. Boron-doped diamond electrooxidation of ethyl paraben: The effect of electrolyte on by-products distribution and mechanisms. J. Environ. Manag. 2017, 195, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Steter, J.R.; Brillas, E.; Sirés, I. On the selection of the anode material for the electrochemical removal of methylparaben from different aqueous media. Electrochim. Acta 2016, 222, 1464–1474. [Google Scholar] [CrossRef]
- Domínguez, J.R.; Muñoz-Peña, M.J.; González, T.; Palo, P.; Cuerda-Correa, E.M. Parabens abatement from surface waters by electrochemical advanced oxidation with boron doped diamond anodes. Environ. Sci. Pollut. Res. 2016, 23, 20315–20330. [Google Scholar] [CrossRef]
- Steter, J.R.; Brillas, E.; Sirés, I. Solar photoelectro-Fenton treatment of a mixture of parabens spiked into secondary treated wastewater effluent at low input current. Appl. Catal. B Environ. 2018, 224, 410–418. [Google Scholar] [CrossRef]
- Seibert, D.; Zorzo, C.F.; Borba, F.H.; de Souza, R.M.; Quesada, H.B.; Bergamasco, R.; Baptista, A.T.; Inticher, J.J. Occurrence, statutory guideline values and removal of contaminants of emerging concern by Electrochemical Advanced Oxidation Processes: A review. Sci. Total Environ. 2020, 748, 141527. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Xiong, Z.; Yao, G.; Lai, B. The electrochemical advanced oxidation processes coupling of oxidants for organic pollutants degradation: A mini-review. Chin. Chem. Lett. 2019, 30, 2139–2146. [Google Scholar] [CrossRef]
- Gomes, J.F.; Leal, I.; Bednarczyk, K.; Gmurek, M.; Stelmachowski, M.; Zaleska-Medynska, A.; Quinta-Ferreira, M.E.; Costa, R.; Quinta-Ferreira, R.M.; Martins, R.C. Detoxification of parabens using UV-A enhanced by noble metals—TiO2 supported catalysts. J. Environ. Chem. Eng. 2017, 5, 3065–3074. [Google Scholar] [CrossRef]
- Zúñiga-Benítez, H.; Peñuela, G.A. Solar lab and pilot scale photo-oxidation of ethylparaben using H2O2 and TiO2 in aqueous solutions. J. Photochem. Photobiol. A Chem. 2017, 337, 62–70. [Google Scholar] [CrossRef]
- Gomes, F.E.; Bergo, P.L.; Trap, M.A.; Spadoto, M.; Galinaro, C.A.; Rodrigues-Filho, E.; Leitão, A.; Tremiliosi-Filho, G. Photolysis of parabens using medium-pressure mercury lamps: Toxicity effects in MCF7, Balb/c 3T3 cells and Ceriodaphnia dubia. Chemosphere 2018, 208, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, M.A.; Ruidíaz-Martínez, M.; Cruz-Quesada, G.; López-Ramón, M.V.; Rivera-Utrilla, J.; Sánchez-Polo, M.; Mota, A.J. Removal of parabens from water by UV-driven advanced oxidation processes. Chem. Eng. J. 2020, 379, 122334. [Google Scholar] [CrossRef]
- Chuang, L.C.; Luo, C.H. Photocatalytic degradation of parabens in aquatic environment: Kinetics and degradation pathway. Kinet. Catal. 2015, 56, 412–418. [Google Scholar] [CrossRef]
- Frontistis, Z.; Antonopoulou, M.; Venieri, D.; Dailianis, S.; Konstantinou, I.; Mantzavinos, D. Solar photocatalytic decomposition of ethyl paraben in zinc oxide suspensions. Catal. Today 2017, 280, 139–148. [Google Scholar] [CrossRef]
- Petala, A.; Frontistis, Z.; Antonopoulou, M.; Konstantinou, I.K.; Kondarides, D.I.; Mantzavinos, D. Kinetics of ethyl paraben degradation by simulated solar radiation in the presence of N-doped TiO2 catalysts. Water Res. 2015, 81, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Petala, A.; Bontemps, R.; Spartatouille, A.; Frontistis, Z.; Antonopoulou, M.; Konstantinou, I.; Kondarides, D.I.; Mantzavinos, D. Solar light-induced degradation of ethyl paraben with CuOx/BiVO4: Statistical evaluation of operating factors and transformation by-products. Catal. Today 2017, 280, 122–131. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, G.; Naushad, M.; Kumar, A.; Kalia, S.; Guo, C.; Mola, G.T. Facile hetero-assembly of superparamagnetic Fe3O4/BiVO4 stacked on biochar for solar photo-degradation of methyl paraben and pesticide removal from soil. J. Photochem. Photobiol. Chem. 2017, 337, 118–131. [Google Scholar] [CrossRef]
- Sousa, S.C.; Cardoso, J.; Monteiro, O. Improved performance of titanate nanostructures for manganese adsorption and posterior pollutants photocatalytic degradation. J. Photochem. Photobiol. A Chem. 2019, 378, 9–16. [Google Scholar] [CrossRef]
- Klementova, S.; Kahoun, D.; Doubkova, L.; Frejlachova, K.; Dusakova, M.; Zlamal, M. Catalytic photodegradation of pharmaceuticals—Homogeneous and heterogeneous photocatalysis. Photochem. Photobiol. Sci. 2017, 16, 67–71. [Google Scholar] [CrossRef]
- Gomes, J.; Lincho, J.; Mazierski, P.; Miodyńska, M.; Zaleska-Medynska, A.; Martins, R.C. Unexpected effect of ozone on the paraben’s mixture degradation using TiO2 supported nanotubes. Sci. Total Environ. 2020, 743, 140831. [Google Scholar] [CrossRef]
- Fernandes, E.; Contreras, S.; Medina, F.; Martins, R.C.; Gomes, J. N-doped titanium dioxide for mixture of parabens degradation based on ozone action and toxicity evaluation: Percursor of nitrogen and titanium effect. Process. Saf. Environ. Prot. 2020, 138, 80–89. [Google Scholar] [CrossRef]
- Fernandes, E.; Martins, R.C.; Gomes, J. Photocatalytic ozonation of parabens mixture using 10% N-TiO2 and the effect of water matrix. Sci. Total Environ. 2020, 718, 137321. [Google Scholar] [CrossRef] [PubMed]
- Zúñiga-Benítez, H.; Aristizábal-Ciro, C.; Peñuela, G.A. Photodegradation of the endocrine-disrupting chemicals benzophenone-3 and methylparaben using Fenton reagent: Optimization of factors and mineralization/biodegradability studies. J. Taiwan Inst. Chem. Eng. 2016, 59, 380–388. [Google Scholar] [CrossRef]
- Domínguez, J.R.; Muñoz, M.J.; Palo, P.; González, T.; Peres, J.A.; Cuerda-Correa, E.M. Fenton advanced oxidation of emerging pollutants: Parabens. Int. J. Energy Environ. Eng. 2014, 5, 1–10. [Google Scholar] [CrossRef]
- Ioannidi, A.; Frontistis, Z.; Mantzavinos, D. Destruction of propyl paraben by persulfate activated with UV-A light emitting diodes. J. Environ. Chem. Eng. 2018, 6, 2992–2997. [Google Scholar] [CrossRef]
- Yang, J.-C.E.; Lin, Y.; Peng, H.-H.; Yuan, B.; Dionysiou, D.D.; Huang, X.-D.; Zhang, D.-D.; Fu, M.-L. Novel magnetic rod-like Mn-Fe oxycarbide toward peroxymonosulfate activation for efficient oxidation of butyl paraben: Radical oxidation versus singlet oxygenation. Appl. Catal. B Environ. 2020, 268, 118549. [Google Scholar] [CrossRef]
- Bekris, L.; Frontistis, Z.; Trakakis, G.; Sygellou, L.; Galiotis, C.; Mantzavinos, D. Graphene: A new activator of sodium persulfate for the advanced oxidation of parabens in water. Water Res. 2017, 126, 111–121. [Google Scholar] [CrossRef]
- Matthaiou, V.; Oulego, P.; Frontistis, Z.; Collado, S.; Hela, D.; Konstantinou, I.K.; Diaz, M.; Mantzavinos, D. Valorization of steel slag towards a Fenton-like catalyst for the degradation of paraben by activated persulfate. Chem. Eng. J. 2019, 360, 728–739. [Google Scholar] [CrossRef]
- Dionisio, D.; Motheo, A.; Sáez, C.; Rodrigo, M. Effect of the electrolyte on the electrolysis and photoelectrolysis of synthetic methyl paraben polluted wastewater. Sep. Purif. Technol. 2019, 208, 201–207. [Google Scholar] [CrossRef]
- Steter, J.R.; Rocha, R.S.; Dionísio, D.; Lanza, M.R.; Motheo, A.J. Electrochemical oxidation route of methyl paraben on a boron-doped diamond anode. Electrochim. Acta 2014, 117, 127–133. [Google Scholar] [CrossRef]
- Dionisio, D.; Santos, L.H.; Rodrigo, M.A.; Motheo, A.J. Electro-oxidation of methyl paraben on DSA®-Cl2: UV irradiation, mechanistic aspects and energy consumption. Electrochim. Acta 2020, 338, 135901. [Google Scholar] [CrossRef]
- Pueyo, N.; Ormad, M.P.; Miguel, N.; Kokkinos, P.; Ioannidi, A.; Mantzavinos, D.; Frontistis, Z. Electrochemical oxidation of butyl paraben on boron doped diamond in environmental matrices and comparison with sulfate radical-AOP. J. Environ. Manag. 2020, 269, 110783. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.E.; de Souza, N.E.; Galinaro, C.A.; Arriveti, L.O.; de Assis, J.B.; Tremiliosi-Filho, G. Electrochemical degradation of butyl paraben on platinum and glassy carbon electrodes. J. Electroanal. Chem. 2016, 769, 124–130. [Google Scholar] [CrossRef]
- Rosales, E.; Buftia, G.; Pazos, M.; Lazăr, G.; Sanromán, M. Ángeles Highly active based iron-carbonaceous cathodes for heterogeneous electro-Fenton process: Application to degradation of parabens. Process. Saf. Environ. Prot. 2018, 117, 363–371. [Google Scholar] [CrossRef]
- Nian, P.; Peng, L.; Feng, J.; Han, X.; Cui, B.; Lu, S.; Zhang, J.; Liu, Q.; Zhang, A. Aqueous methylparaben degradation by dielectric barrier discharge induced non-thermal plasma combined with ZnO-rGO nanosheets. Sep. Purif. Technol. 2019, 211, 832–842. [Google Scholar] [CrossRef]
- Sasi, S.; Rayaroth, M.P.; Devadasan, D.; Aravind, U.K.; Aravindakumar, C.T. Influence of inorganic ions and selected emerging contaminants on the degradation of Methylparaben: A sonochemical approach. J. Hazard. Mater. 2015, 300, 202–209. [Google Scholar] [CrossRef]
- Zanias, A.; Frontistis, Z.; Vakros, J.; Arvaniti, O.S.; Ribeiro, R.S.; Silva, A.M.; Faria, J.L.; Gomes, H.T.; Mantzavinos, D. Degradation of methylparaben by sonocatalysis using a Co–Fe magnetic carbon xerogel. Ultrason. Sonochem. 2020, 64, 105045. [Google Scholar] [CrossRef]
- Gomes, J.F.; Frasson, D.; Pereira, J.L.; Gonçalves, F.J.; Castro, L.M.; Quinta-Ferreira, R.M.; Martins, R.C. Ecotoxicity variation through parabens degradation by single and catalytic ozonation using volcanic rock. Chem. Eng. J. 2019, 360, 30–37. [Google Scholar] [CrossRef]
- Gomes, J.F.; Lopes, A.; Bednarczyk, K.; Gmurek, M.; Stelmachowski, M.; Zaleska-Medynska, A.; Quinta-Ferreira, M.E.; Costa, R.; Quinta-Ferreira, R.M.; Martins, R.C. Effect of noble metals (Ag, Pd, Pt) loading over the efficiency of TiO2 during photocatalytic ozonation on the toxicity of parabens. ChemEngineering 2018, 2, 4. [Google Scholar] [CrossRef]
- Molins-Delgado, D.; Díaz-Cruz, M.S.; Barceló, D. Ecological risk assessment associated to the removal of endocrine-disrupting parabens and benzophenone-4 in wastewater treatment. J. Hazard. Mater. 2016, 310, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.I.; Soares, T.F.; Silva, T.F.; Boaventura, R.A.; Vilar, V.J. Ozone-driven processes for mature urban landfill leachate treatment: Organic matter degradation, biodegradability enhancement and treatment costs for different reactors configuration. Sci. Total Environ. 2020, 724, 138083. [Google Scholar] [CrossRef] [PubMed]
- Buthiyappan, A.; Raman, A.A.A. Energy intensified integrated advanced oxidation technology for the treatment of recalcitrant industrial wastewater. J. Clean. Prod. 2019, 206, 1025–1040. [Google Scholar] [CrossRef]
- Gomes, J.F.; Quinta-Ferreira, M.E.; Costa, R.; Quinta-Ferreira, R.M.; Martins, R.C. Paraben degradation using catalytic ozonation over volcanic rocks. Environ. Sci. Pollut. Res. 2017, 25, 7346–7357. [Google Scholar] [CrossRef] [PubMed]
| Oxidant Species | Oxidation Potential/[V] |
|---|---|
| Hydroxyl (OH) | 2.80 |
| Fluorine (F) | 3.03 |
| Ozone (O3) | 2.07 |
| Sulfate (SO42−) | 2.01 |
| Hydrogen Peroxide (H2O2) | 1.77 |
| Permanganate (MnO42−) | 1.77 |
| Chlorine dioxide (ClO2) | 1.57 |
| Chlorine (Cl2) | 1.36 |
| Dichromate (Cr2O72−) | 1.23 |
| Oxygen (O2) | 1.23 |
| Pollutant | Test Conditions | Results and Conclusions | Reference |
|---|---|---|---|
| Bisphenol A, Bisphenol B, Diamylphthalate, Butylbenzylphthalate, MP, and EP |
|
| [3] |
| MP |
|
| [7] |
| BeP |
|
| [32] |
| MP, EP, PP, BuP, BeP, and p-HBA |
|
| [35] |
| MP, EP, and PP |
|
| [58] |
| MP, EP, PP, BuP, and BeP |
|
| [91] |
| EP |
|
| [92] |
| PP and BuP |
|
| [93] |
| MP, EP and BuP |
|
| [94] |
| EP and BuP |
|
| [95] |
| Pollutant | Catalyst | Test Conditions | Results and Conclusions | Reference |
|---|---|---|---|---|
| Bisphenol A, Bisphenol B, Diamylphthalate, Butylbenzylphthalate, MP, and EP | TiO2 P25 TiO2 Vlp-7000 |
|
| [3] |
| MP, EP, and PP | Metal free graphite-like carbon nitride TiO2 P25 |
|
| [6] |
| MP | TiO2 P25 |
|
| [7] |
| MP | Monolith-supported perovskite |
|
| [31] |
| BeP | TiO2 |
|
| [32] |
| MP, EP, PP, and BuP | I-Bi4O5Br2 |
|
| [33] |
| PP | Al-TiO2 |
|
| [34] |
| MP, EP and PP | TiO2/NT |
|
| [58] |
| MP, EP, PP, BuP, BeP | TiO2 Ag-TiO2 Au-TiO2 Pd-TiO2 Pt-TiO2 |
|
| [91] |
| EP | TiO2 |
|
| [92] |
| EP and BuP | TiO2 P25 |
|
| [95] |
| EP | ZnO TiO2 P25 |
|
| [96] |
| EP | N-TiO2 |
|
| [97] |
| EP | CuOx/BiVO4 |
|
| [98] |
| MP and pesticide | Fe3O4/BiVO4 |
|
| [99] |
| MP | TNW Mn-TNW Mn/TNW |
|
| [100] |
| MP, EP, and PP | Fe ions |
|
| [101] |
| Pollutant | Test Conditions | Results and Conclusions | Reference |
|---|---|---|---|
| MP, EP, PP and BuP |
|
| [23] |
| MP, EP, PP, BuP, and BeP |
|
| [25] |
| MP, EP, PP, BuP and BeP |
|
| [26] |
| MP, EP, PP, BuP and BeP |
|
| [28] |
| MP, EP and PP |
|
| [102] |
| MP, EP, PP, BuP and BeP |
|
| [103] |
| Pollutant | AOP | Catalyst | Test Conditions | Results and Conclusions | Reference |
|---|---|---|---|---|---|
| MP, EP, PP, and BuP | Photolytic ozonation Photocatalytic ozonation Other ozone techniques | TiO2 |
|
| [23] |
| MP, EP, PP, BuP, and BeP | Catalytic ozonation | TiO2 Au-TiO2 Ag-TiO2 Pd-TiO2 Pt-TiO2 |
|
| [26] |
| MP, EP, PP, BuP and BeP | Photocatalytic Ozonation | TiO2 Ag-TiO2 Au-TiO2 Pd-TiO2 Pt-TiO2 |
|
| [27] |
| MP, EP, PP, BuP and BeP | Photolytic Ozonation Photocatalytic Ozonation | ZnO |
|
| [28] |
| MP, EP, and PP | Photocatalytic ozonation | TiO2/NT |
|
| [102] |
| MP, EP, PP, BuP, and BeP | Photocatalytic ozonation | N-TiO2 TiO2 |
|
| [103] |
| MP, EP, PP, BuP, and BeP | Photocatalytic ozonation | N-TiO2 |
|
| [104] |
| Pollutant | AOP | Iron Source | Test Conditions | Results and Conclusions | Reference |
|---|---|---|---|---|---|
| MP, EP, PP, BuP, and BeP | Fenton | Iron (II) sulfate heptahydrated |
|
| [30] |
| 3-Benzophenone and MP | Fenton | Iron (II) chloride tetrahydrate |
|
| [105] |
| MP, EP, PP, and BuP | Fenton | Iron (II) sulfate heptahydrated |
|
| [106] |
| Pollutant | AOP | PS/PMS/H2O2 Source | Test Conditions | Results and Conclusions | Reference |
|---|---|---|---|---|---|
| MP, EP, PP, BuP, BeP, and p-HBA | H2O2 | H2O2 (30%) |
|
| [35] |
| EP | UV/H2 O2UV/PS UV/PMS | H2O2 (30%) Sodium persulfatePotassium monoperoxysulfate |
|
| [73] |
| MP | UV/PS | Sodium persulfate |
|
| [74] |
| PP | UV/PS | Sodium persulfate |
|
| [107] |
| BuP | PMS oxidation | Potassium monoperoxysulfate |
|
| [108] |
| PP | PS oxidation | Sodium persulfate |
|
| [109] |
| PP | PS oxidation | Sodium persulfate |
|
| [110] |
| Pollutant | AOP | Material | Test Conditions | Results and Conclusions | Reference |
|---|---|---|---|---|---|
| PP | Photo-Electrocatalysis | TiO2/NT WO3-TiO2/NT |
|
| [36] |
| MP, EP, PP, BuP, and BeP | Electrochemical oxidation | Ti/Pt anode |
|
| [83] |
| EP | Electrochemical Oxidation | BDD anode |
|
| [85] |
| MP, EP, PP, and BuP | Electrolysis | BDD anode |
|
| [87] |
| MP, EP and PP | Photoelectro-Fenton | BDD anode RuO2 anode |
|
| [88] |
| MP | Electrolysis Photo-electrolysis | BDD anode |
|
| [111] |
| MP | Electrochemical oxidation | BDD anode |
|
| [112] |
| MP | Electrochemical oxidation Photo-electrolysis | DSA-Cl2 of Ti/Ru0.3Ti0.7O2 anode |
|
| [113] |
| BuP | Electrochemical Oxidation | BDD Stainless-steelPlatinum |
|
| [114] |
| BuP | Electrochemical Oxidation | Platinum Glassy carbon |
|
| [115] |
| MP | Electro-FentonAnodic Oxidation | BDD anode |
|
| [116] |
| MP | Dielectric barrier discharge induced non-thermal plasma | ZnO-rGO nanosheets |
|
| [117] |
| Pollutant | AOP | Catalyst | Test Conditions | Results and Conclusions | Reference |
|---|---|---|---|---|---|
| BuP | Photosonochemical | (no catalyst) |
|
| [37] |
| PP | Sonochemical | (no catalyst) |
|
| [38] |
| MP | Sonochemical | (No catalyst) |
|
| [118] |
| MP | Sonocatalysis | Bimetallic Co-Fe carbon xerogel |
|
| [119] |
| Pollutant | Organism | AOP | Results | Reference |
|---|---|---|---|---|
| Bisphenol A, Bisphenol B, Diamylphthalate, Butylbenzylphthalate, Methylparaben, and Ethylparaben |
|
|
| [3] |
| MP, EP, PP, BuP and BeP |
|
|
| [26] |
| MP, EP, PP, BuP, and BeP |
|
|
| [27] |
| MP, EP, PP, BuP, and BeP |
|
|
| [29] |
| MP, EP, PP, BuP, and BeP |
|
|
| [30] |
| MP |
|
|
| [31] |
| MP |
|
|
| [75] |
| MP, EP, PP, BuP and BeP |
|
|
| [91] |
| PP and BuP |
|
|
| [93] |
| MP, EP, and PP |
|
|
| [102] |
| MP, EP, PP, BuP, and BeP |
|
|
| [103] |
| MP, EP, PP, BuP, and BeP |
|
|
| [104] |
| MP, EP, PP, BuP, and BeP |
|
|
| [120] |
| MP, EP, PP, BuP, and BeP |
|
|
| [121] |
| MP, PP, BuP, BeP and 4-benzophenone |
|
|
| [122] |
| Treatment | Contaminant | Associated Costs | Observations | Reference |
|---|---|---|---|---|
| Photocatalysis w/ZnO | MP, EP, Bisphenol A, Bisphenol B, Diamylphthalate and Butyl-benzylphthalate | 103 €/m3 |
| [3] |
| Photocatalysis w/TiO2 P25 | MP, EP, Bisphenol A, Bisphenol B, Diamylphthalate and Butyl-benzylphthalate | 149 €/m3 |
| [3] |
| Photocatalysis w/TiO2 vlp 7000 | MP, EP, Bisphenol A, Bisphenol B, Diamylphthalate and Butyl-benzylphthalate | 285 €/m3 |
| [3] |
| UVC | MP, EP, PP, BuP, BeP and p-HBA | 7 €/gEDC |
| [35] |
| UVC/H2O2 | MP, EP, PP, BuP, BeP and p-HBA | 0.5 €/gEDC |
| [35] |
| UV/H2O2 | EP | 5.93 €/m3 |
| [73] |
| UV/PS | EP | 5.26 €/m3 |
| [73] |
| UV/PMS | EP | 12.28 €/m3 |
| [73] |
| UV/PS | MP | 6.92 €/m3 |
| [74] |
| Electrocatalysis | MP | 0.19–4.59 €/kg |
| [112] |
| Electrocatalysis | MP | 0.03–0.92 €/m3 |
| [112] |
| DBD plasma with ZnO-rGO nanosheets | MP | 0.01–0.14 €/g |
| [117] |
| Photocatalysis | MP | 84 €/g |
| [117] |
| Photocatalysis | EP | 166.25–443.33 €/g |
| [97,117] |
| Ultrasonic | MP | 570–1050 €/g |
| [117,118] |
| Ozonation w/Vulcanic rocks | MP, EP, PP, BeP, and BuP | 0.08 €/m3 |
| [125] |
| Ozonation | MP, EP, PP, BeP, and BuP | 0.26 €/m3 |
| [125] |
| O3 + UVA | MP, EP, PP, BeP, and BuP | 1.91 €/m3 |
| [125] |
| O3 + TiO2 | MP, EP, PP, BeP, and BuP | 0.11 €/m3 |
| [125] |
| O3 + TiO2 + UVA | MP, EP, PP, BeP, and BuP | 1.20 €/m3 |
| [125] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lincho, J.; Gomes, J.; Martins, R.C. Paraben Compounds—Part II: An Overview of Advanced Oxidation Processes for Their Degradation. Appl. Sci. 2021, 11, 3556. https://doi.org/10.3390/app11083556
Lincho J, Gomes J, Martins RC. Paraben Compounds—Part II: An Overview of Advanced Oxidation Processes for Their Degradation. Applied Sciences. 2021; 11(8):3556. https://doi.org/10.3390/app11083556
Chicago/Turabian StyleLincho, João, João Gomes, and Rui C. Martins. 2021. "Paraben Compounds—Part II: An Overview of Advanced Oxidation Processes for Their Degradation" Applied Sciences 11, no. 8: 3556. https://doi.org/10.3390/app11083556
APA StyleLincho, J., Gomes, J., & Martins, R. C. (2021). Paraben Compounds—Part II: An Overview of Advanced Oxidation Processes for Their Degradation. Applied Sciences, 11(8), 3556. https://doi.org/10.3390/app11083556








